Abstract
Background
The use of obstetric early warning systems (OEWS) are recommended as an adjunct to reduce maternal morbidity and mortality. The aim of this review was to document the variation in OEWS trigger thresholds and the quality of information included within accompanying escalation protocols.
Methods
A review of OEWS charts and escalation policies across consultant-led maternity units in the UK (n = 147) was conducted. OEWS charts were analysed for variation in the values of physiological parameters triggering different levels of clinical escalation. Relevant data within the escalation protocols were also searched for: urgency of clinical response; seniority of responder; frequency of on-going clinical monitoring; and clinical setting recommended for on-going care.
Results
The values of physiological parameters triggering specific clinical responses varied significantly between OEWS. Only 99 OEWS charts (67.3%) had an escalation protocol as part of the chart. For 29 charts (19.7%), the only escalation information included was generic, for example to “contact a doctor if triggers”. Only 76 (51.7%) charts detailed the required seniority of responder, 37 (25.2%) the frequency for on-going clinical monitoring, eight (5.4%) the urgency of clinical response and two (1.4%) the recommended clinical setting for on-going care.
Conclusion
The observed variations in the trigger thresholds used in OEWS charts and the quality of information included within the accompanying escalation protocols is likely to lead to suboptimal detection and response to clinical deterioration during pregnancy and the post-partum period. The development of a national OEWS and escalation protocol would help to standardise care across obstetric units.
Keywords: Maternal health, Patient safety, Early warning systems, Patient escalation
Introduction
The use of obstetric early warning systems (OEWS) in UK maternity units was recommended in the 2007 Confidential Enquiry into Maternal and Child Health (CEMACH) report1 as an adjunct to reducing maternal morbidity and mortality.2, 3, 4, 5, 6, 7 Although the report recommended a specific system and chart, and despite subsequent widespread use of OEWS across UK maternity units,4, 6, 7, 8, 9, 10 there remains little consensus regarding the optimum design, incorporated vital sign parameters or how physiological normality is defined.11 This likely leads to variation in clinical practice across UK maternity units.10
In order to have utility, each OEWS is often accompanied by an escalation protocol that details the clinical actions required when the observed value of one or more included parameters reaches a trigger point. These protocols are presented either as an integral part of the OEWS chart or within a separate document. Typically, such protocols include information regarding the i) frequency of on-going clinical monitoring, ii) urgency of a clinical response, iii) required seniority of responder, and iv) need to consider an appropriate setting for on-going care.12 The escalation response is typically graded across three levels (low, medium, high) according to patient risk.
Given the widespread modifications to OEWS in use across the UK,10 it is intuitive that their associated escalation protocols may also vary. The absence of a standardised approach to acutely unwell pregnant and postnatal women is likely to lead to an inconsistent approach to the identification, escalation and subsequent care received12, 13 and possible staff confusion. The strength of the OEWS chart and escalation protocol lies in a standardised, consistent approach to their use. These charts have been validated and selected based on their ability to predict adverse maternal outcomes and prevent further deterioration. Local modifications to these charts and protocols, based on clinical consensus, threaten their utility. Furthermore it is expected that physiological thresholds contained within an OEWS must be consistent with national pregnancy care guidelines.14
The purpose of this study was two-fold. First, we sought to document the thresholds used to trigger different levels of clinical escalation in OEWS used in UK consultant-led maternity departments. Second, we sought to explore staff guidance regarding the escalation of care located within units’ escalation protocols.
Methods
In 2014, the Modified Obstetric Early Warning Systems (MObs) Research Group at Bournemouth University wrote to 194 lead consultant anaesthetists registered with the Obstetric Anaesthetists’ Association (OAA), requesting a copy of the OEWS and associated escalation protocol used in their unit. The methodology used in that study has been previously fully described,11 and the associated analyses of the 120 usable charts received have been published elsewhere.11, 15 An additional set of 27 charts and escalation protocols were obtained independently in 2016 by the UK Audit and Research Collaborative in Obstetrics and Gynaecology’s (UK-ARCOG) deanery representatives. The two sets of charts and escalation protocols were then amalgamated.
Two types of OEWS charts were clinically in use; charts using an aggregate-weighted system and those using a combination of single- and multiple-parameter systems. Aggregate-weighted systems use a defined ‘normal range’ for each physiological parameter. Any measured physiological value outside of this range is allocated a score depending on the degree of physiological disturbance and deviation from ‘normal’. The magnitude of the total score reflects the corresponding trigger response. Charts using a combination of single- and multiple-parameter systems also define a ‘normal range’ and then assign a colour depending on the degree of abnormality of each physiological parameter. A ‘yellow’ is considered mildly/moderately abnormal and a ‘red’ severely abnormal. A response is typically triggered when one red score or two yellow scores are triggered. Throughout this paper we will refer to charts using this system as colour-coded charts.
One member of the research team (JC) undertook a descriptive analysis of each OEWS and escalation protocol to determine the extent of variation. A second reviewer (WPS) analysed 10% of charts (n = 15/147) to validate the initial findings. The parameter thresholds and information within the escalation policies were descriptively analysed and compared to that contained within the Royal College of Physician’s National Early Warning Score (NEWS) and escalation policy, with respect to the following:
-
•
Thresholds used to trigger different levels of clinical escalation for each physiological parameter used in the OEWS (i.e. temperature, respiratory rate, oxygen saturation (SpO2), heart rate, systolic blood pressure (sBP), diastolic blood pressure (dBP));
-
•
Required urgency of clinical response;
-
•
Required seniority of the responding staff;
-
•
Required frequency of on-going clinical monitoring; and
-
•
Recommended clinical setting for managing the patient.
The escalation instructions for each component were categorised according to the level of risk for each woman, corresponding to increasing illness severity, as follows:
-
•
Low risk: one yellow trigger (for colour-coded systems) or a value of 1–3 (for aggregate-weighted scores);
-
•
Medium risk: two yellows or one red trigger or a value of 4–5;
-
•
High risk: greater than two yellows, two or more red triggers or a value of 6 or more.
In addition, we noted whether the escalation protocol was included alongside the OEWS chart or existed as a separate document only.
Details of ethics approval
In line with guidance from the National Health Service (NHS) Health Research Authority, this service evaluation did not require ethical approval. Prior approval was obtained from the OAA survey subcommittee.
Results
A total of 147 OEWS charts were available for analysis (original MObs set = 120; UK-ARCOG = 27). The charts’ locations of origin and whether an escalation protocol was included alongside the OEWS chart are shown in Table 1.
Table 1.
Chart demographics.
| Aggregate-weighted charts | Colour-coded charts | Total | |
|---|---|---|---|
| n/total (%) | n/total (%) | n/total (%) | |
| Location of sampled charts | |||
| England | 45/50 (90.0) | 69/97 (71.1) | 114/147 (77.6) |
| Health Education North East | 3/50 | 4/97 | 7/147 |
| Health Education North West | 9/50 | 4/97 | 13/147 |
| Health Education Yorkshire and the Humber | 4/50 | 7/97 | 11/147 |
| Health Education West Midlands | 8/50 | 10/97 | 18/147 |
| Health Education East Midlands | 5/50 | 2/97 | 7/147 |
| Health Education East of England | 6/50 | 7/97 | 13/147 |
| Health Education Thames Valley | 1/50 | 2/97 | 3/147 |
| Health Education North Central and East London | 1/50 | 5/97 | 6/147 |
| Health Education North West London | 2/50 | 2/97 | 4/147 |
| Health Education South London | 0/50 | 3/97 | 3/147 |
| Health Education Kent, Surry, Sussex | 4/50 | 8/97 | 12/147 |
| Health Education Wessex | 0/50 | 8/97 | 8/147 |
| Health Education South West | 2/50 | 7/97 | 9/147 |
| Scotland | 2/50 (4.0) | 11/97 (11.3) | 13/147 (8.8) |
| Wales | 2/50 (4.0) | 9/97 (9.3) | 11/147 (7.5) |
| Northern Ireland | 0/50 (0) | 5/97 (5.2) | 5/147 (3.4) |
| Other | 1/50 (2.0) | 3/97 (3.1) | 4/147 (2.7) |
| Total | 50 | 97 | 147 |
| Escalation protocol included as part of chart | 42/50 (84.0) | 57/97 (58.8) | 99/147 (67.3) |
Data expressed as number and percentages n/total (%).
Only two thirds of charts (99/147, 67.3%) had an escalation protocol as part of the OEWS chart, with the remainder (48/147, 32.7%) having a separate ‘stand-alone’ escalation protocol document (Table 1). Charts using an aggregate-weighted system were more likely to have an escalation protocol included as part of the OEWS chart (42/50, 84.0%), compared to those using a colour-coded escalation system (57/97, 58.8%). In 29/147 (19.7%) charts the only escalation information included on the charts was to contact a doctor if the woman had observations that triggered either one red or two yellows scores at any one time.
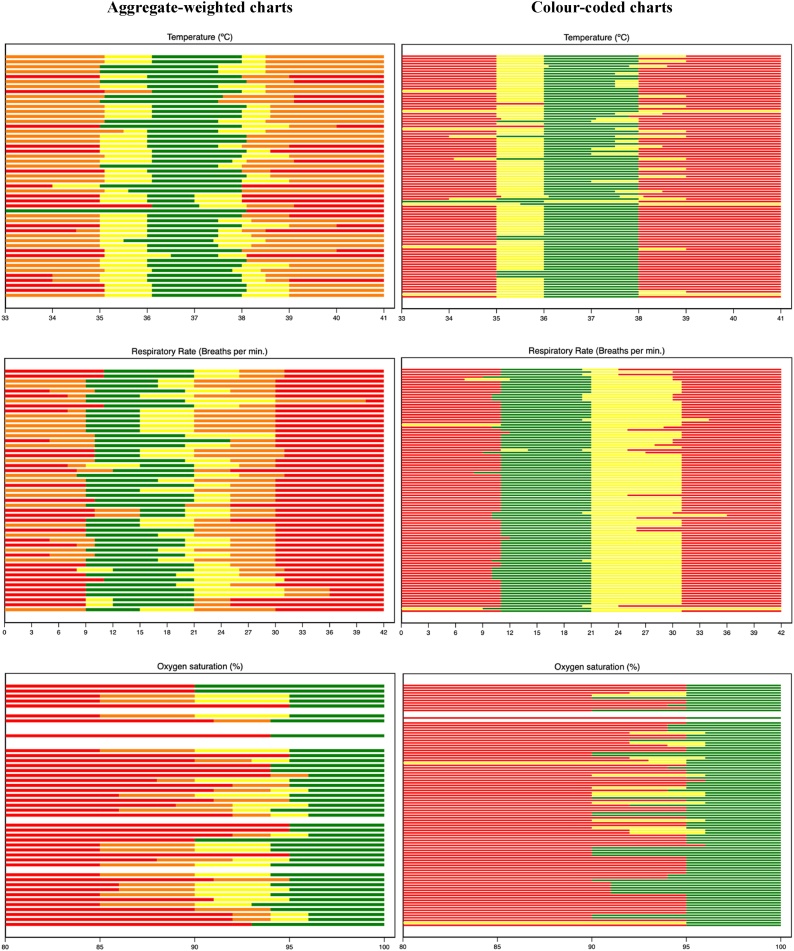
The variation in the trigger values for different physiological parameters and different levels of risk varied greatly between units and depended on the type of OEWS system used (Fig. 1, Fig. 2).
Fig. 1.
Variation in obstetric EWS trigger values for temperature, respiratory rate and SpO2 values. Each line represents an obstetric EWS chart. Green shading represents the ‘normal’ values for each measured parameter; yellow represents ‘mildly abnormal’ values; amber ‘moderately abnormal’ (aggregate-weighted only); and red ‘severely abnormal’. White lines represent charts where there was no data included on the obstetric EWS for that parameter.
Fig. 2.
Variation in obstetric EWS trigger values for heart rate, systolic blood pressure and diastolic blood pressure. Each line represents an obstetric EWS chart. Green shading represents ‘normal’ values for each measured parameter, yellow ‘mildly abnormal’, amber ‘moderately abnormal’ (aggregate-weighted only), red ‘severely abnormal’ and purple ‘extremely abnormal’. White lines represent charts where there was no data included on the obstetric EWS for that parameter.
Generally, units used a normal body temperature range of 36.0 °C–38 °C (99/147, 67.3%). However, in some, values of 36.5 °C, 37.0 °C and 37.5 °C were regarded to be abnormal. A temperature was considered by most units to be severely abnormal when over 38 °C (81/147, 55.1%). In six (4.1%) units a temperature over 40 °C was only considered mildly/moderately abnormal. A temperature below 35 °C was considered severely abnormal in most units (100/147, 68.0%).
A normal respiratory rate range of 11–21 breaths per minute was used by most units (77/147, 52.4%), however, in others (41/147, 27.9%), values between 15–21 were regarded abnormal. Respiratory rates above 31 breaths per minute were considered severely abnormal in 78/147 (53.1%) units. In six units (4.1%), a respiratory rate as high as 33 breaths per minute was only considered to be mildly/moderately abnormal. Most units (88/147, 59.9%) considered a respiratory rate below 11 breaths per minute to be severely abnormal, whilst in 22 units (15.0%), a respiratory rate below 9 breaths per minute was only considered mildly/moderately abnormal.
Several units (15/147, 10.2%) did not use SpO2 as a trigger parameter. Where they were used, SpO2 values above 95% generally considered normal (101/132, 76.5%), although, in some units (17/132, 12.9%), a value of above 90% was used to define normality.
Typically, units used a normal heart rate range of 50–100 beats per minute (87/147, 59.2%), although in several units (5/147, 4.0%) a heart rate of 60 beats per minute was considered abnormally low, whilst a heart rate of 90 beats per minute (18/147, 12.2%) was considered abnormally high. Most units (82/147, 55.8%) considered a heart rate of above 120 beats per minute severely abnormal, whilst 46/147 units (31.3%) considered above 130 beats per minute to be severely abnormal. Heart rates below 40 beats per minute were considered by most units (114/147, 77.6%) to be severely abnormal; however in 33/147 (22.4%) units, a heart rate below 40 beats per minute was only considered to be mildly/moderately abnormal.
Generally, 78/147 (53.1%) units used a normal sBP range of 100–150 mmHg, although several considered sBP values up to 160 mmHg (16/147, 10.9%) and 180 mmHg (4/147, 2.7%) to be normal. A sBP below 90 mmHg was considered to be severely abnormal in 82/147 (55.8%) units; whilst in 39/147 (26.5%) units a sBP below 70 mmHg was regarded as severely abnormal. Several units (10/147, 6.8%) did not use dBP as a trigger. In many units (105/137, 76.6%) a dBP value below 90 mmHg was regarded as normal (note: most of these units did not provide a lower limit for normal dBP values). A dBP above 100 mmHg was considered severely abnormal in 62/137 (45.3%) units. Five units (3.6%) units only considered a dBP above 110 mmHg to be mildly/moderately abnormal.
Table 2 displays the information included within the accompanying escalation protocols, irrespective of whether the protocol formed part of the chart or not. Across each escalation domain, aggregate-weighted OEWS were more likely to include information within their escalation protocol compared to the colour-coded OEWS. Information regarding the required seniority of responder for all risk categories was most frequently included (76/147, 51.7%), followed by frequency for on-going clinical monitoring (37/147, 25.2%) and urgency of clinical response (8/147, 5.4%). Information regarding the recommended clinical setting for on-going care was only included in 2/147 (1.4%) of the escalation protocols; however, this was more likely to be specified for women considered to be at ‘high risk’ (57/123, 46.3%) than those at ‘medium risk’ (23/146, 15.8%) or ‘low risk’ (2/141, 1.4%). On the whole, more information was included for women at ‘medium risk’ than for women at ‘low risk’ and ‘high risk’, although recommendations for clinical setting of care, such as on labour ward or in a high dependency unit, were more likely to be provided for women at ‘high risk’.
Table 2.
Information included within the escalation protocols.
| Low risk | Medium risk | High risk | All risk categories | |
|---|---|---|---|---|
| Urgency of clinical response | ||||
| All charts | 10/142 (7.0) | 82/147 (55.8) | 64/124 (51.6) | 8/147 (5.4) |
| Colour-coded | 5/97 (5.2) | 53/97 (54.6) | 25/76 (32.9) | 2/97 (2.1) |
| Aggregate-weighted | 5/45 (11.1) | 29/50 (58.0) | 39/48 (81.3) | 6/50 (12.0) |
| Seniority of responder | ||||
| All charts | 74/141 (52.5) | 112/147 (76.2) | 94/124 (75.8) | 76/147 (51.7) |
| Colour-coded | 38/97 (39.2) | 67/97 (69.1) | 47/76 (61.8) | 38/97 (39.2) |
| Aggregate-weighted | 36/44 (81.8) | 45/50 (90.0) | 47/48 (97.9) | 38/50 (76.0) |
| Frequency of on-going clinical monitoring | ||||
| All charts | 60/142 (42.3) | 64/147 (43.5) | 41/124 (33.1) | 37/147 (25.2) |
| Colour-coded | 23/97 (23.7) | 27/97 (27.8) | 12/76 (15.8) | 11/97 (11.3) |
| Aggregate-weighted | 37/45 (82.2) | 37/50 (74.0) | 29/48 (60.4) | 26/50 (52.0) |
| Recommended clinical setting of care | ||||
| All charts | 2/141 (1.4) | 23/146 (15.8) | 57/123 (46.3) | 2/147 (1.4) |
| Colour-coded | 0/96 (0) | 10/96 (10.4) | 25/75 (33.3) | 0/97 (0) |
| Aggregate-weighted | 2/45 (4.4) | 12/50 (26.0) | 32/48 (66.7) | 2/50 (4.0) |
Data presented as number (%). Where denominators do not add up to 147 (all charts), 97 (colour-coded) or 50 (aggregate-weighted), these charts did not include that specific risk category in their escalation protocol.
Discussion
Main findings
This analysis of 147 OEWS and their associated escalation protocols from consultant-led units across the UK found significant variation in the thresholds used to trigger different levels of clinical escalation for each physiological parameter and the quality of information included within their escalation protocols. OEWS charts varied significantly not only in terms of what was considered to be normal but also the thresholds chosen to inform the urgency and nature of the required clinical response. The majority of charts sampled provided insufficient information surrounding our four key escalation components, with colour-coded charts typically containing the least information. A third of charts sampled did not have any accompanying information regarding escalation, instead referring to a separate protocol document.
Strengths and limitations
To our knowledge this is the largest and most detailed review of escalation protocols and obstetric early warning systems to date, building upon previous work by Smith et al.11 This study offers a more in-depth analysis of the information included within the escalation protocols. Additionally, we believe it provides a better visual representation of the degree of variation in the thresholds used to trigger different levels of clinical escalation in OEWS. The study has its limitations as not every UK consultant-led maternity unit is represented. However, we believe the significance of the findings is unlikely to change with extra data from the additional units. Similarly, we acknowledge that many of these OEWS and escalation protocols were collected in 2014 and some of these may have already been updated.
Interpretation
The variation demonstrated in the abnormal vital sign thresholds is concerning. Physiological values that would be regarded ‘significantly abnormal’ by some units, were considered only ‘mildly abnormal’ or even ‘normal’ in others. Such variation is likely to be a consequence of local adaptations made to the parameter thresholds and reflects the lack of wider agreement on normal physiological values in pregnancy and the post-partum period. The consequence of these local adaptations is that early signs of deterioration may go undetected with further deterioration remaining unnoticed until the patient is in extremis. Blood pressure triggers in several charts directly contradict the recommended diagnostic thresholds laid out in the updated National Institute for Health and Care Excellence ‘Hypertension in pregnancy’ guidelines.14 Similarly, many of the charts also poorly reflect the recommended parameter thresholds for early identification of maternal sepsis.16
A standardised OEWS could potentially mitigate many of these issues and improve the approach to the recognition and initial management of an acutely unwell obstetric patient but further evidence is needed. The stated benefits of standardisation include reducing the variation in care, improved familiarity for staff moving between hospitals, improved teamwork and communication, and better opportunities for staff training.12, 13 A national EWS (National Early Warning Score, NEWS) has been successfully adopted across the NHS for non-pregnant, adults.12 Despite recommendations made in the 2007 UK CEMACH report1 and more recent calls by the Maternal Critical Care/Enhanced Maternal Care (MCC/EMC) Standards Development Working Group17 for a national OEWS, no such chart currently exists for the whole UK. Instead, charts in use across the UK have large variations in their design and the included physiological parameters ranges,11 and many contain significant design errors.15
Before a national OEWS can be considered it is important to clarify the normal physiological parameter ranges for pregnancy and the postnatal period, and to decide if gestation-specific OEWS need to be considered. Currently there is a paucity of evidence to guide practice in these respects18 although this work is now underway.19, 20 Any standardised OEWS and chart will require rigorous validation before being implemented nationally and will need to reflect national guidelines surrounding hypertension in pregnancy and maternal sepsis. Consideration must also be given as to whether community versions of the OEWS and chart should also be developed using more conservative trigger ranges to take account of the likely subsequent delay associated with arranging transfer from the community to a hospital setting.21
A standardised approach to the recognition of deteriorating women is clearly important, but without effective escalation protocols, maternal care will still be compromised. The lack of detailed information provided to staff within the escalation protocols is extremely concerning. The majority of charts and protocols studied lacked information on the recommended location of care, frequency of on-going monitoring and required urgency of clinical response. These are all crucial considerations when developing a coherent escalation plan and are likely to impact the quality of the clinical response received. Our findings are in line with recent studies of hospital ‘deteriorating patient’ protocols,22, 23, 24 which often lacked detail and which demonstrate significant variation in the instructions provided to clinical staff.
Escalation protocols with too many steps have been labelled too complicated and are seen as a potential barrier to timely escalation,25, 26 therefore in line with the MCC/EMC document17 we believe escalation protocols should only include one intermediate step prior to review by a senior clinician. Additionally the accompanying escalation protocol should be modelled on the one used in NEWS to increase familiarity and reduce the risk of error from staff using the two charts.17 Including the escalation protocol on the OEWS chart itself would seem essential and could improve usability. An interdisciplinary approach to the design and development of the escalation protocol in addition to multi-professional training would maximise engagement and buy in.
Conclusion
We have demonstrated significant variation in the thresholds used to trigger different levels of clinical escalation in OEWS and the staff guidance regarding the escalation of care located within units’ escalation protocols. This variation is likely to lead to disparities in the identification of a deteriorating pregnant woman and the subsequent quality of care she receives. The development of a national OEWS and escalation protocol would help to standardise and improve care across obstetric units.
Conflicts of interests
All members of the MObs group declare that an Obstetric Anaesthetists' Association small project grant, supported by internal resourcing from Bournemouth University, was used to fund the project. JC was supported by a grant from MSD for Mothers. Funds from MSD were provided through its MSD for Mothers program and are the sole responsibility of the authors. MSD for Mothers is an initiative of Merck & Co., Inc., Kenilworth, N.J., U.S.A. GBS was a member of the following groups: Royal College of Physicians of London’s National Early Warning Score Development and Implementation Group; NICE Guideline Development Group on ‘Acutely ill patients in hospital. Recognition of and response to acute illness in adults in hospital’; National Patient Safety Agency Observatory group considering ‘Deterioration not recognised or not acted on’; Department of Health Emergency Care Strategy Team’s ‘Competencies for Recognising and Responding to Acutely Ill Patients in Hospital’. The remaining authors declare no competing interests.
Funding statement
An OAA small project grant (awarded 2nd April 2014), supported by internal resourcing from Bournemouth University, was used to fund the project. JC was supported by a grant from MSD for Mothers. Funds from MSD were provided through its MSD for Mothers program. MSD for Mothers is an initiative of Merck & Co., Inc., Kenilworth, N.J., U.S.A. Neither funder had input into the study design, data collection, data analysis, data interpretation or writing of the report.
Contributors
The Modified Obstetric Early Warning Systems (MObs) Research Group comprises: Debra Bick, Lisa Andrews, Vanora Hundley, Richard Isaacs, Gary Smith, Edwin van Teijlingen, Michael YK Wee, James Cheshire and David Lissauer. All authors (JC, DL, WPS, AT, RI, GBS, VH) conceived the idea and designed the study. JC acquired and analysed the data. WPS acted as second reviewer. JC and DL drafted the manuscript. All authors and members of MObs revised the manuscript and approved the final version of the manuscript for submission.
Acknowledgements
The authors would like to thank the following obstetrics and gynaecology trainees for helping to facilitate data collection at their respective trusts: Kamana Subba, Seuvandhi Gunasekera, Elsa Limura, Rima Dhillon-Smith, Natalie Woodhead, Nowmi Ali, Guy Calcott, Maria Fisher, Edward Harrison, Thomas Coia, Gemma Nightingale, Laura Rylah, Fay Tomlinson, Christina Baker, Sarah Burgess, Louisa Manning, Emma Long, Joana Almeida, Simrit Nijjar, Nicola Ramshaw, Zora Castling, Diana Marcus, Yee Yin Chan, Baian Alhindawi, Tara Lee and Lorraine Sheena Kasaven. We would like to acknowledge the OAA Audit Subcommittee for providing the contact details of the lead consultant anaesthetists. We would also like to thank those anaesthetists and other hospital staff who returned their unit’s early warning system charts and associated escalation protocols. Finally we would like to thank UKARCOG for facilitating the collection of the additional charts and escalation policies.
Appendix A.
The Modified Obstetric Early Warning Systems (MObs) Research Group comprises: Debra Bick (a), Lisa Andrews (b), Vanora Hundley (c), Richard Isaacs (d, e), Gary Smith (e), Edwin van Teijlingen (c), Michael YK Wee (e, f), James Cheshire (g) and David Lissauer (h, i).
a. Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick, Coventry, UK
b. Bournemouth University Clinical Research Unit (BUCRU), Faculty of Health and Social Sciences, Bournemouth University, Bournemouth, UK
c. Centre for Midwifery, Maternal & Perinatal Health, Bournemouth University, Bournemouth, UK
d. Department of Anaesthesia, University Hospital Southampton NHS Foundation Trust, Southampton, UK
e. Centre of Postgraduate Medical Research & Education (CoPMRE), Faculty of Health and Social Sciences, Bournemouth University, Bournemouth, UK
f. Department of Anaesthesia, Poole Hospital NHS Foundation Trust, Poole, UK
g. Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, UK
h. University of Liverpool, Institute of Translational Medicine, University of Liverpool, Crown Street, Liverpool, L69 3BX
i. Malawi-Liverpool-Wellcome Trust Clinical Research Programme, College of Medicine, Blantyre, Malawi
References
- 1.Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer – 2003–2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom; London; 2007. [Google Scholar]
- 2.Umar A., Ameh C.A., Muriithi F., Mathai M. Early warning systems in obstetrics: a systematic literature review. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paternina-Caicedo A., Miranda J., Bourjeily G. Performance of the obstetric early warning score in critically ill patients for the prediction of maternal death. Am J Obstet Gynaecol. 2017;216:e1–8. doi: 10.1016/j.ajog.2016.09.103. [DOI] [PubMed] [Google Scholar]
- 4.Singh S., McGlennan A., England A., Simons R. A validation study of the CEMACH recommended modified early obstetric warning system (MEOWS) Anaesthesia. 2012;67:12–18. doi: 10.1111/j.1365-2044.2011.06896.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh A., Guleria K., Vaid N.B., Jain S. Evaluation of maternal early obstetric warning system (MEOWS chart) as a predictor of obstetric morbidity: a prospective observational study. Eur J Obstet Gynecol. 2016;207:11–17. doi: 10.1016/j.ejogrb.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Carle C., Alexander P., Columb M., Johal J. Design and internal validation of an obstetric early warning score: secondary analysis of the Intensive Care National Audit and Research Centre Case Mix Programme database. Anaesthesia. 2013;68:354–367. doi: 10.1111/anae.12180. [DOI] [PubMed] [Google Scholar]
- 7.Shields L.E., Wiesner S., Klein C., Pelletreau B., Hedriana H.L. Use of maternal early warning trigger tool reduces maternal morbidity. Am J Obstet Gynecol. 2016;214:e1–6. doi: 10.1016/j.ajog.2016.01.154. [DOI] [PubMed] [Google Scholar]
- 8.Edwards S.E., Grobman W.A., Lappen J.R. Modified obstetric early warning scoring systems (MOEWS): validating the diagnostic performance for severe sepsis in women with chorioamnionitis. Am J Obstet Gynecol. 2015;212:e1–8. doi: 10.1016/j.ajog.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Guideline Development Group from the Clinical Strategy and Programmes Division Health Service Executive . 2014. The Irish Maternity Early Warning System (IMEWS) National Clinical Guideline No. 4. [Google Scholar]
- 10.Isaacs R.A., Wee M.Y.K., Bick D.E. A national survey of obstetric early warning systems in the United Kingdom: five years on. Anaesthesia. 2014;69:687–692. doi: 10.1111/anae.12708. [DOI] [PubMed] [Google Scholar]
- 11.Smith G.B., Isaacs R., Andrews L., Wee M.Y.K., Van Teijlingen E., Bick D.E. Vital signs and other observations used to detect deterioration in pregnant women: an analysis of vital sign charts in consultant-led UK maternity units. Int J Obstet Anesth. 2017;30:44–51. doi: 10.1016/j.ijoa.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Royal College of Physicians National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party; London; 2017. [Google Scholar]
- 13.Leotsakos A., Zheng H., Croteau R. Standardization in patient safety: the WHO High 5s project. Int J Qual Health Care. 2014;26:109–116. doi: 10.1093/intqhc/mzu010. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence . 2019. Hypertension in pregnancy: diagnosis and management (NG133) [PubMed] [Google Scholar]
- 15.Isaacs R., Smith G., Gale-Andrews L. Design errors in vital sign charts used in consultant-led maternity units in the United Kingdom. Int J Obstet Anesth. 2019;39:60–67. doi: 10.1016/j.ijoa.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 16.The Uk Sepsis Trust . 2018. Clinical Professional Resources. (Accessed 27 Sept 2018, at https://sepsistrust.org/professional-resources/clinical/) [Google Scholar]
- 17.Royal College of Anaesthetists Care of the critically ill woman in childbirth; enhanced maternal care; London; 2018. [Google Scholar]
- 18.Dennis A.T., Hardy L. Defining a reference range for vital signs in healthy term pregnant women undergoing caesarean section. Anaesth Intensive Care. 2016;44:752–757. doi: 10.1177/0310057X1604400619. [DOI] [PubMed] [Google Scholar]
- 19.Kumar F., Kemp J., Edwards C. Pregnancy physiology pattern prediction study (4P study): protocol of an observational cohort study collecting vital sign information to inform the development of an accurate centile-based obstetric early warning score. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loerup L., Pullon R.M., Birks J. Trends of blood pressure and heart rate in normal pregnancies: a systematic review and meta-analysis. BMC Med. 2019;17:167. doi: 10.1186/s12916-019-1399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown J.P.R. Following NEWS trend: charting progress in obstetrics? Int J Obstet Anesth. 2018;33:1–3. doi: 10.1016/j.ijoa.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Freathy S., Smith G.B., Schoonhoven L., Westwood G. The response to patient deterioration in the UK National Health Service—a survey of acute hospital policies. Resuscitation. 2019;139:152–158. doi: 10.1016/j.resuscitation.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Considine J., Hutchison A.F., Rawson H. Comparison of policies for recognising and responding to clinical deterioration across five Victorian health services. Aust Health Rev. 2018;42:412–419. doi: 10.1071/AH16265. [DOI] [PubMed] [Google Scholar]
- 24.Smith D., Sekhon M., Francis J.J., Aitken L.M. How actionable are staff behaviours specified in policy documents? A document analysis of protocols for managing deteriorating patients. J Clin Nurs. 2019;28:4139–4149. doi: 10.1111/jocn.15005. [DOI] [PubMed] [Google Scholar]
- 25.Chua W.L., Mackey S., Ng E.K.C., Liaw S.Y. Front line nurses’ experiences with deteriorating ward patients: a qualitative study. Int Nurs Rev. 2013;60:501–509. doi: 10.1111/inr.12061. [DOI] [PubMed] [Google Scholar]
- 26.Elliott D., Allen E., Perry L. Clinical user experiences of observation and response charts: focus group findings of using a new format chart incorporating a track and trigger system. BMJ Qual Saf. 2015;24:65–75. doi: 10.1136/bmjqs-2013-002777. [DOI] [PubMed] [Google Scholar]