Abstract
Cardiac arrest is an important public health concern, affecting an estimated 356,500 people in the out-of-hospital setting and 209,000 people in the in-hospital setting each year. The causes of cardiac arrest include acute coronary syndromes, pulmonary embolism, dyskalemia, respiratory failure, hypovolemia, sepsis, and poisoning among many others. In order to tackle the enormous issue of high mortality among sufferers of cardiac arrest, ongoing research has been seeking improved treatment protocols and novel therapies. One of the mechanical devices that has been increasingly utilized for cardiac arrest is venoarterial extracorporeal membrane oxygenation (VA-ECMO). Presently there is only one published randomized controlled trial examining the use of VA-ECMO as part of cardiopulmonary resuscitation (CPR), a process referred to as extracorporeal cardiopulmonary resuscitation (ECPR). Recently there has been significant progress in providing ECPR for refractory cardiac arrest patients. This narrative review seeks to outline the use of ECPR for both in-hospital and out-of-hospital cardiac arrest, as well as provide information on the expected outcomes associated with its use.
Keywords: Cardiac arrest, Extracorporeal membrane oxygenation, ECMO, Extracorporeal cardiopulmonary resuscitation, ECPR, COVID-19
Objective
This narrative review seeks to summarize the utilization of extracorporeal membrane oxygenation (ECMO) in adult patients suffering from in-hospital cardiac arrest (IHCA) or out-of-hospital cardiac arrest (OHCA), as well as provide insight into what outcomes might be expected. Summarizing the current state of ECMO use during cardiopulmonary resuscitation (CPR) for cardiac arrest elucidates the progress that has been made thus far, and identifies areas of potential future research to address the gaps in current knowledge.
Introduction
Cardiac arrest is defined as the cessation of cardiac mechanical activity and circulation, which can be confirmed by the absence of a detectable pulse, unresponsiveness, and absence of normal breathing pattern.1 It has been estimated that each year approximately 356,500 people suffer from OHCA, and 209,000 from IHCA.2 The causes of cardiac arrest are broad, and include: acute coronary syndromes, pulmonary embolism, dyskalemia, respiratory failure, pericardial tamponade, tension pneumothorax, poisoning, sepsis, hypovolemia, hemorrhage, and hypothermia.3 ECMO is one of the mechanical devices that has been increasingly utilized for organ and circulatory support during refractory IHCA and OHCA.4 Such use of ECMO support during initial resuscitation is referred to as extracorporeal cardiopulmonary resuscitation (ECPR).5 The components that make up an ECMO circuit include a blood pump, gas exchange device, conduit tubing that connects to vascular access, and often also includes a heat exchanger for temperature control.6 The standard ECPR circuit for circulatory support during cardiac arrest withdraws blood from a venous cannula (the drainage cannula), pumps the blood through an extracorporeal membrane lung, and returns the newly oxygenated blood to the patient via an arterial cannula (the return cannula). The nomenclature for such is V-A, or V-Ad, if a distal perfusion catheter is also inserted for distal extremity perfusion.7 Certainly, there are times when a more sophisticated set-up is needed for optimal circulatory support, such as adding a second venous drainage catheter to increase cardiac output (VV-A). Another example is after spontaneous cardiac function begins to recover while pulmonary function may still be abnormal, and the heart now is ejecting poorly oxygenated blood which mixes with the extracorporeal oxygenated blood resulting in differential hypoxia requiring additional cephalad systemic oxygenation. This often requires additional return of extracorporeal oxygenated blood to the upper body using a return cannula in an upper body venous site (V-AV).7 The use of ECMO is labor-intensive, requiring specialized equipment and trained providers, and associated with risks such as hemorrhage, vascular injury, renal failure, neurologic injury, and infection among others.[6], 8 As of 2019, the American Heart Association reported insufficient evidence to recommend the routine use of ECPR for patients with cardiac arrest, but that it may be considered to provide rescue organ and circulatory support when conventional CPR (CCPR) fails.5 This narrative review will discuss the current evidence surrounding the use of ECPR in adult patients that experience IHCA or OHCA that is refractory to conventional therapy.
Methods
A comprehensive literature search was performed on June 08, 2020 which included PubMed/MEDLINE, Embase, AccessMedicine, Cochrane Database of Systematic Reviews, DynaMed Plus, UpToDate and Merck Manual. Search terms included Cardiac arrest, IHCA, OHCA, ECMO, ECLS, ECPR, extracorporeal membrane oxygenation, extracorporeal life support, extracorporeal cardiopulmonary resuscitation, and the MeSH terms Heart Arrest and Extracorporeal Membrane Oxygenation. Key references from articles that were reviewed were also obtained and reviewed. Title and abstract screening was performed for 542 unique references, and 199 unique references were fully reviewed. A supplementary literature search in PubMed/MEDLINE was done on September 14, 2020 to include the MeSH terms “COVID-19” OR “severe acute respiratory syndrome coronavirus 2” AND “Extracorporeal Membrane Oxygenation” which identified 95 entries, all of which underwent title and abstract screening. Key references from the reviewed articles were also reviewed, for a total of 40 unique references fully reviewed. An additional 6 unique references were screened and fully reviewed during revision, bringing the total number of unique references screened to 643, and the total number of unique references that underwent full review to 245.
When should ECPR be used?
When guideline-directed resuscitation efforts fail to achieve return of spontaneous circulation (ROSC), an episode of cardiac arrest is deemed refractory and ECPR may be considered.5 However, there is no universally agreed upon timing of when cardiac arrest is refractory. Refractory cardiac arrest (rCA) has been previously defined on the basis of CPR length, number of defibrillations, doses of epinephrine or amiodarone, or a combination of these.9, 10, 11, 12, 13, 14, 15 In the setting of cardiac arrest, a shorter time to initiation of ECMO has been previously associated with favorable neurologic outcome,16, 17, 18 which may make defining cardiac arrest as refractory based upon CPR duration alone excessively limiting. For shockable rhythms it may be better to use defibrillation attempts as the main criteria for defining rCA, though for nonshockable rhythms CPR duration may be the only variable available. Until randomized controlled trials are done to provide firm indications for the use of ECPR, rCA will continue to be defined on the basis of CPR duration or failure to achieve ROSC following multiple defibrillation attempts at the discretion of the ECMO provider. Generally when defining rCA, cardiac arrest that persists despite continuous CCPR lasting 10−30 min or after three attempted defibrillations is considered refractory.
Implementation of ECPR
When initiating ECMO for patients suffering from cardiac arrest, the first step is to have already assembled and trained a dedicated team of experienced providers and trained staff. Key is a rapid response by the team of ECMO specialists. The initial step is rapid but thorough evaluation regarding the appropriateness of instituting ECMO for a particular patient. ECMO “teams” are crucial to facilitate this decision as opposed to a single practitioner. Upon arrival at the patient, a primed circuit and cannulation are the next required steps.
In the setting of thoracotomy, cannulae can be placed directly into the right atrium and aorta. However, cannulae are more often placed peripherally in the vessels of the neck or lower extremities, such as during the emergent use of ECPR for refractory IHCA or OHCA. Patients that are placed on VA-ECMO for refractory cardiac arrest will typically have a drainage cannula placed into the femoral vein, and a return cannula placed into the femoral artery. The placement of a cannula into the appropriate vessel can be accomplished by exposing the vessels via cut down followed by direct puncture of the vessel, or by percutaneous puncture using the Seldinger technique with or without the use of imaging guidance provided by fluoroscopy or ultrasound.6 Cannulation via cut down is usually performed in the intensive care unit in cooperation with an operating team. Percutaneous cannulation can be done in the Emergency Department or in the cardiac catheterization lab to facilitate coronary angiography with coronary intervention as indicated after the patient is stabilized on ECMO, but percutaneous cannulation can also be performed in the operating room, intensive care unit, or as part of specialized prehospital treatment if the appropriate equipment and staff are available. Fluoroscopy and/or ultrasound are helpful for the initial placement and positioning of cannulae, and echocardiography is used to check cardiac function for evidence of recovery before a patient is taken off of ECMO.6
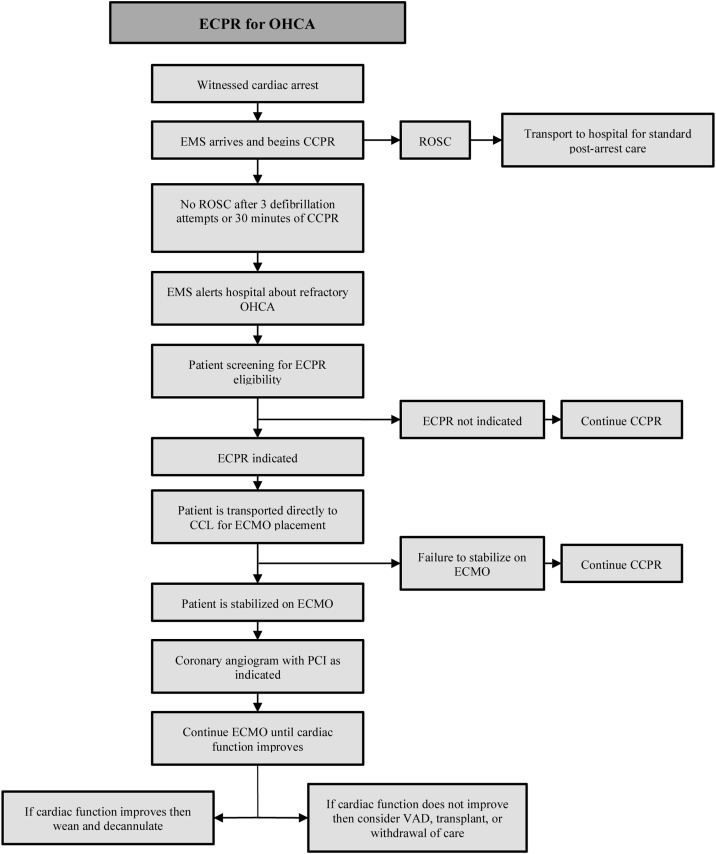
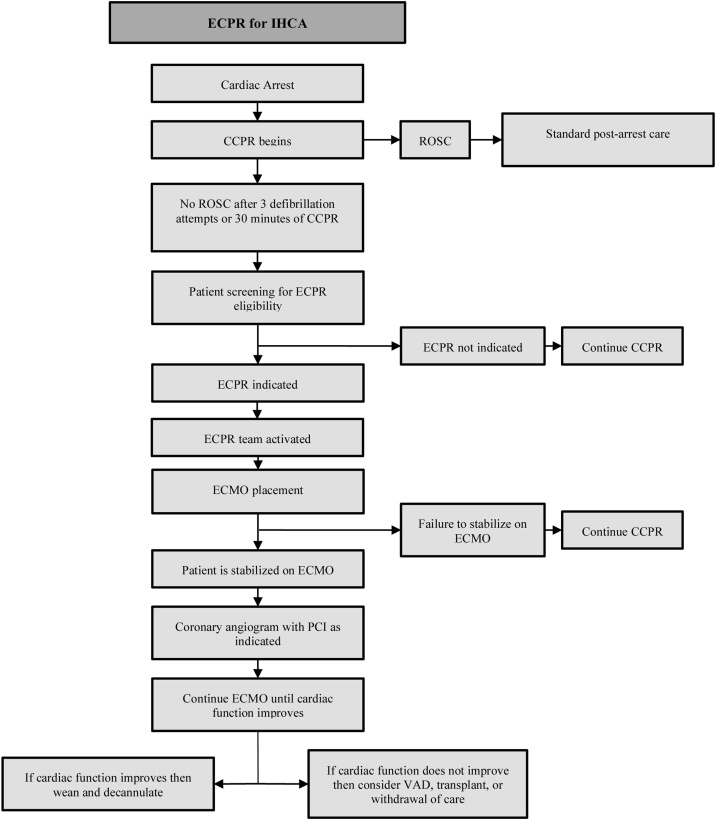
Fig. 1, Fig. 2outline the progression to ECPR.
Fig. 1.
ECPR: Extracorporeal cardiopulmonary resuscitation. OHCA: Out-of-hospital cardiac arrest. EMS: Emergency medical services. CCPR: Conventional cardiopulmonary resuscitation. ROSC: Return of spontaneous circulation. CCL: Cardiac catheterization lab. ECMO: Extracorporeal membrane oxygenation. PCI: Percutaneous coronary intervention. VAD: Ventricular assist device.
Fig. 2.
ECPR: Extracorporeal cardiopulmonary resuscitation. IHCA: In-hospital cardiac arrest. CCPR: Conventional cardiopulmonary resuscitation. ROSC: Return of spontaneous circulation. CCL: Cardiac catheterization lab. ECMO: Extracorporeal membrane oxygenation. PCI: Percutaneous coronary intervention. VAD: Ventricular assist device.
Use with in-hospital cardiac arrest
An observational analysis of adult IHCA in the United States found that between the years 2000 and 2018, less than 1% of reported in-hospital cardiac arrest patients were treated with ECPR.19 Patients in this sample were more likely to receive ECPR for IHCA if they were less than 40 years of age, had a period of pre-arrest hypoperfusion, had congestive heart failure (CHF), or had either a history of myocardial infarction (MI) or MI during the same hospitalization. The presence of shockable initial rhythm was associated with ECPR use, however the majority of patients receiving ECPR in this sample had nonshockable initial rhythms.
Although the use of ECPR occurs in only a small percentage of treated IHCA, its use is increasing and an area of active research and protocol development.20 A systematic review and meta-analysis by D’Arrigo et al. reported that the overall survival to discharge of ECPR-treated IHCA patients was 37.9%, and that 84.4% of survivors had good neurologic outcomes.21 Survival to discharge among ECPR patients in this analysis was much higher than the previously reported survival to discharge rate of 17.0% among IHCA patients treated with CCPR.22, 23 This difference may be due in part to patient selection in the ECPR group such as initial shockable rhythm or fewer comorbidities, however a meta-analysis by Ahn et al comparing the efficacy of ECPR versus CCPR also found that the use of ECPR for IHCA was significantly associated with better survival and neurologic outcome when compared to CCPR, with a reported odds ratio of 2.40 for survival rate and 2.63 for favorable neurologic outcome.24
As additional studies are done, criteria for selecting which IHCA patients receive ECPR is evolving. When strict selection criteria are applied, patients treated with ECPR have higher rates of survival and higher rates of favorable neurologic outcomes.25 ECPR selection criteria have included age, suspected etiology of arrest, initial arrest rhythm, absence of non-cardiac comorbidities, time to initiation of CPR, and duration of CPR.9, 12, 16, 17 While selecting for a population of patients with initial shockable rhythms and no comorbidities may improve reported outcomes, patient populations where the potential benefits outweigh the risks of ECPR should not be unnecessarily excluded.
Meta-analysis of the predictors of favorable outcome in ECPR-treated IHCA showed no significant difference between survivors and non-survivors in terms of age or gender, but found that survivors had significantly lower pre-arrest plasma creatinine levels, a significantly higher likelihood of initial shockable rhythm, significantly shorter low-flow times, significantly lower intra-arrest blood lactate levels before ECPR, and a lower Sequential Organ Failure Assessment (SOFA) score in the first 24 h after ICU admission, when compared with non-survivors.21 When taken together, admitted patients that have low pre-arrest plasma creatinine levels and present with an initial shockable rhythm should be strongly considered for ECPR if the cardiac arrest is refractory to initial resuscitation, and if the decision is made to proceed with ECPR then ECMO should be initiated as soon as possible to minimize low-flow time. During the period of standard CPR prior to ECMO initiation, intra-arrest lactate levels may be measured for prognostication, and if ROSC is achieved then the patient’s SOFA score should be calculated. Factors that may predict a favorable outcome following ECPR for IHCA are summarized in Table 1.
Table 1.
Initial shockable rhythm refers to ventricular fibrillation or pulseless ventricular tachycardia.
| Favorable factors for VA-ECMO for refractory IHCA |
|---|
| Initial shockable rhythm |
| Pre-arrest Serum Creatinine <1.16 mg/dL |
| Duration of CPR before ECPR <33 min |
| Intra-arrest Pre-ECPR Serum Lactate <7.7 mmol/L |
| Post-arrest SOFA score <13 |
| Post-arrest Serum Lactate <8.9 mmol/L |
| Post-arrest Serum Creatinine <1.08 mg/dL within 24 h |
CPR, conventional guideline-directed cardiopulmonary resuscitation; ECPR, extracorporeal cardiopulmonary resuscitation; SOFA score, the calculated Sequential Organ Failure Assessment score.
A single-center study of patients receiving ECPR for IHCA in South Korea found that Body Mass Index (BMI) was not associated with in-hospital mortality, although none of the patients had a BMI of greater than 40 kg/m2 and there was a limited number of patients.26 Despite this study, patients with a BMI ≥ 40 kg/m2 have an increased risk of mortality following IHCA,27 and there is currently no literature focusing on the outcomes of ECPR in these patients. Patients with a BMI ≥ 40 kg/m2 may represent a target population for ECPR as increased body mass can decrease the efficacy of chest compressions during standard CPR,28 however more research must be done.
The increasing use of ECPR has raised concerns over the cost of this treatment option. A decision tree and Markov model were created to calculate Quality-Adjusted Life Years (QALY) in a simulated cohort of patients with refractory IHCA, and found that ECPR could be considered a cost-effective treatment for IHCA in Europe and North America.29 While this model is effective for illustrating that ECPR can be cost-effective from an acute healthcare perspective, more studies should be done to include the costs of long-term care following ECPR for IHCA.
Use with out-of-hospital cardiac arrest
Utilization of ECMO for OHCA has been steadily increasing each year, but still represents only a small fraction of the affected population with only 0.14% utilization in 2008 and 0.69% in 2014 within the United States.30 A registry study in Paris, France indicated that between 2011 and 2018, 4% of patients with OHCA of presumed cardiac etiology with attempted resuscitation received ECPR,31 which reflects larger utilization within a selected population but still a small percentage of OHCA overall.
Use of ECPR for refractory OHCA has been associated with survival rates between 6.9% and 56.0%,15, 32, 33, [34], 35, 37 but the selection criteria between studies varies and may be responsible for the wide range of survival rates. A meta-analysis by Debaty et al. found that initial shockable rhythm, shorter low-flow time, higher arterial pH on admission, and lower serum lactate on admission, were associated with favorable outcomes in OHCA patients undergoing ECPR.36 There was no significant association between age and outcome in this analysis, however many of the included studies that were analyzed had an inclusion criteria which restricted age to <75, <70, or <65 years old. A single-center retrospective analysis in Japan found that for patients who undergo ECPR for refractory OHCA, age ≥70 years was significantly associated with a lower survival rate, and non-significantly associated with poor neurologic outcome,37 but more studies should be done to evaluate age ≥70 years as a contraindication to ECPR.
Bartos et al. also observed significant association between neurologically favorable survival and lower serum lactic acid level, higher arterial pH, higher arterial partial pressure of oxygen (PaO2), and lower arterial partial pressure of carbon dioxide (PaCO2), with each value being obtained upon arrival of the patient. In this cohort, increased duration of CPR was also associated with a higher risk of brain death.38
In a recently published retrospective analysis, OHCA patients that had an arterial pH < 7.03 prior to the initiation of ECPR had unfavorable neurologic outcomes when compared to patients that had pH ≥ 7.03.39 Given the objectivity and reproducibility of arterial blood gas analysis, the authors of the study recommended pH measurement when considering ECPR, because the patients with an arterial pH ≥ 7.03 had a higher probability of favorable neurologic outcome.
Aside from lab values, the cardiac rhythm preceding ECPR initiation should also be monitored. A multi-center observational study examined the relationship between arrest rhythm and neurologic outcome among patients treated with ECPR for OHCA. Ventricular fibrillation (VF) or pulseless ventricular tachycardia (pVT) that was sustained until the initiation of ECPR was significantly associated with favorable neurologic outcome, and patients who initially had VF or pVT but converted to pulseless electrical activity (PEA) or asystole prior to ECPR initiation had no neurologic benefit from ECPR.40 Factors that may predict a favorable outcome following ECPR for OHCA are summarized in Table 2.
Table 2.
Initial shockable rhythm refers to ventricular fibrillation or pulseless ventricular tachycardia.
| Favorable factors for VA-ECMO for refractory OHCA |
|---|
| Initial shockable rhythm |
| Duration of CPR before ECPR <66 min |
| Arterial pH ≥ 7.03 on arrival |
| Serum lactate ≤11.81 mmol/L on arrival |
| Age <70 years |
| Received bystander CPR |
| PaO₂ ≥134 mmHg on arrival |
| PaCO₂ ≤58.5 mmHg on arrival |
| Sustained VF or pVT before ECPR |
CPR, conventional guideline-directed cardiopulmonary resuscitation; ECPR, extracorporeal cardiopulmonary resuscitation; PaO₂, arterial partial pressure of oxygen; PaCO₂, arterial partial pressure of carbon dioxide; VF, ventricular fibrillation; pVT, pulseless ventricular tachycardia.
Although there is high mortality in patients with refractory OHCA, the use of ECMO should not be withheld due to concerns of cost if the cost-effectiveness of ECPR has been demonstrated. A Markov model was developed using the data from two ECMO centers in Sydney, Australia in order to evaluate the cost-effectiveness of ECPR. Based upon the modeling from this data, ECPR for IHCA and OHCA was found to be cost-effective in terms of cost per QALY for Australia, Europe, and the United States.41
The ARREST trial, a phase 2, single center, open label randomized, controlled study of ECMO-facilitated resuscitation in patients suffering out-of-hospital cardiac arrest with refractory ventricular fibrillation was reported in Lancet during 2020.42 This is the first randomized controlled trial for ECPR completed with human participants. Thirty patients were enrolled over an 11-month period (August 2019 to June 2020) with 15 receiving CCPR and 15 receiving early ECMO-facilitated resuscitation, from which there was one patient withdrawal from the study prior to hospital discharge. Patients randomized to ECMO-facilitated resuscitation were transported with ongoing mechanical chest compressions to the catheterization lab at the University of Minnesota. ECMO cannulae were placed in the catheterization lab where ECMO organ and circulatory support was provided and coronary angiography ± PCI were performed. Survival to hospital discharge was achieved in 1/15 (7%) in the control CCPR group versus 6/14 (43%) in the ECPR group. Cumulative survival was significantly greater with ECPR (p < 0.001). The authors noted that the ARREST trial confirms that CCPR alone results in a dismal outcome rate in patients with refractory ventricular fibrillation. However, they believe such positive results are a reflection of the highly orchestrated collaboration and coordinated approach used in this study, including highly specialized and committed intensive care protocols for the lengthy intensive care such patients need.
Pre-hospital ECMO
Using mobile intensive care units in Paris, ECPR has been started in the pre-hospital setting.43 Serial observational data have demonstrated improved survival when aggressive selection criteria are followed, epinephrine is limited to <5 mg, and there is immediate search for the etiology of the cardiac arrest. This formal approach resulted in improving survival from 3% to 38% over time.
Post-ECPR care
All ECPR patients will have some degree of multi-organ dysfunction or even failure.44 Bartos et al., demonstrated that a committed team approach for post-ECPR intensive care was crucial to achieve optimal survival.44 This includes many of the interventional and surgical subspecialities. Each must be willing to take such patients for interventional procedures and/or surgery for a variety of reasons: cardiac, vascular, tracheostomy/feeding tubes, dialysis catheters, bronchoscopy, and endoscopy.
Since massive pulmonary thromboembolism (PTE) may be another possible underlying cause of arrest, patients without identifiable coronary occlusion may also have pulmonary CT angiography performed after ECMO implantation. If massive PTE is diagnosed, catheter thrombectomy ± thrombolysis, or even surgical embolectomy, is indicated.
Furthermore, CT of the head to exclude intracranial causes of arrest such as subarachnoid hemmorhage (SAH) should also be included in the immediate post-ECPR protocol. This examination is also useful because a "catastrophic finding" due to prolonged CPR such as severe cerebral edema with herniation may lead to the termination of active treatment and the consideration of organ transplantation.
Hemorrhage is a common complication when using ECMO for cardiac support in adults, usually occurring at the site of recent surgery or at cannulation sites, but also occurring within the gastrointestinal tract and central nervous system (CNS).[6], 8, 45, 46 In a retrospective analysis, patients undergoing VA-ECMO required a median transfusion of 21 red blood cell (RBC) units, 7 units of fresh frozen plasma (FFP), and 3 units of platelets.45 In this analysis, the presence of a serious bleeding event was not significantly associated with mortality but the amount of RBC transfusion did correlate with worse outcomes.45 The transfusion rates of this study were comparable with the transfusion data of a systematic review which reported median transfusions of 17 RBC units, 15 FFP units, and 5 platelet units in VA-ECMO.47 Sy et al. examined the relationship between anticoagulation targets and the prevalence of bleeding or thromboembolic events, and identified the rates of major bleeding events to be 27% and major thromboembolic events to be 8%, however they were unable to suggest which method of anticoagulation should be used or what the target ranges should be due to low quality of evidence and heterogeneity between studies.47
Limb ischemia is another possible complication of VA-ECMO. In femorally placed cannulae, the arterial return cannula can significantly occlude the femoral artery, decreasing arterial flow to the distal extremity and resulting in ischemia. This issue can be overcome through the placement of a separate perfusion line into the superficial femoral artery distal to the return cannula for antegrade perfusion, or into the posterior tibial artery for retrograde perfusion.6 A meta-analysis by Juo et al. found that patients with a distal perfusion cannula in place had a significantly lower risk of limb ischemia when compared to patients that did not.48
VA-ECMO support increases systemic afterload via the retrograde aortic flow from the arterial return line. The increase in afterload can lead to elevated end-diastolic pressure in the left ventricle (LV) and left atrium (LA), resulting in increased wall stress and myocardial oxygen consumption as well as the risk of developing pulmonary edema.49 While this may not be of concern during the initial resuscitation efforts of ECPR, it can quickly become a complicating issue following the return of an organized cardiac rhythm. To address this, there have been multiple techniques developed to reduce pressure within the LV and/or LA including surgical insertion of catheters into the pulmonary vein or LV apex, or a percutaneous approach with transaortic or transseptal catheterization.50 The use of LV unloading for patients on VA-ECMO for cardiogenic shock has been associated with decreased mortality,51 however there still needs to be studies performed to examine the use of LV unloading in ECPR patients.
Additional adverse events that have been documented to occur during the use of ECMO for cardiac support include infection, renal failure, hyperbilirubinemia, CNS infarction, and mechanical issues of the pump or oxygenator.8
Use of ECPR in COVID-19 patients
In December 2019 an outbreak of viral pneumonia began in China that was found to be caused by a novel coronavirus, now named Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2), with the associated clinical disease referred to as Coronavirus Disease 2019 (COVID-19).52, 53, 54, 55, 56, 57 Since the initial outbreak, COVID-19 has grown into a global pandemic, and as more people have been infected, more details surrounding the illness have been established. Among patients infected with COVID-19, the most common comorbidities have been hypertension, diabetes, and coronary heart disease.52, 53, 54 Coronary heart disease, hypertension, and diabetes have also been identified as significant risk factors for in-hospital death due to COVID-19,53 and a nationwide retrospective analysis in China found that among fatal cases of COVID-19, 56% of patients had hypertension, 26% had diabetes, and 16% had coronary heart disease.56 SARS-CoV-2 has been found to cause a variety of symptoms throughout the body including the cardiovascular system. One study reported cardiac arrhythmias in 16.7% of COVID-19 patients,54 and myocardial injury has been reported in 7.2%–27.8% of COVID-19 patients.54, 57, 58, 59 The cardiovascular effects of COVID-19 continue to be an area of active study, however one aspect of myocardial damage may be due direct viral injury of the myocardium where angiotensin-converting enzyme 2 (ACE2) receptors, the binding site of SARS-CoV-2, have been shown to have relatively high expression.60 A recently published case report describes a patient with confirmed COVID-19 that was placed on VA-ECMO for cardiogenic shock, and underwent endomyocardial biopsy which showed myocardial inflammation on light microscopy and coronavirus particles on electron micrograph.61
A single-center retrospective analysis examined the outcomes of COVID-19 patients in China that suffered IHCA, and found that 87.5% of cases had a respiratory etiology, 94.1% had a nonshockable initial rhythm, ROSC was achieved in 13.2% of patients, 30-day survival rate was 2.9%, and only one patient survived with a favorable neurologic outcome.62 When considering the potential use of ECPR in a COVID-19 patient, issues regarding risk of transmission to staff, timeliness and efficacy of intervention while resuscitation teams are in extensive personal protective equipment (PPE), and the use of resources that may be limited in the setting of a global pandemic must be factored into the evaluation of risks versus benefits.[63], 64 As of September 14, 2020 the use of ECPR only comprised 1% of the ECMO support being used in COVID-19 patients as recorded by the Extracorporeal Life Support Organization (ELSO) Registry.65 The ELSO issued interim guidelines regarding the use of ECPR during the COVID-19 pandemic, stating “At experienced centers, E-CPR may be considered for highly selected non-COVID-19 patients with in-hospital cardiac arrest depending on resource availability.”64 The ELSO guidelines also cautioned the need for careful evaluation of risk-to-benefit ratios before performing ECPR in COVID-19 patients, citing poor outcomes with CCPR in COVID-19 patients, potential contamination of staff, and the use of possibly limited PPE.64
After ECMO
The use of ECMO in patients suffering from refractory cardiac arrest can be an effective bridge to definitive therapy, but is not a treatment on its own. Following the stabilization of a patient on ECMO, the underlying etiology of the cardiac arrest must be corrected. If occlusive coronary artery disease is suspected, the patient should be emergently taken to the cardiac catheterization lab for coronary angiography with percutaneous coronary intervention as indicated. For non-cardiac etiologies, such as dyskalemia or poisoning, the underlying cause should be addressed. As native cardiac function recovers, the level of support provided by ECMO can be gradually weaned down. Cardiac function should be evaluated with echocardiography during a trial off of ECMO support prior to the complete discontinuation of ECMO followed by decannulation6. Patients that do not demonstrate evidence of cardiac recovery after 3–5 days of ECMO support should be considered for ventricular assist device (VAD) placement or heart transplant.6 The requirements of specialized equipment, experienced providers, and potentially challenging disposition means that ECPR is not presently an option for every hospital, but is restricted to the hospitals that can both effectively perform it and manage the patients that are supported by it. Table 3 lists outcomes of patients that underwent ECPR included in this review.
Table 3.
OS: Observational Study. MA: Meta-Analysis. CS: Controlled Study. IHCA: In-Hospital Cardiac Arrest. OHCA: Out-of-Hospital Cardiac Arrest.
| Outcomes of extracorporeal cardiopulmonary resuscitation | |||||||
|---|---|---|---|---|---|---|---|
| Author | Year | Type | IHCA or OHCA | ECPR Sample size | Decannulation rate | Survival to hospital discharge | Survival to hospital discharge with good neurologic outcome |
| Massetti et al.10 | 2005 | OS | Mixed | 40 | – | 8/40 (20.0%) | – |
| Maekawa35 | 2013 | OS | OHCA | 53 | – | 17/53 (32.1%) | 8/53 (15.1%) |
| Stub et al.12 | 2014 | OS | Mixed | 24 | 13/24 (54.2%) | 12/24 (50.0%) | 12/24 (50.0%) |
| Ryu et al.16 | 2015 | OS | Mixed | 227 | – | – | 68/227 (30.0%) |
| Siao et al.9 | 2015 | OS | Mixed | 20 | – | 10/20 (50.0%) | 8/20 (40.0%) |
| Anselmi et al.13 | 2015 | OS | Mixed | 49 | 21/49 (42.9%) | 18/49 (36.7%) | – |
| Choi et al.33 | 2016 | OS | OHCA | 320 | – | 57/320 (17.8%) | 29/320 (9.1%) |
| Yannopoulos et al.15 | 2016 | OS | OHCA | 18 | – | 10/18 (56.0%) | 9/18 (50.0%) |
| Ahn et al.24 | 2016 | MA | Mixed | 872 | – | – | – |
| Thiagarajan8 | 2017 | OS | Mixed | 2,885 | 1,137 (39.4%) | 848/2,885 (29.4%) | – |
| Debaty et al.36 | 2017 | MA | Mixed | 841 | – | – | 125/841 (15.0%) |
| Gil et al.26 | 2017 | OS | IHCA | 200 | 86/200 (43.0%) | 62/200 (31.0%) | 52/200 (26.0%) |
| Lamhaut et al.43 | 2017 | OS | OHCA | 156 | – | – | 21/156 (13.5%) |
| Wengenmayer et al.32 | 2017 | OS | Mixed | 133 | – | 19/133 (14.3%) | – |
| Yukawa et al.17 | 2017 | OS | OHCA | 79 | – | 17/79 (21.5%) | 11/79 (13.9%) |
| Haas et al.34 | 2017 | OS | OHCA | 217 | – | 60/217 (27.6%) | – |
| D’Arrigo et al.21 | 2017 | MA | IHCA | 856 | – | 324/856 (37.9%) | 222/635 (35.0%) |
| Goto et al.37 | 2018 | OS | OHCA | 144 | – | 10/144 (6.9%) | 28/144 (19.4%) |
| Patel et al.30 | 2018 | OS | OHCA | 3650 | – | 39.8% | – |
| Min et al.11 | 2018 | OS | IHCAa | 23 | 7/23 (30.4%) | 7/23(30.4%) | 6/23 (26.1%) |
| Bartos et al.44 | 2018 | OS | OHCA | 68 | – | 31/68 (45.6%) | – |
| Komeyama et al.18 | 2019 | OS | Mixed | 67 | – | – | 20/67 (29.9%) |
| Nakashima et al.40 | 2019 | OS | OHCA | 250 | – | 55/248 (22.8%) | 29/248 (11.7%) |
| Lunz et al.25 | 2020 | OS | Mixed | 423 | – | 102/423 (24.1%) | 88/423 (20.8%) |
| Bartos et al.38 | 2020 | OS | OHCA | 133 | – | – | 52/133 (39.1%) |
| Tonna et al.19 | 2020 | OS | IHCA | 1082 | – | – | – |
| Okada et al.39 | 2020 | OS | OHCA | 260 | – | – | 41/260 (15.8%) |
| Hadaya et al.4 | 2020 | OS | Mixed | 4,348 | – | 1,538/4,348 (35.4%) | – |
| Bougouin et al.31 | 2020 | OS | OHCA | 525 | – | 44/525 (8.4%) | 32/519 (6.2%) |
| Yannopoulos et al.42 | 2020 | CS | OHCA | 15 | – | 6/14 (42.9%) | 6/14 (42.9%) |
This study analyzed the use of ECPR for intraoperative refractory cardiac arrest.
Conclusions
The use of VA-ECMO for the resuscitation of refractory IHCA and OHCA is presently low but is steadily increasing, bringing new data on the outcomes of these ECPR patients. As of 2019 there was insufficient evidence to recommend the routine use of ECPR, however as additional trials are performed this may change in the future. For IHCA patients, the use of ECPR is associated with better survival and neurologic outcomes when compared against standard CPR, and the use of this therapy has been determined to be cost-effective based upon cost per QALY. For OHCA patients, survival rates vary greatly due in part to the lack of standardized inclusion criteria, however survival rates as high as 53% have been reported. This points to the need to establish clear indications and contraindications for the use of ECPR in OHCA. The use of ECPR for OHCA was also determined to be cost-effective based upon cost per QALY. Initial shockable rhythm, shorter low-flow time prior to the initiation of ECMO, and lower serum lactate levels were associated with favorable outcomes in both IHCA and OHCA. Interestingly, an observational study reported that patients with sustained VF/pVT who underwent ECPR had a significantly higher rate of favorable neurologic outcome, but found no neurologic benefit from ECPR for OHCA in patients that presented with an initial shockable rhythm which then converted to PEA/asystole prior to the initiation of ECMO.40 This may reflect progression of ischemic damage, and reinforces the time-sensitive nature of resuscitation. In addition to the lack of well-defined inclusion and exclusion criteria, the use of VA-ECMO for resuscitation faces a number of potential complications including hemorrhage, limb ischemia, pulmonary edema, and mechanical issues of the ECMO circuit. However, innovative solutions such as placement of a distal perfusion cannula and the various LV unloading techniques can overcome some of these obstacles. Additional studies still need to be done to evaluate the routine use of these techniques, and to identify the optimal strategies for maintaining appropriate anticoagulation while minimizing the risk of hemorrhage. Overall, the use of ECPR for the treatment of refractory cardiac arrest in both the in-hospital and out-of-hospital setting appears promising, but is highly dependent on careful patient selection. Continued research into which patients will benefit most from ECPR may eventually lead to its inclusion in routine treatment guidelines.
Future research considerations
There was no identified literature focusing on the outcomes of patients with a BMI ≥ 40 kg/m2 who undergo ECPR for IHCA or OHCA. Studies examining the outcomes of ECPR for patients ≥70 years of age should be done to better evaluate how age should be incorporated into the eligibility criteria of ECPR protocols. There is heterogeneity in studies regarding the optimal anticoagulation strategies for patients undergoing VA-ECMO, and randomized controlled trials should be done to determine the best option. Randomized controlled trials should be done to evaluate the routine use of a distal perfusion cannula in VA-ECMO. Despite the existing evidence to support LV unloading in cardiogenic shock patients, there is a lack of data examining the outcomes of LV unloading in ECPR patients.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Dr. Kern is a consultant and speaker for LivaNova, a manufacteur of an ECMO system. Mr. Klee has no conflicts of interest regarding this manuscript.
CRediT authorship contribution statement
Tyler E. Klee: Conceptualization, Methodology, Investigation, Validation, Writing - original draft, Writing - review & editing, Visualization. Karl B. Kern: Conceptualization, Methodology, Validation, Writing - review & editing, Supervision.
Acknowledgements
None.
References
- 1.Cummins R.O., Chamberlain D.A., Abramson N.S. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the utstein style. A statement for health professionals from a task force of the American heart association, the European resuscitation council, the heart and stroke foundation of Canada, and the Australian resuscitation council. Circulation (New York, N.Y.). 1991;84(2):960–975. doi: 10.1161/01.cir.84.2.960. https://search.datacite.org/works/10.1161/01.cir.84.2.960 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart disease and stroke Statistics—2017 update: a report from the American heart association. Circulation (New York, N.Y.). 2017;135(10):e146–e603. doi: 10.1161/cir.0000000000000485. https://search.datacite.org/works/10.1161/cir.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer DJ, Weingart S, Strayer RJ. Resuscitation and Stabilization. In: Oropello JM, Pastores SM, Kvetan V. Critical Care. McGraw-Hill; Accessed June 07, 2020. https://accessmedicine-mhmedical-com.ezproxy2.library.arizona.edu/content.aspx?bookid=1944§ionid=143515474.
- 4.Hadaya J., Dobaria V., Aguayo E. National trends in utilization and outcomes of extracorporeal support for in- and out-of-hospital cardiac arrest. Resuscitation. 2020;151:181–188. doi: 10.1016/j.resuscitation.2020.02.034. doi: 10.1016/j.resuscitation.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Panchal A., Berg K., Hirsch K. 2019 American heart association focused update on advanced cardiovascular life support: use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: an update to the american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2019;140(24):e881–e894. doi: 10.1161/CIR.0000000000000732. https://search.proquest.com/docview/2314568917 [DOI] [PubMed] [Google Scholar]
- 6.Extracorporeal Life Support Organization . Extracorporeal Life Support Organization (Ann Arbor, MI); 2017. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support.https://www.elso.org/Portals/0/ELSO%20Guidelines%20General%20All%20ECLS%20Version%201_4.pdf Version 1.4. [Google Scholar]
- 7.Broman L.M., Taccone F.S., Lorusso R. The ELSO Maastricht treaty for ECLS nomenclature: abbrevations for cannulation configuration in extracorpeal life support – a position paper of the Extracorporeal Life Support Organization. Critical Care. 2019;23:26. doi: 10.1186/s13054-019-2334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiagarajan R.R., Barbaro R.P., Rycus P.T. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63(1):60–67. doi: 10.1097/MAT.0000000000000475. https://www.ncbi.nlm.nih.gov/pubmed/27984321 [DOI] [PubMed] [Google Scholar]
- 9.Siao F., Chiu C., Chiu C. Managing cardiac arrest with refractory ventricular fibrillation in the emergency department: conventional cardiopulmonary resuscitation versus extracorporeal cardiopulmonary resuscitation. Resuscitation. 2015;92:70–76. doi: 10.1016/j.resuscitation.2015.04.016. https://search.datacite.org/works/10.1016/j.resuscitation.2015.04.016 [DOI] [PubMed] [Google Scholar]
- 10.Massetti M., Tasle M., Le Page O. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thoracic Surg. 2005;79(1):178. doi: 10.1016/j.athoracsur.2004.06.095. http://ats.ctsnetjournals.org/cgi/content/abstract/79/1/178 [DOI] [PubMed] [Google Scholar]
- 11.Min J.J., Tay C.K., Ryu D.K. Extracorporeal cardiopulmonary resuscitation in refractory intra-operative cardiac arrest: An observational study of 12-year outcomes in a single tertiary hospital. Anaesthesia. 2018;73(12):1515–1523. doi: 10.1111/anae.14412. https://onlinelibrary.wiley.com/doi/abs/10.1111/anae.14412 [DOI] [PubMed] [Google Scholar]
- 12.Stub D., Bernard S., Pellegrino V. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2014;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. https://www.clinicalkey.es/playcontent/1-s2.0-S0300957214007515 [DOI] [PubMed] [Google Scholar]
- 13.Anselmi Amedeo, Flécher Erwan, Corbineau H. Survival and quality of life after extracorporeal life support for refractory cardiac arrest: a case series. J Thoracic Cardiovasc Surg. 2015;150(4):947–954. doi: 10.1016/j.jtcvs.2015.05.070. https://www.clinicalkey.es/playcontent/1-s2.0-S0022522315008843 [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka Y., Ikenoue T., Hata N. Hospitals’ extracorporeal cardiopulmonary resuscitation capabilities and outcomes in out-of-hospital cardiac arrest: a population-based study. Resuscitation. 2019;136:85–92. doi: 10.1016/j.resuscitation.2019.01.013. doi: 10.1016/j.resuscitation.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Yannopoulos D., Bartos J.A., Martin C. Minnesota resuscitation consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5(6):n/a. doi: 10.1161/jaha.116.003732. https://search.datacite.org/works/10.1161/jaha.116.003732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu J., Cho Y.H., Sung K. Predictors of neurological outcomes after successful extracorporeal cardiopulmonary resuscitation. BMC Anesthesiol. 2015;15(1):26. doi: 10.1186/s12871-015-0002-3. https://search.datacite.org/works/10.1186/s12871-015-0002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yukawa T., Kashiura M., Sugiyama K., Tanabe T., Hamabe Y. Neurological outcomes and duration from cardiac arrest to the initiation of extracorporeal membrane oxygenation in patients with out-of-hospital cardiac arrest: q retrospective study. Scand J Trauma Resuscitation Emergency Med. 2017;25(1):95. doi: 10.1186/s13049-017-0440-7. https://www.ncbi.nlm.nih.gov/pubmed/28915913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komeyama S., Takagi K., Tsuboi H. The early initiation of extracorporeal life support may improve the neurological outcome in adults with cardiac arrest due to cardiac events. Internal Med. 2019;58(10):1391–1397. doi: 10.2169/internalmedicine.0864-18. https://jlc.jst.go.jp/DN/JLC/92000265558?from=SUMMON [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonna Je, Selzman Ch, Girotra S. Patient and institutional characteristics influence the decision to use extracorporeal cardiopulmonary resuscitation for in-hospital cardiac arrest. Journal of the American Heart Association. 2020;9(9):e015522. doi: 10.1161/JAHA.119.015522. https://www.ncbi.nlm.nih.gov/pubmed/32347147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swol J., Belohlavek J., Haft J.W., Ichiba S., Lorusso R., Peek G.J. Conditions and procedures for in-hospital extracorporeal life support (ECLS) in cardiopulmonary resuscitation (CPR) of adult patients. Perfusion. 2016;31:182–188. doi: 10.1177/0267659115591622. [DOI] [PubMed] [Google Scholar]
- 21.D’Arrigo S., Cacciola S., Dennis M. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Peberdy M.A., Kaye W., Ornato J.P. Cardiopulmonary resuscitation of adults in the hospital: a report of 14 720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. https://search.datacite.org/works/10.1016/s0300-9572(03)00215-6 [DOI] [PubMed] [Google Scholar]
- 23.Girotra S., Nallamothu B.K., Spertus J.A., Li Y., Krumholz H.M., Chan P.S. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–1920. doi: 10.1056/nejmoa1109148. https://search.datacite.org/works/10.1056/nejmoa1109148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn C., Kim W., Cho Y., Choi K., Jang B., Lim T.H. Efficacy of extracorporeal cardiopulmonary resuscitation compared to conventional cardiopulmonary resuscitation for adult cardiac arrest patients: a systematic review and meta-analysis. Sci Rep. 2016;6(1):34208. doi: 10.1038/srep34208. https://www.ncbi.nlm.nih.gov/pubmed/27659306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunz D., Calabrò L., Belliato M. Extracorporeal membrane oxygenation for refractory cardiac arrest: a retrospective multicenter study. Intensive Care Med. 2020;46(5):973–982. doi: 10.1007/s00134-020-05926-6. https://www.ncbi.nlm.nih.gov/pubmed/32052069 [DOI] [PubMed] [Google Scholar]
- 26.Gil E., Na S.J., Ryu J. Association of body mass index with clinical outcomes for in-hospital cardiac arrest adult patients following extracorporeal cardiopulmonary resuscitation. PloS One. 2017;12(4):e0176143. doi: 10.1371/journal.pone.0176143. https://www.ncbi.nlm.nih.gov/pubmed/28423065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahreyar M., Dang G., Waqas Bashir M. Outcomes of in-hospital cardiopulmonary resuscitation in morbidly obese patients. JACC Clin Electrophysiol. 2017;3(2):174–183. doi: 10.1016/j.jacep.2016.08.011. https://search.datacite.org/works/10.1016/j.jacep.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 28.Secombe P.J., Sutherland R., Johnson R. Morbid obesity impairs adequacy of thoracic compressions in a simulation-based model. Anaesth Intensive Care. 2018;46(2):171–177. doi: 10.1177/0310057X1804600205. https://search.informit.org/documentSummary;dn=353137776101993;res=IELHEA [DOI] [PubMed] [Google Scholar]
- 29.Gravesteijn B.Y., Schluep M., Voormolen D.C. Cost-effectiveness of extracorporeal cardiopulmonary resuscitation after in-hospital cardiac arrest: a Markov decision model. Resuscitation. 2019;143:150–157. doi: 10.1016/j.resuscitation.2019.08.024. doi: 10.1016/j.resuscitation.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Patel N.J., Patel N.J., Bhardwaj B. Trends in utilization of mechanical circulatory support in patients hospitalized after out-of-hospital cardiac arrest. Resuscitation. 2018;127:105–113. doi: 10.1016/j.resuscitation.2018.04.007. https://search.datacite.org/works/10.1016/j.resuscitation.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 31.Bougouin W., Dumas F., Lamhaut L. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J. 2020;41(21):1961–1971. doi: 10.1093/eurheartj/ehz753. https://search.proquest.com/docview/2310716456 [DOI] [PubMed] [Google Scholar]
- 32.Wengenmayer T., Rombach S., Ramshorn F. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR) Crit Care (London, England) 2017;21(1):157. doi: 10.1186/s13054-017-1744-8. https://www.ncbi.nlm.nih.gov/pubmed/28637497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi D.S., Kim T., Ro Y.S. Extracorporeal life support and survival after out-of-hospital cardiac arrest in a nationwide registry: a propensity score-matched analysis. Resuscitation. 2016;99:26–32. doi: 10.1016/j.resuscitation.2015.11.013. https://search.datacite.org/works/10.1016/j.resuscitation.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 34.Haas N.L., Coute R.A., Hsu C.H., Cranford J.A., Neumar R.W. Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out-of-hospital cardiac arrest—An ELSO registry study. Resuscitation. 2017;119:56–62. doi: 10.1016/j.resuscitation.2017.08.003. doi: 10.1016/j.resuscitation.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maekawa K., Tanno K., Hase M., Mori K., Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013;41(5):1186–1196. doi: 10.1097/CCM.0b013e31827ca4c8. https://www.ncbi.nlm.nih.gov/pubmed/23388518 [DOI] [PubMed] [Google Scholar]
- 36.Debaty G., Babaz V., Durand M. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. doi: 10.1016/j.resuscitation.2016.12.011. https://search.datacite.org/works/10.1016/j.resuscitation.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 37.Goto T., Morita S., Kitamura T. Impact of extracorporeal cardiopulmonary resuscitation on outcomes of elderly patients who had out-of-hospital cardiac arrests: a single-centre retrospective analysis. BMJ Open. 2018;8(5):e019811. doi: 10.1136/bmjopen-2017-019811. doi: 10.1136/bmjopen-2017-019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartos J., Grunau B., Carlson C. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation. 2020;141(11):877–886. doi: 10.1161/CIRCULATIONAHA.119.042173. https://www.ncbi.nlm.nih.gov/pubmed/31896278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada Y., Kiguchi T., Irisawa T. Association between low pH and unfavorable neurological outcome among out-of-hospital cardiac arrest patients treated by extracorporeal CPR: A prospective observational cohort study in japan. J Intensive Care. 2020;8(1):1–9. doi: 10.1186/s40560-020-00451-6. https://search.proquest.com/docview/2404393108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakashima T., Noguchi T., Tahara Y. Patients with refractory out-of-cardiac arrest and sustained ventricular fibrillation as candidates for extracorporeal cardiopulmonary resuscitation ― prospective multi-center observational study. Circ J. 2019;83(5):1011–1018. doi: 10.1253/circj.cj-18-1257. https://search.datacite.org/works/10.1253/circj.cj-18-1257 [DOI] [PubMed] [Google Scholar]
- 41.Dennis M., Zmudzki F., Burns B. Cost effectiveness and quality of life analysis of extracorporeal cardiopulmonary resuscitation (ECPR) for refractory cardiac arrest. Resuscitation. 2019;139:49–56. doi: 10.1016/j.resuscitation.2019.03.021. https://search.datacite.org/works/10.1016/j.resuscitation.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 42.Yannopoulos D., Bartos J., Raveendran G. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomized controlled trial. Lancet. 2020;396:1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamhut L., Hutin A., Puymirat E. A pre-hospital extracorporeal cardio pulmonar resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: An observational study and propensity analysis. Resuscitation. 2017;117:109–117. doi: 10.1016/j.resuscitation.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Bartos J.A., Carlson K., Carlson C. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. doi: 10.1016/j.resuscitation.2018.08.030. https://search.datacite.org/works/10.1016/j.resuscitation.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 45.Mazzeffi Michael, Greenwood J., Tanaka Kenichi. Bleeding, transfusion, and mortality on extracorporeal life support: ECLS working group on thrombosis and hemostasis. Ann Thoracic Surg. 2016;101(2):682–689. doi: 10.1016/j.athoracsur.2015.07.046. https://www.clinicalkey.es/playcontent/1-s2.0-S0003497515012503 [DOI] [PubMed] [Google Scholar]
- 46.Swol J., Belohlavek J., Brodie D. Extracorporeal life support in the emergency department: a narrative review for the emergency physician. Resuscitation. 2018;133:108–117. doi: 10.1016/j.resuscitation.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Sy E., Sklar M.C., Lequier L., Fan E., Kanji H.D. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care. 2017;39:87–96. doi: 10.1016/j.jcrc.2017.02.014. https://search.datacite.org/works/10.1016/j.jcrc.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 48.Juo Y., Skancke M., Sanaiha Y., Mantha A., Jimenez J.C., Benharash P. Efficacy of distal perfusion cannulae in preventing limb ischemia during extracorporeal membrane oxygenation: a systematic review and meta-analysis. Artif Organs. 2017;41(11):E263–E273. doi: 10.1111/aor.12942. https://search.datacite.org/works/10.1111/aor.12942 [DOI] [PubMed] [Google Scholar]
- 49.Fuhrman B.P., Hernan L.J., Rotta A.T., Heard C.M.B., Rosenkranz E.R. Pathophysiology of cardiac extracorporeal membrane oxygenation. Artif Organs. 1999;23(11):966–969. doi: 10.1046/j.1525-1594.1999.06484.x. https://search.datacite.org/works/10.1046/j.1525-1594.1999.06484.x [DOI] [PubMed] [Google Scholar]
- 50.Xie A., Forrest P., Loforte A. Left ventricular decompression in veno-arterial extracorporeal membrane oxygenation. Ann Cardiothorac Surg. 2019;8(1):9–18. doi: 10.21037/acs.2018.11.07. https://search.datacite.org/works/10.21037/acs.2018.11.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russo J.J., Aleksova N., Pitcher I. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am College Cardiol. 2019;73(6):654–662. doi: 10.1016/j.jacc.2018.10.085. https://search.datacite.org/works/10.1016/j.jacc.2018.10.085 [DOI] [PubMed] [Google Scholar]
- 52.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382(18):1708–1720. doi: 10.1056/nejmoa2002032. https://search.datacite.org/works/10.1056/nejmoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (British edition). 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. https://search.datacite.org/works/10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (British edition). 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. https://search.datacite.org/works/10.1016/s0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen R., Liang W., Jiang M. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. https://search.datacite.org/works/10.1016/s2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. https://search.datacite.org/works/10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tavazzi G., Pellegrini C., Maurelli M. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. https://onlinelibrary.wiley.com/doi/abs/10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shao F., Xu S., Ma X. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kandori K., Narumiya H., Iizuka R. Extracorporeal cardiopulmonary resuscitation should not be performed on confirmed or suspected COVID-19 patients. Resuscitation. 2020;153:6–7. doi: 10.1016/j.resuscitation.2020.05.040. doi: 10.1016/j.resuscitation.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shekar K., Badulak J., Peek G. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66(7):707–721. doi: 10.1097/MAT.0000000000001193. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00002480-202007000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The Extracorporeal Life Support Organization (ELSO): ECMO in COVID-19. Available at: https://www.elso.org/registry/FullCOVID19RegistryDashboard.aspx. Accessed September 14, 2020.