Abstract
Chromanone or Chroman-4-one is the most important and interesting heterobicyclic compound and acts as a building block in medicinal chemistry for isolation, designing and synthesis of novel lead compounds. Structurally, absence of a double bond in chromanone between C-2 and C-3 shows a minor difference from chromone but exhibits significant variations in biological activities. In the present review, various studies published on synthesis, pharmacological evaluation on chroman-4-one analogues are addressed to signify the importance of chromanone as a versatile scaffold exhibiting a wide range of pharmacological activities. But, due to poor yield in the case of chemical synthesis and expensive isolation procedure from natural compounds, more studies are required to provide the most effective and cost-effective methods to synthesize novel chromanone analogs to give leads to chemistry community. Considering the versatility of chromanone, this review is designed to impart comprehensive, critical and authoritative information about chromanone template in drug designing and development.
Keywords: Chroman-4-one, Chromone, Pharmacological activity, Synthesis, Analogues
Introduction
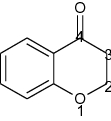
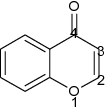
Chroman-4-one is one of the most important heterobicyclic moieties existing in natural compounds as polyphenols and as synthetic compounds like Taxifolin, also known as chromanone or benzo-dihydropyran or benzopyran. Structurally, chroman-4-one is a fusion of benzene nucleus (ring A) with dihydropyran (ring B) which relates to chromane, chromene, chromone and chromenone, but the absence of C2-C3 double bond of chroman-4-one skeleton makes a minor difference (Table 1) from chromone and associated with diverse biological activities [1].
Table 1.
Properties of chromanone and chromone
| Name | Chroman-4-one | Chromone |
|---|---|---|
| IUPAC name | 4-Chromanone | 4-Chromone |
| Other names (Synonym) | Chromanone, 4-chromanone, chroman-4-one dihydrobenzo pyran, benzopyran, 2,3-dihydro-4H-1-benzopyran-4-one | Chromone, 4-Chromone, 4H-Chromen-4-one,4H-1-Benzopyran-4-one |
| Structure |
|
|
| Formula | C9H8O2 | C9H6O2 |
| Formula weight | 148.15 g/mol | 146.14 g/mol |
| Molecular weight | 148.15 g/mol | 146.14 g/mol |
| Density | 1.196 ± 0.06 g/cm3 | 1.248 ± 0.06 g/cm3 |
| Xlog P3 | 1.4 ± 0.24 | 1.4 ± 0.24 |
| Melting point | 36.5 ºC | 59.0 ºC |
|
Composition C H O |
72.96% 5.44% 21.60% |
73.97% 4.14% 21.90% |
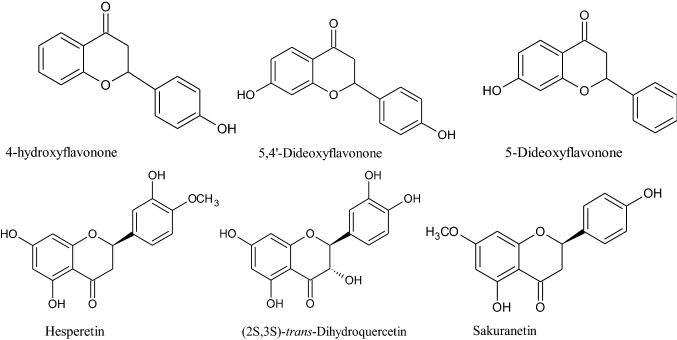
Natural and synthetic chromanone analogs show various biological activities such as anticancer, tumor necrosis factor-α (TNF-α) inhibitors, antivascular, antidiabetic, antioxidant, antimicrobial, antifungal, antiviral, antileishmanial, insecticidal, spasmolytic, analgesic, anti-inflammatory, anticoagulant, estrogenic inhibitor, anti-acetylcholinesterase (AchE) inhibitor, antihuman immunodeficiency virus (HIV), anticonvulsant, antidepressants, anticoronal and antitubercular activity. Due to of these activities, several analogues of chromane are also available in market like tocopherols (vitamin E), taxifolin (antidiabetic), tetrazole (antidiabetic), troglitazone (antidiabetic), ormeloxifene (anticancer) and nebivolol (beta-blocker) [2]. Moreover, several isolated flavanones, flavonols, homoisoflavonoids like naringenin, naringin, myricetin, dihydroquercetin and kaempferol are also under clinical studies [3]. Therefore, among the diverse array of chromane, chroman-4-one/4-chromanone are of considerable interest to researchers due to potency of this clinically useful pharmacophore in the treatment of cancer and several other diseases [4]. Presently, a number of research groups are working in designing and development of more potent and significant chromanone analogues. So, in the present review, recent literature available up to 2020 about chroman-4-one derivative and their pharmacological activities is accrued. Present review article will offer a platform to the researchers in designing and development of novel potent chroman-4-one analogs.
Biological Activities of Chromanone
Anticancer Activity
Discovery and development of novel anticancer agents with potent cytotoxic activity is one of the top priorities in pharmaceutical research. Several limitations like variable efficacy, poor toxicity profile and adverse effects are associated with the usage of currently available chemotherapeutic agents [5]. Generally, cancer can be measured by assessing the degree of mitochondrial impairment and metabolic dysfunctions such as cellular energy supply, cell death signaling, irregulation of metabolic pathways, formation of reactive oxygen species (ROS), compromised enzyme actions, aerobic glycolysis augmented in tumor cells, alterations in lipid metabolism and unbalanced pH [6]. Therefore, to cure such metabolic dysfunctions, naturally occurring flavonoids, flavonols, flavanones (2-phenyl chroman-4-one derivatives) and homoisoflavanones exhibit good anticancer potential along with other pharmacological activities such as anti-inflammatory, antioxidative, antidiabetic, antibacterial, antimicrobial, antifungal, antimutagenic, antiparasitic and anti-HIV [7, 8]. In this regard, the following naturally occurring flavanones such as naringenin [9], naringin [9], sakuranetin [10], eriodictyol [11], calyxin G, deguelin [12] and sterubin [13] have been reported (Figs. 1, 2) to possess potent cytotoxic profile. Moreover, these naturally occurring flavanones can also regulate cellular metabolism, scavenge free radical and suppress proliferation of cancer cells [12, 13]. However, the precise molecular mechanisms of these flavonoids accountable for cytotoxic potential have not been completely elucidated till now. Moreover, poor yield is also a major hurdle to explore the potential of these compounds. So, synthesis of compounds containing chromanone with efficient cytotoxic action might be a privileged approach in searching new targets for cancer treatment [14]. A number of compounds are found to have significant anticancer activity. For instance, several compounds containing chroman-4-one and its natural and synthetic analogs possessing significant anticancer potential against cancer cell lines are enlisted in Table 2 [15].
Fig. 1.
Natural flavanones containing chroman-4-one as parent moiety
Fig. 2.
Chroman-4-one derived structure (under development phase)
Table 2.
Chroman-4-one analogs exhibiting anticancer activity
| Sr. No. | Compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
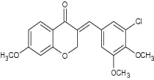
| 1 | (E)-3-benzylidene-7-methoxychroman-4-one derivatives (3-chloro-4,5-dimethoxybenzylidene derivative) |
|
Evaluated against MDA-MB-231 (breast cancer), KB (nasopharyngeal epidermoid carcinoma) and SK-N-MC (human neuroblastoma) cell lines using MTT assay |
Significant inhibition against MDA-MB-231 = 7.56 ± 2.23, KB = 25.04 ± 10.60 and SK-N-MC = 9.64 ± 2.7 Marked as best anticancer agent among the series [16] |
| 2 | (E)-3-(2′-methoxybenzylidene)-4-chromanone |
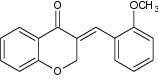
|
Evaluated for antiproliferative activity in HUVEC (human umbilical vein endothelial cells) | Exhibited highest potency against proliferation of endothelial cells with IC50 = 19 µM [17] |
| 3 | 6,7-Methylenedioxy-4-chromanone |
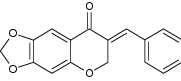
|
Evaluated against three breast cancer cell lines (MCF-7, T47D and MDA-MB-231) | Exhibited highest anticancer activity against tested cell lines (IC50 ≤ 9.3 µg/ml) [18] |
| 4 | 3-Benzylidene-chroman-4-one derivatives |
|
Evaluated against K562, MDA-MB-231 and SK-N-M Cell lines | Exhibited potent anticancer activity with IC50 ≤ 3.86 µg/ml [19] |
| 5 | Hydroxy flavanones |
|
Evaluated against colorectal carcinoma cells such as A549, LLC, AGS, SK-Hepl and HA22T cancer cells | Exhibited potent cytotoxic activity [20] |
| ||||
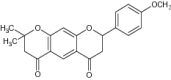
| 6 | Synthetic flavanones (4′,7-dimethoxyflavanone) |
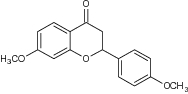
|
Evaluated for antiproliferative activity against MCF-7 (human breast cancer) cells Apoptotic cell population = 34.89% |
Exhibited potent cytotoxic activity [21] |
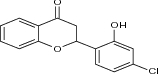
| 7 | Halogenated flavanones 3′,7-dichloroflavanone |
|
Evaluated against MCF-7, LNCaP, PC3, Hep-G2, KB, SK-N-MC cells | Very potent compound (IC50 = 2.9 µM) against MDA-MB-231 cell line than etoposide (reference drug) [22] |
| 3′,6-Dichloroflavanone |
|
|||
| 8 | 7, 8-Methylenedioxyflavanones |
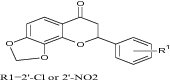
|
Examine cytotoxic potential and apoptosis in human leukemia cells | Exhibit better cytotoxic potency than reference [23] |
| 9 | 2’-Chloro- or 2′-nitro-substituted chroman-4-one compounds |
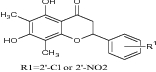
|
Tested for cytotoxic activity against Bel-7402, HL-60, BGC-823 and KB (human cancer cell lines) | Showed significant cytotoxic activity [24] |
| 10 | 7-Methoxyisoflavanone |
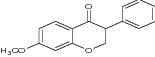
|
Evaluated against HL60 (acute myeloblastic leukemia cells) and PBMC (peripheral blood mononuclear cells) using MTT assay | Displayed highest anticancer activity against HL60 cells [25] |
| Diarylchromanone |
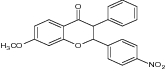
|
|||
| 11 | 3-Arylideneflavanone and chromanone (E, Z isomers) and 3-arylflavones |
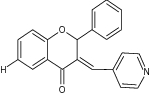
|
Screened against HL-60, NALM-6, WM115 and normal cells HUVEC cancer cell lines |
Z-isomer (chromanone) was found to be more cytotoxic, while E-isomer was entirely inactive IC50 = 0.9 ± 0.2 (HL60), 1.6 ± 0.3 (NALM-6), 6.3 ± 0.5 (WM-115), 5.77 ± 0.17(HUVEC) [26] |
| 12 | 3-Benzylchroman-4-ones |
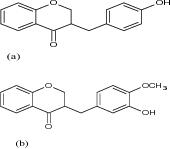
|
Examined against two cancerous cell lines BT549 (human breast carcinoma), HeLa (human cervical carcinoma) and one noncancerous cell line Vero (normal kidney epithelial cells) by MTT assay | 3-Benzylchroman-4-one derivatives (a) was found to be the most active against BT549 (IC50 = 20.1 mM), HeLa cell lines (IC50 = 42.8 mM), whereas 3-benzylchroman-4-one derivatives (b) showed significant activity against HeLa cells (IC50 = 20.45 mM) only [27] |
| 13 | 3-Methenylthiochroman-4-one-l, 1-dioxide |
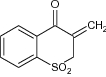
|
Tested against Ehrlich ascites carcinoma tumor growth | 80% inhibition of Ehrlich ascites carcinoma tumor growth in mice was observed [28] |
| 14 | Novel tricyclic heterocyclic chromanone compounds |
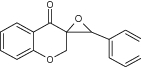
|
Examined against lung, breast and CNS cell lines | Showed significant inhibition against all cell lines tested and marked as active anticancer agent [29] |
| 15 | Spiro [chroman-2, 4′-piperidin]-4-one derivatives |
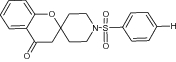
|
Examined cytotoxic activity against three human cancer cell lines; MCF-7 (human breast carcinoma), A2780 (human ovarian cancer) and HT-29 (human colorectal adenocarcinoma) using MTT assay | Spiro [chroman-2, 4′-piperidin]-4-one derivatives with a sulfonyl spacer shows the most potent activity with IC50 = 5.62 ± 1.33 μM (MCF-7) 0.31 ± 0.11 μM (A2780), and 0.47 ± 0.17 μM (HT-29), respectively [30] |
| 16 | Silibinin |
|
Tested against T47D (breast cancer cell lines) by MTT assay | Combination of Silibinin and chrysin may have therapeutic value (combination indices < 1) used in treatment of breast cancer [31] |
| Chrysin |
|
|||
| 17 | 6-Methoxy-3-phenyl chroman-4-one |
|
Tested antiproliferative activity against MCF-7 (breast cancer) and A549 (lung carcinoma) cell lines | Selective Sirtuin 2 (SIRT2) inhibitors exhibited antiproliferative activity for breast cancer and lung carcinoma [7] |
| 3-(Pyridin-3-yl) chroman-4-one |
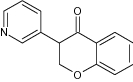
|
|||
| 1,2,4-Oxadiazole containing benzopyran analog |
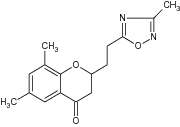
|
|||
| 6,8-Dimethyl-2-[2-(pyridin-3-yl) ethyl]-benzopyran-4-one |
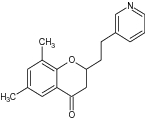
|
|||
| 6-Flurobenzopyran-4-one |
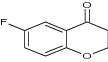
|
|||
| 18 | 2-[(Furan-2-yl) methoxy]-benzopyran-4-one |
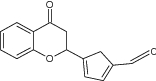
|
Screened against MCF7 mammary adenocarcinoma), HT29 (human colon adenocarcinoma) and A498 (human kidney adenocarcinoma) cell lines using sulforhodamine B dye | Found to possess very potent activity against all the tested cell lines, 7.3 ± 0.3( MCF7), 4.9 ± 0.5 (HT29), 5.7 ± 0.9 (A498) [32] |
| 19 | Chromone-2-carboxamide (series I) and chromane-2,4-dione (series II) derivatives |
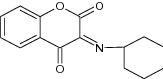
|
Cytotoxic activity on HL-60, MOL T -4, and MCF-7 cancer cell lines | Showed highest cytotoxic activity against 64.6 ± 7.1( HL-60), 68.4 ± 3.9 (MCF7), 33.2 ± 2.1( MOLT-4) [33] |
| 20 | 3-[3/4-(2-aryl-2-oxoethoxy) arylidene] chroman/thiochroman-4-one derivatives |
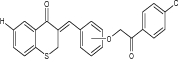
|
Screened against leukemia (L, 4 or 6 cell lines), non-small cell lung cancer (NSCLC, 9 cell lines), melanoma (M, 8 or 9 cell lines), colon cancer (CC, 7 cell lines), central nervous system cancer (CNSC, 6 cell lines), ovarian cancer (OC, 6 or 7 cell lines), prostate cancer (PC, 2 cell lines), renal cancer (RC, 8 cell lines), breast cancer (BC, 6 or 8 cell lines) | 3-[3-(2-(4-Chlorophenyl)-2-oxoethoxy) benzylidene] thiochroman-4-one was found to be most potent and possessed very significant activity against all the tested cancer cell lines [34] |
| 21 | 5′′-Aceto-3′′-(4-bromophenyl)-3′′H,4′H-dispiro[chroman-2′,4-tetrahydropyran-3′,2′′-[1,3,4-thiadiazol]-4′-one |
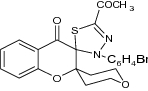
|
Tested against MCF-7 (human breast adenocarcinoma) cell lines | Exhibited significant anticancer potential [35] |
| Trispiro[tetrahydrothiopyran-4,5′-2H-chromano-[3,4-e] [1, 3, 4] oxadithiin-2′,3′′-chroman-2′′4′′′-tetrahydrothiopyran]-4′′-one |
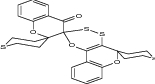
|
|||
| 22 | 2-hydroxy-4-chromanone derivatives |
|
Examined for cytotoxic activity against cancer cells | Potent anticancer agent [36] |
| 23 | Novel noduliprevenone |
|
Produced from the fungus Nodulisporium species, is an endophyte of a Mediterranean alga Tested for cytotoxic activity against 36 cancer cells |
Showed significant cytotoxic activity against tested cell lines [37] |
Antioxidant
Excessive production of ROS, suppression of antioxidants as a result of normal biochemical processes due to the effect of several environmental and endogenous factors result in imbalance of oxidative-antioxidant. The excessive ROS higher than physiological concentrations cause oxidative stress [38, 39]. Oxidative stress affects all cellular functioning which may be responsible for generation of severe diseases like diabetes complications, neurological disease, atherosclerosis, skin lesions, inflammation, rheumatoid arthritis, aging, cardiovascular diseases and cancer [39, 40]. Generally, antioxidants react or debilitate excessive level of ROS [41].
There are a number of isolated flavanones and homoisoflavanones such as silymarin, pinobanksin, silybin, liquiritin, isointricatinol reported for their antioxidant activity in 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging method. Along with isolated components, synthetic benzylidene chromanone derivatives also showed DPPH radical scavenging and ferric reducing antioxidant power (FRAP) [42, 43]. According to structure activity relationship (SAR) of chromanone analogs, C-2 and C-3 substitution with methoxyphenyl, amine derivatives, aromatic groups, benzylidene and cyclohexyl carbamoyl yields more potent antioxidant compounds which can produce equivalent antioxidant activity like vitamin E and Trolox [44] enlisted in Table 3. Therefore, more synthetic work is required to produce new chroman-4-one scaffolds as effective antioxidant compounds which can be used to manage the pathogenesis of diseases in which oxidative stress plays a significant role.
Table 3.
Chroman-4-one analogs exhibiting antioxidant potential
| Sr. No. | Compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | 3-Benzylidene-7-alkoxychroman-4-one derivatives |
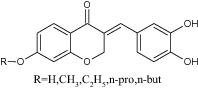
|
Assessed for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, ferric reducing antioxidant power (FRAP) and thiobarbituric acid reactive substances | Showed following inhibition values 24.30 ± 0.51 to 35 ± 0.47 (DPPH), 10.96 ± 0.34 to 14.33 ± 0.52 (FRAP) and Fe2 + µM = 44.62 ± 0.48 to 71.64 ± 0.47 (TBARS) [45] |
| 2 | 6-Hydroxy-7-methoxy-4-chromanone derivatives |
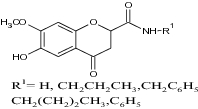
|
(a) Assayed for lipid peroxidation inhibition initiated by Fe2+ and ascorbic acid in rat brain homogenates (b) DPPH scavenging activity |
Exhibited more potent antioxidant activity than vitamin E and Trolox with following values (a) lipid peroxidation inhibition = 176.8 to 300 (b) DPPH = 66.4 to 213.9 [46] |
| 3 | 7-Hydroxy-2-(4- Hydroxy -3-Methoxyphenyl)-Chroman-4-one |
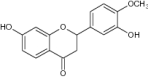
|
Examined antioxidant activity | Reduced oxidative stress, LDL level, Mn-SOD levels and SOD2 gene expression of hyperlipidemic rats by endogenous antioxidant enzymes reduction [47] |
| 4 | 3-(4’-Hydroxybenzyl)-5,7-dihydroxy-6-methyl-8-methoxychroman-4-one |
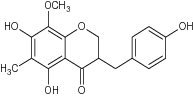
|
Assayed for DPPH radical inhibition | Showed antioxidant property with DPPH inhibition = 5.90 ± 0.150 to 0.64 ± 0.334 [48] |
| 5 | Benzyl-1,2,3-triazolyl hesperetin derivatives |
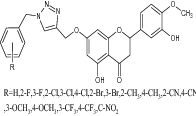
|
Examined antioxidant activity using DPPH and ABTS assays | Significant antioxidant values obtained as follows 30.75 ± 1.965 to 83.57 ± 0.456 (DPPH) 8.545 ± 0.545 to 39.356 ± 0.644 (ABTS), respectively [49] |
|
6 (a) |
Homoisoflavanone7-O-[α-rhamnopyranosyl-(1 → 6)-β-glucopiranoside]-5-hydroxy3-(4-methoxybenzyl)-chroman-4-one |
|
Assayed for antioxidant property against DPPH radical and β-carotene/linoleic acid system | Both compounds (a) and (b) showed respective antioxidant activity with following significant values (a) 273.1 ± 6.2 (DPPH) and (b) 212.4 ± 3.8(DPPH) [50] |
| (b) | 7-O-[α-rhamnopyranosyl-(1 → 6)-β-glucopiranosyl]-5-hydroxy-3-(4′-hydroxybenzyl)-chroman-4-one | |||
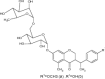
| 7 | Methyl 2-(cyclohexyl carbamoyl)-4-oxochroman-3-carboxylate |
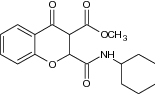
|
Evaluate lipid peroxidation process | Inhibited lipid peroxidation, and has been reported more potent antioxidants than vitamin E and Trolox [51] |
| 8 | Chroman-4-one derivative |
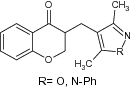
|
Examined DPPH inhibition | Showed DPPH inhibition with significant values [51] |
| 9 |
Isointricatinol (7,8-dihydroxy-3-(4’methoxybenzyl) chroman-4-one) |
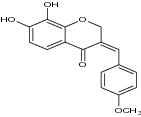
|
Assayed free radical scavenging effect against DPPH and ABTS radicals | Showed 85.50 mM (DPPH) and 44.13 mM (ABTS) values signify good antioxidant potential. [52] |
| 10 |
Liquiritin (7-hydroxy-2-[4-[3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl] oxyphenyl]-chroman-4-one |
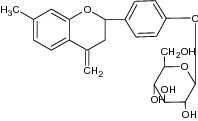
|
Examined for inhibition of enzymes superoxide anion (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) in mice | Showed neuroprotective effect against focal cerebral ischemia/reperfusion (I/R) by inhibition of SOD (75.11 ± 5.80 U/mg), CAT(7.62 ± 0.48U/mg) and GSH-Px (887.24 ± 79.23U/mg) [53] |
| 11 | Silybin |
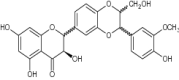
|
Evaluated for inhibition of Cyclic voltammetry (CV), DPPH scavenging and microsomal lipid Peroxidation (LPx) | Exhibited antioxidant potential with significant inhibition of CV (524 Epa mV), DPPH (1745 ± 65) and LPx (33.6 ± 1.2). [54] |
| 12 | 7-Methoxy-3-(4-methoxyphenyl)-chroman-4-one |
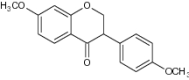
|
Examined for antioxidative capacity | Exhibited protective effects against cardiovascular defects, cancer and other diseases due to its high antioxidant capacity [55] |
| 13 | Pinobanksin |
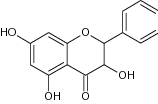
|
Antioxidant capacities were analyzed by HAT, SET-PT and SPLET mechanisms |
Showed significant antioxidant effect Among all the compounds, mainly 7–OH group contributes to the antioxidant activities [56] |
| 14 | 7,8-Dihydroxy-3-[(3,4-dihydroxyphenyl) methylene] chroman-4-one |
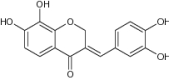
|
Antioxidant activity by NBT superoxide scavenging and DPPH free radical scavenging inhibition |
NBT = 8.5 µM DPPH = 4.5 µM [57] |
| 15 | Silymarin |
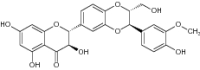
|
Antioxidant activity reported against DPPH |
Approximately 33% Inhibition of DPPH [58] |
Anti-inflammatory Activity
Localized protective reaction to infection or injury well known as inflammatory reaction plays a significant role in the pathological mechanism of various inflammation-related diseases like cancer, Alzheimer, diabetes, atherosclerosis and cardiovascular disorders. Knowledge of etiology and mechanism of inflammation progression can prevent further development of diseases [59].
For treatment of inflammation, diverse range of isolated homoisoflavanones, dihydroflavonols such as dihydromyricetin, hesperitin, stilbin and silybin containing chromanone pharmacophore are reported with vivid bioactivity and play an exclusive role in discovery and development of new anti-inflammatory drugs [60]. Among the isolated compounds from natural sources, chroman-4-one containing stilbin and silybin are used clinically as anti-inflammatory drugs. Moreover, these isolated compounds further pave the way for synthesis of new anti-inflammatory drugs via inhibition of cyclooxygenase 2 (COX-2). 4-Homisoflavonones enlisted in Table 4 developed as chroman-4-one analogs were found to have significant inhibition for COX 2 receptor binding in range of 0 ± 5.4 to 0 ± 21.3(% activity) [54, 61]. Likewise, a new chromanone, violacein A produced from Streptomyces violaceoruber emerged as potential therapeutic for treatment of inflammation-related disease by suppressing NF-kB (nuclear factor kappa light chain enhancer of activated B cells) signaling pathways. Moreover, it can be used for the treatment of other diseases like cancer [62]. Generally, SAR studies revealed that C-2 substitution with hydroxybenzylidine, arylidene, hydroxyphenyl, pyridine-3-yl and fluorophenyl displayed the best anti-inflammatory activity with significant inhibition. So, exploring the substitutions at C-3, 6, 7 and 8th of chromanone nucleus yields more efficient anti-inflammatory compounds which can compete isolated phytochemicals as well as existing anti-inflammatory drugs [60, 62]
Table 4.
Chroman-4-one analogs exhibiting anti-inflammatory activity
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | Hesperetin derivatives |
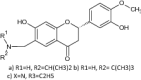
|
Anti-inflammatory activity was tested against IL-6, TNF-a cells | Exhibited significant anti-inflammatory activity by decreasing IL-6, TNF-a with minus log P values –0.26 (a), 0.76 (b) and − 0.75(c), respectively [63] |
| 2 | 4′-O-Demethylophiopogonanone E |
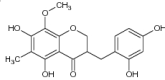
|
Inhibited pro-inflammatory cytokine ILβ, IL-6 and phosphorylation of ERK1/2 and JNK in MAPK signaling pathways to decrease NO and cytokines production | IC50 value for pro-inflammatory cytokine: ILβ = 32.5 ± 3.5 μg/mL, IL-6 = 13.4 ± 2.3 μg/mL which showed potent anti-inflammatory activity [59] |
| 3 | Novel (E)-5,7-dimethoxy-3-(4’-hydroxybenzylidene) chroman-4-one |
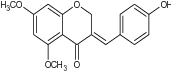
|
Tested in croton oil-induced edema in animal study | Showed significant anti-inflammatory potential [64] |
| 4 | 3-Arylidene-7-methoxychroman-4-one |
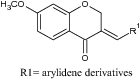
|
Evaluated in Carrageenan induced paw edema in rats | Exhibited significant anti-inflammatory activity [65] |
| 5 | 3,5,7-Trihydroxy-2-(4′-fluorophenyl) chroman-4-one |
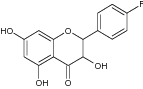
|
Tested for anti-inflammatory activity in IL-1β, IL-6, and TNF-α cells |
Decreased IL-1β, IL-6, and TNF-α cells inflammation These 3 derivatives showed most significant anti-inflammatory activity and cytotoxicity [65] |
| 3,5,7-Trihydroxy-2-(pyridin-3-yl) chroman-4-one |
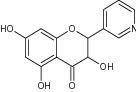
|
|||
| 3,5,6,7-Tetrahydroxy-2-(3′,4′-dihydroxyphenyl) chroman-4-one |
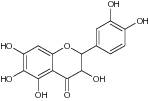
|
|||
| 6 | (R)-3-(3,4-Dihydroxybenzyl)-7-hydroxy-5-methoxychroman-4-one |
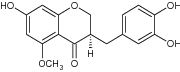
|
Evaluated for COX-2 receptor binding | Showed significant anti-inflammatory activity against COX-2 with % activity = 0 ± 5.4 [61] |
| (E)-3-(3,4-dihydroxybenzylidene)-7-hydroxy-5-methoxychroman-4-one |
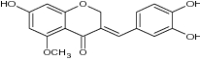
|
% activity = 0 ± 8.3 [61] | ||
| 1,3,6-Trihydroxy-2-methoxy-8-methylxanthen-9-one |
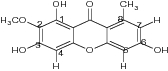
|
% activity = 0 ± 21.3 | ||
| Ovatifolionone acetate |
|
|||
| % activity = 0 ± 7.8 | ||||
| 7 | Pruinosanone A |
|
Inhibited inducible nitric oxide synthase (iNOS) protein expression | Showed anti-inflammatory potential with inhibition value 1.96 mM [66] |
| 8 | Myricitrin |
|
Significantly inhibited 5-lipoxygenase (5-LO) | Potent anti-inflammatory activity with IC50 = 7.8 ± 0.2 µM [67] |
| 9 | Violacin A (Streptomyces violaceoruber) |
|
Suppressed NF-kB signaling pathways | A potential therapeutic candidate for the treatment of inflammation-related disorders [62] |
| 10 | 7-Dimethylamino-4-chromanone |
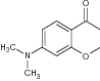
|
Tested for anti-inflammatory potential | Showed anti-inflammatory activity [68] |
Antidiabetic Activity
Diabetes mellitus represented as hyperglycemia is increasing globally at very significant pace and has become a complex chronic metabolic disease associated with diabetic complications like diabetic neuropathy and nephropathy [69]. A number of potent antidiabetic drugs like metformin, glucotrol, tolbutamide, acarbose, pioglitazone, gliptins, liraglutide are available in the market, but long-term use of the antidiabetic drugs may cause serious side effects, and moreover, these drugs are less effective in management of associated diabetic complications [70]. In this regard, a number of isolated compounds as natural flavonols, flavanones and homoisoflavanones containing chromanone pharmacophore such as naringenin, hesperitin, dihydroquercetin, eriodictyol, sakuranetin, aromadendrin, butin, pinocembrin, nymphaeol A, sophoraflavanone G and sterubin (Fig. 3) are already reported for their excellent potency in the treatment of diabetic mellitus and diabetes associated complications [2]. These chromanone containing flavanones treat diabetes through inhibition of receptors like glucagon-like peptide 1 (GLP-1), dipeptidyl peptidase-IV (DPP4), peroxisome proliferator receptor gamma (PPAR-γ), Alpha glucosidase (α-glucosidase), phosphatidylinositol 3-kinase (PI3K) in both in vivo and in vitro experimentation [71]. Moreover, several synthetic chromanone analogs are also reported (enlisted in Table 5) for antidiabetic potential via α-glucosidase inhibition and DPPH radical scavenging activity. Zhu et al. 2019 isolated various chromanone analogs from the seeds of Psoralea corylifolia and evaluated for diglyceride acyltransferase (DGAT), protein-tyrosine phosphatase 1B(PTP1B) and α-glucosidase activity. Out of theses isolated compounds, (2S)-7-methoxy-6-(2-hydroxy-3-methyl but-3-en-1-yl)-2-(4-hydroxyphenyl)chroman-4-one, (2S)-4′-hydroxyl-7-hydroxy methylene-6-(2′′,3′′-epoxy-3′′- methyl butyl) flavanone and bavachinone B exhibited good antidiabetic effect by inhibition of DGAT, PTP1B and α-glucosidase. Takao K. et al. introduced benzylidene-4-chromanone derivatives as the lead compound for the development of novel α-glucosidase inhibitors as well as significant antioxidative agent due to its potential in significantly inhibiting the level of DPPH. By the virtue of SAR studies, substitution at C-2, 3, 6 and 7th position of parent chromanone provide effective antidiabetic drugs which might help in the synthesis of more efficient novel compounds for the cure of diabetes and its complications [72, 73]. But, the research and published data about the synthetic chromanone containing medicinal compounds as antidiabetic are very less as compared to isolated flavanones, flavonols and homoisoflavanones. Therefore, a very effective synthetic approach is required for the synthesis and evaluation of more chromanone analogs as antidiabetics [72, 74].
Fig. 3.
Natural chromanones exhibiting antidiabetic activity
Table 5.
Chroman-4-one analogs exhibiting antidiabetic activity
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | [(E)-8-(3,7-dimethylocta-2,6-dienyl)-5,7-dihydroxy-2-(4-hydroxyphenyl) chroman-4-one)] |
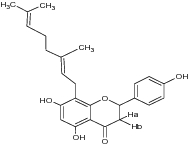
|
Evaluated for α-glucosidase inhibition | Exhibited antidiabetic activity with α-glucosidase inhibition [75] |
|
2 (a) |
(2S)- 7- methoxy—6- (2- hydroxy—3- methyl but- 3- en—1- yl)- 2- (4- hydroxyphenyl) chroman-4- one |
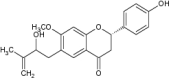
|
Isolated from seeds of Psoralea corylifolia Examined for DGAT, PTP1B and α- glucosidase inhibition |
Showed antidiabetic activity inhibiting DGAT, PTP1B and α- glucosidase with significant values [73] |
| (b) | (2S)-4′-hydroxyl—7-hydroxy methylene-6- (2′′,3′′- epoxy—3′′- methyl butyl) flavanone |
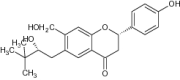
|
||
| (c) | Bavachinone B |
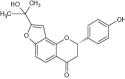
|
||
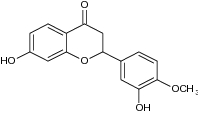
| 3 | 7-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-chroman-4-one |
|
Tested for antidiabetic activity | Increased insulin and decreased blood glucose level, HOMA-IR value and PEPCK gene expression in diabetic rats [76] |
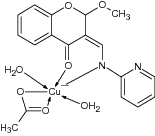
| 4 | 2-Methoxy-4-chromanones ligated Cu (II) |
|
Evaluated for α-glucosidase assay | Showed highest α-glucosidase inhibition with IC50 = 0. 060 ± 0.3 mM and displayed antidiabetic potential [77] |
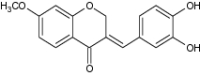
| 5 | 3-Benzylidene-4-chromanone derivatives |
|
Assayed for DPPH radical scavenging and α-glucosidase inhibition | Showed significant antioxidant potential [72] |
| 6 | 5,7-Dimethoxy-3-(2′-hydroxybenzyl)-4-chromanone |
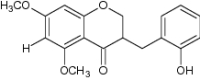
|
Evaluated for antidiabetic activity | Improved postprandial hyperglycemia in streptozotocin-induced diabetic mice by inhibiting carbohydrate digesting enzymes [78] |
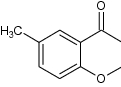
| 7 | 6-Methyl-4-chromanone |
|
in vitro evaluation for antidiabetic effect | Showed good antidiabetic effect using the glucose uptake by isolated rat hemi diaphragm (in vitro model) [79] |
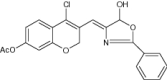
| 8 | 2H-Chromenylphenyloxazolones derivatives |
|
Evaluated for free radical scavenging and α-glucosidase inhibitory activity | Exhibited antidiabetic activity with antioxidant effect [80] |
Antibacterial and Antifungal Agents
Increased prevalence of pathogenic microbial infections necessitates the need for new antibacterial agents with distinct mechanism of action and broad spectrum of activity over a wide range of bacterial and fungal strains. Considering this viewpoint, several naturally occurring flavonoids and heterobicyclic compounds containing chromanone pharmacophore were found to have diverse activities ranging from antibacterial to antiviral activity. But these compounds were found to have two major limitations like poor yield and resistance against the general bacterial and fungal strains such as Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Aspergillus niger, Fusarium oxysporum, Penicillium italicum, Pythium ultimum, Sclerotinia sclerotium, Phytophthora capsici, E. Faecalis, C. albicans, C. Krusei, C. Glabrata and A. fumigates [81]. Therefore, there is a need for new synthetic antibacterial and antifungal compounds containing chromanone moiety.
It was found that substitution at C-2 with pyrazol-4-yl derivatives, methoxyphenyl, alkyl, vinyl, hydroxyl methyl and chlorophenyl group displayed broad spectrum antibacterial activity against tested bacterial strains, whereas azolyl and benzylidene derivatization yields good antifungal agents as shown in Table 6 [82]. Moreover, C-2, 3, 4, 6 and 7 substitutions also predicted efficient antibacterial and antifungal compounds with broad spectrum activity as well as low or no resistance. Likewise, most important series of benzylidene derivatives C-3-substituted 3-(benzo[1,3]dioxol-5-ylmethylene)-7-hydroxychroman-4-one, a 3-benzylidene-4-chromanone exhibited significant antibacterial activity against gram positive and gram negative bacteria, while 3-azolyl-4-chromanone phenyl hydrazones exhibited antifungal potential against C. albicans, S. cerevisiae, A. niger and M. gypseum pathogens [83]. Hence, more exhaustive approach for the chromanone scaffold may lead medical chemist to synthesize more potent antibacterial and antifungal compounds with maximum efficacy and minimum resistance.
Table 6.
Chroman-4-one analogs exhibiting antibacterial and antifungal values
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
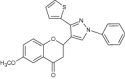
| 1 | 2-(1-Phenyl-3-(2-thienyl)-1H-pyrazol-4-yl) chroman-4-one derivatives |
|
Showed antibacterial activity evaluated against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and Escherichia coli using ampicillin (reference drug) -zone of inhibition (mm) measured using cup plate agar diffusion method | S. aureus = 32 mm B. subtilis = 15 mm P. aeruginosa = 11 mm Escherichia coli = 33 mm [84] |
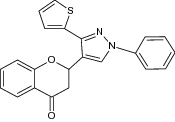
|
Exhibited antifungal activity against Aspergillus niger, Fusarium oxysporum and Penicillium italicum using griseofulvin (standard drug) | A. niger = 14 mm F. oxysporum = 30 mm P. italicum = 20 mm [84] | ||
| 2 | 2,2-Dimethylchroman-4-one derivatives |
|
Nutrient agar media and Potato Dextrose Agar (PDA) medium used for the measurement of antibacterial and antifungal potential as in zone of inhibition (mm) Exhibited antibacterial and antifungal activities |
B. subtilis = 31.5 mm, S. aureus = 20.5 mm K. pneumonia = 34.8 mm E. coli = 33.8 mm A. niger = 10.2 mm F. oxysporum = 17.5 mm [85] |
|
B. subtilis = 33 mm, S. aureus = 20 mm K. pneumonia = 33.5 mm E. coli = 30.5 mm A. niger = 15 mm F. oxysporum = 14.5 mm [85] | |||
| 3 | 6-Chloro-2-vinyl chroman-4-one |
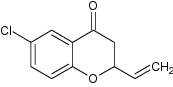
|
Showed antimicrobial activity against Bacillus Subtilis, Escherichia coli and Staphylococcus aureus-Vinyl group at carbon C-2 is necessary for the biological activity and Chloro group increases the activity | B. Subtilis = 4.17lg/mL E. coli = 4.17lg/mL S. aureus = 7.30lg/mL [86] |
| 4 | 2-Hydroxymethyl-chroman-4-one |
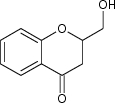
|
Exhibited antifungal activity against P. ultimum, S. sclerotiorum and P. capsici It was also used as intermediate for the synthesis of more effective benzopyranones |
ED50 ppm values for P. ultimum = 54.99 ppm S.sclerotiorum = 52.03 ppm P. capsici = 35.77 ppm [87] |
| 5 | Aposphaerin A and B |
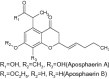
|
These are natural potent antibiotics evaluated for MRSA (methicillin resistant S. aureus) assay | Exhibited significant activity against methicillin resistant S. aureus (MRSA) [88] |
| 6 | 3-Phenylsulfonyl-3-(2-propenyl) chroman-4- one |
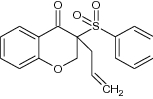
|
Evaluated for antimicrobial activity | Exhibited potent antimicrobial activity [89] |
| 7 | 5,7–Dihydroxy-3-(4-methoxybenzyl)-8-methyl chroman-4-one |
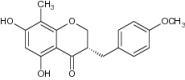
|
Isolated from the rhizomes of Polygonatum verticillatum-Antimicrobial activity assay using nutrient agar plate medium | Displayed significant antimicrobial activity via inhibition of B. subtilis, S. aureus and E. coli [90] |
| 8 | 2-(2,6-Dimethylhept-5-en-1-yl)-5,7-dihydroxychroman-4-one |
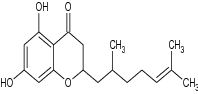
|
Antibacterial evaluation against E. Faecalis, S. aureus MSSA, S. aureus MRSA and E. coli (µg/ml) | E. Faecalis = 6.25(µg/ml) S. aureus MSSA = 3.13 (µg/ml), S. aureus MRSA = 3.13 (µg/ml) E. coli = 1.5 µg/ml [91] |
| 9 | 1-(2-(4-Chlorophenyl)-6-methoxychroman-4-ylidene)-2-phenylhydrazine |
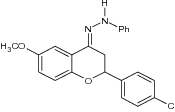
|
Exhibited potent antimicrobial activity against S. aureus, E. coli, C. Albicans and Aspergillus niger using agar diffusion technique | S. aureus = 15.5 ± 0.7 mm E. coli = 21.5 ± 0.7 mm C. albicans = 20.0 ± 0.0 mm A. niger = 20.0 ± 0.0 mm [92] |
| 10 | 3-(Benzo-[1, 3] dioxol-5-ylmethylene)-7-hydroxychroman-4-one, a 3-benzylidene-4-chromanone |
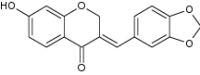
|
Evaluated against gram-negative bacteria | Significant antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Bacillus sphaericus, Klebsiella aerogenes, C. violaceum, Pseudomonas aeruginosa [92] |
| 3-benzylidene-4-chromanones derivatives |
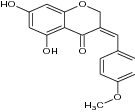
|
Tested antibacterial activity against S. aureus | S. aureus = 0.52 mM | |
| 11 | (2S,3R,4R,5R,6S)-2-(((2S,3R,4R,5S,6R)-4,5-dihydroxy-2-(((S)-5-hydroxy-2-(4-hydroxyphenyl) spiro [chroman-4,2′- [1, 3] dioxolan]-7-yl) oxy)-6-(hydroxymethyl) tetrahydro-2H-pyran-3-yl) oxy)-6-methyltetrahydro-2H-pyran-3,4,5-triol |
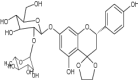
|
Exhibited antibacterial activity for gram-positive and gram-negative bacteria using broth micro-dilution method | S. aureus = 0.125 mg/mL E. coli = No inhibition P. aeruginosa = 0.25 mg/mL S. aureus (MRSA Positive) = 0.125 mg/mL |
| Also showed antioxidant potential | IC50 = 18.7 μg/mL [82] | |||
| 12 | (Spiro[chroman-2,1’-cyclohexan]-4-one) semi carbazone derivative |
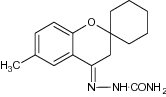
|
Antifungal activity evaluated against C. albicans, C. Krusei GO3, C. Glabrata HO5 and A. fumigates strains | Observed a clear zone of inhibition against C. Krusei GO3 strain [93] |
| 13 | Novel spiro chromanone–aurone hybrids (Z)-2′-(3,4-Dimethoxybenzylidene) spiro{cyclo-hexane-1,7′-furo[3,2-g] chromene}-3′,5′(2′H,6′H)-dione |
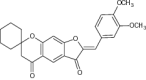
|
Exhibited high antibacterial activity for a number of gram + ve and –ve bacteria’s using streptomycin and amphotericin B (as standard drugs) | B. subtilis = 21.0 S. aureus = 19.5 P. aeruginosa = 22.5 E. coli = 21.0 A. flavus = 4.5 F. oxysporum = 6.5[94] |
| 14 | 2-MBT(2-mercaptobenzothiazole) with chroman-4-one moiety derivatives |
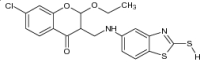
|
Evaluated for antimicrobial potential against S. aureus, B. subtilis, M. tuberculosis, C. albicans and S. cerevisiae strains | Showed potent antimicrobial activity with significant inhibition [95] |
| 15 | Novel flavanone derivatives |
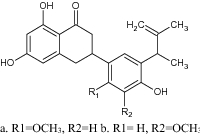
|
Antibacterial activity against S. aureus strains including MRSA |
MIC value = 16 µg/mL More potent than standard drug oxacillin [96] |
| 16 | Spiro[2-benzoyl-cyclohexyl-4,5-diphenylpyrrolidine-3,3’-chroman-4-one] |
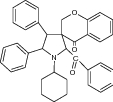
|
Evaluated for antifungal and antibacterial activities | Showed significant inhibition against tested pathogen strains [97] |
| 17 | 3-Azolyl-4-chromanone phenylhydrazones |
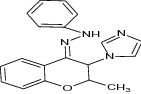
|
Tested against C. albicans, S. cerevisiae, A. niger and M. gypsum pathogens | MICs values (μg /mL) are: C. albicans = 8 μg/mL, S. cerevisiae = 16 μg/mL, A. niger = 16 μg/mL, M. gypseum = 16 μg/mL Exhibited Antifungal potential [83] |
| 18 | 5,7-Dihydroxy-2-[4-(3-methyl-but-2-enyloxy) ~ phenyl] chroman-4-one{selinone) |
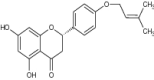
|
Antifungal activity against C. albicans | Showed significant inhibition [98] |
| 19 | (E)-5,7-dimethoxy-3-benzylidene-4-chromanone |
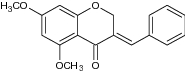
|
Antifungal activity against C. albicans | Showed significant antifungal activity (MIC50 ± 25 mM) [81] |
Anti TB Agents
Among the available treatments for tuberculosis, first-line and second-line antituberculosis (TB) drugs, DOTS (directly observed treatment short-course) is one of the most competent multidrug effective strategy developed by WHO against Mycobacterium tuberculosis. However, the success rate for cure of TB patients struggles to achieve 85% [99]. Therefore, more research is required for the development of novel potent antimycobacterial agents with promising efficacy and less side effects. From the series of heterobicyclic compounds, isolated as well as synthetic chroman-4-one analogs were reported to inhibit M. tuberculosis [100, 101]. Chromanone analogs having significant potential for the treatment of TB with greater efficacy and less side effects are enlisted in Table 7. This will provide us a new framework in designing and development of chromanone derivatives and their potent multidrug combinations like spiro chromanones as novel antiTB agents [102].
Table 7.
Chroman-4-one analogs exhibiting antitubercular activity
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
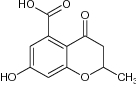
| 1 | 7-Hydroxy-2-methyl-4-oxo-3,4-dihydro-2H-benzopyran-5-carboxylic acid |
|
Evaluated for M. tuberculosis | Potential inhibitors of M. tuberculosis (MbtI) with IC50 = 55.8 µM [103] |
| 2 | (E)-3-(dibenzo [b, d] furan-2-ylmethylene)-6-fluorochroman-4-one |
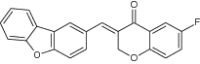
|
Examined for M. tuberculosis H37Rv Inhibition | Inhibited M. tuberculosis H37Rv with MIC 12.5 µg/mL and considered as most potent antitubercular agents [104] |
| (E)-3-(dibenzo [b, d] furan-2-ylmethylene)-6-bromochroman-4-one |
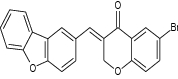
|
|||
| 3 | Chromane-4-one analogs |
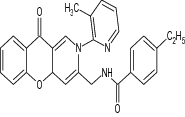
|
Tested anti TB activity against M. tuberculosis and MDR-TB in vitro |
in vitro study revealed anti TB activity against M. tuberculosis and MDR-TB with MICs of 0.22 and 0.07 μg/mL, respectively, and decreased the bacterial load in lung and spleen tissues with log10 = 1.11 and 2.94 [105] |
| 4 | 7-Hydroxy-(E)-3-[(2-fluorophenyl) methylene] chroman-4-one |
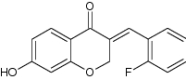
|
Screened against Mycobacterium smegmatis mc2 155 for anti TB potential | Exhibited significant inhibition as efflux pump inhibitors against Mycobacterium smegmatis mc2 155 [106] |
| 7-Hydroxy-(E)-3-[(4-methoxyphenyl) methylene] chroman-4-one |
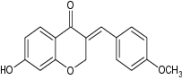
|
|||
| 7-Hydroxy-(E)-3-[(3-allyloxyphenyl) methylene] chroman-4-one |
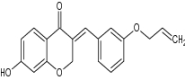
|
|||
| 5 | Triazolyl methoxy flavanones |
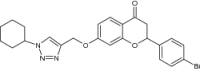
|
Examined for mycobacterial FAS-II and PknG inhibition |
Inhibited mycobacterial FAS-II (IC50 = 20.3 µM) and PknG The % growth inhibition was 75.1 at 100 µM. [107] |
| 6 | Amino alcohol-linked spiro chromones |
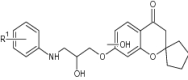
|
Tested for M. tuberculosis H37Rv (ATCC27294) cells growth | Inhibited M. tuberculosis H37Rv (ATCC27294) with MIC value of 3.13 µg/mL [108] |
| 7 | 3-(4′-Methoxybenzyl)-7,8-methylenedioxy-chroman-4-one |
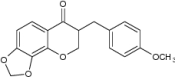
|
Evaluated for M. phlei, M. aurum, M. fortuitum and M. smegmatis inhibition | Displayed antimycobacterial activity against M. phlei (16 µ g/mL), M. aurum (32 µ g/mL), M. fortuitum (128 µ g/mL) and M. smegmatis (256 µ g/mL) [109] |
| 8 | Bis-spiro chromanones |
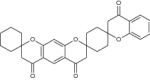
|
Examined against strain H37Rv (ATCC 27,294) for antimycobacterial potential | Exhibited antimycobacterial activity against H37Rv (ATCC 27,294) strain with MIC = 3.125 μg/mL [110] |
Antiviral Agents
A number of viral diseases in human ranging from mild upper respiratory infection to fatal cardiac and neurological illnesses are caused by numerous picornaviruses particularly rhinoviruses (HRVs) and enteroviruses (EVs). A diverse range of effective antipicornavirus agents are developed and some are in clinical phase study [111]. Literature evidence has established that natural and synthetic flavonoids containing chromane obstruct the replication step of picornavirus and further prevent the decapsidation of infected viral segments and release of corresponding RNA within cells [112]. Tait S et al. (2006) examined antiviral activity of homoisoflavonoids (3-benzylchroman-4-ones derivatives) on enteroviruses replication, and these chromanone analogs showed significant activity against coxsackie virus B1, B3, B4, A9, echovirus 30 and substitution at C-3 of parent chromanone exhibited good efficacy against HRVs growth. Moreover, introduction of thiopyran at C-2 by Hegab, MI et al. (2015) showed considerable antiviral activity against strained adenovirus type 7 with significant inhibition as shown in Table 8. So, following these substitutions, more antiviral chromanone analogs may be designed and synthesized with significant antiviral activity [35, 113].
Table 8.
Chroman-4-one analogs exhibiting antiviral activity
| Sr. No. | Name | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | 5′′-Aceto-3′′-phenyl-3′′H,4′H-dispiro[chroman-2′,4-tetrahydropyran-3′,2′′-[1,3,4-thiadiazol]-4′-one |
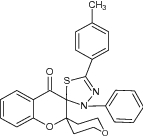
|
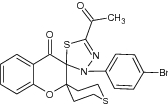
Examined for adenovirus type-7 inhibition for antiviral activity | Showed considerable antiviral activity against strained adenovirus type 7 with significant inhibition [35] |
| 5′′-Aceto- 3′′-(4-bromophenyl)- 3′′H, 4′H-dispiro[chroman-2′,4-tetrahydrothiopyran-3′,2′′-[1,3,4-thiadiazol]-4′-one |
|
|||
| 2 | Chroman-4-one derivatives |
|
Evaluated against human rhinovirus (HRV) 1B and 14 inhibition | Displayed antiviral potency against tested HRV 1B and 14 strains [114] |
| 3 | 3-Benzylchroman-4-one’s derivatives |
|
Tested for antiviral activity | Showed antiviral activity against Coxsackie virus B1, B3, B4, A9 and echovirus 30 [115] [113] |
Anti-HIV Compounds
Chromanone derivatives have attained much potential against treatment of Acquired Immune Deficiency Syndrome (AIDS) caused by HIV-1 [116]. In 1994, an isolated calanolide A and inophyllums from calophyllum genus reported as a novel anti-HIV chemotype inhibited HIV-1-specific nonnucleoside RT [117]. Furthermore, an enantioselective designing of 2, 3-dimethyl-4-chromanone ring attachment was attempted for the synthesis of potent calophyllum coumarins as anti-HIV 1 and it showed significant effect which can compete with currently available protease inhibitors (atazanavir, ritonavir, lopinavir, nelfinavir, saquinavir etc.) approved by Food and Drug Administration (FDA) [118, 119].
Dawood et al. (2005) tested a series of 3-benzylidene derivatives of chromanone and substitution of thio (S) group at the place of oxo(-O) at Ist position displayed impressive anti-HIV activity. Consequently, SAR studies revealed that C-2 and 6 substitution with oxo propionic acid and benzyl group exhibited anti-HIV activity by inhibition of HIV-1 IN strand transfer with good inhibitory values as shown in Table 9. So, more research and development on chromanone analogs can be a prominent approach for synthesis of novel HIV1 inhibitors against the treatment of AIDS [120].
Table 9.
Chroman-4-one analogs exhibiting anti-HIV activity
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | Calanolide A |
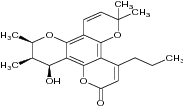
|
Examined AZT-resistant strains for anti-HIV potency | Showed significant anti-HIV activity against AZT-resistant strains [118] [121] |
| 2 |
1,3-Diketo acids, new chromone and chromanone derivatives |
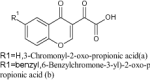
|
Evaluated for anti-HIV activity | Exhibited anti-HIV activity by inhibition of HIV-1 IN strand transfer (IC50, µM = 168.2 and 100.6 for a and b respectively) [122] |
| 3 | 3-Benzylidene derivatives of chromanone |
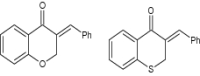
|
Tested for anti-HIV effect | Showed significant anti-HIV activity [120] |
Antileishmanial Agents
A group of parasites of genus Leishmania causes tropical disease known as Leishmaniasis, which spreads in living beings by the bite of infected sandflies [123]. These flies have a multifarious life cycle which exhibits an amastigote phase in the host cell as well as a promastigote phase in vector flies. A number of clinical representations, especially cutaneous, visceral and mucosal forms, attain the most serious grade for Leishmaniasis [124]. Although diverse treatment for Leishmaniasis or cutaneous leishmaniasis (CL) exists, less potency, toxicity and cost issues are some of the significant limitations associated with the present treatment options. So, development of more effective novel compounds against Leishmaniasis remains an important stratagem. A wide range of natural flavonoids having chromanone pharmacophore and synthetic oxygenated heterocyclic compounds such as hydrazones, thiosemicarbazones and semicarbazones have been reported to exhibit antileishmanial potential. Among these, semicarbazone and thiosemicarbazone derivatives of flavanone and thioflavanone could be considered a potential privileged structure [125]. Using this approach, derivatization of chroman-4-one and thiochroman-4-ones with acyl hydrazones resulted in significant enhancement of antileishmanial potential [126]. As thiochroman-4-one derivatives showed immense similarity with the chroman-4-one compounds so could be considered as potential scaffold with wide range of bioactivity like antiviral, antitumoral, antimalarial, antibacterial and antileishmanial [127]. Therefore, more research in thiochromanone derivatization is required for more novel antileishmanial compounds, and some relevant compounds are reported in Table 10.
Table 10.
Chroman-4-one analogs exhibiting antileishmanial activity
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | Thiochroman-4-one’s derivatives |
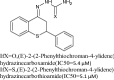
|
Evaluated for in vitro antileishmanial activity against the intracellular amastigote form of Leishmania panamensis | These compounds showed good antileishmanial activity with significant inhibition [128] |
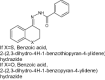
| 2 | Benzoic acid, 2-(2,3-dihydro-4H-1-benzopyran-4-ylidene) hydrazide |
|
In vitro evaluation using macrophage intracellular mastigotes of Leishmania (Viannia) panamensis and L. (V) braziliensis by flow cytometry antileishmanial activity | Showed combined actions of antileishmanial with inflammatory and wound healing properties [129] |
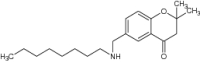
| 3 | 2,2-Dimethyl-6-(octyl amino) methyl chroman-4-one |
|
Evaluated in romastigotes, axenic amastigotes and Leishmania-infected macrophages | Exhibited antileishmanial potency with IC50 = 24.6 ± 0.4 μM [130] |
Anti-Acetyl Cholinesterase (AchE) Agents for Alzheimer Treatment
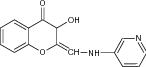
Alzheimer is a complicated neurodegenerative disease that poses severe threat to human health and characterized by memory loss, behavioral abnormalities and cognitive deficits [131]. Alzheimer’s Association reported that the number of patients suffering from Alzheimer’s disease (AD) is now approximately 47 million and this figure is projected to increase around 100 million by 2050 entire over the world [132]. So, demand of a better treatment for AD around the world is a challenge for the medical science.
Polyphenolic compounds like flavonoids, flavones, isoflavone exhibit broad range of biological properties including inhibition of AchE enzymes and its catalytic steps [133]. In this review, we included a number of chroman-4-one containing flavonoids which exhibit AchE inhibition potential [134]. This study presents that presence of an amino group, piperidinyl of chromanone derivatives and novel dithiocarbamates show potent AchE inhibition in comparison with the standard drugs like Tacrine (Table 11). Therefore, designing and development of new effective chromanone hybrids as AchE inhibitors provide a new framework to the treatment of AD and other neurodegenerative disorders [135].
Table 11.
Chroman-4-one analogs exhibiting anti-AchE inhibition
| Sr. No. | Name of compound | Structure | Evaluation | Inhibition |
|---|---|---|---|---|
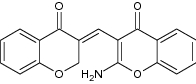
| 1 | Chromone-chromanone hybrid |
|
Evaluated for acetylcholine esterase (AchE) inhibition assay | An amino group on the 2-position of chromone ring showed potent AchE inhibition against the reference drug, Tacrine with IC50 = 0.27 µM [135] |
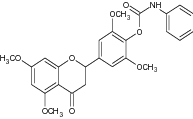
| 2 | Flavonoid derivative with carbamate moiety |
|
Evaluated for AchE inhibition and anti-amnestic potential | Exhibited AchE inhibitory activity with IC50 = 9.9 ± 1.6 mM [136] |
| 3 | Chroman-4-one derivative with the piperidinyl ethoxy side chain and 4-hydroxybenzylidene substitution |
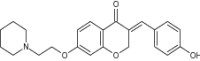
|
Evaluated for AchE activity and docking for finding effective binding pockets | Showed most potent anti-acetylcholinesterase activity (IC50 = 1.18 μM) and also showed remarkable interactions with the binding pockets of the ChE enzymes against Butrylcholinesterase (BuchE) and AchE, in the molecular docking study [137] |
| 4 | Novel chromanone-dithiocarbamate hybrids (n = 5) |
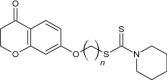
|
Evaluated for AchE-induced Aβ aggregation inhibition | A novel hybrid with n = 5 showed best activity to inhibit AchE (IC50 = 0.10 μM) and AchE-induced Aβ aggregation (33.02% at 100 μM) and could effectively inhibit self-induced Aβ aggregation (38.25%at 25 μM) [138] |
| 5 | (E)-3-(3-Hydroxy-4-(piperidin-1-ylmethyl) benzylidene)-6,7-dimethoxychroman-4-one |
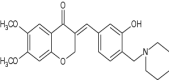
|
Selective AchE and MAO-B dual inhibitors of homoisoflavonoid Mannich bases were screened against Alzheimer’s disease | (E)-3-(3-Hydroxy-4-(piperidin-1-ylmethyl) benzylidene)-6,7-dimethoxychroman-4-one showed excellent AchE and MAO-B inhibitory activities (IC50 = 2.49 ± 0.08 nM and 1.74 ± 0.0581lM, respectively), good self- and Cu2+−induced Ab1-42 aggregation inhibitory potency, antioxidant activity, biometal chelating ability and high BBB permeability. [139] |
Anticonvulsants
Epilepsy is a chronic neurological disease in which patient suffers from seizures, transient attacks that affect at least 70 million people around the world. A number of therapeutic agents are available in the market as antiepileptic drugs such as phenytoin, phenobarbital, benzodiazepines, carbamazepine, ethosuximide and sodium valproate exhibit acceptable management on seizures, but resistance is observed in 30–40% epilepsy patients [140]. Therefore, MDT (multiple-drug therapies) is preferred for the control of seizures. But still no MDT or single drug can prevent the development or treat epilepsy for longer time interval without development of resistance [141]. Considering this viewpoint, more potent compounds which can cure or prevent epilepsy with high efficacy and less side effects are need of the hour.
A series of chroman-4-one derivatives such as azolylchromanones, azolylchromanone oximes and imidazolyl chromanone oxime ethers were tested for their anticonvulsant potential in lithium, pilocarpine induced seizure and pentylenetetrazol (PTZ) kindling model of epilepsy [142]. Among these tested compounds, some analogs (Enlisted in Table 12) showed good anticonvulsant activity with significant seizure latency and seizure duration. SAR studies showed that presence of azolyl ring at 3-postion, halogen (Chloro) group at the 7-position and/or an alkyl (especially methyl) group at the 2-position of the chromane ring resulted in an enhancement of anti-seizure efficiency in O-(2,4-dichlorobenzyl) oxime series and no significant difference in efficacy was observed for both (Z)- and (E)-isomers against the seizure durations and seizure latency [143]. Therefore, effective report of promising chroman-4-one analogs (Table 11) in epileptic models can predict potential clinical worth in treatment of adequate epileptic disorder.
Table 12.
Chroman-4-one analogs exhibiting anticonvulsant activity
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | 7-Chloro-3-(1H-imidazol-1-yl) chroman-4-one |
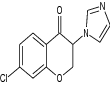
|
Investigated for both anticonvulsive and antiepileptogenic properties using lithium pilocarpine induced seizure and PTZ-induced kindling models | Effective against lithium and pilocarpine induced status epilepticus with seizure latency = 32.12 ± 3.04 and seizure duration = 12.50 ± 1.87, whereas in PTZ-kindling model of epilepsy, seizure latency and seizure index were observed as 3.75 ± 0.14 and 3.43 ± 0.14, respectively [144] |
| 2 | 3-(1H-1,2,4-triazol-1-yl) chroman-4-one |
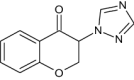
|
Evaluated for PTZ-kindling model of epilepsy |
Produced significant action in delaying seizures as well as effective protection against PTZ-induced seizures (seizure latency = 22.00 ± 4.11 min) and deaths [145] |
| 3 | Imidazolyl chromanone oxime |
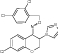
|
Tested in PTZ-kindling model of epilepsy | Exhibited anticonvulsant activity with Seizure latency (s) = 715 ± 153 Seizure duration (s) = 40.3 ± 4.7 |
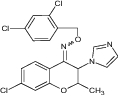
|
Seizure latency(s) = 776 ± 97 Seizure duration (s) = 342 ± 4.9 [143] |
Antidepressants
Depression, a mental disorder, seeks a major concern in medical practice, and it is more common in patient than reported. Its generation is associated with amino acid metabolism in which tryptophan, an essential amino acid metabolizes amines and releases various amine neurotransmitters like serotonin, epinephrine, norepinephrine (NE), melanin and dopamine (DA) controlling the autonomic nervous system (ANS) and central nervous system (CNS) functioning under the catalysis of flavoenzyme monoamine oxidases (MAOs). It exists as in two isomeric forms MAO-A and MAO-B, from which MAO-A selectively metabolizes NE, epinephrine, 5-HT, whereas MAO-B specifically deactivates β-phenethylamine, benzylamine and inactivated by the following inhibitors such as rasagiline and selegiline with many side effects and resistance [146, 147]. So, new MAOs inhibitors are required for developing new antidepressant drugs.
For the development of more effective novel MAO inhibitors, the chromanone scaffolds from natural and synthetic sources displayed a promising effect and exhibited their potency for MAO-B inhibition selectively [148]. In particular, chromanone derivatives substituted with benzyloxy group at C-7 are more potent MAO-B inhibitors and after searching of more chromanone derivatives, DSP-1053, a novel compound examined for serotonin reuptake in vivo with 5-HT1A partial agonistic activity and inhibited serotonin reuptake and considered as fast antidepressant drug in clinical practice (Table 13) [149]. Consequently, after reviewing the clinical data of DSP-1053, a probability comes out that more research in chromanone compounds might add a new class of antidepressant drugs.
Table 13.
Chroman-4-one analogs displaying antidepressant potential
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | (E)-3-Heteroarylidenechroman-4-ones |
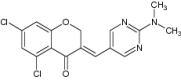
|
In vitro investigation to inhibit the enzymatic action of both human monoamine oxidase (hMAO) isoforms such as hMAO-A and B | Showed highest hMAO-B potency (IC50 = 10.58 nM) and selectivity (SI > 9452) with respect to the standard inhibitor selegiline (IC50 = 19.60 nM, IC50 > 3431) [150] |
| 2 | Chroman-4-one derivatives |
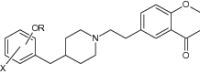
|
Evaluated for the inhibition of serotonin transporter (SERT) and 5-HT1A receptor | Exhibit as novel dual inhibitors of the SERT/5-HT1A receptors [151] |
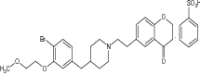
| 3 | DSP-1053 (Novel compound) |
|
Examined for serotonin reuptake Inhibition with 5-HT1A partial agonistic activity in in vivo study |
Inhibited serotonin transporter with an IC50 value of 2.74 ± 0.41 nmol/L and had an intrinsic activity for 5-HT1A receptors of 70.0 ± 6.3% -A novel serotonin reuptake inhibitor showed fast antidepressant effect [149] |
| 4 | 7-((4-Fluorobenzyl) oxy) chroman-4-one |
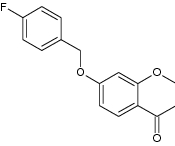
|
Evaluated for hMAO-B inhibitory activity in vitro | Inhibit MAO-B inhibition with IC50 = 8.62 nM and SI > 11,627.9-fold-Showed low neurotoxicity in SH-SY5Y cells in vitro-Used to treat neurodegenerative disease [152] |
| 5 | (E)-3-(4-methoxybenzylidene)-7-(3-(piperidin-1-yl) propoxy) chroman-4-one |
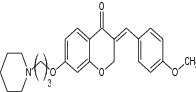
|
Evaluation for dual acetyl cholinesterase (AchE) and monoamine oxidase (MAO-B) inhibition | Inhibition of AchE and hMAO-B enzymes activities, with IC50 value of 3.94 and 3.44 mM, respectively [147] |
Insecticides
As per the literature, it is reported that for the development and metamorphosis regulation in insect, mainly 20-hydroxy-ecdysone and juvenile hormones are necessary and play promoting roles in endocrine systems of insects as growth regulators [153]. In 1988, 1-tert-Butyl-1, 2-dibenzoyl hydrazine, first non-steroidal ecdysone agonist, was reported. Further, a variety of ecdysone agonists were identified among them diacyl hydrazine compounds produced significant response for the growth and regulation of the insect structure [154, 155]. In 2007, Zhao et al. have introduced new design of insecticidal agents as chromanone derivatives of diacylhydrazines in which chromafenozide group was attached on a chromanone moiety instead of chromane structure (Table 14). This study provided significant evidence that chromanone derivatives of diacylhydrazines exhibited more potency for insecticidal activity as compared to reference compound ANS-118 (commercial insecticide containing chroman moiety) [156].
Table 14.
Insecticidal evaluation of chroman-4-one analogs
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | Chromanone derivatives of diacylhydrazines |
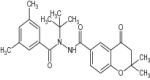
|
Tested killing property against Mythima separata | Showed insecticidal potency against Mythima separata [156] |
| 2 | Rotenone |
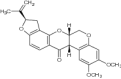
|
Evaluated for insecticide activity | Exhibited impressive insecticidal effect [157] |
Diagnostic agents
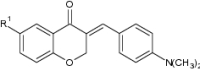
Ganand et al. synthesized a series of (E)-3-benzylidenechroman-4-one’s derivatives (homoisoflavonoids) and evaluated for their diagnostic imaging potential for pathogenesis of AD by targeting β-amyloid (Aβ) plaques. In vitro studies revealed that (E)-3-(4-methoxybenzylidene)-6-bromo-chroman-4-one and (E)-3-(4-dimethylamino-benzylidene)-6-bromo-chroman-4-one derivatives exhibited high binding affinities to Aβ plaques with 9.98 and 9.10 nM (Ki values), respectively, as compared to [125I] 2-(4′- dimethylaminophenyl)-6-iodoimidazo[1,2-α] pyridine (IMPY) (reference compound) [1, 158], whereas fluorescent staining test (applied on the brain sections of AD patients) and biodistribution data revealed that (E)-3-(4-dimethylamino-benzylidene) derivatives can selectively label Aβ plaques in brain sections and [125I]-radio labeled compound showed adequate brain uptake for brain scanning (Table 15) [1]. Chroman-4-one derivatives may be helpful diagnostic imaging agent for early finding of Aβ plaques in AD brain. Hence, further research and development of novel chromanone derivatives for their diagnostic potential creates a great interest in the field of medicinal chemistry.
Table 15.
Chroman-4-one analogs evaluated as diagnostic agents
| Sr. No. | Name of compound | Structure | Evaluation | Inference |
|---|---|---|---|---|
| 1 | (E)-3-(4-Methoxybenzylidene)-6-bromo-chroman-4-one |
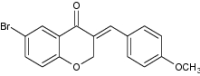
|
Diagnosis of Alzheimer’s disease | Exhibited high binding affinities to Aβ plaques with 9.98 and 9.10 nM (Ki values), respectively, against [125I] IMPY (reference compound) Helpful diagnostic imaging agent for early finding of Aβ plaques in Alzheimer’s disease brain [158] |
| (E)-3-(4-Dimethylamino-benzylidene)-6-bromo-chroman-4-one |
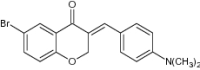
|
|||
| Iodine labeled 3-benzylidenechroman-4-one’s derivative, R1 = 125I |
|
Miscellaneous
Antidengue
M. M. V. Ramana et al. (2015) reported a docking study of isolated flavanones and designed chroman-4-one compounds against NS2B/NS3 protease (dengue virus protein). As per literature survey, naringenin and pinocembrin are known to have effective potential as antidengue agent with good dock score. Similarly, in-silco experimentation revealed the antidengue activity of isolated eriodictyol having Leu 149 and Asn 152 hydrogen bonding and 2-(2, 4-dihydroxy-6-methylphenyl)-5, 7-dihydroxychroman-4-one [designed compound] also showed interactions with Asp 75 and Asn 152 with a comparable glide score -7.31 that characterizes its antidengue activity [159].
Antiparasitic
Chroman-4-one analogs 6-hydroxy-2-(3-hydroxyphenyl) chroman-4-one, 6-hydroxy-2-(4-hydroxyphenyl) chroman-4-one and 2-(3,4-dihydroxyphenyl)-6-hydroxychroman-4-one displayed antiparasitic activity by targeting pteridine reductase-1 and showed significant inhibition against T. brucei and L. infantum at 10 µM and 50 µM. Therefore, more research on chroman-4-one derivatives as antiparasitic may provide a new platform for the development of new antiparasitic agents. [160].
Anti-aging
Chroman-4-one derivatives were used as active compound in cosmetic preparations for care, improvement and refreshment of texture of the skin and hairs, for treatment of skin as well as hair-related defects like inflammation, allergies or wound healing process. Furthermore, cosmetic formulations containing vitamin A and its derivatives like retinol esters, retinoic acid have shown their action on the differentiation process of epithelial cells and therefore used against psoriasis, acne, skin spotting, wrinkles and discoloration. Therefore, chroman-4-one scaffolds have significant cosmetic value along with pharmacological activities [160].
Anticoronal
Kwon Dur-Han, et al. proposed inhibitory potential of an isolated flavanone, quercetin-7-rhamnoside against viral propagation. Furthermore, experimentation showed effective results against inhibition of coronavirus via specific inhibition of PEDV (porcine epidemic diarrhea virus) proliferation in Vero cells with good inhibitory activity = -7.143 CC50/IC50 = > (100 g/mL) as compared to standard antiviral drug (ribavirin). Accordingly, in vitro inhibiting activity of quercetin-7-rhamnoside against PEDV, a number of flavonoids such as quercetin, luteolin, apigenin showed effective inhibition in vero cells. In spite of PEDV inhibition, quercetin-7-rhamnoside also exhibited antiviral potential against porcine transmissible gastroenteritis virus (TGEV) with inhibition value > 1.58 [CC50/IC50 = > (100 g/mL/63.3 µg/ml)] and porcine respiratory coronavirus (PRCV) with inhibition value > 1.67 [CC50/IC50 = > (100 g/mL/59.8 µg/ml)]. Therefore, quercetin-7-rhamnoside displayed better antiviral inhibition potential against three types of coronaviruses like TGEV, PEDV and PRCV than reference drug (ribavirin) in vitro. In conclusion, more experimentation on chromanone containing flavonoids as anticoronal for the inhibition of other classes of corona virus might be led a new frame for drug discovery and development [161]
Conclusion
Chroman-4-one/chromanone pharmacophore is a privileged scaffold in medicinal research, consisting of two rings in which 2, 3-dihydro-γ-pyranone fused with an aromatic benzene nucleus and derivatization at 2, 3 and 4-positions of chromanone skeleton yields more effective families of flavonoids like 3-benzylidene-chromanones, spirochromanone, hydrazones, oximes, flavanones, homisoflavonones and isoflavanones. This structural diversification of chromanone occupied an important role in pharmaceutical field as they owe numerous biological activities like anticancer, antioxidant, antidiabetic, anti-inflammatory, antiviral, antitubercular, antibacterial, antifungal, antiparasitic, anti-AchE, anticonvulsant, anti-HIV and antileishmanial properties. As chromanone scaffolds exhibit numerous potent biological activities, even some derivatives are novel like DSP-1053 (novel serotonin reuptake inhibitor and fast antidepressant), calanolide A (anti-HIV), Silibinin and chrysin(anticancer), taxifolin (antidiabetic), tetrazole (antidiabetic), troglitazone (antidiabetic), ormeloxifene (anticancer) and nebivolol (beta-blockers); nevertheless, the market proportion of potent chromanone analogs is fewer. Therefore, for future prospective, more consideration is required for designing and developing potent synthetic chromanone analogs which may provide better therapeutic value.
References
- 1.Emami S, Ghanbarimasir Z. Recent advances of chroman-4-one derivatives: synthetic approaches and bioactivities. Eur. J. Med. Chem. 2015;93:539–563. doi: 10.1016/j.ejmech.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 2.Panche AN, Diwan AD, Chandra R. Flavonoids: an overview. J. Nutr. Sci. 2016;5(47):1–15. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christophe Carola H., Ralf Rosskopf M.: Use of chroman-4-one derivatives.: United States Patent application publication., US 2010/0028278 A1,1, 1–24 (2010).
- 4.Cotelle N. Role of flavonoids in oxidative stress. Curr. Top. Med. Chem. 2001;I(2001):569–590. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- 5.Jaracz S, Chen J, Kuznetsova LV, et al. Recent advances in tumor-targeting anticancer drug conjugates. Biomed. Pharmacother. 2005;13:5043–5054. doi: 10.1016/j.bmc.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 6.Schindler R, Mentlein R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J. Nutr. 2006;136:1477–1482. doi: 10.1093/jn/136.6.1477. [DOI] [PubMed] [Google Scholar]
- 7.Piyush K, Kuldeep S, Azizur RM, et al. A review of benzopyran derivatives in pharmacotherapy of breast cancer. Asian J. Pharm. Clin. Res. 2018;11(7):43–46. doi: 10.22159/ajpcr.2018.v11i7.26017. [DOI] [Google Scholar]
- 8.Raj V, Lee J. 2H/4H-Chromenes-A versatile biologically attractive scaffold. Front. Chem. 2020;8:623. doi: 10.3389/fchem.2020.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felgines C, Texier O, Morand C, et al. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279(6):1148–1154. doi: 10.1152/ajpgi.2000.279.6.G1148. [DOI] [PubMed] [Google Scholar]
- 10.Kodama O. Sakuranetin, a flavanone ultraviolet-irradiated phytoalexin from rice leaves. Phytochemistry. 1992;31(11):3807–3809. doi: 10.1016/S0031-9422(00)97532-0. [DOI] [Google Scholar]
- 11.Ley JP. Evaluation of bitter masking flavanones from herba santa (Eriodictyon californicum (H. & A.) Torr., Hydrophyllaceae ) J. Agric. Food Chem. 2005;53:6061–6066. doi: 10.1021/jf0505170. [DOI] [PubMed] [Google Scholar]
- 12.Kumar D, Sharma P, Singh H, et al. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017;7(59):36977–36999. doi: 10.1039/C7RA05441F. [DOI] [Google Scholar]
- 13.Gordo J, Cabrita E, Oliva A, et al. Thymus mastichina: chemical constituents and their anti-cancer activity. Nat. Prod. Commun. 2012;7(11):1491–1494. [PubMed] [Google Scholar]
- 14.Lopez-Lazaro M. Flavonoids as anticancer agents: structure-activity relationship study. Curr. Med. Chem. - Anti-Cancer Agents. 2002;2(6):691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- 15.Kopustinskiene DM, Jakstas V, Savickas A, et al. Flavonoids as anticancer agents. Nutrients. 2020;12(2):1–24. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noushini S, Alipour E, Emami S, et al. Synthesis and cytotoxic properties of novel (E)-3-benzylidene-7- methoxychroman-4-one derivatives. DARU J. Pharm. Sci. 2013;21(1):1–10. doi: 10.1186/2008-2231-21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanova L, Varinska L, Pilatova M, et al. Cyclic chalcone analogue KRP6 as a potent modulator of cell proliferation: An in vitro study in HUVECs. Mol. Bio. Rep. 2013;40(7):4571–4580. doi: 10.1007/s11033-013-2547-x. [DOI] [PubMed] [Google Scholar]
- 18.Alipour E, Mousavi Z, Safaei Z, et al. Synthesis and cytotoxic evaluation of some new[1,3]dioxolo[4,5-g]chromen-8-one derivatives. DARU J. Pharm. Sci. 2014;22:41. doi: 10.1186/2008-2231-22-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahram L. Synthesis and in vitro cytotoxic activity of novel chalcone-like agents. Iran J. Basic Med. Sci. 2013;16:1155–1162. [PMC free article] [PubMed] [Google Scholar]
- 20.Shen S, Ko H, Tseng S, et al. Structurally related antitumor effects of flavanones in vitro and in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004;197:84–95. doi: 10.1016/j.taap.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Choi EJ, Lee JI, Kim G. Effects of 4 ’, 7-Dimethoxyflavanone on cell cycle arrest and apoptosis in human breast cancer MCF-7 Cells. Arch. Pharm. Res. 2011;34(12):2125–2130. doi: 10.1007/s12272-011-1216-7. [DOI] [PubMed] [Google Scholar]
- 22.Safavi M, Esmati N, Kabudanian S, et al. Halogenated flavanones as potential apoptosis-inducing agents: synthesis and biological activity evaluation. Eur. J. Med. Chem. 2012;58:573–580. doi: 10.1016/j.ejmech.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Orlikova B. Methylenedioxy flavonoids: assessment of cytotoxic and anti-cancer potential in human leukemia cells. Eur. J. Med. Chem. 2014;84:173–180. doi: 10.1016/j.ejmech.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Shi L, Feng XE, Rong J, et al. Synthesis and biological activity of flavanone derivatives. Bioorg. Med. Chem. Lett. 2010;20(18):5466–5468. doi: 10.1016/j.bmcl.2010.07.090. [DOI] [PubMed] [Google Scholar]
- 25.Kanagalakshmi K, Premanathan M, Priyanka R, et al. Synthesis, anticancer and antioxidant activities of 7-methoxyisoflavanone. Eur. J. Med. Chem. 2010;45(6):2447–2452. doi: 10.1016/j.ejmech.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Kupcewicz B, Balcerowska-Czerniak G, Małecka M, et al. Structure-cytotoxic activity relationship of 3-arylideneflavanone and chromanone (E, Z isomers) and 3-arylflavones. Bioorganic Med. Chem. Lett. 2013;23(14):4102–4106. doi: 10.1016/j.bmcl.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 27.Simon L, Abdul Salam AA, Kumar S, et al. Synthesis, anticancer, structural, and computational docking studies of 3-benzylchroman-4-one derivatives. Bioorg. Med. Chem. Lett. 2017;27(23):5284–5290. doi: 10.1016/j.bmcl.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Holshouser MH, Loeffler JL. Synthesis and antitumor testing of 3-methenylthiochroman-4-one-l,1 -dioxide. J. Pharm. Sci. 1982;71(6):715–717. doi: 10.1002/jps.2600710630. [DOI] [PubMed] [Google Scholar]
- 29.Ei-fotooh A. Synthesis of novel tricyclic heterocyclic compounds as potential anticancer agents using chromanone and thiochromanone as synthons. Indian J. Chem. -Section B. 2003;42(august):1985–1993. [Google Scholar]
- 30.Abdelatef SA, El-Saadi MT, Amin NH, et al. Synthesis and anticancer screening of novel spiro[chroman-2,4’- piperidin]-4-one derivatives with apoptosis-inducing activity. J. Appl. Pharm. Sci. 2018;8(1):009–16. [Google Scholar]
- 31.Maasomi ZJ, Soltanahmadi YP, Dadashpour M, et al. Synergistic anticancer effects of silibinin and chrysin in T47D breast cancer cells. Asian Pacific J. Cancer Prev. 2017;18(5):1283–1287. doi: 10.22034/APJCP.2017.18.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murti Y, Mishra P. Synthesis and evaluation of flavanones as anticancer agents. Indian J. Pharm. Sci. 2014;76(2):163–166. [PMC free article] [PubMed] [Google Scholar]
- 33.Gaspar A, Mohabbati M, Cagide F, et al. Searching for new cytotoxic agents based on chromen-4-one and chromane-2,4-dione scaffolds. Res. Pharm. Sci. 2019;14(1):74–83. doi: 10.4103/1735-5362.251855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demirayak S, Yurttas L, Gundogdu-Karaburun N, et al. New chroman-4-one/thiochroman-4-one derivatives as potential anticancer agents. Saudi Pharm. J. 2017;25(7):1063–1072. doi: 10.1016/j.jsps.2017.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegab, M.I., Morsy. E, Mageed. A.E. et al.: Synthesis and characterization of new 3′′,5′′-diaryl-3′′H,4′H-dispiropyran/thiopyran[4,2′-chroman-3′,2′′-[1,3,4-thiadiazol]-4′-one derivatives and related compounds as anticancer and antiviral agents, Phosphorus, Sulfur, and Silicon and the Related Elements. 190(11):00–00 (2015).
- 36.Siddiqui ZN, Farooq FA. Practical one pot synthesis of novel 2-hydroxy-4-chromanone derivatives from 3-formylchromone. J. Chem. Sci. 2012;124(5):1097–1105. doi: 10.1007/s12039-012-0300-y. [DOI] [Google Scholar]
- 37.Pontius A, Krick A, Kehraus S, et al. Noduliprevenone: A novel heterodimeric chromanone with cancer chemopreventive potential. Chem. Eur. J. 2008;14:9860–9863. doi: 10.1002/chem.200801574. [DOI] [PubMed] [Google Scholar]
- 38.Bolwell GP, Wojtaszek P. Mechanisms for the generation of reactive oxygen species in plant defence: a broad perspective. Physiol. Mol. Plant Pathol. 1997;51(6):347–366. doi: 10.1006/pmpp.1997.0129. [DOI] [Google Scholar]
- 39.Chen Q, Vazquez EJ, Moghaddas S, et al. Production of reactive oxygen species by mitochondria: central role of complex III. J. Biol. Chem. 2003;278(38):36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 40.Miyake Y, Yamamoto K, Tsujihara N, et al. Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids. 1998;33(7):689–695. doi: 10.1007/s11745-998-0258-y. [DOI] [PubMed] [Google Scholar]
- 41.Gholamian-Dehkordi N, Luther T, Asadi-Samani M, et al. An overview on natural antioxidants for oxidative stress reduction in cancers; a systematic review. Immunopathol. Persa. 2017;3(2):12. doi: 10.15171/ipp.2017.04. [DOI] [Google Scholar]
- 42.Karak P. Biological activities of flavonoids: an overview. Int. J. Pharm. Sci. Res. 2019;10(4):1567–1574. [Google Scholar]
- 43.Chen X, Mukwaya E, Wong MS, et al. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014;52:655–660. doi: 10.3109/13880209.2013.853809. [DOI] [PubMed] [Google Scholar]
- 44.Huyut Z, Beydemir S, Gulcin I. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. 2017;2017:7616791. doi: 10.1155/2017/7616791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foroumadi A, Samzadeh-Kermani A, Emami S, et al. Synthesis and antioxidant properties of substituted 3-benzylidene-7-alkoxychroman-4-ones. Bioorganic Med. Chem. Lett. 2007;17(24):6764–6769. doi: 10.1016/j.bmcl.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 46.Lee H, Lee K, Jung JK, et al. Synthesis and evaluation of 6-hydroxy-7-methoxy-4-chromanone- and chroman-2-carboxamides as antioxidants. Bioorganic Med. Chem. Lett. 2005;15(11):2745–2748. doi: 10.1016/j.bmcl.2005.03.118. [DOI] [PubMed] [Google Scholar]
- 47.Ayunda RD, Prasetyastuti HP. Effect of 7-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-chroman-4-one on level of mangan-superoxide dismutase (Mn-sod) and superoxide dismutase 2 (SOD2) gene expression in hyperlipidemia rats. Indones. J. Pharm. 2019;30(3):180–186. doi: 10.14499/indonesianjpharm30iss3pp180. [DOI] [Google Scholar]
- 48.Wang D, Zeng L, Li D, et al. Antioxidant activities of different extracts and homoisoflavanones isolated from the Polygonatum odoratum. Nat. Prod. Res. 2013;27(12):1111–1114. doi: 10.1080/14786419.2012.701212. [DOI] [PubMed] [Google Scholar]
- 49.Mistry B, Patel RV, Keum Y. Access to the substituted benzyl-1, 2, 3-triazolyl hesperetin derivatives expressing antioxidant and anticancer effects. Arab. J. Chem. 2017;10(2):157–166. doi: 10.1016/j.arabjc.2015.10.004. [DOI] [Google Scholar]
- 50.Calvo MI. Homoisoflavanones from Ledebouria floribunda. Fitoterapia. 2009;80(2):96–101. doi: 10.1016/j.fitote.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Hu H, Chen X, Sun K, et al. Silver-catalyzed radical cascade cyclization toward 1,5-/1,3-dicarbonyl heterocycles: an atom-/step-economical strategy leading to chromenopyridines and isoxazole-/pyrazole-containing chroman-4-ones. Org. Lett. 2018;20(19):6157–6160. doi: 10.1021/acs.orglett.8b02627. [DOI] [PubMed] [Google Scholar]
- 52.Roy SK, Agrahari UC, Gautam R, et al. Isointricatinol, a new antioxidant homoisoflavonoid from the roots of Caesalpinia digyna Rottler. Nat. Prod. Res. 2012;26(8):690–695. doi: 10.1080/14786419.2010.548813. [DOI] [PubMed] [Google Scholar]
- 53.Sun YX, Tang Y, Wu AL, et al. Neuroprotective effect of liquiritin against focal cerebral ischemia/reperfusion in mice via its antioxidant and antiapoptosis properties. J. Asian Nat. Prod. Res. 2010;12(12):1051–1060. doi: 10.1080/10286020.2010.535520. [DOI] [PubMed] [Google Scholar]
- 54.Gazak R, Svobodova A, Psotova J, et al. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorganic Med. Chem. 2004;12(21):5677–5687. doi: 10.1016/j.bmc.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 55.Peng ZY, Liu XY, Yang YL, et al. 7-Methoxy-3-(4-methoxyphenyl) chroman-4-one. Acta Cryst. 2012;E68:250. [Google Scholar]
- 56.Zheng YZ, Deng G, Chen DF, et al. Theoretical studies on the antioxidant activity of pinobanksin and its ester derivatives: effects of the chain length and solvent. Food Chem. 2018;240:323–329. doi: 10.1016/j.foodchem.2017.07.133. [DOI] [PubMed] [Google Scholar]
- 57.Siddaiah V, Maheswara M, Venkata Rao C, et al. Synthesis, structural revision, and antioxidant activities of antimutagenic homoisoflavonoids from Hoffmanosseggia intricata. Bioorg. Med. Chem. Lett. 2007;17(5):1288–1290. doi: 10.1016/j.bmcl.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Hadaruga DI, Hadaruga NG. Antioxidant activity of hepatoprotective silymarin and silybum marianum L. extract. Chem. Bull. 2009;54(68):104–107. [Google Scholar]
- 59.Zhao JW, Chen DS, Deng CS, et al. Evaluation of anti-inflammatory activity of compounds isolated from the rhizome of Ophiopogon japonicas. BMC Complement Altern. Med. 2017;17(1):1–12. doi: 10.1186/s12906-016-1539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ali KA, Abdelhafez NAA, Ragab EA, et al. Design and synthesis of novel fused heterocycles using 4-chromanone as synthon. Russ. J. Gen. Chem. 2015;85(12):2853–2860. doi: 10.1134/S107036321512035X. [DOI] [Google Scholar]
- 61.Waller CP, Thumser AE, Langat MK, et al. COX-2 inhibitory activity of homoisoflavanones and xanthones from the bulbs of the southern african ledebouria socialis and ledebouria ovatifolia (Hyacinthaceae: Hyacinthoideae) Phytochemistry. 2013;95(6):284–290. doi: 10.1016/j.phytochem.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Ma J, Cao B, Chen X, et al. Violacin A, a new chromanone produced by Streptomyces violaceoruber and its anti-inflammatory activity. Bioorganic Med. Chem. Lett. 2018;28(5):947–951. doi: 10.1016/j.bmcl.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 63.Wang QQ, Shi JB, Chen C, et al. Hesperetin derivatives: Synthesis and anti-inflammatory activity. Bioorganic Med. Chem. Lett. 2016;26(5):1460–1465. doi: 10.1016/j.bmcl.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 64.Shaikh MM, Kruger HG, Bodenstein J, et al. Anti-inflammatory activities of selected synthetic homoisoflavanones. Nat. Prod. Res. 2012;26(16):1473–1482. doi: 10.1080/14786419.2011.565004. [DOI] [PubMed] [Google Scholar]
- 65.Hu C, Zhou Z, Xiang Y, et al. Design, synthesis and anti-inflammatory activity of dihydroflavonol derivatives. Med. Chem. Res. 2018;27(1):194–205. doi: 10.1007/s00044-017-2054-z. [DOI] [Google Scholar]
- 66.Zheng C, Wang L, Han T, et al. Pruinosanones A-C, anti-inflammatory isoflavone derivatives from Caragana pruinosa. Sci Rep. 2016;6:2–9. doi: 10.1038/srep31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winekenstadde D, Angelis A, Waltenberger B, et al. Phytochemical profile of the aerial parts of sedum sediforme and anti-inflammatory activity of myricitrin. Nat. Prod. Commun. 2015;10(1):83–88. [PubMed] [Google Scholar]
- 68.Wright, George C., Goldenberg, Marvin M.: 7-Dimethylamino-4-chromanoneUnited StatesMorton-Norwich Products, Inc. (Norwich, NY), 4108872 (1978).
- 69.Edwards JL, Vincent AM, Cheng HT, et al. Diabetic neuropathy: Mechanisms to management. Pharmacol. Therap. 2008;2008:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unnikrishnan MK, Veerapur V, Nayak Y, et al. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoids. Polyphenols Hum. Health Dis. 2013;2013:143–161. [Google Scholar]
- 71.Adams GL, Velazquez F, Jayne C, et al. Discovery of chromane propionic acid analogues as selective agonists of GPR120 with in vivo activity in rodents. ACS Med. Chem. Lett. 2017;8(1):96–101. doi: 10.1021/acsmedchemlett.6b00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takao K, Yamashita M, Yashiro A, Sugita Y. Synthesis and biological evaluation of 3-Benzylidene-4-chromanone derivatives as free radical scavengers and α-Glucosidase inhibitors. Chem. Pharm. Bull. 2016;64(8):1203–1207. doi: 10.1248/cpb.c16-00327. [DOI] [PubMed] [Google Scholar]
- 73.Zhu, G.; Luo, Y.; Xu, X., et al.: Anti-diabetic compounds from the seeds of Psoralea corylifolia. Fitoterapia 139,(2019) [DOI] [PubMed]
- 74.Kocevar N, Glavac I, Kreft S. Flavonoidi. Farm Vestn. 2007;58(4):145–148. [Google Scholar]
- 75.Lotulung PDN, Fajriah S, Sundowo A, et al. Anti diabetic flavanone compound from the leaves of Artocarpus communis. Indones. J. Chem. 2010;9(3):505–508. doi: 10.22146/ijc.21524. [DOI] [Google Scholar]
- 76.Sadewa AH. Effect of 7-hydroxy-2- (4-hydroxy-3-methoxy-phenyl ) - chroman-4-one ( swietenia macrophylla king seed ) on retinol binding protein-4 and phosphoenolpyruvate carboxykinase gene expression in type 2 diabetic rats. Rom. J. Diabetes Nutr. Metab. Dis. 2016;23(3):255–265. [Google Scholar]
- 77.Sneha Jose E, Philip JE, Shanty AA. Novel class of mononuclear 2-methoxy-4-chromanones ligated Cu (II), Zn (II), Ni (II) complexes: synthesis, characterisation and biological studies. Inorganica Chim. Acta. 2018;478(I):155–165. doi: 10.1016/j.ica.2018.03.023. [DOI] [Google Scholar]
- 78.Park, J.; Seo, Y.; Han, J.: in vitro and alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 863(September),(2019) [DOI] [PubMed]
- 79.Ravi S, Sadashiva CT, Tamizmani T. In vitro glucose uptake by isolated rat hemi-diaphragm study of Aegle marmelos correa root. Bangladesh J. Pharmacol. 2009;4(1):65–68. [Google Scholar]
- 80.Raju BC, Tiwari AK, Kumar JA, et al. α-Glucosidase inhibitory antihyperglycemic activity of substituted chromenone derivatives. Bioorg. Med. Chem. 2010;18(1):358–365. doi: 10.1016/j.bmc.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 81.Shaikh MM, Kruger HG, Smith P, et al. Crystal structure and potent antifungal activity of synthetic homoisoflavanone analogues. J. Pharm. Res. 2013;6(1):1–5. [Google Scholar]
- 82.Tiratsuyan SG, Hovhannisyan AA, Karapetyan AV, et al. Synthesis and biological activities of novel pyridazine derivatives. Mor. J. Chemi. 2016;63(5):698–704. [Google Scholar]
- 83.Ayati A, Falahati M, Irannejad H, et al. Synthesis, in vitro antifungal evaluation and in silico study of 3-azolyl-4-chromanone phenylhydrazones. DARU J. Pharm. Sci. 2012;20(46):1–7. doi: 10.1186/2008-2231-20-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dongamanti A, Naji HH, Bommidi VL, et al. Microwave-assisted one-pot synthesis and antimicrobial evaluation of 2-(1-phenyl-3-(2-thienyl)-1H-pyrazol-4-yl)chroman-4-one derivatives. Heterocycl. Commun. 2016;22(5):259–264. doi: 10.1515/hc-2016-0027. [DOI] [Google Scholar]
- 85.Ashok D, Kumar RS. Solvent-free microwave-assisted synthesis and biological evaluation of aurones and flavanones based on 2, 2-dimethylchroman-4-one. Chem. Heterocycl. Compd. 2016;52(7):453–459. doi: 10.1007/s10593-016-1917-4. [DOI] [Google Scholar]
- 86.Hoettecke N, Rotzoll S, Albrecht U. Synthesis and antimicrobial activity of 2-alkenylchroman-4-ones, 2-alkenylthiochroman-4-ones and 2-alkenylquinol-4-ones. Bioorg. Med. Chem. 2008;16(24):10319–10325. doi: 10.1016/j.bmc.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 87.Kang JG, Shin SY, Kim MJ, et al. Isolation and anti-fungal activities of 2-hydroxymethyl-chroman-4-one produced by Burkholderia sp. MSSP. J. Antibiot. 2004;57(11):726–731. doi: 10.7164/antibiotics.57.726. [DOI] [PubMed] [Google Scholar]
- 88.Albrecht U, Lalk M, Langer P. Synthesis and structure-activity relationships of 2-vinylchroman-4-ones as potent antibiotic agents. Bioorg. Med. Chem. 2005;13(5):1531–1536. doi: 10.1016/j.bmc.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 89.Sriram D, Srinivasan S, Santhosh KC. 3-phenylsulfonyl-3-(2-propenyl)chroman-4-one. Acta Crystallogr. Cryst Struct. Commun. 1997;53(6):793–794. doi: 10.1107/S0108270197001261. [DOI] [Google Scholar]
- 90.Sharma S, Patial V, Singh D, et al. Antimicrobial homoisoflavonoids from the Rhizomes of Polygonatum verticillatum. Chem. Biodivers. 2018;15(12):1800430. doi: 10.1002/cbdv.201800430. [DOI] [PubMed] [Google Scholar]
- 91.Feng L, Maddox MM, Alam MZ, et al. Synthesis, structure-activity relationship studies, and antibacterial evaluation of 4-chromanones and chalcones, as well as olympicin A and derivatives. J. Med. Chem. 2014;57(20):8398–8420. doi: 10.1021/jm500853v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Albogami AS, Alkhathlan HZ, Saleh TS, et al. Microwave-assisted synthesis of potent antimicrobial agents of flavanone derivatives. Orient J. Chem. 2014;30(2):435–443. doi: 10.13005/ojc/300205. [DOI] [Google Scholar]
- 93.Pawar MJ, Burungale B, Karale BK. Synthesis and antimicrobial activity of spiro [chromeno [4, 3- thiadiazoline compounds. ARKIVOC: Online. J. Org. Chem. 2009;2009:97–107. [Google Scholar]
- 94.Ramana Kishore N, Ashok D, Sarasija M, et al. Microwave-assisted synthesis of novel spirochromanone–aurone hybrids and their antimicrobial activity. Russ. J. Gen. Chem. 2018;88(5):1015–1019. doi: 10.1134/S1070363218050298. [DOI] [Google Scholar]
- 95.Alsarahni A, Eldeen ZM, Alkaissi E, et al. Synthesis and structural elucidation of amino acetylenic and thiocarbamates derivatives for 2-mercaptobenzothiazole as antimicrobial agents. Int. J. Pharm. Sci. 2017;9(2):192. [Google Scholar]
- 96.Qing-hui XU, Ji-zhen LI, Jiang-hua HE, et al. Synthesis of novel flavanone derivatives and their anti Staphylococcus aureus evaluation. Chem. Res. Chin. Univ. 2013;29(4):695–698. doi: 10.1007/s40242-013-2519-7. [DOI] [Google Scholar]
- 97.Thinagar S, Velmurugan D, Amalraj RR. Crystal structure of spiro [2-benzoyl-cyclohexyl-4, 5-diphenylpyrrolidine-3,3’-chroman-4-one] Cryst Res. Technol. 2000;35(8):979–986. doi: 10.1002/1521-4079(200008)35:8<979::AID-CRAT979>3.0.CO;2-F. [DOI] [Google Scholar]
- 98.Agnes Kenez LJ, et al. A simple synthesis of selinone, an antifungal component of monotes engleri. Heterocycl. Commun. 2002;8(6):543–548. [Google Scholar]
- 99.Kaufmann, S. H. E., van Helden P.: Handbook of tuberculosis: Clinics, Diagnostics. Wiley-VCH, therapy and epidemiology. Weinheim (2008).
- 100.Saengchantara ST, Wallace TW. Chromanols, chromanones, and chromones. Nat. Prod. Rep. 1986;3:465–475. doi: 10.1039/np9860300465. [DOI] [Google Scholar]
- 101.Alvey L, Prado S, Saint-joanis B, et al. Diversity-oriented synthesis of furo [3, 2- f ] chromanes with antimycobacterial activity. Eur. J. Med. Chem. 2009;44:2497–2505. doi: 10.1016/j.ejmech.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 102.Wu M, Peng C, Chen I, et al. Antitubercular chromones and flavonoids from Pisonia aculeata. J. Nat. Prod. 2011;5(74):976–982. doi: 10.1021/np1008575. [DOI] [PubMed] [Google Scholar]
- 103.Pini E, Poli G, Tuccinardi T, et al. New chromane-based derivatives as inhibitors of mycobacterium tuberculosis salicylate synthase: Preliminary biological evaluation and molecular modeling studies. Molecules. 2018;23(7):1–14. doi: 10.3390/molecules23071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yempala T, Sriram D, Yogeeswari P, et al. Molecular hybridization of bioactives: synthesis and antitubercular evaluation of novel dibenzofuran embodied homoisoflavonoids via Baylis-Hillman reaction. Bioorganic Med. Chem. Lett. 2012;22(24):7426–7430. doi: 10.1016/j.bmcl.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 105.Asif MA. Brief overview on recent advances in the development of anti-tubercular compounds containing different heterocyclic ring systems. Appl. Microbiol. Open Access. 2016;02(04):1–14. doi: 10.4172/2471-9315.1000122. [DOI] [Google Scholar]
- 106.Roy SK, Kumari N, Gupta S, et al. 7-Hydroxy-(E)-3-phenylmethylene-chroman-4-one analogues as efflux pump inhibitors against Mycobacterium smegmatis mc2 155. Eur. J. Med. Chem. 2013;66:499–507. doi: 10.1016/j.ejmech.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 107.Anand N, Singh P, Sharma A, et al. Synthesis and evaluation of small libraries of triazolylmethoxy chalcones, flavanones and 2-aminopyrimidines as inhibitors of mycobacterial FAS-II and PknG. Bioorg. Med. Chem. 2012;20(17):5150–5163. doi: 10.1016/j.bmc.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 108.Mujahid M, Gonnade RG, Yogeeswari P, et al. Synthesis and antitubercular activity of amino alcohol fused spirochromone conjugates. Bioorg. Med. Chem. Lett. 2013;23(5):1416–1419. doi: 10.1016/j.bmcl.2012.12.073. [DOI] [PubMed] [Google Scholar]
- 109.Donnell G, Bucar F, Gibbons S. Phytochemistry and antimycobacterial activity of Chlorophytum inornatum. Phytochemistry. 2006;67:178–182. doi: 10.1016/j.phytochem.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 110.Dongamanti A, Aamate VK, Devulapally MG, et al. Bis-spirochromanones as potent inhibitors of Mycobacterium tuberculosis: synthesis and biological evaluation. Mol. Divers. 2017;21(4):999–1010. doi: 10.1007/s11030-017-9779-y. [DOI] [PubMed] [Google Scholar]
- 111.Lee H, Kr D, Kim JH.: ( 12 ) United States Patent. Vol. 2. 1–5(2011).
- 112.Gaikwad MS, Mane AS, Hangarge RV, et al. Synthesis of newer selenadiazoles and thiadiazoles from their chroman-4-one precursors. Indian J. Chem. Sect. B. 2003;42(January):189–191. [Google Scholar]
- 113.Tait S, Laura A, Desideri N, Fiore L. Antiviral activity of substituted homoisoflavonoids on enteroviruses. Antiviral Res. 2006;72:252–255. doi: 10.1016/j.antiviral.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Conti C, Proietti L, Desideri N. Design, synthesis and in-vitro evaluation of novel chroman-4-one, chroman, and 2 H-chromene derivatives as human rhinovirus capsid-binding inhibitors. Bioorg Med Chem. 2011;19(24):7357–7364. doi: 10.1016/j.bmc.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 115.Quaglia MG, Desideri N, Bossu E, et al. Enantioseparation and anti-rhinovirus activity of 3-Benzylchroman-4-ones. Chirality. 1999;500:495–500. doi: 10.1002/(SICI)1520-636X(1999)11:5/6<495::AID-CHIR23>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 116.Kang Y, Mei Y, Du Y, Jin Z. Total synthesis of the highly potent anti-HIV natural product daurichromenic acid along with its two chromane derivatives, rhododaurichromanic acids A and B. Organic Lett. 2003;5(23):4481–4484. doi: 10.1021/ol030109m. [DOI] [PubMed] [Google Scholar]
- 117.Rama Rao AV, Galtonde AS, et al. A conclse selective of chlral of the chromanol wolety of anti-HIV agent calanolide A. Tetrahedron Lett. 1994;35(34):6347–6350. doi: 10.1016/S0040-4039(00)73429-0. [DOI] [Google Scholar]
- 118.Ishikawa T, Oku Y, Tanaka T, et al. An approach to anti-HIV-1 active Calophyllum coumarin synthesis: an enantioselective construction of 2,3-dimethyl-4-chromanone ring by quinineassisted intramolecular Michael-type addition. Tetrahedron Lett. 1999;40(19):3777–3780. doi: 10.1016/S0040-4039(99)00607-3. [DOI] [Google Scholar]
- 119.Pokorna J, Machala L, Rezacova P, Konvalinka J. Current and novel inhibitors of HIV protease. Viruses. 2009;1:1209–1239. doi: 10.3390/v1031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dawood KM. Regio and stereoselective synthesis of bis-spiropyrazoline-5,3′- chroman(thiochroman)-4-one derivatives via bis-nitrilimines. Tetrahedron. 2005;61(22):5229–5233. doi: 10.1016/j.tet.2005.03.083. [DOI] [Google Scholar]
- 121.Sekino E, Kumamoto T, Tanaka T, et al. Concise synthesis of anti-HIV-1 Active (+)-Inophyllum B and (+)-Calanolide A by application of (-)-quinine-catalyzed intramolecular oxo-michael addition. J. Org. Chem. 2004;69(8):2760–2767. doi: 10.1021/jo035753t. [DOI] [PubMed] [Google Scholar]
- 122.Park JH, Lee SU, Kim SH, et al. Chromone and chromanone derivatives as strand transfer inhibitors of HIV-1 integrase. Arch. Pharm. Res. 2008;31(1):1–5. doi: 10.1007/s12272-008-1111-z. [DOI] [PubMed] [Google Scholar]
- 123.Nagle AS, Khare S, Kumar AB, et al. Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem. Rev. 2014;114:11305–11347. doi: 10.1021/cr500365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat. Publ. Gr. 2011;9(8):604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 125.Boer MD, Argaw D, Jannin J, et al. Leishmaniasis impact and treatment access. Clin Microbiol. Infect. 2011;17(10):1471–1477. doi: 10.1111/j.1469-0691.2011.03635.x. [DOI] [PubMed] [Google Scholar]
- 126.Cywin CL, Firestone RA, Mcneil DW, et al. The design of potent hydrazones and disulfides as cathepsin S inhibitors. Bioorg. Med. Chem. 2003;11:733–740. doi: 10.1016/S0968-0896(02)00468-6. [DOI] [PubMed] [Google Scholar]
- 127.Das P, De T, Chakraborti T. Leishmania donovani secretory serine protease alters macrophage inflammatory response via COX-2 mediated PGE-2 production. Indian J. Biochem. Biophys. 2014;51(December):542–551. [PubMed] [Google Scholar]
- 128.Id EV, Echeverri F, Upegui YA, et al. Hydrazone derivatives enhance antileishmanial activity of thiochroman-4-ones. Molecules. 2018;23(70):1–12. doi: 10.3390/molecules23010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Upegui Y, Rios K, Quinones W, et al. Chroman-4-one hydrazones derivatives: synthesis, characterization, and in vitro and in vivo antileishmanial effects. Med. Chem. Res. 2019;28(12):2184–2199. doi: 10.1007/s00044-019-02446-x. [DOI] [Google Scholar]
- 130.Castro H, Aguiar PD, Cardoso S, et al. Synthesis and evaluation of novel chromanone and quinolinone analogues of uniflorol as anti-leishmanial agents. Heliyon. 2020;6(March):1–11. doi: 10.1016/j.heliyon.2020.e03614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roberson ED, Mucke L. 100 years and counting: Prospects for defeating Alzheimer’s disease. Science. 2006;80(314):781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Association A. Alzheimer ’ s disease facts and figures. Alzheimer’s and Dementia. 2019;15(3):321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 133.Jung M, Park M. Acetylcholinesterase inhibition by flavonoids from agrimonia pilosa. Molecules. 2007;12:2130–2139. doi: 10.3390/12092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen Y, Zhang J, Sheng R, et al. Synthesis and biological evaluation of novel flavonoid derivatives as dual binding acetylcholinesterase inhibitors. J. Enzyme Inhib. Med. Chem. 2009;24(April):372–380. doi: 10.1080/14756360802187885. [DOI] [PubMed] [Google Scholar]
- 135.Parveen M, Malla AM, Yaseen Z, et al. Synthesis, characterization, DNA-binding studies and acetylcholinesterase inhibition activity of new 3-formyl chromone derivatives. J. Photochem. Photobiol. B Biol. 2014;130:179–187. doi: 10.1016/j.jphotobiol.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 136.Anand P, Singh B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013;36(4):375–399. doi: 10.1007/s12272-013-0036-3. [DOI] [PubMed] [Google Scholar]
- 137.Shamsimeymandi R, Pourshojaei Y, Eskandari K, et al. Design, synthesis, biological evaluation, and molecular dynamics of novel cholinesterase inhibitors as anti-Alzheimer’s agents. Arch. Pharm. 2019;352:7. doi: 10.1002/ardp.201800352. [DOI] [PubMed] [Google Scholar]
- 138.Jiang, N.; Ding, J.; Liu, J., et al.: Novel chromanone-dithiocarbamate hybrids as multifunctional AchE inhibitors with β-amyloid anti-aggregation properties for the treatment of Alzheimer’s disease. Bioorg. Chem. 89(May),(2019) [DOI] [PubMed]
- 139.Li Y, Qiang X, Luo L, et al. Multitarget drug design strategy against Alzheimer’s disease: Homoisoflavonoid mannich base derivatives serve as acetylcholinesterase and monoamine oxidase B dual inhibitors with multifunctional properties. Bioorg. Med. Chem. 2017;25(2):714–726. doi: 10.1016/j.bmc.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 140.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 141.Sabers A, Gram L. Newer anticonvulsants comparative review of drug interactions and adverse effects. Drugs. 2000;60(1):23–33. doi: 10.2165/00003495-200060010-00003. [DOI] [PubMed] [Google Scholar]
- 142.Gentry JR, Hill C, Malcolm R. New Anticonvulsants: a review of applications for the management of substance abuse disorders. Ann. Clin. Psychiatry. 2002;14(4):233–245. doi: 10.3109/10401230209147462. [DOI] [PubMed] [Google Scholar]
- 143.Emami S, Kebriaeezadeh A, Ahangar N, et al. Imidazolylchromanone oxime ethers as potential anticonvulsant agents : Anticonvulsive evaluation in PTZ-kindling model of epilepsy and SAR study. Bioorg. Med. Chem. Lett. 2011;21(2):655–659. doi: 10.1016/j.bmcl.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 144.Kebriaeezadeh A, Emami S, Ebrahimi M. Anticonvulsant and antiepileptogenic effects of azolylchromanones on lithium pilocarpine induced seizures and pentylenetetrazole-kindling model of epilepsy. Biomed. Pharmacother. 2008;62:208–211. doi: 10.1016/j.biopha.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 145.Emami S, Kebriaeezadeh A, Zamani J, Shafiee A. Azolylchromans as a novel scaffold for anticonvulsant activity. Bioorganic Med. Chem. Lett. 2006;16:1803–1806. doi: 10.1016/j.bmcl.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 146.Tang SW, Helmeste DM, Leonard BE. Antidepressant compounds: a critical review. Depression Psychopathol. Pharmacother. 2010;27:1–19. [Google Scholar]
- 147.Wang Y, Sun Y, Guo Y, et al. Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimer ’ s disease : synthesis, pharmacological analysis and molecular modeling of homoisoflavonoid derivatives Dual functional cholinesterase and MAO inhibitors for the treatment. J. Enzyme Inhib. Med. Chem. march. 2015;2015:1475–6374. doi: 10.3109/14756366.2015.1024675. [DOI] [PubMed] [Google Scholar]
- 148.Dupuy JM, Ostacher MJ, Huffman J, et al. A critical review of pharmacotherapy for major depressive disorder. Int. J. Neuropsychopharmacol. 2011;14:1417–1431. doi: 10.1017/S1461145711000083. [DOI] [PubMed] [Google Scholar]
- 149.Kato T, Matsumoto Y, Yamamoto M, et al. DSP-1053, a novel serotonin reuptake inhibitor with 5-HT 1A partial agonistic activity, displays fast antidepressant effect with minimal undesirable effects in juvenile rats. Pharmacol. Res. Perspect. 2015;3(3):1–17. doi: 10.1002/prp2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Desideri N, Fioravanti R, Biava M, et al. (E)-3-Heteroarylidenechroman-4-ones as potent and selective monoamine oxidase-B inhibitors Nicoletta. Eur. J. Med. Chem. 2016;3:1–30. doi: 10.1016/j.ejmech.2016.03.081. [DOI] [PubMed] [Google Scholar]
- 151.Seifert T, Malo M, Kokkola T, et al. Chroman-4-one- and chromone-based sirtuin 2 inhibitors with antiproliferative properties in cancer cells. J. Med. Chem. 2014;57:9870–9888. doi: 10.1021/jm500930h. [DOI] [PubMed] [Google Scholar]
- 152.Lan, J.S., Xie, S.S., Huang, M., et al.: Chromanones: selective and reversible Monoamine Oxidase B inhibitors with nanomolar potency. Med. chem. comm.1–33(2015).
- 153.Toya T, Fukasawa H, Masui A, Endo Y. Potent and selective partial Ecdysone agonist activity of chromafenozide in Sf9 Cells. Biochem. Biophys. Res. Commun. 2002;292:1087–1091. doi: 10.1006/bbrc.2002.6771. [DOI] [PubMed] [Google Scholar]
- 154.Wing KD. 5849, a nonsteroidal Ecdysone agonist: effects on a drosophila cell line. Science. 1988;241(4864):467–469. doi: 10.1126/science.3393913. [DOI] [PubMed] [Google Scholar]
- 155.Michaell G. Recent advances in our knowledge of ecdysteroid biosynthesis in insects and crustaceans. Insect. Biochem. Molec. Biol. 1994;24(2):115–132. doi: 10.1016/0965-1748(94)90078-7. [DOI] [Google Scholar]
- 156.Zhao P, Li J, Yang G. Synthesis and insecticidal activity of chromanone and chromone analogues of diacylhydrazines. Bioorg. Med. Chem. 2007;15:1888–1895. doi: 10.1016/j.bmc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 157.Dev S, Koul O. Insecticides of natural origin. The Netherlands: Harwood Academic Publishers; 1997. [Google Scholar]
- 158.Gan C, Zhao Z, Nan D, et al. Homoisoflavonoids as potential imaging agents for b -amyloid plaques in Alzheimer ’ s disease. Eur. J. Med. Chem. 2014;76:125–131. doi: 10.1016/j.ejmech.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 159.Ramana MMV, Ranade PB, Betkar RR, et al. Flavanones : Potential antidengue targets in silico approach. J. Chem. Pharm Res. 2015;7(9):469–474. [Google Scholar]
- 160.Pisa FD, Landi G, Iacono L, et al. Chroman-4-one derivatives targeting pteridine reductase 1 and showing anti-parasitic activity. Molecules. 2017;22(426):1–16. doi: 10.3390/molecules22030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kwon, D.H., Choi, W.J., Lee, C.H., Kim, J.H., Kim, M.B.: Flavonoid compound having an antiviral activity.2. United States Patent, 1–5 (2011).