Abstract
Adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide (formerly called AF-2364), is a nonhormonal male contraceptive, since it effectively induces reversible male infertility without perturbing the serum concentrations of follicle stimulating hormone (FSH), testosterone, and inhibin B based on studies in rats and rabbits. Adjudin was shown to exert its effects preferentially by perturbing the testis-specific actin-rich adherens junction (AJ) at the Sertoli–spermatid interface known as apical ectoplasmic specialization (apical ES), thereby effectively inducing spermatid exfoliation. Adjudin did not perturb germ cell development nor germ cell function. Also, it had no effects on Sertoli cell–cell AJ called basal ectoplasmic specialization (basal ES), which, together with tight junction constitute the blood-testis barrier (BTB), unless an acute dose of adjudin was used. Adjudin also did not perturb the population of spermatogonial stem cells nor Sertoli cells in the testis. However, the downstream signaling protein(s) utilized by adjudin to induce transient male infertility remains unexplored. Herein, using adult rats treated with adjudin and monitored changes in the phenotypes across the seminiferous epithelium between 6 and 96 h in parallel with the steady-state protein levels of an array of signaling and cytoskeletal regulatory proteins, recently shown to be involved in apical ES, basal ES and BTB function. It was shown that adjudin exerts its contraceptive effects through changes in microtubule associated proteins (MAPs) and signaling proteins mTORC1/rpS6 and p-FAK-Y407. These findings are important to not only study adjudin-mediated male infertility but also the biology of spermatogenesis.
Keywords: MAP, microtubule, testis, spermatogenesis, Sertoli cells, adjudin, cadmium chloride
In the mammalian testis, extensive remodeling of cell junctions take place in the seminiferous epithelium during the epithelial cycle to support spermatogenesis (1–4). This is necessary to accommodate the transport of preleptotene spermatocytes across the blood-testis barrier (BTB), but also developing spermatids across the seminiferous epithelium, so that spermatozoa transformed from step 19 spermatids in rat testes can be released into the tubule lumen at spermiation (5–7). This thus requires the rapid breakdown (ie, depolymerization or shrinkage) and reassembly (ie, polymerization), with intermittent stabilization, of cytoskeletons, most notably actin- and microtubule (MT)-based cytoskeletons, and referred to cytoskeletal dynamics (8, 9). These 2 cytoskeletons are most prominent at the testis-specific Sertoli-spermatid and Sertoli cell–cell interface known as the apical and basal ectoplasmic specialization (ES), which both cytoskeletons lay adjacent to each other to support apical ES and basal ES function (3, 10, 11).
Mammalian target of rapamycin (mTOR) is a nonreceptor Ser/Thr protein kinase found in virtually all mammalian cells, which regulates multiple cellular processes, most notably cell energy status, including cell survival, apoptosis, blood-testis barrier dynamics, and others (12–14). When mTOR binds to regulatory-associated protein of mTOR (Raptor) or rapamycin insensitive companion of mTOR (Rictor), each serves as an adaptor protein, this creates either the mammalian target of rapamycin complex 1 (mTORC1) or the mTORC2 signaling complex, respectively (13, 15). Studies have shown that mTORC1 exerts its effects to modulate multiple cellular events, including BTB dynamics, via rpS6 (16) and Atk1/2 signaling pathway in vitro (17–19) and in vivo (20–22) through changes in the organization of actin- and MT-based cytoskeletons. On the other hand, p-FAK-Y407 plays a crucial role of regulating BTB dynamics in the testis of rats (23, 24) and humans (25) through cytoskeletal organization, and have recently shown to be likely involved in the mTORC1/rpS6/Akt1/2 signaling pathway (26). Collectively, these findings have shown that the mTORC1/rpS6/Akt1/2 and p-FAK-Y407 are the putative signaling proteins involved in modulating Sertoli cell cytoskeletal function to modify BTB function and spermatogenesis.
Studies in epithelia, including the seminiferous epithelium in the testis, have shown that MT-associated proteins (MAPs), most notably structural MAPs (eg, MAP1a), are crucial to stabilize MTs and actin filaments to support cytoskeletal function (27, 28). Other MAPs include end-binding proteins that associate with the plus (+) or minus (-) ends of MTs to stabilize MTs and modulate MT dynamics (29–32), motor proteins that drive the transport of germ cells and cargoes along the MT-based tracks (33–35), proteins that polymerize or cleave/severe MTs (eg, katanin, spastin, fidgetin) (36, 37), and MT nucleators (38, 39) that are also necessary to confer cytoskeletal function. Most importantly, these MAPs, such as +TIPs (MT+ end-tracking proteins, eg, End-binding Protein 1 [EB1] (32)), -TIPs (MT- end-targeting proteins, eg, calmodulin-regulated spectrin-associated protein 2 [CAMSAP2] (31)), and dynein 1 (35) (an MT- end-directed motor protein), that support MT function are also involved in conferring actin cytoskeletal dynamics, including the testis, as noted in recent reports (21, 22, 31, 32, 40). In brief, these studies illustrate the intimate functional relationship between the 2 cytoskeletons to support spermatogenesis. However, the downstream signaling proteins (or underlying mechanism) by which these MAPs regulate spermatogenesis through their effects on actin- and MT-cytoskeletons in the testis remains to be better understood. On the other hand, the involved signaling proteins and the signaling cascades utilized by adjudin to mediate germ cell exfoliation remain unexplored. Since studies in the last decade have identified several signaling proteins that are involved in modulating BTB, cell junction dynamics, and spermatogenesis (41–43), we sought to examine if these signaling proteins are also utilized by adjudin to affect spermatogenesis. Herein, we utilized the pharmaceutical and toxicant models based on a series of coherent experiments using adult rats treated with nonhormonal male contraceptive adjudin and the environmental toxicant cadmium chloride (CdCl2) for studies. We sought to unravel the involvement of several structural MAPs, the mTORC1/rpS6 and the p-FAK-Y407 signaling proteins in adjudin-mediated changes in cytoskeletal organizations of MTs and F-actin. These changes, in turn, impeded germ cell adhesion, leading to germ cell exfoliation from the testis, causing transient infertility (44). More important, findings reported here provide insightful information regarding the role of MAPs, mTORC1/rpS6, and p-FAK-Y407 in supporting cytoskeletal organization to sustain spermatogenesis.
Materials and Methods
Animals
Adult Sprague-Dawley rats at ~250 gm body weight (b.w.) were obtained from Charles River Labs (Kingston, New York). All adult rats were housed at the Rockefeller University Comparative Bioscience Center in groups of 2 animals per cage for at least 48 hours prior to their use. The use of animals for the experiments reported here, involving the use of adjudin and CdCl2 outlined in the “Materials and Methods” section, was approved by the Rockefeller University (RU) Institutional Animal Care and Use Committee with Protocol Number 18-043-H. The use of CdCl2, an environmental hazardous material, for our studies was also approved by RU Laboratory Safety and Environmental Health (RU LS&EH). Rats in groups of n = 4–6, including control, were euthanized at specified time points by CO2 asphyxiation in a euthanasia animal chamber approved by the RU LS&EH using slow displacement of chamber air (20–30%/minute) from a CO2 gas tank with a built-in regulator. All rats were kept at 20oC ± 1oC with a 12-hour light, 12-hour dark cycle, and with free access to standard rat chow and water.
Antibodies
Antibodies used for the various experiments to obtain corresponding data reported here were purchased from different vendors (see Table S1, which is located in a digital research materials repository (45)). Each of these antibodies was earlier characterized in our laboratory and described in published reports, as noted in corresponding sections in this report, including their specificity, working dilutions, their specific use for either immunoblotting (IB), immunofluorescence analysis, or both, and Research Resource Identifiers (RRID) (Table S1). Also, these antibodies cross-reacted with the corresponding proteins in rats, as noted by the manufacturers. Antibodies from Abcam (Cambridge, Massachusetts) included: α-tubulin (46), GAPDH (47), detyrosinated α-tubulin (48), Prickle 1 (49), ß-tubulin (50), acetylated α-tubulin (51), and MAP1a (52). Antibodies from Cell Signaling Technology (Danvers, Massachusetts) included: rpS6 (53), p-rpS6-S235/S236 (54), p-rpS6-S240/S244 (55), mTOR (56), and Raptor (57). Antibody from BD Biosciences (San Jose, California) included Eps8 (58). Antibodies from Invitrogen (Carlsbad, California) included: p-FAK-Y407 (59) and p-FAK-Y397 (60). Antibodies from Santa Cruz Biotechnology (Dallas, Texas) included actin (61), EB1 (62, 63), FAK (64), rabbit IgG-HRP (65), and mouse IgG-HRP (66). Antibodies from Proteintech Group (Rosemont, Illinois) included: MAP2 (67), MT-affinity regulating kinase 4 (MARK4) (68), CAMSAP2 (69), and MARK2 (70). Antibodies from Thermo Fisher Scientific (Fair Lawn, New Jersey) included: mouse IgG Alexa Fluor 488 (71), mouse IgG Alexa Fluor 555 (72), rabbit IgG Alexa Fluor 488 (73), and rabbit IgG Alexa Fluor 555 (74). Antibody from Millipore Sigma (Burllington, Massachusetts) was tyrosinated α-tubulin (75). Antibody from Sigma-Aldrich (Allentown, Pennsylvania) was Arp3 (76).
Treatment of rats with adjudin or cadmium chloride
Adjudin, 1-(2,4-dichorobenzyl)-1H-indazole-3-carbohydrazide, synthesized as earlier described with a purity of >99.5% (77) was suspended in 0.05% methylcellulose (ie, 0.05 gm/100 ml MilliQ water; Sigma-Aldrich, St Louis, Missouri) at 20 mg adjudin/ml, stirring at room temperature for ~3 hours, which was then stored at 4oC until use within 1 week, as earlier described (77, 78). Rats were treated with this adjudin suspension via oral gavage at a single dose of 50 mg/kg b.w. In some experiments, rats were also treated with CdCl2 via intraperitoneal injection (i.p.) by dissolving CdCl2 in 0.9% saline (0.9 gm sodium chloride per 100 ml MilliQ water) at 10 mg/ml at a dose of 3 mg/kg b.w., as described (79). Animals were then sacrificed by CO2 asphyxiation in groups of 4 to 6 rats at specified time points so that at least n = 3 testes from different rats were used for each experiment. Testes were snap-frozen in liquid nitrogen and stored at -80oC until use, either for immunoblot analysis or to obtain frozen sections for immunofluorescence analysis. In experiments for histological analysis (for hematoxylin and eosin staining) or immunofluorescence analyses, testes were fixed in either modified Bouin’s fixative (80) (for cadmium-treated samples) or modified Davidson fixative (81) (for adjudin-treated samples), as earlier described (31, 32, 40) and embedded in paraffin to obtain 5-µm thick sections using a microtome.
Protein lysate preparation, protein estimation, and IB analysis
Testis lysates were obtained by suspending tissue blocks snap-frozen in liquid nitrogen and stored at -80oC earlier in lysis buffer (50 mM tris [pH 7.4 at 22°C] containing 0.15 M NaCl, 2 mM EGTA, 1% Nonidet P-40 [v/v], and 10% glycerol [v/v]) supplemented with protease and phosphatase inhibitors freshly added to the lysis buffer as described (31, 32). If necessary, tissue blocks were trimmed into smaller pieces by using a pair of autoclaved scissors. Tissues suspended in lysis buffer were subjected to sonication to obtain homogenates (twice, 8 seconds each, with 1 minute in between, in ice) with a Cole-Parmer ultrasonic processor (Model CPX 130PB; Cole-Parmer, Chicago, Illinois). Homogenates were centrifuged at 16 000 g for 1 hour at 4°C to obtain the clear supernatant as protein lysates. Protein concentration in lysates was estimated using Bio-Rad detergent compatible (DC) protein assay kits (Bio-Rad Laboratories, Hercules, California), with BSA as a standard. Immunoblotting was performed using 30 µg of protein per sample for each lane by SDS-PAGE, proteins in gels were electroblotted onto BioRad nitrocellulose membrane previously soaked in MilliQ water for 1-2 min before its use, and the levels of proteins in each blot were assessed by chemiluminescence using a GE ImageQuant LAS-4000 Mini-Luminiscent Image Analyzer and quantified by using Image Quant (Version 1.3) software package, with in-house enhanced chemiluminescence kits as described (82). GAPDH, β-actin, and/or α-tubulin served as protein loading control for IB analysis. To avoid interexperimental variations, all samples within an experimental group, including treatment and control samples, were analyzed simultaneously in a single experimental session. The IB data shown here were results of a representative experiment from n = 3 independent experiments, which yielded similar results.
Immunofluorescence microscopy using paraffin sections
To visualize the co-localization of MAP1a, CAMSAP2, EB1 and α- or β-tubulin (staining for MTs) in the seminiferous epithelium of adult rats, testes were fixed in modified Davidson’s fixative (consisting of 30% of formaldehyde [37%], 15% ethanol, 5% glacial acetic acid, and 50% double-distilled H2O) or modified Bouin’s fixative overnight. Thereafter, testes were embedded in paraffin, and cross-sections (~5-µm thick) were obtained with a microtome. For histology or IF, paraffin was removed by xylene, to be followed by hydration using graded ethanol, and then washed in PBS (10 mM sodium phosphate, 0.15 M NaCl [pH 7.4] at 22°C) (thrice), and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, Missouri) in PBS for 10 minutes. After permeabilization, sections were washed in PBS, blocked with 1% BSA in PBS (w/v) for 30 minutes at room temperature, and then incubated with the corresponding primary antibody (Table S1) (45) diluted in PBS overnight at 4°C. Afterwards, slides were washed in PBS and then incubated with the corresponding secondary antibody (mouse or rabbit IgG-Alexa Fluor 555 for red fluorescence, mouse or rabbit IgG-Alexa Fluor 488 for green fluorescence; Invitrogen, Carlsbad, California) diluted in PBS for 1 hour at room temperature. To visualize cell nuclei, sections were incubated with 4’,6-diamidino-2-phenylindole (DAPI). Testes sections on slides were then washed and mounted in ProLong Gold Antifade mounting medium (ThermoFisher Scientific, Waltham, Massachusetts). Fluorescence images were captured using a Nikon Eclipse 90i fluorescence microscope system and Nikon NIS Elements 3.2 Imaging Software package (Nikon Instruments Inc, Melville, New York). All images shown in this report, including IF, using either paraffin or frozen sections, and histological analysis, were representative findings of an experiment from n = 3 independent experiment using different testes, which yielded similar results.
Histological analysis
Histological analysis was performed using testes fixed in either modified Bouin’s fixative (80) (Polysciences, Warrington, Pennsylvania) (Fig. 1) or modified Davidson’s fixative (81, 83) (Fig. 2), embedded in paraffin. After deparaffinization, 5-µm sections were obtained in a microtome, mounted on microscopic slides, and stained with hematoxylin and eosin. For histological analysis, at least 100 cross-sections from each testis of n = 3 rats (to a total of 300 randomly cross-sections of testes) were randomly selected and examined to assess for abnormality following adjudin or cadmium treatment. Histological analysis of testes to identify defects of spermatogenesis are noted and summarized in Fig. 1 (long-term adjudin exposure, see list items [1] to [5]) and Fig. 2(short-term adjudin exposure, see list items [1] to [6]), which included: (1) reduction of tubule diameter by ~25% to 50% due to germ cell loss, (2) germ cell exfoliation that led to tubules devoid of germ cells until the epithelium contained only Sertoli cells and spermatogonia; (3) presence of multinucleated round spermatids, which are signs of Sertoli cell injury, as noted in other toxicant models (84–86); (4) loss of Sertoli cell polarity wherein cell nuclei were no longer restrictively localized near the basement membrane, as noted in controls, but moved further away from the basement membrane; (5) loss of elongate/elongated spermatid polarity wherein spermatid heads no longer pointed to the basement membrane but aligned parallel to or against the basement membrane; and (6) entrapment of elongated spermatids to the epithelium after spermiation had occurred. These observations were consistent with an earlier report (44). Also, defects in spermatid polarity induced by adjudin have been carefully evaluated in an earlier report by electron microscopy (87), and were consistent with the noted observation in Fig. 1. We did not test the function of the sperm recovered from rats by day 254 following adjudin, since earlier studies from our laboratory based on fertility tests had shown that these rats were fertile, producing offspring without notably pathological defects (78).
Figure 1.
Histological analysis to illustrate the reversible antifertility effects of adjudin in adult rats. In this study, adult rats treated with a single dose of adjudin at 50 mg/kg b.w. at time 0 (Ctrl, control) (n = 4 rats per time points, including Ctrl). Thereafter, rats were euthanized at specified time points, as noted. Testes were processed for histological analysis. By 6D, elongating/elongated spermatids were no longer detected across the seminiferous epithelium in >90% of the tubules, due to germ cell exfoliation, and multiple nucleated spermatids were noted (white arrows). Virtually, all round spermatids and spermatocytes had undergone exfoliation from the epithelium by 20D. By 20, 40, and 98D, Sertoli cells (black arrows) and spermatogonia (yellow arrows) were the cells found in the epithelium. Spermatogonia began to resume spermatogenesis, as noted in the seminiferous epithelium of testes by 128D, since spermatocytes were notes in some tubules (blue arrows). By 163D, >70% of the tubules had round and elongating spermatids, and the seminiferous epithelium was virtually indistinguishable from control testes by 254D in >98% of the tubules, with Sertoli cells (black arrows), spermatocytes (blue arrows), round spermatids (brown arrows), and elongate spermatids (red arrows). Red blood cells (erythrocytes) (green arrow) were also noted in a microvessel in the interstitium. Scale bar, 250 µm, applied for all micrographs (boxed in black) except for insets; 80 µm in enlarged image in inset boxed in blue; 100 µm in enlarged image in inset boxed in green; 60 µm in enlarged image in inset boxed in red. Abbreviations: b.w., body weight; D, day.
Figure 2.
Histological analysis to depict phenotypic changes across the seminiferous epithelium of tubules following adjudin treatment. In order to identify the downstream signaling proteins utilized by adjudin to induce reversible male infertility, we sought to use testes from adult rats (n = 4 rats per time point) treated with adjudin for just 6, 24, and 96 hours, long before extensive phenotypic changes were detected across the seminiferous epithelium as noted in Fig. 1. While cross-sections of testes from adjudin treated rats at 6 hours (h) did not display any notable changes at low magnification (see left panel) and meiosis (annotated by red bracket) was noted in the tubules, closer examination of these tubules illustrated notable defects in spermatogenesis. For instance, defects in spermatid polarity wherein the sperm heads no longer pointed to the basement membrane were detected (yellow arrows), such that many of them laid parallel to, or 180o away from, the basement membrane (annotated by dashed greenline) in many tubules by 6, 24, and 96 hours. Defects in Sertoli cell polarity were noted since some Sertoli cell nuclei were no longer located near the base of the tubule but found almost in the midsection of the seminiferous epithelium (blue arrow). Also, a considerable number of elongated spermatids were found embedded deep inside the epithelium when spermiation had occurred (green arrows), as noted in tubules by 6 and 24 hours. Furthermore, multinucleated round spermatids were noted (red arrows) in the epithelium. Scale bar, 250 µm in the first column, which applied to all micrographs of the same panel; 80 µm in the second column; 60 µm in inset, which applied to micrographs in all insets.
For IF using frozen sections of testes and actin staining
For staining of filamentous (F)-actin or other markers (eg, p-rpS6-S240/S244) testes snap-frozen in liquid nitrogen were cut in a cryostat (-20°C) at 7-µm thick and mounted onto positively charged slides. Sections were then fixed in 4% paraformaldehyde (in PBS [wt/vol]) for 10 minutes, washed in PBS (10 mM NaH2PO4, pH 7.4, at 22°C, containing 0.15 M NaCl), and permeabilized with 0.1% Triton X-100 in PBS for 10 minutes. Thereafter, sections were washed in PBS, blocked with 1% BSA for 30 minutes at room temperature, and then incubated with corresponding primary antibody diluted in PBS overnight at 4°C. Thereafter, slides were washed in PBS and then incubated with secondary antibody (mouse or rabbit IgG-Alexa Fluor 555, red fluorescence) or Alexa Fluor 488 (green fluorescence)-phalloidin conjugate (to visualize F-actin) (Table S1) (45) diluted in PBS for 1 hour at room temperature. To visualize cell nuclei, sections were also incubated with DAPI. Testes sections on slides were then washed and mounted in ProLong Gold Antifade mounting medium. Fluorescence images were examined in a Nikon Eclipse 90i fluorescence microscope, and acquired with a Nikon Ds-Qi1Mc or DsFi1 digital camera using Nikon NIS Elements AR 3.2 Software Package (Nikon, Tokyo, Japan), and saved in a TIFF format. Image overlays were performed using Adobe Photoshop CS6 (Adobe, San Jose, California). All images shown in this report were untouched images and as originally acquired.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 Software Package (GraphPad Software). Data presented are the mean ± SD of n = 3–4 independent experiments of 3–4 different rat testes. All samples within an experiment group, including control, were analyzed simultaneously to avoid inter-experimental variations for both IB and IF experiments. Data were subjected to 2-way analysis of variance (ANOVA) (for multigroup comparisons) and/or analyzed by Student’s t-test (for 2-group comparisons). P < 0.05 was considered statistically significant.
Results
Adjudin is capable of causing transient male infertility by inducing reversible germ cell exfoliation from the testis
As noted in Fig. 1, treatment of adult rats (n = 4 rats per time point) with a single dose of adjudin (formerly known as AF-2364) at 50 mg/kg b.w. (via oral gavage) was capable of inducing extensive germ cell exfoliation from the seminiferous epithelium, consistent with earlier findings from our laboratory (44, 78). Adjudin is a nonhormonal male contraceptive, since its treatment in adult rats that led to transient male infertility did not alter serum FSH, LH, and testosterone (88), nor inhibin B, levels (89). By day 6, virtually no elongating/elongated spermatids were found in >98% of the tubules, and multinucleated spermatids were noted in virtually all tubules examined (see white arrows in low- and high-magnified images on sections of 6D testes) (Fig. 1), which were defective round spermatids to be phagocytosed by Sertoli cells, as noted in testes following Sertoli cell injury (84, 85, 90). By day 20, 40, and 98, only Sertoli cells and some spermatogonia were found in the seminiferous epithelium across cross-sections of all tubules examined (Fig. 1). Thereafter, spermatogenesis resumed, and by day 128, spermatocytes were noted in >80% of the tubules (Fig. 1). More importantly, over 90% of the tubules had signs of robust spermatogenesis by day 163, and these rats became fully fertile by day 254 following treatment (Fig. 1) based on fertility tests, as earlier reported (78). While the findings noted in Fig. 1 had demonstrated unequivocally the efficacy of adjudin as a potential male contraceptive, the involving downstream signaling proteins (or underlying mechanism(s)) by which it induced germ cell exfoliation remained unknown. Two subsequent studies had reported that this adjudin-mediated germ cell loss from the testis was the result of defects in the actin-based cytoskeleton, most notably the disruptive spatial expression of actin regulatory proteins Eps8, formin 1, and Arp3 vs defects in MT cytoskeleton due to the disruptive expression of MT regulatory proteins EB1 and MARK4 (91, 92). For instance, Eps8 and formin 1 were necessary to confer apical and basal ES integrity by maintaining actin filament bundles at the ES via the action of the actin barbed end capping and bundling protein Eps8, and actin linear nucleation protein formin 1. Their actions were in contrast to Arp3 which conferred branched actin polymerization to support rapid conversion of actin filaments from a bundled and a branched/unbundled configuration to provide plasticity to the ES (93). On the other hand, adjudin perturbed the spatial expression of EB1 and MARK4, which were shown to maintain MT stability and to induce MT shrinkage/catastrophe, respectively (8). Nonetheless, detailed information regarding cytoskeletal disorganization induced by adjudin, in particular the involving downstream signaling proteins, remained unknown. Herein, we sought to use a short-term adjudin exposure animal model wherein rats treated with the drug at 50 mg/kg b.w., via oral gavage, were terminated at 6 hours and 24 hours vs 96 hours when distinctive phenotypes across the seminiferous epithelium were not considerably noted as such by 6 hours (Fig. 2). Yet, some defects that would be overlooked in the initial studies were detected as early as 6 hours. Besides mild elongating/elongated spermatids loses from the epithelium were detected, elongated spermatids had defects in their polarity such that their heads were not pointed to the basement membrane but deviated by at least 90o from their intended orientation (Fig. 2 yellow arrows). This defect of spermatid polarity induced by adjudin reported here is consistent with 2 earlier reports when sections of testes were examined by electron microscopy and high resolution microscopy, since adjudin perturbed the expression of cell polarity proteins such as Par3 and Par6 (44, 87). Sertoli cells also had defects in polarity since the nuclei of some of the Sertoli cells no longer restrictively localized near the basement membrane but aligned in midsection of the epithelium (Fig. 2, blue arrowhead). Furthermore, many elongated spermatids were trapped deep inside the seminiferous epithelium when spermiation had occurred, as seen in tubules by 24 hours (Fig. 2, green arrows). A large number of multinucleated spermatids were noted by 96 hours, as well as the obvious depletion of elongate spermatids and some spermatocytes (Fig. 2), consistent with findings noted in Fig. 1 when day 6 was the first time point that was examined in the long-term study.
Adjudin perturbs the expression and distribution of MAPs in the testis
In this report, we focused on a group of MAPs to examine if adjudin exerted its effects through this protein group. This included structural MAPs (eg, MAP1a, MAP2), signaling MAPs (eg, MARK 2, MARK4), and regulatory MAPs (eg, MT end-binding proteins such as CAMSAP2, a -TIP, and EB1 [a +TIP]), which are known to be involved in regulating MT, but also actin dynamics based on studies in many epithelia (8, 27, 28, 30, 94), including seminiferous epithelium in the testis (31, 32, 40). For instance, structural MAPs are known to bind to MTs to maintain MT stability. On the other hand, MARKs are Ser/Thr protein kinases, capable of phosphorylating MAPs to induce their detachment from MTs, thereby destabilizing MTs that lead to MT catastrophe (28). For -TIPs (eg, CAMSAP2) and +TIPs (eg, EB1), they induce MT shrinkage/catastrophe and stability, respectively (94). Adjudin was found to cause a notably disruptive change in the spatial expression of MAP1a, which is known to promote MT, but also F-actin, stability by binding onto MTs and also F-actin (8, 27) across the seminiferous epithelium in adult rat testes (Fig. 3, left panel). More importantly, considerable downregulation on the expression of MAP2, MARK2, MARK4, and CAMSAP2 were noted by IB analysis (Fig. 3, right panel). Yet, a considerable surge in the expression of MAP1a was detected, consistent with the IF data seen in Fig. 3, illustrating these 2 structural MAPs, namely MAP1a and MAP2, may have entirely different functions to modulate MTs in the testis. On the other hand, there was a gradual reduction on the expression of detyrosinated α-tubulin (to stabilize MTs (95)) but no change on acetylated α-tubulin (to stabilize MTs (95)) or tyrosinated α-tubulin (to make MTs more dynamic (95), likely to promote MT catastrophe (Fig. 3, right panel)). Indeed, a disruptive distribution concomitant with a declining expression of CAMSAP2 was noted across the seminiferous epithelium as early as 6 hours after adjudin treatment (Fig. 4). A similar pattern of disruptive localization of EB1 across the seminiferous epithelium was also noted even though the overall expression of EB1 did not alter (Fig. 4), consistent with IB data (Fig. 3).
Figure 3.
Changes in the distribution and expression of MAPs in the testis during adjudin-mediated defects in spermatogenesis. Using cross-sections of testes from rats treated with adjudin, MTs (visualized by α-tubulin staining, which together with ß-tubulin create the α-/ß-tubulin oligomers and are the building blocks of MTs) appeared as track-like structures that stretched across the seminiferous epithelium, aligned perpendicular to the basement membrane (annotated by the dashed white line), as noted in control testes. One of the structural MAPs called MAP1a, which is known to promote MT stability by binding onto MTs (27) was shown to co-localize with MTs. After adjudin treatment, within 6 hours, MTs were considerably defragmented, which was worsened by 24 and 96 hours. MAP1a also followed the same trend as MTs regarding its distribution across the epithelium, wherein its distribution was considerably defragmentated. Results noted here are representative images of an experiment from n = 3 independent experiments, which yielded similar results. MAP1a expression across the epithelium based on IF was also considerably upregulated, consistent with the IB data (shown on the right panel), using corresponding specific antibodies (Table S1). Except for MAP1a, all other MAPs, including MAP2, MARK2, MARK4, CAMSAP 2 (but not EB1, which had no changes in expression), and detyrosinated α-tubulin (which conferred MT stabilization (95)), but not acetylated α-tubulin nor tyrosinated α-tubulin (which conferred MT stability and MT destabilization, respectively (95)) were notably downregulated in the testes following short-term adjudin treatment. IBs were shown on the right panel from a representative experiment. Bar graphs below the IBs were composite data, with each bar representing a mean ± SD of n = 3 independent experiments with replicate IBs for each time point in each experiment. Scale bar, 60 µm, which applies to all other micrographs. ***, P < 0.005 by ANOVA. Abbreviations: ANOVA, analysis of variance; IB, immunoblot analysis; IF; immunofluorescence analysis; MAP1a, microtubule associated protein 1a; MAPs, microtubule-associated proteins; MTs, microtubules.
Figure 4.
Changes in the distribution of 2 regulatory MAPs, CAMSAP2 (a -TIP) and EB1 (a +TIP), in the testis during adjudin-mediated defects in spermatogenesis. MT dynamics, namely polymerization, stabilization, and shrinkage/catastrophe, are regulated by the concerted efforts of 2 regulatory MAPs, namely CAMSAP2 (31) (a -TIP) and EB1 (32) (a +TIP), in the testis, which promote MT depolymerization/shrinkage and stability, respectively (94). In control testes, CAMSAP2 and EB1 that associated with the MT minus (-) end and MT plus (+) end, respectively, co-localized with MT (visualized by α-tubulin staining), were almost superimposable, and stretched across the entire seminiferous epithelium and aligned perpendicular to the basement membrane (annotated by dashed white line). By 6 hours after adjudin treatment when defects across the epithelium were not notable based on gross histological analysis (Fig. 2), distinctive defects in MTs as well as CAMSAP2 and EB1 were remarkably noted. This pattern of defragmentation was more obvious by 24 and 96 hours, both CAMSAP2 and EB1 were almost collapsed, similar to the MTs. As such, the disruptive expression and localization of CAMSAP2 and EB1 failed to support MT function, facilitating MT shrinkage and catastrophe. Results noted here are representative images of an experiment from n = 3 independent experiments which yielded similar results. Scale bar, 60 µm, which applies to all other micrographs in the same panel. Abbreviations: MAPs, microtubule-associated proteins; MT, microtubule.
Treatment of rats with CdCl2 perturbs the expression and distribution of MAPs
We next examined whether the changes noted using the pharmaceutical/drug model were specific to the nonhormonal male contraceptive adjudin. In this study, adult rats (n = 4 rats per time point) were treated with CdCl2, an environmental toxicant known to induce testis injury that leads to germ cell exfoliation, at 3 mg/kg b.w. (via i.p.), as earlier reported (79, 96–98). As noted in Fig. 5, the cadmium-induced testis injury was notably detected within 16 hours and became more obvious by 24 hours, with considerable thinning of the seminiferous epithelium due to germ cell loss from the testis. This is due to defects in MT organization across the seminiferous epithelium wherein MT-based tracks were truncated by as early as 6 hours following cadmium treatment and by 16 to 24 hours, MTs no longer stretched across the epithelium but virtually collapsed (Fig. 5). These changes were possibly due to disruptive spatial distribution of the MAPs, such as MAP1a and CAMSAP2 (Fig. 5), analogous to the patterns noted in the testes of adjudin-treated rats, as noted in Figs. 3 and 4.
Figure 5.
Changes in the distribution of 2 regulatory MAPs, MAP1a and CAMSAP2, in the testis during cadmium-induced testis injury. Here, we next assessed if changes in the distribution of MAPs across the epithelium following adjudin treatment as noted in Figs. 3 and 4 were not results of drug-mediated responses specific to adjudin, but possibly physiological responses in the testis to a pharmaceutical agent (eg, adjudin) that perturbed spermatogenesis. Using an established model of cadmium-induced testis injury, changes in the distribution of 2 MAPs, namely MAP1a and CAMSAP2, across the epithelium during cadmium-induced epithelial damage were examined. Interestingly, during cadmium-induced epithelial thinning due to germ cell exfoliation, changes in the distribution of MAP1a and CAMSAP2 were found to be parallel to MTs (visualized by α-tubulin) following adjudin treatment, consistent with findings noted in Figs. 3 and 4. Results noted here are representative images of an experiment from n = 3 independent experiments, which yielded similar results. Scale bar, 80 µm, which applies to other micrographs in the same panel. Abbreviations: MAPs, microtubule-associated proteins; MTs, microtubules.
Adjudin induces germ cell exfoliation through an activation of the mTORC1/rpS6 and p-FAK-Y397/p-FAK-Y407 signaling pathways
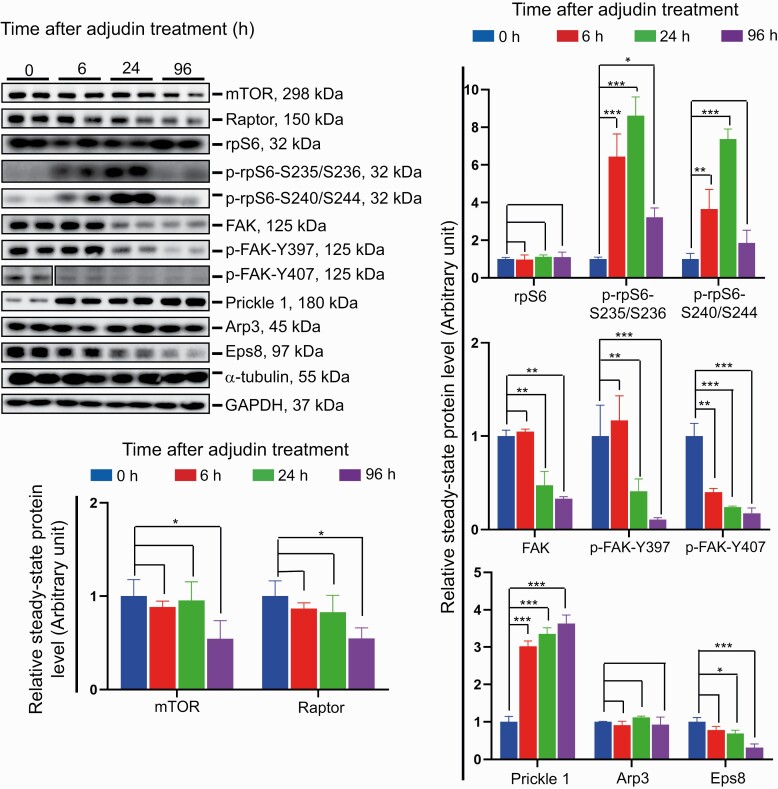
Using the short-term adjudin exposure model (Fig. 2), it was noted that adjudin induced a mild but statistically significant downregulation of mTOR (a Ser/Thr nonreceptor protein kinase (12, 13)) and Raptor (13, 99) expression by 96 hours (Fig. 6). It is noted that the binding of Raptor to mTOR creates mTORC1, an important signaling complex known to regulate the energy status and multiple physiological processes of mammalian cells (12, 15, 100). While the steady-state protein level of rpS6, the downstream regulator of mTORC1 and a phosphorylation inducible protein translation regulator (16), in the testis was not affected, its 2 activated/phosphorylated forms, namely p-rpS6-S235/S236 and p-rpS6-S240/S244, recognized by the corresponding specific antibody (Table S1) (45) were considerably upregulated by 6 hours and persisted distinctively, but transiently, through 24 hours, before it returned to the normal level as noted in control testes by 96 hours (Fig. 6). More importantly, adjudin downregulated the expression of FAK, but most notably p-FAK-Y397 and p-FAK-Y407 (Fig. 6), isoforms of which both are known regulators of BTB dynamics (24). Adjudin treatment also considerably activated the expression of planar cell polarity (PCP) protein Prickle 1, which is known to form a putative complex with Vangl2 to regulate spermatid PCP to support spermatogenesis by modulating MT- and actin-based cytoskeletons in the testis (101) (Fig. 6). Adjudin also downregulated the expression of Eps8 (Fig. 6), an actin barbed end capping and bundling protein at the ES in the testis (102). However, adjudin had no effects on the steady-state level of Arp3, which is known to induce branched actin polymerization (Fig. 6), converting the bundling actin filaments at the ES to assume a branched/unbundled configuration to support ES degeneration (103). However, adjudin considerably altered the spatial distribution of Arp3 at the apical ES, the cell adhesion site by which elongated spermatids anchored onto the Sertoli cells in the seminiferous epithelium (Fig. 7). For instance, within 6 hours after adjudin treatment, Arp3 no longer robustly expressed only at the concave (ventral) side of spermatid head at the apical ES as bulb-like structures noted in control testes, but diffused away from the site toward the back of spermatid heads (Fig. 7). These notable changes in Arp3 distribution at the apical ES were also accompanied by similar changes in F-actin organization (Fig. 7), since Arp3 together with Arp2 created the Arp2/3 complex when activated by N-WASP, which is a crucial protein complex to induce actin polymerization (104). Additionally, the robust increase in p-rpS6-S240/S244 expression following adjudin treatment noted in Fig. 6 was further examined by IF (Fig. 7). It was noted that it was p-rpS6-S240/S244 that was robustly and restrictively expressed at the tip of spermatid heads at the apical ES in control testes that had diffused away from the apical ES site and was noted by 6 hours (Fig. 7). By 6 and 24 hours, it was noted that a considerable surge in its expression was detected at the basal ES/BTB near the basement membrane (annotated by the white dashed line) (Fig. 7), consistent with IB data noted in Fig. 6.
Figure 6.
Adjudin-induced epithelial defects that impede spermatogenesis are through changes in the mTORC1/rpS6 and FAK signaling pathway. Studies by IB using corresponding specific antibodies (Table S1) (45), adjudin-induced epithelial damage that perturbed spermatogenesis was associated with changes in the expression of a number of signaling proteins, which were recently shown to be involved in modulating actin- and MT-based cytoskeletal functions (8, 9). As noted, adjudin treatment affected mTORC1 signaling complex expression, involving a downregulation of mTOR, Raptor, and a considerable, but transient, upregulation of p-rpS6-S235/S236 and p-rpS6-S240/S244. It also induced considerable downregulation of FAK, in particular the steady-state levels of the phosphorylated (and activated) forms of FAK: p-FAK-Y397 and p-FAK-Y407. Also noted is a time-dependent upregulation of the PCP protein Prickle 1 and downregulation of the actin-barbed end-capping and bundling protein Eps8. Representative IB data is noted in the left (top) panel, and composite data is shown in the bar graphs, with each bar representing a mean ± SD of n = 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; by ANOVA. Abbreviations: ANOVA, analysis of variance; FAK, focal adhesion kinase; IB, immunblot analysis; MT, microtubule; PCP, planar cell polarity.
Figure 7.
Adjudin-induced epithelial defects that impede spermatogenesis also perturb F-actin organization through changes in actin regulatory proteins Arp3 and p-rpS6. In control testes, F-actin appeared as bulb-like structures robustly expressed at the apical ES, mostly at the concave (ventral) side of spermatid heads. F-actin also expressed near the basement membrane at the basal ES and BTB site in stage VII tubules. F-actin co-localized with both the branched actin polymerization protein Arp3 and the p-rpS6-S240/S244 signaling protein (an activated form of rpS6), downstream of the mTORC1 signaling protein in regulating testes as noted in control testes. Within 6 hours after adjudin treatment, long before phenotypic changes across the seminiferous epithelium were noted, there were changes in the spatial expression of Arp3 (diffusing away from the concave side of spermatid heads) at the apical ES. For instance, p-rpS6-S240/S244 no longer robustly expressed at the concave side of spermatid heads at the apical ES but diffused away from the site at 6 hours. This disorganized expression of F-actin and Arp3 thus perturbed the organization of actin filaments at both apical and basal ES, impeding spermatogenesis. Results noted here are representative images of an experiment from n = 3 independent experiments, which yielded similar results. Scale bar, 40 µm, which applies to other micrographs in the same panel. Abbreviations: BTB, blood-testis barrier; ES, ectoplasmic specialization, a testis-specific adherens junction type.
In summary, the findings noted in Figs. 6 and 7 have conclusively demonstrated that adjudin exerts its damaging effects to the testis through a signaling cascade involving signaling proteins mTORC1/rpS6 and p-FAK-Y397/p-FAK-Y407 and also MAPs (Fig. 3).
Discussion
Adjudin is a potent nonhormonal male contraceptive drug in both rats (44, 105) and rabbits (106) with excellent efficacy and reversibility (44). It passed all the standard acute toxicity studies, mutagenesis, and chromosome aberration studies conducted by licensed toxicologists, but a relatively narrow margin between its safety and efficacy was discovered in a 29-day subchronic toxicity study in adult rats (89). For instance, the no-observed-adverse-effect (NOAEL) of adjudin in female rats (n = 10 adult female rats) was at 50 mg/kg b.w. per day for 29 days. However, the NOAEL could not be determined in male rats (n = 10 adult rats), since 3 of these rats had signs of skeletal muscle atrophy, and 1 rat had signs of liver damage even though all rats displayed extensive seminiferous epithelial damage in the testis with germ cell loss from virtually all tubules, except for spermatogonia (89, 107). While work is in progress in our laboratory to identify the next generation adjudin-based drugs based on structure-function-toxicity modifications to reduce systemic toxicity, we sought to identify the regulatory signaling proteins and the underlying cascade utilized by adjudin to induce germ cell exfoliation. If this information is known, a better approach can be found not just for new nonhormonal male contraceptive development with minimal side effects, it also offers new therapeutic approaches to intervene in unexplained male infertility with unidentified etiology, such as nonobstructive azoospermia. Also, if the downstream signaling cascade(s) utilized by adjudin are known, there may be other potential targets to intervene in male fertility or to correct male infertility. For instance, recent reports have shown that the testis produces several bioactive peptides endogenously in the seminiferous epithelium through proteolytic cleavage of local constituent proteins at the apical ES and basement membrane (42, 108). These include F5-peptide from the laminin-γ3 chains at the apical ES (109, 110), and NC1-peptide from collagen α3 (IV) chains (111, 112) as well as LG3/4/5-peptide from laminin-α2 chains (113–115) in the basement membrane (42). Subsequent studies have shown that these peptides exert their differential effects downstream through distinctive signaling proteins, which are capable of modifying drug (eg, adjudin) permeation across the Sertoli cell BTB, and also spermatogenesis (21, 22, 40, 116). These include p-rpS6-S235/S236 and p-rpS6-S240/S244 of p-rpS6, the downstream signaling protein of mTORC1 (115, 117) and p-FAK-Y407 (109, 110). These studies have also demonstrated that the combined administration of adjudin with these peptides (eg, F5-peptide) and their downstream signaling proteins (eg, p-rpS6-S235/S236/S240/S244 or its quadruple phosphomimetic, and thus constitutively active, mutant, namely p-rpS6-S235E/S236E/S240E/S244E-mutant) could considerably modify adjudin diffusion (or permeation) across the BTB (21, 22, 40). These observations also illustrate the possibility of considerably widening the margin between the safety and efficacy of adjudin if an adjudin-conjugated bioactive peptide or one of its downstream signaling protein can effectively modify the drug permeation across the tissue barrier. In this report, we sought to examine if adjudin per se had any effects on some of the signaling proteins recently shown to be crucial regulators of spermatogenesis. Indeed, it was shown that p-rpS6-S235/S236, p-rpS6-S240/S244, pFAK-Y407, p-FAK-Y397, and several MAPs are involved in the adjudin-mediated defects in spermatogenesis, which ultimately perturb the actin and MT cytoskeletons. The net result leads to the eventual disruption of germ cell adhesion onto the seminiferous epithelium and germ cell exfoliation. These findings will have considerable impact to not only design next-generation potent drugs to modify blood-tissue barrier functions beyond the BTB, but to manipulate spermatogenesis, either for male contraceptive purposes or to improve male fertility. For instance, as noted in this report, the environmental toxicant cadmium also exerts damaging effects on spermatogenesis via disruptive changes in the spatial expression of MAP1a and CAMSAP2, analogous to adjudin. Thus, an approach that alleviates the disruption of these proteins would likely rescue the testis from cadmium-induced testis injury. Based on the findings in this report, which coupled with data from several in vivo (38, 65, 66, 67) and in vitro (17, 18, 23, 25) studies, the likely signaling proteins and the signaling cascades utilized by adjudin to perturb actin and MT cytoskeletons, thereby inducing germ cell exfoliation, are depicted in Fig. 8. Undoubtedly, this model will be rapidly updated when more data are available, but it provides helpful information upon which functional experiments can be designed. For instance, can manipulation of p-FAK-Y407 and/or mTORC2/rpS6 signaling function be used to alleviate the disruptive effects of adjudin on spermatogenesis via the corresponding knockdown or overexpression (or the use of inhibitors and/or agonists) of these signaling proteins?
Figure 8.
A schematic illustrating the signaling cascade utilized by adjudin to induce germ cell exfoliation from the seminiferous epithelium. Based on data in this report, the adjudin-mediated defects in spermatogenesis, including germ cell exfoliation as noted in the seminiferous epithelium, are likely involved changes in the expression of multiple MAPs, an activation of mTORC1/p-rpS6, and a downregulation of p-FAK-Y407. Abbreviation: MAPs, microtubule-associated proteins.
In this context, it is of interest to note that in studies using a genetic model of specific knockout (KO) of mTOR, namely Mtorflox/flox;Amhr2cre/+ mice, in Sertoli cells by downregulating mTOR expression in the testis led to a phenotype that shared many of the similarities in the testes (118) following adjudin treatment. These included adult-onset testicular atrophy associated with seminiferous epithelial damage, loss of Sertoli cell polarity, and germ cell exfoliation, and many tubules were devoid of advanced germ cells (118). More importantly, the conditional KO of mTOR in Sertoli cells also led to a surge in the expression of p-rpS6-S240/S244 in the testis of these mice (118). Unsurprisingly, earlier studies have shown that overexpression of rpS6-WT (wild type, ie, full-length rpS6 cDNA) cloned into the mammalian expression vector pCI-neo in the testis in vivo led to transient BTB disruption and a surge in the steady-state protein level of p-rpS6, and the testis was associated with seminiferous epithelial damage and germ cell exfoliation (20). Furthermore, the use of a quadruple phosphomimetic mutant of p-rpS6, namely p-rpS6-S235E/S236E/S240E/S244E (17), for its overexpression in the testis was even more potent than overexpressing rpS6-WT in the testis to induce BTB remodeling and seminiferous epithelial damage with notable germ cell exfoliation (20, 40). Thus, these observations are in perfect agreement with the data reported herein that downregulation of mTOR and Raptor (ie, mTORC1) induced by adjudin that led to a transient surge in p-rpS6 expression through an increase in p-rpS6-S235/S236 and p-rpS6/S240/S244 would induce extensive epithelial damages. These include defects in spermatid and Sertoli cell polarity, exfoliation of spermatids, spermatocytes, and round spermatids from the epithelium. Nonetheless, it is cautious to note that the increased rpS6 protein phosphorylation at S235/S236 and S240/S244 could involve other protein kinases such as protein kinase A, protein kinase C, and others. Furthermore, additional work is required to confirm if adjudin directly inhibits mTORC1 activity.
On the other hand, the expression of p-FAK-Y397 and p-FAK-Y407 detected by IBs were considerably downregulated following adjudin treatment, within 6 to 24 hours, before notably gross phenotypes were detected in the seminiferous epithelium. Earlier studies have shown that p-FAK-Y397 is crucial to support apical ES integrity via its effects to maintain F-actin organization at the site (119–121). For instance, overexpression of a constitutively active p-FAK-Y397E-mutant in the testis was found to delay the timely remodeling of F-actin at the apical ES site, thereby elongated spermatids failed to complete their release at spermiation in stage VIII tubules but were persistently detected in stage IX-X tubules (121). On the other hand, p-FAK-Y407 is crucial to support basal ES/BTB integrity by conferring proper nucleation of actin filaments at the site (24). Overexpression of p-FAK-Y407E, a phosphomimetic mutant of p-FAK-Y407, in Sertoli cells cultured in vitro, was found to rescue Sertoli cell epithelium from perfluorooctanesulfonate (PFOS)-mediated cell injury by promoting Sertoli cell tight junction (TJ)-barrier function (23). More importantly, using primary cultures of human Sertoli cells, overexpression of a human p-FAK-Y407E mutant was capable of blocking PFOS-mediated cell injury by maintaining the F-actin organization across the Sertoli cell cytosol in the presence of PFOS through proper distribution of actin regulatory proteins such as Eps8 and Arp3 (25). Taken collectively, the findings reported herein thus suggest that adjudin likely exerts its effects to perturb spermatogenesis through 2 signaling cascades, 1 is based on the mTORC1/rpS6 signaling pathway and the other 1 is involving p-FAK-Y397 and p-FAK-Y407, as noted in Fig. 8. However, these signaling proteins are likely working in concert with the MAPs that modulate MT organization. This possibility is supported by findings that there was a surge in the expression of MAP1a, a structural MAP, and considerable downregulation of MARK2 and MARK4. These changes are likely to be a physiological response by the testis during the assault of adjudin in the testis to promote MT stability wherein MAP1a would bind onto the MT to maintain MT stability (27), which coupled with the downregulation of MARK2 and MARK4 to delay the detachment of MAPs (eg, MAP1a) from MT to further confer their stability (28). Nonetheless, these protective physiological measures failed to maintain proper MT organization, since there was considerable downregulation of -TIPs (eg, CAMSAP2) and the MT-stabilizing detyrosinated α-tubulin. Their changes were coupled with the disruptive distribution of +TIPs (eg, EB1, even though the EB1 steady-state protein level was not altered, as shown by IB following adjudin treatment) led to MT shrinkage and catastrophe, as noted in the immunofluorescence analyses. In brief, the likely signaling proteins and cascade utilized by adjudin to induce cytoskeletal dysfunction, as depicted in Fig. 8, based on findings in this report and recent findings, will serve as a helpful guide in future studies to explore the use of these signaling proteins and MAPs to develop novel male contraceptives and treatments (or management) of male infertility.
Acknowledgements
Financial Support: This work was supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD056034 to C.Y.C.), National Key Research and Development Program of China (2018YFC1003500 to S.F.), the National Natural Science Foundation of China (NSFC) (81730042 to R.G.), the China Shenzhen Science Technology and Innovative Commission (SZSTI) (SZSTI-JCYJ20180508152336419 to C.K.C.W.). L.W. was supported by a China Pharmaceutical University World Explorer Study Abroad Scholarship.
Author Contributions: C.Y.C. conceived the study; C.Y.C. and L.W. designed the research; L.W., M.Y., H.L., S.W., and C.Y.C. performed research; R.G., C.K.C.W., B.S., F.S., and C.Y.C. contributed new reagents/analytic tools; L.W. and C.Y.C. performed data analysis; L.W. and C.Y.C. performed the in vivo animal experiments; L.W. and C.Y.C. prepared all figures; and C.Y.C. wrote the paper. All authors read and approved the final manuscript. L.W and M.Y. contributed equally to the completion of this work.
Additional Information
Conflict of Interest: The authors declare that there is no conflict of interest.
Data Availability
Some data generated or analyzed during this study are included in this published article (eg, image files, gel analysis data, and composite data reported in Figs. 1–7, or in the data repositories listed in the References section, such as data deposited at https://figshare.com listed in (45) with Doi: 10.6084/m9.figshare.12949331. Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: intercellular junctions and contacts between germs cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc Res Tech. 2010;73(4):409–494. [DOI] [PubMed] [Google Scholar]
- 2. Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73(4):241–278. [DOI] [PubMed] [Google Scholar]
- 3. Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. [DOI] [PubMed] [Google Scholar]
- 4. Cheng CY, Mruk DD. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In: Sertoli Cell Biology, 2nd Edition. Ed. Griswold, M.D., Amsterdam, Elsevier; 2015:333–383. [Google Scholar]
- 5. O’Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4(2):e979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1(1):14–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64(1):16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang L, Yan M, Wu S, et al. Microtubule cytoskeleton and spermatogenesis – lesson from studies of toxicant models. Toxicol Sci. 2020;177(2):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Yan M, Wu S, et al. Actin binding proteins, actin cytoskeleton and spermatogenesis – lesson from toxicant models. Reprod Toxicol. 2020;96(6):76–89. [DOI] [PubMed] [Google Scholar]
- 10. Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol. 2016;59(11):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong EW, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778(3):692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–162. [DOI] [PubMed] [Google Scholar]
- 14. Mok KW, Mruk DD, Cheng CY. Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the “Yin” and “Yang” effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. Int Rev Cell Mol Biol. 2013;301:291–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li N, Cheng CY. Mammalian target of rapamycin complex (mTOR) pathway modulates blood-testis barrier (BTB) function through F-actin organization and gap junction. Histol Histopathol. 2016;31(9):961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyuhas O. Ribosomal protein S6 phosphorylation: four decades of research. Int Rev Cell Mol Biol. 2015;320:41–73. [DOI] [PubMed] [Google Scholar]
- 17. Mok KW, Mruk DD, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Akt-mediated effects on MMP-9. J Cell Sci. 2014;127(Pt 22):4870–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mok KW, Chen H, Lee WM, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Arp3-mediated actin microfilament organization in rat Sertoli cells. An in vitro study. Endocrinology. 2015;156(5):1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mok KW, Mruk DD, Silvestrini B, Cheng CY. rpS6 Regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology. 2012;153(10):5036–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li SYT, Yan M, Chen H, et al. mTORC1/rpS6 regulates blood-testis barrier dynamics and spermatogenetic function in the testis in vivo. Am J Physiol Endocrinol Metab. 2018;314(2):E174–E190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao BP, Li L, Yan M, Ge R, Lian Q, Cheng CY. Regulation of BTB dynamics in spermatogenesis – insights from the adjudin toxicant model. Toxicol Sci. 2019;172(1):75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mao B, Li L, Yan M, et al. F5-Peptide and mTORC1/rpS6 effectively enhance BTB transport function in the testis – lesson from the adjudin model. Endocrinology. 2019;160(8): 1832–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wan HT, Mruk DD, Wong CK, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr(407): an in vitro study. Endocrinology. 2014;155(1):249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lie PP, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci U S A. 2012;109(31):12562–12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen H, Gao Y, Mruk DD, et al. Rescue of PFOS-induced human Sertoli cell injury by overexpressing a p-FAK-Y407E phosphomimetic mutant. Sci Rep. 2017;7(1):15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao Y, Chen H, Xiao X, et al. Perfluorooctanesulfonate (PFOS)-induced Sertoli cell injury through a disruption of F-actin and microtubule organization is mediated by Akt1/2. Sci Rep. 2017;7(1):1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bodakuntla S, Jijumon AS, Villablanca C, Gonzalez-Billault C, Janke C. Microtubule-associated proteins: structuring the cytoskeleton. Trends Cell Biol. 2019;29(10): 804–819. [DOI] [PubMed] [Google Scholar]
- 28. Ramkumar A, Jong BY, Ori-McKenney KM. ReMAPping the microtubule landscape: how phosphorylation dictates the activities of microtubule-associated proteins. Dev Dyn. 2018;247(1):138–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16(12):711–726. [DOI] [PubMed] [Google Scholar]
- 30. Mao BP, Ge R, Cheng CY. Role of microtubule +TIPs and -TIPs in spermatogenesis – insights from studies of toxicant models. Reprod Toxicol. 2020;91(1):43–52. [DOI] [PubMed] [Google Scholar]
- 31. Mao BP, Li L, Ge R, et al. CAMSAP2 is a microtubule minus-end targeting protein that regulates BTB dynamics through cytoskeletal organization. Endocrinology. 2019;160(6):1448–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the sertoli cell blood-testis barrier in male rats: an in vitro study. Endocrinology. 2015;156(2):680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10(10):682–696. [DOI] [PubMed] [Google Scholar]
- 34. Bhabha G, Johnson GT, Schroeder CM, Vale RD. How dynein moves along microtubules. Trends Biochem Sci. 2016;41(1):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wen Q, Tang EI, Lui WY, et al. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am J Physiol Endocrinol Metab. 2018;315(5):E924–E948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodson HV, Jonasson EM. Microtubules and microtubule-associated proteins. Cold Spring Harb Perspect Biol. 2018;10(6):a022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McNally FJ, Roll-Mecak A. Microtubule-severing enzymes: from cellular functions to molecular mechanism. J Cell Biol. 2018;217(12):4057–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roostalu J, Surrey T. Microtubule nucleation: beyond the template. Nat Rev Mol Cell Biol. 2017;18(11):702–710. [DOI] [PubMed] [Google Scholar]
- 39. Tovey Corinne A, Conduit Paul T. Microtubule nucleation by γ-tubulin complexes and beyond. Essays Biochem. 2018;62(6):765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yan M, Li L, Mao BP, et al. mTORC1/rpS6 signaling complex modifies BTB transport function – an in vivo study using the adjudin model. Am J Physiol Endocrinol Metab. 2019;317(5):E121–E138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wen Q, Tang EI, Gao Y, et al. Signaling pathways regulating blood-tissue barriers - Lesson from the testis. Biochim Biophys Acta Biomembr. 2018;1860(1):141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu S, Yan M, Ge R, Cheng CY. Crosstalk between Sertoli and germ cells in male fertility. Trends Mol Med. 2020;26(2):215–231. [DOI] [PubMed] [Google Scholar]
- 43. Mao B, Bu T, Mruk D, Li C, Sun F, Cheng CY. Modulating the blood-testis barrier towards increasing drug delivery. Trends Pharmacol Sci. 2020;41(10):690–700. [DOI] [PubMed] [Google Scholar]
- 44. Cheng CY. Toxicants target cell junctions in the testis – insights from the indazole-carboxylic acid model. Spermatogenesis 2014;4(2):e981485 (DOI: 10.4161/21565562.2014.9814895). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang L, Cheng CY. Endocrinology en-2020-00638-R1 (DOI: 10.6084/m9.figshare.12949331) 2020; https://figshare.com/articles/figure/Supplemental_Figure_S1_to_Wang_L_et_al_Endocrinology_MS_en-2020-00638-R1/12949331.
- 46.RRID:AB_2241126.
- 47.RRID:AB_2107448.
- 48.RRID:AB_869990.
- 49.RRID:AB_443207.
- 50.RRID:AB_2210370.
- 51.RRID:AB_448182.
- 52.RRID:AB_10903974.
- 53.RRID:331355.
- 54.RRID:AB_916156.
- 55.RRID:AB_10694233.
- 56.RRID:AB_2105622.
- 57.RRID:AB_561245.
- 58.RRID:AB_397544.
- 59.RRID:AB_2533708.
- 60.RRID:AB_1500096.
- 61.RRID:AB_2714189.
- 62.RRID:AB_10989267.
- 63.RRID:AB_2141629.
- 64.RRID:AB_2300502.
- 65.RRID:AB_634837.
- 66.RRID:AB_634824.
- 67.RRID:AB_2137880.
- 68.RRID:AB_2140610.
- 69.RRID:AB_2068826.
- 70.RRID:AB_2140752.
- 71.RRID:AB_2534088.
- 72.RRID:AB_141780.
- 73.RRID:AB_2576217.
- 74.RRID:AB_2535850.
- 75.RRID:AB_261811.
- 76.RRID:AB_476749.
- 77. Cheng CY, Silvestrini B, Grima J, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65(2):449–461. [DOI] [PubMed] [Google Scholar]
- 78. Cheng CY, Mruk D, Silvestrini B, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72(4):251–261. [DOI] [PubMed] [Google Scholar]
- 79. Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117(Pt 5):783–798. [DOI] [PubMed] [Google Scholar]
- 80. Tang EI, Xiao X, Mruk DD, et al. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012;2(2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM. Fixation of testes and eyes using a modified Davidson’s fluid: comparison with Bouin’s fluid and conventional Davidson’s fluid. Toxicol Pathol. 2002;30(4):524–533. [DOI] [PubMed] [Google Scholar]
- 82. Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: an inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1(2):121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lanning LL, Creasy DM, Chapin RE, et al. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol Pathol. 2002;30(4):507–520. [DOI] [PubMed] [Google Scholar]
- 84. Creasy DM. Evaluation of testicular toxicity in safety evaluation studies: the appropriate use of spermatogenic staging. Toxicol Pathol. 1997;25(2):119–131. [DOI] [PubMed] [Google Scholar]
- 85. Creasy DM. Pathogenesis of male reproductive toxicity. Toxicol Pathol. 2001;29(1):64–76. [DOI] [PubMed] [Google Scholar]
- 86. Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2014;4(2):e979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wong EW, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A. 2008;105(28):9657–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64(5):1500–1508. [DOI] [PubMed] [Google Scholar]
- 89. Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12(11):1323–1328. [DOI] [PubMed] [Google Scholar]
- 90. Murphy CJ, Richburg JH. Implications of Sertoli cell induced germ cell apoptosis to testicular pathology. Spermatogenesis 2014;4(2):e979110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tang EI, Lee WM, Cheng CY. Coordination of actin- and microtubule-based cytoskeletons supports transport of spermatids and residual bodies/phagosomes during spermatogenesis in the rat yestis. Endocrinology. 2016;157(4):1644–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li L, Tang EI, Chen H, et al. Sperm release at spermiation is regulated by changes in the organization of actin- and microtubule-based cytoskeletons at the apical ectoplasmic specialization – a study using the adjudin model. Endocrinology. 2017;158(12):4300–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435(3):553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9(4):309–322. [DOI] [PubMed] [Google Scholar]
- 95. Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206(4):461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49(4):840–849. [DOI] [PubMed] [Google Scholar]
- 97. Setchell BP, Waites GM. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47(1):81–86. [DOI] [PubMed] [Google Scholar]
- 98. Wong CH, Mruk DD, Siu MK, Cheng CY. Blood-testis barrier dynamics are regulated by {alpha}2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146(4):1893–1908. [DOI] [PubMed] [Google Scholar]
- 99. Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014;36(12):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bockaert J, Marin P. mTOR in brain physiology and pathologies. Physiol Rev. 2015;95(4):1157–1187. [DOI] [PubMed] [Google Scholar]
- 101. Chen H, Xiao X, Lui WY, Lee WM, Cheng CY. Vangl2 regulates spermatid planar cell polarity through microtubule (MT)-based cytoskeleton in the rat testis. Cell Death Dis. 2018;9(3):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. Faseb J. 2009;23(8):2555–-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107(25):11411–11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Romero S, Le Clainche C, Gautreau AM. Actin polymerization downstream of integrins: signaling pathways and mechanotransduction. Biochem J. 2020;477(1):1–21. [DOI] [PubMed] [Google Scholar]
- 105. Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60(2):146–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hu GX, Hu LF, Yang DZ, et al. Adjudin targeting rabbit germ cell adhesion as a male contraceptive: a pharmacokinetics study. J Androl. 2009;30(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35(1):86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li H, Liu S, Wu S, Li L, Ge R, Cheng CY. Bioactive fragments of laminin and collagen chains: lesson from the testis. Reproduction. 2020;159(3):R111–R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs 2012;3(11):1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gao Y, Mruk DD, Lui WY, Lee WM, Cheng CY. F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis. Oncotarget. 2016;7(39):64203–64220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wong EW, Cheng CY. NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis. 2013;3(2): e25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chen H, Mruk DD, Lee WM, Cheng CY. Regulation of spermatogenesis by a local functional axis in the testis: role of the basement membrane-derived noncollagenous 1 domain peptide. Faseb J. 2017;31(8):3587–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li L, Mao B, Wu S, et al. Endogenously produced LG3/4/5-peptide protects testes against toxicant-induced injury. Cell Death Dis. 2020;11(6):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gao Y, Mruk D, Chen H, Lui WY, Lee WM, Cheng CY. Regulation of the blood-testis barrier by a local axis in the testis: role of laminin α2 in the basement membrane. Faseb J. 2017;31(2):584–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gao Y, Chen H, Lui WY, Lee WM, Cheng CY. Basement membrane laminin α2 regulation of BTB dynamics via its effects on F-actin and microtubule cytoskeletons is mediated tThrough mTORC1 signaling. Endocrinology. 2017;158(4):963–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wu S, Yan M, Li L, et al. mTORC1/rpS6 and spermatogenic function in the testis-insights from the adjudin model. Reprod Toxicol. 2019;89(10):54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Su W, Cheng CY. Cdc42 is involved in NC1 peptide-regulated BTB dynamics through actin and microtubule cytoskeletal reorganization. Faseb J. 2019;33(12):14461–14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Boyer A, Girard M, Thimmanahalli DS, et al. mTOR regulates gap junction alpha-1 protein trafficking in Sertoli cells and is required for the maintenance of spermatogenesis in mMice. Biol Reprod. 2016;95(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144(5):2141–2163. [DOI] [PubMed] [Google Scholar]
- 120. Beardsley A, Robertson DM, O’Donnell L. A complex containing alpha6beta1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190(3):759–-770. [DOI] [PubMed] [Google Scholar]
- 121. Wan HT, Mruk DD, Li SY, et al. p-FAK-Tyr(397) regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab. 2013;305(6): E687–E699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some data generated or analyzed during this study are included in this published article (eg, image files, gel analysis data, and composite data reported in Figs. 1–7, or in the data repositories listed in the References section, such as data deposited at https://figshare.com listed in (45) with Doi: 10.6084/m9.figshare.12949331. Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.