Abstract
Aim
To describe the impact of extracorporeal membrane oxygenation (ECMO) assisted CPR (E-CPR) on cerebral oxygen delivery during in-hospital cardiac arrest (IHCA).
Methods
Retrospective case series from a tertiary academic medical center. Regional cerebral oxygen saturation (rSO2) was measured continuously using cerebral oximetry in six patients who experienced IHCA. During CPR, the time of E-CPR initiation was recorded, and rSO2 values were subsequently analyzed for a period beginning 5 min before and ending 2.5 min after the initiation of E-CPR.
Results
The average rSO2 value in the 2.5 min period following E-CPR initiation increased by 20.8% as compared to the 5-min period before E-CPR initiation.
Conclusions
ECMO can be employed in parallel with cerebral rSO2 monitoring during CPR for adult IHCA patients. E-CPR is associated with rapid and significant increases in brain oxygen delivery.
Keywords: Cardiac arrest, Resuscitation, ECMO, CPR, Ischemic brain injury, Brain oxygen delivery
Introduction
Cardiac arrest (CA) is common, with an estimated 347,000 out-of-hospital and 209,000 in-hospital arrests occurring annually in US adults.1 Survival rates remain low, at approximately 25% and 10% respectively.1 Survival is often associated with moderate to severe neurological impairment at hospital discharge in 40% of patients.1 This reflects a two-step process of ischemia and reperfusion injury.2 As the magnitude of secondary injury after CA is proportional to the magnitude of the ischemic burden during cardiopulmonary resuscitation (CPR),2, 3, 4, 5 any process that enhances oxygen delivery during CPR could limit ischemic and secondary injuries and improve survival and neurological outcomes.
Regional cerebral oxygen saturation (rSO2) can be monitored in real-time using near-infrared spectroscopy (NIRS).6 Our group has utilized this technology for a number of studies, including a multicenter prospective study of 183 IHCAs, which demonstrated that higher rSO2 during CPR is independently associated with improved rates of ROSC and survival with favorable neurological outcomes at hospital discharge.7 These findings have been validated by a meta-analysis of over 2400 subjects,8 and suggest that the mechanism by which rSO2 is associated with improved CA survival and neurological outcomes may be through its attenuation of ischemia. We have also explored methods to augment oxygen delivery beyond conventional CPR during CA.9, 10 In a study of 34 patients, we found that mechanical-CPR is associated with >20% higher rSO2 than manual CPR.10 In another study of 36 patients, we tested the impact of standard dose epinephrine on augmentation of rSO2,9 and found that epinephrine (1 mg IV) during CPR produces a small increase in rSO2.9 Although the use of veno-arterial extracorporeal membrane oxygenation (ECMO) during CPR (E-CPR) to enhance oxygen delivery is becoming increasingly common across U.S. hospitals,11 few studies have examined the impact of E-CPR on brain oxygen delivery.12, 13, 14
To further investigate the relationship between E-CPR and rSO2 in the setting of CA, additional investigations are needed. We thus sought to examine the impact of E-CPR on cerebral oxygenation, with the hypothesis that E-CPR will enhance cerebral oxygen delivery beyond other previously studied methods of rSO2 augmentation.
Materials and methods
This case series reports on a retrospective convenience sample of adult patients (≥18 years) who experienced IHCA (cessation of heartbeat and respiration for ≥5 min) during working hours (Monday–Friday, 9am–5pm) at a single tertiary medical center. Data were collected as part of quality improvement (QI) initiatives and the use of these data was authorized by the Stony Brook Hospital Institutional Review Board prior to the start of data collection. From December 2012 to December 2016, E-CPR was initiated by physician recommendation in 10 patients, and continuous rSO2 monitoring was recorded for 6 of those patients.
Prior to the start of E-CPR, each patient received ACLS in accordance with AHA guidelines. The rSO2 values were measured using a portable oximeter with a single adhesive sensor attached to either side of the patient’s forehead (Equanox 7600, Nonin Medical, Plymouth, MN). Cerebral oximetry monitoring began during CPR, prior to the initiation of ECMO, and was continuous throughout CPR until either ROSC was achieved, or until the cessation of resuscitative efforts.
The oximeter calculated one rSO2 measurement every 4 s. Research staff at the scene of CA used an event-marking feature on the cerebral oximetry device to indicate the time that ECMO was initiated. ROSC was identified when the patient had a palpable pulse, and sustained ROSC was defined as a palpable pulse lasting more than 20 min.
Statistical analysis
Continuous rSO2 data were arranged so that time 0 represented the initiation of E-CPR. To account for the time needed for catheter placement before achieving full ECMO support, the first 2.5 min of data following the ECMO initiation event mark were excluded from the final analyses. Excluding rSO2 measurements from this time period allowed for the elimination of artifact produced during catheter placement, and allowed for greater standardization in the comparison of pre-ECMO data to true post-ECMO data for each subject.
For each subject, mean rSO2 values were calculated for the 5 min period immediately before the ECMO initiation time mark, and the 2.5−5 min period after full ECMO support was achieved. All pre-ECMO and post-ECMO data were represented as the mean ± standard deviation (SD). A Shapiro-Wilk test supported values being drawn from a normal distribution; therefore, a paired t-test was used to compare the differences between the pre-ECMO and post-ECMO values. Statistical analyses were performed using SAS. P values of <0.05 were considered to be of statistical significance.
Results
Patient demographics
Demographic data were collected from the medical record, including age, sex, and chronic disease burden using the Charlson Comorbidity Index.15 Summary statistics of patients’ demographic and clinical characteristics are shown in Table 1. There was no identifiable cause of CA (i.e. PE, massive hemorrhage, etc.) for any of the six patients in the series. CA was presumed to be an end result of multi-system disease. None of the patients survived to hospital discharge.
Table 1.
Demographic and clinical data for six patients who received E-CPR and rSO2 monitoring.
| Age (years) | Sex | Charleson Comorbidity Index | Cardiac Arrest Location | Initial Rhythm | Duration of CPR (min) | CA Outcome | Final Disposition | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 56 | F | 1 | CCU | PEA | 11 | ROSC > 20 mins | In-Hospital Death |
| Patient 2 | 76 | M | 3 | Diagnostic/Intervention | Asystole | 61 | ROSC > 20 mins | In-Hospital Death |
| Patient 3 | 71 | M | 4 | Inpatient Floors | Asystole | 102 | No ROSC | In-Hospital Death |
| Patient 4 | 75 | M | 5 | CCU | PEA | 18 | ROSC > 20 mins | In-Hospital Death |
| Patient 5 | 49 | F | 3 | ED | PEA | 16 | ROSC > 20 mins | In-Hospital Death |
| Patient 6 | 48 | M | 5 | CCU | PEA | 19 | No ROSC | In-Hospital Death |
CA, Cardiac Arrest; ROSC, Return Of Spontaneous Circulation; ED, Emergency Department; CCU, Coronary Care Unit.
rSO2 changes
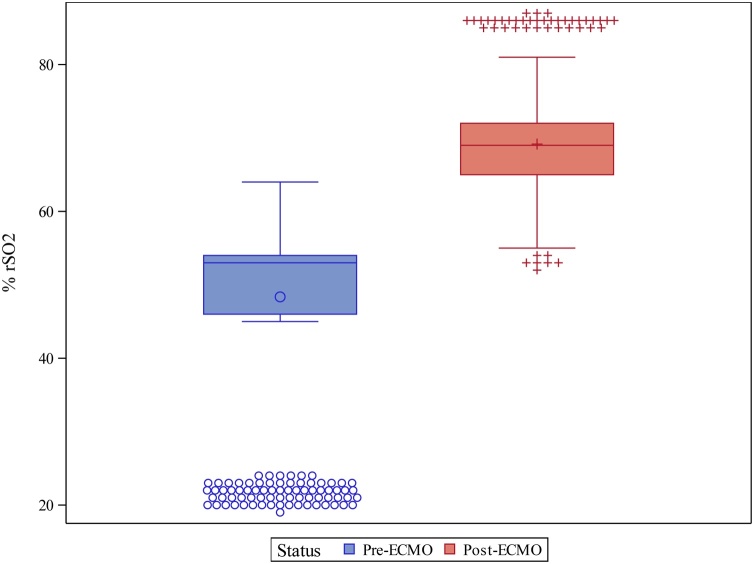
A Student t-test revealed that mean rSO2 was significantly higher following ECMO compared to baseline pre-ECMO values (69.2 ± 8.99 vs. 48.5 ± 12.86, p = 0.008) (see Fig. 1). A paired t-test revealed a significant increase in rSO2 following ECMO initiation for each individual patient (all p < 0.05), with an average increase of 20.8 ± 12.10% observed when comparing the mean pre- and post-ECMO values (see Fig. 2).
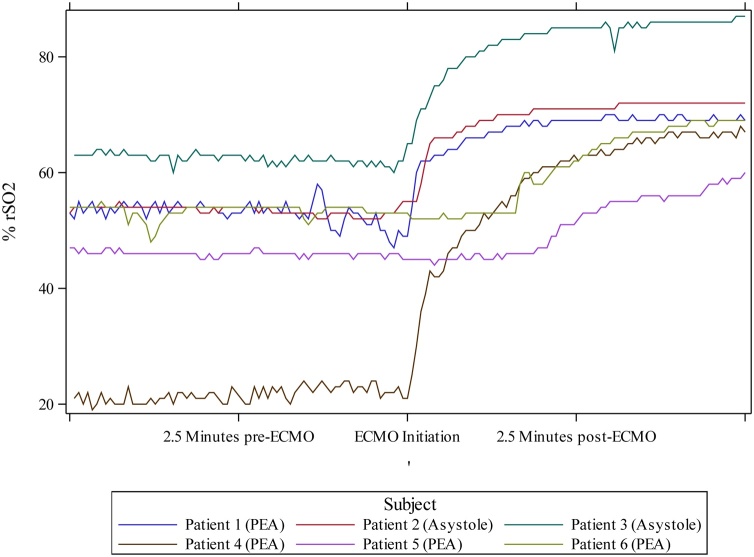
Fig. 1.
Cerebral oximetry measurements for each patient graphed over time. Serial cerebral oximeter recordings for each patient were aligned, with the event-marked time of ECMO-initiation centered on the x-axis. The 5 min before and after the ECMO-initiation time mark are included on the graph. ECMO, extracorporeal membrane oxygenation. % rSO2, regional oxygen saturation.
Fig. 2.
Changes in mean rSO2 values before and after initiation of E-CPR. Pre-ECMO mean % rSO2 during a 5 min period before initiation of ECMO. Post-ECMO mean % rSO2 during the 2.5–5 min period after the initiation of ECMO.
Discussion
We demonstrated that E-CPR is associated with rapid and significant increases in brain oxygen delivery beyond conventional CPR. E-CPR leads, on average, to a nearly 21% increase in brain oxygen delivery—a more substantial increase as compared to previously studied interventions using epinephrine or mechanical CPR.9, 10
Our data is consistent with other published studies. A single-patient case report by Koyama et al. described tissue oxygenation index (TOI) monitoring during IHCA E-CPR, and found a marked increase in cerebral TOI after the initiation of ECMO.12 Another single-patient case report published by Taccone and colleagues found that rSO2 increased from 30% to 75% following initiation of ECMO in the treatment of an OHCA.13 Indeed, it is not surprising that an intervention aimed at improving blood oxygenation would also result in increased availability of oxygen for delivery to end-organ tissues.
It is presumed that the mechanism by which E-CPR enhances brain oxygen delivery is multifactorial. While ECMO can enhance mean arterial pressure (MAP), it can also improve the partial pressure of oxygen in the blood. These will both contribute to reducing the initial ischemic burden and enable E-CPR to attenuate the severe inflammatory response syndrome that often follows CA. At the onset of CA, ischemia leads to excitotoxicity and disruption to the blood-brain barrier. This can result in cerebral edema and increased intracranial pressure, which can further disrupt synaptic structures and inflammation.2 Reperfusion after ROSC also leads to oxygen free radical injury and the accumulation of activated platelets and neutrophils in microvessels, impairing cerebral blood flow (CBF) and contributing to worsening cerebral ischemia, which leads to further inflammation.2 Overall, systemic inflammation peaks approximately three hours after ROSC, ultimately contributing to multi-organ failure and death in the post-CA period.16, 17, 18 Interestingly, in a swine study, Zhang et al. found that pigs that received E-CPR showed significantly reduced levels of IL-1, IL-1β, IL-6, TNFα, TGFβ, and other pro-inflammatory cytokines, as compared to pigs that received conventional CPR.19 This study suggests that E-CPR has the potential to improve survival rates and neurological outcomes in CA patients via its attenuation of inflammation.19 This study also further highlights the importance of optimizing oxygen delivery during CPR, without assuming that brain damage is inevitable.
The present report is limited in that it evaluates only six cases, selected by convenience sampling methods. Furthermore, the management of each patient’s CA was variable prior to the initiation of ECMO, and ECMO was not reliably initiated early within the CA. Implementing E-CPR earlier in the process of CA may lead to earlier optimization of vital organ oxygen delivery and may therefore lead to higher rates of ROSC and survival with improved neurological outcomes. Still, findings from this extended case series suggest that even with delayed initiation, E-CPR may improve cerebral rSO2 levels significantly and rapidly, reducing ischemic insult to the brain. Incorporating pre- and post-ECMO arterial blood gas levels in future studies will help to further explore the mechanisms by which E-CPR improves rSO2. Regardless, our findings reinforce the feasibility of providing E-CPR with concomitant rSO2 monitoring, and suggest that E-CPR may be a viable intervention to attenuate ischemic injury and improve patient outcomes following CA.
Conclusion
Studying the impact of E-CPR on brain resuscitation through largescale, randomized control trials will help to determine the impact of E-CPR on CA survival and neurological outcomes. Findings from these prospective studies are likely to guide future resuscitative efforts and the development of improved standard of care practices in the treatment of CA patients.
Financial support
The study was supported with funds provided by The Department of Medicine at New York University Grossman School of Medicine. The study sponsor did not participate in study design, analysis and interpretation of results or the writing of the manuscript. The cerebral oximetry equipment was provided by Nonin Medical, however the company had no other role in the study.
Conflicts of interest
There are no conflicts of interest to report.
CRediT authorship contribution statement
Emma Roellke: Conceptualization, Data curation, Visualization, Writing - original draft, Writing - review & editing. Sam Parnia: Conceptualization, Methodology, Funding acquisition, Supervision. Jignesh Patel: Data curation, Writing - review & editing. Steven Friedman: . Amanda Mengotto: Conceptualization, Data curation, Writing - original draft.
Contributor Information
Emma Roellke, Email: Emma.Roellke@nyulangone.org.
Sam Parnia, Email: Sam.Parnia@nyulangone.org.
Jignesh Patel, Email: Jinesh.Patel@stonybrookmedicine.edu.
Steven Friedman, Email: Steven.Friedman2@nyulangone.org.
Amanda Mengotto, Email: Amanda.Mengotto@einsteinmed.org.
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Chalkias A., Xanthos T. Post-cardiac arrest brain injury: pathophysiology and treatment. J Neurol Sci. 2012;315:1–8. doi: 10.1016/j.jns.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Neumar R.W., Nolan J.P., Adrie C., Aibiki M., Berg R.A., Böttiger B.W. Post–cardiac arrest syndrome. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 4.Basu S., Liu X., Nozari A., Rubertsson S., Miclescu A., Wiklund L. Evidence for time-dependent maximum increase of free radical damage and eicosanoid formation in the brain as related to duration of cardiac arrest and cardio-pulmonary resuscitation. Free Radic Res. 2003;37:251–256. doi: 10.1080/1071576021000043058. [DOI] [PubMed] [Google Scholar]
- 5.Negovsky V.A. The second step in resuscitation—the treatment of the ‘post-resuscitation’ disease. Resuscitation. 1972;1:1–7. doi: 10.1016/0300-9572(72)90058-5. [DOI] [PubMed] [Google Scholar]
- 6.Tobias J.D. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Dev. 2006;3:235–243. doi: 10.1586/17434440.3.2.235. [DOI] [PubMed] [Google Scholar]
- 7.Parnia S., Yang J., Nguyen R., Ahn A., Zhu J., Inigo-Santiago L. Cerebral oximetry during cardiac arrest: a multicenter study of neurologic outcomes and survival∗. Crit Care Med. 2016;44:1663–1674. doi: 10.1097/CCM.0000000000001723. [DOI] [PubMed] [Google Scholar]
- 8.Cournoyer A., Iseppon M., Chauny J.M., Denault A., Cossette S., Notebaert E. Near-infrared spectroscopy monitoring during cardiac arrest: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:851–862. doi: 10.1111/acem.12980. [DOI] [PubMed] [Google Scholar]
- 9.Deakin C.D., Yang J., Nguyen R., Zhu J., Brett S.J., Nolan J.P. Effects of epinephrine on cerebral oxygenation during cardiopulmonary resuscitation: a prospective cohort study. Resuscitation. 2016;109:138–144. doi: 10.1016/j.resuscitation.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Parnia S., Nasir A., Ahn A., Malik H., Yang J., Zhu J. A feasibility study of cerebral oximetry during in-hospital mechanical and manual cardiopulmonary resuscitation*. Crit Care Med. 2014;42:930–933. doi: 10.1097/CCM.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 11.Holberg M.J., Geri G., Wiberg S., Guerguerian A., Donnino M.W., Nolan J.P. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: a systematic review. Resuscitation. 2018;131:91–100. doi: 10.1016/j.resuscitation.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyama Y., Mizutani T., Marushima A., Sonobe A., Shimojo N., Kawano S. Cerebral tissue oxygenation index using near-infrared spectroscopy during extracorporeal cardio-pulmonary resuscitation predicted good neurological recovery in a patient with acute severe anemia. Intern Med. 2017;56:2451–2453. doi: 10.2169/internalmedicine.7826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taccone F.B., Fagnoul D., Rondelet B., Vincent J., Backer D. Cerebral oximetry during extracorporeal cardiopulmonary resuscitation. Crit Care. 2013;17:409. doi: 10.1186/cc11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehara N., Hirose T., Shiozaki T., Wakai A., Nishimura T., Mori N. The relationship between cerebral regional oxygen saturation during extracorporeal cardiopulmonary resuscitation and the neurological outcome in a retrospective analysis of 16 cases. J Intensive Care. 2017;5:20. doi: 10.1186/s40560-017-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Oda Y., Tsuruta R., Kasaoka S., Inoue T., Maekawa T. The cutoff values of intrathecal interleukin 8 and 6 for predicting the neurological outcome in cardiac arrest victims. Resuscitation. 2009;80:189–193. doi: 10.1016/j.resuscitation.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Adrie C., Adib-Conquy M., Laurent I., Monchi M., Vinsonneau C., Fitting C. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 18.Showemaker W.C., Appel P.L., Kram H.B. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med. 1988;16:1117–1120. doi: 10.1097/00003246-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Li C., Yuan X., Ling J., Zhang Q., Liang Y. ECMO attenuates inflammation response and increases ATPase activity in brain of swine model with cardiac arrest compared with CCPR. Biosci Rep. 2019;39 doi: 10.1042/BSR20182463. BSR20182463. [DOI] [PMC free article] [PubMed] [Google Scholar]