Abstract
Aim
We plan to conduct a randomised clinical trial among people likely to witness opioid overdose to compare the educational effectiveness of point-of-care naloxone distribution with best-available care, by observing participants’ resuscitation skills in a simulated overdose. This mixed methods feasibility study aims to assess the effectiveness of recruitment and retention strategies and acceptability of study procedures.
Methods
We implemented candidate-driven recruitment strategies with verbal consent and destigmatizing study materials in a family practice, emergency department, and addictions service. People ≥16 years of age who are likely to witness overdose were randomized to point-of-care naloxone distribution or referral to an existing program. We evaluated participant skills as a responder to a simulated overdose 3–14 days post-recruitment. Retention strategies included flexible scheduling, reminders, cash compensation and refreshments. The primary outcome was recruitment and retention feasibility, defined as the ability to recruit 28 eligible participants in 28 days, with <50% attrition at the outcome simulation. Acceptability of study procedures and motivations for participation were assessed in a semi-structured interview.
Results
We enrolled 30 participants over 24 days, and retained 21 participants (70%, 95%CI 56.7–100). The most common motivation for participation was a desire to serve the community or loved ones in distress. Participants reported that study procedures were acceptable and that the outcome simulation provided a supportive and affirming environment.
Conclusion
The planned trial is ready for implementation. Recruitment and retention is feasible and study processes are acceptable for people who are likely to witness overdose. (Registration: NCT03821649).
Keywords: Feasibility trial, Pilot trial, Randomized control trial, Opioid overdose, Simulation, Resuscitation, Prehospital care, Bystanders, Substance use, Overdose education and naloxone distribution, Study recruitment, Study retention, Harm reduction, Emergency medicine, Public health, Addiction medicine
Background
Overdose education and naloxone distribution (OEND) programs train people who are likely to witness opioid overdose to respond with effective first aid interventions, including the administration of the opioid antagonist naloxone. OEND programs are known to reduce opioid-related deaths and enhance knowledge and opioid overdose response skills.1, 2, 3
We plan to conduct a randomised clinical trial (RCT) to compare the effectiveness of a novel overdose response training tool with the current best-available OEND program. The novel tool includes a brief training video and naloxone kit designed for implementation and distribution in clinical settings, while the best-available alternative consists of referral to a local pharmacy or harm reduction agency for OEND. The planned trial will recruit participants in emergency department, family practice and addictions medicine settings and evaluate the effectiveness of the intervention by observing participants’ performance in a simulated overdose emergency.
The planned trial raises a range of feasibility and logistical challenges. The planned trial aims to recruit participants including people who are themselves at risk of opioid overdose and anyone who is likely to witness overdose, and both patients and visitors in the clinical environment. There is little precedent for trial recruitment in this population and setting, or for the use of resuscitation simulations as an outcome assessment tool among people who are likely to witness opioid overdose.4 Challenges with participant recruitment and retention are widely reported among studies seeking to enroll people who use drugs.5, 6, 7 Before conducting a full-scale trial, a feasibility study was necessary to determine the effectiveness of our recruitment and retention strategy, and the acceptability of study procedures.
Objectives
The primary objective was to establish if recruitment and retention of participants was feasible for the planned RCT. The secondary objectives were to (A) assess the participant recruitment rate in each recruitment site, (B) compare participant retention rates in the experimental and control arms, and (C) describe implementation challenges and opportunities for study process improvements.
Methods
We conducted a mixed methods feasibility study for a proposed parallel design RCT. We developed, registered, and published a feasibility study protocol based on Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines, adapted to feasibility studies (ClinicalTrials.gov NCT03821649).8, 9 The proposed RCT is described in detail elsewhere (ClinicalTrials.gov NCT04740099), and the feasibility study replicated all of the proposed RCT procedures, with the exception of the sample size and data analysis. There were no scientific protocol amendments or deviations. This report adheres to the CONSORT Extension for Pilot and Feasibility Studies (Supplement A: Reporting Checklist).10
The study was approved by research ethics boards at St. Michael’s Hospital (#17-280), Toronto Public Health (#2017-09), and the University of Toronto (#RIS37212). This study is part of the Surviving Opioid Overdose with Naloxone Education and Resuscitation (SOONER) Project, which combines participatory co-design and RCT methods to design and evaluate a novel OEND kit and reduce the stigma of opioid use and overdose.9
Participants
Participants were individuals presenting to the St. Michael’s Hospital Department of Family and Community Medicine, Addictions Medicine Service (inpatient or ambulatory care clinic), the Emergency Department, or the hospital-affiliated Inner City Family Health Team. Individuals were eligible for participation if they were at least 16 years of age and at elevated risk of opioid overdose or likely to witness opioid overdose according to criteria adapted from 2015 American Heart Association Guidelines (Table 1).11 Participants could be patients, or visitors accompanying a patient. Detailed inclusion and exclusion criteria are provided elsewhere.9
Table 1.
Eligibility criteria.
| Eligibility Criteria (individuals were eligible for the trial if they were a patient or visitor at a study recruitment site, > = 16 years of age, and met any one or more of the criteria below): | N = 30 No (%) |
|---|---|
|
10 (33.3) |
|
11 (36.7) |
|
12 (40) |
|
0 (0) |
|
16 (53.3) |
|
15 (50.0) |
|
14 (46.7) |
Recruitment, consent and retention
To reduce selection biases and ensure that all potentially eligible candidates were made aware of the study, participants were recruited using a “candidate-driven” strategy. All patients and visitors presenting to the recruitment settings were given an information card indicating that a study was underway for people who take opioids or are in contact with people who take opioids, and inviting them to join the study if they were interested. Peer workers, nurses and physicians could also refer candidates to research personnel. Patients and visitors who expressed interest in participating were approached by study personnel to explain procedures and obtain consent. The study used a verbal consent process supported by written materials. All study materials were designed based on participatory approaches using plain, destigmatizing and supportive language and formatting.12, 13 Given the variability in operations and work processes in the different recruitment sites, study materials were customized and adapted to meet the needs of each study site. Customizations were made at numerous points in the recruitment process, from recruitment card distribution to the timing of research interactions within the clinical encounter. For example, in the family medicine setting, cards were distributed at registration, and participant recruitment usually occurred after the clinical encounter. In the emergency department, cards were distributed at a variety of touchpoints in the process of care based on the patient’s presentation and recruitment occurred during waiting periods in the clinical encounter.
We implemented a multi-pronged strategy to support participant retention. Participants were asked to provide telephone, email, and text message contact details, and contact information for friends, family, case workers or other members of their social network who could assist with follow-up. Outcome assessment and follow-up appointments were scheduled flexibly and with sufficient time to reschedule if necessary. Study personnel provided multiple follow-up phone calls, letters and text message reminders. Each participant was provided a consistent point of contact among study personnel from enrolment to study completion. Participants received cash at each study visit to a maximum of CA$75 for completing all study visits (∼CA$25/h), transit tokens, food, and refreshments.9
Randomisation
Participants were assigned to the control or experimental arm a with 1:2 allocation, stratified by site. Study staff developed and implemented the allocation sequence and completed the randomisation using RedCap software at Unity Health Toronto.14 Since the control arm follows existing processes of care, we chose unbalanced allocation to gather additional information about the experimental arm. Eligible and consenting participants who presented together in the same clinical encounter (e.g. a patient and visitor) were assigned to the same study arm to reduce contamination.
Interventions
Participants randomized to the experimental arm received purpose-designed point-of-care brief overdose first aid training and a naloxone kit containing two doses of intranasal naloxone (Narcan™, Adapt Pharmaceuticals) with administration instructions. Study personnel showed participants a video on how to identify a life-threatening opioid overdose, activate 911, administer intranasal naloxone, perform chest compressions, reassess, and continue chest compressions until paramedics arrive. This video and training materials are embargoed while the trial is underway to minimize interference with the study itself.
Control arm participants received best-available care including referral to an existing local OEND program at a harm reduction service or retail pharmacy, facilitated with a custom-designed referral card. In the emergency department, this was supplemented with a pre-existing on-site OEND program delivered by nursing staff.
All participants were scheduled to engage as a responder in a high-fidelity overdose simulation 3 to 14 days post-recruitment. The scenario was co-designed and reviewed by the study’s community advisers who have lived experience with opioid overdose. This simulation recreated a life-threatening opioid overdose, where a manikin model was found in an apartment with no apparent signs of life. The scenario would proceed with a deterioration to cardiac arrest and death without prompt intervention. Participants were provided with a simulated naloxone kit equivalent to the one they would have received through their training. A telephone enabled simulated calls with a 911 dispatcher. The simulation was video recorded.
Two paramedic assessors with expertise in prehospital care reviewed all simulation recordings, conducted independent and blinded assessments of the simulation recordings, and assigned a global assessment (pass or fail) to each simulation. A lead investigator adjudicated discrepancies. Assessors scored participant performance on first aid skills using a validated checklist modified for overdose scenarios.15
Staff provided a standardized video briefing prior to the simulation to orient participants and instruct them to respond as if the situation were real. The simulation was followed by a structured simulation debrief.16 Participants completed an interviewer-administered questionnaire at enrolment and after the simulation to assess knowledge, confidence and willingness to respond to overdose including the validated Opioid Overdose Knowledge and Attitudes Scales (OOKS and OOAS).17 Participants completed a semi-structured interview concerning their opioid use and overdose experiences, stigma, and experiences with study recruitment and participation (Supplement B: Interview Guide). We conducted a thematic analysis of the participants’ motivations for participation in the study and their study experiences, particularly in the simulation.18 We incorporated feedback received from site staff into refinements to the study’s implementation procedures.
Participants who reported injection drug use or were close contacts of people who injected drugs were asked to complete the OOKS and OOAS again at 3 months following enrolment. Other studies have used this time interval with similar populations using the OOKS and OOAS as outcome measures. This visit was included in the study to permit comparison with the existing literature.19, 20
Feasibility outcomes
The primary outcome for this feasibility study was recruitment and retention feasibility, defined as the ability to recruit approximately 28 eligible participants in 28 recruitment days, and <50% attrition at the study’s primary outcome assessment at 3–14 days. We computed that at least 19 of 28 participants would need to be retained to exclude retention rates of <50% based on a one-sided 95% confidence interval for the binomial distribution.9 We set these feasibility thresholds based on available resources to continue study processes and complete a timely definitive trial.
Secondary feasibility outcomes included (A) the proportion of eligible participants who declined or did not consent to participate, and (B) the proportion of participants who attended the outcome simulation but did not complete the simulation or withdrew from the study. Tertiary feasibility outcomes were the acceptability of recruitment, retention and outcome assessment procedures as reported by study participants in a semi-structured interview, or through informal feedback gathered from participants and site staff.9
Outcomes of the planned trial
The primary outcome of the planned trial is the proportion of resuscitation failures as observed in the simulation scenario occurring 3–14 days after randomisation. The secondary outcome was the proportion of satisfactory performance on each of the eight first aid skills: (1) recognise the emergency, (2) position the victim, (3) activate emergency medical services, (4) administer naloxone (prepare device, administer correctly), (5) hand placement, (6) chest compressions rate and depth, (7) continue compressions until end of simulation and (8) order of operations and organisation. The tertiary outcomes are participants’ scores on the OOKS and OOAS at the time of the outcome simulation and at 3-months after randomisation. Outcome data for the planned trial were gathered and recorded to ensure the feasibility of study processes, but not analysed or reported in the context of the feasibility study.
Role of the funding source
The project was funded by the Canadian Institutes of Health Research (CIHR) grant #148817 and the Canadian Research Initiative in Substance Misuse. The Canadian Centre on Substance Use and Addiction (CCSA) contributed to project workshops and community engagement. Funders had no role in study design, implementation or interpretation.
Results
Recruitment and retention
We enrolled 30 participants during 113 h of recruitment activities, conducted over 24 days between January 28 and May 28, 2019. Table 1 shows the percent of participants meeting each of the study’s eligibility criteria. Table 2 provides socio-demographic characteristics of participants. All participants were included in the feasibility outcomes analyses.
Table 2.
Participant demographics.
| Category | Overall N = 30 |
|---|---|
| Age (median [IQR]) | 40.5 [33.0−53.2] |
| Self-reported gender no. (%) | |
| Female | 10 (33.3) |
| Male | 18 (60.0) |
| Other | 2 (6.7) |
| Born in Canada no. (%) | 25 (83.3) |
| Self-reported ethnicity/race no. (%) | |
| Blacka | 6 (20.0) |
| First Nations | 8 (26.7) |
| Indigenous | 13 (43.3) |
| Othera | 3 (10.0) |
| Housing n (%) | |
| Boarding home | 3 (10.0) |
| Group home | 12 (40.0) |
| Homeless/on street | 6 (20.0) |
| Renting | 6 (20.0) |
| Otherb | 3 (10.0) |
| Education no (%) | |
| Elementary | 8 (26.7) |
| High School | 8 (26.7) |
| University/College | 12 (40.0) |
| Graduate/Professional | 6 (6.7) |
| Previous first aid, CPR or OEND training | 18 (62.1) |
| Previously witnessed overdose | 11 (36.6) |
Categories collapsed to protect small cell sizes. “Black” included participants who identified as any of “Black – African”, “Black – Carribean” and “Black – North American”. “Other” includes any participant who chose “other” as their self-reported ethnicity/race, or who chose any other category not listed here.
Categories collapsed to protect small cell sizes. Includes “correctional facility/jail,” “own home,” “shelter/hostel” and “supportive housing”.
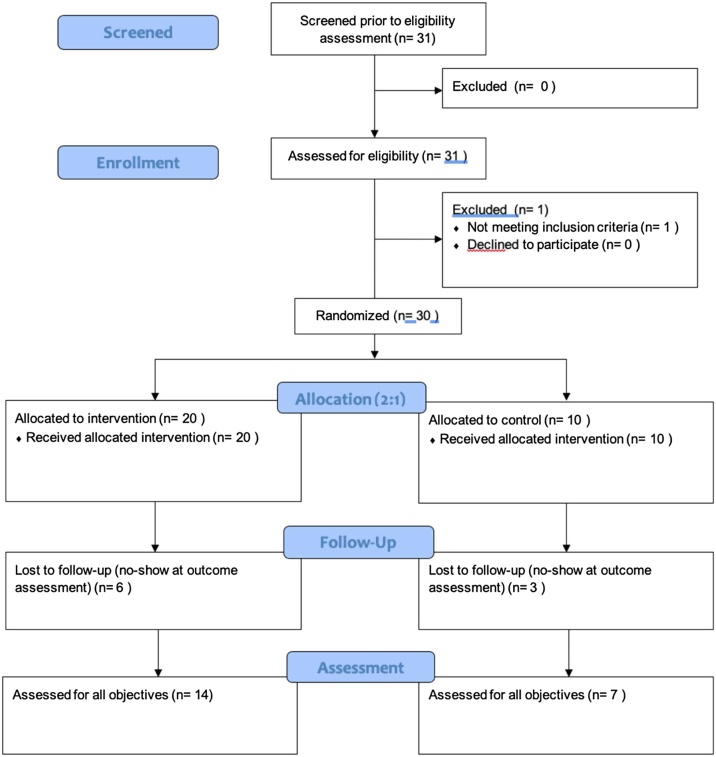
One individual asked to speak to study staff based on their interest in the study, but did not meet eligibility criteria. There were no eligible candidates who discussed the study with research staff but declined to participate (Fig. 1). On three occasions, individuals presented together and were recruited and randomized together (two pairs, one trio).
Fig. 1.
CONSORT feasibility diagram.
Table 3 provides opioid use characteristics among the participants who reported using opioids themselves. The majority of participants who reported taking non-prescription opioids used fentanyl (90.9%). The majority of participants who reported taking prescription opioids used opioids prescribed for opioid agonist therapy (methadone 64.3%). The majority of participants took opioids one or more times per day (76%).
Table 3.
Opioid use information among participants who use opioids (N = 16).
| Participants who take prescription opioids | 14 |
| Methadone or buprenorphine (%)a | 10 (71.5) |
| Morphine (%) | 4 (28.6) |
| Othera | 3 (21.4) |
| Participants who take non-prescription opioids | 11 |
| Fentanyl (%) | 10 (90.9) |
| Heroin (%) | 5 (45.5) |
| Other (%) | 4 (36.4) |
| Injection opioid use (%) | 7 (43.8) |
| Number of opioids used (%) | |
| 1 | 5 (31.2) |
| 2–3 | 8 (50.0) |
| 4 or more | 3 (18.8) |
| Frequency of opioid use | Non-prescription | Prescription |
|---|---|---|
| n = 11 | n = 14 | |
| n (%) | n (%) | |
| Multiple times per day | 4 (36.4) | 4 (28.6) |
| Every day or most daysa | 3 (27.2) | 10 (71.4) |
| A few times a week | 4 (36.4) | 0 (0.0) |
| Rarely or few times a month | 0 (0.0) | 0 (0.0) |
Categories merged to protect small cell sizes.
We retained 21 participants at the outcome simulation, corresponding with a retention rate of 70% (one-sided 95% CI 56.7–100). Table 4 provides participant retention numbers by study site and for the experimental and control arms of the study. One participant withdrew from the study after they viewed the briefing video and simulation room. They indicated that the choice to withdraw was related to personal discomfort and the appearance of the physical space. Of the 16 participants who were eligible for the 3-month follow-up visit, 4 (25%) were retained.
Table 4.
Recruitment and retention by study site and randomisation.
| Site | ||
|---|---|---|
| Recruited (N = 30) n(%) |
Completed Simulation (N = 21) n (% of recruited) |
|
| Family medicine | 12 (40) | 10 (83.3) |
| Addiction service | 10 (33.3) | 6 (60.0) |
| Emergency department | 8 (26.7) | 5 (62.5) |
| Randomisation | ||
| Experimental | 20 | 14 (70.0) |
| Control | 10 | 7 (70.0) |
Acceptability and refinement of procedures
Participants discussed their experience of engaging with the study in three domains: motivation for participation, study process experiences, and simulation experiences. Each of these domains and the dominant themes expressed by participants are provided in Table 5.
Table 5.
Participant experience of study processes representative quotes.
| Domain and theme | Representative participant quotes |
|---|---|
| Domain 1: motivation for participation | |
| Helping behaviours and altruism |
|
| Clinician referral |
|
| Compensation supports other factors |
|
| Domain 2: study process experiences | |
| Supportive environment |
|
| Reminders |
|
| Stigma |
|
| Domain 3: simulation experiences | |
| Benefit of hands-on experiences |
|
| Challenging and emotional responses |
|
| Comfortable, affirming environment |
|
Participants expressed a wide range of motivators for engaging in the study. Most commonly, participants expressed that they perceived overdose response as a helping behaviour and participated to serve their community or loved ones in distress. Despite the candidate-driven recruitment, several participants emphasized the importance of a referring clinician in their decision to participate. Others indicated that although they chose to participate on the basis of its educational or altruistic value, the cash compensation helped to support participation.
Many participants emphasized that the study processes and study personnel were pleasant and caring, and created a supportive environment for study participants. Participants indicated that reminders were helpful and contributed to retention. No participants indicated that the reminders were inappropriately intrusive or frequent. One participant raised concerns about a stigmatizing experience with security personnel while awaiting research staff in the hospital lobby (Table 5). This experience was managed as an opportunity for quality improvement and expanding our community of support for the trial within the hospital. Study staff reached out to inform hospital security about the study and engage them in the project’s ongoing implementation. We also implemented an emotional support and crisis plan for any participants in distress throughout the study.
In response to questions about their experiences in the simulation, most participants indicated that they felt that the simulation established a comfortable and affirming environment to practice hands-on overdose response skills. In particular, some participants emphasized that the simulation elicited challenging emotions, sometimes related to experiences of responding to overdose. These responses were largely coupled with the assertion that hands-on experience and real emotions are necessary to build experience, skills, and self-efficacy (Table 5). Study staff reported that nearly all participants indicated that they valued the simulation experience and felt that it built confidence in their overdose response skills.
Clinical and administrative personnel offered feedback concerning study processes, leading to quality improvements and staff education. For example, in one setting staff expressed concern about distributing materials at registration, asking patients and visitors if they use opioids or are in frequent contact with people who use opioids. This prompted an opportunity to collaboratively engage site staff in the study’s anti-stigma strategies, and process refinements whereby research personnel were permitted to approach patients directly to discuss naloxone distribution.
Data for the planned trial
Data collection and analysis procedures for the simulation, study interviews and questionnaires were refined through the feasibility study. A technical problem resulted in 1 unsuccessful simulation recording and data loss for this participant. Procedure checks were implemented to prevent this from recurring. All other simulations were recorded and assessed by two independent reviewers, confirming the operability of study databases and feasibility of these study processes. Study interviews and questionnaire data was collected per-protocol. Data for the outcomes of the planned trial were not analysed for the feasibility study.
Discussion
Our findings demonstrate that people who use opioids or are likely to witness overdose can be successfully recruited to a RCT concerning OEND, and retained to participate in an overdose simulation. Our findings demonstrate that the recruitment and retention strategy is adaptable for successful participant engagement in emergency department, family practice and addictions medicine settings. In these settings, the recruitment and retention strategy was successful in recruiting a sample of participants with diverse ethno-racial, gender, housing and educational backgrounds among a target population of people who take opioids or are likely to witness opioid overdose. Insufficient reporting of socio-demographic data is a gap in Canadian research concerning the opioid crisis.21 The collection of identifiers is a study strength, and reinforces that our recruitment and retention strategy serves a population including structurally marginalized groups. Study participants were predominantly people who take fentanyl or methadone and who take opioids one or more times per day. Our recruitment strategy may be less effective for individuals who take opioids sporadically.
Collaboration with community members in the study design is a strength that contributed to the success and feasibility of the study. Participants indicated that the study procedures and their participation as a responder in an opioid overdose simulation were broadly acceptable and safe. These findings are valuable because adverse experiences in health care and research are common among people who take drugs. For all staff involved in participant recruitment and study implementation, we invested in training and aptitudes to redress the stigma of opioid use and ensure a supportive, affirming, and positive research experience for participants. These experiences support the feasibility of the overall study. Feedback from study participants and site staff have also contributed to process improvements and troubleshooting.
Our findings are aligned with other studies regarding effective recruitment and retention strategies for research participants who use opiate drugs.5, 6, 7 Simplified verbal consent processes, multimodal reminders, and flexible study visit scheduling have also been shown to support recruitment and retention in other research contexts and populations.22, 23, 24, 25 Our findings reinforce existing evidence that cash incentives can be used to support participants in a non-coercive manner.26, 27 Although many of our participants were recruited through conventional clinician referrals, our candidate-driven recruitment method is a novel strategy and may reduce selection biases. The acceptability of study processes reflects the community-based and participatory approaches used to design and implement this research.28
Although our recruitment and retention methods may be used in other settings and marginalized populations, our study occurred in a well-resourced metropolitan academic setting. Although elements of our recruitment and retention strategy may be adaptable to a variety of contexts, our overall findings may not be generalizable to other settings or populations. The feasibility of our recruitment and retention strategy, and the acceptability of our study processes cannot guarantee the overall viability of the planned trial. Only one individual who expressed interest in discussing the study with recruitment staff was found to be ineligible, so it is possible that other eligible candidates were not informed about the study. Despite our candidate-driven recruitment methods, perceptions in the clinical environment regarding individual risks of opioid overdose may introduce unmeasured selection biases.
The SOONER Trial is feasible and acceptable to study participants and therefore ready for deployment.
Conflicts of interest
Aaron Orkin: Member of the American Red Cross Scientific Advisory Committee, First Aid Subcouncil; Member of the International Liaison Committee on Resuscitation First Aid Task Force; Expert Witness for the Office of the Chief Coroner of Ontario regarding an inquest into the prehospital death of a person experiencing opioid overdose.
Leigh Chapman: Membership of an advocacy organization - Toronto Overdose Prevention Society.
Curtis Handford: Funded Primary Care Clinical Lead position mid-east Toronto sub-region of Toronto Central Local Health Integration Network; also funded position of Deputy Chief, Department of Family and Community Medicine.
Peter Jüni: Received research grants to the institution from Appili Therapeutics, Astra Zeneca, Biotronik, Biosensors International, Eli Lilly, The Medicines Company, and honoraria to the institution for participation in advisory boards and/or consulting from Amgen, Ava and Fresenius. Serve as an unpaid member of the steering group or executive committee of trials funded by Abbott Vascular, Astra Zeneca, Biotronik, Biosensors, St. Jude Medical, Terumo and The Medicines Company.
Pamela Leece: Collaborator on Canadian Institutes of Health Research-funded evaluation of the Ontario naloxone program and pharmacy naloxone program, and Canadian Research Initiative in Substance Misuse, Naloxone Best Practices group.
All other authors declared no conflicts of interest.
Funding
This project was funded by a project grant from the Canadian Institutes of Health Research (CIHR) grant #148817 and Canadian Research Initiative in Substance Misuse. The Canadian Centre on Substance Use and Addiction (CCSA) also contributed to project workshops and community engagement.
CRediT authorship contribution statement
Aaron Orkin: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing (lead), Software, Resources and project administration (supporting). Mercy Charles: Investigation, Project administration (equal), Methodology (supporting), Writing - review & editing (supporting). Kristine Norris: Investigation, Project administration (equal), Methodology (supporting), Writing - review & editing (supporting). Rekha Thomas: Investigation, Project administration (equal), Methodology (supporting), Writing - review & editing (supporting). Leigh Chapman: Methodology (supporting), Validation (supporting), Writing - review & editing (supporting). Amy Wright: Methodology (supporting), Validation (supporting), Writing - review & editing (supporting). Douglas Campbell: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Curtis Handford: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Michelle Klaiman: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Shaun Hopkins: Conceptualization (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Rita Shahin: Conceptualization (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Kevin Thorpe: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Software (lead), Supervision (supporting), Writing - review & editing (supporting). Peter Jüni: Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Supervision (supporting), Writing - review & editing (supporting). Janet Parsons: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Kate Sellen: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Nick Goso: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Richard Hunt: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Supervision (supporting), Writing - review & editing (supporting). Pamela Leece: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Writing - review & editing (supporting). Laurie Morrison: Conceptualization (supporting), Data curation (supporting), Formal analysis (supporting), Investigation (supporting), Methodology (supporting), Resources (supporting), Supervision (supporting), Writing - review & editing (supporting). Vicky Stergiopoulos: Conceptualization (supporting), Methods (supporting) Resources (supporting). Suzanne Turner: Conceptualization (supporting), Investigation (supporting). Carol Strike: Conceptualization (equal), Data curation (supporting), Formal analysis (supporting), Funding acquisition (equal), Investigation (equal), Methodology (equal), Project administration (supporting), Resources (lead), Software (supporting), Supervision (lead), Validation (supporting), Visualization (supporting), Writing - original draft preparation (supporting), Writing - review & editing (supporting).
Acknowledgements
The investigators are particularly grateful to the SOONER Community Advisory Committee for their engagement and direction on this project, and to the staff, volunteers, and patients in all of the recruitment sites including the St. Michael’s Hospital Department of Family and Community Medicine, Addictions Medicine Team, and Department of Family and Community Medicine, and Inner City Health Associates and the Inner City Family Health Team. Thank you to Toronto Public Health and particularly the staff and volunteers at The Works for their contributions to this project, and to the staff of the Allan Waters Family Simulation Program at the Li Ka Shing Knowledge Institute, St. Michael’s Hospital, for supporting the outcome assessment and simulation processes. Thank you to Ian Drennan and Courtney Troung for assessing the simulation videos, Shunjun (Diana) Yan for her contributions to data cleaning, and to Dr. Ross Upshur for his contributions as Dr. Orkin’s doctoral supervisor. The SOONER Investigators acknowledge Ruby Sniderman and Audra Stitt for their contributions to project administration and coordination and Dr. Dan Werb for his contributions to study design. We acknowledge Adapt Pharmaceuticals for providing intranasal naloxone devices and simulation placebo devices for this phase of the study, Laerdal International for providing a high-fidelity simulation manikin, MadeGood foods for providing snacks for study participants, and Priority Dispatch Corporation for supporting the study’s simulation design.
Contributor Information
Aaron M. Orkin, Email: aaron.orkin@mail.utoronto.ca.
Mercy Charles, Email: Mercy.charles@unityhealth.to.
Kristine Norris, Email: kristine.norris@unityhealth.to.
Rekha Thomas, Email: rekha.thomas@unityhealth.to.
Leigh Chapman, Email: lchapman474@gmail.com.
Amy Wright, Email: a5wright@ryerson.ca.
Douglas M. Campbell, Email: douglas.campbell@unityhealth.to.
Curtis Handford, Email: curtis.handford@unityhealth.to.
Michelle Klaiman, Email: michelle.klaiman@unityhealth.to.
Shaun Hopkins, Email: shopkins@toronto.ca.
Rita Shahin, Email: rshahin@toronto.ca.
Kevin Thorpe, Email: kevin.thorpe@utoronto.ca.
Peter Jüni, Email: peter.juni@utoronto.ca.
Janet Parsons, Email: parsonsj@smh.ca.
Kate Sellen, Email: ksellen@faculty.ocadu.ca.
Nick Goso, Email: ngoso@faculty.ocadu.ca.
Richard Hunt, Email: rhunt@faculty.ocadu.ca.
Pamela Leece, Email: pamela.leece@oahpp.ca.
Laurie J. Morrison, Email: laurie.morrison@unityhealth.to.
Vicky Stergiopoulos, Email: vicky.stergiopoulos@camh.ca.
Suzanne Turner, Email: suzanne.turner@mail.utoronto.ca.
Carol Strike, Email: carol.strike@toronto.ca.
References
- 1.Walley A.Y., Xuan Z., Hackman H.H., Quinn E. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irvine M.A., Kuo M., Buxton J.A. Modelling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction. 2019;114:1602–1613. doi: 10.1111/add.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark A.K., Wilder C.M., Winstanley E.L. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8:153–163. doi: 10.1097/ADM.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi L., Green T.C., Bowman S.E., Ray M.C., McKenzie M.S., Rich J.D. 2017. Patient simulation for assessment of layperson management of opioid overdose with intranasal naloxone in a recently released prisoner cohort. Simulation in healthcare. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott C.K. A replicable model for achieving over 90% follow-up rates in longitudinal studies of substance abusers. Drug Alcohol Depend. 2004;74:21–36. doi: 10.1016/j.drugalcdep.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neale J., Tompkins C.N., McDonald R., Strang J. Improving recruitment to pharmacological trials for illicit opioid use: findings from a qualitative focus group study. Addiction. 2018;113:1066–1076. doi: 10.1111/add.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers K., Webb A., Frantz J., Randall M. What does it take to retain substance-abusing adolescents in research protocols? Delineation of effort required, strategies undertaken, costs incurred, and 6-month post-treatment differences by retention difficulty. Drug Alcohol Depend. 2003;69:73–85. doi: 10.1016/s0376-8716(02)00252-1. [DOI] [PubMed] [Google Scholar]
- 8.Chan A.-W., Tetzlaff J.M., Altman D.G. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orkin A., Campbell D., Handford C. Protocol for a mixed-methods feasibility study for the surviving opioid overdose with naloxone education and resuscitation (SOONER) randomised control trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldridge S.M., Chan C.L., Campbell M.J. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavonas E.J., Drennan I.R., Gabrielli A. 2015. Part 10: special circumstances of resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. [DOI] [PubMed] [Google Scholar]
- 12.Donetto S., Pierri P., Tsianakas V., Robert G. Experience-based co-design and healthcare improvement: realizing participatory design in the public sector. Des J. 2015;18:227–248. [Google Scholar]
- 13.Freire K., Sangiorgi D. Service design and healthcare innovation: from consumption to co-production to co-creation. Service design and service innovation conference: Linköping Electronic Conference Proceedings. 2010:39–50. [Google Scholar]
- 14.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitfield R.H., Newcombe R.G., Woollard M. Reliability of the Cardiff Test of basic life support and automated external defibrillation version 3.1. Resuscitation. 2003;59:291–314. doi: 10.1016/s0300-9572(03)00246-6. [DOI] [PubMed] [Google Scholar]
- 16.Eppich W., Cheng A. Promoting excellence and reflective learning in simulation (PEARLS): development and rationale for a blended approach to health care simulation debriefing. Simul Healthc. 2015;10:106. doi: 10.1097/SIH.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 17.Williams A.V., Strang J., Marsden J. Development of opioid overdose knowledge (OOKS) and attitudes (OOAS) scales for take-home naloxone training evaluation. Drug Alcohol Depend. 2013;132:383–386. doi: 10.1016/j.drugalcdep.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Braun V., Clarke V. 2012. Thematic analysis. [Google Scholar]
- 19.Lott D.C., Rhodes J. Opioid overdose and naloxone education in a substance use disorder treatment program. Am J Addict. 2016;25:221–226. doi: 10.1111/ajad.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams A.V., Marsden J., Strang J. Training family members to manage heroin overdose and administer naloxone: randomized trial of effects on knowledge and attitudes. Addiction. 2014;109:250–259. doi: 10.1111/add.12360. [DOI] [PubMed] [Google Scholar]
- 21.Belzak L., Halverson J. The opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can. 2018;38:224–233. doi: 10.24095/hpcdp.38.6.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varner C., McLeod S., Nahiddi N., Borgundvaag B. Text messaging research participants as a follow-up strategy to decrease emergency department study attrition. CJEM. 2017:1–6. doi: 10.1017/cem.2016.408. [DOI] [PubMed] [Google Scholar]
- 23.Flory J., Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 24.Beardsley E., Jefford M., Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol. 2007;25:e13–14. doi: 10.1200/JCO.2006.10.3341. [DOI] [PubMed] [Google Scholar]
- 25.Watson J.M., Torgerson D.J. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol. 2006;6:34. doi: 10.1186/1471-2288-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Festinger D.S., Marlowe D.B., Dugosh K.L., Croft J.R., Arabia P.L. Higher magnitude cash payments improve research follow-up rates without increasing drug use or perceived coercion. Drug and alcohol dependence. 2008;96:128–135. doi: 10.1016/j.drugalcdep.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Festinger D.S., Marlowe D.B., Croft J.R., Dugosh K.L., Mastro N.K., Lee P.A. Do research payments precipitate drug use or coerce participation? Drug Alcohol Depend. 2005;78:275–281. doi: 10.1016/j.drugalcdep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Wallerstein N.B., Duran B. Using community-based participatory research to address health disparities. Health Promot Pract. 2006;7:312–323. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]