Abstract
The novel coronavirus disease 2019 (COVID-19) pandemic has lasted over 1 year and will not disappear in a short time. There is no specific remedy against the virus as yet. Vaccination is thus far one of the most important strategies for preventing COVID-19. Cancer patients with COVID-19 have a higher mortality because of immunosuppression. Immune checkpoint inhibitors (ICIs) are a novel anticancer strategy for blocking inhibitory pathways, which are related to the immune response. There is a question regarding whether COVID-19 vaccination and ICI treatment impact each other in cancer patients. This review explores both sides of the relationship between ICI treatment and COVID-19 vaccination and suggests good efficacy and safety of ICI treatment after COVID-19 vaccination as well as little impact on the virus protection and toxicity associated with COVID-19 vaccination during ICI treatment.
Keywords: : cancer, COVID-19, efficacy, immune checkpoint inhibitor, vaccination
Lay abstract
The novel coronavirus disease 2019 (COVID-19) pandemic has lasted over 1 year. Vaccination is a promising strategy for preventing COVID-19. Cancer patients are prone to infection with COVID-19, and these patients have high mortality. Immune checkpoint inhibitors (ICIs) are a novel anticancer strategy. Whether COVID-19 vaccination and ICI treatment impact each other in cancer patients remains unknown. This review explores both sides of the relationship between ICI treatment and COVID-19 vaccination and suggests good efficacy and safety of ICI treatment after COVID-19 vaccination as well as little impact on the virus protection and toxicity associated with COVID-19 vaccination during ICI treatment.
It has been over 1 year since the novel coronavirus disease 2019 (COVID-19) pandemic broke out and spread globally, with more than 176.8 million infections worldwide and 3.8 million cumulative deaths as of June 15, 2021 [1]. There continue to be many uncertainties in 2021, one of which is whether COVID-19 will become a long-term global or regional epidemic and infectious disease. Although the pandemic situation remains dire, medical staff and scientific researchers have made unremitting efforts and developed various treatments for COVID-19, including specific drugs for killing or inhibiting the virus, preventing viral infection and strengthening human immunity. However, there are still no specific treatments showing a definite effect. Therefore, vaccine development is one of the most important strategies for fighting this disease and is uniquely positioned to prevent further viral spread and epidemic recurrence in the world's population. At least 237 candidate vaccines are currently in preclinical and clinical development [2]. Candidate vaccines are categorized based on their components and range from traditional whole-pathogen vaccines to various new-generation vaccines. Traditional whole-pathogen vaccines include live-attenuated vaccines and inactivated vaccines. The new-generation vaccines include mRNA vaccines, plasmid DNA vaccines, viral vector-based vaccines and nonpathogenic bacterial vector-based vaccines [3]. During this pandemic, it is crucial to reconsider thoughtfully the treatment and protection of patients with malignancy, as these patients are highly susceptible to COVID-19 infection and have a higher mortality rate [4,5]. Immunosuppression status is considered to be a crucial reason for the higher incidence of COVID-19 in cancer patients [6]. On the one hand, cancer patients are in a state of impaired natural immunity; after a series of chemotherapy, radiotherapy, surgical and other anticancer treatments, the body's immunity and physical status get even weaker. Immune checkpoint inhibitors (ICIs) are a kind of novel anticancer treatment that work by blocking inhibitory pathways. ICI treatment is currently an important regimen for cancer patients with melanoma, lung cancer, etc. In treating malignancies, ICIs result in longer-term survival, but they can also cause some side effects, such as rashes, gastrointestinal reactions, thyroid dysfunction, pneumonitis and colitis [7,8]. Many oncology societies have proposed guidelines that recommend categorizing patients into high, medium and low priority groups and managing the course of the disease accordingly [9].
In addition, ICI treatment is related to the immune response, including humoral and cellular immunity, cytokine release, immune molecule changes and even autoimmunity. There is also a question of whether COVID-19 vaccination and ICI treatment impact each other in cancer patients. Could COVID-19 vaccination impact the efficacy and safety of ICI treatment? Likewise, could ICI treatment affect the virus protection and toxicity associated with COVID-19 vaccination? All these questions are discussed in this review.
Current ICI treatment for cancer patients with COVID-19
Some preliminary data have revealed that cancer patients with SARS coronavirus 2 (SARS-CoV2) infection have a high probability of mortality [10,11]. Anticancer treatment, such as chemotherapy or surgery, might increase the severity of infection and risk of death in SARS-CoV2-infected patients. ICI treatment has become one of main treatments for different tumor types (e.g., melanoma, lung cancer, renal cancer and Hodgkin's lymphoma). Unfortunately, there is insufficient scientific evidence to confirm the potential interaction between ICI treatment and COVID-19 vaccination, so ICI treatment in cancer patients with COVID-19 infection is a controversial topic [12].
ICI treatment activates CD8+ T cells and elevates levels of cytokines such as IL-6 and IFN-γ to kill tumor cells [13,14]. In patients with COVID-19, the causes of death are acute respiratory distress syndrome and multiple organ failure, which is induced by high levels of interleukin, interferon and other cytokines [15]. By contrast, ICI treatment is also used in patients with viral infections, including HIV, HBV and HCV, and the toxicity and efficacy are similar to that seen in patients without viral infection [16]. CD8+ T cells are the major cell type to resist viral infection, and ICI treatment can effectively reactivate exhausted CD8+ T cells [17]. The inflammatory cytokines secreted by CD8+ T cells might enhance the antiviral ability of the body and eliminate COVID-19; hence, ICI treatment might be helpful for defeating viral infection in patients. Immune interstitial lung disease (iILD) is a common and fatal adverse event associated with ICI treatment. The incidence rate of iILD ranges from 2.5–5% with ICI monotherapy to 7–10% with combination ICI therapy [18]. When cancer patients with COVID-19 suffer from iILD, it is likely to aggravate lung damage. Moreover, the salvage treatment with iILD is different from that used in COVID-19 pneumonitis, which makes the situation worse [19]. Nevertheless, the incidence of iILD is relatively rare, and there is insufficient evidence to suggest that iILD increases in the presence of COVID-19. The clinical outcome of patients receiving ICI treatment with COVID-19 infection is controversial, and there has been no prospective study in this field to date. In this review, studies with small sample size as well as retrospective studies will be discussed in the following sections.
Robilotti et al. reported on 31 out of 423 cancer patients with COVID-19 who were treated with ICIs and showed that ICI treatment was a risk factor for developing severe disease [20]. Yang et al. reported on 205 cancer patients with COVID-19, of whom seven received ICI treatment, with the result of four survivals and three deaths [21]. Among these, four patients were treated within 4 weeks prior to SARS-CoV2 infection and two died. Wu et al. reported on 11 cancer patients who received prior ICI treatment and were confirmed to have COVID-19 [22]. Among these, seven developed severe COVID-19 infection, whereas four had nonsevere infection. Six out of the seven patients with severe infection received three or more cycles of ICI treatment, whereas only one of the four patients with nonsevere infection received three cycles. Therefore, severity of infection and mortality in cancer patients with COVID-19 might be associated with the number of cycles of ICI treatment. A multicenter clinical study that included 105 cancer patients with COVID-19 and 536 age-matched noncancer patients with COVID-19 was reported by Dai et al. [23]. Six of the 105 cancer patients receiving ICI treatment tended to have a high proportion of critical symptoms (four of six patients) and high mortality (two of six patients). Lee et al. reported that mortality in 800 cancer patients with COVID-19 was associated with age, sex and comorbidities [24]. Previous treatment within 4 weeks, including chemotherapy, hormonal therapy, radiotherapy and ICI treatment, did not affect mortality. Luo et al. reported that in 69 lung cancer patients with COVID-19, ICI treatment was not associated with a high risk of disease deterioration when adjusting for smoking status [25]. Szabados et al. reported on four of 74 genitourinary cancer patients treated with ICIs who were infected with SARS-CoV2 and indicated that all four patients were alive 32–45 days after diagnosis [26]. Yekeduz et al. reported on a 75-year-old female COVID-19 patient with metastatic malignant melanoma and many complications, such as hypertension, diabetes mellitus type 2, atrial fibrillation, coronary artery disease and chronic obstructive pulmonary disease, who completed 27 cycles of ICI treatment [27]. These cases suggest that cancer patients with COVID-19 might be suitable for ICI treatment.
All the aforementioned clinical studies involving ICI-treated cancer patients and SARS-CoV-2 infection are summarized in Table 1. Although the evidence is not sufficient and controversial, the authors of the current review hypothesize that appropriate ICI treatment is acceptable in cancer patients infected with COVID-19, except in those with severe disease. However, some questions remain to be solved. First, when is the best intervention time point for ICI treatment after cancer patients are infected with COVID-19? Second, who is the appropriate candidate for ICI treatment? Third, how do we distinguish ICI-associated pneumonia from COVID-19? In addition, individualized assessment of the risks and benefits in patients with COVID-19 will determine whether ICI treatment is likely to provide the greatest benefit.
Table 1. Summary of studies reporting on immune checkpoint inhibitor treatment in cancer patients with coronavirus disease 2019.
| Outcome | Study | Patients (n) | Observed outcome | Survival rate, % (n) | Ref. |
|---|---|---|---|---|---|
| Unfavorable | Robilotti et al. | 31 | Risk factor | – | [20] |
| Yang et al. | 7 | Four survivals and three deaths | 57.14 (4) | [21] | |
| Wu et al. | 11 | Seven with development of severe disease | 63.64 (7) | [22] | |
| Dai et al. | 6 | Four with critical symptoms and two deaths | 66.66 (4) | [23] | |
| Favorable | Lee et al. | 44 | No effect on mortality | 77.27 (34) | [24] |
| Luo et al. | 69 | No association with increased risk | 76.81 (53) | [25] | |
| Szabados et al. | 4 | Survival | 100 (4) | [26] |
Impact of COVID-19 vaccination on efficacy & safety of ICI treatment in cancer patients
Currently, at least 283 COVID-19 vaccine candidates are registered worldwide, of which about ten are undergoing phase III clinical trials [28]. Preliminary results have demonstrated that the COVID-19 vaccines have acceptable safety and high efficacy [29]. However, for cancer patients undergoing ICI treatment, direct evidence regarding COVID-19 vaccination is limited, and whether COVID-19 vaccination is suitable remains unknown. Some studies related to vaccination and ICI use in cancer patients might give us some hints.
One study demonstrated that rotavirus vaccines display both immunostimulatory and oncolytic properties in activating NF-κB and overcoming resistance to ICI treatment, and the combination of rotavirus vaccines and ICI treatment could generate durable tumor-specific immunity [30]. The INVIDIa study reported that cancer patients who underwent influenza vaccination had more cycles of ICI treatment (median 20.5 vs six cycles) and obtained improved overall survival compared with those who did not undergo vaccination (72 vs 62 months) [31]. The study also demonstrated better 1-year overall survival in the non-small-cell lung cancer subgroup (86.7 vs 66.7%).
Some physicians are concerned about whether vaccination impacts the safety of ICI treatment by increasing the incidence of immune-related adverse events (irAEs). Since vaccination may overload the immune system and trigger a ‘cytokine storm,’ severe toxicity may damage the organs and even lead to death. However, in real-world studies, the results have been controversial. A retrospective study compared the incidence of irAEs in cancer patients who received both pembrolizumab and the influenza vaccine with those who received pembrolizumab alone [32]. Interestingly, the vaccinated group had a lower incidence of irAEs of any grade (25.7 vs 40.2%) and a longer median irAE-free duration (not reached vs 28 months) compared with the nonvaccinated group.
Some studies have reported that the incidence of irAEs is not significantly different between cancer patients who receive ICI treatment plus the influenza vaccine and those who receive ICI treatment alone. Wijn et al. found no significant difference in the incidence of irAEs between influenza-vaccinated and nonvaccinated groups (26 vs 22%) in non-small-cell lung cancer patients treated with nivolumab [33]. The incidence of severe irAEs was also not significantly different between the two groups (7 vs 4%). Chong et al. reported that the incidence of irAEs was similar between anti-PD-1 plus the influenza vaccine and anti-PD-1 alone groups, though it was higher in patients who received combined anti-PD-1 (nivolumab) and anti-CTLA-4 (ipilimumab) treatment [34]. A small cohort study reported an acceptable incidence of irAEs after administering inactivated influenza vaccine to cancer patients treated with ICIs [35]. However, Laubli et al. showed that influenza vaccination could increase the incidence of irAEs in ICI treatment, with approximately 52% experiencing all grades of irAEs and 26% experiencing severe grade 3/4 irAEs in a study of 23 lung cancer patients receiving ICI treatment combined with the influenza vaccine [36]. Similarly, a retrospective study reported by Chong et al. at the 2018 American Society of Clinical Oncology meeting found that all grades of irAEs were more common in patients receiving combined ICI therapy (anti-PD-1 and ipilimumab) than in those receiving anti-PD-1 alone (43 vs 18%) [37]. In addition, the occurrence time of irAEs in the combined treatment group was significantly earlier than that in the single treatment group. A recent study reported a preliminary result in 134 cancer patients who received ICI treatment and two doses of a COVID-19 vaccine (BNT162b2), noting that the side effect profile between the healthy controls and the cancer patients was similar except for muscle pain [38]. Moreover, irAEs in cancer patients were the same as those observed prior to COVID-19 vaccination.
The accumulated evidence suggests that COVID-19 vaccination may improve the efficacy of ICI treatment in cancer patients, and the side effects associated with COVID-19 vaccination may be ignored in cancer patients treated with single ICIs. The only concern is that the use of combined ICI therapy (anti-PD-1, anti-PD-L1, anti-CTLA-4) may increase the incidence of irAEs. These patients should be carefully assessed when they plan to undergo COVID-19 vaccination.
Impact of ICI treatment on virus protection & toxicity associated with COVID-19 vaccination
With regard to the current severe epidemic situation, COVID-19 vaccination is no doubt the most effective preventive measure. There are at least 283 COVID-19 vaccine candidates with different antiviral mechanisms currently registered [39]. A recombinant COVID-19 viral vector vaccine from China may provide both humoral immunity and cellular immunity [40]. As a result, COVID-19 vaccination is an effective measure for preventing SARS-CoV-2 infection in cancer patients. In the era of ICI treatment for cancer patients, oncologists and scientists question whether ICI treatment affects the virus protection and toxicity associated with COVID-19 vaccination.
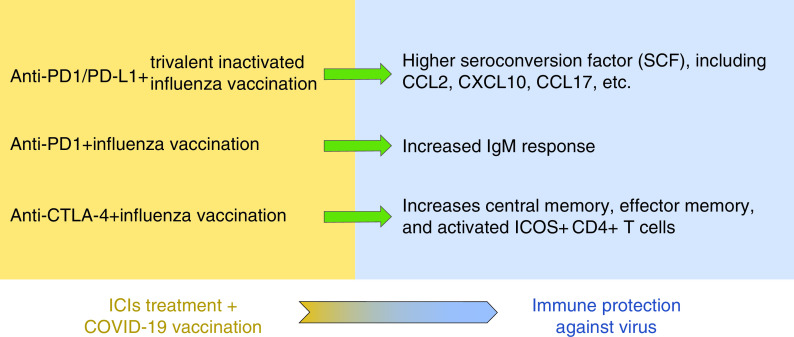
In 2018, Laubli et al. found that seropositivity was slightly, though nonsignificantly, lower in 23 metastatic non-small-cell lung cancer patients treated with anti-PD-1/PD-L1 and trivalent inactivated influenza virus vaccine compared with healthy controls [36]. Interestingly, the seroconversion factors, including CCL2, CXCL10 and CCL17, were significantly higher in the cancer patients compared with the age-matched healthy controls. Similarly, Weber et al. [41] found that ipilimumab increased central memory and effector memory and activated ICOS+ CD4+ T cells but not ICOS+ CD8+ T cells or FoxP3+ CD4+ regulatory T cells, which is consistent with the results observed by Laubli et al. [36]. At the 2017 American Society of Clinical Oncology meeting, Kanaloupitis et al. demonstrated a significant increase in IgM response after influenza vaccination in patients receiving anti-PD-1 treatment [42]. Based on these findings, scientists hypothesized that cancer patients had much greater immune stimulation compared with healthy people because of the relatively low baseline level in cancer patients [36]. Although no study has yet been conducted on the effect of ICI treatment and COVID-19 vaccination, on the basis of similar studies, the authors of the current review suggest that patients receiving ICI treatment may undergo COVID-19 vaccination, and that this may increase the efficacy of the vaccine to some extent (Figure 1).
Figure 1. Treating cancer patients with immune checkpoint inhibitors may increase the efficacy of COVID-19 vaccines.
ICI: Immune checkpoint inhibitor; COVID-19: Coronavirus disease 2019.
Since both ICI treatment and vaccination for COVID-19 stimulate the body's immune response, the authors question whether COVID-19 vaccination might increase the incidence of irAEs with ICI treatment. To date, there are no data demonstrating the direct answer. Therefore, the authors referred to studies involving the use of other vaccines during ICI treatment. Erickson et al. found that acute viral lower respiratory tract infection causes rapid pulmonary CD8+ cytotoxic T lymphocyte functional impairment via PD-1/PD-L1 signaling [43]. Blocking PD-1 signaling prevents CD8+ cytotoxic T lymphocyte impairment, reduces viral titers during primary infection and enhances the protection of immunized mice against infection. Additionally, PD-L1 expression on CD8+ T cells is negatively correlated with T-cell proportions in patients infected with A(H1N1)pdm09 [44]. These results are similar to those seen in research from Erickson et al. [45]. Taken together, these data indicate that ICIs may improve the efficacy of COVID-19 vaccines while having little impact on the toxicity associated with COVID-19 vaccination.
Conclusion
To date, no COVID-19 vaccination guidelines indicate whether cancer patients should be vaccinated or only certain subgroups should be. However, based on previous studies involving vaccines and ICI treatment, this review suggests good efficacy and safety of ICI treatment after COVID-19 vaccination as well as improvement in virus protection and little impact on the toxicity associated with COVID-19 vaccination during ICI treatment. Our opinion is consistent with statements from the European Society for Medical Oncology and the American Society of Clinical Oncology regarding COVID-19 vaccination in patients with cancer [46,47]. We suggest that cancer patients who intend to receive a COVID-19 vaccine follow the instructions of immunologists and oncologists instead of paying attention to rumors on the internet or nonprofessionals. Nevertheless, whether ICI treatment or a COVID-19 vaccine is used should be carefully considered based on an individual cancer patient's specific situation. Evidence regarding COVID-19 vaccination in cancer patients receiving ICIs or ICI treatment after COVID-19 vaccination still needs to be verified by clinical trials.
Future perspective
Nevertheless, we believe that this question will be answered correctly as prospective trials are conducted and similar concerns will be accurately predicted with the continuous development of molecular biotechnology and genetic engineering techniques in the next 5–10 years.
Summary points.
Cancer patients are prone to infection with coronavirus disease 2019 (COVID-19) and have high mortality.
Appropriate immune checkpoint inhibitor (ICI) treatment is acceptable in cancer patients infected with COVID-19, except in those with severe disease.
COVID-19 vaccination may improve the efficacy of ICI treatment in cancer patients.
The side effects of COVID-19 vaccination may be ignored in cancer patients treated with single ICIs, though there is a concern in those receiving combined ICI therapy.
ICI treatment may improve virus protection and have little impact on the toxicity associated with COVID-19 vaccination.
Author contributions
Conception and design: Z Xu, Y He and J Sun. Administrative support: J Sun. Provision of study materials or patients: B Luo, J Li and X Hou. Collection and assembly of data: Q Yang, Y Zhou and J Ye. Data analysis and interpretation: X Wu, Y Feng and T Hu. Manuscript writing: B Luo, J Li, X Hou, Q Yang, Y Zhou, J Ye, X Wu, Y Feng, T Hu, Z Xu, Y He and J Sun. Final approval of manuscript: B Luo, J Li, X Hou, Q Yang, Y Zhou, J Ye, X Wu, Y Feng, T Hu, Z Xu, Y He and J Sun.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81972858), the Clinical Research Project of Army Medical University (2018XLC1010), and the Chongqing Science and Technology Leading Talents Program (cstccxljrc201910), and the Research Projects of the Joint Logistics Support Force of PLA (TKTJKY2020003, TKTJKY2020029 and TKTJKY2020136).
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors did not use any human tissues or organs, and they state that they have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations to protect their right and privacy.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.WHO. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int
- 2.WHO. Draft landscape of COVID-19 candidate vaccines (2021). www.who.int/publications/m/item/draft-landscape-of- covid-19-candidate-vaccines
- 3.Wang J, Peng Y, Xu H, Cui Z, Williams RO 3rd. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech 21(6), 225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Zhu F, Xie L et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 31(7), 894–901 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323(18), 1775–1776 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am. J. Clin. Oncol. 43(6), 452–455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest 154(6), 1416–1423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieux-Klotz C, Dior M, Damotte D et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target. Oncol. 12(3), 301–308 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. Curr. Oncol. Rep. 22(5), 53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W, Guan W, Chen R et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 21(3), 335–337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 21(4), e180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambichler T, Reuther J, Scheel CH, Susok L, Kern P, Becker JC. Cancer and immune checkpoint inhibitor treatment in the era of SARS-CoV-2 infection. Cancers (Basel) 12(11), 3383 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shergold AL, Millar R, Nibbs RJB. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol. Res. 145, 104258 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Turgeon GA, Weickhardt A, Azad AA, Solomon B, Siva S. Radiotherapy and immunotherapy: a synergistic effect in cancer care. Med. J. Aust. 210(1), 47–53 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Kaur S, Bansal R, Kollimuttathuillam S et al. The looming storm: blood and cytokines in COVID-19. Blood Rev. 46, 100743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah NJ, Al-Shbool G, Blackburn M et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J. Immunother. Cancer 7(1), 353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller BC, Sen DR, Al Abosy R et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20(3), 326–336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 20(1), e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabro L, Peters S, Soria JC et al. Challenges in lung cancer therapy during the COVID-19 pandemic. Lancet Respir. Med. 8(6), 542–544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robilotti EV, Babady NE, Mead PA et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 26(8), 1218–1223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K, Sheng Y, Huang C et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 21(7), 904–913 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Chu Q, Zhang H et al. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun. (Lond.) 40(8), 374–379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai M, Liu D, Liu M et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 10(6), 783–791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee LY, Cazier JB, Angelis V et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395(10241), 1919–1926 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 10(8), 1121–1128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabados B, Abu-Ghanem Y, Grant M, Choy J, Bex A, Powles T. Clinical characteristics and outcome for four SARS-CoV-2-infected cancer patients treated with immune checkpoint inhibitors. Eur. Urol. 78(2), 276–280 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yekeduz E, Dursun B, Aydin GC et al. Clinical course of COVID-19 infection in elderly patient with melanoma on nivolumab. J. Oncol. Pharm. Pract. 26(5), 1289–1294 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 396(10262), 1595–1606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383(27), 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shekarian T, Sivado E, Jallas AC et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci. Transl. Med. 11(515), eaat5025 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Bersanelli M, Giannarelli D, Castrignano P et al. Influenza vaccine indication during therapy with immune checkpoint inhibitors: a transversal challenge. The INVIDIa study. Immunotherapy 10(14), 1229–1239 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Failing JJ, Ho TP, Yadav S et al. Safety of influenza vaccine in patients with cancer receiving pembrolizumab. JCO Oncol. Pract. 16(7), e573–e580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijn DH, Groeneveld GH, Vollaard AM et al. Influenza vaccination in patients with lung cancer receiving anti-programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur. J. Cancer 104, 182–187 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin. Infect. Dis. 70(2), 193–199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwynn ME, Deremer DL, Saunders KM, Parikh J, Bollag RJ, Clemmons AB. Immune-mediated adverse events following influenza vaccine in cancer patients receiving immune checkpoint inhibitors. J. Oncol. Pharm. Pract. 26(3), 647–654 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Laubli H, Balmelli C, Kaufmann L et al. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J. Immunother. Cancer 6(1), 40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong CR, Park V, Harding JJ, Brite J, Kamboj M. Safety of influenza vaccination in patients undergoing immunotherapy treatment for advanced cancer. J. Clin. Oncol. 36(Suppl. 15), e15073–e15073 (2018). [Google Scholar]
- 38.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 22(5), 581–583 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. COVID-19 vaccine tracker and landscape. www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 40.Zhu FC, Li YH, Guan XH et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395(10240), 1845–1854 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber JS, Hamid O, Chasalow SD et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J. Immunother. 35(1), 89–97 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Kanaloupitis DK, Chandran A, Ralph A, Thompson R, Hallmeyer S. Safety and efficacy of concurrent administration of influenza vaccine in patients undergoing anti-PD-1 immunotherapy. J. Clin. Oncol. 35(Suppl. 15), e14607–e14607 (2017). [Google Scholar]
- 43.Erickson JJ, Gilchuk P, Hastings AK et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J. Clin. Invest. 122(8), 2967–2982 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valero-Pacheco N, Arriaga-Pizano L, Ferat-Osorio E et al. PD-L1 expression induced by the 2009 pandemic influenza A(H1N1) virus impairs the human T cell response. Clin. Dev. Immunol. 2013, 989673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson JJ, Rogers MC, Hastings AK, Tollefson SJ, Williams JV. Programmed death-1 impairs secondary effector lung CD8(+) T cells during respiratory virus reinfection. J. Immunol. 193(10), 5108–5117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ESMO. COVID-19 vaccination in cancer patients: ESMO statements. www.esmo.org/covid-19-and-cancer/covid-19-vaccination
- 47.American Society of Clinical Oncology. COVID-19 vaccines & patients with cancer. www.asco.org/asco-coronavirus-resources/covid-19-vaccines-patients-cancer