Abstract
Aim
Compare vasopressin to a second dose of epinephrine as rescue therapy after ineffective initial doses of epinephrine in diverse models of pediatric in-hospital cardiac arrest.
Methods
67 one- to three-month old female swine (10−30 kg) in six experimental cohorts from one laboratory received hemodynamic-directed CPR, a resuscitation method where high quality chest compressions are provided and vasopressor administration is titrated to coronary perfusion pressure (CoPP) ≥20 mmHg. Vasopressors are given when CoPP is <20 mmHg, in sequences of two doses of 0.02 mg/kg epinephrine separated by minimum one-minute, then a rescue dose of 0.4 U/kg vasopressin followed by minimum two-minutes. Invasive measurements were used to evaluate and compare the hemodynamic and neurologic effects of each vasopressor dose.
Results
Increases in CoPP and cerebral blood flow (CBF) were greater with vasopressin rescue than epinephrine rescue (CoPP: +8.16 [4.35, 12.06] mmHg vs. + 5.43 [1.56, 9.82] mmHg, p = 0.02; CBF: +14.58 [-0.05, 38.12] vs. + 0.00 [-0.77, 18.24] perfusion units (PFU), p = 0.005). Twenty animals (30%) failed to achieve CoPP ≥20 mmHg after two doses of epinephrine; 9/20 (45%) non-responders achieved CoPP ≥20 mmHg after vasopressin. Among all animals, the increase in CBF was greater with vasopressin (+14.58 [-0.58, 38.12] vs. 0.00 [-0.77, 18.24] PFU, p = 0.005).
Conclusions
CoPP and CBF rose significantly more after rescue vasopressin than after rescue epinephrine. Importantly, CBF increased after vasopressin rescue, but not after epinephrine rescue. In the 30% that failed to meet CoPP of 20 mmHg after two doses of epinephrine, 45% achieved target CoPP with a single rescue vasopressin dose.
Keywords: Cardiac arrest, Cardiopulmonary resuscitation, Pediatrics, Coronary perfusion pressure, Cerebral blood flow, Vasopressin, Epinephrine
Introduction
Pediatric in-hospital cardiac arrest (IHCA) occurs in 1.4-6% of children admitted to pediatric intensive care units (PICUs).1 Less than half of these children survive to hospital discharge and many have new functional morbidities post-arrest.2 During cardiopulmonary resuscitation (CPR), coronary perfusion pressure (CoPP), the difference between the aortic pressure and the right atrial (RA) pressure during the relaxation phase of chest compressions (“diastole”), is a major determinant of achieving return of spontaneous circulation (ROSC)3 and surviving to hospital discharge.4 Vasopressors are therefore given during CPR to increase systemic vascular resistance and thereby increase diastolic blood pressure (DBP) and CoPP.5 However, vasopressors may have adverse neurologic effects during CPR, with particular concern that epinephrine decreases cerebral blood flow.[6], [7], [8]
Our group developed and investigated the use of hemodynamic-directed CPR (HD-CPR), using systolic blood pressure-guided chest compression force and CoPP-guided vasopressor administration to improve outcomes.9 In numerous pre-clinical studies, this HD-CPR strategy led to higher coronary and cerebral perfusion pressures, higher rates of survival, superior neurologic outcomes, and improved mitochondrial respiration in the heart and brain as compared to standard, guideline-based CPR.[10], [11], [12], [13], [14], [15] The vasopressor strategy employed in HD-CPR requires vasopressors to be administered in a protocolized manner if the CoPP is <20 mmHg. An initial dose of 0.02 mg/kg epinephrine is given as dictated by CoPP, followed by a minimum duration of one minute, and a second dose of 0.02 mg/kg epinephrine if CoPP is <20 mmHg. After an additional one-minute minimum duration, 0.4 Units/kg of vasopressin is administered if CoPP remains <20 mmHg. Despite the efficacy of HD-CPR, the specific physiologic effects of vasopressin rescue have not been explicitly studied.
Therefore, the primary objective of this study was to compare the physiologic responses to a rescue dose (second dose) of epinephrine with a rescue dose of vasopressin. We hypothesized that CoPP and CBF would increase more following vasopressin than epinephrine.7 Additionally, we sought to characterize the physiologic response to vasopressin in a group of animals that failed to achieve CoPP ≥20 mmHg after either of two doses of epinephrine (i.e., epinephrine non-responders). We hypothesized that many of these epinephrine “non-responders” would have increases in CoPP and CBF following a single dose of vasopressin.
Materials and methods
Study design and data sources
This was a retrospective analytic study of data from laboratory experiments utilizing HD-CPR in porcine models of pediatric IHCA. All animals with HD-CPR as the resuscitation method were eligible for analysis, with exclusion criteria as fewer than 2 doses of epinephrine or no vasopressin administered. Animals did not require CBF data for inclusion.
Data collection
The Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee approved all experimental protocols, which were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Comprehensive descriptions of animal preparation, anesthetic, and surgical methods are available in previous publications.[10], [11], [12], [13], [14], [15], [16], [17] Briefly, female Yorkshire swine were anesthetized and mechanically ventilated. To broadly study the pediatric age range, 1-month-old (∼10 kg) and 3-month-old (∼30 kg) swine were utilized. Vascular catheters were placed and high-fidelity pressure transducers were advanced to the RA, pulmonary artery (PA), and aorta for continuous hemodynamic measurements, confirmed with fluoroscopy and pressure waveform transduction. Before and during the experimental protocol, the electrocardiogram (ECG), aortic pressure, RA pressure, PA pressure, pulse oximetry, and end-tidal carbon dioxide (ETCO2) values and waveforms were displayed and recorded. Coronary perfusion pressure was automatically calculated and displayed in real time by subtracting the RA pressure from the aortic pressure.10 A CPR quality-recording defibrillator (Zoll R Series Plus; Zoll Medical Corporation) was used during CPR and recorded chest compression rate (per minute) and depth (centimeters). A subset of animals underwent invasive neuromonitoring with a PeriFlux laser Doppler (Perimed, Inc.) monitor to measure CBF, located in the superficial cerebral cortex.
Experimental protocol
Injury Period: Animals underwent one of the following injuries:
-
1)
Primary ventricular fibrillation (VF) cardiac arrest (3-month-old swine): VF was electrically induced and left untreated for seven minutes, followed by a minimum of 10 min of HD-CPR and defibrillation.[11], [15]
-
2)
Asphyxia-associated cardiac arrest (1- and 3-month-old swine): Asphyxia was induced by clamping the endotracheal tube for 7 min, following which VF was induced and HD-CPR commenced.[10], [12], [13], [14] The induction of VF ensured a consistent 10-minute CPR period as effective CPR after asphyxia-induced cardiac arrest alone typically results in ROSC within 2–4 minutes.[18], [19]
-
3)
Lipopolysaccharide (LPS)-induced shock-associated IHCA (3-month-old swine): Animals received 45 min of an intravenous LPS infusion to induce shock, followed by induction of VF to ensure an adequate cardiac arrest period in which to study HD-CPR.17 A subset of these animals received nitric oxide (iNO) therapy.
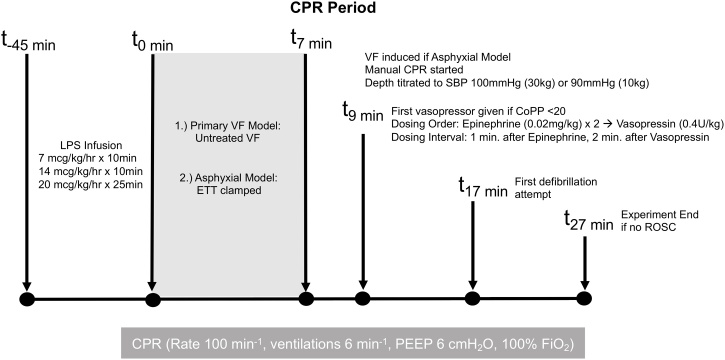
Resuscitation Period: In all subjects, chest compressions were provided with a target rate of 100 per minute guided by metronome. Per our established HD-CPR algorithm (Fig. 1), chest compression depth was titrated to maintain a systolic blood pressure (SBP) of 90 mmHg for 10 kg swine and 100 mmHg for 30 kg swine. Vasopressors were given by protocol, as needed, to maintain a goal CoPP of ≥20 mmHg. Beginning two minutes into CPR, epinephrine was administered (0.02 mg/kg as recommended for swine models[20], [21], [22]) if CoPP <20 mmHg during mid-diastole for at least three consecutive chest compressions. If CoPP was <20 mmHg at least one minute after first epinephrine dose or at any subsequent time, an additional dose of epinephrine was administered. If CoPP was <20 mmHg at least one minute after second epinephrine dose or any subsequent time, vasopressin (0.4 U/kg) was administered. The cycle restarted two minutes following vasopressin; these intervals are based on previous data about the pharmacodynamics effects of these vasoconstrictors during CPR.23 Only the first cycle was included for analysis.
Fig. 1.
Hemodynamic-directed CPR protocol.
CPR = cardiopulmonary resuscitation; ETT = endotracheal tube; VF = ventricular fibrillation; HD = hemodynamic-directed; SBP = systolic blood pressure; CoPP = coronary perfusion pressure; ROSC = return of spontaneous circulation
Data collection and processing
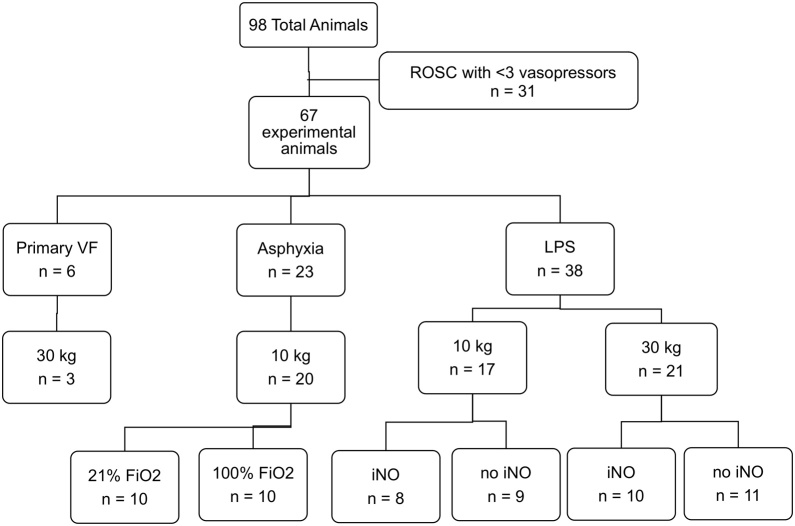
All physiologic measurements were recorded at 1000 Hz (PowerLab, ADInstruments, Inc.). Vasopressor administration was recorded in real-time. The one minute preceding each vasopressor dose, the one minute following each epinephrine dose, and the two minutes following each vasopressin dose were divided into 15-second data epochs. Mean values for each physiologic parameter were calculated for each epoch. Data were analyzed from only the first cycle of vasopressors (i.e., epinephrine, epinephrine, vasopressin) for each experiment to minimize confounding. For comparison of physiologic response between rescue vasopressors after initial epinephrine dose failed to sustain the target CoPP, the second dose of epinephrine was compared to the rescue vasopressin dose. Data from 98 experiments were compiled; 31 subjects did not meet inclusion criteria because they did not receive vasopressin during the course of the original experiments. The remaining 67 evaluable subjects constituted the total cohort (Fig. 2).
Fig. 2.
Study population enrollment and characteristics.
Statistical analyses
The primary analysis compared the physiologic change following vasopressin to that observed following the second dose of epinephrine. The primary outcomes were changes in CoPP and CBF. All measurements were treated as non-normally distributed after a Shapiro-Wilk analysis was performed and were reported as medians with interquartile ranges and compared using non-parametric analyses. For each physiologic parameter, the mean of the four epochs preceding each vasopressor dose was utilized as a baseline measurement. The maximum value among the epochs following each particular vasopressor dose was utilized in order to best describe the peak vasopressor effect. To avoid overlap with subsequent vasopressor administrations, four epochs (i.e., 1 min) were evaluated following each epinephrine dose and eight epochs (i.e., 2 min) were evaluated following vasopressin. The change in each of these measurements from pre- to post-vasopressor administration were calculated and compared between the second dose of epinephrine and the dose of vasopressin with Wilcoxon signed-rank tests.
For the secondary analyses, an epinephrine CoPP threshold non-responder cohort was defined a priori as animals that failed to have any 15-second mean CoPP ≥20 mmHg after either dose of epinephrine. These subjects were characterized as vasopressin responders if their CoPP was ≥20 mmHg in any 15-second epoch during the two minutes after vasopressin administration. The above analyses were repeated in subgroups of epinephrine responders, epinephrine non-responders, and in epinephrine non-responders that responded to vasopressin.
In prospectively planned supplemental analyses, the change in CoPP following the second dose of epinephrine to a priori response targets (≥3 mmHg; ≥5 mmHg; ≥7 mmHg; ≥10 mmHg) were utilized as alternative definitions of epinephrine responsiveness. The number of responders and non-responders in each of these categories was summarized, as were the number of vasopressin responders (according to the same definition) among each epinephrine non-responder population. Statistical analyses were performed with the R statistical software (R core 2018). An α value of ≤0.05 was considered statistically significant.
Results
Characterization of vasopressor responsiveness
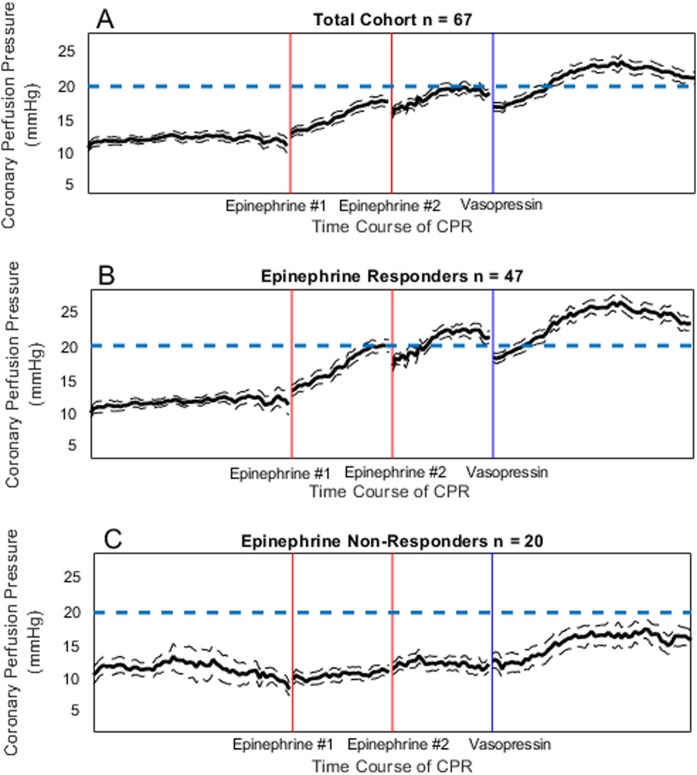
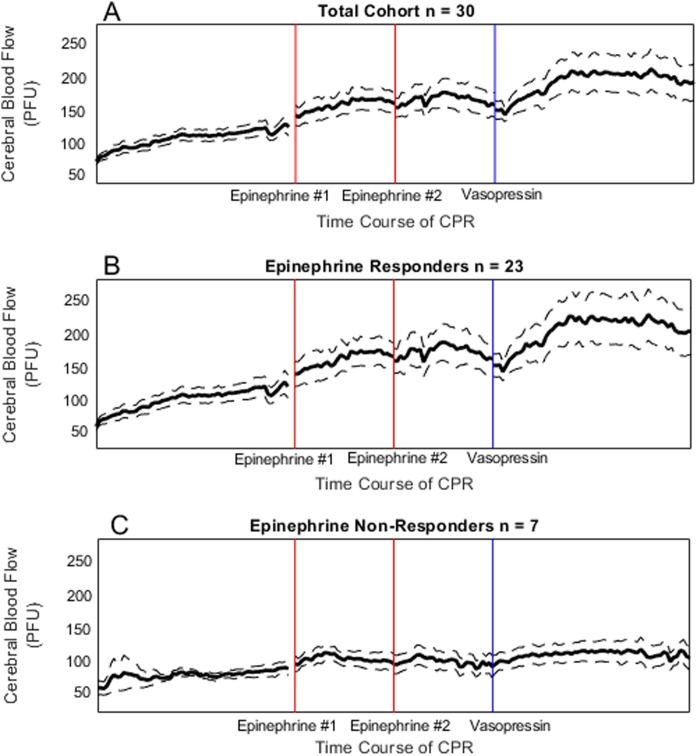
Total cohort: In the final study cohort (n = 67), the mean baseline CBF prior to cardiac arrest was 348.16 ± 38 PFU (SEM), which dropped to 0 PFU in all animals at the start of CPR. There was a significant increase in CoPP and CBF after both doses of epinephrine and after vasopressin (Supplemental Table 1; Fig. 3, Fig. 4). Vasopressin increased CoPP (+8.16 [4.35, 12.06] mmHg vs. + 5.43 [1.56, 9.82] mmHg; p = 0.02), mean PA pressure (+4.05 [0.42, 7.80] mmHg vs. + 0.04 [-0.80, 2.98] mmHg; p < 0.001) and CBF (+14.58 [−0.05, 38.12] PFU vs. + 0.00 [-0.77, 18.24] PFU, p = 0.01) more than the second dose of epinephrine (Table 1).
Fig. 3.
(a) Coronary perfusion pressure during cardiac arrest in the total cohort (n = 67). (b) Coronary perfusion pressure during cardiac arrest in a population of epinephrine non-responders (n = 20), defined by coronary perfusion pressure < 20 mmHg after both doses of epinephrine. (c) Coronary perfusion pressure during cardiac arrest in a sub-population of subjects who did not achieve coronary perfusion pressure ≥ 20 mmHg after epinephrine but did achieve ≥ 20 mmHg after vasopressin (n = 9).
legend: The x-axis reflects time during CPR. Time is discontinuous in the figure because vasopressors were delivered on an as-needed basis when CoPP <20 mmHg. The first two vertical lines represent the first and second epinephrine doses and the third vertical line represents the vasopressin dose. The one minute of data following each epinephrine dose and the two minutes of data following the vasopressin dose are depicted. The dashed, horizontal line depicts the CoPP goal of 20 mmHg.
Fig. 4.
(a) Cerebral blood flow during cardiac arrest in the total cohort of subjects with evaluable cerebral blood flow data (n = 30). (b) Cerebral blood flow during cardiac arrest in the total cohort of subjects with evaluable cerebral blood flow data who were epinephrine non-responders (n = 23), defined by coronary perfusion pressure < 20 mmHg after both doses of epinephrine. (c) Cerebral blood flow during cardiac arrest in the total cohort of subjects with evaluable cerebral blood flow data, who did not achieve coronary perfusion pressure ≥ 20 mmHg after epinephrine but did achieve ≥ 20 mmHg after vasopressin (n = 7).
legend: “The x-axis reflects time during CPR discontinuously because of vasopressors were delivered on an as-needed basis when CoPP <20 mmHg. The first two vertical lines represent the first and second epinephrine doses and the third vertical line represents the vasopressin dose. The one minute of data following each epinephrine dose and the two minutes of data following the vasopressin dose are depicted. The dashed, horizontal line depicts the CoPP goal of 20 mmHg.
Table 1.
Comparison of the effects of the second dose of epinephrine in a.) the total cohort (n = 67), b.) epinephrine responders (n = 47), and c.) epinephrine non-responders (n = 20).
| Epinephrine #2 Δ | Vasopressin Δ | p | |
|---|---|---|---|
| Total Cohort (n = 67) | |||
| CoPP | 5.43 (1.56–9.82) | 8.16 (4.35–12.06) | 0.02 |
| CBF | 0.00 (−0.77 – 18.24) | 14.58 (−0.05–38.12) | 0.01 |
| MAP | 5.90 (1.98–13.82) | 8.62 (3.90–13.16) | 0.21 |
| Mean PA | 0.04 (−0.80 – 2.98) | 4.05 (0.42–7.80) | <0.001 |
| RAP | 0.94 (0.43–1.61) | 0.70 (0.19–1.39) | 0.25 |
| ETCO2 | −0.18 (−2.37 – 2.83) | −0.19 (−1.5 – 0.98) | 0.74 |
| Epinephrine Responders (n = 47) | |||
| CoPP | 6.89 (2.46–10.65) | 8.24 (4.18–13.99) | 0.21 |
| CBF | 0.065 (−0.51 – 22.67) | 16.03 (−0.15–44.8) | 0.04 |
| MAP | 9.00 (3.32–14.62) | 9.02 (4.40–13.99) | 0.60 |
| Mean PA | 0.23 (−0.65 – 2.98) | 4.26 (0.66–8.39) | 0.001 |
| RAP | 0.91 (0.43–1.38) | 0.81 (0.91–1.44) | 0.64 |
| ETCO2 | −0.02 (−2.11 – 2.93) | −0.18 (−1.31 – 0.98) | 0.63 |
| Epinephrine Non-responders (n = 20) | |||
| CoPP | 2.76 (1.28–6.58) | 7.64 (4.86–11.02) | 0.22 |
| CBF | −0.28 (−2.33 – 6.91) | 14.58 (3.55–34.90) | 0.045 |
| MAP | 2.53 (0.91–7.29) | 6.84 (3.68–10.34) | 0.16 |
| Mean PA | −0.13 (−1.71 – 2.37) | 2.45 (0.44–6.59) | 0.01 |
| RAP | 1.19 (0.49–2.05) | 0.70 (0.22–1.29) | 0.23 |
| ETCO2 | −0.82 (−2.86 – 1.29) | −0.47 (−1.87 – 0.90) | 0.95 |
Vasopressor effects were defined as the difference between pre-vasopressor mean and post-vasopressor maximum. Medians are reported with interquartile ranges. These differences were compared using Wilcoxon signed rank statistical tests.
CoPP = coronary perfusion pressure (mmHg); CBF = cerebral blood flow (PFU); MAP = mean arterial pressure (mnHg); Mean PA = mean pulmonary artery pressure (mmHg); RAP = right atrial pressure (mmHg); EtCO2 = end tidal carbon dioxide (mmHg).
Epinephrine responders: In the 47 animals who achieved a CoPP of ≥20 mmHg after one of the first two doses of epinephrine, vasopressin rescue generated a greater rise in CBF (0.065 [-0.51, 22.67] PFU vs. + 16.03 [-0.15, 44.8] PFU, p = 0.04), and mean PA pressure (0.23 [-0.65, 2.98] mmHg vs. + 4.26 [0.66, 8.39] mmHg, p = 0.001).
Epinephrine non-responders: In 20/67 (29.9%) experiments, neither dose of epinephrine resulted in any epoch with CoPP ≥ 20 mmHg. Nine of these 20 (45%) achieved a CoPP of ≥20 mmHg within 2 min after a single dose of rescue vasopressin, and 6/9 (67%) achieved return of spontaneous circulation. Among these 20 epinephrine non-responders, the change in CBF of -0.28 [-2.33, 6.91] PFU after the epinephrine rescue was significantly less than after vasopressin rescue +14.58 [3.55–34.90] PFU (p = 0.045) (Table 1; Fig. 4). The change in CoPP was not significantly different between rescue doses of epinephrine, +2.76 [1.28, 6.58] mmHg, and vasopressin, +7.64 [4.86, 11.02] mmHg, (p = 0.22) (Table 1; Fig. 3c).
Additional characterizations of epinephrine responsiveness
The degrees of responsiveness to epinephrine based on change in CoPP are depicted in Supplemental Table 2. Thirty-one subjects had an increase in CoPP ≥ 3 mmHg to either dose of epinephrine. Among the 36 of 67 (54%) animals with a CoPP rise of ≤ 3 mmHg after the second dose of epinephrine, 20 (56%) had ≥ 3 mmHg increase after vasopressin. This is less than the 47 animals defined as epinephrine responders by the primary criteria of achieving CoPP ≥20 mmHg.”
Discussion
In this large, retrospective, analytic study of swine treated with HD-CPR across several diverse pathophysiologic models of IHCA (i.e., asphyxia, ventricular fibrillation, endotoxemia), the increases in coronary perfusion pressure and importantly, cerebral blood flow were consistently greater after vasopressin rescue than after rescue with a second dose of epinephrine. In the 30% of animals who failed to achieve the a priori hemodynamic goal CoPP of 20 mmHg after two doses of epinephrine (i.e. epinephrine non-responders), CBF increased more and nearly half attained the CoPP goal of 20 mmHg after a single “rescue” dose of vasopressin. While prior published data do not support the routine use of vasopressin as a substitute for epinephrine or combined with epinephrine,24 our data support the consideration of vasopressin as a potential rescue therapy when epinephrine does not achieve adequate CoPP response.
In the total cohort (n = 67), vasopressin had significantly greater effect on CoPP, a value that directly correlates with ROSC,3 than the preceding rescue dose of epinephrine. The magnitude of the difference in CoPP response between vasopressin and epinephrine was greatest in epinephrine non-responders (+7.64 [4.86, 11.02] mmHg vs. + 2.76 [1.28, 6.58] mmHg), though this difference did not reach statistical significance. This improvement in CoPP is consistent with prior work showing significantly higher CoPP after a combination of vasopressin and epinephrine than after either medication alone.25
To ensure these findings were robust and potentially translatable to a practical clinical setting, we sought to further characterize the response to the second dose of epinephrine and offer alternative definitions of epinephrine responsiveness based upon thresholds of CoPP rising ≥ 3 mmHg, ≥5 mmHg, ≥7 mmHg, or ≥10 mmHg after the second dose of epinephrine (Supplemental Table 2). Less than half of animals (31/67, 46%) achieved an increase in CoPP ≥ 3 mmHg, demonstrating heterogeneity in the hemodynamic response to epinephrine and also that some animals with a poor response to epinephrine do, in fact, respond to vasopressin (Fig. 3c).
Mean PA pressure increased more following vasopressin than epinephrine in the total cohort and in both epinephrine responders and epinephrine non-responders. This observation was somewhat unexpected as vasopressin is known to increase systemic vascular resistance without the same degree of pulmonary vasoconstriction as is observed with epinephrine.[26], [27] Therefore, we hypothesize that these higher PA pressures after vasopressin are due to the generation of more pulmonary blood flow rather than an increase in pulmonary vascular resistance. Future studies will seek to directly measure pulmonary vascular resistance or pulmonary blood flow to further elucidate the mechanism behind these findings.
Vasopressin also increased CBF more than the rescue dose of epinephrine in all responder groups (Table 1). The second “rescue” dose of epinephrine did not increase median CBF (Fig. 4). Vasopressor effects on the cerebral vasculature are complex and highly variable, with high doses of vasopressors at risk to decrease rather than increase perfusion to critical areas of brain.28 Epinephrine has potential detrimental cerebral effects, and higher rescue doses of epinephrine have been shown to induce cerebral vasoconstriction in animal studies.6 These effects can include decreased cerebral oxygenation[7], [29] and impaired cerebral microvascular blood flow,29 with a recent study showing that epinephrine-induced hemodynamic increases in MAP and CPP did not translate into improved cerebral oxygen tension or metabolism.30 In addition, the large, randomized clinical trial PARAMEDIC II suggested that cardiac arrest survivors treated with epinephrine had more severe neurologic impairment than those without.31
Vasopressin may have fewer adverse effects on the cerebral microvasculature. Previous porcine translational models of cardiac arrest have demonstrated greater CBF, higher cerebral pH, lower PCO2, and lower cerebral oxygen extraction after vasopressin vs. epinephrine,32 and improved rates of survival with good neurologic outcomes.33 Other porcine studies have shown vasopressin to be more effective in raising cerebral blood flow either alone34 or in combination with epinephrine.[35], [36] A randomized clinical trial showed that vasopressin combined with epinephrine and steroids, compared to epinephrine alone, may increase survival to hospital discharge with favorable neurological status.37 However, vasopressin remains a Class IIb recommendation in adults and Class Indeterminate recommendation in children by current resuscitation guidelines.24
Recent translational studies have shown that the effects of epinephrine are both short-lived[30], [38] and diminish with subsequent boluses.[39], [40] Our data also show that the effect of the second dose of epinephrine is diminished compared to the first dose on systemic (Fig. 3) and cerebral (Fig. 4) hemodynamics. However, the timing of vasopressor administration in this HD-CPR protocol (i.e. repeat vasopressor dose as soon as 60 s post-epinephrine dosing) precludes assessment of the duration of epinephrine effect. These pharmacodynamic characteristics of epinephrine during CPR, short duration of effect and and diminishing returns, may have implications for optimal vasopressor management during CPR. Perhaps the longer-acting vasopressin may be a reasonable alternative as a rescue medication, especially when provided in response to hemodynamic effect (e.g., for patients with invasive arterial blood pressure monitoring).
Most pediatric cardiac arrests occur in ICUs and many of these patients have arterial catheters in place at the time of arrest,41 thus one could determine which patients fail physiologic response to initial epinephrine boluses. Prior negative adult human randomized controlled trials have randomized initial vasopressin therapy vs epinephrine therapy42 or combined initial vasopressin-epinephrine vs epinephrine therapy,43 but meta-analyses of combined routine vasopressin-epinephrine therapies have only shown promise for out of hospital adult asystolic arrest.44 Vasopressin in these trials was administered in a standardized manner without regard to hemodynamic responses, so it is possible that there would be increased efficacy when administered to achieve diastolic blood pressure goals that are associated with improved outcomes.4 Cumulatively, these existing studies and the present data support the conduct of head-to-head prospective studies comparing CBF and neurologic outcome with rescue vasopressin compared to rescue standard dose epinephrine (e.g. usual care) in a physiology-directed fashion, stratified by initial host response to epinephrine.
This study has limitations. First, its pre-clinical nature could limit translatability to the bedside. However, experiments were conducted in established translational porcine models of cardiac arrest, subjects underwent consistent and standardized injuries, received consistent and highly protocolized resuscitations to minimize variability, and had closed systems of invasive monitoring for hemodynamic and neurologic data collection, with paired analyses allowing effect comparisons within a single subject. In addition, these laboratory models are relevant to patients in the ICU setting with arterial catheters in place at the time of cardiac arrest. Second, we cannot exclude the possibility that vasopressin’s effects were partly augmented by the preceding administration of epinephrine with this experimental design. Conversely, vasopressin was provided later in the resuscitation effort, which may have limited its pharmacodynamic effect. Third, as this was an observational analysis of animals receiving HD-CPR, we did not compare vasopressin “rescue” to “rescue” with a third dose of epinephrine. Because these animals had less response to the second dose of epinephrine than to the first dose (Fig. 3a, Fig. 4), and subsequent boluses of epinephrine have diminishing effects,[39], [45] it is likely that the differences would have been more marked if we compared a third dose of epinephrine to vasopressin as the third vasopressor dose.
Conclusions
In translational models of CPR, rescue doses of vasopressin increased coronary perfusion pressure and cerebral blood flow significantly greater than rescue doses of epinephrine. Our findings support the future evaluation of rescue vasopressin doses to selected patients who do not respond adequately to epinephrine during CPR.
Conflicts of interest
Financial support was provided through Russell Raphaely Endowed Chair funds at the Children’s Hospital of Philadelphia and grant funding through the NIH National Heart, Lung, and Blood Institute (R01HL141386 & K23HL148541) and Eunice Kennedy Shriver National Institute for Child Health and Human Development (R21HD089132).
Unrelated disclosures include the following: Dr. Morgan receives grant funding from the NIH and serves on the American Heart Association (AHA) Emergency Cardiovascular Care Committee. Dr. Berg receives grant funding from the NIH. Dr. Sutton receives grant funding from the NIH, serves on the AHA Emergency Cardiovascular Care Committee, and is Vice Chair of the AHA Get with the Guidelines-Resuscitation Pediatric Task Force. Dr. Kilbaugh receives grant funding from the National Institutes of Health (NIH) and the Department of Defense. Dr Nadkarni receives unrestricted grant funding from the NIH, AHRQ, Zoll Medical, American Heart Association, Laerdal Medical, Nihon Kohden, Inc and serves as a volunteer committee member for the American Heart Association, International Liaison Committee on Resuscitation, Citizen CPR Foundation, and Society for Critical Care Medicine.
The authors have disclosed that they do not have any conflicts of interest.
All authors have made substantial contributions to the conception and design of the study, data acquisition, interpretation or analysis, or drafting of the article. All authors have provided final approval of the version submitted.
Financial support
-
-
Children’s Hospital of Philadelphia Department of Anesthesiology and Critical Care Medicine
-
-
National Institutes of Health National Heart, Lung, and Blood Institute R01HL141386 & K23HL148541
-
-
National Institutes of Health Eunice Kennedy Shriver National Institute for Child Health and Human Development R21HD089132
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resplu.2020.100050.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Berg R.A., Nadkarni V.M. Incidence and Outcomes of Cardiopulmonary Resuscitation in PICUs. Crit Care Med. 2016;44:798–808. doi: 10.1097/CCM.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe H.A., Sutton R.M. Functional outcomes among survivors of pediatric in-hospital cardiac arrest are associated with baseline neurologic and functional status, but not with diastolic blood pressure during CPR. Resuscitation. 2019;143:57–65. doi: 10.1016/j.resuscitation.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paradis N.A., Martin G.B. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 4.Berg R.A., Sutton R.M., Reeder R.W. Association Between Diastolic Blood Pressure During Pediatric In-Hospital Cardiopulmonary Resuscitation and Survival. Circulation. 2018;137:1784–1795. doi: 10.1161/CIRCULATIONAHA.117.032270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg R.A., Sanders A.B., Kern K.B. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 6.Gedeborg R., Silander H.C., Ronne-Engstrom E., Rubertsson S., Wiklund L. Adverse effects of high-dose epinephrine on cerebral blood flow during experimental cardiopulmonary resuscitation. Crit Care Med. 2000;28:1423–1430. doi: 10.1097/00003246-200005000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Ristagno G., Sun S., Tang W., Castillo C., Weil M.H. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145–2149. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 8.Ristagno G., Tang W., Sun S., Weil M.H. Cerebral cortical microvascular flow during and following cardiopulmonary resuscitation after short duration of cardiac arrest. Resuscitation. 2008;77:229–234. doi: 10.1016/j.resuscitation.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Chopra A.S., Wong N., Ziegler C.P., Morrison L.J. Systematic review and meta-analysis of hemodynamic-directed feedback during cardiopulmonary resuscitation in cardiac arrest. Resuscitation. 2016;101:102–107. doi: 10.1016/j.resuscitation.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Morgan R.W., Kilbaugh T.J., Shoap W. A hemodynamic-directed approach to pediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation. 2017;111:41–47. doi: 10.1016/j.resuscitation.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naim M.Y., Sutton R.M., Friess S.H. Blood Pressure- and Coronary Perfusion Pressure-Targeted Cardiopulmonary Resuscitation Improves 24-Hour Survival From Ventricular Fibrillation Cardiac Arrest. Crit Care Med. 2016;44 doi: 10.1097/CCM.0000000000001859. e1111-e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton R.M., Friess S.H., Naim M.Y. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190:1255–1262. doi: 10.1164/rccm.201407-1343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lautz A.J., Morgan R.W., Karlsson M. Hemodynamic-Directed Cardiopulmonary Resuscitation Improves Neurologic Outcomes and Mitochondrial Function in the Heart and Brain. Crit Care Med. 2019;47 doi: 10.1097/CCM.0000000000003620. e241-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton R.M., Friess S.H., Bhalala U. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84:696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friess S.H., Sutton R.M., Bhalala U. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41:2698–2704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan R.W., French B., Kilbaugh T.J. A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: Diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation. 2016;104:6–11. doi: 10.1016/j.resuscitation.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan R.W., Sutton R.M., Karlsson M. Pulmonary Vasodilator Therapy in Shock-associated Cardiac Arrest. Am J Respir Crit Care Med. 2018;197:905–912. doi: 10.1164/rccm.201709-1818OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg R.A., Hilwig R.W., Kern K.B., Babar I., Ewy G.A. Simulated mouth-to-mouth ventilation and chest compressions (bystander cardiopulmonary resuscitation) improves outcome in a swine model of prehospital pediatric asphyxial cardiac arrest. Crit Care Med. 1999;27:1893–1899. doi: 10.1097/00003246-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Berg R.A., Hilwig R.W., Kern K.B., Ewy G.A. "Bystander" chest compressions and assisted ventilation independently improve outcome from piglet asphyxial pulseless "cardiac arrest". Circulation. 2000;101:1743–1748. doi: 10.1161/01.cir.101.14.1743. [DOI] [PubMed] [Google Scholar]
- 20.Berg R.A., Otto C.W., Kern K.B. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med. 1994;22:282–290. doi: 10.1097/00003246-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Brown C.G., Werman H.A., Davis E.A., Hobson J., Hamlin R.L. The effects of graded doses of epinephrine on regional myocardial blood flow during cardiopulmonary resuscitation in swine. Circulation. 1987;75:491–497. doi: 10.1161/01.cir.75.2.491. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez R., Urbano J., Botran M. Adrenaline, terlipressin, and corticoids versus adrenaline in the treatment of experimental pediatric asphyxial cardiac arrest. Pediatr Crit Care Med. 2014;15 doi: 10.1097/PCC.0000000000000127. e280-7. [DOI] [PubMed] [Google Scholar]
- 23.Lindner K.H., Prengel A.W., Pfenninger E.G. Vasopressin improves vital organ blood flow during closed-chest cardiopulmonary resuscitation in pigs. Circulation. 1995;91:215–221. doi: 10.1161/01.cir.91.1.215. [DOI] [PubMed] [Google Scholar]
- 24.Panchal A.R., Berg K.M., Hirsch K.G. 2019 American Heart Association Focused Update on Advanced Cardiovascular Life Support: Use of Advanced Airways, Vasopressors, and Extracorporeal Cardiopulmonary Resuscitation During Cardiac Arrest: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2019;140 doi: 10.1161/CIR.0000000000000732. e881-e94. [DOI] [PubMed] [Google Scholar]
- 25.Mayr V.D., Wenzel V., Voelckel W.G. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation. 2001;104:1651–1656. doi: 10.1161/hc3901.095896. [DOI] [PubMed] [Google Scholar]
- 26.Tayama E., Ueda T., Shojima T. Arginine vasopressin is an ideal drug after cardiac surgery for the management of low systemic vascular resistant hypotension concomitant with pulmonary hypertension. Interact Cardiovasc Thorac Surg. 2007;6:715–719. doi: 10.1510/icvts.2007.159624. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg L., Liao Q., Steen S. The effects of epinephrine/norepinephrine on end-tidal carbon dioxide concentration, coronary perfusion pressure and pulmonary arterial blood flow during cardiopulmonary resuscitation. Resuscitation. 2000;43:129–140. doi: 10.1016/s0300-9572(99)00129-x. [DOI] [PubMed] [Google Scholar]
- 28.Thorup L., Koch K.U., Upton R.N., Ostergaard L., Rasmussen M. Effects of Vasopressors on Cerebral Circulation and Oxygenation: A Narrative Review of Pharmacodynamics in Health and Traumatic Brain Injury. J Neurosurg Anesthesiol. 2019 doi: 10.1097/ANA.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 29.Ristagno G., Tang W., Huang L. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med. 2009;37:1408–1415. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- 30.Putzer G., Martini J., Spraider P. Effects of different adrenaline doses on cerebral oxygenation and cerebral metabolism during cardiopulmonary resuscitation in pigs. Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Perkins G.D., Ji C., Deakin C.D. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2018;379:711–721. doi: 10.1056/NEJMoa1806842. [DOI] [PubMed] [Google Scholar]
- 32.Prengel A.W., Lindner K.H., Keller A. Cerebral oxygenation during cardiopulmonary resuscitation with epinephrine and vasopressin in pigs. Stroke. 1996;27:1241–1248. doi: 10.1161/01.str.27.7.1241. [DOI] [PubMed] [Google Scholar]
- 33.Wenzel V., Lindner K.H., Krismer A.C. Survival with full neurologic recovery and no cerebral pathology after prolonged cardiopulmonary resuscitation with vasopressin in pigs. J Am Coll Cardiol. 2000;35:527–533. doi: 10.1016/s0735-1097(99)00562-8. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel V., Lindner K.H., Baubin M.A., Voelckel W.G. Vasopressin decreases endogenous catecholamine plasma concentrations during cardiopulmonary resuscitation in pigs. Crit Care Med. 2000;28:1096–1100. doi: 10.1097/00003246-200004000-00031. [DOI] [PubMed] [Google Scholar]
- 35.Voelckel W.G., Lurie K.G., McKnite S. Comparison of epinephrine and vasopressin in a pediatric porcine model of asphyxial cardiac arrest. Crit Care Med. 2000;28:3777–3783. doi: 10.1097/00003246-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Voelckel W.G., Lurie K.G., McKnite S. Effects of epinephrine and vasopressin in a piglet model of prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2002;30:957–962. doi: 10.1097/00003246-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Mentzelopoulos S.D., Malachias S., Chamos C. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310:270–279. doi: 10.1001/jama.2013.7832. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien C.E., Santos P.T., Reyes M. Association of diastolic blood pressure with survival during paediatric cardiopulmonary resuscitation. Resuscitation. 2019;143:50–56. doi: 10.1016/j.resuscitation.2019.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosrati R., Lin S., Mohindra R., Ramadeen A., Toronov V., Dorian P. Study of the Effects of Epinephrine on Cerebral Oxygenation and Metabolism During Cardiac Arrest and Resuscitation by Hyperspectral Near-Infrared Spectroscopy. Crit Care Med. 2019;47 doi: 10.1097/CCM.0000000000003640. e349-e57. [DOI] [PubMed] [Google Scholar]
- 40.Mavroudis C.D., Ko T.S., Morgan R.W. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit Care. 2020;24:583. doi: 10.1186/s13054-020-03297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berg R.A., Sutton R.M., Holubkov R. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41:2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel V., Krismer A.C., Arntz H.R. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]
- 43.Gueugniaud P.Y., David J.S., Chanzy E. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359:21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 44.Mentzelopoulos S.D., Zakynthinos S.G., Siempos I., Malachias S., Ulmer H., Wenzel V. Vasopressin for cardiac arrest: meta-analysis of randomized controlled trials. Resuscitation. 2012;83:32–39. doi: 10.1016/j.resuscitation.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Wenzel V., Lindner K.H., Krismer A.C., Miller E.A., Voelckel W.G., Lingnau W. Repeated administration of vasopressin but not epinephrine maintains coronary perfusion pressure after early and late administration during prolonged cardiopulmonary resuscitation in pigs. Circulation. 1999;99:1379–1384. doi: 10.1161/01.cir.99.10.1379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.