Abstract
Background
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has been used as extracorporeal cardiopulmonary resuscitation (ECPR) to support further resuscitation efforts in patients with cardiac arrest, yet its clinical effectiveness remains uncertain.
Objectives
This study reviews the role of ECPR in contemporary resuscitation care compared to no ECPR and/or standard care, e.g. conventional CPR, and quantitatively summarize the rates of long-term neurologically intact survival after adult in-hospital cardiac arrest (IHCA) or out-of-hospital cardiac arrest (OHCA).
Methods
We searched the following databases on January 31 st, 2020: CENTRAL, MEDLINE, Embase, and Web of Science. We followed PRISMA guidelines and used PICO format to summarize the research questions. Risk of bias was assessed using the ROBINS-I tool. Pooled risk ratios (RRs) for each outcome of interest were calculated. Quality of evidence was evaluated according to GRADE guidelines.
Results
Six cohort studies were included, totaling 1750 patients. Of these, 530 (30.3%) received the intervention, and 91 (17.2%) survived with long-term neurologically intact survival. ECPR compared to no ECPR is likely associated with improved long-term neurologically intact survival after cardiac arrest in any setting (risk ratio [RR] 3.11, 95% confidence interval [CI] 2.06–4.69; p < 0.00001) (GRADE: Very low quality). Similar results were found for long-term neurologically intact survival after IHCA (RR 3.21, 95% CI 1.74–5.94; p < 0.0002) (GRADE: Very low quality) and OHCA (RR 3.11, 95% CI 1.50–6.47; p < 0.002) (GRADE: Very low quality). Long-term time frames for neurologically intact survival (three months to two years) were combined into a single category, defined a priori as a Glasgow-Pittsburgh cerebral performance category (CPC) of 1 or 2.
Conclusions
VA-ECMO used as ECPR is likely associated with improved long-term neurologically intact survival after cardiac arrest. Future evidence from randomized trials is very likely to have an important impact on the estimated effect of this intervention and will further define optimal clinical practice. Review registration: PROSPERO CRD42020171945.
Keywords: ECPR, Extracorporeal cardiopulmonary resuscitation, Extracorporeal life support, Cardiac arrest
Introduction
Extracorporeal cardiopulmonary resuscitation (ECPR), or cardiopulmonary resuscitation (CPR), assisted by veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is a method of temporary mechanical circulatory support based on utilization of an extracorporeal membrane oxygenation (ECMO) system.1, 2, 3 All VA-ECMO circuits consist of a venous cannula, usually placed in the right or left common femoral vein for extraction, while an arterial cannula is usually placed in the right or left femoral artery for infusion, and a membrane oxygenator where gas exchange occurs is connected to a centrifugal pump with a heat exchanger.3, 4, 5, 6, 7
Neither the guidelines of the American Heart Association (AHA) nor those of the European Resuscitation Council (ERC) recommend the routine use of ECPR for cardiac arrest (Class IIb, LOE C-LD).8, 9, 10, 11 However, ECMO-facilitated resuscitation has been increasingly used to assist early return of perfusion, supporting further resuscitation in order to mitigate the multi-organ dysfunction that accompanies in-hospital cardiac arrest (IHCA) and out-of-hospital cardiac arrest (OHCA).12, 13, 14, 15 Currently, 129,037 patients are enrolled in the January 2020 Extracorporeal Life Support Organization (ELSO) Registry database, including 2387 adults with ECPR who survived to discharge (or transfer), with the number of cases in which VA-ECMO was used as ECPR increasing in the last decade. Overall survival to discharge/transfer after the use of ECPR for cardiac arrest was 29.0%.12
Outcomes of early deployment of VA-ECMO as ECPR for IHCA or OHCA in prior research have varied greatly among a range of study designs that include case series, case-control, and cohort studies. This approach has been associated with a 2- to 4-fold (8.0%–15.0% to 30.0%–45.0%) increase in patient-centered outcomes, including survival to discharge and neurologically intact survival.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 An unexplored outcome of this approach is long-term neurologically intact survival in patients with cardiac arrest who respond poorly to the current standard of care.45, 46, 47, 48, 49, 50 Thus, identifying the rates of neurologically intact survival and optimal clinical practice in this patient population remains a high priority, as is the role of early ECMO-facilitated resuscitation therapy for cardiac arrest.8, 9, 10, 11
Objectives
This study reviewed the role of ECPR in contemporary resuscitation care compared to no ECPR. A further objective was to quantitatively summarize the rates of long-term neurologically intact survival after adult cardiac arrest in any setting (in-hospital or out-of-hospital).
Materials and methods
This meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) and in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.51 Our review protocol was drafted and revised as necessary, before being registered in the International Prospective Register of Systematic Reviews (CRD42020171945) (https://www.crd.york.ac.uk/PROSPERO/).
The review question was formulated following the PICO framework (P-Populations/People/Patient/Problem, I-Intervention(s), C-Comparison, O-Outcome) and the Question Statement.52 Question: Among adults (≥ 16 years) resuscitated from IHCA or OHCA (P) and treated with ECPR (I), compared to no ECPR and/or conventional CPR (C), what are the rates of long-term neurologically intact survival (O)?
Criteria for considering studies for this review
Types of study
All studies employing patient‐level randomization or cluster randomization comparing ECPR vs. no ECPR and/or conventional CPR were considered for inclusion. We also considered observational analytic studies (cohort and case-control studies) with an appropriate control group published between January 1 st, 2000, and January 31 st, 2020. A preliminary review suggested there would not be any relevant articles prior to the year 2000. We excluded any other type of study design.
Types of participant
We considered for inclusion adults suffering IHCA or OHCA, with resuscitation attempted by a bystander or healthcare provider. We excluded studies considering IHCA or OHCA due to trauma, hypothermia, and toxic substances, as the core interventions provided by healthcare providers (CPR and early defibrillation) are unlikely to be of significant benefit in such circumstances. We also excluded studies considering IHCA/OHCA in pediatrics and pregnancy. The exclusions were meant to reduce heterogeneity in the population while maintaining generalizability to most patients suffering cardiac arrest.
Types of intervention
We considered for inclusion studies comparing ECMO using pump-driven venous-arterial (VA) circuits vs. no ECPR and/or conventional CPR.
Types of outcome measure
Primary outcomes
The primary outcomes of interest were long-term neurologically intact survival after IHCA and OHCA, after IHCA, and after OHCA. Long-term time frames for neurologically intact survival (three months to two years) were combined into a single category, defined a priori as a Glasgow-Pittsburgh cerebral performance category (CPC) of 1 or 2, as measured by any validated scale.53
Search methods for identification of studies
Electronic searches
We used the PRESS (Peer Review of Electronic Search Strategies) checklist to develop the research strategy.54 We searched the following databases on January 31 st, 2020: The Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library (Issue 1 of 12, January 2020), MEDLINE (PubMed) (2000 to January 2020), Embase (Ovid) (2000 to January 2020), and Web of Science (2000 to January 2020), followed by a supplementary search on May 12th to ascertain that no new literature was published in the interim. We used relevant keywords and controlled vocabulary (e.g. medical subject headings). We also applied filters for MEDLINE and Embase terms to optimize search performance, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions.55 We adapted the search strings devised for MEDLINE for use in searching other databases (Appendix A). All clinical studies published in English as full-text articles in indexed journals were considered for inclusion regardless of publication or publication status.
Searching other resources
We searched the following clinical trial registries for ongoing/unpublished randomized clinical trials (RCTs) on January 31 st, 2020: The National Institutes of Health ongoing clinical trials register (www.clinicaltrial.gov) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/). We searched the reference lists of included studies for further references and the abstracts of conference proceedings of the AHA and the ERC. We also increased reliance on web-based searching to identify additional studies.
Data collection and analysis
Selection of studies
Two investigators independently screened the titles and abstracts of all retrieved citations against the inclusion criteria. Studies that met the criteria were independently reviewed by the investigators. We used EndNote (X9.3, Clarivate Analytics) to manage the collected publications. Disagreements regarding inclusion/exclusion were resolved via discussion or by the decision of a third independent investigator. We used a Kappa coefficient to measure interrater reliability to determine the degree of agreement between the two investigators collecting studies for eligibility. The formula was entered into Microsoft Excel. In the case of a Kappa of 0.01–0.80 the third independent investigator reviewed all excluded full-text articles for eligibility to ensure optimized sensitivity.
Data extraction and management
Data extraction was performed using a standardized Excel form. It focused on identifying information on sample participants (demographic characteristics), study methods (setting, intervention, method of delivery), clinical parameters, outcome measures, and complications or adverse events. Any disagreements were resolved by discussion or by the decision of the third independent investigator.
Assessment of risk of bias
Two investigators independently assessed the methodological qualities of each study using the Cochrane Collaboration Risk of in Non-randomized Studies of Interventions (ROBINS-I) tool.56 Using this tool, seven domains are investigated for potential risk of bias, judged via signaling questions. Bias was assessed per study; each bias domain and overall risk of bias were classified as low, moderate, serious, or critical risk. Disagreements were resolved via discussion or by the decision of the third independent investigator.56, 57
Measures of treatment effect
We calculated the risk ratios (RRs) and associated 95% confidence intervals (CIs) for each study to measure neurological outcomes, which were grouped into the categories of favorable neurological outcome (CPC) of 1 or 2 so they could be adapted for the meta‐analysis. Pooled data were analyzed using the Mantel-Haenszel method and a two-sided p-value < 0.05 was considered statistically significant. We planned to use a random‐effects model with appropriate caution in interpretation in the event of moderate or high heterogeneity; otherwise, we use the fixed‐effect model. The pooled estimates of effect in the random‐effects model presented the average effect of unadjusted long-term neurologically intact survival after IHCA and OHCA, IHCA, or OHCA treated by ECPR compared to no ECPR and/or conventional CPR. All analysis was performed using Review Manager (RevMan 5.3)58 and followed the recommendations given in the Cochrane Handbook for Systematic Reviews of Interventions.57
Dealing with missing data
We planned to contact authors of included studies in the event that not all relevant data were presented in the text of a study. Missing relevant statistical parameters and variance measures were calculated if data permitted.
Assessment of heterogeneity
We assessed heterogeneity across studies by inspecting the detailed clinical characteristics of the included studies. We evaluated the presence and degree of heterogeneity using the Mantel‐Haenszel Chi2 test and the I2 statistic for each outcome. We considered statistically significant heterogeneity a p-value ≤ 0.10 for the Mantel‐Haenszel Chi2 test or values > 50% using the I2 statistic.57
Assessment of reporting biases
We planned to use a funnel plot to assess publication bias and test for funnel plot asymmetry if more than 10 studies were included.59
Subgroup analysis
We planned to perform subgroup analyses if sufficient data were available (i.e. from three or more studies). Our subgroup analysis of interest included the following variables: time interval from onset of cardiac arrest to initiation of CPR (no-flow time ≤ 5 min or > 5 min); time interval from initiation of CPR to return of spontaneous circulation (ROSC) by ECMO-facilitated resuscitation or termination of resuscitation (low flow time ≤ 60 min or > 60 min).60
Sensitivity analysis
We planned to perform a sensitivity analysis for primary outcomes if a sufficient number of studies reported outcomes, to determine if a high risk of bias affected results. We planned to perform sensitivity analyses by excluding studies with high risk of bias and studies with unclear risk of bias. We also planned to perform sensitivity analyses comparing fixed‐effect pooled estimates or 95% CIs vs. random‐effects pooled estimates or 95% CIs.
Summary of findings
We created a summary of findings table for the outcomes of interest. We used GRADE principles (Grades of Recommendation, Assessment, Development and Evaluation) to appraise the quality/certainty of evidence associated with specific outcomes.61, 62 The quality of a body of evidence reflects within-study risk of bias, directness of evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias. Evidence quality for each specific outcome was classified as high, moderate, low, or very low quality. We used the methods recommended in the Cochrane Handbook and GRADEpro GDT for these analyses.57, 63
Results
Study selection
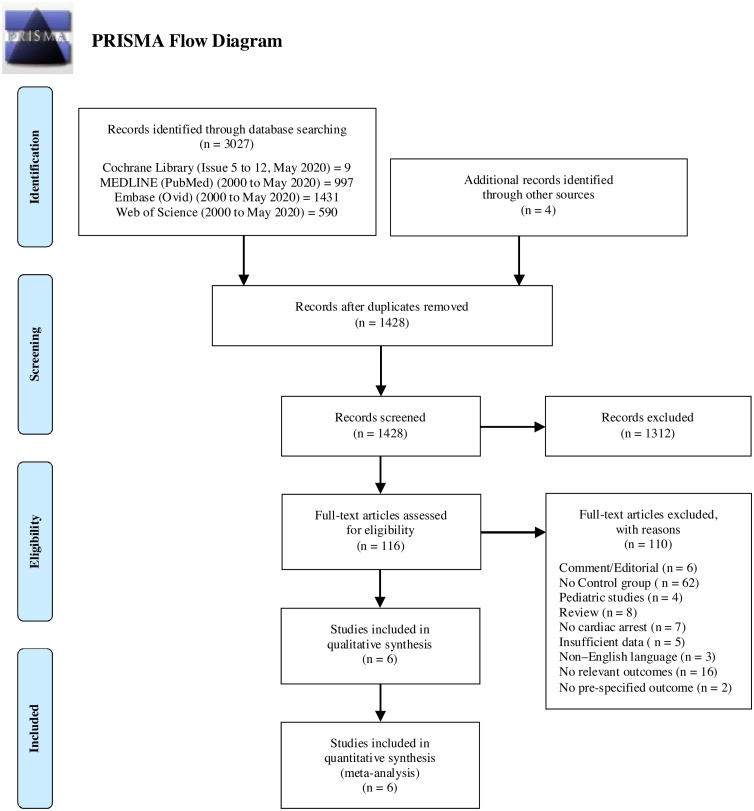
The initial search returned nine citations from CENTRAL, 997 citations from MEDLINE, 1431 citations from Embase, and 550 citations from Web of Science. Additional papers were identified by searching Google Scholar or through bibliographic review. After duplicates were eliminated, 1428 citations remained for screening, of which 116 were eligible for full-text review. We excluded 110 papers at this stage, because they did not meet the study inclusion criteria after review of the text. These studies are listed in Appendix B. The interrater reliability between the reviewers of positive agreement was 0%, negative agreement was 94%, kappa was 0.33. Fig. 1 shows a PRISMA diagram of the study selection process. We included six cohort studies comprising 1750 participants in the review. Of these, 530 participants (30.3%) received the intervention; 91 (17.2%) survived with long-term neurologically intact survival.45, 46, 47, 48, 49, 50 Three studies were in an out‐of‐hospital setting,45, 46, 47 two took place in‐hospital,48, 49 and one had both in‐hospital and out‐of‐hospital components.50 Table 1, Table 2, Table 3, Table 4 provide an overview of included studies. The search of clinicaltrials.gov identified several ongoing clinical trials of ECPR for cardiac arrest; overviews of these are provided in Appendix C.
Fig. 1.
PRISMA flow diagram for systematic review of the use of ECPR vs. no ECPR and/or conventional CPR on long-term neurologically intact survival after adult cardiac arrest in any setting (in-hospital or out-of-hospital).
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097.
Table 1.
Characteristics of studies included in the review.
| Authors, year, country | Study design | Years of inclusion | No. of pts | Inclusion criteria/Criteria for ECPR | Exclusion criteria/Contraindication for ECPR | Primary endpoints/Additional endpoints |
|---|---|---|---|---|---|---|
| Chen et al. 200848 Taiwan | A single-center propensity-matched, prospective, cohort study. | 2004–2006 | 172 | Age 18–75 years, witnessed cardiac arrest, CPR for > 10 min, cardiac etiology. Only patients who underwent witnessed arrest of cardiac origin and CPR duration (defined as the interval from beginning CPR to ROSC or death) for more than 10 min were recruited in the study cohort. | CPR < 10 min, known severe irreversible brain damage, terminal malignancy, a traumatic origin with uncontrolled bleeding; non-cardiac arrest, signed DNR order. | Survival to hospital discharge and analysis was by intention to treat. Additional endpoints: ROSC, 24 -hs, 3-days, 14-days, 30-days, and 6-months survival. |
| Kim et al. 201445 South Korea | A single-center propensity-matched, retrospective, cohort study based on a prospective cohort. | 2006–2013 | 499 | Age ≥ 18 years, sudden cardiac arrest with presumed correctable causes, witnessed cardiac arrest with or without bystander CPR, no-flow time (expected to be short, even for unwitnessed cardiac arrest). ECPR team was activated if above criteria were met and patient required prolonged CPR > 10 min as in-hospital CPR duration or when recurrently arrested in the ED after achieving sustained ROSC for at least 20 min. | Cardiac arrest due to a clearly uncorrectable cause, presence of a terminal illness or malignancy, suspected traumatic origin of arrest; no informed consent from family. | CPC of 1 or 2 at 3 months post-cardiac arrest. To find indications for predicting good neurological outcome according to CPR duration and the optimal duration of CPR before considering ECPR. Additional endpoints: Cause of death at 3-months. |
| Maekawa et al. 201346 Japan | A post hoc analysis of data from a single-center prospective, cohort study, including propensity score matching. | 2000–2004 | 162 | Age ≥ 16 years, CPR duration > 20 min, witnessed, presumed cardiac origin. ECPR was initiated if ROSC did not occur or could not be maintained during transportation, if the patient was assessed to have good activities of daily life before cardiac arrest, and if the cardiac arrest was clinically presumed as cardiac in origin by the patient’s information reported by paramedics and rapid echocardiographic examination. | Previously signed DNR order, pronounced dead before hospital arrival. Contraindication for ECPR: Non-cardiac cause of arrest. Cardiac arrest was presumed to be of cardiac origin unless it was known or likely to have been caused by trauma, submersion, hypothermia, drug overdose, asphyxia, exsanguination, or any other noncardiac cause including intracranial hemorrhage, acute aortic dissection, and terminal malignancy. | Favorable neurologic status at 3-months after cardiac arrest. Determine potential predictors that can identify candidates for ECPR among patients with OHCA. Additional endpoints: ED survival. |
| Sakamoto et al. 201447 Japan | A multi-center prospective, cohort study. | 2008–2011 | 451 | VF/VT on the initial electrocardiogram, cardiac arrest on arrival to hospital with or without prehospital ROSC, arrival at hospital within 45 min of the emergency call or the cardiac arrest, no ROSC for 15 min after hospital arrival in spite of ongoing CPR. | Age < 20 or > 75 years, poor level of activities of daily living prior to arrest, arrest of non-cardiac origin (i.e. trauma, drug intoxication, primary cerebral disorder, aortic dissection, terminal phase of cancer), core temperature < 30 °C, no informed consent from patient representatives. | Favorable neurologic status at 1-month and 6-months after OHCA, defined as the Glasgow-Pittsburgh CPC of score of 1 or 2. |
| Shin et al. 201349 South Korea | A single-center propensity-matched, retrospective, cohort study. | 2003–2009 | 406 | Prolonged arrest and no ROSC within 10–15 min after initiation of CPR, when ROSC could not be maintained due to recurrent arrest, or when recovery without ECMO support was unlikely due to known severe left ventricular dysfunction or coronary artery disease despite relatively short CPR duration. | Age > 80 years, previous severe neurological damage, current intracranial hemorrhage, malignancy in the terminal stage, arrest of traumatic origin with uncontrolled bleeding, arrest of septic origin, irreversible multi-organ failure leading to cardiac arrest, and patients who signed DNR orders. Patients with CPR duration of less than 10 min, unwitnessed arrest. | Survival at 2-years and neurological outcomes. Neurological outcome was defined by the Modified Glasgow Outcome Score. Additional endpoints: Survival analysis for neurological outcomes at 6-months; 2-years follow-up was conducted for all survivors. |
| Siao et al. 201550 Taiwan | A single-center retrospective, cohort study. | 2011–2013 | 60 | Age 18–75 years, cardiac arrest with initial VF and CPR initiated within 5 min (no-flow duration < 5 min), refractory VF defined as VF resistant to at least 3 defibrillations, 3 mg of epinephrine, 300 mg of amiodarone, and no ROSC achieved after CPR for more than10 min. | Severe head trauma or severe acute active bleeding, severe sepsis, VF that developed during resuscitation for initial asystole or pulseless electrical activity, terminal stage of malignancy, any history of severe neurological deficits (including dementia, intracranial hemorrhage, or ischemic stroke and bedridden state). | Survival to discharge and neurologically intact survival; also looked at 1-year survival to discharge and favorable neurological outcome. Additional endpoints: ROSC. |
Abbreviations: CPR = cardiopulmonary resuscitation; CPC = cerebral performance category; ECLS = extracorporeal life-support; ECMO = extracorporeal membrane oxygenation; ECPR = extracorporeal cardiopulmonary resuscitation; ED = emergency department; DNR = do-not-resuscitate; OHCA = out-of-hospital cardiac arrest; ROSC = return of spontaneous circulation; VF = ventricular fibrillation; VT = ventricular tachycardia.
Notes: All studies compared ECPR vs. no ECPR while Shin et al. compared ECPR attempt vs. no ECPR attempt. Sakamoto et al. compared emergency departments with ECPR vs. emergency departments with no ECPR.
Table 2.
Demographic and baseline clinical characteristics of the ECPR group and the CCPR group of studies included.
| Patient groups (n) |
Age (mean ± [SD]/median [IQR]) |
Male, n (%) |
Witnessed arrest, n (%) |
Bystander CPR, n (%) |
Arrest to CPR (min)* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, year, country | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR |
| Chen et al. 200848 Taiwan | 59 | 113 | 57.4 ± 12.5 | 60.3 ± 13.3 | 50 (85) | 73 (65) | 59 (100) | 113 (100) | Not applicable | Not applicable | … | … |
| Kim et al. 201445 South Korea | 55 | 444 | 53 (41–68) | 69 (56–77) | 41 (75) | 285 (64) | 43 (78) | 328 (74) | 23 (42) | 151 (34) | 7 (0–13) | 8 (5–12) |
| Maekawa et al. 201346 Japan | 53 | 109 | 54 (47–60) | 71 (59–80) | 44 (83) | 79 (73) | … | … | 29 (55) | 42 (39) | 6 (2–9) | 7 (3–10) |
| Sakamoto et al. 201447 Japan | 258 | 193 | 56 (NR) | 58 (NR) | 235 (90) | 172 (89) | 186 (72) | 151 (78) | 127 (49) | 90 (46) | … | … |
| Shin et al. 201349 South Korea | 85 | 321 | 59.9 ± 15.3 | 61.6 ± 14.2 | 53 (62) | 201 (63) | 85 (100) | 321 (100) | Not applicable | Not applicable | … | … |
| Siao et al. 201550 Taiwan | 20 | 40 | 54.5 ± 11.9 | 60.3 ± 11.2 | 18 (90) | 28 (70) | … | … | Not applicable | Not applicable | 1–4.5 | … |
Abbreviations: CPR = cardiopulmonary resuscitation; CCPR = conventional cardiopulmonary resuscitation; ECPR = extracorporeal cardiopulmonary resuscitation.
Notes: Total percentages refer to studies with available data and continuous variables reported as mean ± standard deviation (SD) or as median interquartile range (IQR). Proportions - No. (%) of studies performing propensity score matching refer to the unmatched pre-arrest and post-arrest clinical characteristics and outcomes.
Notes: None of the patients received mechanical cardiopulmonary resuscitation (mCPR).
*Reported as the interval from collapse to initiation of CPR or no-flow duration.
Table 3.
Baseline clinical characteristics and variables of the ECPR group and the CCPR group of studies included.
| Asystole, n (%) |
PEA, n (%) |
VF/VT, n (%) |
CPR duration (min) |
CPR to ECMO duration (min) |
ROSC (ROSB), n (%) |
Presume cardiac etiology, n (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, year, country | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR |
| Chen et al. 200848 Taiwan | 13 (22) | 31 (27) | 17 (29) | 46 (41) | 29 (42) | 36 (32) | 53 ± 37 | 43 ± 31 | … | … | 55 (93) | 63 (56) | 59 (100) | 113 (100) |
| Kim et al. 201445 South Korea | 14 (26) | 268 (60) | 10 (18) | 91(21) | 31 (56) | 85 (19) | 62 (47–89) | 35 (21–50) | 1.5 (0.6–6.4)* | … | 44 (80) | 212 (48) | 49 (89) | 267 (62) |
| Maekawa et al. 201346 Japan | … | … | … | … | 32 (60) | 24 (22) | 49 (41–59) | 56 (47–66) | … | … | … | … | … | … |
| Sakamoto et al. 201447 Japan | … | … | … | … | 258 (100) | 193 (100) | … | … | … | … | … | … | 226 (87) | 150 (77) |
| Shin et al. 201349 South Korea | 10 (12) | 47 (15) | 50 (59) | 201 (63) | 25 (29) | 73 (23) | 42 ± 26 | 41 ± 37 | … | … | 64 (75) | 167 (52) | 63 (74) | 182 (57) |
| Siao et al. 201550 Taiwan | … | … | … | … | 20 (100) | 40 (100) | 70 ± 50 | 34 ± 18 | 49 ± 43.9 | … | 19 (95) | 19 (48) | 16 (80) | 21 (53) |
Abbreviations: CPR = cardiopulmonary resuscitation; CCPR = conventional cardiopulmonary resuscitation; ECLS = extracorporeal life support; ECPR = extracorporeal cardiopulmonary resuscitation; ECMO = extracorporeal membrane oxygenation; PEA = pulseless electrical activity; ROSB = return of spontaneous heartbeat; ROSC = return of spontaneous circulation; VF = ventricular fibrillation; VT = ventricular tachycardia.
Notes: Total percentages refer to studies with available data and continuous variables reported as mean ± SD or as median IQR.
Studies reporting in-hospital cardiac arrest did not report collapsed-time to CPR though it was considered to be minimal as per inclusion criteria.
CPR duration was defined as the interval between initiation of CPR and ROSC or death in the CCPR group, and as the interval between initiation of CPR and ECLS implantation in the ECPR group. Return of spontaneous heartbeat was identified by echocardiography in the ECPR group and by palpable central pulse or peripheral arterial pulse in the CCPR group.
*The median time interval from ROSC to ECPR implantation was 1.5 (range 0.6–6.4) hours.
Table 4.
Baseline clinical characteristics and outcomes of the ECPR group and the CCPR group of studies included.
| Serum lactate/Arterial pH (mean ± [SD]/median [IQR]) |
Coronary angiography, n (%) |
Reperfusion therapy/PCI/CABG, n (%) |
ACS/AMI, n (%) |
Therapeutic hypothermia, n (%) |
Neurological outcome (CPC 1–2) at discharge, 30-day and/or long-term neurological outcome, n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, year, country | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR | ECPR | CCPR |
| Chen et al. 200848 Taiwan | Lactate: NR | Lactate: NR | … | … | 26 (44) | 6 (6) | 37 (63) | 80 (71) | No applied | No applied | Discharge: 14 (24) | Discharge: 12 (11) |
| pH: 12.0 | pH: 3.7 | 30-day: 14 (24) | 30-day: 12 (11) | |||||||||
| (2.4–39.7)* | (1.1–20)* | 1-year: 9 (15) | 1 year: 10 (9) | |||||||||
| Kim et al. 201445 South Korea | Lactate: 17.7 | Lactate: 10.8 | 39/44 | 11/15 | 29 (94) | 3 (100) | 36/52 (69) | 9/52 (17) | 17 (31) | 71 (16) | Discharge: 8 (14) | Discharge: 36 (8) |
| (8.8–16.0)† | (7.3–14.0)† | (89) | (73)‡ | 30-day: 8 (15) | 30-day: 36 (8) | |||||||
| pH: 6.98 | pH: 6.94 | 3-months: 8 (15) | 3-months: 36 (8) | |||||||||
| (6.86–7.05)† | (8.8–16.0)† | |||||||||||
| Maekawa et al. 201346 Japan | Lactate: NR | Lactate: NR | … | … | 21 (40)§ | 6 (6)§ | … | … | 26 (49)‖ | 7 (6)‖ | Discharge: NR | Discharge: NR |
| pH: NR | pH: NR | 30-day: NR | 30-day: NR | |||||||||
| 3 months: 15 (28) | 3-months: 5 (5) | |||||||||||
| Sakamoto et al. 201447 Japan | Lactate: NR | Lactate: NR | 157/177 (89)‖ | 25/37 | 97/177 (55)‖ | 21/37 | 165 (64) | 115 (59) | 162/167 (92) | 20/37 (54) | Discharge: NR | Discharge: NR |
| pH: NR | pH: NR | (68)‖ | (57)‖ | 30-day: 32 (12) | 30-day: 3 (1.6) | |||||||
| 6-months: 29 (11) | 6-months: 5 (3) | |||||||||||
| Shin et al. 201349 South Korea | Lactate: NR | Lactate: NR | … | … | 18 (21)§ | 11 (3)§ | 38 (45) | 82 (26) | No applied | No applied | Discharge: NR | Discharge: NR |
| pH: NR | pH: NR | 30-day: 24 (28)# | 30-day: 24 (8)# | |||||||||
| 2-years: 22 (26)# | 2-years: 22 (7)# | |||||||||||
| Siao et al. 201550 Taiwan | Lactate: 8.90 (2.29) | Lactate: 8.25 (2.29) | … | … | 12 (60)§,¶ | 16 (40)§,¶ | 12 (60) | 16 (40) | 9 (45)|| | 9 (23)|| | Discharge: 8 (40) | Discharge: 3 (8) |
| pH: NR | pH: NR | 30-day: NR | 30-day: NR | |||||||||
| 1-year: 8 (40) | 1-year: 3 (8) | |||||||||||
Abbreviations: ACS = acute coronary syndrome; AMI = acute myocardial infarction; CPR = cardiopulmonary resuscitation; CCPR = conventional cardiopulmonary resuscitation; CPC = cerebral performance category; ECPR = extracorporeal cardiopulmonary resuscitation; ECMO = extracorporeal membrane oxygenation; GABC = coronary artery bypass grafting; PCI = percutaneous coronary intervention; pH = measured acid-base balance of the blood; ROSB = return of spontaneous heartbeat; ROSC = return of spontaneous circulation.
Notes: Total percentages refer to studies with available data.
Neurologically intact survival (i.e. long-term [three months to two years]) were combined into a single category.
* Available maximal lactic acid in 24 -h period.
† Measured in 48 ECPR patients and 332 CCPR patients.
‡ In 15 suspected ACS patients with ROSC (≥ 20 min).
§ Reported as primary PCI.
‖ The contents of treatments given to 214 patients (92% of 177 patients in the ECPR group and 54% of 37 patients in the CCPR group), who were alive at 24 h after cardiac arrest. The frequencies of introducing TH and performing intra-aortic balloon pump were significantly higher in the ECPR group.
¶ Emergency coronary angiography was performed by cardiologist if acute myocardial infarction was suspected.
|| Therapeutic hypothermia was considered when the patients remain comatose after ROSB (ECPR group) or ROSC (CCPR group) and decided by the ICU attending physicians.
# Minimal neurological impairment was defined as a Modified Glasgow Outcome Score (MGOS) ≥ 4.
Included studies
Two studies were prospective cohort,47, 48 one study performed post hoc analysis of a previously published, prospective single-center study,46 and the last three were retrospective cohort.45, 46, 47, 48, 49, 50 Four studies performed propensity score matched analysis45, 46, 47, 48, 49 and two used a logistic regression analysis.47, 50 Two studies were conducted in South Korea,45, 49 two in Taiwan,48, 50 and two in Japan.47, 46 Years of patient inclusion ranged from 2000 to 2013. Eligibility criteria for ECPR varied across studies; an overview is provided in Appendix D. The average age of patients exposed to the intervention ranged from 53 to 60 and 58 to 69 in the control group. In the ECPR group 90.0% of patients were male vs. 70.0% in the control group. Witnessed arrest was present in 457 of 474 patients (96.4%) in the ECPR group and 933 of 1071 patients (87.1%) in the control group. Bystander CPR was performed in 179 of 366 patients (47.3%) in the ECPR group and in 283 of 366 patients (77.3%) in the control group. The initial documented heart rhythm of ventricular fibrillation was reported in 117 of 252 patients (44.4%) in the ECPR group and in 218 of 987 patients (22.1%) in the control group. Overall collapse to ECPR times was not reported. Three studies reported no-flow duration (collapse to initial CPR),45, 46, 47, 48, 49, 50 one reported CPR to ECMO duration,50 and another reported time interval from ROSC to ECMO implantation.45 Two studies reported neurologically intact survival at three months,45, 47 one at six months,47 two at one year,48, 50 and one at two years.49 The rates of long-term neurologically intact survival after IHCA and OHCA, IHCA, or OHCA of ECPR-treated patients were 15.5%, 22.7%, and 12.3%, respectively. Conversely, the rates of long-term neurologically intact survival after IHCA and OHCA, IHCA, or OHCA treated with conventional CPR were 6.2%, 6.8%, and 5.9%, respectively.45, 46, 47, 48, 49, 50 Five studies defined favorable neurological outcome as a Cerebral Performance Category score of 1–2.45, 46, 47, 48, 49, 50 One study defined minimal neurological impairment as a Modified Glasgow Outcome Score (MGOS) ≥ 4.49 No studies specifically assessed adverse events. An overview of the clinical course, complications, and circuit configuration and setup is provided in Table 5, Table 6.
Table 5.
Clinical course and complications of the ECPR group and the CCPR group of studies included.
| Patients (n) |
Weaned off cardiac assist device (ECMO) (%) |
Bridge to short/long term IABP/VAD or HTP (%) |
Bleeding (%) |
Peripheral vessel complications (%) |
Blood transfusions (pRBC/FFP) (%) |
Duration of ECMO (hrs) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, year, country | ECLS | CCPR | ECLS | CCPR | ECLS | CCPR | ECLS | CCPR | ECLS | CCPR | ECLS | CCPR | ECLS | CCPR |
| Chen et al. 200848 Taiwan | 59 | 113 | 29 (49)* | Not applicable | IABP: NR | … | … | … | … | … | pRBC: NR | … | 110 ± 28 | … |
| VAD: 5.1 | FFP: NR | |||||||||||||
| HTP: 8.1 | ||||||||||||||
| Kim et al. 201445 South Korea | 55 | 444 | 8 (15)† | Not applicable | IABP: NR | … | 27‡ | … | … | … | pRBC§ | … | 43.6 | … |
| VAD: 5.1 | FFP: NR | (29.7–92.8)† | ||||||||||||
| HTP: 8.1 | ||||||||||||||
| Maekawa et al. 201346 Japan | 53 | 109 | … | Not applicable | IABP: 51 | IABP: 9.2 | 33‖ | … | 15.4 | … | pRBC: NR | … | … | … |
| VAD: NR | FFP: NR | |||||||||||||
| HTP: NR | ||||||||||||||
| Sakamoto et al. 201447 Japan | 260 | 194 | … | Not applicable | IABP: 63 | IABP: 12 | …¶ | … | … | … | pRBC: NR | … | … | … |
| VAD: NR | FFP: NR | |||||||||||||
| HTP: NR | ||||||||||||||
| Shin et al. 201349 South Korea | 85 | 321 | … | Not applicable | IABP: NR | VAD: 0.0 | … | … | … | … | pRBC: NR | … | … | … |
| VAD: 0.0 | HTP: 0.9 | FFP: NR | ||||||||||||
| HTP: 2.4 | ||||||||||||||
| Siao et al. 201550 Taiwan | 20 | 40 | … | Not applicable | IABP: 50 | IABP: 25 | … | … | … | … | pRBC: NR | … | 79.7 ± 35.1 | … |
| VAD: NR | FFP: NR | |||||||||||||
| HTP: NR | ||||||||||||||
Abbreviations: CCPR = conventional cardiopulmonary resuscitation; ECMO = extracorporeal membrane oxygenation; ECPR = extracorporeal cardiopulmonary resuscitation; FFP = fresh frozen plasma; HTP = heart transplant; IABP = intra-aortic balloon pumping; pRBC = packed red blood cells; VAD = ventricular assist device.
NotesTotal percentages refer to studies with available data and continuous variables reported as mean ± SD or as median IQR.
* Weaning, defined as successful separation from extracorporeal life-support without mortality in 12 h, was not attempted until 72 h after initiation. Ventricular assist device and heart transplantation were alternatives in the absence of contraindications when weaning was unsuccessful in 5–7 days.
† Successful weaning and duration of ECMO in patients with a good neurological outcome (measured as a CPC score of 1 or 2).
‡ Reported bleeding at access site 27.3%, leg ischemia 6.8%, circuit failure 0%, intracranial hemorrhage/stroke 2.3%, and acute kidney injury 1.9%.
§ Amount of transfused pRBC5 (3–12) in patient with a CPC 1, 2 (n = 8) and pRBC: 5 (1–10) in patient with a CPC 3–5 (n = 44).
‖ Reported bleeding at access site 32.7%, leg ischemia requiring reperfusion 15.4%, unsuccessful cannulation 1.9%, infection 7.7%, and compartment syndrome requiring fasciotomy 1.9%.
¶ During the study period, several ECMO-related complications were reported. Bleeding and hematoma of insertion sites were relatively common. Other rare complications were vascular injury, catheter infection, limb ischemia, gastrointestinal bleeding, hemolysis, and stroke. Transfusion of pRBC and FFP were performed but total percentages were not reported.
Table 6.
Configuration and component set-up of the extracorporeal membrane oxygenation system of studies included.
| Authors, year, country | Centrifugal pump | Cannulation procedure and strategy | Arterial catheter | Venous catheter | Anterograde reperfusion catheter | Initiation of pump flow rate | ACT aim therapeutic range |
|---|---|---|---|---|---|---|---|
| Chen et al. 200848 Taiwan | Bio-Pump, Medtronic, Anaheim, USA | Percutaneous femoral cannulation was preferred in most cases | Not specified | Not specified | Yes* | 50–100 mL/kg/min | 160–180 s (220 s during weaning) |
| Kim et al. 201445 South Korea | Twin-pulse life support (T-PLS), New Heartbio, Korea Capiox Emergency Bypass System, Terumo Corp, Tokyo, Japan | Percutaneous femoral artery and vein using the Seldinger technique | 15–17 Fr | 21–23 Fr | … | 2.5–3.0 L/min | 200–220 s |
| Maekawa et al. 201346 Japan | Capiox Emergency Bypass System, Terumo Corp, Tokyo, Japan | Percutaneous femoral artery and vein cannulation. Femoral cut down procedures were not performed | 15–17 Fr | 19–21 Fr | As necessary | 50–60 mL/min/kg | … |
| Sakamoto et al. 201447 Japan | Several types of centrifugal pumps were used | Percutaneous femoral artery and vein (or any other method) | Not specified | Not specified | As necessary | Maximal flow rate (target: 4 L/min or above) | 1.5–2.5 times normal |
| Shin et al. 201349 South Korea | Capiox Emergency Bypass System, Terumo Corp, Tokyo, Japan | Percutaneously in a majority of case or surgically in challenging cases | 14–21 Fr | 21–28 Fr | Yes† | 2.2 L/min/BSA (m2)‡ | … |
| Siao et al. 201550 Taiwan |
Bio-Pump, Medtronic, Anaheim, USA | Femoral cannulation in the emergency department | Not specified | Not specified | … | A minimum flow of 2 L/min | 180–220 s |
Abbreviations: ACT = activated clotting time; BSA = body surface area.
Notes: Only one study reported unsuccessful cannulation or if cannulation strategy was performed by emergent cannulation, cannulation guidance by ultrasound or combination of ultrasound and fluoroscopy guided cannulation. This study used ultrasound-guided catheter insertion in the emergency department and fluoroscope-guided catheter insertion in the catheterization room.45
* No bridging tube between the arterial and venous lines was applied. To avoid possible distal malperfusion an antegrade reperfusion catheter for distal limb perfusion was applied when the mean pressure of the superficial femoral artery was below 50 mmHg.
† A bypass catheter was inserted into the femoral artery to facilitate distal limb perfusion in the event of leg ischemia after arterial cannulation.
‡ The flow rate was set above 2.2 L/min/body surface area (m2) initially, and was adjusted subsequently to maintain a mean arterial pressure above 65 mm Hg.
Risk of bias in included studies
We present details of our risk of bias judgments in the risk of bias summary table. Based on the ROBINS-I tool, all studies were deemed to have an overall serious risk of bias, which could also be considered critical, with confounding being the primary source of bias. Additional details of bias assessments using the ROBINS-I tool are provided in eTable 1 in the Supplementary Contents.
Primary outcomes
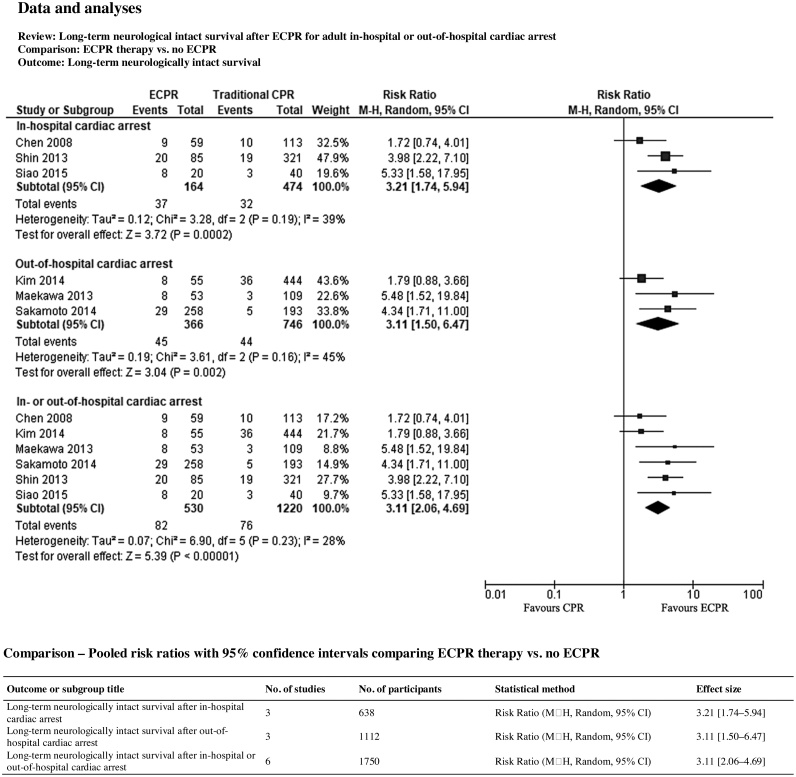
Long-term neurologically intact survival after cardiac arrest occurred in 82 of 530 patients (15.5%) in the ECPR group and in 76 of 1220 patients (6.2%) in the control group. Statistical heterogeneity among these studies was not significant according to the Chi2 test and the I2 statistic (Chi2 = 6.90, degrees of freedom (df) = 5, p-value 0.23, I2 = 28%). The pooled results for cardiac arrest in any setting show that ECPR compared to no ECPR and/or conventional CPR is likely associated with improved long-term neurologically intact survival after IHCA and OHCA (RR 3.11, 95% CI 2.06–4.69, p < 0.00001; 6 studies, 1750 participants; an increase from 62 to 194 per 1000, 95% CI 128–292; Fig. 2) (GRADE: Very low quality; downgraded for serious risk of bias).45, 46, 47, 48, 49, 50
Fig. 2.
Forest plot of comparison of long‐term neurological intact survival after adult cardiac arrest. Abbreviations: CI = confidence interval; event = number of patient with outcomes; total = number of participants at risk; df = degree of freedom; I2 = indicate the percentage of total variation across the studies that is due to heterogeneity rather than change; M—H = stands for the Mantel-Haenszel method. The result and its 95% CI are presented by a diamond, with the risk ratio (95% CI) and its statistical significance given alongside. Squares or diamonds to the right of the solid vertical line indicate benefit with the intervention (ECPR) over the comparator group (no ECPR), but this is conventionally significant (p < 0.05) only if the horizontal line or diamond does no overlap the solid vertical line. Neurologically intact survival (i.e. long-term [three months to two years]) were combined into a single category.
Long-term neurologically intact survival after IHCA occurred in 37 of 164 patients (22.6%) in the ECPR group and in 32 of 474 patients (6.8%) in the control group. Statistical heterogeneity among these studies was moderate according to the Chi2 test and the I2 statistic (Chi2 = 3.28, df = 2, p-value 0.19, I2 = 39%). The pooled results for in-hospital arrest show that ECPR compared to no ECPR and/or conventional CPR is likely associated with improved long-term neurologically intact survival after IHCA (RR 3.21, 95% CI 1.74–5.94, p < 0.0002; 3 studies, 638 participants; an increase from 68 to 217 per 1000, 95% CI 117–401; Fig. 2) (GRADE: Very low quality; downgraded for serious risk of bias).48, 49, 50
Long-term neurologically intact survival after OHCA occurred in 45 of 366 patients (12.3%) in the ECPR group and in 44 of 746 patients (5.9%) in the control group. Statistical heterogeneity among these studies was moderate according to the Chi2 test and the I2 statistic (Chi2 = 3.61, df = 2, p-value 0.16, I2 = 45%). The pooled results for out-of-hospital arrest showed that ECPR compared to no ECPR and/or conventional CPR is likely associated with improved long-term neurologically intact survival after OHCA (RR 3.11, 95% CI 1.50–6.47, p < 0.002; 3 studies, 1112 participants; an increase from 59 to 183 per 1000, 95% CI 88–382; Fig. 2) (GRADE: Very low quality; downgraded for serious risk of bias).45, 46, 47
Assessment of reporting biases
We did not test for publication bias using a funnel plot or other analytical methods because fewer than 10 studies were included.59
Summary of findings table and GRADE assessment
We created a summary of findings table for the outcomes of interest. Based on the GRADE criteria, the overall quality of the evidence was graded very low quality. We downgraded the overall quality to very low due to a serious risk of bias. A summary of findings and GRADE assessment is provided in eTable 2 in the Supplementary Contents.
Subgroup analysis
All studies included participants with non‐traumatic cardiac arrest; however, few studies reported subgroup data according to time periods (i.e. no-flow time, low flow time). We performed no pre-defined subgroup analyses or investigations of heterogeneity due to insufficient data.
Sensitivity analysis
We were unable to perform the planned sensitivity analyses due to the small number of included studies.
Discussion
We identified a limited number of studies comparing the use of ECPR vs. no ECPR and/or conventional CPR in terms of long-term neurologically intact survival after adult IHCA or OHCA. Six cohort studies met our inclusion criteria, totaling 1750 cardiac arrest patients. Of these, 530 (30.3%) received the intervention; 91 (17.2%) survived with long-term neurologically intact survival.45, 46, 47, 48, 49, 50 The studies were non-randomized, prospective or retrospective cohorts; some used propensity score matching.45, 46, 48, 49 Some of the studies provided adjusted data; however, the included covariates were not sufficient to significantly reduce the risk of bias. Current evidence for the use of ECPR for cardiac arrest is limited by clinical heterogeneity and paucity of evidence at the level of RCTs, but we identified that the evidence is more in favor of ECPR than the comparator group (conventional CPR). There was a moderate degree of heterogeneity in our pooled analyses. We used a random-effects model for pooled analyses to account for these differences. No currently available RCT has investigated ECMO in the context of ECPR for cardiac arrest, though several are ongoing, as noted on the International Clinical Trials Registry Platform. Our analysis suggests that the risk of bias for individual studies was serious, which could also be considered critical. Based on GRADE criteria, the overall quality of evidence was very low across all outcomes, with the quality of evidence downgraded for serious risk of bias, with confounding being the primary source of bias.
The International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support Task Force recently performed a systematic review comparing the use of ECPR vs. manual or mechanical CPR for adults and children following cardiac arrest. The results of the studies included in their review were mixed and the quality of evidence was overall assessed as very low and at high risk of bias.64 Subsequently, the ILCOR has suggested ECPR may be considered a rescue therapy for selected patients with cardiac arrest when conventional CPR fails in settings where this can be implemented (weak recommendation, very low certainty of evidence).64 The inclusion criteria of our review differ from the ILCOR review as we restricted our analyses to long-term neurologically intact survival and we separated and pooled the data after adult cardiac arrest in any setting (in-hospital or out-of-hospital). In our review the overall individual and pooled estimates and 95% CIs for the random-effects model examining long-term neurologically intact survival after IHCA and OHCA, IHCA, or OHCA are more in favor of ECPR than conventional CPR.45, 46, 47, 48, 49, 50 Our analysis suggests that the treatment effect (or effect size) is consistent with the findings of most primary studies.45, 46, 47, 48, 49, 50 These results are consistent with previous systematic reviews and meta-analyses, meaning that the strategy of ECPR has resulted in functionally favorable survival rates ranging from 10% to 45%,65, 66, 67, 68, 69, 70, 71, 72, 73 provided that cardiac arrest patients present with initial shockable cardiac rhythm, shorter CPR duration, higher admission arterial pH, and lower admission serum lactate level.14, 65 The ELSO Registry reports 29.0% survival to discharge/transfer in those treated with ECPR. This indicates the current clinical interest in ECPR for cardiac arrest patients.12 ECPR thus seems to be a valuable option in selected cases.10, 11
Patients receiving ECPR in this review were more likely to be male, younger than 75 years, have potentially reversible conditions, suffer from acute coronary syndrome, and to undergo emergency cardiac catheterization and, when necessary, coronary revascularization.45, 46, 47, 48, 49, 50 All of these factors are known to be associated with increased survival to discharge and neurologically intact survival. Outcomes of ongoing RCTs will clarify the role of ECPR in this particular population. When ECMO is used as ECPR, efforts should be made to select patients who would benefit from the intervention and to minimize the duration of CPR to ECMO flow, as both are critical determinants of favorable outcomes.14, 65 ECMO should be initiated expeditiously in potentially reversible cases of refractory IHCA or OHCA, regardless of whether ROSC has been achieved, with manual and/or mechanical chest compressions to facilitate return of ROSC and to support further resuscitation efforts, including early coronary angiography and percutaneous coronary intervention (PCI) in selected patients with a suspected or obvious cardiac cause of cardiac arrest.10, 11 Further effort should be made to evaluate bundle treatment options used during ECPR, including coronary catheterization laboratory (CCL) (i.e. PCI, coronary artery bypass grafting),16, 17 CCL and target temperature management (TTM),18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 CCL and intra-aortic balloon pump (IABP),24 and CCL, IABP, and TTM.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 The development of practices associated with success, like creating large, feasible, regional or state-wide integrated resuscitation networks similar to the Minnesota Resuscitation Consortium should be a priority (i.e. the University of Minnesota refractory VF/VT ECPR protocol for OHCA), with the goal of overcoming knowledge gaps and improving outcomes in individuals who would have otherwise died (Appendix E).26, 27
Limitations
Our review should be interpreted in the context of certain limitations. This review was limited to articles from four databases published in English between January 1st, 2000, and January 31st, 2020. This review aimed to identify the most recent and relevant articles, and therefore we excluded older publications; our preliminary review suggested there would be no relevant articles prior to 2000. While we took steps to ensure all relevant articles were included, it is possible some were missed due to the selection of databases, search terms, and language limitations. Therefore, there is an acknowledged risk of bias in article selection and interpretation. Most of the included studies were non-randomized and prospective or retrospective cohort design, single-center, and had high risk of bias, in particular confounding bias, potentially limiting internal validity. There was an attempt to adjust for confounding factors in the design and/or analysis of each study, yet the observed association between exposure and outcome is still dominated by residual confounding effects, potentially limiting our analysis. A major limitation of this study is the pooling unadjusted results of some heterogeneous outcomes. Furthermore, all studies were from Asia, thus are unlikely to reflect systems of care in North America and Europe, limiting comparability. Outcomes reported among controls are also lower than those in other developed countries, where the rate of bystander CPR is considerably higher than in Asia. All of which potentially limits applicability, generalizability, external validity, and possible considerations for indirectness. Our ability to draw conclusions is thus severely limited by the quality of the primary data. The well-recognized weaknesses of observational studies mean that no reliable conclusions can be drawn from the primary data, which carries a high risk of bias and may yield precise but spurious results when combined.73 This thus suggests the importance of high quality research into the feasibility and patient-centered outcomes of using ECPR in novel settings, such as via EMS-based or ED-based large randomized trials, to further investigate the effectiveness of ECPR for cardiac arrest.
Since this review was accepted, in October 2020, we searched for recent studies on the topic. The Advanced Reperfusion Strategies for Refractory Cardiac Arrest (ARREST) trial has recently been published. The trial evaluated the initiation of ECMO in the cardiac catheterization laboratory compared with Advanced cardiac life support (ACLS) among patients with OHCA and refractory VF/VT.74 The primary outcome, survival to hospital discharge, occurred in 43.0% of the ECMO group, compared to 7.0% of the standard ACLS group (posterior probability = 0.9861). The secondary outcome, survival to 6 months, occurred in 43.0% of the ECMO group, compared to 0.0% of the standard ACLS group (p = 0.0063). The ARREST trial showed that early implementation of ECMO was superior to standard ACLS at improving survival of people who suffered refractory VF/VT OHCA.74 This trial paves the way for further research into advanced targeted therapies and advanced cardiac care.
Conclusions
VA-ECMO used as ECPR is a contemporary resuscitation approach that is likely associated with improved long-term neurologically intact survival after adult cardiac arrest. Using GRADE methodology we conclude that the quality of evidence is very low. We further conclude that evidence from randomized trials is very likely to have an important impact on the estimated effect of this intervention and will further define optimal clinical practice.
Author contributions
DM, LM, and WA were responsible for the study conception and design. DM and LM were responsible for data abstraction, analysis, and interpretation of the data. DM performed the statistical analysis and drafted the original manuscript. All authors reviewed and approved the final version of the manuscript. All authors meet ICMJE authorship criteria. DM takes responsibility for the integrity of the data, the accuracy of the data analysis and for the paper as a whole.
Declaration of competing interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no specific grant support from any funding agency in the commercial or not-for-profit sector. No sponsors had any role in the study design, implementation, data collection, analysis or preparation of this manuscript.
Acknowledgments
The authors would like to thank the library staff at the Veterans Affairs Caribbean Healthcare System Library Service for assistance with producing the search strategy.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resplu.2020.100045.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Douglas J.E., Schuerer D.J., Kolovos N.S., Boyd K.V., Coopersmith C.M. Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest. 2008;134:179–184. doi: 10.1378/chest.07-2512. [DOI] [PubMed] [Google Scholar]
- 2.Sidebotham D., McGeorge A., McGuinness S., Edwards M., Willcox T., Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory disease in adults: Part 1—overview of extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2009;23:886–892. doi: 10.1053/j.jvca.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Conrad S.A., Broman L.M., Taccone F.S. The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support: a position paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med. 2018;198:447–451. doi: 10.1164/rccm.201710-2130CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappalardo F., Montisci A. What is extracorporeal cardiopulmonary resuscitation? J Thorac Dis. 2017;9:1415–1419. doi: 10.21037/jtd.2017.05.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagawa E. Extracorporeal cardiopulmonary resuscitation for adult cardiac arrest patients. World J Crit Care Med. 2012;1:46–49. doi: 10.5492/wjccm.v1.i2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McRae K., de Perrot M. Principles and indications of extracorporeal life support in general thoracic surgery. J Thorac Dis. 2018;10:S931–S946. doi: 10.21037/jtd.2018.03.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keebler M.E., Haddad E.V., Choi C.W. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail. 2018;6:503–516. doi: 10.1016/j.jchf.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 8.de Caen A.R., Berg M.D., Chameides L. Part 12: pediatric advanced life support: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S526–42. doi: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maconochie I.K., Bingham R., Eich C. European Resuscitation Council Guidelines for Resuscitation 2015: Section 6. Paediatric life support. Resuscitation. 2015;95:223–248. doi: 10.1016/j.resuscitation.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Link M.S., Berkow L.C., Kudenchuk P.J. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S444–64. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 11.Soar J., Nolan J.P., Böttiger B.W. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Extracorporeal Life Support Organization. ECLS Registry Report: International Summary. https://www.elso.org/. Accessed January 2020.
- 13.Thiagarajan R.R., Barbaro R.P., Rycus P.T. ELSO Member Centers: Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 14.Debaty G., Babaz V., Durand M. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. doi: 10.1016/j.resuscitation.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Wengenmayer T., Rombach S., Ramshorn F. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR) Crit Care. 2017;21:157. doi: 10.1186/s13054-017-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tazarourte K., Sapir D., Laborne F.X. Refractory cardiac arrest in a rural area: mechanical chest compression during helicopter transport. Acta Anaesthesiol Scand. 2013;57:71–76. doi: 10.1111/j.1399-6576.2012.02759.x. [DOI] [PubMed] [Google Scholar]
- 17.Shinar Z., Bellezzo J., Paradis N. Emergency department initiation of cardiopulmonary bypass: a case report and review of the literature. J Emerg Med. 2012;43:83–86. doi: 10.1016/j.jemermed.2011.06.134. [DOI] [PubMed] [Google Scholar]
- 18.Haneya A., Philipp A., Diez C. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83:1331–1337. doi: 10.1016/j.resuscitation.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Leick J., Liebetrau C., Szardien S. Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin Res Cardiol. 2013;102:661–669. doi: 10.1007/s00392-013-0580-3. [DOI] [PubMed] [Google Scholar]
- 20.Stub D., Bernard S., Pellegrino V. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Fagnoul D., Taccone F.S., Belhaj A. Extracorporeal life support associated & with hypothermia and normoxemia in refractory cardiac arrest. Resuscitation. 2013;84:1519–1524. doi: 10.1016/j.resuscitation.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Lebreton G., Pozzi M., Luyt C.E. Out-of-hospital extra-corporeal life support implantation during refractory cardiac arrest in a half-marathon runner. Resuscitation. 2011;82:1239–1242. doi: 10.1016/j.resuscitation.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Putzer G., Mair B., Hangler H., Strohle M., Mair P. Emergency extracorporeal life support after prolonged out-of-hospital cardiac arrest. J Cardiothorac Vasc Anesth. 2014;28:1036–1038. doi: 10.1053/j.jvca.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Shin J.S., Lee S.W., Han G.S., Jo W.M., Choi S.H., Hong Y.S. Successful extracorporeal life support in cardiac arrest with recurrent ventricular fibrillation unresponsive to standard cardiopulmonary resuscitation. Resuscitation. 2007;73:309–313. doi: 10.1016/j.resuscitation.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Lamhaut L., Jouffroy R., Soldan M. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation. 2013;84:1525–1529. doi: 10.1016/j.resuscitation.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Yannopoulos D., Bartos J.A., Martin C. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yannopoulos D., Bartos J.A., Raveendran G. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–1117. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 28.Avalli L., Maggioni E., Formica F. Favorable survival of in-hospital compared to out-of-hospital refractory cardiac arrest patients treated with extracorporeal membrane oxygenation: an Italian tertiary care centre experience. Resuscitation. 2012;83:579–583. doi: 10.1016/j.resuscitation.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Wang C.H., Chou N.K., Becker L.B. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;85:1219–1224. doi: 10.1016/j.resuscitation.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Nagao K., Kikushima K., Watanabe K. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ J. 2010;74:77–85. doi: 10.1253/circj.cj-09-0502. [DOI] [PubMed] [Google Scholar]
- 31.Le Guen M., Nicolas-Robin A., Carreira S. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care. 2011;15 doi: 10.1186/cc9976. R29.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mojoli F., Venti A., Pellegrini C. Hospital survival and long term quality of life after emergency institution of venoarterial ECMO for refractory circulatory collapse. Minerva Anestesiol. 2013;79:1147–1155. [PubMed] [Google Scholar]
- 33.Mochizuki K., Imamura H., Iwashita T., Okamoto K. Neurological outcomes after extracorporeal cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest: a retrospective observational study in a rural tertiary care center. J Intensive Care. 2014;2:33. doi: 10.1186/2052-0492-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagawa E., Dote K., Kato M. Should we emergently revascularize occluded coronaries for cardiac arrest? Rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605–1613. doi: 10.1161/CIRCULATIONAHA.111.067538. [DOI] [PubMed] [Google Scholar]
- 35.Choi D.S., Kim T., Ro Y.S. Extracorporeal life support and survival after out-of-hospital cardiac arrest in a nationwide registry: A propensity score-matched analysis. Resuscitation. 2016;99:26–32. doi: 10.1016/j.resuscitation.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Poppe M., Weiser C., Holzer M. The incidence of “load&go” out-of-hospital cardiac arrest candidates for emergency department utilization of emergency extracorporeal life support: a one-year review. Resuscitation. 2015;91:131–136. doi: 10.1016/j.resuscitation.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Blumenstein J., Leick J., Liebetrau C. Extracorporeal life support in cardiovascular patients with observed refractory in-hospital cardiac arrest is associated with favourable short and long-term outcomes: a propensity-matched analysis. Eur Heart J Acute Cardiovasc Care. 2016;5:13–22. doi: 10.1177/2048872615612454. [DOI] [PubMed] [Google Scholar]
- 38.Pozzi M., Koffel C., Armoiry X. Extracorporeal life support for refractory out-of- hospital cardiac arrest: should we still fight for? A single-centre, 5-year experience. Int J Cardiol. 2016;204:70–76. doi: 10.1016/j.ijcard.2015.11.165. [DOI] [PubMed] [Google Scholar]
- 39.Schober A., Sterz F., Herkner H. Emergency extracorporeal life support and ongoing resuscitation: a retrospective comparison for refractory out-of-hospital cardiac arrest. Emerg Med J. 2017;34:277–281. doi: 10.1136/emermed-2015-205232. [DOI] [PubMed] [Google Scholar]
- 40.Shin T.G., Choi J.H., Jo I.J. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011;39:1–7. doi: 10.1097/CCM.0b013e3181feb339. [DOI] [PubMed] [Google Scholar]
- 41.Chen J.S., Ko W.J., Yu H.Y. Analysis of the outcome for patients experiencing myocardial infarction and cardiopulmonary resuscitation refractory to conventional therapies necessitating extracorporeal life support rescue. Crit Care Med. 2006;34:950–957. doi: 10.1097/01.CCM.0000206103.35460.1F. [DOI] [PubMed] [Google Scholar]
- 42.Kagawa E., Inoue I., Kawagoe T. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest treated with cardiopulmonary resuscitation with extracorporeal life support. Resuscitation. 2010;81:968–973. doi: 10.1016/j.resuscitation.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 43.Venturini J.M., Retzer E., Estrada J.R. Mechanical chest compressions improve rate of return of spontaneous circulation and allow for initiation of percutaneous circulatory support during cardiac arrest in the cardiac catheterization laboratory. Resuscitation. 2017;115:56–60. doi: 10.1016/j.resuscitation.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Debaty G., Moustapha I., Bouzat P. Outcome after severe accidental hypothermia in the French Alps: a 10-year review. Resuscitation. 2015;93:118–123. doi: 10.1016/j.resuscitation.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Kim S.J., Jung J.S., Park J.H., Park J.H., Hong Y.S., Lee S.W. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out-of-hospital cardiac arrest: a propensity-matched study. Crit care. 2014;18:535. doi: 10.1186/s13054-014-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maekawa K., Tanno K., Hase M., Mori K., Asai Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med. 2013;41:1186–1196. doi: 10.1097/CCM.0b013e31827ca4c8. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto T., Morimura N., Nagao K. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y.S., Lin J.W., Yu H.Y. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372:554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 49.Shin T.G., Jo I.J., Sim M.S. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol. 2013;168:3424–3430. doi: 10.1016/j.ijcard.2013.04.183. [DOI] [PubMed] [Google Scholar]
- 50.Siao F.Y., Chiu C.C., Chiu C.C.W. Managing cardiac arrest with refractory ventricular fibrillation in the emergency department: conventional cardiopulmonary resuscitation versus extracorporeal cardiopulmonary resuscitation. Resuscitation. 2015;92:70–76. doi: 10.1016/j.resuscitation.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Moher D., Liberati A., Tetzlaff J., Altman D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 52.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobs I., Nadkarni V., Bahr J. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation. Resuscitation. 2004;63:233–249. doi: 10.1016/j.resuscitation.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 54.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Lefebvre C., Manheimer E., Glanville J. 2011. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. The Cochrane Collaboration. [Google Scholar]
- 56.Sterne J.A., Hernán M.A., Reeves B.C. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higgins J.P., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. handbook.cochrane.org. Available from. [Google Scholar]
- 58.The Cochrane Collaboration; 2014. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre. [Google Scholar]
- 59.Rucker G., Schwarzer G., Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine. 2008;27:746–763. doi: 10.1002/sim.2971. [DOI] [PubMed] [Google Scholar]
- 60.Sawyer K.N., Kurz M.C. Caution when defining prolonged down time in out of hospital cardiac arrest as extracorporeal cardiopulmonary resuscitation becomes accessible and feasible. Resuscitation. 2014;85:979–980. doi: 10.1016/j.resuscitation.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Guyatt G.H., Oxman A.D., Kunz R., Vist G.E., Falck-Ytter Y., Schunemann H.J. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guyatt G.H., Oxman A.D., Vist G. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 63.McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 14 February 2020. Hamilton (ON): McMaster University (developed by Evidence Prime).
- 64.Holmberg M.J., Geri G., Wiberg S. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: a systematic review. Resuscitation. 2018;131:91–100. doi: 10.1016/j.resuscitation.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Arrigo S., Cacciola S., Dennis M. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Kim S.J., Kim H.J., Lee H.Y., Ahn H.S., Lee S.W. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: a meta-analysis. Resuscitation. 2016;102:106–116. doi: 10.1016/j.resuscitation.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 67.Ouweneel D.M., Schotborgh J.V., Limpens J. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Medicine. 2016;42:1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ortega-Deballon I., Hornby L., Shemie S.D., Bhanji F., Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation. 2016;101:12–20. doi: 10.1016/j.resuscitation.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 69.Wang G.N., Chen X.F., Qiao L. Comparison of extracorporeal and conventional cardiopulmonary resuscitation: a meta-analysis of 2260 patients with cardiac arrest. World J Emerg Med. 2017;8:5–11. doi: 10.5847/wjem.j.1920-8642.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J., Ma Q., Zhang H., Liu S., Zheng Y. Predictors of survival and neurologic outcome for adults with extracorporeal cardiopulmonary resuscitation. Medicine. 2018:97. doi: 10.1097/MD.0000000000013257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beyea M.M., Tillmann B.W., Iansavichene A.E., Randhawa V.K., Aarsen K.V., Nagpal A.D. Neurologic outcomes after extracorporeal membrane oxygenation assisted CPR for resuscitation of out-of-hospital cardiac arrest patients: a systematic review. Resuscitation. 2018;130:146–158. doi: 10.1016/j.resuscitation.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 72.Miraglia D., Miguel L.A., Alonso W. Extracorporeal cardiopulmonary resuscitation for in‐ and out‐of‐hospital cardiac arrest: systematic review and meta-analysis of propensity score‐matched cohort studies. JACEP Open. 2020;4:342–361. doi: 10.1002/emp2.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egger M., Schneider M., Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140–144. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yannopoulos D., Bartos J., Raveendran G. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020 doi: 10.1016/s0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.