Abstract
Aim
We explored the potential for over-compression from current paediatric chest compression depth guidelines using chest computed tomography(CT) images of a large, heterogenous, Asian population.
Methods
A retrospective review of consecutive children, less than 18-years old, with chest CT images performed between from 2005 to 2017 was done. Demographic data were extracted from the electronic medical records. Measurements for internal and external anterior-posterior diameters (APD) were taken at lower half of the sternum. Simulated chest compressions were performed to evaluate the proportion of the population with residual internal cavity dimensions less than 0 mm (RICD < 0 mm, representing definite over-compression; with chest compression depth exceeding internal APD), and RICD less than 10 mm (RICD < 10 mm, representing potential over-compression).
Results
592 paediatric chest CT studies were included for the study. Simulated chest compressions of one-third external APD had the least potential for over-compression; no infants and 0.3% children had potential over-compression (RICD < 10 mm). 4 cm simulated chest compressions led to 18% (95% CI 13%–24%) of infants with potential over-compression, and this increased to 34% (95% CI 27%–41%) at 4.4 cm (upper limit of “approximately” 4 cm; 4 cm + 10%). 5 cm simulated compressions resulted in 8% (95% CI 4%–12%) of children 1 to 8-years-old with potential over-compression, and this increased to 22% (95% CI 16%–28%) at 5.5 cm (upper limit of “approximately” 5 cm, 5 cm + 10%).
Conclusion
In settings whereby chest compression depths can be accurately measured, compressions at the current recommended chest compression of approximately 4 cm (in infants) and 5 cm (in young children) could result in potential for over-compression.
Keywords: Cardiopulmonary resuscitation, Chest compression, Computed tomography, Pediatrics
Introduction
High quality chest compressions of sufficient depth remain one of the key factors to improve survival and neurobehavioural outcomes following cardiac arrest.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Current International Liaison Committee on Resuscitation (ILCOR) paediatric chest compression depth recommendations advocates chest compression to be at least one-third external anterior-posterior diameter (APD), or approximately 4 cm in infants and 5 cm in children.1, 2, 3, 4, 5, 6, 7, 8 This recommendation was informed by retrospective observational paediatric studies,12, 13 paediatric chest computed tomography (CT) studies,14, 15 and extrapolation from adult data.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Chest compression should ideally be to the point where no further cardiac output is achievable with increased compression depth, without causing damage to the internal organs.14, 15, 16, 17
Some adult studies have observed potential harm in over-compression,17 which has not previously been studied in the paediatric population. Recently published chest CT studies suggested that for Asian children, current paediatric chest compression recommendations may be too deep in the pre-adolescent age group.18, 19, 20 For older Asian children and adolescents, it is unknown how age cut-offs for the use of adult life support guidelines might affect the potential for over-compression. Prior findings of paediatric chest CT studies on the potential for over-compression were limited by the relatively small datasets for infants, and potential ethnic differences in body habitus between paediatric populations worldwide. We hypothesised that current paediatric chest compression depth guidelines might result in significant over-compression in Asian infants, children and adolescents. We explored this radiologically by using an Asian tertiary paediatric hospital’s large databank of paediatric chest CT images.
Our primary aim was to explore the proportion of Asian infants, children and adolescents with potential for over-compression by simulating chest compressions on chest CT images at target depths and age-groups recommended by current paediatric chest compression guidelines. Our secondary aim was to compare how the minor variations in paediatric chest compression depth targets and age-group definitions using selected international, regional and local paediatric chest compression guidelines might result in potential over-compression in Asian infants, children and adolescents radiologically.
Methods
Setting and study design
Singapore has a heterogenous population with Chinese, Malays, Indians, and other minority races. This study was a retrospective, quantitative study of chest CT scans performed on patients aged below 18-years of age for medical purposes in KK Women’s and Children’s Hospital in Singapore from 2005 to 2017, with Institutional Review Board approval with waiver of informed consent (Reference Number 2017/3131). We used a convenience sample of a large database of consecutive paediatric chest CTs as there were no prior studies in our local population (Singapore) that studied the potential of over-compression using current paediatric chest compression guidelines.
Inclusion and exclusion criteria
All chest CT scans of consecutive patients below 18-years of age performed from 1 January 2005 to 31 December 2017 were obtained via the institutional electronic database records with measurements done using built-in Vue Motion Image Viewer® were included in the study.
Repeat chest scans of patients were excluded if they were performed within two months of the index chest CT scan. Chest CT studies were also evaluated and excluded if they had pathologies which may affect measurements. These included studies with significant chest wall deformities, significant plural or intrathoracic pathologies, and technical limitations (inability to identify chest borders or landmarks). The patients’ demographic data were extracted from electronic databases based on the date of CT scans conducted.
Data collection and measurements
Epidemiological data were extracted from the electronic medical records during the period when the scans were performed.
This study employed the same methodology used in previous landmark papers for identifying CT chest landmarks and measurements.14, 15 Please refer to Fig. 1 and Appendix A (supplementary material).
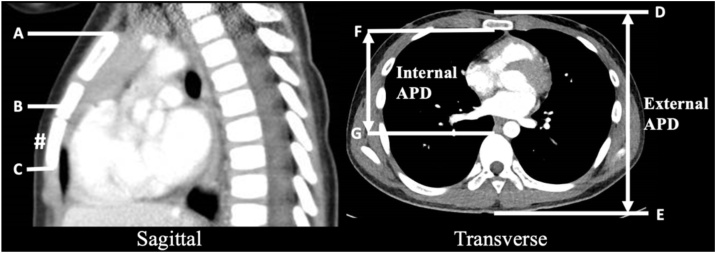
Fig. 1.
Measurements taken from axial and sagittal CT images14, 15, 16, 18, 19, 20.
Mid-sternum (B) is the midpoint between superior edge of bony sternum (A) and inferior edge of bony sternum (C) on sagittal CT image.
Lower half of the sternum (#) is the lower half of the sternum which lies between B and C on sagittal CT image.
External anterior-posterior diameter (APD) is the distance between the skin anterior to the sternum (D) and the skin overlying the tip of the spinous process (E) on axial CT image.
Internal anterior-posterior diameter (APD) is the distance between posterior margin of the sternum (F) and the anterior margin of the vertebral body (G) on axial CT image.
Simulated chest compression: Compression simulated at a specific depth, measured from anterior chest wall (D) on axial CT image.
Using CT chest imaging of a heterogenous, Asian, paediatric population, we measured the external and internal APD at the middle of the lower-half of the sternum4, 5, 6, 7, 8 and compared them with current paediatric chest compression depth guidelines. The internal APD represented the internal chest cavity (between sternum and anterior vertebra) which could be potentially compressible.
Training and audit of radiological measurements by non-radiologist investigators
The non-radiologist investigators were guided by a senior radiologist on taking the measurements for data collection. An audit using a random sampling of 10% of data collected were cross-referenced with a senior radiologist to ensure that their measurements correlated adequately.
Assessment of potential for over-compression radiologically
To assess the potential for over-compression, we studied the residual internal cavity dimension (RICD) which was the residual chest depth after a simulated compression (as determined by the methodology in Appendix A). Based on prior radiological studies, CT scans of patients with RICD of less than 10 mm (RICD < 10 mm) were simulated considered probably too deep as this could potentially result in internal organ injuries.14, 15, 16, 18, 19, 20 Simulated chest compressions that exceeded the internal chest cavity size (internal APD) were reported as RICD less than 0 mm (RICD < 0 mm) which represented definite over-compression. We prospectively defined RICD < 10 mm as “potential over-compression” and RICD<0 mm as “definite over-compression”.
The recommended age cut-off for considering using adult basic life support guidelines based on resuscitation guidelines from Australia and New Zealand Resuscitation Committee on Resuscitation (ANZCOR)6 and Singapore Resuscitation and First Aid Council (SRFAC)7, 9 was 8 years old. On the other hand, American Heart Association (AHA)4 and the European Resuscitation Council (ERC)5 suggested that adult chest compression guidelines could be considered if the paediatric cardiac arrest victims had signs of puberty. Since there is an age range for puberty, we arbitrarily standardised this to be synonymous with more than 12 years of age.
To study our primary aim, we analysed the proportion of infants with potential for over-compression at simulated chest compression depths at one-third external APD, 3 cm, 4 cm, and 5 cm at the lower half of the sternum. For children above one-years-old, simulated chest compression depth at one-third external APD, 4 cm, 5 cm, and 6 cm were used to evaluate evaluated the proportion of children with potential for over-compression (RICD < 10 mm) or definite over-compression (RCID < 0 mm). Two age sub-groups of a) “1−8 years” and “>8 years”, and b) “1−12 years” and “>12 years” in children were used for subgroup analysis.
For our secondary aim, we used selected paediatric basic resuscitation guidelines from North America,4 Europe,5 Australia,6 Singapore,7, 9 Japan,10 and South Korea11 to evaluate the number of patients with potential over-compression (RICD < 10 mm) and definite over-compression (RICD < 0 mm) when simulated chest compressions were delivered to achieve these recommended chest compression depth targets (range). Based on prior paediatric chest compression target studies,21 “approximately” was set at plus or minus 10% (±10%) of the set target depth. The potential for over-compression was evaluated at simulated chest compression depth using these guidelines. This would be approximately one-third external APD (±10%), one-third external to less than half external APD for all paediatric patients. For infants, absolute simulated chest compression depth range of 3–4 cm, and approximately 4 cm (4 cm ± 10%, i.e. 3.6–4.4 cm) were evaluated. For children above one-year-old, simulated chest compression depth range of 4–5 cm, approximately 5 cm (5 cm ± 10%, i.e. 4.5–5.5 cm), 4–6 cm, and 5–6 cm were evaluated.
Statistical analysis
Demographic and clinical characteristics were summarised by mean and standard deviation (SD) for continuous variables with approximately normal distribution, and frequency and percentage for categorical variables.
For the audit, intra-class correlation coefficient methodology was used to assess correlation within a random 10% sample of films, to assess if non-radiologist investigators’ measurements correlated sufficiently with an “expert” (senior radiologist).
Potential over-compression (RICD < 10 mm) and definite over-compression (RICD < 0 mm) were summarised by frequency, percentage and the corresponding 95% confidence interval based on various paediatric resuscitation guidelines for infants and children as defined above. Comparison of mean internal and external APD between age groups of “1−8” and “1−12 years” and between “>8 years” and “>12 years” was achieved by using mixed-effects linear regression. Similarly, mixed-effects logistic regression has been adopted to compare potential over-compression (RICD < 10 mm) and definite over-compression (RICD < 0 mm) at various simulated chest compression depths between age groups of “1−8 years” and “1−12 years” and between “>8 years” and “>12 years”.
All statistical analyses were performed using Stata/SE 16.1 (StataCorp. LP, College Station, TX USA) with a two-sided test of 5% statistical significance.
Results
Study population
A total of 978 chest CT scans (700 scans of children and 278 scans of infants) were reviewed. 301 scans for children and 85 scans for infants were rejected due to chest wall deformities (pectus excavatum, pectus carinatum, and thoracic masses), or inability to identify chest borders or landmarks on the CT scans. 592 CT scans for 399 children and 193 scans for infants were finally included for the study.
Demographic data and chest depth measurements
Of the chest CT scans of 193 infants and 399 children, 107 (55.4%) of the infants and 201 (50.4%) of the children were male (Table 1). Other demographic data of the studied population were described in Table 1.
Table 1.
Demographics and clinical characteristics of patients.
| Infants (<1-year-old) (n = 193) | Children (≥1-year-old) (n = 399) | |
|---|---|---|
| Age | ||
| Age (years) for children, mean (SD, range) | – | 8.53 (4.95, 1−17) |
| Age distribution for children, n (%) | ||
| 1−8 years | – | 197 (49.4) |
| >8−12 years | – | 69 (17.3) |
| >12 years | – | 133 (33.3) |
| Gender, n (%) | ||
| Female | 86 (44.6) | 198 (49.6) |
| Male | 107 (55.4) | 201 (50.4) |
| Race, n (%) | ||
| Chinese | 96 (49.7) | 254 (63.7) |
| Malay | 43 (22.3) | 72 (18.0) |
| Indian | 23 (11.9) | 34 (8.5) |
| Others | 31 (16.1) | 39 (9.8) |
| Computed tomographic chest dimensions | ||
| External APD at lower half of sternum (mm), mean (SD) | 101.89 (14.10) | 155.34 (28.51) |
| Internal APD at lower half of sternum (mm), mean (SD) | 57.54 (8.13) | 80.69 (13.86) |
Legend: APD – anterior-posterior diameter, SD – standard deviation.
For infants, the mean external and internal APD at the lower half of the sternum were 101.89 mm (SD 14.10) and 57.54 mm (SD 8.13) respectively (Table 1). In children 1-year and older, the mean external and internal APD at the lower half of the sternum were 155.34 (SD 28.51) and 80.69 mm (SD 13.86) respectively.
The internal and external APD measurements at the lower half of the sternum for age subgroups of children one-year-old (“1−8years” vs “1−12 years” and “>8 years” vs “>12 years” were reported in Table 2. There was statistically significant difference between the age subgroups in children for all APD measurements.
Table 2.
Summary of internal and external APD at lower half of sternum for children by age group.
| 1−8 years | 1−12 years | p value | >8 years | >12 years | p Value | |
|---|---|---|---|---|---|---|
| Mean internal APD, mm (SD) | 72.01 (8.77) | 75.11 (10.97) | <0.001 | 89.15 (12.62) | 91.85 (12.21) | <0.001 |
| Mean external APD, mm (SD) | 134.07 (13.51) | 141.61 (20.67) | <0.001 | 176.09 (23.57) | 182.81 (21.29) | <0.001 |
Legend: APD – anterior-posterior diameter, SD – standard deviation.
Mean one-third external APD of infants was 33.96 mm (SD 4.70). The mean one-third external APD in children 1−8 years compared to 1−12 years were 44.69 mm (SD 4.50) and 47.20 mm (SD 6.89) respectively. For children “>8 years” compared to children “>12 years”, the mean one-third external APD were 58.70 mm (SD 7.86) and 60.94 mm (SD 7.10) respectively.
Correlation between radiological measurements in the audit sample made by non-radiologist investigators and senior radiologist
The mean difference between measurements for external APD was −1.91 mm (intra-class correlation coefficient of 0.98, 95%CI 0.91 to 0.99) and −2.91 mm for internal APD (intra-class correlation coefficient of 0.78, 95%CI 0.37 to 0.93), which demonstrated moderate to excellent correlation.
Potential for over-compression at relative and absolute chest compression depths
When chest compressions of one-third external APD were simulated at the lower half of the sternum, no infants and only 1 child (0.3%) had potential over-compression; none had definite over-compression (Table 3).
Table 3.
Residual internal chest dimension (RICD) after simulated chest compression at lower half of sternum.
| Infants (<1-year-old) | RICD<10 mm, n (%, 95%CI) | RICD<0 mm, n (%, 95%CI) | ||||||||
| Simulated chest compression depth 1/3 external APD | 0 (0, 0−2) | 0 (0, 0−2) | ||||||||
| 3 cm | 2 (1, 0.1−4) | 0 (0, 0−2) | ||||||||
| 4 cm | 35 (18, 13−24) | 2 (1, 0.1−4) | ||||||||
| 5 cm | 120 (62, 55−69) | 35 (18, 13−24) | ||||||||
| Children (≥1-year-old) | RICD < 10 mm, n (%, 95%CI) | RICD < 0 mm, n (%, 95%CI) | RICD < 10 mm, n (%, 95%CI) | RICD < 0 mm, n (%, 95%CI) | RICD < 10 mm, n (%, 95%CI) | RICD < 0 mm, n (%, 95%CI) | RICD < 10 mm, n (%, 95%CI) | RICD < 0 mm, n (%, 95%CI) | ||
| Children age group 1 | Children age group 2 | |||||||||
| Simulated chest compression depth | 1−8 years (n = 197) | >8 years (n = 202) | 1−12 years (n = 266) | >12 years (n = 133) | ||||||
| 1/3 external APD | 1 (1, 0.01−3) | 0 (0, 0−2) | 0 (0, 0−2) | 0 (0, 0−2) | 1 (0.4, 0.01−2) | 0 (0, 0−1) | 0 (0, 0−3) | 0 (0, 0−3) | ||
| 4 cm | 2 (1, 0.1−4) | 0 (0, 0−2) | 0 (0, 0−2) | 0 (0, 0−2) | 2 (1, 0.1−3) | 0 (0, 0−1) | 0 (0, 0−3) | 0 (0, 0−3) | ||
| 5 cm | 15 (8, 4−12) | 2 (1, 0.1−4) | 0 (0, 0−2) | 0 (0, 0−2) | 15 (6, 3−9) | 2 (1, 0.1−3) | 0 (0, 0−3) | 0 (0, 0−3) | ||
| 6 cm | 81 (41, 34−48) | 15 (8, 4−12) | 10 (5, 2−9) | 0 (0, 0−2) | 88 (33, 27−39) | 15 (6, 3−9) | 3 (2, 0.5−6) | 0 (0, 0−3) | ||
Legend: APD – anterior-posterior diameter, RICD – residual internal chest dimension.
Values are n (%, 95% CI).
Percentages are in bold if more than ≥5% of age group.
A simulated 4 cm chest compression led to 35 infants (18%,95%CI 13% to 24%) with potential over-compression, of which 2 infants (1%, 95%CI 0.1% to 4%) had definite over-compression (Table 3). The number of infants with both potential and definite over-compression increased significantly beyond 4 cm.
For children above 1-year-old, a simulated 5 cm chest compression led to 15 children (8%, 95%CI 4% to 12%) with potential over-compression and 2 children (1%, 95%CI 0.1% to 4%) with definite over-compression both age groups 1−8 years and 1−12 years old.
Simulated chest compressions at selected resuscitation council guidelines compression depth targets
A comparison of target chest compression depth targets using selected national resuscitation council guidelines were used to look at the proportion of the studied paediatric population with potential for over-compression and definite over-compression (Table 4).
Table 4.
Simulated chest compression and associated over-compression using pediatric basic life support guidelines from selected resuscitation councils.
| Basic life support guidelines | Pediatric2, 3, 4, 16, 18 | Pediatric17 | Infant16 | Infant2, 3, 4 | Child16, 18 | Child2, 3, 4/Adult18 | Adult16 | Adult5 |
| AHA/ANZCOR/ERC/Korea/Singapore | Japan, ANZCOR | Singapore | AHA/ANZCOR/ERC | Singapore Korea | AHA/ANZCOR/ERC: Pediatric - Korea: Adult |
Singapore | AHA/ANZCOR/ERC/Japan | |
| Age group | 1/3 to <1/2 ext APDa(≥1/3 ext APD) | Approximately 1/3 ext APD (±10%) | 3 to 4 cm | Approximately 4 cm (±10%) (3.6 to 4.4 cm) | 4 to 5 cm | Approximately 5 cm (±10%) (4.5 to 5.5 cm) | 4 to 6 cm | 5 to 6 cm |
| Infants <1 year old | ||||||||
| RCID <10 mm | 0 (0, 0−2) to 141 (73, 66−79) | 0 (0, 0−2) to 3 (2, 0.3−4) | 2 (1, 0.1−4) to 35 (18, 13−24) | 15 (8, 4−12) to 65 (34, 27−41) | – | – | – | – |
| RCID <0 mm | 0 (0, 0−2) to 8 (4, 2−8) | 0 (0, 0−2) to0 (0, 0−2) | 0 (0, 0−2) to 2 (1, 0.1−4) | 1 (1, 0.01−3) to 8 (4, 2−8) | – | – | – | – |
| Children 1–8 years old | ||||||||
| RCID <10 mm | 1 (1, 0.01−3) to 157 (80, 73−85) | 0 (0, 0−2) to2 (1, 0.1−4) | – | – | 2 (1, 0.1−4) to 15 (8, 4−12) | 3 (2, 0.3−4) to 43 (22, 16−28) | 2 (1, 0.1−4) to 81 (41, 34−48) | 15 (8, 4−12) to 81 (41, 34−48) |
| RCID <0 mm | 0 (0, 0−2) to 25 (13, 8−18) | 0 (0, 0−2) to 0 (0, 0−2) | – | – | 0 (0, 0−2) to 2 (1, 0.1−4) | 0 (0, 0−2) to 2 (1, 0.1−4) | 0 (0, 0−2) to 15 (8, 4−12) | 2 (1, 0.1−4) to 15 (8, 4−12) |
| Children 1−12 years old | ||||||||
| RCID <10 mm | 1 (0.4, 0.01−2) to 213 (80, 75−85) | 0 (0, 0−1) to 2 (1, 0.1−3) | – | – | 2 (1, 0.1−3) to 15 (6, 3−9) | 3 (1, 0.2−3) to 44 (17, 12−22) | 2 (1, 0.1−3) to 88 (33, 27−39) | 15 (6, 3−9) to 88 (33, 27−39) |
| RCID <0 mm | 0 (0, 0−1) to 45 (17, 13−22) | 0 (0, 0−1) to 0 (0, 0−1) | – | – | 0 (0, 0−1) to 2 (1, 0.1−3) | 0 (0, 0−1) to 2 (1, 0.1−3) | 0 (0, 0−1) to 15 (6, 3−9) | 2 (1, 0.1−3) to 15 (6, 3−9) |
| Children >8 years old | ||||||||
| RCID <10 mm | 0 (0, 0−2) to 171 (85, 79−89) | 0 (0, 0−2) to 3 (1, 0.3−4) | – | – | 0 (0, 0−2) to 0 (0, 0−2) | 0 (0, 0−2) to 4 (2, 1−5) | 0 (0, 0−2) to 10 (5, 2−9) | 0 (0, 0−2) to 10 (5, 2−9) |
| RCID <0 mm | 0 (0, 0−2) to 74 (37, 30−44) | 0 (0, 0−2) to 0 (0, 0−2) | – | – | 0 (0, 0−2) to 0 (0, 0−2) | 0 (0, 0−2) to 0 (0, 0−2) | 0 (0, 0−2) to 0 (0, 0−2) | 0 (0, 0−2) to 0 (0, 0−2) |
| Children >12 years old | ||||||||
| RCID <10 mm | 0 (0, 0−3) to 115 (86, 79−92) | 0 (0, 0−3) to 3 (2, 0.5−6) | – | – | 0 (0, 0−3) to 0 (0, 0−3) | 0 (0, 0−3) to 3 (2, 0.5−6) | 0 (0, 0−3) to 3 (2, 0.5−6) | 0 (0, 0−3) to 3 (2, 0.5−6) |
| RCID <0 mm | 0 (0, 0−3) to 54 (41, 32−49) | 0 (0, 0−3) to 0 (0, 0−3) | – | – | 0 (0, 0−3) to 0 (0, 0−3) | 0 (0, 0−3) to 0 (0, 0−3) | 0 (0, 0−3) to 0 (0, 0−3) | 0 (0, 0−3) to 0 (0, 0−3) |
Legend: AHA – American Heart Association, ANZCOR - Australian and New Zealand Committee on Resuscitation. ERC – European Resuscitation Council, ext APD - external anterior-posterior diameter, RICD<10 mm - residual internal chest dimension less than 10 mm representing possible over-compression, RICD<0 mm - residual internal chest dimension less than 0 mm representing definite over-compression.
Values are n (%, 95% CI).
Percentages are in bold if more than ≥5% of population.
Current ILCOR, AHA and ERC pediatric chest compression guidelines1, 2, 3, 4, 5, 6, 7 recommended compression depth target of “at least” one-third external APD for all paediatric ages who are pre-pubertal, without an upper limit. We had set an upper limit of “less than one-half external APD” based on previous literature and recommendations1, 2, 3, 4, 5, 6, 7, 10, 11.
Simulated chest compressions of “approximately” one-third external APD had the least potential for over-compression across all age groups. At its upper acceptable limit of 10%, none had definite over-compression and 8 infants and children (1.3%) had definite over-compression.
At the upper limit of simulated chest compression of “approximately 4 cm” (4 + 10%cm) in infants, 4% had definite over-compression (compression depth exceeded internal APD) and 34% had potential for over-compression.
Simulated compressions at the upper limit of “approximately 5 cm” (5 + 10%cm), resulted in 1% of younger children (1−8 years) with potential for over-compression and 22% with definite over-compression. The proportion of children 1−12years with potential for over-compression, when using the upper limits of absolute chest depth compression targets (5.5 cm) were lower than the 1 to 8-years-age group, P < 0.001.
A simulated chest compression of 6 cm (upper limit of recommended adult chest compression depth), resulted in 10 children who were >8 years (5%), compared to 3 children >12 years (2%) with potential for over-compression. The odds of potential for over-compression for children >12-years-old was 34% lower than that for children>8-years-old when a simulated chest compression depth of 6 cm was performed (odds ratio, OR 0.66, 95% CI 0.42 to 1.05; P = 0.083).
Discussion
In this radiological study, we looked at potential for over-compression in Asian infants and children using CT chest imaging and found that generally there was a significant proportion of our Asian study population with potential for over-compression when absolute chest compression depth targets of “approximately 4 cm” and “approximately 5 cm” were simulated in infants and children 8-years or less, respectively. These findings were consistent with other Asian paediatric studies,18, 19, 20 which alluded that a compression depth of 5 cm and beyond may be too deep for young Asian children; especially those 8-years old and below.
When relative chest compression depth target of one-third external APD was used, the potential for over-compression across all age groups was the lowest. However, most paediatric chest compression guidelines recommend a relative chest compression target depth of “at least” one-third external APD and not “approximately”.
The use of Asian paediatric chest compression depth guidelines (Japan, Korea, and Singapore), being more conservative than ILCOR, AHA, ERC and ANZCOR appeared to result in less potential for over-compression in our study population. Amongst these guidelines, the compression depth target of “approximately one-third external APD”, “3 to 4 cm” in infants, and “4–5 cm” in younger children had least potential for over-compression in our Asian study population.
At 6 cm, the upper limit of adult chest compression depth target, there was increased proportion of Asian children of “>8 years” compared to “>12 years” with potential for over-compression. This suggested that the use of adult chest compression guidelines may be more appropriate in adolescents more than 12-years-old. This would appear to support current ILCOR, AHA and ERC age cut-off for basic life support by community rescuers by visual identification of “signs of puberty”, which would be more likely to occur after 12-years of age.
While there were concerns that the Asian paediatric population may be smaller in habitus in terms of chest dimensions, we did not find this in our study. Mean one-third external APD at the lower half of the sternum in our study population of Asian infants and children 1 to 8-years-old was 33.96 mm (SD 4.70) and 44.69 mm (SD 4.50) respectively. These were similar to prior published studies on non-Asian paediatric populations that found one-third external APD were approximately 3.4 cm in infants and 4.4 cm in children 1 to 8-years-old.15, 21, 22 The finding that the chest measurements were not substantially lower in our study population as compared to other paediatric populations suggested that the potential for over-compression may be an issue for some infants and young children across all populations. We were not able to find direct studies on the associations of ethnicity on chest compression depth or chest size over the compression landmark. There were two studies from Britain and the United States, involving school going children (5–11-years-old) that investigated how ethnicity and chest size affected spirometry.23, 24 While not directly applicable, as the measurements were not made at the chest compression landmark, and not over the sternum (where there would be minimal soft tissue), they reported data which showed less than 1 cm (0.2–0.9 cm) difference between the mean external APD of Asians and non-Asians in their local population.
We were unable to infer what the optimal chest compressions targets for Asian paediatric population, especially for infants and younger children, due to the limitations of a radiological study. It is notable that a large adult out-of-hospital cardiac arrest study25 found a trend towards lower survival with chest compression depths >4.56 cm (maximum survival after adjusting for confounders was in the depth interval of 4.03–5.53 cm with peak, 4.56 cm); suggesting that the 2010 American Heart Association cardiopulmonary resuscitation guideline target for adults could potentially be too deep. With this observation that the peak survival was at a mean chest compression depth of 4.56 cm in adults, it would be important to clinically validate current recommended paediatric chest compression depth targets, especially for infants and younger children. A forensic study in adults observed increased rates of chest compression-related injuries if compressions of more than 6 cm were delivered.17 There are, currently, no published forensic paediatric studies looking at this. Interestingly, in an experimental study using piglets model simulating toddlers, haemodynamic-guided chest compressions led to mean chest compression depths at 4 cm and had better survival outcomes compared to when chest compressions were randomised to receive standardised chest compressions of 5 cm (target chest compression depth for toddlers).26 Earlier clinical studies12, 13 on paediatric chest compression depths that informed current paediatric chest compression guidelines were based on single-sensor cardiopulmonary resuscitation (CPR) quality monitoring devices that could have over-estimated compression depths. The single-sensors used to obtain compression depth in the landmark paediatric clinical studies could have been affected by compression on non-firm surfaces and patient movement. Recent observational studies21, 27 using more advanced CPR feedback devices with dual sensors (anterior and posterior) reported that pediatric chest compression depth targets were rarely achieved in clinical practice. The newer dual-sensors measure the difference between the sensors rather than gross movement of the sensor and the patient. While these more recent studies showed that paediatric chest compressions rarely achieve recommended chest compression depth targets, with increasing use of more advanced CPR feedback devices with dual sensors, coupled with CPR coaching under CPR quality improvement programmes, improved compliance to current recommended absolute chest compressions depth targets were demonstrated to be realistically achievable.28, 29
However, our new results suggest that in settings that allow for accurate compression depth monitoring, there could be increased potential for over-compression in Asian infants and younger children less than 8 years of age if chest compression went beyond 4 cm and 5 cm respectively, even if they were within the upper acceptable limits of +10%.
Amongst the various paediatric life support guidelines, compression depth targets of “approximately one-third external APD” appeared to have the least potential for over-compression in our study population. However, relative chest compression depth targets can only be grossly estimated, and it is unknown if they can be realistically targeted with sufficient accuracy in practice. In the settings when chest compression can be accurately monitored and targeted during CPR, an absolute range of “3 to 4 cm” in infants, and “4 to 5 cm” in younger children appeared to result in relatively less potential for over-compression. These findings need to be validated and their clinical implications studied in clinical trials to better inform future paediatric chest compression guidelines.
Strengths
Our study has a large sample size with 592 paediatric CT scans analysed and the largest sample size for patients below the age of 1 (193 CT scans) in an Asian population.
Limitations
This study could not address the issue of potential for “under-compression” or “optimal compression” depth in the paediatric population. Optimal chest compression depth should be based on clinical trials and clinically important outcomes. We have thus considered the findings of the study to be relevant in settings where compression depth can be accurately measured, for example, with CPR dual-sensor feedback devices.
CT scans are usually performed with arms raised at end-inspiration in contrast to patient undergoing CPR who have arms lowered and placed at the sides of the torso. Although there could be differences between the chest dimension measured during inspiration and expiration in adult studies,30 and CPR is performed throughout the respiratory cycle, measurements used in this project were at 1 standardized point in maximal inspiration. Of note, paediatric cardiac arrest victims whose arms are by their side have approximately 5–10 compressions delivered during exhalation compared to inhalation.21 Thus, our study is conservative and would be likely to underestimate (not overestimate) potential for over-compression.
In addition, the patients whose CT scans were reviewed usually have underlying medical conditions, hence their growth might have been affected causing their chest dimensions to be smaller than the chest dimensions in healthy children. However, ill patients are also more likely to receive chest compressions than the healthy population, thus our findings may still be relevant and generalisable.
The potential for chest compression causing internal injuries when RICD is less than 10 mm is not evidence-based but based on expert opinions and consensus.14, 15, 16, 18, 19, 20 It remains unknown if chest compression depths that result in RICD less than 10 mm but more than 0 mm, could lead to actual injury or harm, especially in infants. However, the paediatric myocardium, notably in infants, tend to be less elastic31 and the more pliable paediatric chest wall may allow greater transmission of blunt force to intrathoracic structures.32 Thus, we also conservatively reported RICD<0 mm in our assessment of potential for over-compression.
Conclusions
Chest compression depth at “approximately” one-third external anterior-posterior diameter had the least potential for over-compression across all paediatric age-groups. In situations whereby chest compression depth can be accurately measured and targeted, compression depth at the upper limit of approximately 4 cm (4.4 cm) in infants and approximately 5 cm (5.5 cm) in younger children could result in significant potential for over-compression.
The authors have full access to all aspects of the research and writing process, and take final responsibility for the paper. The contributions of the authors are as follow:
Dr Gene Ong conceptualized, designed, co-ordinated the study, supervised the data collection, contributed significantly to writing of the initial draft of the manuscript, and critically reviewed and revised the manuscript.
Mr. Aloysius Ang assisted in the design of the study, designed the data collection instruments, collected data, contributed to the writing of the initial draft of the manuscript, critically reviewed, and revised the manuscript.
Mr. Amirzeb collected data, contributed significantly to the writing of the initial draft of the manuscript, and critically reviewed and revised the manuscript.
Miss Fong and Mr. Tan collected data, critically reviewed, and revised the manuscript.
Miss Chen and Dr Chan carried out the data analysis for this study, critically reviewed, and revised the manuscript.
Dr Tang PH assisted in the audit and review of the radiological data, reviewed, and revised the manuscript.
Drs Ng KC, Ian Maconochie and Pek JH critically reviewed and revised the manuscript.
Dr Vinay Nadkarni contributed significantly to concept, critically reviewed and revised the manuscript.
All authors have revised the material critically for important intellectual content and approved the final version to be submitted.
There are no financial disclosures.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resplu.2021.100112.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Maconochie I.K., Aickin R., Hazinski M.F. Pediatric Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2020;142:S140–S184. doi: 10.1161/CIR.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 2.de Caen A.R., Maconochie I.K., Aickin R. Part 6: Paediatric basic life support and paediatric advanced life support: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation. 2015;132:S177–203. doi: 10.1161/CIR.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 3.Considine J., Gazmuri R.J., Perkins G.D. Chest compression components (rate, depth, chest wall recoil and leaning): a scoping review. Resuscitation. 2020;146:188–202. doi: 10.1016/j.resuscitation.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Atkins D.L., Berger S., Duff J.P. Part 11: Paediatric Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S519–25. doi: 10.1161/CIR.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 5.Maconochie I.K., Bingham R., Eich C. European Resuscitation Council Guidelines for Resuscitation 2015: Section 6. Paediatric life support. Resuscitation. 2015;95:223–248. doi: 10.1016/j.resuscitation.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 6.2017. Australian and New Zealand Committee on Resuscitation ANZCOR guideline 12.1, Introduction to paediatric advanced life support. June 2017. (Accessed 15 July 2020, at https://www.nzrc.org.nz/assets/Guidelines/Paed-ALS/All-Paed-ALS-guidelines-June-2017.pdf) [Google Scholar]
- 7.Ong G.Y., Chan I., Ng A. Singapore paediatric resuscitation guidelines 2016. Singapore Med J. 2017;58:373–390. doi: 10.11622/smedj.2017065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hingley S., Booth A., Hodgson J., Langworthy K., Shimizu N., Maconochie I. Concordance between the 2010 and 2015 Resuscitation Guidelines of International Liaison Committee of Resuscitation Councils (ILCOR) members and the ILCOR Consensus of Science and Treatment Recommendations (CoSTRs) Resuscitation. 2020;151:111–117. doi: 10.1016/j.resuscitation.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Lim S.H., Wee F.C., Chee T.S. Basic cardiac life support: 2016 Singapore guidelines. Singapore Med J. 2017;58:347–353. doi: 10.11622/smedj.2017063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Japan Resuscitation Council Resuscitation Guidelines . 2015. Chapter 1. Basic Life Support, March 2016. (Accessed 15 July 2020, at https://www.japanresuscitationcouncil.org/wp-content/uploads/2016/02/20121005_BLS.pdf) [Google Scholar]
- 11.Lee J.S., Ahn J.Y., Kim D.K. Part 5. Paediatric basic life support: 2015 Korean Guidelines for Cardiopulmonary Resuscitation. Clin Exp Emerg Med. 2016;3:S39–S47. doi: 10.15441/ceem.16.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton R.M., French B., Niles D.E. 2010 American Heart Association Recommended Compression Depths During Paediatric In-hospital Resuscitations are Associated with Survival. Resuscitation. 2014;85:1179–1184. doi: 10.1016/j.resuscitation.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton R.M., Case E., Brown S.P. A quantitative analysis of out-of-hospital paediatric and adolescent resuscitation quality–a report from the ROC epistry-cardiac arrest. Resuscitation. 2015;93:150–157. doi: 10.1016/j.resuscitation.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao P.C., Chiang W.C., Yang C.W. What is the correct depth of chest compression for infants and children? A radiological study. Paediatrics. 2009;124:49–55. doi: 10.1542/peds.2008-2536. [DOI] [PubMed] [Google Scholar]
- 15.Braga M.S., Dominguez T.E., Pollock A.N. Estimation of optimal CPR chest compression depth in children by using computer tomography. Paediatrics. 2009;124:e69–e74. doi: 10.1542/peds.2009-0153. [DOI] [PubMed] [Google Scholar]
- 16.Meyer A., Nadkarni V., Pollock A. Evaluation of the Neonatal Resuscitation Program’s recommended chest compression depth using computerised tomography imaging. Resuscitation. 2010;81:544–548. doi: 10.1016/j.resuscitation.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Hellevuo H., Sainio M., Nevalainen R. Deeper chest compression - more complications for cardiac arrest patients? Resuscitation. 2013;84:760–765. doi: 10.1016/j.resuscitation.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.H., Lee J.H., Cho K.W. Verification of the optimal chest compression depth for children in the 2015 American Heart Association Guidelines: Computed Tomography Study. Pediatr Crit Care Med. 2018;19:e1–e6. doi: 10.1097/PCC.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 19.Jin S.Y., Oh S.B., Kim Y.O. Estimation of optimal paediatric chest compression depth by using computed tomography. Clin Exp Emerg Med. 2016;3:27–33. doi: 10.15441/ceem.16.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.H., Han S.K., Na J.U. Current guideline of chest compression depth for children of all ages may be too deep for younger children. Emerg Med Int. 2019;2019 doi: 10.1155/2019/7841759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niles D.E., Duval-Arnould J., Skellett S. Characterization of paediatric in-hospital cardiopulmonary resuscitation quality metrics across an international resuscitation collaborative. Pediatr Crit Care Med. 2018;19:421–432. doi: 10.1097/PCC.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 22.Sutton R.M., Niles D., Nysaether J. Pediatric CPR quality monitoring: analysis of thoracic anthropometric data. Resuscitation. 2009;80:1137–1141. doi: 10.1016/j.resuscitation.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Lum S., Bountziouka V., Sonnappa S. Lung function in children in relation to ethnicity, physique and socioeconomic factors. Eur Respir J. 2015;46:1662–1671. doi: 10.1183/13993003.00415-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whittaker A.L., Sutton A.J., Beardsmore C.S. Are ethnic differences in lung function explained by chest size? Arch Dis Child Fetal Neonatal Ed. 2005;90:F423–8. doi: 10.1136/adc.2004.062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stiell I.G., Brown S.P., Nichol G. What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitation of adult patients? Circulation. 2014;130:1962–1970. doi: 10.1161/CIRCULATIONAHA.114.008671. [DOI] [PubMed] [Google Scholar]
- 26.Morgan R.W., Kilbaugh T.J., Shoap W. A hemodynamic-directed approach to paediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation. 2017;111:41–47. doi: 10.1016/j.resuscitation.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton R.M., Wolfe H., Nishisaki A. Pushing harder, pushing faster, minimizing interruptions… but falling short of 2010 cardiopulmonary resuscitation targets during in-hospital paediatric and adolescent resuscitation. Resuscitation. 2013;84:1680–1684. doi: 10.1016/j.resuscitation.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C.-Y., Hsia S.-H., Lee E.-P. Effect of audiovisual cardiopulmonary resuscitation feedback device on improving chest compression quality. Sci Rep. 2020;10:398. doi: 10.1038/s41598-019-57320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng A., Duff J.P., Kessler D. International Network for Simulation-based Paediatric Innovation Research and Education (INSPIRE) CPR. Optimizing CPR performance with CPR coaching for paediatric cardiac arrest: a randomised simulation-based clinical trial. Resuscitation. 2018;132:33–40. doi: 10.1016/j.resuscitation.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Nozoe M., Mase K., Tsutou A. Regional chest wall volume changes during various breathing maneuvers in normal men. J Jpn Phys Ther Assoc. 2011;14:12–18. doi: 10.1298/jjpta.Vol14_002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marijianowski M.M., van der Loos C.M., Mohrschladt M.F., Becker A.E. The neonatal heart has a relatively high content of total collage and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol. 1994;23:1204–1208. doi: 10.1016/0735-1097(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 32.Alemayehu H., Aguayo P. Paediatric Blunt Thoracic Trauma. J Pediatr Intensive Care. 2015;4:35–39. doi: 10.1055/s-0035-1554987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.