ABSTRACT
The combination of coronavirus disease 2019 (COVID-19) pneumonia and pulmonary–renal syndrome due to ANCA‐associated vasculitis (AAV) poses diagnostic uncertainty and a therapeutic dilemma. According to current limited knowledge of COVID-19, the application of commonly used drugs in AAV, cyclophosphamide (CYC) and rituximab (RTX), must be weighed carefully in active COVID-19 infection. We report a case of a 52-year-old male patient with concurrent severe COVID-19 pneumonia and acute relapse of pulmonary–renal syndrome due to AAV after recent RTX maintenance dose. The patient presented with severe hypoxaemia, complete B-cell depletion and severe acute respiratory syndrome coronavirus 2 viraemia. He was successfully treated with therapeutic plasma exchange employing COVID-19 convalescent plasma.
Keywords: ANCA vasculitis, intensive care, plasma exchange, plasmapheresis, pneumonia, rituximab, SARS-CoV-2
BACKGROUND
In the absence of infection or known severe acute respiratory syndrome coronavirus 2 (SARS-CoV‐2) exposure, the American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) recommend continuation of ongoing immunosuppressive therapy in patients with systemic rheumatologic diseases [1]. Although scientific evidence is sparse, an exception to this recommendation might be treatment with B-cell directed therapy, for example rituximab (RTX) [2]. RTX specifically depletes CD20-positive B lymphocytes, hampering protective antibody-mediated immunity following infection and vaccination. Case reports and observational studies indicate staggering poor prognosis of coronavirus disease 2019 (COVID-19) in patients treated with RTX for rheumatologic diseases [3]. Early on during the pandemic, experts speculated that patients with RTX-induced hypogammaglobulinaemia and severe COVID-19 could profit from treatment with COVID-19 convalescent plasma (CCP) [4].
Herein, we report a case of a 52-year-old male patient with severe COVID-19 pneumonia and acute relapse of ANCA‐associated vasculitis (AAV) 18 days after the first maintenance dose of RTX according to MAINRITSAN protocol. The patient was successfully treated with therapeutic plasma exchange (TPE) substituting CCP.
CASE REPORT
On admission to our intensive care unit (ICU), the patient had a 2-week history of haemoptysis, fever and shortness of breath. A chest computed tomography scan showed left mid-zone consolidation, resembling both patterns found in COVID-19 peak stage 3 and pulmonary–renal syndrome associated with AAV. COVID-19 nasopharyngeal swabs tested SARS-CoV-2 RNA positive. In addition, SARS-CoV-2 RNAemia was detected, indicating a higher risk for severe COVID-19 [5]. Routine bloods tests were performed. Pertinent values are outlined in Suppl. Table 1. quick sequential organ failure assessment (qSOFA) on admission was 0, APACHE and SOFA score 17 and 6, respectively. High-flow nasal oxygen (HFNO) was initiated immediately due to severe hypoxaemia. Elevated MPO-ANCA levels of 533 RE/mL confirmed the relapse of AAV. Immunophenotyping of lymphocytes showed complete B-cell depletion.
Treatment with high-dose glucocorticoids (GCs) (250 mg prednisolone daily) was initiated. TPE was performed five times on consecutive days, processing 4 L plasma during each treatment and substituting CCP. According to previous studies [6], two units of high titer CCP (quality A, ‘high titer’ containing SARS-CoV-2 immunoglobulin G (IgG) >3 (Euroimmun ELISA) and/or neutralizing activity >250) were transfused at the end of each TPE. The other plasma used in TPE were low titer CCP (quality B, SARS-CoV-2 IgG and neutralizing activity >50, quality C: SARS-CoV-2 IgA/IgG antibodies with or without detection of neutralizing antibodies). CCP was manufactured with authorization of the local government and according to recommendations of the German end European Union (EU) authorities (EU Guidance [7]). In total, 79 CCPs were transfused (18 quality A, 14 quality B and 47 quality C).
We were able to generate a sufficiently high titer of anti-SARS-CoV-2 antibody to control COVID-19 pneumonia early after admission (Figure 1). In addition, we achieved sufficient clearing of ANCA and pro-inflammatory cytokines (Supplementary data, Figure 1A). Defervescence occurred 2 days after ICU admission. On day 6, the patient received 1000 mg of CYC and was weaned off the HFNO. The patient was transferred to the regular ward on day 9 and discharged on day 16. The patient received 120 mg prednisolone daily on discharge with continued tapering. SARS-CoV-2 IgG titers increased to day 14 and remained high for 1 month of follow-up, indicating active humoural immune response even under persisting B-cell depletion.
FIGURE 1:
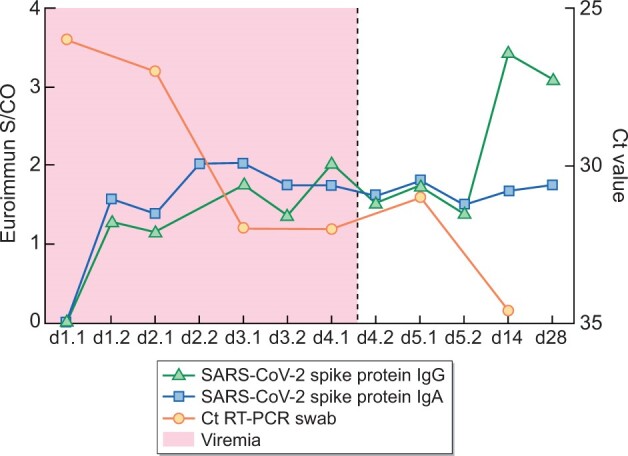
Time course of SARS-CoV-2 spike protein IgG and IgA, Ct value of RT-PCR nasal swab and viremia.
DISCUSSION
There is still uncertainty over which ongoing immunosuppressive regimens pose an additional risk in case of SARS-CoV-2 infection. While some biologicals and small molecules showed no harmful or even beneficial effects (e.g. tocilizumab and baricitinib) in COVID-19, RTX has been associated with poor outcomes [3, 8]. For patients with COVID-19 under RTX, at present no therapeutic strategy is defined.
Our patient not only presented with severe COVID-19 pneumonia after recent application of RTX, but in addition, suffered from an acute relapse of AAV necessitating immediate therapeutic intervention.
In AAV GC pulse plus immunosuppressive therapy comprising RTX or CYC or a combination of both are effective in reducing mortality and end-stage renal disease [9]. The use of TPE had been supported by former trials for rapid control of disease activity. In the latest large randomized trials, however, the additional benefits of TPE were limited [10]. Hence, TPE is no longer an inherent part of induction therapy in AAV.
In the present case of acute AAV, severe COVID-19 pneumonia and depletion of B cells after RTX, which implies hampered humoural immune response to SARS-CoV-2, we opted for TPE with CCP as the substitute. The patient's condition rapidly improved during the 5 days of TPE. Strikingly, even in the absence of detectable B cells, the patient was able to generate SARS-CoV-2-specific IgG 2–3 weeks after infection. We show that TPE with CCP was safe and feasible in COVID-19 pneumonia in an immunocompromised patient, and most likely improved the clinical outcome.
PATIENT CONSENT
We declare no competing interests. The patient provided informed consent for publication of this case report.
FUNDING
Collection and manufacturing of Covid-19 convalescent plasma was supported by EU (EU ESI grant DE 101019967).
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank B. Böll, D.A. Eichenauer, A. Shimabukuro-Vornhagen and J. Neuhann for valuable discussion of the case and manuscript.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
Data is available by request to corresponding authors.
Contributor Information
Matthias Kochanek, Department of Internal Medicine, Faculty of Medicine and University Hospital Cologne, Center for Integrated Oncology Aachen Bonn Cologne Dusseldorf (CIO ABCD), University of Cologne, Cologne, Germany.
Jorge Garcia Borrega, Department of Internal Medicine, Faculty of Medicine and University Hospital Cologne, Center for Integrated Oncology Aachen Bonn Cologne Dusseldorf (CIO ABCD), University of Cologne, Cologne, Germany.
Laura Beckmann, Department of Internal Medicine, Faculty of Medicine and University Hospital Cologne, Center for Integrated Oncology Aachen Bonn Cologne Dusseldorf (CIO ABCD), University of Cologne, Cologne, Germany.
Julia Neuhann, Faculty of Medicine and University Hospital Cologne, Transfusion Medicine (CIO), University Hospital of Cologne, Cologne, Germany.
Birgit S Gathof, Faculty of Medicine and University Hospital Cologne, Transfusion Medicine (CIO), University Hospital of Cologne, Cologne, Germany.
Veronica Di Cristanziano, Faculty of Medicine and University Hospital Cologne, Institute of Virology, Cologne, Germany.
Henning Hagmann, Department II of Internal Medicine, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne Cluster of Excellence on Cellular Stress Responses in Ageing-Associated Diseases, Center for Molecular Medicine Cologne, Cologne, Germany.
REFERENCES
- 1. Landewe RB, Machado PM, Kroon F et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis 2020; 79: 851–858 [DOI] [PubMed] [Google Scholar]
- 2. Schulze-Koops H, Krueger K, Vallbracht I et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2021; 80: e67. [DOI] [PubMed] [Google Scholar]
- 3. Loarce-Martos J, García-Fernández A, López-Gutiérrez F et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int 2020; 40: 2015–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta P, Porter JC, Chambers RC et al. B-cell depletion with rituximab in the COVID-19 pandemic: where do we stand? Lancet Rheumatol 2020; 2: e589–e590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eberhardt KA, Meyer-Schwickerath C, Heger E et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID-19 patients. Viruses 2020; 12: 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li L, Zhang W, Hu Y et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA 2020; 324: 460–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. An EU programme of COVID-19 convalescent plasma collection and transfusion. https://ec.europa.eu/health/sites/default/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf (10 March 2021, date last accessed)
- 8. Sarzi-Puttini P, Marotto D, Caporali R et al. Prevalence of COVID infections in a population of rheumatic patients from Lombardy and Marche treated with biological drugs or small molecules: A multicentre retrospective study. J Autoimmun 2021; 116: 102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McAdoo SP, Medjeral-Thomas N, Gopaluni S et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant 2019; 34: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walsh M, Merkel PA, Peh CA et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 2020; 382: 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available by request to corresponding authors.


