Delirium is an important public health problem. It is independently associated with worse clinical outcomes, including persistent cognitive impairment, increased mortality, and greater risk of institutionalization [1]. The prevalence of delirium is high in the intensive care unit (ICU), occurring in up to 70% of the sickest patients requiring mechanical ventilation [1]. Early studies in hospitalized patients with coronavirus disease 2019 (COVID-19) report delirium rates of 20–30%, which increase to 60–70% in severe illness [1]. An international multicenter cohort study that included 69 adult ICUs across 14 countries of 2088 COVID-19 patients reported that over a 21-day period, delirium had a prevalence of 55% and lasted a median of 3 days (IQR, 2–6 days) [2], which is more common and prolonged than that in non-COVID cohorts.
Despite its frequency, the pathophysiology of delirium remains poorly understood. Multiple factors are associated with delirium, many of which are coincident with critical illness (e.g., mechanical ventilation), and therefore clinical-pathological correlation has been difficult to prove as it is challenging to disentangle delirium attributable risk from the impact of general critical illness itself. Current evidence suggests that medications (e.g., benzodiazepines), systemic inflammation and/or the acute stress response may contribute to cerebral metabolic insufficiency, neuroinflammation (e.g., glial activation), neurotransmitter imbalances and deficiencies in neurological substrate and/or failure of network connectivity [3], ultimately leading to the development of delirium.
It was initially presumed that higher rates of delirium might be the result of direct neuronal SARS-CoV-2 infection. SARS-CoV-2 is a neurotropic virus that has the potential to enter the central nervous system (CNS) via angiotensin converting enzyme 2 (ACE2) receptors in the olfactory bulb [4]. Herpes simplex virus 1 infects the olfactory bulb and then the brain to cause encephalitis. Animal models have shown that some coronaviruses, including SARS-CoV, can do the same. However, in a single-cell RNA sequencing gene expression analysis of human biopsy samples, later confirmed in mouse models where deeper olfactory bulb tissue could be examined, ACE2 receptors were found in vascular cells (e.g., pericytes and immune cells of the macrophage/monocyte lineage) and not neurons [5]. Further, the SARS-CoV-2 virus has rarely been isolated from samples of cerebral spinal fluid (CSF), suggesting that viral replication within neurons is of less importance than other potential mechanisms such as immune-mediated damage within the CNS.
Indeed, the high proportion of delirium in patients with COVID-19 associated critical illness is likely due to microvascular disease and inflammatory mechanisms. Severe coagulopathy and vascular endothelial dysfunction leading to small vessel occlusions and microhemorrhages, evident on magnetic resonance imaging, contributed to delirium rates of greater than 80% in 150 patients with COVID-19-related critical illness admitted to two centres in France [6]. In mouse models, SARS-CoV-2 infection induces a hypermetabolic state and resultant hypoxic local environment, suggesting an underlying association between viral infection and ischemic infarcts [7]. Findings of territorial ischemic lesions (n = 6; 14%), activated microglia (n = 37; 86%) and infiltration by cytotoxic T-lymphocytes (n = 34; 79%) were seen in the post-mortem evaluation of 43 patients who died with SARS-CoV-2, 12 of whom died in ICUs [8]. A distinct microbleed phenomenon in cerebral white matter has been previously described in patients with general critical illness [9], the pathogenesis of which was attributed to hypoxemia from acute respiratory failure. Further work is needed to determine the SARS-CoV-2 dependent and independent factors or conditions responsible for microvascular and inflammatory changes seen in delirium.
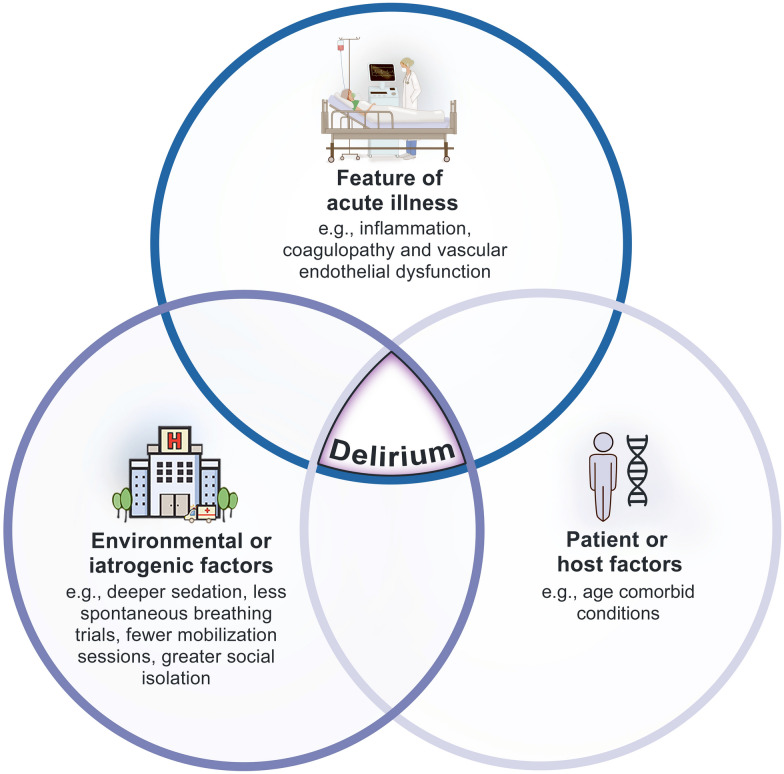
Delirium remains a key independent predictor of cognitive impairment and dementia incidence at least 3 months following hospitalization [10]. Certain ICU-specific delirium phenotypes (e.g., hypoxic and septic) have been associated with greater risk of long-term cognitive impairment [11]. These states may perpetuate chronic neuroinflammation and neurotoxicity [12]. Work also is underway to examine how SARSCoV2 might contribute to prolonged cognitive impairment post hospitalization. Similar neurotropic viruses such as Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS-CoV-1) coronaviruses have been shown to trigger the formation of Lewy bodies which are present in a range of neurologic diseases including Parkinson’s dementia. In a post-mortem analysis of the brains of rhesus macaques infected with SARS-CoV-2 virus, in the midbrain region was infiltrated by T-lymphocytes, activated microglia and intracellular Lewy bodies [13]. Such observations might herald a higher proportion or severity of critical illness induced long-term cognitive impairment or accelerated dementia (Fig. 1).
Fig. 1.
Risk factors for COVID-19-associated delirium
Apart from exploring the biologic mechanisms of SARS-COV-2 related delirium, it is also crucial to consider the importance of changes to standard ICU clinical practices during COVID-19. Resources have been strained (e.g., higher nurse: patient ratios) and there has been widespread reduction in family visitations. There have been reports of deeper levels of sedation, fewer spontaneous breathing trials, and limited mobility sessions in patients with COVID-19 as compared to other critically ill patients. In a worldwide two-day prevalence study of ABCDEF bundle practices across 212 ICUs in 38 countries amidst the first wave of the pandemic, rates of adherence to individual bundle items ranged from 16% for family engagement to 62% for sedation assessment [14]. This is in comparison to pre-pandemic rates of 67% and 89%, for family engagement and sedation assessment respectively [15]. Difficulties in reaching standards of care during times of strain may contribute both to increase rate of delirium and worsened long-term sequelae.
Long-term post-ICU follow-up will be instrumental to understand the full spectrum of health consequences from COVID-19-associated critical illness. Although outpatient clinics dedicated to follow-up are opening at many academic institutions, especially where large numbers of SARS-CoV-2 outbreaks have occurred, there is no standardized follow-up for survivors and their families. Creating such programs will enable standardized assessment of long-term cognitive outcomes in a representative population of critical illness survivors. Further, COVID-19 critical illness survivors potentially represent a relatively homogenous sub population with common mechanisms and offer an incredible opportunity to map out survivor biology and epidemiology, whilst generating evidence using trials to inform clinical care.
Author contributions
MEW wrote initial draft of manuscript; DFM and MSH revised for critical content. All authors approved the final version of manuscript.
Declarations
Conflicts of interest
The authors have no relevant conflict of interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilcox ME, Girard TD, Hough CL. Delirium and long-term cognition in critically ill patients. BMJ. 2021;2021:373. doi: 10.1136/bmj.n1007. [DOI] [PubMed] [Google Scholar]
- 2.Pun BT, Badenes R, la HerasCalle G, Orun OM, Chen W, Raman R, Simpson BK, Wilson-Linville S, HinojalOlmedillo B, de la VallejoCueva A, van der Jagt M, Navarro Casado R, Leal Sanz P, Orhun G, Ferrer Gomez C, Nunez Vazquez K, Pineiro Otero P, Taccone FS, GallegoCurto E, Caricato A, Woien H, Lacave G, Neal HR, Peterson SJ, Brummel NE, Girard TD, Ely EW, Pandharipande PP, Group C-ICIS Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, Slooter AJC, Ely EW. Delirium. Nat Rev Dis Primers. 2020 doi: 10.1038/s41572-41020-00223-41574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brunink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rosler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Kortvelyessy P, Reinhold D, Siegmund B, Kuhl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 5.Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6:31. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, Studer A, Radosavljevic M, Kummerlen C, Monnier A, Boulay C, Fafi-Kremer S, Castelain V, Ohana M, Anheim M, Schneider F, Meziani F. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, van Dijk D, Mane S, Gunel M, Ring A, JaffarKazmi SA, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas JL, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, Dottermusch M, Heinemann A, Pfefferle S, Schwabenland M, Magruder DS, Bonn S, Prinz M, Gerloff C, Puschel K, Krasemann S, Aepfelbacher M, Glatzel M. Neuropathology of patients with in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanou EM, Coutinho JM, Shannon P, Kiehl TR, Levi MM, Wilcox ME, Aviv RI, Mandell DM. Critical illness-associated cerebral microbleeds. Stroke. 2017;48:1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg TE, Chen C, Wang Y, Jung E, Swanson A, Ing C, Garcia PS, Whittington RA, Moitra V. Association of delirium with long-term cognitive decline: a meta-analysis1. JAMA Neurol. 2020;77(11):1373–1381. doi: 10.1001/jamaneurol.2020.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, Hughes CG, Chandrasekhar R, Pun BT, Boehm LM, Elstad MR, Goodman RB, Bernard GR, Dittus RS, Ely EW. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6:213–222. doi: 10.1016/S2213-2600(18)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 13.Philippens IHCHM, Böszörményi KP, Wubben JA, Fagrouch ZC, van Driel N, Mayenburg AQ, Lozovagia D, Roos E, Schurink B, Bugiani M, Bontrop RE, Middeldorp J, Bogers WM, de Geus-Oei L-F, Langermans JAM, Stammes MA, Verstrepen BE, Verschoor EJ. SARSCoV2 causes brain inflammation and induces Lewy body formation in macaques. bioRxiv. 2021 doi: 10.1101/2021.02.23.432474. [DOI] [Google Scholar]
- 14.Balas MC, Pun BT, Pasero C, Engel HJ, Perme C, Esbrook CL, Kelly T, Hargett KD, Posa PJ, Barr J, Devlin JW, Morse A, Barnes-Daly MA, Puntillo KA, Aldrich JM, Schweickert WD, Harmon L, Byrum DG, Carson SS, Ely EW, Stollings JL. Common challenges to effective ABCDEF bundle implementation: the ICU liberation campaign experience. Crit Care Nurse. 2019;39:46–60. doi: 10.4037/ccn2019927. [DOI] [PubMed] [Google Scholar]
- 15.Morandi A, Piva S, Ely EW, Myatra SN, Salluh JIF, Amare D, Azoulay E, Bellelli G, Csomos A, Fan E, Fagoni N, Girard TD, Heras La Calle G, Inoue S, Lim CM, Kaps R, Kotfis K, Koh Y, Misango D, Pandharipande PP, Permpikul C, Cheng Tan C, Wang DX, Sharshar T, Shehabi Y, Skrobik Y, Singh JM, Slooter A, Smith M, Tsuruta R, Latronico N. Worldwide survey of the “Assessing pain, both spontaneous awakening and breathing trials, choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment” (ABCDEF) bundle. Crit Care Med. 2017;45:e1111–e1122. doi: 10.1097/CCM.0000000000002640. [DOI] [PMC free article] [PubMed] [Google Scholar]