Abstract
Background
Newly-developed suction-based airway clearance devices potentially provide a novel way to improve outcome in patients with foreign body airway obstruction. We conducted a randomised controlled crossover manikin trial to compare the efficacy and usability of two of these devices with abdominal thrusts.
Methods
We randomised participants from a UK medical school to one of six groups which determined the order in which participants attempted the three techniques (abdominal thrusts; LifeVac, Nesconset, New York, USA; Dechoker, Concord North Carolina, USA). Randomisation was performed using an online randomisation system. Following brief training, participants sought to remove a foreign body airway obstruction from a manikin using the allocated technique. The primary outcome was successful removal of the foreign body. Usability was assessed in a questionnaire following the three simulations.
Results
We randomised and analysed data from 90 participants (58% male; 86% aged 18−29 years). Compared with abdominal thrusts, successful foreign body airway obstruction removal was achieved more frequently in manikins in the LifeVac group (odds ratio 47.32, 95% CI 5.75–389.40) but not in the Dechoker group (odds ratio 1.22, 95% CI 0.60–2.47). The usability of LifeVac and abdominal thrusts were generally evaluated more positively than the Dechoker.
Conclusion
In this manikin study, we found that, compared with abdominal thrusts, the success rate for foreign body airway obstruction removal was higher in the LifeVac group but not in the Dechoker group.
Keywords: Airway obstruction, Choking, Basic life support, Anti-choking device, Randomised controlled trial, Simulation
Introduction
Foreign body airway obstruction (FBAO) is an important cause of mortality and morbidity, particularly in the very young and old.1, 2, 3 Each year, FBAO is responsible for almost 2,000 ambulance calls in London and approximately 250 UK deaths.1, 3
Current treatment for FBAO is based on a step-wise approach, that incorporates techniques including coughing, back blows, abdominal thrusts, and chest thrusts/compressions.4 Abdominal thrusts are reserved for severe cases of FBAO that are not relieved by back blows, due to associated risk of thoracic, vascular and gastro-oesophageal injury.5 Evidence supporting specific interventions is limited, such that current treatment recommendations are based predominantly on case series and expert opinion.5, 6
The risks associated with current treatments for FBAO have driven interest in alternative strategies for FBAO removal. In recent years, new suction-based airway clearance devices have been developed in which manual suction is applied via a face mask to relieve FBAO. A recent systematic review of these devices identified published data for only one device.7 Available studies for this device were limited to manikin studies, cadaver studies, and clinical case series. Based on the limited data published to date, the International Liaison Committee on Resuscitation has decided that it would be premature to make a recommendation for or against the use of devices, and highlighted the urgent need for further research.6
To date, no study has compared these devices with standard care.7 The efficacy and usability of new devices, in comparison with standard care, are important factors in determining whether a medical device should be adopted in practice. In view of the current absence of evidence in relation to this important issue, we identified the specific need for research in this area.
Methods
We conducted an open-label, randomised controlled crossover manikin trial to compare the efficacy and usability of two suction-based airway clearance devices (LifeVac, Nesconset, New York, USA; Dechoker, Concord, North Carolina, USA) with the abdominal thrust.
The LifeVac comprises a facemask attached to compressible bellows. To use the device, the mask is held over the choking patient’s mouth and nose, and then the handle of the bellows is pressed downwards and sharply pulled upwards.8 The Dechoker comprises a facemask attached to an oropharyngeal tube attached to a large cylinder with a plunger. To generate negative pressure, the plunger is pulled backwards sharply.9 Both devices are promoted as being straightforward to use.10, 11
The trial protocol was finalised before the start of the study. The study was reviewed and approved by the University of Warwick Biomedical & Scientific Research Ethics Committee (reference 108/18–19). Written informed consent was obtained from all participants. No changes were made to the trial protocol following commencement.
Setting and participants
The study was conducted in the Medical School at the University of Warwick. We included university staff and students that could communicate in English and who provided written informed consent to participate. We excluded individuals who had a physical disability that precluded use of the devices.
Randomisation
Following confirmation of eligibility and provision of written informed consent we randomised participants in an equal ratio to one of six groups that determined the order in which they completed the three interventions. Details of the groups and corresponding order are included in figure one and the electronic Supplement (Table S1). The randomisation sequence was developed using an online system using a fixed block size of six by a researcher that was not involved in participant recruitment.12 For randomisation, we used an online randomisation system to maintain allocation concealment.13 Following randomisation, participants were informed only of the intervention that they would be requested to complete next in the sequence.
Interventions and study process
The researcher showed the participant a short information video on how to deliver the first intervention. For the LifeVac and Dechoker, we extracted key information from manufacturer training videos freely available on the internet.10, 11 For abdominal thrusts, we extracted information from a video on foreign body airway obstruction developed by a UK first aid charity.14 Participants were not given the opportunity to handle the device or practice any technique prior to the simulated scenario.
For the scenario, participants were informed that a 25-year old male was eating steak at a restaurant when they suddenly began to cough and pointing to their throat. Back slaps had been attempted, but these were ineffective. For the patient, we used a manikin (Choking Charlie, Laerdal Medical AS, Stavanger, Norway) with a simulated food bolus sited in the manikin’s throat, as per manufacturer instructions. The participant was then to perform the allocated intervention. To ensure consistency across interventions, participants were permitted only to use the allocated intervention. Participants were given up to four-minutes to remove the obstruction.
After the first scenario, we adopted the same procedure for subsequent interventions. There was no break between attempting interventions. Following scenario three, participants completed a questionnaire on device usability. It was not possible to blind either the research participant or outcome assessor to treatment allocation.
Outcomes
The primary study outcome was successful removal of the foreign body airway obstruction within four-minutes. This was defined as the removal of the simulated food bolus from the manikin’s mouth. The four-minute period was timed by a single researcher with a stopwatch.
The secondary efficacy outcome was time to FBAO removal. A single researcher present during the scenario measured the time in seconds from the start of the scenario to the point that the FBAO exited the manikin’s mouth using a stopwatch. Secondary usability outcomes were captured in a survey completed at the end of the three scenarios. For each device, participants were asked to rank five statements on a scale of 1 (strongly disagree) to 10 (strongly agree). These statements were: I understood how to use the device; the device was easy to learn; the device was easy to use; I felt confident using this device; and I would feel confident using this device in a real-life emergency.
Sample size
We selected a sample size of 90 participants. In the absence of any preliminary data to provide insights in to expected effect size, our sample size was chosen based on the time frame available for data collection and the size of the pool of potential participants.
Statistical methods
We describe categorical data as number and frequency. We describe all continuous data as median and interquartile range to reflect the type of data collected. For our primary outcome (successful removal), we first assessed for a group, period or carryover effect, using a mixed-effects binary logistic regression model. In the absence of such effects, we used the same model framework to estimate the effect in removing the foreign body airway obstruction for both LifeVac and Dechoker, compared with abdominal thrusts. Participants were included as a random-effect in the model. The analysis was not adjusted for any covariates.
For time to removal, we visualised data using a Kaplan-Meier survival curve. As indicated by the crossed curves, violation of the proportional hazards assumption precluded use of a cox proportional hazard model or ordinal regression. Weighted log-rank tests were not used as the crosses occurred at different time points. The proportional odds assumption was assessed by the test of parallel lines. As such, we categorised time to removal in to five groups based on time to removal (group 1: 0−59 seconds, group 2: 60−119 seconds, group 3: 120−179 seconds, group 4: 180−239 seconds, and group 5: not successfully removed). We then adopted the same modelling strategy described for our primary outcome to compare groupings (group one v all other groups; groups one/two v all other groups, etc).
For usability outcomes, we compared across all three groups using Friedman’s test. In the event that the overall test was statistically significant (p < 0.05), we compared differences between pairs of groups (LifeVac v Abdominal thrusts; LifeVac v Dechoker; Dechoker v Abdominal thrusts) using the Wilcoxon signed-rank test.
The analyses were conducted on a per-protocol basis. We present model results as odds ratio and 95% confidence interval (CI) and reported p values for the non-parametric test results. All primary statistical tests were two-sided with a pre-specified significance level of 0.05. Pairwise comparisons of the usability outcomes were two-sided with a Bonferroni correction applied to account for multiple testing, such that pairwise level of significance was 0.017 (0.05 divided by three). We undertook analyses using SPSS (version 26.0, IBM Corp, Armonk, New York) and STATA (version 16.0, StataCorp, College Station, Texas).
Results
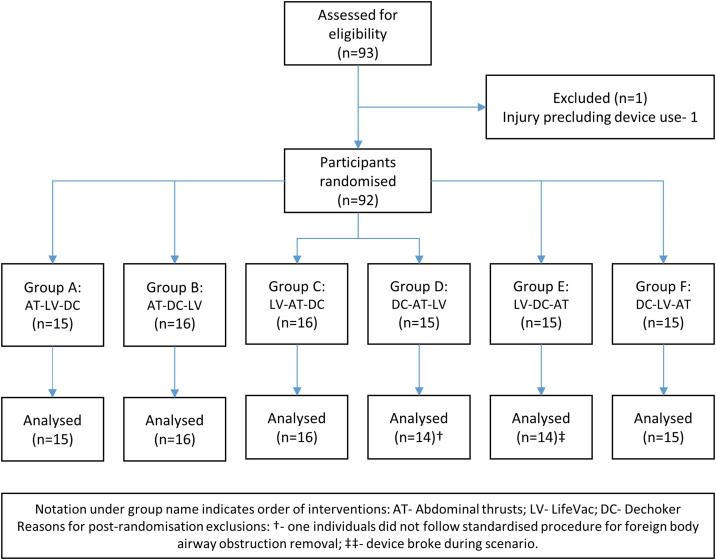
In October 2019, 93 individuals were screened for study participation, of which 92 participants were eligible, provided written informed consent and were randomised (Fig. 1). In two cases, participants did not complete all three tests correctly, such that they were not included in the analysis. Data from 90 individuals were available for analysis.
Fig. 1.
CONSORT participant flow diagram.
Most participants were male (n = 52, 58%), aged 18−29 (n = 77, 86%), and a medical student (n = 86, 96%) (Table 1). Most participants had previously attended a first aid course (n = 85, 94%). Few participants had previously seen a LifeVac or Dechoker device. Participant characteristics were similar across the study groups (Supplementary appendix Table S2).
Table 1.
Participant characteristics.
| All (n = 90) | |
|---|---|
| Age (years)-n(%)a | |
| 18−29 | 77 (85.6%) |
| 30−39 | 8 (8.9%) |
| 40−49 | 2 (2.2%) |
| 50−59 | 2 (2.2%) |
| Sex- male-n (%)a | 52 (58.4%) |
| Role- n (%) | |
| Student-medical | 86 (95.6%) |
| Student-other | 0 (0%) |
| Staff | 4 (4.4%) |
| Attended first aid course- Yes-n (%) | 85 (94.4%) |
| Real-life experience of FBAO management-n (%) | |
| None | 72 (80.0%) |
| Back slaps | 15 (16.7%) |
| Back slaps/abdominal thrusts | 3 (3.3%) |
| Previously seen Life-Vac-n (%) | 6 (6.7%) |
| Previously seen Dechoker-n (%) | 3 (3.3%) |
One participant declined to answer.
For the primary outcome, the FBAO was successfully removed in 99% cases with LifeVac, 74% cases with Dechoker, and 71% cases with abdominal thrusts (Table 2). The odds of successful removal was significantly higher in the LifeVac group than abdominal thrusts (odds ratio 47.32, 95% CI 5.75–389.40), but was not significantly higher in the Dechoker group compared with abdominal thrusts (odds ratio 1.22, 95% CI 0.60–2.47).
Table 2.
Study outcomes.
| Between group comparisons (odds ratio (95% confidence interval)) |
|||||
|---|---|---|---|---|---|
| LifeVac | Dechoker | Abdominal thrust | LifeVac v abdominal thrusts | Dechoker v abdominal thrusts | |
| FBAO removal success-n (%) | 89 (98.9%) | 67 (74.4%) | 64 (71.1%) | 47.32 (5.75–389.40) | 1.22 (0.60–2.47) |
| Time to removal- n (%) | |||||
| Group 1: 0−59 seconds | 74 (82.2%) | 40 (44.4%) | 60 (66.7%) | 2.39a (1.17–4.88) | 0.38a (0.20 – 0.72) |
| Group 2: 60−119 seconds | 13 (14.4%) | 14 (15.6%) | 2 (2.2%) | 13.53b (3.83–47.86) | 0.67b (0.36–1.25) |
| Group 3: 120−179 seconds | 1 (1.1%) | 6 (6.7%) | 1 (1.1%) | 24.95c (5.17–120.50) | 0.83c (0.42–1.65) |
| Group 4: 180−239 seconds | 1 (1.1%) | 7 (7.8%) | 1 (1.1%) | 47.32d (5.75–389.40) | 1.22d (0.60–2.47) |
| Unsuccessful (Group five) | 1 (1.1%) | 23 (25.6%) | 26 (28.9%) | ||
Comparison of group 1 v groups 2–5.
Comparison of groups 1–2 v groups 3–5.
Comparison of groups 1–3 v groups 4–5.
Comparison of groups 1–4 v group 5.
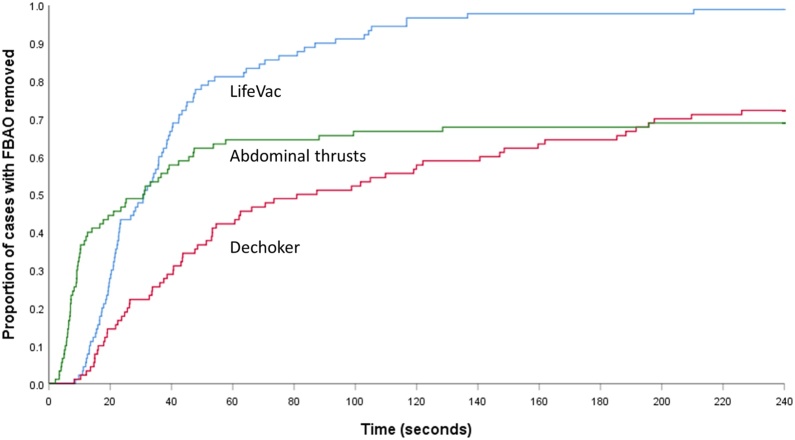
For time to removal, Fig. 2 shows the timing of success across groups. The crossed curves indicate the violation of proportional hazards assumption. Removal in less than one-minute occurred in 82% cases using LifeVac, 44% cases using Dechoker and 67% using abdominal thrusts. After the first minute, the FBAO was successfully removed in 17% cases using LifeVac, 30% cases using Dechoker, and 4% cases using abdominal thrusts. Across group comparisons, Lifevac was consistently superior to abdominal thrusts. For Dechoker, comparison of group one (removal in less than one minute) with subsequent time periods showed Dechoker to be less efficacious than abdominal thrusts (odds ratio 0.38, 95% CI 0.20 to 0.72). This effect was not observed in subsequent time point comparisons.
Fig. 2.
Time to removal of foreign body for study interventions.
Participants reported that they understood how to use all three techniques (Table 3). For all other usability outcomes, we observed statistically significant differences across the three groups. The LifeVac consistently outperformed the Dechoker device, whilst comparisons between the other two groups (LifeVac v Abdominal thrusts; Dechoker v Abdominal thrusts) were mixed. Reported confidence using techniques in real-life was highest in the abdominal thrust group, although between group comparisons showed abdominal thrusts were not superior to the LifeVac.
Table 3.
usability outcomes.
| LifeVac median (IQR) | Dechoker median (IQR) | Abdominal thrust median (IQR) | p-valuea | P-value for comparison between groupsb |
|||
|---|---|---|---|---|---|---|---|
| LifeVac v Dechoker | LifeVac v abdominal thrusts | Dechoker v abdominal thrusts | |||||
| Understand how to use technique | 9.0 (7.0–10.0) | 9.0 (7.0–10.0) | 9.0 (8.0–10.0) | 0.115 | – | – | – |
| Technique easy to lean | 9.0 (8.0–10.0) | 8.0 (6.0–9.0) | 9.0 (7.0–10.0) | <0.001 | 0.007 | 0.47 | 0.015 |
| Technique easy to use | 9.0 (6.0–10.0) | 6.0 (4.0–8.3) | 7.0 (5.0–9.0) | <0.001 | <0.001 | 0.013 | 0.08 |
| Confident using technique | 8 (6.0–9.0) | 6.0 (2.0–8.0) | 7.5 (5.0–9.0) | <0.001 | <0.001 | 0.50 | <0.001 |
| Confidence using technique in real-life emergency | 7.0 (5.5–9.0) | 5.0 (1.0–8.0) | 8.0 (5.0–9.0) | <0.001 | <0.001 | 0.84 | <0.001 |
IQR, interquartile range.
p-values based on 90 comparisons except confidence using technique in real-life emergency (89 comparisons).
p-values based on 90 comparisons except confidence using technique in real-life emergency- LifeVac v Dechoker (89 comparisons); confidence using technique in real-life emergency-DeChoker v Abdominal thrusts (89 comparisons).
Discussion
In this manikin randomised crossover trial of 90 participants, we identified that use of LifeVac resulted in both quicker FBAO removal and greater overall success. Dechoker was not superior to abdominal thrusts. Success rates in the LifeVac group were reflected across usability outcomes.
The successful removal of the FBAO without harm to the patient is the primary aim of all FBAO treatments. Following their first description in 1974 and despite early controversy, abdominal thrusts have become a core component of FBAO guidelines.4, 15, 16 However, abdominal thrust success rates are challenging to determine as data are limited to case series. In our study, a population of predominantly medical students that had previously undertaken a first aid course achieved a success rate of 71%. The most robust clinical report of abdominal thrusts effectiveness reported a FBAO removal success rate of 79%, although this is likely an over-estimate due to selection bias and recall bias.15 In contrast to suction-based airway clearance devices, a key advantage of abdominal thrusts is that they require no additional equipment to perform. Modifications have been described for use in patients that are unable to stand.17
For the two devices (LifeVac and Dechoker), published data on success rates are very limited.7 A systematic review identified no published peer-reviewed studies of the Dechoker device.7 In a manikin study of LifeVac, participants achieved a 94% success rate with one attempt and a 100% success rate with three attempts.18 A cadaver study of LifeVac reported a 98% success rate on the first attempt, and a 100% success rate with two attempts.19 The overall success rate for the LifeVac of 99% in our study is broadly consistent with these previous studies.
A key issue with these devices is that their use may distract the rescuer from other techniques, such as back slaps, abdominal thrusts and chest thrusts. The successful removal of an FBAO using devices relies on the generation of sufficient negative pressure, which is dependent on achieving an effective facemask seal. Previous research highlights the challenge of achieving an adequate seal with a face mask, particularly when using a one-handed technique.20, 21, 22 Our study recruited in a medical school such that most participants were medical students and may have a greater awareness of the importance and technique for generating an adequate seal than the general public.
The key difference between the Dechoker and LifeVac is that the DeChoker incorporates an oropharyngeal tube. Theoretically, the tube should focus the generated negative pressure to a specific location to facilitate FBAO removal. However, in our study, the LifeVac was superior to the Dechoker both in terms of overall success rates and time to removal. In the clinical setting, an important concern is that the insertion of the orophrangeal tube component of the Dechoker has parallels with a blind finger sweep, which are associated with harms such as soft tissue injury and the risk of inadvertent FBAO translocation making it more difficult to remove.23, 24, 25
Our study has a number of important limitations. Firstly, manikin studies provide an important way to test the efficacy of FBAO interventions using standardised processes. However, generalisability to the clinical setting is limited as it is not possible to recreate the fidelity of a time-critical clinical event. Secondly, our simulated obstruction was a small hard spherical object. Performance of different techniques will likely vary with obstructions of different consistencies and size. Thirdly, we recruited participants from a medical school which is reflected in the demographics of participants including the high proportion that had previously attended a first aid course. This may not be reflective of the general population. Fourthly, we were unable to blind either study participants or outcome assessors, which may have contributed to performance or detection bias.
Fifthly, the training for each intervention was relatively brief and did not allow participants the opportunity to practice. We used key components of manufacturer training information in our participant training videos. Based on this training, participants reported that they understood how to use study techniques. It is not known whether additional, more intense training may have influenced study results. Finally, we asked participants to continue using the same technique for the four-minute scenario. In contrast, clinical guidelines recommend alternating techniques if a specific technique does not quickly lead to successful FBAO removal.4
Conclusion
In this manikin study, we found evidence that individuals using the LifeVac were more successful in removing a simulated foreign body airway obstruction than individuals using abdominal thrusts. We did not find evidence of improved success by individuals using the Dechoker, compared with individuals using abdominal thrusts. Further research in the clinical setting is needed to understand the potential role of suction-based airway clearance devices in the management of FBAO.
Funding
GDP is supported as an NIHR Senior Investigator and by the NIHR Applied Research Centre (ARC) West Midlands, UK. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflict of interests
KC is an associate editor of Resuscitation Plus. The remaining authors have no conflicts of interest to declare.
CRediT authorship contribution statement
Emma Patterson: Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Ho Tsun Tang: Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Chen Ji: Formal analysis, Writing - review & editing, Supervision. Gavin D. Perkins: Conceptualization, Methodology, Formal analysis, Resources, Writing - review & editing, Supervision. Keith Couper: Conceptualization, Methodology, Formal analysis, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resplu.2020.100067.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Pavitt M.J., Nevett J., Swanton L.L. London ambulance source data on choking incidence for the calendar year 2016: an observational study. BMJ Open Respir Res. 2017;4 doi: 10.1136/bmjresp-2017-000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Injury Facts. Preventable death and death rates per 100,000 population in the home and community by cause and age group, United States, 2017. [Available from: https://injuryfacts.nsc.org/home-and-community/home-and-community-overview/deaths-in-the-home-and-community-by-age-group-and-cause/, accessed 14th January 2020].
- 3.Office for National Statistics. Choking related deaths registered in England and Wales, 2014 to 2017. [Available from https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/009342chokingrelateddeathsregisteredinenglandandwales2014to2017, accessed 2nd November 2020].
- 4.Perkins G.D., Handley A.J., Koster R.W. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation. 2015;95:81–99. doi: 10.1016/j.resuscitation.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Couper K., Abu Hassan A., Ohri V. Removal of foreign body airway obstruction: A systematic review of interventions. Resuscitation. 2020;156:174–181. doi: 10.1016/j.resuscitation.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Olasveengen T.M., Mancini M.E., Perkins G.D. Adult basic life support: international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2020;156:A35–A79. doi: 10.1016/j.resuscitation.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunne C.L., Peden A.E., Queiroga A.C., Gomez Gonzalez C., Valesco B., Szpilman D. A systematic review on the effectiveness of anti-choking suction devices and identification of research gaps. Resuscitation. 2020;153:219–226. doi: 10.1016/j.resuscitation.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 8.LifeVac Europe Ltd. General information: LifeVac Europe Ltd; [Available from: https://www.lifevac.eu/lifevac-pamphlet.pdf, accessed 9th September 2020].
- 9.Dechoker. How to Use Dechoker Anti-Choking Device (ACD) [Available from: https://www.dechoker.com/pages/dechoker-usage-instructions, accessed 9th September 2020].
- 10.LifeVac. LifeVac Training 2018 [Available from: https://www.youtube.com/watch?v=aqizWSL2ndA, accessed 9th September 2020].
- 11.Dechoker. How it Works: Dechoker, Anti-Choking Device 2018 [Available from: https://www.youtube.com/watch?v=MYVyoBrAIK4, accessed 9th September 2020].
- 12.Sealed Envelope Ltd. Create a blocked randomisation list. [Available from: https://www.sealedenvelope.com/simple-randomiser/v1/lists, accessed 2nd November 2020].
- 13.Sealed Envelope Ltd. Simple randomisation service. [Available from: https://www.sealedenvelope.com/simple-randomiser/v1/, accessed 2nd November 2020].
- 14.St John Ambulance. What To Do When Someone Is Choking - First Aid Training - St John Ambulance [Available from: https://www.youtube.com/watch?v=PA9hpOnvtCk accessed 9th September 2020].
- 15.Redding J.S. The choking controversy: critique of evidence on the Heimlich maneuver. Crit Care Med. 1979;7:475–479. [PubMed] [Google Scholar]
- 16.Heimlich H. Pop goes the cafe coronary. Emerg Med. 1974;6:154–155. [Google Scholar]
- 17.Blain H., Bonnafous M., Grovalet N., Jonquet O., David M. The table maneuver: a procedure used with success in four cases of unconscious choking older subjects. Am J Med. 2010;123(1150):e7–9. doi: 10.1016/j.amjmed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Lih-Brody L., Singer M., Brody E. 382 Lifevac: a novel device for the resuscitation of the adolescent choking victim. Ann Emergency Med. 2017;70:S149–S150. [Google Scholar]
- 19.Juliano M., Domingo R., Mooney M.S., Trupiano A. Assessment of the LifeVac, an anti-choking device, on a human cadaver with complete airway obstruction. Am J Emergency Med. 2016;34:1673–1674. doi: 10.1016/j.ajem.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 20.Otten D., Liao M.M., Wolken R. Comparison of bag-valve-mask hand-sealing techniques in a simulated model. Ann Emergency Med. 2014;63:6–12. doi: 10.1016/j.annemergmed.2013.07.014. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerstein N.S., Carey M.C., Braude D.A. Efficacy of facemask ventilation techniques in novice providers. J Clin Anesthesia. 2013;25:193–197. doi: 10.1016/j.jclinane.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Hart D., Reardon R., Ward C., Miner J. Face mask ventilation: a comparison of three techniques. J Emergency Med. 2013;44:1028–1033. doi: 10.1016/j.jemermed.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Abder-Rahman H.A. Infants choking following blind finger sweep. J Pediatr. 2009;85:273–275. doi: 10.2223/JPED.1892. [DOI] [PubMed] [Google Scholar]
- 24.Hartrey R., Bingham R.M. Pharyngeal trauma as a result of blind finger sweeps in the choking child. J Accid Emergency Med. 1995;12:52–54. doi: 10.1136/emj.12.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gjoni D., Mbamalu D., Banerjee A., James K.K. An unusual complication of an attempt to open the airway in a choking child. Br J Hospital Med. 2009;70:595. doi: 10.12968/hmed.2009.70.10.44630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.