Abstract
Aim
This study investigates the potentially adverse association between extracorporeal cardiopulmonary resuscitation (ECPR) after cardiac arrest on weekends versus weekdays.
Methods
Single-centre, retrospective, stratified (weekday versus weekend) analysis of 318 patients who underwent in-hospital ECPR after out-of-hospital and in-hospital cardiac arrest (OHCA/IHCA) between 01/2008 and 12/2018. Weekend was defined as the period between Friday 17:00 and Monday 06:59.
Results
Seventy-three patients (23%) received ECPR during the weekend and 245 arrests (77%) occurred during the weekday. Whereas survival to discharge did not differ between both groups, long-term survival was significantly lower in the weekend group (p = 0.002). In the multivariate analysis, independent risk factors associated with hospital mortality were no flow time (OR 1.014; 95% CI 1.004–1.023) and serum lactate prior ECPR (OR 1.011; 95% CI 1.006–1.012), whereas each unit serum haemoglobin above average had a protective effect on in-hospital mortality (OR 0.87; 95% CI 0.79–0.96). New onset kidney failure requiring renal replacement therapy occurred more often in the weekend group (30.1% versus 18.4%; p = 0.04). One third of patients experienced complications regardless ECPR was initiated at weekdays or weekends.
Conclusion
Extracorporeal cardiopulmonary resuscitation at weekends adversely seems to impact long-term survival regardless timing (dayshift/nightshift). Duration of CPR and serum lactate prior ECPR were demonstrated as independent risk factors for in-hospital mortality. As ECPR at weekends could not be shown to be an independent outcome predictor a thorough analysis of clinical events subsequent to this intervention is warranted to understand long-term consequences of ECPR initiation after cardiac arrest.
Keywords: ECPR, Weekend effect, Long-term survival
Introduction
Sudden cardiac death remains a major source of mortality in developed economies despite recent improvements in primary and secondary prevention.1 The incidence varies between 50–100 per 100,000 in the general population2 and a recent report estimated 292,000 in-hospital cardiac arrests in US adults.3 Extracorporeal life support, realized as veno-arterial extracorporeal membrane oxygenation (ECMO), has been gradually incorporated in cardiopulmonary resuscitation4, 5 and recent meta-analyses suggest improved survival to discharge in patients who received ECPR compared to conventional CPR (CCPR).6, 7, 8 Nevertheless, there is inconclusive evidence to either support or refute ECPR for in- and out-of hospital cardiac arrest.9, 10 Survival after in-hospital cardiac arrest is affected by time of day and day of week where survival rates were lower during nights and weekends even when adjusted for potentially confounding patient, event and hospital characteristics.11 A recent meta-analysis showed that hospital inpatients admitted during weekends may have a higher mortality rate compared to those admitted during weekdays.12
Schopka et al. expanded available evidence by investigating survival after ECPR on weekends in a single Korean centre13 and demonstrated lower survival and resuscitation quality including higher cannulation failure and complication rate.
Overall evidence for the association of adverse outcome after ECPR at weekends and timing is sparse. Although many institutional ECMO programs are set up with a 24/7 service and dedicated resources, short-term outcome may not be associated with initial timing of ECLS, regardless day of the week.
We analysed the outcome of 318 patients after witnessed IHCA/OHCA who underwent ECPR in our institution between January 2009 and December 2018.
Methods
Patients
A retrospective analysis of 318 patients who received ECPR by means of femoral percutaneous access between January 2009 and December 2018 was performed to analyse outcome and identify potential risk factors for adverse outcome.
Decision-making on ECPR at the index institution is made on a case-by-case basis. Age per se is no contraindication, but ECPR is not considered in patients with advanced basic life support for less than 15 min, known irreversible brain injury, known terminal malignancy, trauma with uncontrolled bleeding, unwitnessed circulatory arrest and in patients who declared to wish no life-prolonging mechanical support.
Percutaneous cannulation was performed by an ECMO-experienced intensivist at bedside, in the emergency room (for OHCA) or in the cardiac catheter laboratory using a modified Seldinger technique. A preliminary ultrasound assessment of both groins was done in the vast majority of all cases. In every patient catecholamine support via continuous infusion was already started during CPR.
Long-term follow-up was obtained via personal phone contact once a year and subsequent documentation in our institutional database.
Institutional Review Board approval was obtained prior data analysis (No. 15-101-0051) and the need for informed consent was waived due to the retrospective design.
Definitions
Weekend was defined as the time from Friday 17:00 to Monday 06:59. Nightshift was defined as the time between 17:00 and 06:59 regardless the day of the week. Cardiac arrest was the first arrest event during the index hospitalisation. CPR to pump-on time was the interval from the first chest compression or defibrillation until beginning of extracorporeal circulation.
Cerebral performance categories (CPC) scale was used to assess neurologic outcome at hospital discharge, whereas CPC 1 is a return to normal cerebral function and normal living and CPC 5 indicates brain death.
Lactate clearance in percent after 24 h was estimated as [(serum lactateinitial – serum lactate24 h)/serum lactate24 h] × 100. Cannulation related problems refer to difficult vascular access with multiple punctures, multiple uses of disposables and cannulation site bleeding during cannulation.
ECLS equipment and ECLS management
Equipment from four manufacturers (Maquet Cardiopulmonary GmbH, Xenios AG, LivaNova PLC and Zoll Medical Corporation) was used during the study period. The institutional management of patients on ECLS was described previously.14 Distal limb perfusion and routine limb monitoring with NIRS was utilized in selected patients since September 2011.
Statistical analysis
Data were analysed with Stata SE 16.0 (StataCorp. LLC; College Station, TX, USA). Continuous data were presented as mean with standard deviation if normally distributed. Non-normally distributed continuous data were presented as median with interquartile range (25th–75th percentile). Normality was estimated graphically with quantile-quantile plots and formally with the Shapiro–Wilk test.
Categorical data were presented as frequencies and percentage. Intergroup comparisons for normally distributed continuous data were done with Student’s t-test and for non-normally distributed data with the rank sum test. Differences including confidence intervals between medians were assessed by quantile regression. Categorical data in n × k contingency tables were analysed with the chi-square test (Pearson) and for 2 × 2 tables with Fisher’s exact test. Chi-squared test for trend was used to determine a (linear) trend among ordered proportions.
Survival as time-to-event data was analysed with the Kaplan–Meier estimate and the logrank test was used to estimate differences in survival times. Univariate and multivariate logistic regression was used to identify predictor variables for in-hospital mortality. Calibration and discriminatory performance were tested with the Hosmer-Lemeshow goodness of fit test and ROC analysis.
All tests were two-sided and a p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
We analysed 318 patients who experienced cardiac arrest and received extracorporeal cardiopulmonary resuscitation between January 2009 and December 2018. We observed a steady increase in ECPR between 2009 with only 15 procedures and 2018 with 64 procedures. Almost every fourth patient (73/318; 23%) received ECPR during the weekend. Survival to discharge after cardiac arrest was lowest (≈30%) compared to other indications for the use of veno-arterial ECMO during the index timeframe.
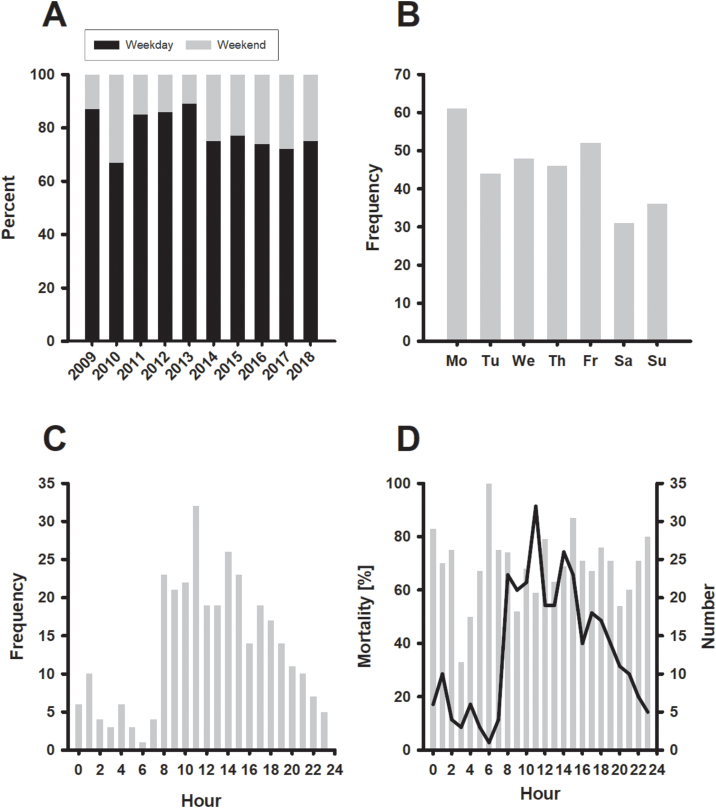
Fig. 1 summarizes the relative distribution of ECPR on weekdays versus weekends (Panel A). Although slight variation over time was observed, the proportion of ECPR on weekends was stable at around 30% since 2013. As shown in Panel B, most procedures occurred on Mondays (n = 61; 19.2%) followed by Fridays (n = 52; 16.3%) and Wednesdays (n = 48; 15%). The distribution by hour showed a sharp increase at 08:00, peaking at 11:00 before declining until midnight.
Fig. 1.
Panel A: Proportion of patients who received ECPR during weekday and weekend (Friday 17:00 to Monday 06:59) stratified by years 2009 to 2018. Panel B: Number of patients who underwent ECPR stratified by weekday. Panel C: Number of ECPR patients by hour. Panel D: Mortality (grey bars) by hour. The solid line represents the total number of patients, who received ECPR in the index hour.
Table 1 summarizes the patient characteristics prior ECPR. The arterial pH was higher in patients in the weekday group (mean difference +0.10; 95% CI 0.045 to 0.173). A prior cardiac surgical procedure was more often present in the weekday group (22.4% versus 8.2%; p = 0.006). Although not statistically significant, the time between CPR start and Pump-on was shorter in the weekday group (minus 8 min; 95% CI -17 to 1).
Table 1.
Patient characteristics.
| Variable | Weekday (n = 245) | Weekend (n = 73) | P-value |
|---|---|---|---|
| Age (years) | 58.3 (49.7; 67.1) | 55.5 (47; 65.6) | 0.18 |
| Gender (n; %) | |||
| Female | 68 (28%) | 15 (21%) | 0.29 |
| Male | 177 (72%) | 58 (79%) | |
| Time CPR start to Pump On (min) | 42 (IQR 26–65) | 58 (IQR 34–73) | 0.02 |
| OHCA | 50 (IQR 33–75) | 60 (IQR 47–65) | 0.21 |
| IHCA | 36 (IQR 22–60) | 51 (IQR 31–78) | 0.01 |
| Weight (kg) | 80 (IQR 70; 95) | 80 (IQR 70; 90) | 0.60 |
| Body mass index (kg × m−2) | 28.3 ± 6.5 | 27.3 ± 4.9 | 0.16 |
| ECPR during | |||
| Dayshift (n; %) | 191 (78%) | 47 (64.4%) | 0.02 |
| Nightshift (n; %) | 54 (22%) | 26 (35.6%) | |
| Major indications for ECPR (n; %) | |||
| Acute myocardial infarction | 98 (40 %) | 35 (47.9 %) | 0.52 |
| Pulmonary embolism | 27 (11 %) | 9 (12.3 %) | |
| Ventricular fibrillation | 27 (11 %) | 8 (11 %) | |
| Dilated Cardiomyopathy | 15 (6.1%) | 4 (5.5%) | |
| Other | 78 (31.8 %) | 17 (23.3 %) | |
| Prior cardiac surgery (n; %) | 55 (22.4%) | 6 (8.2%) | 0.006 |
| SOFA score | 15 (12; 18) | 15 (14; 17) | 0.81 |
| LIS score | 2.6 ± 0.9 | 2.8 ± 0.7 | 0.06 |
| Out of hospital cardiac arrest | 99 (40.4 %) | 21 (28.8 %) | 0.08 |
| Renal replacement therapy (n; %) | 31 (12.6%) | 6 (8.2%) | 0.40 |
| Number of failed organs (n; %) | |||
| 1 | 166/243 (68.3%) | 42/72 (58.3%) | 0.28 |
| 2 | 66/243 (27.2%) | 24/72 (33.3%) | |
| >2 | 11/243 (4.5%) | 6/72 (8.3%) | |
| Patient cardiopulmonary and laboratory values at the time of ECMO start | |||
| Lactate (mmol × l−1) | 12.21 ± 5.33 | 13,1 ± 6,11 | 0.25 |
| Norepinephrine; standardized (μg × kg−1 × min−1 × m−2) | 0.23 (IQR 0; 0.47) | 0.21 (IQR 0; 41) | 0.75 |
| Epinephrine; standardized (μg × kg−1 × min−1 × m-2) | 0.15 (IQR 0; 0.37) | 0.14 (IQR 0; 0.26) | 0.67 |
| Mean arterial pressure (mm Hg) | 42 ± 13 | 39 ± 12 | 0.09 |
| Hemoglobin (g × dl−1) | 10.1 ± 2.7 | 10.7 ± 2.5 | 0.07 |
| Tidal volume (ml) | 456 (IQR 400; 500) | 456 (IQR 400; 513.5) | 0.53 |
| PEEP (mbar) | 9.7 ± 3.4 | 10.5 ± 3.3 | 0.14 |
| FiO2:O2 Ratio | 73 (IQR 55; 126) | 67 (IQR 57; 85.5) | 0.40 |
| Arterial paO2 (mmHg) | 70 (IQR 54; 106) | 67 (IQR 57; 85.5) | 0.68 |
| Arterial paCO2 (mmHg) | 49 (IQR 41.5; 66) | 62 (IQR 47.5; 77) | 0.01 |
| Arterial pH | 7.14 ± 0.18 | 7.03 ± 0.20 | <0.001 |
| LDH (U/l) | 390 (IQR 263; 619) | 466 (IQR 326; 678) | 0.12 |
| CRP (mg × dl−1) | 10 (IQR 3; 46) | 7 (IQR 3; 19) | 0.20 |
| Prothrombin time (%) | 53 (IQR 38; 74) | 56 (IQR 31.5; 72.5) | 0.58 |
| D-Dimer (mg × l−1) | 19 (IQR 6; 35) | 30 (IQR 9; 35) | 0.09 |
| Thrombocytes (n × nl−1) | 161 (IQR 116; 216) | 163 (IQR 112; 238) | 0.81 |
| Plasma-free hemoglobin (mg × dl−1) | 289 (IQR 151; 585) | 367 (IQR 228; 564) | 0.43 |
| Bilirubin (mg × dl−1) | 1.2 ± 3.0 | 0.58 ± 0.5 | 0.006 |
| GOT (U × l−1) | 112 (IQR 45; 257) | 155 (IQR 85; 257) | 0.12 |
Clinical outcome
Table 2 displays clinical outcome data for both groups. Median extracorporeal support did not differ, and it was 2 days. Fig. 1 Panel D shows long-term mortality versus hour of ECPR initiation. Mortality follows a similar pattern as the frequency of ECPR runs (Fig. 1 Panel C). Long-term mortality was significantly higher in patients with ECPR on a weekend compared ECPR on a weekday (87.7% vs. 73.5%; p = 0.01). The mortality rate per 1000 days was 2.0 (95% CI 1.7–2.4) for patients who received ECPR at weekdays, compared to 4.3 deaths (95% CI 3.3–5.5) per 1000 days for those at weekends.
Table 2.
Clinical outcome.
| Variable | Weekday n = 245) | Weekend (n = 73) | P-value |
|---|---|---|---|
| Days on ECLS | 2 (IQR 1; 4) | 2 (IQR 1; 4) | 0.91 |
| Length of Hospital stay (days) | 27 (IQR 21; 43) | 45 (IQR 23; 67) | 0.15 |
| Overall Mortality (n; %) (in-hospital and during follow-up) |
180 (73.5%) | 64 (87.7%) | 0.011 |
| Mortality in patients w/o cardiac surgery (n; %) | 140/190 (73.7%) | 60/67 (89.5%) | 0.006 |
| Mortality in patients with prior cardiac surgery (n; %) | 40/55 (72.7%) | 4/6 (66.7%) | >0.99 |
| 30-Day mortality (n; %) | 156 (63.7%) | 55 (75.3%) | 0.07 |
| 60-Day mortality (n; %) | 164 (66.9%) | 55 (75.3%) | 0.20 |
| Survival to index hospital discharge (n; %) | 81 (33%) | 19 (26%) | 0.31 |
| Cause of death (n; %) | |||
| Cerebral hypoxia | 73/164 (44.5%) | 26/56 (46.4%) | 0.55 |
| Low Cardiac Output | 28/164 (17.1%) | 13/56 (23.2%) | |
| Multi organ failure | 24/164 (14.6%) | 4/56 (7.1%) | |
| Bleeding | 17/164 (10.4%) | 4/56 (7.1%) | |
| Cerebral Performance Category at hospital discharge (n; %) | |||
| CPC 0 | 9/81; 11.1% | 0 | 0.45 |
| CPC 1 | 56/81; 69.1% | 15/19; 78.9% | |
| CPC 2 | 11/81; 13.6% | 2/19; 10.5% | |
| CPC 3 | 4/81; 4.9% | 1/19; 5.3% | |
| CPC 4 | 1/81; 1.2% | 1/19; 5.3% | |
| Peak NSE (μg × l−1) | 71 (IQR 45; 159) | 89 (IQR 42; 191) | 0.72 |
| Lactate (mmol × l−1) 24 h after arrest | 7.33 ± 5,88 | 6.88 ± 5.55 | 0.65 |
| Lactate ‘clearance’ (%) | 46% (IQR 13; 73) | 54% (IQR 27; 73) | 0.34 |
| Lactate clearance (n; %) | |||
| Positive | 145 (75.5%) | 35 (83.3%) | 0.32 |
| Negative | 47 (24.5%) | 7 (16.7%) | |
| Peak Plasma-free hemoglobin (mg × l−1) | 321 (IQR 168; 614) | 428 (IQR 226; 668) | 0.14 |
| New onset acute kidney failure with RRT (n; %) | 45 (18.4%) | 22 (30.1%) | 0.04 |
| RBC transfusions (n) | |||
| 0 | 111/245 | 35/73 | 0.15 |
| 1 < 3 | 34/245 | 12/73 | |
| 3 < 5 | 21/245 | 9/73 | |
| 5 < 10 | 46/245 | 8/73 | |
| >10 | 33/245 | 9/73 | |
| FFP transfusions (n) | |||
| 0 | 165/244 | 54/73 | 0.43 |
| 1 < 3 | 6/244 | 4/73 | |
| 3 < 5 | 18/244 | 2/73 | |
| 5 < 10 | 32/244 | 8/73 | |
| >10 | 23/244 | 5/73 | |
| Platelet transfusions > 1 concentrate (n; %) | 66/241 (27.3%) | 11/73 (15.0%) | 0.04 |
| Mean number of membrane oxygenators | 1.14 ± 0.46 | 1.19 ± 0.54 | 0.45 |
| Complications (n; %) | 87 (35.5%) | 24 (32.8 %) | 0.78 |
| Cannulation site bleeding | 9/87 (10.3%) | 4/24 (16.7%) | 0.47 |
| Limb ischemia | 22/87 (25.3%) | 4/24 (16.7%) | 0.43 |
| Failed distal limb perfusion | 6/87 (6.9%) | 3/24 (12.5%) | 0.4 |
| GI-bleeding | 3/87 (3.4%) | 4/24 (16.7%) | 0.04 |
| HIT | 5/87 (5.8%) | 1/24 (4.2%) | >0.99 |
| Other | 42/87 (55.5%) | 8/24 (33.3%) | 0.1 |
| Cannulation related problems (n, %) | 79/245 (32.2%) | 19/73 (26%) | 0.39 |
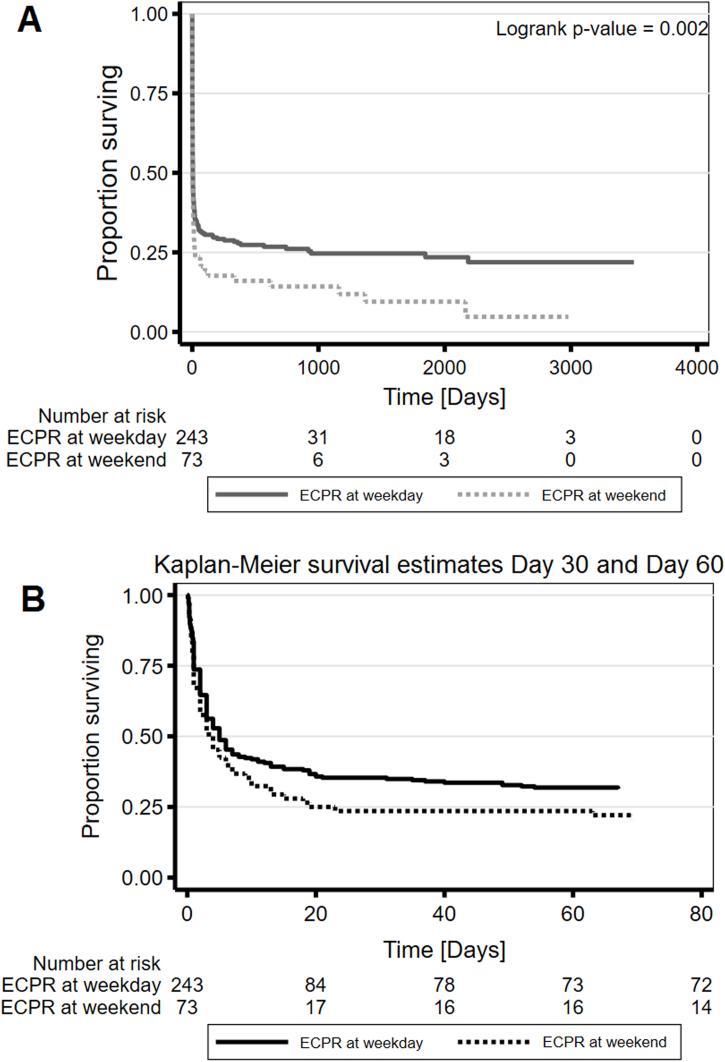
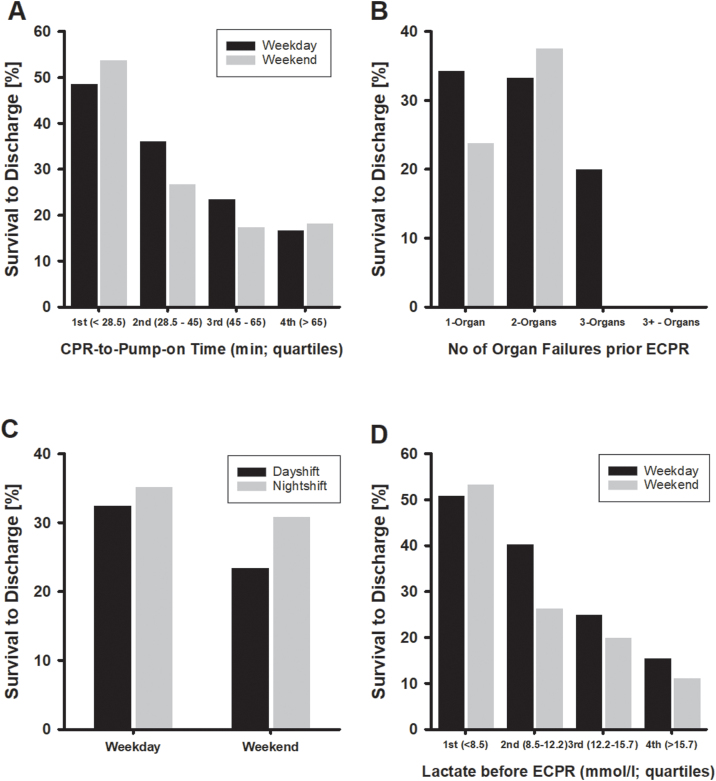
Fig. 2 illustrates a significant difference in long-term mortality between both groups (p = 0.002), whereas 30-day mortality did not differ. Fig. 3 shows survival to discharge in relation to CPR-to-Pump-on time, number of organ failures prior ECPR, day-/nightshift distinction and serum lactate before ECPR. Survival to discharge was inversely associated with CPR-to Pump-on time regardless weekday/weekend distinction (Panel A). Around 50% survival occurred in the group of patients with up to 28.5 min (95% CI 25–30; 1st quartile) CPR-to Pump-on time, whereas 20% survival was observed in those patients with >65 min until extracorporeal support was initiated. Serum lactate prior ECPR inversely correlated with survival to discharge (Panel D) with survival to discharge of around 50% in patients with serum lactate values <855 mmolxl−1 (1st quartile).
Fig. 2.
Panel A: Long-term mortality stratified by weekday/weekend. There is a significant difference in mortality (logrank p-value = 0.002). Panel B: In-hospital mortality within the first 60 days. About 50% mortality occurs within the first 5 days after initiation of ECPR. OR for day 30 is 1.74 (95% CI 0.94–3.35) with a p-value = 0.07, OR for day 60 is 1.50 (95% CI 0.80–2.91) with a p-value = 0.20.
Fig. 3.
Panel A: Survival to discharge versus CPR-to-ECPR time (in quartiles) stratified by weekday/weekend. Panel B: Survival to discharge versus number of organ failures stratified by weekday/weekend. There was no survival to discharge if more than three organs failed. Panel C: Survival to discharge versus day-/nightshift stratified by weekday/weekend. Panel D: Survival to discharge versus serum lactate prior ECPR stratified by weekday/weekend.
New onset acute kidney failure with renal replacement therapy occurred more often in patients with ECPR at the weekends (30.1% vs. 18.4%; p = 0.04). Acute kidney failure occurred in 24/100 (24%) of survivors to discharge and was not statistically significant different compared to non-survivors (43/218; 19.8%). Regardless of timing, survivors to discharge had a significantly higher median lactate clearance after 24 h after ECPR initiation compared to non-survivors (64% vs. 39%; p < 0.001). Adverse neurological outcome (CPC categories 3 and 4) occurred in 6.1% of patients in the weekday and in 10.6% of patients in the weekend group (Table 2). Cannulation-related problems were observed in almost one third of patients regardless timing.
Risk factors for in-hospital mortality
Table 3 summarizes the results from uni- and multivariate logistic regression. Time of CPR until initiation of ECLS (Odds ratio 1.014; 95% CI 1.004–1.023) and serum lactate prior ECLS (Odds ratio 1.011; 95% CI 1.006–1.012) were identified as two independent risk factors for adverse outcome, whereas serum haemoglobin level before ECLS showed a protective effect (OR 0.87; 95% CI 0.79 to 0.96). Each minute of CPR above average until extracorporeal support starts adds 1.4% hospital mortality if adjusted for serum lactate and haemoglobin and each mg per decilitre serum lactate above average adds 1.1% hospital mortality after adjustment. On average, non-survivors had 14.9 additional minutes CPR (95% CI 6.9–23; p < 0.001) until extracorporeal support started. Each gram per decilitre above average reduces hospital mortality by 13.1% if adjusted for serum lactate and time of CPR to pump-on.
Table 3.
Factors potentially associated with hospital mortality.
| Predictor variables | Unadjusted - univariate |
Adjusted - multivariate |
P-value | ||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Time CPR – Pump ona | 1.02 | 1.00–1.03 | 1.014 | 1.004–1.023 | 0.004 |
| Lactate prior ECPRa | 1.01 | 1.00–1.02 | 1.011 | 1.006–1.012 | <0.001 |
| MAP prior ECPRa | 0.98 | 0.96–0.99 | |||
| pH prior ECPRb | 0.02 | 0.004–0.14 | |||
| Arterial pCO2b | 1.00 | 0.99–1.02 | |||
| D-Dimer prior ECPR | 1.03 | 1.01–1.05 | |||
| Haemoglobin prior ECPRa | 0.91 | 0.83–1.00 | 0.869 | 0.786–0.961 | 0.007 |
| ECPR on weekend | 1.40 | 0.78–2.52 | |||
| Prior cardiac surgery | 0.77 | 0.58–1.03 | |||
| Presence of complications | 1.38 | 0.83–2.30 | |||
| New onset acute kidney failure | 0.78 | 0.44–1.38 | |||
| Serum bilirubin | 1.2 | 0.92–1.2 | |||
| Cannulation related issues | 1.43 | 0.84–2.42 | |||
Multivariate model: Goodness-of-fit was acceptable (Hosmer-Lemeshow p-value 0.40 (χ2 = 2.91) and moderate discriminatory performance in the ROC-analysis (AUC = 0.72; 95% CI 0.66 to 0.78).
Variables included in the first multivariate logistic model.
Not included in multivariate logistic model due to strong negative correlation between pH and lactate.
Discussion
This retrospective single-centre analysis from an ELSO Centre of Excellence showed impaired long-term survival of patients who underwent ECPR at weekends. Survival to discharge and reported complications were similar in both groups and statistically not significant different. From this perspective, this analysis clearly contrasts the finding from Schopka et al. from 2016 who found significantly higher mortality and complications in patients with ECPR at weekends.13 If adjusted for ECPR-related variables, the odds ratios for survival to discharge were not significantly different. The authors concluded that ECPR-related variables and not baseline comorbidity may have contributed to the lower survival in the weekend group. To date, the Korean single-centre registry analysis is the only published report on the impact of the ‘weekend effect’ on ECPR patients. Fagnoul et al. summarized available data for ECPR in out-of-hospital cardiac arrest settings and emphasised the impact of arrest time to ECMO start15 and a 10-year comparative analysis of veno-arterial ECMO for refractory cardiac arrest in 131 patients revealed a mortality of 82.4%.16 We could confirm a recent finding from a Swedish cohort study17 with 72 patients with cardiac arrest prior to veno-arterial ECMO where arterial lactate was identified as an independent risk factor (OR 1.15; 95% CI 1.01–1.31) for 90-day mortality. These findings were in line with the results of a systemic review and meta-analysis by d’Arrigo et al.18 We are also in line with numerous previous publications that showed CPR-to Pump-on time as an independent risk factor for hospital mortality; regardless weekday/weekend timing. Our findings indicate that differences in infrastructure may be crucial. Whereas Schopka et al.13 demonstrated impaired quality of care at weekends and during night-time, our centre established ECPR teams with physicians and perfusionists trained and experienced in extracorporeal support. These teams are available at a 24/7 service level regardless day of the week. This approach facilitates a high level of care resulting in low rates of cannulation failure and constant survival to hospital discharge rates.
The effect of haemoglobin level on survival to discharge after ECPR has been investigated by several groups,19, 20, 21 but results are heterogeneous. Whereas the protective effect of haemoglobin disappeared in multivariate analysis,19 a Korean study20 with 115 patients with cardiac arrest identified lower haemoglobin levels before ECPR as an independent risk factor for poor neurological outcome (OR 1.5; 95% CI 1.07–2.10). Although the SOS-Kanto study demonstrated the association of favourable neurological outcome in cardiac arrest with higher pre-arrest haemoglobin values, the potential association of haemoglobin and outcomes in ECPR patients requires further investigation. There is no uniform definition on low or lower haemoglobin values and often hospitalized patients underwent interventions or surgeries with volume administration before arrest. From a simplified physiological point of view it is easy to assume that the extend of ischemia and reperfusion injury during and after cardiac arrest should depend on oxygen delivery capacities but up to now there is no conclusive scientific evidence for that. Our cohort includes patients with previous cardiac surgery and others with haemorrhagic and septic shock and therefore the reported protective effect of haemoglobin deserves careful interpretation.
The impaired long-term survival in patients with ECPR at weekends remains challenging to explain and a gap in available clinical evidence calls for further research. A recent analysis of more than 12,000 paediatric patients with cardiac arrest showed after adjustment for potential confounders that ECPR during nights (23:00 to 06:59) was associated with impaired survival to discharge, whereas no effect of timing on outcome between weekdays and weekends was observed.22 Another study demonstrated that outcomes were not significantly worse for paediatric patients undergoing ECLS cannulation during off-hours (19:00–07:00) compared to normal weekday working hours.23
Multiple extracerebral organ dysfunction after return of spontaneous circulation in post cardiac arrest syndrome is associated with long-term outcome24 and gross healthcare related expenses.25 In our cohort neither survival to discharge nor cerebral performance were statistically significant between both groups. This finding might be explained, at least partially, by the fact that early in-hospital mortality mainly depends on therapy withdrawal and cerebral hypoxia. Thus, similar CPR to Pump-on times and immediately available ECMO teams and thus similar short-term outcomes can be expected regardless timing of ECPR. Long-term outcome mainly depends on multi-organ dysfunction and failure because of prolonged shock and stabilization during the initial period after arrest. Differences in long-term outcome may be explained by differences in early patient care during weekends compared to weekdays. Even in institutions with sophisticated infrastructure and a 24/7 ECPR service, variations in experience among personnel, for example at intensive care units and rapid availability of diagnostic and laboratory tests at weekends have a relevant impact on long-term organ function.
Our data indicate the importance of organ dysfunction, e.g. renal impairment, but further research with focus on early care is required to disentangle the weekend effect on long-term outcome.
Conclusions
We conclude that ECPR after cardiac arrest at weekends, defined as the period between Friday 17:00 and Monday 06:59, results in lower long-term survival compared to patients who receive ECPR at weekdays and regardless the distinction between night- and dayshift care. As only time of CPR until extracorporeal support and serum lactate prior ECMO but not timing of ECPR itself have been identified as independent risk factors for hospital mortality a thorough analysis of clinical care and events along the entire care pathway is warranted to comprehend long-term consequences of ECPR after cardiac arrest.
Ethics
Institutional Review Board approval was obtained prior data analysis (No. 15-101-0051) and the need for informed consent was waived due to the retrospective design.
Conflicts of interest
C. Diez is faculty member at the University Medical Centre Regensburg and currently a full-time employee of the Getinge AB, Sweden.
All other authors have no conflicts of interest related to this work.
CRediT authorship contribution statement
Dirk Lunz: Investigation, Writing - original draft, Supervision, Project administration. Daniele Camboni: Investigation, Writing - original draft, Visualization, Supervision. Alois Philipp: Investigation, Project administration. Bernhard Flörchinger: Investigation, Writing - review & editing. Armando Terrazas: Writing - review & editing, Data curation. Thomas Müller: Investigation, Writing - review & editing. Christof Schmid: Supervision, Project administration. Claudius Diez: Validation, Formal analysis, Software, Supervision, Writing - original draft.
Acknowledgements
We would like to thank all perfusionists at our institution for their dedicated work. In addition, we would like to thank all intensive care personnel including nurses for their daily work with ECMO patients. This study would not have been possible without their support and passion.
References
- 1.Deo R., Albert C.M. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125:620–637. doi: 10.1161/CIRCULATIONAHA.111.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishman G.I., Chugh S.S., Dimarco J.P. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmberg M.J., Ross C.E., Fitzmaurice G.M. Annual Incidence of Adult and Pediatric In-Hospital Cardiac Arrest in the United States. Circ: Cardiovasc Qual Outcomes. 2019;12 [PMC free article] [PubMed] [Google Scholar]
- 4.Shin T.G., Choi J.H., Jo I.J. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011;39:1–7. doi: 10.1097/CCM.0b013e3181feb339. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y.S., Lin J.W., Yu H.Y. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang G.N., Chen X.F., Qiao L. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: a meta-analysis of 2 260 patients with cardiac arrest. World J Emerg Med. 2017;8:5–11. doi: 10.5847/wjem.j.1920-8642.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twohig C.J., Singer B., Grier G., Finney S.J. A systematic literature review and meta-analysis of the effectiveness of extracorporeal-CPR versus conventional-CPR for adult patients in cardiac arrest. J Intensive Care Soc. 2019;20:347–357. doi: 10.1177/1751143719832162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouweneel D.M., Schotborgh J.V., Limpens J. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42:1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmberg M.J., Geri G., Wiberg S. Extracorporeal Cardiopulmonary Resuscitation for Cardiac Arrest: A Systematic Review. Resuscitation. 2018;131:91–100. doi: 10.1016/j.resuscitation.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Liu C., Huang J. Clinical efficacy of extracorporeal cardiopulmonary resuscitation for adults with cardiac arrest: meta-analysis with trial sequential analysis. BioMed Res Int. 2019;2019:6414673. doi: 10.1155/2019/6414673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peberdy M.A., Ornato J.P., Larkin G.L. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 12.Pauls L.A., Johnson-Paben R., McGready J., Murphy J.D., Pronovost P.J., Wu C.L. The weekend effect in hospitalized patients: a meta-analysis. J Hosp Med. 2017;12:760–766. doi: 10.12788/jhm.2815. [DOI] [PubMed] [Google Scholar]
- 13.Lee D.S., Chung C.R., Jeon K. Survival after extracorporeal cardiopulmonary resuscitation on weekends in comparison with weekdays. Ann Thoracic Surg. 2016;101:133–140. doi: 10.1016/j.athoracsur.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 14.Schopka S., Philipp A., Hilker M. Clinical course and long-term outcome following venoarterial extracorporeal life support-facilitated interhospital transfer of patients with circulatory failure. Resuscitation. 2015;93:53–57. doi: 10.1016/j.resuscitation.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Fagnoul D., Combes A., De Backer D. Extracorporeal cardiopulmonary resuscitation. Curr Opin Crit Care. 2014;20:259–265. doi: 10.1097/MCC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 16.Pozzi M., Armoiry X., Achana F. Extracorporeal life support for refractory cardiac arrest: a 10-year comparative analysis. Ann Thoracic Surg. 2019;107:809–816. doi: 10.1016/j.athoracsur.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Fux T., Holm M., Corbascio M., van der Linden J. Cardiac arrest prior to venoarterial extracorporeal membrane oxygenation: risk factors for mortality. Crit Care Med. 2019;47:926–933. doi: 10.1097/CCM.0000000000003772. [DOI] [PubMed] [Google Scholar]
- 18.D’Arrigo S., Cacciola S., Dennis M. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.W., Han K.S., Park J.S., Lee J.S., Kim S.J. Prognostic indicators of survival and survival prediction model following extracorporeal cardiopulmonary resuscitation in patients with sudden refractory cardiac arrest. Ann Intensive Care. 2017;7:87. doi: 10.1186/s13613-017-0309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu J.A., Cho Y.H., Sung K. Predictors of neurological outcomes after successful extracorporeal cardiopulmonary resuscitation. BMC Anesthesiol. 2015;15:26. doi: 10.1186/s12871-015-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.group S-Ks Relationship between the hemoglobin level at hospital arrival and post-cardiac arrest neurologic outcome. Am J Emerg Med. 2012;30:770–774. doi: 10.1016/j.ajem.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Bhanji F., Topjian A.A., Nadkarni V.M. Survival rates following pediatric in-hospital cardiac arrests during nights and weekends. JAMA Pediatr. 2017;171:39–45. doi: 10.1001/jamapediatrics.2016.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez K.W., Dalton B.G., Weaver K.L., Sherman A.K., St Peter S.D., Snyder C.L. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32:665–669. doi: 10.1007/s00383-016-3901-6. [DOI] [PubMed] [Google Scholar]
- 24.Roberts B.W., Kilgannon J.H., Hunter B.R. Association between elevated mean arterial blood pressure and neurologic outcome after resuscitation from cardiac arrest: results from a multicenter prospective Cohort Study. Crit Care Med. 2019;47:93–100. doi: 10.1097/CCM.0000000000003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pekkarinen P.T., Backlund M., Efendijev I. Association of extracerebral organ failure with 1-year survival and healthcare-associated costs after cardiac arrest: an observational database study. Crit Care. 2019;23:67. doi: 10.1186/s13054-019-2359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]