Abstract
Objectives
We evaluated the feasibility of optimising coronary perfusion pressure (CPP) during cardiopulmonary resuscitation (CPR) with a closed-loop, machine-controlled CPR system (MC-CPR) that sends real-time haemodynamic feedback to a set of machine learning and control algorithms which determine compression/decompression characteristics over time.
Background
American Heart Association CPR guidelines (AHA-CPR) and standard mechanical devices employ a “one-size-fits-all” approach to CPR that fails to adjust compressions over time or individualise therapy, thus leading to deterioration of CPR effectiveness as duration exceeds 15–20 min.
Methods
CPR was administered for 30 min in a validated porcine model of cardiac arrest. Intubated anaesthetised pigs were randomly assigned to receive MC-CPR (6), mechanical CPR conducted according to AHA-CPR (6), or human-controlled CPR (HC-CPR) (10). MC-CPR directly controlled the CPR piston’s amplitude of compression and decompression to maximise CPP over time. In HC-CPR a physician controlled the piston amplitudes to maximise CPP without any algorithmic feedback, while AHA-CPR had one compression depth without adaptation.
Results
MC-CPR significantly improved CPP throughout the 30-min resuscitation period compared to both AHA-CPR and HC-CPR. CPP and carotid blood flow (CBF) remained stable or improved with MC-CPR but deteriorated with AHA-CPR. HC-CPR showed initial but transient improvement that dissipated over time.
Conclusion
Machine learning implemented in a closed-loop system successfully controlled CPR for 30 min in our preclinical model. MC-CPR significantly improved CPP and CBF compared to AHA-CPR and ameliorated the temporal haemodynamic deterioration that occurs with standard approaches.
Keywords: CPR, Cardiopulmonary resuscitation, OHCA, Mechanical CPR, Machine learning, Personalized medicine, Porcine, Haemodynamics, Refractory VF
List of abbreviations
- ACD
Active Compression Decompression
- AHA
American Heart Association
- AHA-CPR
CPR executed according to AHA guidelines
- ANOVA
analysis of variance
- AUC
area under the curve
- CBF
carotid blood flow
- CPP
coronary perfusion pressure
- CPR
cardiopulmonary resuscitation
- HC-CPR
human-controlled CPR
- ITD
impedance threshold device
- LINR
linear regression
- LQR
linear-quadratic regulator
- LUCAS
Lund University Cardiac Arrest System
- MC-CPR
machine-controlled CPR
- OHCA
out-of-hospital cardiac arrest
- RA
right atrium
- ROSC
return of spontaneous circulation
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Introduction
More than 350,000 cases of out-of-hospital cardiac arrest (OHCA) occur in the United States every year, resulting in approximately 320,000 deaths.1,2 The majority of these patients receive cardiopulmonary resuscitation (CPR) for more than 25–30 min.3,4 However, manual or mechanical CPR lasting longer than 15–20 min is profoundly ineffective, with almost 100% mortality in patients requiring more than 35–40 min of CPR.4, 5, 6, 7, 8, 9 For patients with refractory ventricular fibrillation (VF)/ventricular tachycardia (VT) OHCA, extracorporeal CPR provides stabilisation capabilities associated with improved neurologically favourable survival.7 With prompt identification and treatment, coronary stenosis or occlusion can be potentially reversed by advanced perfusion/reperfusion strategies.9, 10, 11 Despite these crucial advances, outcomes are strongly associated with the patient’s perfusion state,7 and 55–60% of these patients still die, mostly from severe brain injury caused by the inherent inability of standard CPR to maintain oxygen delivery to the brain over prolonged periods of time.12, 13, 14 Our goal is to increase the rate of neurologically favourable survival in patients with refractory OHCA.
Quality of CPR is a critical determinant of survival,15,16 and we recently showed that the CPR compression characteristics associated with survival in OHCA patients vary between individuals and vary throughout resuscitation.17,18 However, the current American Heart Association (AHA) recommendations for CPR use a “one-size-fits-all” approach that does not adapt to the individual variability or time-variation of CPR and fails to maintain viability after 30 min of resuscitation.7 To achieve our goal, we must optimise CPR performance and “personalise” compression/decompression therapy.
Machine learning and automatic control are powerful means to optimise and individualise medical processes. Their use has been proposed in various applications, including seizure reduction,19 neuromuscular control,20 and glycaemic control.21 We are therefore investigating the use of machine learning and automated control (referred to colloquially as “artificial intelligence”) to create an individualised CPR method that applies dynamic strategies by changing compression characteristics during CPR.
In previous studies, we showed that machine learning algorithms can predict coronary perfusion pressure (CPP) with as little as 1 mmHg of error.22 Now we transition to a closed-loop system. We investigated two methods of closed-loop CPR: (1) machine-controlled CPR (MC-CPR), the first known closed-loop system of CPR operated by machine learning prediction and control algorithms built into computer software; and (2) human-controlled CPR (HC-CPR), which was operated only by the brain of a physician tasked with monitoring invasive pressures during CPR and titrating CPR compression/decompression in an effort to optimise perfusion, but without any distinctive rules.
In this study, we used our previously established porcine model of cardiac arrest to compare these two methods to a static CPR device which executed AHA guidelines precisely (AHA-CPR). The primary measures of CPR efficacy in this study were carotid blood flow (CBF) and coronary perfusion pressure (CPP). We hypothesised that applying static AHA guidelines during prolonged CPR would be suboptimal compared to closed-loop control, whether controlled by the brain of a human operator or by a machine applying machine learning and control algorithms. We further hypothesised that the MC-CPR group may outperform the human-controlled HC-CPR group as CPR duration approaches 30 min.
Methods
Preparatory phase
This study was approved by the University of Minnesota Institutional Animal Care and Use Committee. A total of 24 naïve farm-raised female pigs (54 ± 5 kg) were prepared under anaesthesia. Data recording and animal handling procedures have been previously described.13,23,24 The pigs were anaesthetised with intramuscular ketamine and xylazine (5 ml of 100 mg/ml dose and 1-3 mg/kg, respectively) followed by isoflurane at a dose of 1-1.4%. Animals were ventilated with a tidal volume of 8 ml/kg using room air volume control ventilation (Narkomed, Draeger Medical, Telford, Pennsylvania). Arterial blood gas levels were obtained at baseline and every 5 min until 30 min of CPR. Animal temperature was measured with an oesophageal temperature probe, and normothermia (37 ± 0.5 C) was maintained. Vascular access was obtained percutaneously in the femoral artery and in the right external jugular vein. The internal carotid artery was accessed by cut-down, and an ultrasonic flow probe (Transonic, Ithaca, New York) was adhered to the artery. Carotid blood flow (CBF) was recorded at baseline and every 5 min until 30 min of CPR. Central aortic blood pressure and right atrial (RA) pressure were measured with Millar catheters (Millar Instruments, Houston, Texas) placed in the descending thoracic aorta and right external jugular sheath, respectively. Measurements from these Millar catheters were used for calculation of CPP as the difference between diastolic blood pressure and RA pressure in spontaneous circulation, and the difference between decompression phase aortic pressure and RA pressure during CPR. Haemodynamic pressures were continuously recorded in LabVIEW (LabVIEW 2015, National Instruments, Austin, Texas).
Before the beginning of the study, 24 animal identification numbers were put into a list that subsequently randomised them to the 3 arms (HC-CPR, AHA-CPR, and MC-CPR) in a 2:1:1 allocation. The veterinary technicians and the researchers that were responsible for animal preparation were blinded to the study arm. An intention to treat strategy was strictly followed during analysis and no crossovers were allowed after randomisation. Two of the 12 animals in the HC-CPR group died during the preparatory phase from profound vasoplegia. Thus, a total of 22 animals received CPR. A higher number of animals was given to HC-CPR due to the a priori anticipation of higher standard deviation in the behaviour of a human operator.
VF induction and CPR
Following surgical preparation, isoflurane administration ceased for 3 min. At the end of 3 min, VF was electrically induced using a pacing wire. Once VF was initiated, ventilation stopped, and no treatment was provided for 3 min to mimic the earliest time of first responder arrival and initiation of CPR. Then, all animals received CPR with a mechanical CPR device and an impedance threshold device (ITD) as follows:
-
•
AHA-CPR: A mechanical CPR device (Lund University Cardiac Arrest System [LUCAS]) was used to administer CPR with precise and accurate adherence to AHA-CPR depths (set at 5.3 cm of compression and 0 cm decompression at 100 compressions/min).13,25,26
-
•
HC-CPR: In this group, a human operator was able to view the CPP waveform in real-time and was tasked to maximise CPP by using a custom active compression-decompression CPR piston. This was a specially designed piston that enabled real-time adjustment of compression and decompression amplitude.13,25, 26, 27 A maximum of 6 cm for compression amplitude and 7.0 cm for decompression amplitude was allowed. Compressions were supplied at 100 beats per minute with a 50% compression-decompression duty cycle. The operator of the piston in this group was a physician who was instructed to optimise and improve CPP during CPR via the direct control of compression and decompression amplitudes. The physician operator followed their medical and physiologic intuition, and had continuous access to view the CPP on a screen in order to modify their choices in whatever way they saw appropriate. The physician was told that they were part of an experiment to compare human-controlled CPR against AHA guidelines in order to provide positive reinforcement. They were unaware that they would be compared to MC-CPR and were blinded to AHA-CPR results. Using this group, we were able to compare the performance of a human physician’s brain against the performance of MC-CPR’s machine learning and control algorithms.
-
•
MC-CPR: the same custom CPR piston used for HC-CPR was applied in the MC-CPR group, except the amplitudes of compression and decompression were instead controlled by the outputs of machine learning and control algorithms running within Matlab software (Mathworks, Natick, Massachusetts) and communicating to the piston. The algorithms are briefly described in the next section, and more detail may be found in the Mathematical Supplement.
All animals received 30 min of CPR by one of the methods above. Ventilations were delivered at 10 respirations/min and 8 ml/kg of tidal volume. Epinephrine was delivered per standard protocol with 0.014 mg/kg per 5-min intravenous dose starting at minute 10 of CPR. Animals were defibrillated up to three times at the end of CPR. If defibrillations were unsuccessful, the animal was declared dead. In case of return of spontaneous circulation (ROSC), animals were monitored for up to 30 min and then euthanised.
Closed-loop method of machine-controlled CPR
An overview of the flow of information in MC-CPR is displayed in Fig. 1. Live CPP data as well as the amplitudes of compression and decompression were continuously streamed to the computer which housed all algorithms. Continuous waveforms were converted into single measurements of mean CPP, compression amplitude, and decompression amplitude such that data was calculated on a cycle-by-cycle basis.
Fig. 1.
Flow of Information During Closed-loop Control
Waveforms of the piston’s distance from the chest and the CPP output from the animal were discretised to a cycle-by-cycle basis before being provided to the algorithms. The predictive algorithm (LINR) was used within the framework of the control algorithm (LQR), but they can be conceptualised as passing information in a stepwise manner as depicted. The user of the CPU sets the target CPP for an instant of time and gradually increases Q until the controller reaches the target, then increases the target itself. If the controller was overshooting, or if external forces were being applied to the animal, such as IV injections, R would be increased in order to minimise inappropriate reactions from the controller. CPP, coronary perfusion pressure; CPR, cardiopulmonary resuscitation; CPU, central processing unit; LQR, linear-quadratic regulator; LINR, linear regression; Q, quadratic characteristics of the controller; R, regulator characteristics of the controller.
In general, closed-loop MC-CPR consisted of an algorithm for prediction of CPP and an algorithm for control of the piston. Together, these computer algorithms were used to calculate the optimal amplitudes of CPR to send to the piston for execution. Of note, the algorithms did require some information from a user at the computer; they required the user to specify what mean CPP was the desired “target” for CPR and to set the intensity of the controller in reaching that target (Q), as well as how quickly the controller could react to changes in CPP (R). Since it has been reported in literature that a CPP >15 mm Hg is required to achieve ROSC in the setting of cardiac arrest,28 the lower limit “target” for MC-CPR was a CPP of at least 15 mm Hg. To be confidently beyond this threshold, we aimed for a target of 20–25 mmHg. Once this target was achieved, excessive resuscitation efforts were moderated by the algorithms of MC-CPR. A detailed description of the algorithms of MC-CPR is also included (Mathematical Supplement).
Data analysis
CPR haemodynamics, distance waveforms, and force waveforms measured from the head of the custom piston were recorded by LabVIEW and analysed with Matlab. CBF was quantified by taking a 10-second average of the ultrasonic flow waveform every 5 min, including negative values of flow. CPP was quantified as an average over the decompression phase of CPR only. The thoracic compliance of each animal during CPR was calculated from the force and distance waveforms, as described in the Mathematical Supplement.
Statistical analyses were performed using Stata (StataCorp, College Drive, Texas) software. The temporal evolution of CPP and CBF during CPR was quantified by a linear fit of their minute-by-minute values. To assess the effect of the treatment arm on the measured haemodynamic and metabolic variables, we performed a two-way Analysis of Variance (ANOVA) with minute of CPR and study arm as entered parameters. Lastly, the area under the curve (AUC), a parameter highly correlated with ROSC,28 was calculated for CPP in each animal. Linear fit and AUC comparison between MC-CPR and the other groups were performed with two-way ANOVA.
Results
No differences between groups were observed in the baseline haemodynamic measurements.
Compression characteristics
Fig. 2 summarises the amplitudes of compression and decompression among groups. Compression amplitude was 53 mm for AHA-CPR, 47 ± 8 mm for MC-CPR, and 43 ± 5 mm for HC-CPR. Decompression amplitude was 51 ± 8 mm for MC-CPR, 42 ± 5 mm for HC-CPR, and 0 mm AHA-CPR. HC-CPR and MC-CPR led to active decompression and shallower compression amplitudes compared with AHA-CPR. MC-CPR depths varied more throughout time compared to the other methods.
Fig. 2.
Amplitudes of Compression and Decompression
Values are means with standard deviations represented by shading. MC-CPR showed the greatest variation in compression and decompression depths. AHA, American Heart Association recommendations; HC-CPR, human-controlled CPR; MC-CPR, machine-controlled CPR.
Haemodynamic pressures and ROSC rates
Table 1 shows the baseline and mean values of all haemodynamics calculated for each 5-min interval over the 30-min CPR period. Diastolic and systolic blood pressures were significant between groups (p < 0.05). CPP over the 30 min of CPR was also significantly different between groups (p < 0.001), and the disparity between MC-CPR and the other two groups increased as time approached 30 min (Fig. 3). After the initial drop from baseline, CPP consistently increased for MC-CPR and decreased for AHA-CPR, whereas for HC-CPR the CPP increased during the first 15 min and then decreased. Fig. 3 shows the minute-by-minute values of CPP, with temporal evolution quantified by a linear fit. Animals treated with MC-CPR had a positive slope (+0.36 ± 0.17 mmHg/min) while declining slopes were observed in those treated with AHA-CPR (–0.26 ± 0.15 mmHg/min) and HC-CPR (–0.01 ± 0.05 mmHg/min) (p = 0.004).
Table 1.
Assessments and results over 30 min of CPR.
| Signal |
Minutes of CPR |
ANOVAa |
||||||
|---|---|---|---|---|---|---|---|---|
| Group | Baseline | 0-5 | 5-10 | 10-15 | 15-20 | 20-25 | 25-30 | p-value |
| CPP, mmHg | ||||||||
| AHA-CPR | 60 ± 7 | 15 ± 2 | 14 ± 2 | 12 ± 3 | 9 ± 3 | 9 ± 3 | 8 ± 3 | <0.001 |
| HC-CPR | 68 ± 5 | 14 ± 1 | 19 ± 1 | 21 ± 1 | 19 ± 1 | 17 ± 1 | 15 ± 1 | |
| MC-CPR | 55 ± 7 | 13 ± 1 | 18 ± 1 | 20 ± 3 | 21 ± 3 | 22 ± 3 | 22 ± 3 | |
| CBF, % of baseline | ||||||||
| AHA-CPR | 100 | 44 ± 4 | 33 ± 4 | 24 ± 6 | 18 ± 6 | 15 ± 6 | 11 ± 5 | 0.035 |
| HC-CPR | 100 | 26 ± 3 | 27 ± 2 | 22 ± 3 | 21 ± 3 | 18 ± 3 | 16 ± 3 | |
| MC-CPR | 100 | 23 ± 2 | 23 ± 2 | 22 ± 2 | 19 ± 3 | 22 ± 4 | 24 ± 3 | |
| Systolic aortic BP (compression), mmHg | ||||||||
| AHA-CPR | 107 ± 7 | 79 ± 5 | 70 ± 5 | 70 ± 8 | 62 ± 11 | 56 ± 12 | 53 ± 13 | 0.0066 |
| HC-CPR | 113 ± 6 | 62 ± 3 | 70 ± 4 | 73 ± 5 | 70 ± 5 | 66 ± 5 | 62 ± 5 | |
| MC-CPR | 102 ± 7 | 56 ± 6 | 59 ± 2 | 60 ± 5 | 60 ± 6 | 65 ± 7 | 67 ± 8 | |
| Diastolic aortic BP (decompression), mmHg | ||||||||
| AHA-CPR | 70 ± 6 | 28 ± 4 | 26 ± 4 | 24 ± 5 | 19 ± 6 | 18 ± 6 | 17 ± 6 | <0.001 |
| HC-CPR | 78 ± 5 | 28 ± 1 | 33 ± 1 | 37 ± 2 | 34 ± 2 | 31 ± 2 | 27 ± 2 | |
| MC-CPR | 65 ± 6 | 26 ± 2 | 30 ± 1 | 33 ± 4 | 34 ± 5 | 36 ± 5 | 37 ± 5 | |
| Systolic RA (compression), mmHg | ||||||||
| AHA-CPR | 5 ± 1 | 152 ± 25 | 152 ± 25 | 150 ± 32 | 130 ± 30 | 110 ± 22 | 104 ± 21 | <0.001 |
| HC-CPR | 4 ± 1 | 79 ± 6 | 85 ± 6 | 86 ± 5 | 85 ± 6 | 91 ± 9 | 107 ± 23 | |
| MC-CPR | 4 ± 1 | 73 ± 8 | 75 ± 6 | 85 ± 11 | 74 ± 7 | 81 ± 7 | 81 ± 8 | |
| Diastolic RA (decompression), mmHg | ||||||||
| AHA-CPR | 10 ± 1 | 13 ± 2 | 12 ± 2 | 12 ± 2 | 10 ± 3 | 9 ± 3 | 9 ± 3 | <0.001 |
| HC-CPR | 10 ± 1 | 14 ± 1 | 14 ± 1 | 16 ± 1 | 15 ± 1 | 14 ± 1 | 12 ± 1 | |
| MC-CPR | 10 ± 1 | 13 ± 2 | 12 ± 1 | 13 ± 2 | 13 ± 2 | 14 ± 2 | 15 ± 2 | |
| pCO2, mmHg | ||||||||
| AHA-CPR | 38 ± 1 | 40 ± 6 | 37 ± 8 | 40 ± 10 | 49 ± 17 | 34 ± 2 | 87 ± 1 | 0.002 |
| HC-CPR | 42 ± 1 | 37 ± 2 | 33 ± 2 | 34 ± 4 | 28 ± 3 | 35 ± 3 | 45 ± 10 | |
| MC-CPR | 39 ± 2 | 35 ± 4 | 29 ± 2 | 27 ± 2 | 28 ± 3 | 27 ± 2 | 28 ± 3 | |
| pO2, mmHg | ||||||||
| AHA-CPR | 126 ± 9 | 86 ± 4 | 87 ± 15 | 84 ± 16 | 59 ± 15 | 70 ± 4 | 42 ± 10 | 0.031 |
| HC-CPR | 107 ± 4 | 86 ± 8 | 94 ± 7 | 82 ± 9 | 84 ± 8 | 71 ± 8 | 75 ± 8 | |
| MC-CPR | 110 ± 6 | 79 ± 8 | 102 ± 4 | 89 ± 5 | 117 ± 40 | 85 ± 16 | 85 ± 2 | |
| SaO2, % | ||||||||
| AHA-CPR | 99 ± 1 | 96 ± 2 | 85 ± 12 | 82 ± 13 | 73 ± 18 | 90 ± 2 | 2 ± 1 | 0.044 |
| HC-CPR | 98 ± 1 | 94 ± 2 | 96 ± 1 | 92 ± 2 | 93 ± 2 | 84 ± 5 | 85 ± 9 | |
| MC-CPR | 99 ± 1 | 95 ± 1 | 97 ± 1 | 96 ± 2 | 95 ± 3 | 93 ± 4 | 86 ± 3 | |
| Force, lb | ||||||||
| HC-CPR | – | 25 ± 1 | 21 ± 1 | 19 ± 2 | 18 ± 2 | 18 ± 2 | 17 ± 1 | 0.264 |
| MC-CPR | – | 22 ± 3 | 22 ± 1 | 20 ± 1 | 19 ± 1 | 20 ± 1 | 19 ± 2 | |
| Compliance, (m^-1) | ||||||||
| HC-CPR | – | 39 ± 1 | 42 ± 1 | 42 ± 1 | 41 ± 1 | 40 ± 1 | 40 ± 1 | <0.001 |
| MC-CPR | – | 40 ± 3 | 46 ± 4 | 46 ± 4 | 46 ± 4 | 46 ± 6 | 47 ± 6 | |
Values are means ± standard error calculated for 5-min intervals over the 30-min CPR period.
AHA, American Heart Association recommendations; ANOVA, analysis of variance; BP, blood pressure; CBF, carotid blood flow; CPP, coronary perfusion pressure; CPR, cardiopulmonary resuscitation; HC-CPR, human-controlled CPR; MC-CPR, machine-controlled CPR; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; RA, right atrium; SaO2, oxygen saturation of arterial blood.
Two-way ANOVA with Study Arm and minute of CPR inserted as model parameters.
Fig. 3.
Mean Coronary Perfusion Pressure for animals receiving CPR based on AHA Recommendations vs Human-Controlled CPR vs Machine-Controlled CPR
CPP for each minute of CPR is plotted along with the linear curves used to quantify the rate of change of CPP per minute. AHA, American Heart Association; CPP, coronary perfusion pressure; CPR, cardiopulmonary resuscitation; HC-CPR, human-controlled CPR; MC-CPR, machine--controlled CPR.
The areas under the CPP curve (AUC) were calculated for every animal. The mean AUC of HC-CPR (520 ± 27 mmHg∗sec) and MC-CPR (570 ± 68 mmHg∗sec) were significantly greater than that of AHA-CPR (332 ± 72 mmHg∗sec) (p = 0.011), indicating that AHA-CPR led to less cardiac perfusion. This agreed with the rates of ROSC observed after defibrillation: 50% for HC-CPR, 50% for MC-CPR, and only 17% for AHA-CPR.
Carotid blood flow
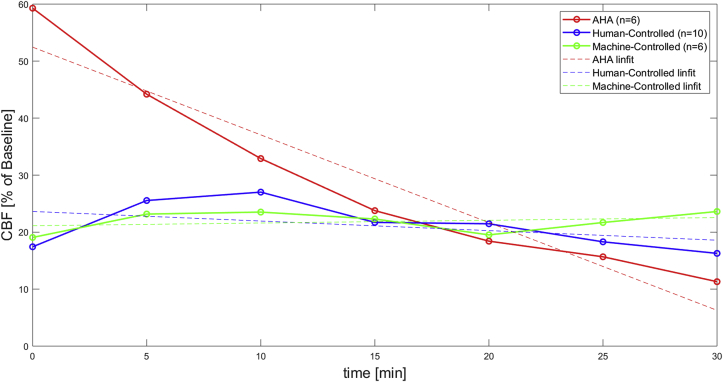
The trends in CPP over the course of CPR paralleled trends in cerebral blood flow (measured as CBF), as displayed in Fig. 4 and Table 1. The minute-by-minute CBF was significantly different between groups (p = 0.035). AHA-CPR had the highest CBF at initiation of CPR (59% of baseline flow at minute 0, Fig. 4), correlating with a higher initial compression depth based on protocol. However, the AHA-CPR group showed a steep drop in CBF over the first 15 min, whereas levels increased or were maintained for HC-CPR and MC-CPR. The rate of change in CBF quantified by linear fit showed a negative slope of –1.5 ± 0.3%/min for AHA-CPR, a negative slope of –0.2 ± 0.2%/min for HC-CPR, and a nearly flat slope of 0.0 ± 0.2%/min for MC-CPR. Both the MC-CPR and HC-CPR slopes were significantly more positive than the slope of AHA-CPR (p = 0.002). Together with the CPP findings, these data suggest that MC-CPR can maintain vital organ perfusion and ameliorate haemodynamic deterioration for 30 min of CPR.
Fig. 4.
Mean Carotid Blood Flow of AHA vs Human-Controlled vs Machine-Controlled CPR
CBF was measured every 5 min of CPR and expressed as a percentage of baseline. CBF was further analysed by linearly fitting the data. CBF, carotid blood flow; CPR, cardiopulmonary resuscitation.
Arterial blood gases
As shown in Table 1, values for arterial partial pressure of CO2 initially decreased or remained relatively stable throughout CPR in the MC-CPR and HC-CPR groups but varied widely in the AHA-CPR group. Partial pressure of oxygen was variable in all groups. Oxygen saturation of arterial blood was lower for AHA-CPR than for MC-CPR and HC-CPR, especially during the last 5 min of CPR. The arterial partial pressure of CO2, the arterial pressure of O2, and the arterial oxygen saturation were significantly different between groups (p < 0.05 for all).
Chest wall compliance
Force during CPR could not be calculated for AHA-CPR because the LUCAS is a proprietary device without accessible sensors. Thus, force and thoracic compliance were only calculated for HC-CPR and MC-CPR. Compliance values were higher for MC-CPR than for HC-CPR (p < 0.001) as displayed in Table 1.
Respiratory control variation
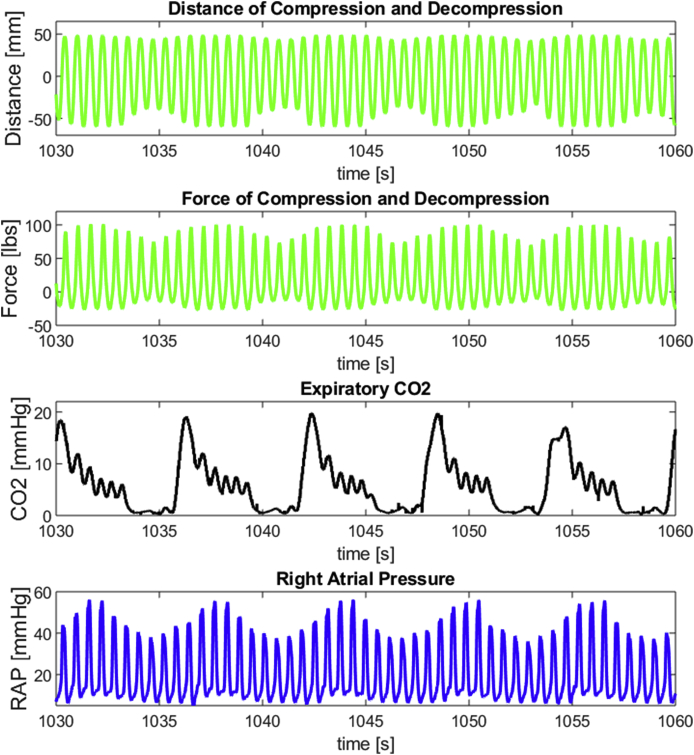
The arterial blood gas results and perfusion results were complimented by an observation that the MC-CPR group had CPR amplitudes, and consequently forces, which varied with the respiratory cycle. As illustrated in Fig. 5, MC-CPR algorithms were able to anticipate when breaths were occurring based on changes in the CPP driven by RA pressure.
Fig. 5.
Closed-loop Feedback of Coronary Perfusion Pressure to the Prediction and Control Algorithms of Machine-Controlled CPR
Closed-loop feedback resulted in forces and amplitudes of CPR which automatically oscillated with respirations. CO2, carbon dioxide CPP, coronary perfusion pressure; RAP, right atrial pressure.
Discussion
This study shows that machine learning algorithms can be deployed successfully to predict CPR performance and control compression/decompression parameters, improving haemodynamic and perfusion measurements over 30 min of CPR. We found that MC-CPR ameliorated, if not eliminated, the natural decline in both coronary and cerebral perfusion (measured using the surrogates CPP and CBF) which were observed in the AHA-CPR group in the present study and have been historically appreciated in prolonged CPR following AHA guidelines.4,6,28 Thus, contrary to current established belief, it is possible to maintain CPR quality for extended periods of resuscitation. The improvement of vital organ perfusion by MC-CPR was further corroborated by an improved ROSC rate; however, the study was not powered for survival.
In comparing HC-CPR to MC-CPR, it is important to acknowledge that machine learning can lead to interventions which cannot be rationalised by current scientific knowledge. MC-CPR was the only group which changed depth and force of compression with the respiratory cycle (Fig. 5). Oscillating force and amplitude with the respiratory cycle may have contributed to the discrepancy between MC-CPR and HC-CPR, but we can only speculate on the physiologic mechanism. The oscillation was not due to a hard-coded input to the control algorithms of MC-CPR but was instead an observed result of MC-CPR’s capacity to predict and anticipate changes in CPP influenced by RA pressure. It is possible that this oscillation led to less pulmonary trauma and avoided pulmonary vasoconstriction by allowing better alveolar gas distribution. Since thoracic compliance was also greater in MC-CPR than in HC-CPR, it is also possible that MC-CPR’s oscillation was able to mechanically condition the thorax, harnessing the thoracic pump which has been theorised to be a source of blood flow in CPR.29,30
Animals in both the HC-CPR and MC-CPR groups received considerably lower compression depth than the AHA-recommended CPR. In addition, the immediate feedback of CPP in both HC-CPR and MC-CPR led to the independent selection— by human operator or algorithm control, respectively— of active decompression for the optimisation of CPR. It was unsurprising that both the human operator and the machine algorithms chose active decompression when immediate CPP feedback was available, since active compression-decompression (ACD) + ITD CPR has been shown to be superior to standard CPR or ACD alone.31 However, it was surprising that MC-CPR performed better than HC-CPR. MC-CPR chose a lower compression depth compared to AHA-CPR and chose a higher decompression depth than HC-CPR on average. While both active decompression groups (MC-CPR and HC-CPR) had significantly elevated diastolic RA pressures, likely representing greater active filling, it remains the most remarkable that— even when compared to immediate CPP assessment and response by a physician operator with access to active decompression in the HC-CPR group— MC-CPR still performed better in maximising CPP.
In addition, our results show that MC-CPR has the potential to account for inter- and intra-patient variability. Inter-patient variability was indicated in Fig. 2, as the automated algorithms were able to vary CPR depths in an effort to find the optimal depths in the long term. Intra-patient variability was exhibited in the time-variance of perfusion during prolonged CPR, displayed in Fig. 3, Fig. 4.
Refractory OHCA patients undergo mechanical CPR for 45–60 min or more,7 and their dismal prognosis will continue if new ways to optimise prolonged CPR are not implemented. While HC-CPR was better than AHA-CPR as a method of titrating CPR amplitude to maximise CPP initially, HC-CPR is entirely dependent on the mind of the operator and has low reproducibility. Thus, HC-CPR is not generalisable, limiting its clinical applicability. In contrast, MC-CPR not only displayed the most promise in maintaining perfusion but is also a method that can be implemented in an automated mechanical device in the future. We envision MC-CPR as a future method of not only personalising compression/decompression amplitude, but also compression rate, compression-to-decompression duty cycle, and compression location on the chest. Since the layering of more control variables will exponentially increase the complexity of control, in the long run “personalisation” of controls can only be feasibly achieved by a computer-controlled method like MC-CPR.
The study has limitations. First, CPP cannot be directly measured in the field. Methods for non-invasive surrogate measurements of perfusion will be vital to the development of robust closed-loop CPR systems which are clinically applicable. End tidal CO2, real-time VF ECG waveform analysis, and thoracic compliance are promising measures (some of which have already been correlated to ROSC outcomes32,33) that could be researched independently or in combination as substitutes for CPP. Second, as depicted in Fig. 1, a computer user must supervise and tune the MC-CPR algorithm. Future research will involve eliminating this necessity by learning the process of tuning through a higher-level algorithm so that no supervisor of the computer would be necessary to ensure safe and effective operation of MC-CPR. Third, our study was performed in a porcine model with the use of an ITD. These animal experiments show promise, and subsequent studies will be done to expand on these preliminary results and refine MC-CPR for use in more scenarios, such as without the application of an ITD, before integration into clinical practice. Finally, we evaluated only haemodynamic improvement and not survival. We have previously shown that haemodynamic improvements during the course of CPR lead to better survival outcomes in the same model and in translational clinical trials.17,25,31,34, 35, 36, 37, 38
Conclusion
MC-CPR improved CPP and CBF over the course of 30 min of CPR compared to AHA guidelines CPR and HC-CPR. MC-CPR sustained high perfusion pressures throughout 30 min of CPR and ameliorated the haemodynamic deterioration expected to occur with standard approaches. To our knowledge, this is the first report to show that machine learning methods can control CPR for extensive periods in a preclinical setting. Identification of additional CPR parameters to control, as well non-invasive or less invasive surrogates of CPP for feedback are targets of future investigation.
Funding sources
The funding source for this work was the Robert Eddy Endowment for Resuscitation Medicine awarded to Dr. Demetris Yannopoulos.
CRediT authorship contribution statement
Pierre S. Sebastian: Conceptualization, Investigation, Methodology, Writing - original draft, Software, Formal analysis, Supervision. Marinos N. Kosmopoulos: Conceptualization, Investigation, Methodology, Writing - original draft, Formal analysis, Project administration. Manan Gandhi: Conceptualization, Investigation, Methodology, Software, Formal analysis, Project administration, Supervision. Alex Oshin: Conceptualization, Investigation, Methodology, Software, Formal analysis. Matthew D. Olson: Software, Methodology, Supervision. Adrian Ripeckyj: Investigation, Methodology. Logan Bahmer: Investigation, Methodology. Jason A. Bartos: Conceptualization, Methodology, Writing - review & editing, Supervision. Evangelos A. Theodorou: Conceptualization, Methodology, Resources, Project administration, Funding acquisition, Supervision. Demetris Yannopoulos: Conceptualization, Methodology, Writing - original draft, Visualization, Resources, Project administration, Funding acquisition, Supervision.
Declaration of competing interest
None of the authors had any conflicts of interest or financial disclosures to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2020.100021.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Zive D.M., Schmicker R., Daya M. Survival and variability over time from out of hospital cardiac arrest across large geographically diverse communities participating in the Resuscitation Outcomes Consortium. Resuscitation. 2018;131:74–82. doi: 10.1016/j.resuscitation.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Grunau B., Puyat J., Wong H. Gains of continuing resuscitation in refractory out-of-hospital cardiac arrest: a model-based analysis to identify deaths due to intra-arrest prognostication. Prehosp Emerg Care. 2018;22:198–207. doi: 10.1080/10903127.2017.1356412. [DOI] [PubMed] [Google Scholar]
- 4.Goto Y., Funada A., Goto Y. Relationship between the duration of cardiopulmonary resuscitation and favorable neurological outcomes after out-of-hospital cardiac arrest: a prospective, nationwide, population-based cohort study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagao K., Nonogi H., Yonemoto N. Duration of prehospital resuscitation efforts after out-of-hospital cardiac arrest. Circulation. 2016;133:1386–1396. doi: 10.1161/CIRCULATIONAHA.115.018788. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds J.C., Grunau B.E., Rittenberger J.C., Sawyer K.N., Kurz M.C., Callaway C.W. Association between duration of resuscitation and favorable outcome after out-of-hospital cardiac arrest: implications for prolonging or terminating resuscitation. Circulation. 2016;134:2084–2094. doi: 10.1161/CIRCULATIONAHA.116.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartos J.A., Grunau B., Carlson C. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation. 2020;141:877–886. doi: 10.1161/CIRCULATIONAHA.119.042173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yannopoulos D., Bartos J.A., Aufderheide T.P. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;139:e530–e552. doi: 10.1161/CIR.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 9.Yannopoulos D., Bartos J.A., Raveendran G. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017;70:1109–1117. doi: 10.1016/j.jacc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 10.Spaulding C.M., Joly L.M., Rosenberg A. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 11.Yannopoulos D., Bartos J.A., Martin C. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaway C.W., Donnino M.W., Fink E.L. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yannopoulos D., Bartos J.A., George S.A. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves short term survival in a porcine model of ischemic refractory ventricular fibrillation. Resuscitation. 2017;110:6–11. doi: 10.1016/j.resuscitation.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartos J.A., Carlson K., Carlson C. Surviving refractory out-of-hospital ventricular fibrillation cardiac arrest: critical care and extracorporeal membrane oxygenation management. Resuscitation. 2018;132:47–55. doi: 10.1016/j.resuscitation.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Yannopoulos D., Aufderheide T.P., Abella B.S. Quality of CPR: an important effect modifier in cardiac arrest clinical outcomes and intervention effectiveness trials. Resuscitation. 2015;94:106–113. doi: 10.1016/j.resuscitation.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Cheskes S., Schmicker R.H., Rea T. The association between AHA CPR quality guideline compliance and clinical outcomes from out-of-hospital cardiac arrest. Resuscitation. 2017;116:39–45. doi: 10.1016/j.resuscitation.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Duval S., Pepe P.E., Aufderheide T.P. Optimal combination of compression rate and depth during cardiopulmonary resuscitation for functionally favorable survival. JAMA cardiology. 2019;4(9):900–908. doi: 10.1001/jamacardio.2019.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiyama A., Duval S., Nakamura Y., Yoshihara K., Yannopoulos D. Impedance threshold device combined with high-quality cardiopulmonary resuscitation improves survival with favorable neurological function after witnessed out-of-hospital cardiac arrest. Circ J. 2016;80:2124–2132. doi: 10.1253/circj.CJ-16-0449. [DOI] [PubMed] [Google Scholar]
- 19.Brar H.K., Exarchos I., Pan Y., Theodorou E., Mahmoudi B. Seizure reduction using model predictive control. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:3152–3155. doi: 10.1109/EMBC.2018.8512911. [DOI] [PubMed] [Google Scholar]
- 20.Valero-Cuevas F.J., Hoffmann H., Kurse M.U., Kutch J.J., Theodorou E.A. Computational models for neuromuscular function. IEEE Rev. Biomed. Eng. 2009;2:110–135. doi: 10.1109/RBME.2009.2034981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovorka R., Kremen J., Blaha J. Blood glucose control by a model predictive control algorithm with variable sampling rate versus a routine glucose management protocol in cardiac surgery patients: a randomized controlled trial. J Clin Endocrinol Metab. 2007;92:2960–2964. doi: 10.1210/jc.2007-0434. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi M., Pan Y., Theodorou E., Sebastian P., Olson M., Yannopoulos D. vol. 51890. American Society of Mechanical Engineers; 2018. Learning to predict coronary perfusion pressure during cardiopulmonary resuscitation. (Dynamic Systems and Control Conference). [Google Scholar]
- 23.Segal N., Matsuura T., Caldwell E. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation. 2012;83:1397–1403. doi: 10.1016/j.resuscitation.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartos J.A., Matsuura T.R., Sarraf M. Bundled postconditioning therapies improve hemodynamics and neurologic recovery after 17 min of untreated cardiac arrest. Resuscitation. 2015;87:7–13. doi: 10.1016/j.resuscitation.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripeckyj A., Kosmopoulos M., Shekar K. Sodium nitroprusside-enhanced cardiopulmonary resuscitation improves blood flow by pulmonary vasodilation leading to higher oxygen requirements. JACC Basic Transl Sci. 2020;5:183–192. doi: 10.1016/j.jacbts.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuura T.R., Bartos J.A., Tsangaris A. Early effects of prolonged cardiac arrest and ischemic postconditioning during cardiopulmonary resuscitation on cardiac and brain mitochondrial function in pigs. Resuscitation. 2017;116:8–15. doi: 10.1016/j.resuscitation.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartos J.A., Voicu S., Matsuura T. Role of epinephrine and extracorporeal membrane oxygenation in the management of ischemic refractory ventricular fibrillation. a randomized trial in pigs. JACC Basic Transl Sci. 2017;2:244–253. doi: 10.1016/j.jacbts.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds J.C., Salcido D.D., Menegazzi J.J. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care. 2010;14:78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halperin H.R., Tsitlik J.E., Guerci A.D. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation. 1986;73:539–550. doi: 10.1161/01.cir.73.3.539. [DOI] [PubMed] [Google Scholar]
- 30.Rudikoff M.T., Maughan W.L., Effron M., Freund P., Weisfeldt M.L. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation. 1980;61:345–352. doi: 10.1161/01.cir.61.2.345. [DOI] [PubMed] [Google Scholar]
- 31.Aufderheide T.P., Frascone R.J., Wayne M.A. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoene P., Coult J., Murphy L. Course of quantitative ventricular fibrillation waveform measure and outcome following out-of-hospital cardiac arrest. Heart Rhythm. 2014;11:230–236. doi: 10.1016/j.hrthm.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 33.Segal N., Metzger A.K., Moore J.C. Correlation of end tidal carbon dioxide, amplitude spectrum area, and coronary perfusion pressure in a porcine model of cardiac arrest. Phys Rep. 2017;5 doi: 10.14814/phy2.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz J., Segal N., Kolbeck J., McKnite S., Caldwell E., Yannopoulos D. Sodium nitroprusside enhanced cardiopulmonary resuscitation (SNPeCPR) improves vital organ perfusion pressures and carotid blood flow in a porcine model of cardiac arrest. Resuscitation. 2012;83:374–377. doi: 10.1016/j.resuscitation.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzger A.K., Herman M., McKnite S., Tang W., Yannopoulos D. Improved cerebral perfusion pressures and 24-hr neurological survival in a porcine model of cardiac arrest with active compression-decompression cardiopulmonary resuscitation and augmentation of negative intrathoracic pressure. Crit Care Med. 2012;40:1851–1856. doi: 10.1097/CCM.0b013e318246b9ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yannopoulos D., Tang W., Roussos C., Aufderheide T.P., Idris A.H., Lurie K.G. Reducing ventilation frequency during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respir Care. 2005;50:628–635. [PubMed] [Google Scholar]
- 37.Yannopoulos D., Nadkarni V.M., McKnite S.H. Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation. 2005;112:803–811. doi: 10.1161/CIRCULATIONAHA.105.541508. [DOI] [PubMed] [Google Scholar]
- 38.Yannopoulos D., McKnite S., Aufderheide T.P. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2005;64:363–372. doi: 10.1016/j.resuscitation.2004.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.