Abstract
Aim
Targeted temperature management (TTM) in post-resuscitation care has changed dramatically over the last two decades. However, uptake across Australian and New Zealand (NZ) intensive care units (ICUs) is unclear. We aimed to describe post-resuscitation care in our region, with a focus on TTM, and to gain insights into clinician’s opinions about the level of evidence supporting TTM.
Methods
In December 2017, we sent an online survey to 163 ICU medical directors in Australia (n = 141) and NZ (n = 22).
Results
Sixty-one ICU medical directors responded (50 from Australia and 11 from NZ). Two respondents were excluded from analysis as their Private ICUs did not admit post-arrest patients. The majority of remaining respondents stated their ICU followed a post-resuscitation care clinical guideline (n = 41/59, 70%). TTM was used in 57 (of 59, 97%) ICUs, of these only 64% had a specific TTM clinical guideline/policy and there was variation in the types of patients treated, temperatures targeted (range = 33–37.5 °C), methods for cooling and duration of cooling (range = 12–72 h). The majority of respondents stated that their ICU (n = 45/57, 88%) changed TTM practice following the TTM trial: with 28% targeting temperatures >36 °C, and 23 (of 46, 50%) respondents expressed concerns with current level of evidence for TTM. Only 38% of post-resuscitation guidelines included prognostication procedures, few ICUs reported the use of electrophysiological tests.
Conclusions
In Australian and New Zealand ICUs there is widespread variation in post-resuscitation care, including TTM practice and prognostication. There also seems to be concerns with current TTM evidence and recommendations.
Keywords: Heart arrest, Resuscitation, Post-resuscitation care, Target temperature management, Survey
Introduction
Targeted temperature management (TTM) is recommended in international guidelines to reduce the neurological injury that occurs after cardiac arrest.1 The active cooling of patients post-cardiac arrest was first recommended by the International Liaison Committee on Resuscitation (ILCOR) in 20032, following the publication of two seminal randomised control trials.3,4 At that time, international guidelines recommended cooling patients to a therapeutic hypothermia (TH) range of 32–34 °C.5,6 However, subsequent international surveys suggested institutional uptake of TH for cardiac arrest was low (<50%),7, 8, 9 with a lack of policy, information, resources, equipment and expertise identified as barriers.10, 11, 12
The recommended targeted temperature for this treatment was expanded, from a range of 32–34 °C to 32–36 °C,13,14 following results from the Targeted Temperature Management Trial (TTM trial) in 201315 −which found no difference in patient outcomes within this temperature range. International surveys conducted immediately following the TTM trial publication suggest more ICUs have adopted cooling post-cardiac arrest (91%–94%), but that implementation of TTM varied greatly.16,17 Understanding why this variation in practice is occurring is vital to informing future research and guideline recommendations.
Current post-resuscitation practice, including TTM practice, in our region of Australia and New Zealand is unknown. Although, recent temperature data in our region suggest a large change in practice has occurred following the TTM trial,18,19 and current practice may not be following the Australian and New Zealand Committee on Resuscitation (ANZCOR) post-resuscitation guidelines.20 In this study, we surveyed ICU Medical Directors in Australia and New Zealand with the aim of describing elements of post-resuscitation care, including any changes in practice following publication of the TTM Trial and to examine their opinions on the current level of evidence for TTM.
Method
In December 2017, we conducted an online survey of medical directors for all ICUs registered in the Australian and New Zealand Intensive Care Society (ANZICs). Completion of the survey was voluntary and the study was approved by the Monash University Human Ethics Committee.
ICU Medical Directors were emailed an invitation to participate, with the exclusion of specialty hospitals, and the survey was completed on Survey Monkey. A second email was sent if a response was not received within four weeks. The questionnaire consisted of multiple choice and open-ended responses. Questions were derived from the findings of previous work,7, 8, 9 and on advice from experts in resuscitation from the Australian Resuscitation Outcomes Consortium (Aus-ROC) and the ANZICS Research Committee. Prior to release, the questionnaire was evaluated for face validity among Aus-ROC investigators and piloted first at hospitals associated with these investigators. We also validated responses against the clinical guidelines if these were provided by the ICU Medical Directors.
Data analysis was descriptive, and comparisons were made using the Fisher’s exact test. Content analysis was performed by one investigator (JB) on the open-ended response regarding the current level of evidence for TTM. Statistical analysis was performed in Stata with a p-value <0.05 considered statistically significant.
Results
Sixty-one ICU medical directors responded to the survey (response rate = 37%) – with representation from both countries (50 in Australia and 11 in NZ), all categories of ICUs and all states and regions (Table 1).
Table 1.
Characteristics of participating and non-participating ICUs.
| Characteristic | Overall N = 163 |
Participating N = 61 |
Non-participating N = 102 |
|---|---|---|---|
| Tertiary level | 38 (23%) | 17 (28%) | 21 (21%) |
| Private | 56 (34%) | 16 (26%) | 40 (39%) |
| Metropolitan | 30 (18%) | 7 (12%) | 23 (23%) |
| Rural/regional | 34 (21%) | 19 (31%) | 15 (15%) |
| Paediatric ICU | 5 (3%) | 2 (3%) | 3 (3%) |
| Country | |||
| Australia | 141 (87%) | 50 (82%) | 91 (89%) |
| New Zealand | 22 (13%) | 11 (18%) | 11 (11%) |
| Australian State/Territory | |||
| Victoria | 36 (25%) | 10 (20%) | 26 (28%) |
| New South Wales | 51 (36%) | 16 (32%) | 35 (38%) |
| Queensland | 26 (18%) | 12 (24%) | 14 (15%) |
| Western Australia | 12 (8%) | 5 (10%) | 7 (8%) |
| South Australia | 11 (8%) | 3 (6%) | 8 (9%) |
| Tasmania | 3 (2%) | 1 (2%) | 2 (2%) |
| ACT | 2 (1%) | 2 (4%) | 0 (0%) |
| Northern Territory | 2 (1%) | 1 (2%) | 1 (1%) |
| ICU beds | |||
| 3-9 | 23 (38%) | ||
| 10-19 | 22 (36%) | ||
| 20-30 | 9 (15%) | ||
| >30 | 7 (11%) | ||
The majority of respondents (n = 59, 97%) were from ICUs admitting cardiac arrest patients. Of these, 41 respondents (69%) stated their ICU used a post-resuscitation clinical guideline (Table 2). The majority of these respondents reported using clinical guidelines that specified haemodynamic parameters, but few guidelines (19/50, 38%) included prognostication procedures. Few respondents reported (the use of somatosensory evoked potential (SSEP, 9/50) or electroencephalography (EEG, 16/50) to inform prognostication.
Table 2.
Post-resuscitation practice in participating ICUs.
| Characteristic | Participating n = 61 (%) |
|---|---|
| Follows a Post-Resuscitation guideline | 41/59 (69) |
| Pre-defined targets for MAP | 27/41 (66) |
| Pre-defined targets for pCO2 | 25/41 (61) |
| Pre-defined targets for pO2 | 26/41 (63) |
| Pre-defined targets for glucose | 26/41 (63) |
| TTM usage | (n = 51) |
| TTM clinical guideline | 33/51 (64) |
| TTM pre-ICU | 9/51 (18) |
| Duration | |
| 12 h | 2 (4) |
| 24 h | 35 (68) |
| 28 h | 3 (6) |
| 36 h | 3 (6) |
| 48 h | 4 (8) |
| 60 h | 1 (2) |
| 72 h | 1 (2) |
| unsure | 2 (4) |
| Cooling devices | |
| Inductiona | (n = 51) |
| Ice packs | 29 (57) |
| Cold fluid | 27 (53) |
| Cooling blankets | 36 (71) |
| Endovascular | 5 (10) |
| Other (cooling device, mattress, paracetamol) | 4 (8) |
| Maintenancea | (n = 51) |
| Ice packs | 20 (39) |
| Cold fluid | 13 (25) |
| Cooling blankets | 38 (75) |
| Endovascular | 6 (12) |
| Other (cooling device, mattress) | 3 (6) |
More than one response allowed.
TTM was used in 97% (n = 57/59) of ICUs as a component of post-resuscitation care. Two respondents reported TTM was not administered in their ICUs, and stated this was because of a lack of expertise and resources (rural ICU respondent), and because of the lack of scientific evidence (tertiary metropolitan ICU respondent).
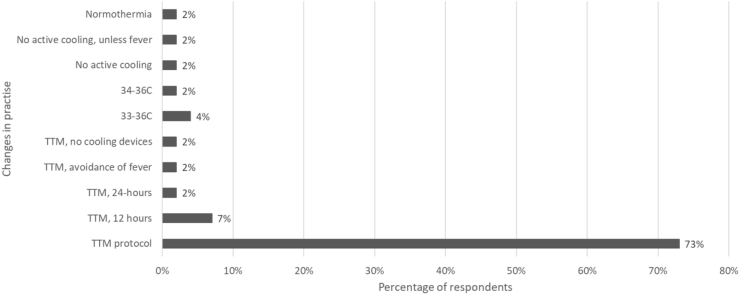
Of the 57 ICUs providing TTM, 51 (89%) provided details of TTM practice. Following the TTM trial publication, the majority of ICUs (n = 45/51, 88%) changed their target temperature practice (Fig. 1). This change was not associated with hospital type (88% teaching vs 88% non-teaching) or location (89% metropolitan vs. 88% rural, p = 0.62), and was reported by 100% of New Zealand and 85% of Australian respondents. Only 19% (n = 10) reported restricting TTM to a subset of patients, the majority of these ICUs (n = 9) restricted TTM to patients with a shockable rhythm, with one ICU restricting based on witnessed status.
Fig. 1.
Changes to Target Temperature Management following the TTM RCT 2013 publication.
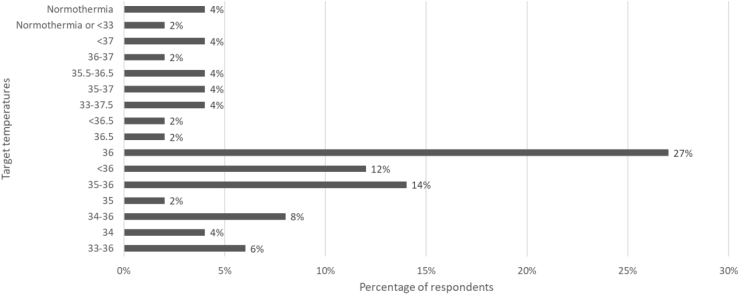
The majority of respondents reported changing to the TTM RCT protocol. However, there was a wide range of target temperatures reported, with 28% of ICUs targeting temperatures greater than 36 °C (92% of this subset were teaching hospitals and 100% were in Australia) and 6% were targeting normothermia (Fig. 2). The duration of TTM varied between 12 and 72 h, with the majority (69%) reporting a 24-h treatment window. The ICUs used various cooling methods, with the most common method being cooling blankets (71%).
Fig. 2.
Target Temperatures reported by ICU Medical Directors.
Almost half (n = 23/46) of the respondents expressed concerns with the current level of evidence for TTM. Specific themes of their concern were: whether the evidence was sufficient; design flaws in the existing randomised controlled trials (RCTs); and, applicability of RCTs to practice (Table 3).
Table 3.
Themes, and supporting quotes, identified in response to the question “Do you have any concerns with the current level of evidence for TTM post cardiac arrest?”.
| TTM | Supporting quotes |
|---|---|
| Insufficient evidence |
“The TTM trial needs to be replicated. There are issues with the time to significantly different temps and also the bias of clinicians observing neurology in patients with hypothermia induced delayed excretion of sedative agents.” “Not much evidence- really just lack of harm”. “Too few studies”. |
| Design flaws in trials |
“Temperature management in ICU is too late.” “All studies have major weakness/design flaws that limit clinical utility.” “Non-inferiority trial would be better with a RCT designed to see if TTM is better than TH management” “Suggests hypothermia useful. Never tested whether avoidance of hyperthermia is as efficacious.” “There is no conclusive evidence about its efficacy.” “The "evidence’ from TTM Nielsen study did not test the upper limit of temperature that can be tolerated without any harm.”. |
| Applicability to practice |
“Still not sure about the < 36. Currently doing an audit to see how much normothermia occurs. When targeted 32–33, no normothermia during cooling phase.” “Seems to change very frequently and is not uniformly applied.” |
Discussion
Our online survey of Australian and New Zealand ICU medical directors indicates a wide variation in the delivery of post-resuscitation care. There has been a large shift in TTM practice, with the majority of respondents stating their ICU had changed practice following the TTM trial. However, our data also shows that not all ICUs are following the current ANZCOR guideline,20 with 28% of ICUs targeting temperatures over 36 °C.
Our data provides further evidence supporting the recent report from the Australian and New Zealand Intensive Care Society (ANZICS) Adult Patient Database. Salter et al.19 found a significant shift in the lowest and highest recorded temperatures in the first 24 h of ICU in cardiac arrest patients in the period following publication of the TTM trial. Similar changes have also been reported in ICU admissions in the UK.21 Alarmingly, in both these studies, OHCA mortality was trending down the decade preceding the publication of the TTM trial, but increased for the first time in the years following. This may be explained by a combination of difficulties in implementing a 36 °C protocol,18 and potentially by a misinterpretation of the TTM trial as a negative study and an abandonment of this treatment all together. It is also possible that this change in mortality reflects a wider spread change in attitude and practice to post-resuscitation care, including earlier prognostication. However, most before-and-after studies have not reported changes in other aspects of care (e.g. coronary angiogram),18,22,23 and the large registry studies suggest no change in time in ICU either overall or in non-survivors19,21 There is a growing body of evidence showing significant decreases in TTM administration following the TTM trial,24, 25, 26 and our data suggest over a quarter of ICUs are now targeting normothermic temperatures.
Despite recommendations from leading bodies, such as ILCOR27 and Resuscitation Councils,20 and the widespread adoption of TTM, there appears to be divide among the medical community concerning the current level of evidence for TTM28 which may explain why some ICUs are now practicing outside of guideline recommendations. Key themes expressed by half of our respondents surround insufficient evidence, efficacy of treatment and applicability to clinical practice. Such concerns were also seen internationally from clinicians following publication of the TTM trial.29,30 Some respondents in our study described the “normothermia” seen in the 36 °C arm of the TTM trial. The TTM trial temperature curves show mean temperatures of approximately 36 °C, but the 95% confidence intervals extend into the “normothermic” range. However it is important to note that no patients in the TTM trial experienced fever in the first 24-h, but subsequent implementation of the 36 °C protocol into clinical practise has seen significant increases in patients with fever in some studies.18,19 This was also seen in the recent HYPERION trial in non-shockable arrests. Although, not specifically reported, the confidence intervals of the temperature curves in the normothermic group in HYPERION were within the febrile range.31 The TTM-2 trial,32 which is has recently completed recruitment, will compare therapeutic hypothermia at 33 °C with normothermia and early treatment of fever (≥37.8 °C), may allay some the concerns with the current level of evidence.
Our study also demonstrated other variations in TTM treatment and post-resuscitation care–including the duration of TTM, use of cooling devices, prognostication and use of specific a post-resuscitation care clinical guideline. Similar variations have been reported in other international ICU surveys.17,33 The variation seen in duration and cooling methods most likely reflects those used in clinical trials. For example, the duration of TTM varies in trials between 123 and 7234 h, as there not currently any clear evidence of the optimal TTM duration in adults27 or children.35 There is also no clear consensus on cooling devices, although a recent review suggests some methods may be superior to others.36 Although we only asked limited questions about prognostication, we found only one-third included prognostication in their clinical guideline and few used SSEP and EEG. These findings require further investigation to uncover what other tests are currently in widespread use. There is also the need for large audit of care in our region to examine whether the variation in post-resuscitation care, particularly care that does not adhere to guideline recommendations, impacts on patient outcomes. This has been reported elsewhere,37 and may be needed in our region to change future practice. Alternately, trials investigating the standardisation of post-resuscitation care across ICUs,38 or transport to cardiac arrest centres,39 may be worth investigating to ensure patients receive optimal care.
Our study has a number of limitations. The survey was conducted in December 2017, and may not reflect current practice and may only reflect practice in Australian and New Zealand. The response rate was less than ideal, and there are some differences in the characteristics of the responding ICUs (Table 1). Also not all participants answered all questions on the survey, and our survey may be subject to responder bias (i.e. those who changed practice may have been more likely to respond). Responses were checked against post-resuscitation clinical guidelines/protocols when these were provided.
Despite these limitations, our study provides important data on post-arrest care in Australia and New Zealand ICUs. Our data indicates areas requiring further research, such as prognostication, as well as a shift and wide variation in TTM practice. Our study also identifies current concerns of the ICU medical specialists, which will need to be overcome to achieve a standardisation of TTM post-resuscitation care.
Funding
This study was funded by a grant from the Australian College of Critical Care Nurses. JB and DS are funded by Heart Foundation Fellowships. JF is funded by a National Health and Medical Research Council (NHMRC) Grant.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2020.100002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Stub D., Bernard S., Duffy S.J., Kaye D.M. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123:1428–1435. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 2.Nolan J.P., Morley P.T., Hoek T.L., Hickey R.W. Advancement life support task force of the international Liaison committee on R. Therapeutic hypothermia after cardiac arrest. An advisory statement by the advancement life support task force of the international Liaison committee on resuscitation. Resuscitation. 2003;57:231–235. doi: 10.1016/s0300-9572(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 3.Bernard S.A. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.ECC Committee S. Task forces of the American Heart association. 2005 American Heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112:IV1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 6.Nolan J.P. European Resuscitation Council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation. 2005;67(Suppl 1):S39–S86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy J., Green R.S., Stenstrom R., Committee C.C.C. The use of induced hypothermia after cardiac arrest: a survey of Canadian emergency physicians. CJEM. 2008;10:125–130. doi: 10.1017/s1481803500009830. [DOI] [PubMed] [Google Scholar]
- 8.Kliegel A., Gamper G., Mayr H. Therapeutic hypothermia after cardiac arrest in Lower Austria--a cross-sectional survey. Eur J Emerg Med. 2011;18:105–107. doi: 10.1097/MEJ.0b013e32833d46b2. [DOI] [PubMed] [Google Scholar]
- 9.Skulec R., Dostalova G., Kovarnik T., Linhart A., Seblova J. Therapeutic hypothermia in cardiac arrest survivors: a survey of practice in the Czech Republic. Resuscitation. 2008;77:419–420. doi: 10.1016/j.resuscitation.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Abella B.S., Rhee J.W., Huang K.N., Vanden Hoek T.L., Becker L.B. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64:181–186. doi: 10.1016/j.resuscitation.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Bigham B.L., Dainty K.N., Scales D.C., Morrison L.J., Brooks S.C. Predictors of adopting therapeutic hypothermia for post-cardiac arrest patients among Canadian emergency and critical care physicians. Resuscitation. 2010;81:20–24. doi: 10.1016/j.resuscitation.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Merchant R.M. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34:1935–1940. doi: 10.1097/01.CCM.0000220494.90290.92. [DOI] [PubMed] [Google Scholar]
- 13.Kleinman M.E. Part 5: adult basic life support and cardiopulmonary resuscitation quality: 2015 American Heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S414–S435. doi: 10.1161/CIR.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 14.Nolan J.P. European resuscitation Council and European society of intensive care medicine guidelines for post-resuscitation care 2015: section 5 of the European resuscitation Council guidelines for resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen N. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 16.Deye N. Changes in cardiac arrest patients’ temperature management after the 2013 "TTM" trial: results from an international survey. Ann Intensive Care. 2016;6:4. doi: 10.1186/s13613-015-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford A.H. Management of cardiac arrest survivors in UK intensive care units: a survey of practice. J Intensive Care Soc. 2016;17:117–121. doi: 10.1177/1751143715615151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bray J.E. Changing target temperature from 33 degrees C to 36 degrees C in the ICU management of out-of-hospital cardiac arrest: a before and after study. Resuscitation. 2017;113:39–43. doi: 10.1016/j.resuscitation.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Salter R. Changes in temperature management of cardiac arrest patients following publication of the target temperature management trial. Crit Care Med. 2018;46:1722–1730. doi: 10.1097/CCM.0000000000003339. [DOI] [PubMed] [Google Scholar]
- 20.Australian and New Zealand Committee on Resuscitation 2016. https://resusorgau/guidelines/ Last accessed: 27/08.2019.
- 21.Nolan J.P. Increasing survival after admission to UK critical care units following cardiopulmonary resuscitation. Crit Care. 2016;20:219. doi: 10.1186/s13054-016-1390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casamento A. A comparison of therapeutic hypothermia and strict therapeutic normothermia after cardiac arrest. Resuscitation. 2016;106:83–88. doi: 10.1016/j.resuscitation.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Johnson N.J. Targeted temperature management at 33 versus 36 degrees: a retrospective cohort study. Crit Care Med. 2020;48:362–369. doi: 10.1097/CCM.0000000000004159. [DOI] [PubMed] [Google Scholar]
- 24.Abazi L. Long-term survival in out-of-hospital cardiac arrest patients treated with targeted temperature control at 33 degrees C or 36 degrees C: a national registry study. Resuscitation. 2019;143:142–147. doi: 10.1016/j.resuscitation.2019.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Khera R. Hospital variation in the utilization and implementation of targeted temperature management in out-of-hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004829. [DOI] [PubMed] [Google Scholar]
- 26.Lascarrou J.B. Temporal trends in the use of targeted temperature management after cardiac arrest and association with outcome: insights from the Paris Sudden Death Expertise Centre. Crit Care. 2019;23:391. doi: 10.1186/s13054-019-2677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnino M.W. Temperature management after cardiac arrest: an advisory statement by the advanced life support task force of the international Liaison committee on resuscitation and the American Heart association emergency cardiovascular care committee and the Council on cardiopulmonary, critical care, perioperative and resuscitation. Resuscitation. 2016;98:97–104. doi: 10.1016/j.resuscitation.2015.09.396. [DOI] [PubMed] [Google Scholar]
- 28.Taccone F.S., Picetti E., Vincent J.L. High quality targeted temperature management (TTM) after cardiac arrest. Crit Care. 2020;24:6. doi: 10.1186/s13054-019-2721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stub D. Targeted temperature management after cardiac arrest. N Engl J Med. 2014;370:1358. doi: 10.1056/NEJMc1401250. [DOI] [PubMed] [Google Scholar]
- 30.Varon J., Polderman K. Targeted temperature management after cardiac arrest. N Engl J Med. 2014;370:1358–1359. doi: 10.1056/NEJMc1401250. [DOI] [PubMed] [Google Scholar]
- 31.Lascarrou J.B. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381:2327–2337. doi: 10.1056/NEJMoa1906661. [DOI] [PubMed] [Google Scholar]
- 32.Dankiewicz J. Targeted hypothermia versus targeted Normothermia after out-of-hospital cardiac arrest (TTM2). A randomized clinical trial – rationale and design. Am Heart J. 2019;217:23–31. doi: 10.1016/j.ahj.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Storm C. Use of target temperature management after cardiac arrest in Germany--a nationwide survey including 951 intensive care units. Resuscitation. 2014;85:1012–1017. doi: 10.1016/j.resuscitation.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Fink E.L. 24 vs. 72 hours of hypothermia for pediatric cardiac arrest: a pilot, randomized controlled trial. Resuscitation. 2018;126:14–20. doi: 10.1016/j.resuscitation.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buick J.E. Paediatric targeted temperature management post cardiac arrest: a systematic review and meta-analysis. Resuscitation. 2019;139:65–75. doi: 10.1016/j.resuscitation.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 36.Calabro L. Effect of different methods of cooling for targeted temperature management on outcome after cardiac arrest: a systematic review and meta-analysis. Crit Care. 2019;23:285. doi: 10.1186/s13054-019-2567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stub D. Association between hospital post-resuscitative performance and clinical outcomes after out-of-hospital cardiac arrest. Resuscitation. 2015;92:45–52. doi: 10.1016/j.resuscitation.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Sunde K. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Yeung J., Matsuyama T., Bray J., Reynolds J., Skrifvars M.B. Does care at a cardiac arrest centre improve outcome after out-of-hospital cardiac arrest? - a systematic review. Resuscitation. 2019;137:102–115. doi: 10.1016/j.resuscitation.2019.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.