Abstract
Aim
Pseudo-pulseless electrical activity (pseudo-PEA) is a global hypotensive ischemic state with retained coordinated myocardial contractile activity and an organized ECG with no clinically detectable pulses. The role of standard external chest compressions (CPR) and its associated intrinsic hemodynamics remains unclear in the setting of pseudo-PEA. We undertook an experimental trial to compare epinephrine alone versus epinephrine with CPR in the treatment of pseudo-PEA.
Methods
Using a porcine model of hypoxic pseudo-PEA, we randomized 12 Yorkshire male swine to resuscitation with epinephrine only (control) (0.0015 mg/kg) versus epinephrine plus standard CPR (intervention). Animals who achieved return of spontaneous circulation (ROSC) were stabilized, fully recovered to hemodynamic and respiratory baseline, and rearrested up to 6 times. Primary outcome was ROSC defined as a sustained systolic blood pressure (SBP) of 60 mmHg for 2 min. Secondary outcomes included time to ROSC, coronary perfusion pressure (CoPP), and end-tidal carbon dioxide (ETCO2).
Results
Among 47 events of pseudo-PEA in 12 animals, we observed significantly higher proportion of ROSC when treatment included CPR (14/21 – 67%) compared to epinephrine alone (4/26 – 15%) (p = 0.0007). CoPP, aortic pressures and ETCO2 were significantly higher, and right atrial pressures were lower in the intervention group.
Conclusions
In a swine model of hypoxia-induced pseudo-PEA, epinephrine plus CPR was associated with improved intra-arrest hemodynamics and higher probability of ROSC. Thus, epinephrine plus CPR may be superior to epinephrine alone in the treatment of patients with pseudo-PEA.
Keywords: Cardiac arrest, Resuscitation, Swine model, Pseudo pulseless electrical activity, Epinephrine, Cardiopulmonary resuscitation, CPR
Introduction
Cardiac arrest survival outcomes remain poor, particularly for patients with non-shockable rhythms such as pulseless electrical activity (PEA) or asystole. In contrast with the survival rates of up to 30% seen in patients with ventricular fibrillation (VF) or pulseless ventricular tachycardia, the aggregated outcomes of patients with PEA and asystole, indicate survival rates of less than 2.5%.1, 2, 3, 4
The pathophysiologic basis of PEA remains poorly understood. Therapies such as epinephrine or external chest compressions as a component of cardiopulmonary resuscitation (CPR), are generically applied to all patients in the PEA.5 This may not be the optimal approach, as preclinical and clinical research conducted more than 30 years ago established that there are significant hemodynamic differences within the spectrum of patients categorized under PEA.6, 7 Within this heterogeneous group, pseudo-pulseless electrical activity (pseudo-PEA) has been recognized as a global hypotensive ischemic state with retained coordinated myocardial contractile activity and an organized ECG, with no clinically detectable pulses.7, 8, 9 The use of echocardiography during cardiac arrest has revealed that pseudo-PEA may be present in over 50% of patients with presumed PEA,10, 11, 12 and in one large prospective study, myocardial contractions in echocardiography, was the variable most associated with survival at all time-points including hospital discharge.13
While it is known that patients with pseudo-PEA have a better prognosis compared to true PEA, an optimal treatment strategy has not been determined. Prior work has shown that synchronization of chest compressions with the residual native systole may result in hemodynamic improvement, most notably coronary perfusion pressure (CoPP). It is possible that standard dyssynchronous CPR may be detrimental to hemodynamics and resuscitation outcomes. 14 Furthermore, retrospective clinical data has shown that pseudo-PEA patients treated with vasopressors and no chest compressions, may have better outcomes.15 To better establish optimal treatment strategies for pseudo-PEA, we undertook a pilot study comparing the effect of epinephrine alone (EPI) versus epinephrine plus chest compressions (EPI + CPR) in the treatment of hypoxic pseudo-PEA. Our hypoxic porcine model of pseudo-PEA may best be considered a mimic of hypoxic arrest using 100% O2 and epinephrine for resuscitation. Our hypothesis was that the outcome with respect to ROSC is not improved by addition of chest compressions.
Methods
All procedures described in this study were conducted in accordance with the guidelines of the National Research Council of the National Academies and with the approval of the Dartmouth College Institutional Animal Care and Use Committee.
Animal model preparation
Thirteen farm raised adolescent Yorkshire male swine (21–25 kg) were fasted overnight with free access to water and then sedated with Midazolam (0.5 mg/kg) SQ followed by SQ ketamine (30 mg/kg). After endotracheal intubation, sedation was maintained with oxygen (1–3 L/min) and isoflurane (0.5–4%). Following surgical procedures anesthesia was transitioned to an infusion of Midazolam (0.6–1.6 mg/kg/h) and Ketamine (20–50 mg/kg/h) for the remainder of the study. Volume-controlled mechanical ventilation was provided (GE Datex-Ohmeda Modulus SE, Madison, WI) with tidal volume (TV) of 15–20 cc/kg and ventilation rate (VR) of 8–20 breaths per minute. FIO2 was 100% during the initial phase of preparation and reduced to 30% shortly thereafter and kept until hypoxic injury was initiated (throughout the baseline phase). Ventilation rate and TV were titrated to maintain normocapnia (end-expiratory partial pressure of CO2 of 35–45 mmHg) as measured continuously by in-line capnometry (CO2SMO, Novametrix, Wallingford, CT). Arterial blood gases (I-Stat, Abbott Point of Care, Princeton, NJ) were analyzed to confirm adequate baseline ventilation. Gas concentrations were measured using an oxygen concentration analyzer (Oxygen Analyzer S-3A/II, Applied Electrochemistry, VMETEK). Monitoring throughout the experiments included ECG, ETCO2, and arterial blood pressure. Animals were secured in a supine position and given normal saline at a rate of 10 ml/kg per hour through a vein and titrated to maintain a central venous pressure (CVP) of ∼5 mmHg. Using ultrasound-guided percutaneous technique, micromanometer catheters (SPR-350 Millar Instruments, Houston, TX) with a lumen were placed into (1) the right atrium (RA) via the femoral vein, and (2) the descending aorta through the femoral artery for continuous pressure measurements. Fluoroscopy was used to confirm optimal position of catheters and unfractionated heparin (100 units/kg) was given to prevent catheter clotting. Analog outputs of the physiological parameters were digitized and stored using a 16-channel computerized data-acquisition system at a sampling rate of 1000 Hz (Powerlab 16SP, ADInstruments, Castle Hill, Australia). Raw data channels included surface and transesophageal ECG, aortic pressure (AoP), RA pressure and ETCO2. A transesophageal echocardiography (TEE) probe was inserted after endotracheal intubation and kept in place throughout the experiment for continuous imaging of the heart (Philips 21369A/T6210 Omni-Plane II Trans-Esophageal, Phillips Healthcare, Bothell, WA, USA). In preparation for the induction of pseudo-PEA, isoflurane was gradually discontinued and animals were converted to continuous intravenous anesthesia using ketamine (50 mcg/kg/min) and fentanyl (0.45 mcg/kg/min). The IV anesthesia protocol was maintained 15 min to allow isoflurane washout and to establish a stable level of continuous IV anesthesia prior to initiation of the hypoxia protocol.
Induction of pseudo-PEA and intervention protocol
Point of care arterial blood gases and other basic tests including pH, pCO2, pO2, base excess, HCO3, TCO2, O2 percent saturation, Na (mmol/L), K (mmol/L), ionized calcium (iCa), glucose, hematocrit, and hemoglobin were measured at baseline and after each episode of pseudo-PEA (i-STAT, Abbott Point of Care, Abbott Park, IL). Once adequate anesthesia had been confirmed, animals were paralyzed using vecuronium (1.0 mg/kg) to minimize gasping.16 Baseline data were measured before any episode of pseudo-PEA occurred and after resuscitation from subsequent pseudo-PEA events.
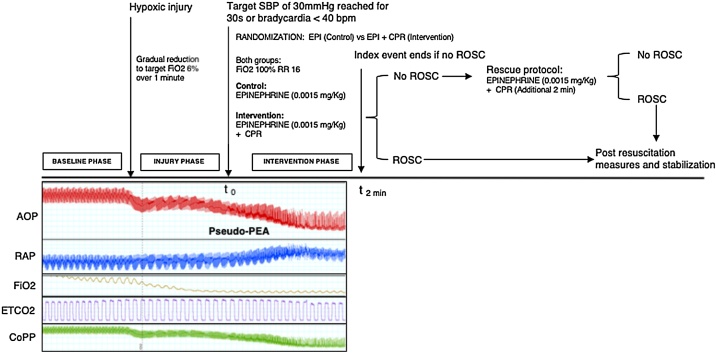
Animals were ventilated with a progressively hypoxic gas mixture of O2/N2 decreasing the concentration of O2 to a target FIO2 of 6% to induce pseudo-PEA. Onset of pseudo-PEA was defined by the following criteria; (1) sustained aortic SBP = <30 mmHg for 30 s recorded by the aortic catheter or the animal became bradycardic (HR < 40), (2) presence of an organized cardiac rhythm in ECG, and (3) presence of organized myocardial contractions visualized with continuous TEE. After onset of pseudo-PEA, animals in both groups were ventilated with a FIO2 of 100% and VR 16, and given a single bolus dose of epinephrine IV (0.0015 mg/kg). Animals in the EPI + CPR (intervention) group received also mechanical chest compressions using a piston-driven device (Thumper, Michigan Instruments, Grand Rapids, MI) delivered at a rate of 100 compressions per minute and depth titrated to compress 1/3 of the antero-posterior chest depth. After 2 min CPR was interrupted for 4 s to analyze arterial pressures, ECG rhythm and myocardial activity on TEE. ROSC was defined as SBP > 60 mmHg without CPR for more than 20 min. If ROSC was detected at any point during CPR, compressions were terminated and the event was concluded. If no ROSC was achieved at the 2-min interval in either group, the event was considered finished (outcome registered as no ROSC) and the animal was subsequently given a trial of resuscitation (rescue protocol), for another 2 min with a new dose of epinephrine IV (0.0015/kg) and CPR with the goal to rescue the animal for subsequent experiments. Animals successfully resuscitated either during the 2-min endpoint or during the rescue period, were allowed full respiratory and hemodynamic recovery of at least 20 min of a sustained SBP above 65 mmHg between events. Recovery was defined as normalization of all pressures to the baseline pressures, as well as normalization of arterial blood gas parameters including pH, CO2, O2, HCO3 and lactate. A new pseudo-PEA event was only started if the animal was hemodynamically stable in baseline pressures and had normal arterial blood gas. Fig. 1 summarizes the experimental protocol.
Fig. 1.
Experimental protocol.
Sample size calculation
Sample size was based on prior pilot experiments from our laboratory involving the same asphyxia-induced porcine model of pseudo-PEA. Based our estimated treatment effect we needed a total of 20 experiments in each group assuming α = 0.05 and 75% power to detect
an absolute difference of 40% in ROSC (20% vs 60%). Based on our pilot data, under similar conditions in this porcine model we estimated an average of 4 experiments per animal and 10% pre-randomization attrition rate, therefore 13 animals were planned.
Statistical analysis and outcomes
The primary outcome was ROSC. Secondary outcomes included time to ROSC, and hemodynamics, including mean CoPP, DBP and ETCO2 during resuscitation phase as known predictors of resuscitation success. The CoPP was defined as the average difference between the aortic pressure and RA pressure during the release phase of chest compression, and was calculated with PowerLab (ADInstruments, Chelmsford, MA). Continuous hemodynamic waveform data was collected during baseline, injury, intervention and recovery periods using PowerLab (ADInstruments, Chelmsford, MA). For analysis, we averaged all data into 15-s epochs using a custom script (MATLAB; MathWorks, Inc., Natick, MA). Coronary perfusion pressure was calculated by subtracting the RA pressure from the AoP during mid-diastole between subsequent chest compressions. During mid-diastole, the average of all samples (100 Hz) of RA pressure was subtracted from the average of all samples of aortic pressure to calculate CoPP. For statistical analyses, 2 sets of analyses were performed: (1) the unit of measure was the individual pig (e.g. the 12 initial experiments), and (2) the unit of measure was the experiment (initial + all subsequent experiments). To determine differences in ROSC between intervention arms, the Fisher’s exact test was used. For hemodynamic parameters, a 2-factor analysis of variance (ANOVA) in repeated measures was performed where intervention arm (Epi + CPR) was a factor and time (baseline, injury, intervention) was the repeated measure. Post-hoc pairwise comparisons were performed using t-tests with the pooled variance. Hemodynamic parameters are presented as means with 95% confidence intervals. Probabilities less than .05 were considered statistically significant. All statistical analyses were performed using SAS statistical software (version 9.4, SAS Institute, Cary NC).
Results
Baseline characteristics
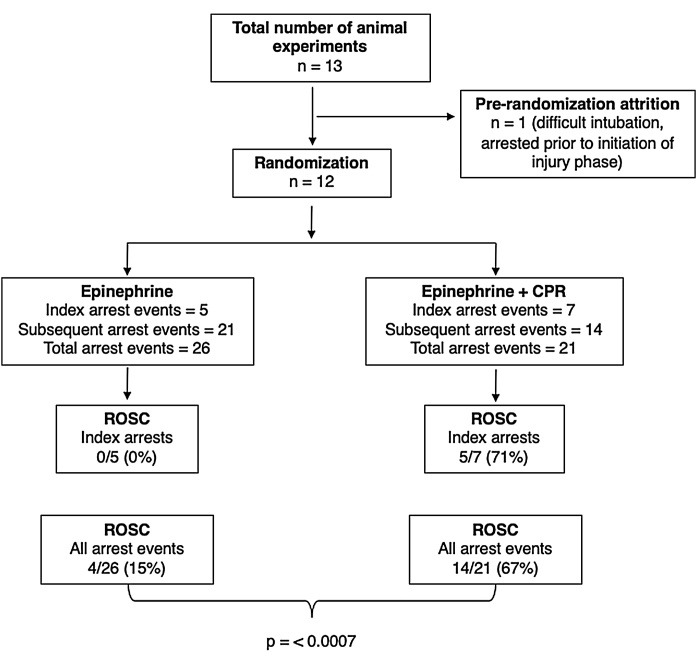
One of the 13 animals prepared for the study had an episode of hypoxemia and arrested prematurely during endotracheal intubation and was therefore not included in the protocol. All other 12 animals underwent randomization. The number of experimental resuscitation events per animal was variable, with a minimum of 1 and a maximum of 6 events of pseudo-PEA. The summary of experiments conducted in each animal including treatment allocation and outcome for each event are provided in Supplementary Table 1. A total of 47 pseudo-PEA arrest events were completed in the 12 animals, with 5 initial episodes of pseudo-PEA in the EPI group and 7 in the EPI + CPR group (Fig. 2). There were no significant differences in baseline characteristics including weight, arterial blood gases and hemodynamics during preparation phase. There were also no differences in hemodynamics at the end of injury phase (onset of pseudo-PEA) between experimental groups (Table 1). There were no episodes of spontaneous re-arrest or any other immediate complications during the experiments.
Fig. 2.
Study flowchart.
Table 1.
Experimental animal baseline characteristics.
| N = 47 events | Epi (n = 26) | Epi + CPR (n = 21) | P Value |
|---|---|---|---|
| Weight, kg | 22.7 (1.3) | 23 (1.4) | 0.45 |
| pH baseline | 7.34 (0.09) | 7.32 (0.11) | 0.5 |
| PCO2 baseline, mmHg | 47.9 (5.9) | 50.7 (6.9) | 0.14 |
| pO2 baseline, mmHg | 278.3 (113.9) | 223.6 (100.9) | 0.09 |
| HCO3 baseline, meq/L | 26.2 (3.3) | 34 (31.3) | 0.26 |
| Glucose baseline, mmol/L | 157.7 (79.9) | 138 (59.2) | 0.33 |
| Baseline hemodynamics | |||
| Heart rate, bpm | 111.3 (16.1) | 111.0 (20.6) | 0.85 |
| Mean aortic systolic pressure, mmHg | 72.7 (8.9) | 78.0 (14.5) | 0.27 |
| Mean aortic diastolic pressure, mmHg | 53.4 (8.8) | 57.8 (13.8) | 0.32 |
| Mean right atrial pressure, mmHg | 8.1 (2.3) | 7.6 (2.7) | 0.98 |
| End-tidal carbon dioxide, mmHg | 21.5 (4.1) | 23.6 (5.9) | 0.03 |
| Prior to onset therapy in Pseudo-PEA | |||
| Heart rate, bpm | 71.4 (18.7) | 82.2 (20.9) | 0.32 |
| Mean aortic systolic pressure, mmHg | 30.0 (8.6) | 34.9 (10.8) | 0.53 |
| Mean aortic diastolic pressure, mmHg | 18.1 (2.9) | 18.7 (3.1) | 0.50 |
| Mean right atrial pressure, mmHg | 9.7 (2.0) | 10.1 (2.1) | 0.43 |
| End-tidal carbon dioxide, mmHg | 14.8 (4.8) | 16.8 (7.2) | 0.04 |
| Rate of re-arrest events (%) | 21/26 (81) | 14/21 (67) | 0.13 |
Abbreviations: epi, epinephrine; CPR, cardiopulmonary resuscitation; bpm, beats per minute; PEA, pulseless electrical activity.
Experimental outcomes
All 47 pseudo-PEA events, including the 12 initial experiments, met the established pseudo-PEA criteria of SBP < 30 mmHg. Ten events (all within subsequent experiments) met both criteria for pseudo-PEA; SBP < 30 mmHg and HR < 40. We observed significantly higher rate of ROSC when treatment included CPR (EPI + CPR; intervention), with 5/7 (71%) vs 0/5 (0%) in the EPI group (control) during the initial arrest event (n = 12) (p = 0.028). When comparing ROSC in all 47 events of pseudo-PEA, we also found a significantly higher ROSC in the EPI + CPR group with 14/21 (67%) vs 4/26 (15%) in the EPI group (p = 0.0007). Among episodes with ROSC, we did not find significant difference when comparing the mean time to ROSC between the two experimental groups (Table 2).
Table 2.
Comparison of outcomes.
| Epi (n = 26) | Epi + CPR (n = 21) | P Value | |
|---|---|---|---|
| Rate ROSC All Arrests (%) | 4/26 (15) | 14/21 (67) | 0.0007 |
| Rate ROSC Index Arrest (%) | 0/5 (0) | 5/7 (71) | 0.028 |
| Rate ROSC Subsequent Arrests (%) | 5/21 (24) | 9/14 (64) | 0.033 |
| Mean Time to ROSC All Arrests (SD) | 96 s (32.9) | 83.3 s (28.3) | 0.16 |
| Mean Time to ROSC Index Arrests (SD) | – | 82.8 s (23.9) | – |
| Mean Time to ROSC Subsequent Arrests (SD) | 96 s (32.9) | 83.6 s (31.8) | 0.26 |
Abbreviations: epi, epinephrine; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; SD, standard deviation.
Intra-arrest hemodynamics
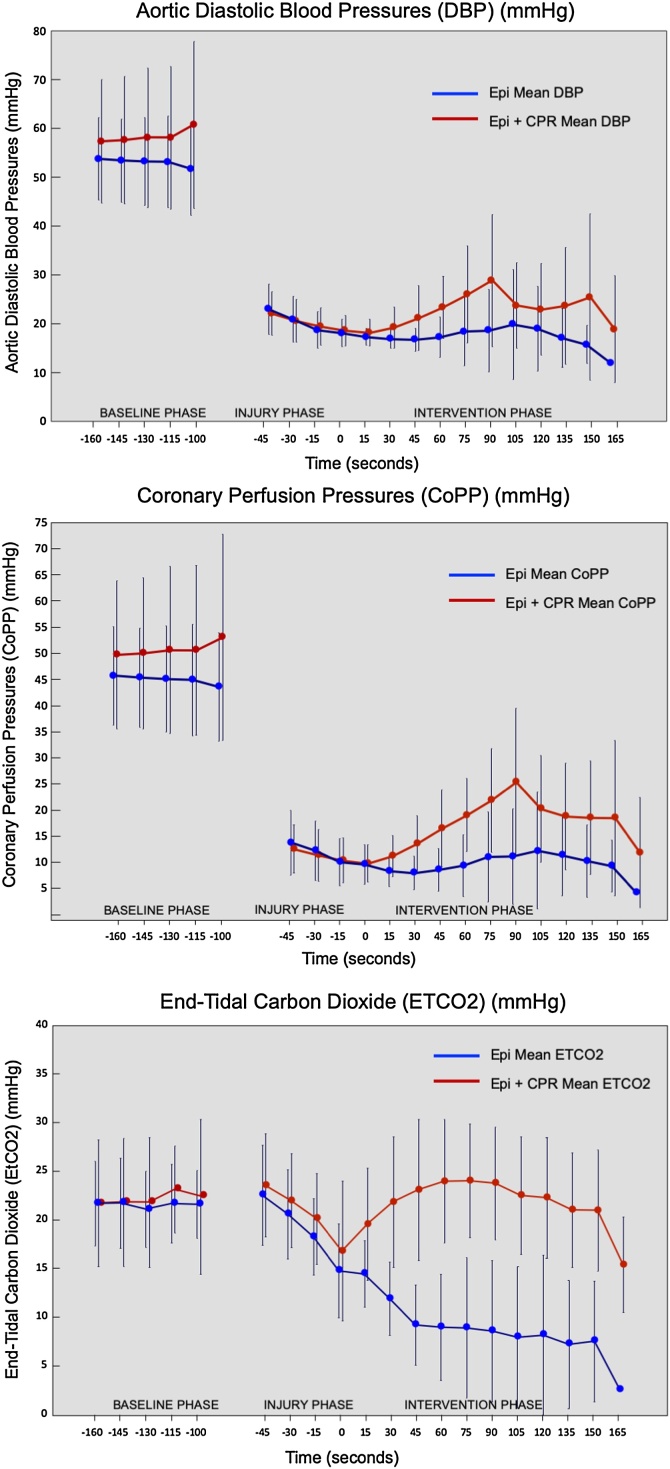
The mean CoPP in the EPI + CPR group was 17.25 mmHg (±6) compared to 8.7 mmHg (±5.8) in the EPI group. Mean RA pressure was 8.7 mmHg (±2.3) in the EPI group compared to 6.3 mmHg (±2.6) in the EPI + CPR group (p < 0.001). Mean ETCO2 was 23.3 mmHg (±5.4) in the EPI + CPR group compared to 10 (±5.3) in the EPI group (Table 3). Fig. 3 shows a comparison of hemodynamic parameters over time between the two experimental groups during baseline, injury and intervention phases.
Table 3.
Hemodynamics during protocol.
| N = 47 events | Epi (n = 26) | Epi + CPR (n = 21) | P Value |
|---|---|---|---|
| Intra-arrest hemodynamics | |||
| Mean CoPP, mmHg | 8.7 (5.8) | 17.25 (6) | <0.001 |
| Mean aortic SBP, mmHg | 29.0 (14.4) | 82.0 (13.2) | <0.001 |
| Mean aortic DBP, mmHg | 17.3 (5.6) | 23.7 (5.8) | <0.01 |
| Mean RAP, mmHg | 8.7 (2.3) | 6.3 (2.6) | <0.001 |
| End-tidal carbon dioxide, mmHg | 10.0 (5.3) | 23.3 (5.4) | <0.001 |
Abbreviations: epi, epinephrine; CPR, cardiopulmonary resuscitation; CoPP, coronary perfusion pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; RAP, right atrial pressure.
Fig. 3.
Hemodynamic parameters during baseline and resuscitation periods.
Discussion
In a porcine model of pseudo-PEA, CPR in addition to epinephrine increases the rate of ROSC during hypoxia-induced pseudo-PEA. Furthermore, the coronary and aortic pressures as well as ETCO2, is higher when CPR is performed, suggesting better myocardial and systemic perfusion with this resuscitation strategy. To our knowledge, this is the first study to compare these two treatment strategies. If replicated, these observations may indicate that systematic evaluation of the role of both epinephrine and chest compressions in patients could be indicated.
Paradis et al. were among the first to discover that PEA is a heterogenous entity with various degrees of hemodynamics parameters despite the absence of a clinically detectable pulse.7, 8 In the following decades, the introduction of transthoracic and transesophageal echocardiography during cardiac arrest resuscitation has confirmed such variability by demonstrating various levels of myocardial contractions in patients with PEA,10, 11, 12 and shown that patients with pseudo-PEA have better chances of ROSC and survival as compared to those with PEA, with either disorganized myocardial activity or no contractions at all.13
In line with those findings, a recent large multi-center study in pediatric cardiac arrests has demonstrated that children with initial resuscitation rhythm of bradycardia and poor perfusion have significantly better outcomes including higher likelihood of survival to discharge (OR 2.31) and favorable neurological outcome (OR 2.21) when compared to cardiac arrests with pulseless rhythms.17
Despite the likely differences in both the hemodynamics and the myocardial activity of these patients, current resuscitation algorithms for non-shockable rhythms such as asystole and PEA provide “one-size-fits-all” recommendations that include epinephrine and chest compressions as they mainstay therapy. All patients receive the same bundle, regardless of their underlying physiology and their hemodynamic response (or lack thereof) to these therapies. Identification of the optimal treatment in pseudo-PEA is particularly important in light of the increase in the proportion of patients with PEA as initial rhythm in all cardiac arrests.5, 18 In contrast with shockable rhythms where defibrillation provides a highly effective and mechanism-specific treatment, in PEA resuscitation is centered on the identification of reversible causes, and attempting to increase myocardial perfusion while maintaining adequate cerebral and systemic perfusion.19 To that end, it has been well established that myocardial perfusion represents the most important determinant of resuscitation success. 20, 21
In agreement with these principles, in this study we found a significantly higher mean CoPP in the intervention group compared to the epinephrine only group. Consistent with this result, we measured higher RA pressures intra-arrest in the absence of CPR. Overall, our results suggest that there is decreased forward flow when no CPR is performed. These findings are consistent with data from both animal and human studies conducted in late 1980s and 1990s which established that CoPP was the principal determinant of myocardial perfusion and that, specifically, the generation of a CoPP of at least 15 mmHg is required for successful resuscitation.8, 20 Our results support the conclusion that epinephrine alone may not be sufficient to generate this CoPP threshold. However, an alternative explanation to this finding could be that the dose of epinephrine used in this study (0.0015 mg/kg) may be insufficient to achieve the threshold of CoPP required for ROSC. Future studies could evaluate higher dosing or goal-directed (e.g. DBP or CoPP-guided) dosing of epinephrine.
While the role of epinephrine as a therapy that can help increase the probability of ROSC in pseudo-PEA seems clear, 22 the effectiveness of chest compressions in these patients has been reasonably questioned. Because residual left ventricular function is a key feature of pseudo-PEA, there is a further concern that standard CPR unsynchronized to the native myocardial function may actually interfere with ventricular filling and have a deleterious effect on overall cardiac output and myocardial perfusion.14 Based on that concern, some have proposed an approach to pseudo-PEA analogous to cardiogenic shock, with use of vasopressors such as epinephrine, and no chest compression.15
Our hypoxic model of pseudo-PEA may best be considered a mimic of cardiac arrests associated with acute hypoxia. Such patients frequently have residual hemodynamics on echocardiography. Following intubation and application of 100% oxygen, a subgroup of these patients may still be without detectable pulses. Catecholamine such as epinephrine may be helpful in this setting, leaving the question of whether to apply chest compressions. It seems reasonable that clinicians will withhold chest compressions with systolic blood pressures about 60 mmHg. But in the setting of hemodynamics similar to those we have studied, SBPs below 30 mmHg, particularly in settings without invasive hemodynamic monitoring, clinicians would be likely initiating chest compressions. Our results support that approach. In this experimental study, we have confirmed that, even if not performed in synchrony with the native myocardial contractions, CPR contributes to lower RA pressures and yield higher diastolic aortic blood pressures, which in turn translate into higher CoPP, compared to epinephrine alone.
Based on our clinical observations performing transesophageal echocardiography (TEE) during cardiac arrest resuscitations,12 we have confirmed that PEA represents a spectrum of disease with different degrees of myocardial contractility, that begins with a severe form of cardiogenic shock and ends with asystole. Pseudo-PEA would be located somewhere along this spectrum, and the optimal treatment for individual patients may depend on where they are on this spectrum. If this approach is more effective, then a critical question is at which point do we switch between therapeutic strategies? Furthermore, assuming such a transition zone, this begs the question; which parameter or combination of them (e.g. SBP, DBP, ETCO2, myocardial activity in echocardiography, etc.) will be most reliable to make this determination? A recent editorial by Harper et al addressed the question of the timing at which chest compressions should be initiated in patients with refractory hypotension in the operative setting, and highlight the important knowledge gaps in regards to our understanding of the hemodynamics and physiology of the continuum from hypotension and shock, to cardiac arrest.23 As we continue our work to better understand this problem and evaluate tools that can help us better characterize this transition from shock to PEA (e.g. ETCO2, TEE, etc.), it is important that we simultaneously work on understanding the hemodynamic effects and impact in outcomes that currently available therapies have in this spectrum of disease.
There are important limitations of this research. We had a relatively small sample of animals, which we tried to address by serial arrest events in each animal in order to increase the number of events. While this approach allowed us to be adequately powered for our primary outcome of ROSC and obtain sufficient hemodynamic data to further characterize the effects of the two tested strategies, it prevented us from looking at short term survival and neurological outcomes.
Subsequent work should include larger number of experiments to allow comparison of index arrests only and to evaluate survival differences. Another implication of our approach of serial arrests is that after the index cardiac arrest event, subsequent events may be more or less likely to result in ROSC depending on the effects of the first hypoxic injury or the resuscitation phase (e.g. epinephrine and CPR effects) in the myocardium. We have attempted to minimize these potential effects by waiting for complete hemodynamic and metabolic recovery (blood gas) before initiating a new event. Our hypoxia-induced pseudo-PEA model is juvenile without the comorbidities that are usually present in adult patients with cardiac arrest.
Lastly, this model represents an early phase of PEA cardiac arrest and therefore the effect of the therapies trialed here may not necessarily be applicable or have the same effects in later (e.g. refractory) phases of cardiac arrest. Future studies should evaluate the optimal treatment strategy for pseudo-PEA in a model of prolonged pseudo-PEA or refractory cardiac arrest.
Conclusions
This pre-clinical trial suggests that in a swine model of hypoxia induced pseudo-PEA, epinephrine plus CPR may be superior to treatment with epinephrine alone. These results should serve as a proof of concept and provide a framework in the development of further preclinical and clinical research that can help optimize the resuscitation of patients with pseudo-PEA.
Author contributions
FT, NAP and BSA contributed to the conceptualization and design of the study. FT, CC, ALL, WJH, KLM and NAP carried out the experiments. FT, FS, CC, ALL, WJH, KLM, WPL, NAP and BSA performed data analyses and all authors participated from the discussion and interpretation of results. All authors contributed significantly to the writing and editing of the manuscript. All authors have approved the final version of the manuscript.
Funding
This investigation was funded by a research grant awarded to Dr. Teran from the ZOLL Foundation(# 74774258.1).
Conflict of interest
Dr. Paradis holds U. S. Patent 7,645,247 “A device for synchronizing the parameters of resuscitative therapies to residual myocardial activity during cardiopulmonary resuscitation,” which has been licensed to ZOLL, Inc. Dr. Paradis is also a scientific consultant. All other authors declare that they have no competing interests.
Acknowledgements
This work was presented at the 2019 Resuscitation Science Symposium in Philadelphia, PA, on November 17, 2019. Dr. Teran has received research funding from Zoll Foundation and from the Emergency Medicine Foundation; Dr. Abella has received funding from National Institutes of Health, Patient-Centered Outcomes Research Institute, Physio-Control and the American Heart Association. Dr. Abella has received consulting and speaking honoraria from Becton Dickinson and Stryker Medical. The authors declare no conflicts of interest specifically related to this manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resplu.2021.100110.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Daya M.R., Schmicker R.H., Zive D.M. Out-of-hospital cardiac arrest survival improving over time: results from the Resuscitation Outcomes Consortium (ROC) Resuscitation. 2015;91:108–115. doi: 10.1016/j.resuscitation.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichol G., Thomas E., Callaway C.W. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wissenberg M., Lippert F.K., Folke F. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA. 2013;310:1377–1384. doi: 10.1001/jama.2013.278483. [DOI] [PubMed] [Google Scholar]
- 4.Høybye M., Stankovic N., Holmberg M., Christensen H.C., Granfeldt A., Andersen L.W. In-hospital vs. out-of-hospital cardiac arrest: patient characteristics and survival. Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Myerburg R.J., Halperin H., Egan D.A. Pulseless electric activity: definition, causes, mechanisms, management, and research priorities for the next decade: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2013;128:2532–2541. doi: 10.1161/CIRCULATIONAHA.113.004490. [DOI] [PubMed] [Google Scholar]
- 6.Niemann J.T., Rosborough J.P., Ung S., Criley J.M. Coronary perfusion pressure during experimental cardiopulmonary resuscitation. Ann Emerg Med. 1982;11:127–131. doi: 10.1016/s0196-0644(82)80236-9. [DOI] [PubMed] [Google Scholar]
- 7.Paradis N.A., Martin G.B., Rivers E.P. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. doi: 10.1001/jama.1990.03440080084029. [DOI] [PubMed] [Google Scholar]
- 8.Paradis N.A., Martin G.B., Goetting M.G., Rivers E.P., Feingold M., Nowak R.M. Aortic pressure during human cardiac arrest. Identification of pseudo-electromechanical dissociation. Chest. 1992;101:123–128. doi: 10.1378/chest.101.1.123. [DOI] [PubMed] [Google Scholar]
- 9.Larabee T.M., Paradis N.A., Bartsch J., Cheng L., Little C. A swine model of pseudo-pulseless electrical activity induced by partial asphyxiation. Resuscitation. 2008;78:196–199. doi: 10.1016/j.resuscitation.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Bocka J.J., Overton D.T., Hauser A. Electromechanical dissociation in human beings: an echocardiographic evaluation. Ann Emerg Med. 1988;17(5):450–452. doi: 10.1016/S0196-0644(88)80234-8. [DOI] [PubMed] [Google Scholar]
- 11.Flato U.A.P., Paiva E.F., Carballo M.T., Buehler A.M., Marco R., Timerman A. Echocardiography for prognostication during the resuscitation of intensive care unit patients with non-shockable rhythm cardiac arrest. Resuscitation. 2015;92:1–6. doi: 10.1016/j.resuscitation.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Teran F., Dean A.J., Centeno C. Evaluation of out-of-hospital cardiac arrest using transesophageal echocardiography in the emergency department. Resuscitation. 2019;137:140–147. doi: 10.1016/j.resuscitation.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Gaspari R., Weekes A., Adhikari S. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation. 2016;109:33–39. doi: 10.1016/j.resuscitation.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Paradis N.A., Halperin H.R., Zviman M., Barash D., Quan W., Freeman G. Coronary perfusion pressure during external chest compression in pseudo-EMD, comparison of systolic versus diastolic synchronization. Resuscitation. 2012;83:1287–1291. doi: 10.1016/j.resuscitation.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Gaspari R., Weekes A., Adhikari S. A retrospective study of pulseless electrical activity, bedside ultrasound identifies interventions during resuscitation associated with improved survival to hospital admission. A REASON Study. Resuscitation. 2017;120:103–107. doi: 10.1016/j.resuscitation.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Yang L., Weil M.H., Noc M., Tang W., Turner T., Gazmuri R.J. Spontaneous gasping increases the ability to resuscitate during experimental cardiopulmonary resuscitation. Crit Care Med. 1994;22:879–883. doi: 10.1097/00003246-199405000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Morgan R.W., Reeder R.W., Meert K.L. Survival and hemodynamics during pediatric cardiopulmonary resuscitation for bradycardia and poor perfusion versus pulseless cardiac arrest. Crit Care Med. 2020:881–889. doi: 10.1097/CCM.0000000000004308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobb L.A., Fahrenbruch C.E., Olsufka M., Copass M.K. Changing incidence of out-of-hospital ventricular fibrillation, 1980-2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 19.Berg K.M., Soar J., Andersen L.W. Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:S92–S139. doi: 10.1161/CIR.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 20.Kern K.B., Ewy G.A., Voorhees W.D., Babbs C.F., Tacker W.A. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 21.Raessler K.L., Kern K.B., Sanders A.N., Tacker W.A., Ewy G.A. Aortic and right atrial systolic pressures during cardiopulmonary resuscitation: A potential indicator of the mechanism of blood flow. Am Heart J. 1988;115:1021–1029. doi: 10.1016/0002-8703(88)90071-3. [DOI] [PubMed] [Google Scholar]
- 22.Paradis N.A., Wenzel V., Southall J. Pressor drugs in the treatment of cardiac arrest. Cardiol Clin. 2002;20:61–78. doi: 10.1016/S0733-8651(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 23.Stephens T.J., Peden C.J., Haines R. Hospital-level evaluation of the effect of a national quality improvement programme: time-series analysis of registry data. BMJ Qual Saf. 2020;29:623–635. doi: 10.1136/bmjqs-2019-009537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.