Abstract
Background
mRNA SARS-CoV-2 vaccines are administered to 2 million individuals per day in the United States under US Food and Drug Administration emergency use authorization.
Methods
Observational cohort study of hospital employees who received their first SARS-CoV-2 mRNA vaccination between 14 December 2020 and 8 January 2021, including employees who reported onset of an injection site reaction ≥48 hours after administration of their first or second dose to an employee hotline.
Results
Thirteen female employees who received the mRNA-1273 vaccine (Moderna) during the first 3 weeks of the SARS-CoV-2 vaccine rollout at San Francisco General Hospital reported a pruritic rash at the injection site appearing 3 -9 days after receipt of their initial dose. Five had milder or similar reactions with earlier onset after the second dose. One additional female employee reported this delayed reaction only after the second dose. None reported serious adverse events or had symptoms severe enough to seek medical attention. These cases represented 1.1% of the 1275 female employees who received their first mRNA-1273 dose and 2.0% of the 557 who were aged 31 -45 years during this initial vaccine rollout. None of 675 males who initiated mRNA-1273 or 3612 employees of any sex who initiated BNT162b (Pfizer) vaccination during this period reported delayed-onset reactions.
Conclusions
These results suggest that delayed-onset, injection site pruritic rashes after mRNA-1273 SARS-CoV-2 vaccine administration, lasting up to 1 week, occur commonly in females, do not lead to serious sequela, and should not deter receipt of the second vaccine dose.
Keywords: adverse effect, COVID-19, injection site reaction, SARS-CoV-2, vaccine
1.1% of 1275 female employees and 2.0% of those aged 31–45 years receiving the mRNA-1273 SARS-CoV-2 vaccine developed a benign, delayed-onset, pruritic injection site rash not reported by any males or any BNT162b vaccine recipients during hospital vaccine rollout.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinations, primarily mRNA types, are administered to 2 million individuals per day in the United States to prevent coronavirus disease 2019 (COVID-19) [1]. Due to the COVID-19 public health emergency, these vaccines were approved under an emergency use authorization (EUA) without long-term safety monitoring data. Continued monitoring of adverse events following vaccination will be critically important.
When employees at San Francisco General Hospital began receiving mRNA SARS-CoV-2 vaccines, the Occupational Health Service (OHS) COVID-19 Emergency Response Team established a vaccine hotline reporting system, and all employees vaccinated at the hospital were encouraged to call the hotline to report any subsequent severe or unusual adverse events. In early January 2021, this hotline began receiving calls from employees complaining of a large, red, itchy rash at the injection site of the mRNA-1273 vaccine (Moderna), which had appeared several days after vaccination and all initial local symptoms had resolved. The public briefing document submitted to the US Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee in support of Moderna’s EUA application for mRNA-1273 on 17 December 2020 did mention local reactions persisting beyond 7 days in 3.7% of vaccine recipients and 1.3% of placebo recipients in the pivotal phase 3 trial that led to EUA approval. However, it did not provide morphologic features, demographic data, time of onset, or the risk of such reactions after the second dose in participants who reacted to the first [2]. Thus, to further characterize such delayed reactions reported to the OHS vaccine hotline, staff began obtaining a structured history and requesting photographs of skin abnormalities from these and subsequent employees who reported delayed-onset symptoms at the injection site. Here, we describe the clinical features, incidence, and demographic characteristics of this unique adverse event in a cohort of hospital employees.
METHODS
Employees working at San Francisco General Hospital began receiving the BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) SARS-CoV-2 mRNA vaccines on 14 December 2020 and 23 December 2020, respectively. Our analysis includes the cohort of all employees who received their initial BNT162b2 or mRNA-1273 vaccination at the hospital between 14 December 2020 and 8 January 2021. At the time of vaccination, staff administering the vaccine specifically advised employees that local symptoms were to be expected with onset during the first 2 days after vaccine administration and instructed them to call an OHS vaccine hotline if they had severe or unusual sides effects after vaccination. A case was defined as an employee who reported a cutaneous injection site reaction to OHS with the onset occurring at least 2 full days (48 hours) after vaccination. OHS created a standard work algorithm for providers to systematically collect and document clinical information from any employee who reported onset of injection site symptoms that began 2 or more days after vaccine administration. This algorithm included asking the employee to send a photograph of the involved skin to OHS and notifying 1 of 2 hotline physicians (M. A. J. or Z. M.). In addition, a minimum of 10 days after the second vaccine dose was administered to each case, they were asked to complete a questionnaire by telephone or secure email in order to validate and complete the clinical history that hotline providers had documented. These questionnaires were completed a median 21 days (range, 14–25) after the second vaccine dose was administered. The size of the delayed injection site reaction was determined by 1 of the investigators (M. A. J.) from a photograph, if provided, or from the employee’s own estimate.
All cases provided informed consent, as required by the University of California–San Francisco institutional review board (UCSF-IRB), for any information in their medical record, including photographs, to be included in this report. One employee with this reaction also enrolled in a separate UCSF-IRB–approved research protocol and underwent skin biopsy.
Using a database established by the hospital performance improvement team for vaccination record-keeping, we obtained the total number of employees who received BNT162b2 or mRNA-1273 between 14 December 2020 and 8 January 2021, stratified within subgroups by vaccine received, self-identified sex, and the age groups of 18–30, 31–45, 46–65, and >65 years.
Statistical Analyses
Cumulative incidence of the delayed injection site reaction was calculated for the overall cohort and subgroups stratified by vaccine received (mRNA-1273 vs BNT162b), self-identified gender (female vs male), and age group (18–30 vs 31–45 vs 46–65 vs >65 years). Rates of the reaction were compared between groups using the 2-sided χ2 or Fisher exact test. Rates of the reaction to the second dose among employees who had the reaction to the first dose were compared between those who received the second dose in the same arm vs those who received it in the opposite arm using the 2-sided Fisher exact test. All analyses were performed using Stata Version 16.1 (Statacorp LLC), and P < .05 was deemed significant for all tests.
RESULTS
Incidence
Between 14 December 2020 and 8 January 2021, 5567 employees received their first dose of an mRNA COVID-19 vaccine at San Francisco General Hospital, with 1955 receiving mRNA-1273 (1275 females and 675 males) and 3612 receiving BNT162b (2350 females and 1249 males). Among the 1275 female employees who received their first dose of mRNA-1273 during this period, the cumulative incidence of reporting a delayed injection site reaction to either the first or second mRNA-1273 dose was 1.1% (95% confidence interval [CI]: .6% to 1.8%). Among the subgroup of 557 female recipients aged 31–45 years, the incidence was 2.0% (95% CI: .99% to 3.51%). There were no reported delayed injection site reactions among the 675 male recipients of mRNA-1273 or the 3612 employees who received BNT162b.
Rates of the delayed injection site reaction were significantly higher among recipients of mRNA-1273 compared with recipients of BNT162b (0.7% vs 0%; P < .001) and among female compared with male recipients of mRNA-1273 (1.1% vs 0%; P = .004). Among female recipients of mRNA-1273, there was a trend toward a higher rate among those aged 31–45 years (18–30, 0.5% vs 31–45, 2.0% vs 46–65, 0.4% vs >65, 0%; P = .10).
Clinical Characteristics
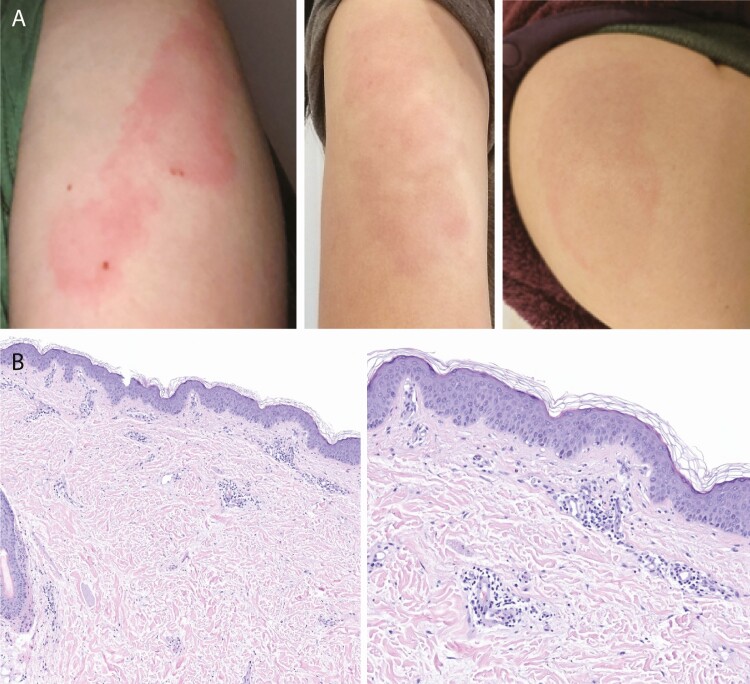
Thirteen employees, all females aged 28–55 years, reported a delayed injection site reaction that involved erythema and pruritus that began 2 or more days after their initial mRNA-1273 vaccination. Figure 1A shows several examples of the morphology, and Table 1 provides individual case clinical details. The dimensions of involved skin ranged from 5 × 5 cm to 15 × 8 cm, appeared 3–9 (median 7) days after the first dose was administered, and lasted 2–7 (median 4) days. Pain or tenderness was reported by 7, swelling or induration by 9, and lymphadenopathy by 2. Three employees reported using an over-the-counter medication to relieve pruritus symptoms (2 used an oral antihistamine and 1 a low-potency topical steroid).
Figure 1.
A, Employee-submitted photographs of delayed injection site reactions following the first dose of mRNA-1273. B, A punch biopsy of the skin demonstrated a largely perivascular lymphocytic infiltrate with little epidermal change (left). There is increased spacing of reticular dermal collagen bundles, which suggests associated dermal edema. At higher magnification (right), interstitial neutrophils and eosinophils can be seen. The microscopic findings resemble an urticarial hypersensitivity dermatitis.
Table 1.
Clinical Characteristics and Course of Delayed Injection Site Reaction to mRNA-1273
| Number/Sex/ Age, years | Atopic or Allergic History | Hormone Use | Onset, days | Duration, days | Rash Dimensions, cm | Erythema | Pain | Swelling | Pruritus | Lymphadenopathy | Over-the-Counter Medication Used | Second Vaccine Arm | Reaction to Second Dose | Onset of Second Reaction, days | Duration of Second Reaction, days | Reaction Severity Relative to First Dose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/41 | N | N | 8 | 2 | 5 × 5 | Y | Y | N | Y | N | N | Opposite | Y | 3 | 2 | Less |
| 2/F/34 | Y | Y | 8 | 3 | 10 × 10 | Y | N | Y | Y | N | N | Opposite | N | N/A | N/A | N/A |
| 3/F/45 | N | N | 5 | 4 | 15 × 8 | Y | N | Y | Y | N | N | Opposite | N | N/A | N/A | N/A |
| 4/F/34 | N | N | 7 | 6 | 15 × 8 | Y | Y | Y | Y | N | N | Opposite | N | N/A | N/A | N/A |
| 5/F/31 | N | N | 7 | 4 | 10 × 8 | Y | N | Y | Y | Y | N | Same | N | N/A | N/A | N/A |
| 6/F/42 | N | N | 7 | 3 | 5 × 5 | Y | N | N | Y | N | N | Same | N | N/A | N/A | N/A |
| 7/F/41 | Y | N | 9 | 3 | 10 × 8 | Y | Y | N | Y | N | Y (diphenhydramine by mouth) | Unknown | N | N/A | N/A | N/A |
| 8/F/41 | N | N | 4 | 7 | 10 × 8 | Y | Y | Y | Y | Y | N | Same | Y | 2 | 5 | Less |
| 9/F/42 | Y | N | 6 | 5 | 13 × 8 | Y | N | Y | Y | N | Y (topical hydrocortisone) | Opposite | Y | 2 | 6 | Less |
| 10/F/39 | Y | Y | 8 | 2 | 15 × 8 | Y | Y | N | Y | N | N | Opposite | N | N/A | N/A | N/A |
| 11/F/35 | Y | Y | 8 | 6 | 10 × 10 | Y | N | Y | Y | N | N | Same | Y | 2 | 3 | Less |
| 12/F/55a | N | N | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Same | Y | 2 | 3 | N/A |
| 13/F/55 | N | N | 7 | 7 | 10 × 10 | Y | Y | Y | Y | N | N | Opposite | N | N/A | N/A | N/A |
| 14/F/28 | N | N | 3 | 6 | 8 × 5 | Y | Y | Y | Y | N | Y (cetirizine by mouth) | Same | Y | 2 | 7 | Same |
Abbreviation: N/A, not applicable.
aEmployee number 12 only had a reaction to the second vaccine dose.
Only 5 of these 13 individuals had a delayed reaction to the second dose, which started 2–3 days after injection and was less severe in 4 and of equal severity in 1. Reactions to the second dose were of similar duration to initial dose reactions (2–7 days). One additional female employee (number 12 in Table 1) who had no delayed reaction to the first mRNA-1273 injection reported a mild reaction that began 2 days after the second injection. Three of 5 employees who had this delayed reaction after the first dose and received their second injection in the same arm as the first had another delayed reaction after the second dose (cumulative incidence, 0.60; 95% CI: .17 to 1.03) compared with 2 of 7 who received their second injection in the opposite arm (cumulative incidence, 0.29; 95% CI: –.04 to .62). Thus, individuals who had this reaction after their first dose and received the second injection in the same arm as their first had 2.10 times the risk (95% CI: .53 to 8.29) of another delayed reaction compared with those who received the second injection in the opposite arm, a nonsignificant trend (P = .2763).
Demographic and Potential Medical Cofactors
Demographic characteristics of the entire cohort and the 14 cases (age and gender) are provided in Table 2. Race/ethnicity data were not available for the entire cohort. Potential medical cofactors that might affect the risk of having this reaction are listed in Table 1. An atopic or allergic history (see the Methods section and Supplementary Materials for details) was reported by 5 employees. Among the 12 participants of child-bearing age, only 3 reported using an oral or implantable hormonal contraceptive at the time of initial vaccination.
Table 2.
Demographic Characteristics of Vaccinated Employee Cohort and Cases of Delayed Injection Site Reaction
| BNT162b Recipients | mRNA-1273 Recipients | |||
|---|---|---|---|---|
| Demographic Category | Total | Cases | Total | Cases |
| Female, age in years | 2350 | 0 | 1275 | 14 |
| 18–30 | 298 | 0 | 213 | 1 |
| 31–45 | 1081 | 0 | 557 | 11 |
| 46–65 | 914 | 0 | 480 | 2 |
| >65 | 56 | 0 | 25 | 0 |
| Male, age in years | 1249 | 0 | 675 | 0 |
| 18–30 | 132 | 0 | 71 | 0 |
| 31–45 | 504 | 0 | 281 | 0 |
| 46–65 | 535 | 0 | 286 | 0 |
| >65 | 76 | 0 | 36 | 0 |
| Nonbinary, age in years | 13 | 0 | 5 | 0 |
| 18–30 | 1 | 0 | 1 | 0 |
| 31–45 | 8 | 0 | 2 | 0 |
| 46–65 | 3 | 0 | 2 | 0 |
| >65 | 1 | 0 | 0 | 0 |
Age data were missing for 1 female recipient and 2 male recipients of the BNT162b vaccine and for 1 male recipient of the mRNA-1273 vaccine.
Histopathology
One case (number 14 in Table 1) had a skin biopsy done 4 days after the second mRNA-1273 dose was administered, while erythema and pruritus were still present. The punch biopsy specimen demonstrated a superficial and deep lymphohistiocytic infiltrate with scattered admixed interstitial neutrophils and eosinophils (Figure 1B). The overlying epidermis demonstrated very subtle spongiosis. A small segment of unaffected subcutis was included in the specimen. The histopathologic findings were most compatible with an urticarial hypersensitivity dermatitis.
DISCUSSION
In a cohort of 5567 healthcare system employees, who received their first dose of an mRNA SARS-CoV-2 vaccine to prevent COVID-19 over a 3-week period shortly after the FDA granted EUAs for the administration of these vaccines, we report the incidence as well as clinical and demographic characteristics of an erythematous, pruritic delayed local injection site reaction to mRNA-1273 (Moderna) or BNT162b2 (Pfizer). We found such delayed skin reactions were reported by 1.1% of all female employees who received the mRNA-1273 and, when stratified by age, 2% of females aged 31–45 years. No males who received mRNA-1273 and no employees of either sex who received BNT162b2 reported such reactions. The onset of these reactions occurred 3–9 days (median, 7) after the first dose of mRNA-1273, consistent with 2 recent case series reports [3, 4]. Since an estimated 30 million American women in the 31- to 45-year age group are eligible for vaccination to prevent COVID-19 [5], it is imperative to accurately define the characteristics of these reactions and reassure the public that they resolve within a week and are not associated with medically significant adverse events.
A published subsequent analysis of more than 14 000 mRNA-1273 recipients in the US phase 3 trial reported delayed injection site reactions in 0.8% of vaccine recipients after the first dose and 0.2% after the second dose, but few clinical details were provided [6]. A “delayed” reaction in this subsequent analysis was defined as an adverse event that began ≥8 days after injection. Given that our employees reported the onset of this local cutaneous reaction a median 7 days after receiving their first mRNA-1273 dose and 2 days after receiving their second dose, using this definition would have led to substantial underreporting in our cohort. In fact, we suspect the incidence we observed of this reaction is actually an underestimate of true incidence because we relied on employees to self-report it to a hotline. Many healthcare professionals, in particular, may not have experienced these symptoms as alarming enough to warrant reporting.
Our OHS staff’s initial concern after receiving the first reports of this vaccine reaction was whether employees might experience a more severe reaction to their second dose, a concern the general public might reasonably share. However, among the 13 employees who reported a delayed reaction after their first mRNA-1273 injection, only 5 reported a delayed reaction after their second; and 4 of these 5 rated the second reaction as less severe than the first. These findings corroborate those of Blumenthal et al who found that only 8 of 12 individuals with this delayed reaction after the first dose of mRNA-1273 had a delayed skin reaction after the second dose, and all 8 reactions were mild [3]. Given widespread public concern about the safety of vaccines to prevent COVID-19 [7], it is important for public health agencies and providers to educate the public about the possibility of these delayed injection site reactions to mRNA-1273 and reassure them that they represent a benign process that does not preclude receiving the second vaccination dose. Since we observed a trend toward less risk of this reaction after the second dose when it was given in the opposite arm from the first dose (similar to the trend observed by Blumenthal et al [3]), it would be reasonable to suggest to individuals who have this reaction to mRNA-1273 after the first dose that they choose the opposite arm for their second dose. Future trials of mRNA vaccines that require more than 1 dose should also record and analyze whether local reactions differ after the second dose based on whether it is administered in the same or opposite arm as the first dose.
The histopathology in our 1 case who underwent skin biopsy demonstrated findings compatible with a urticarial hypersensitivity reaction. Similar findings were reported in a single skin biopsy from a patient with this delayed injection site reaction by Blumenthal et al [3]. Though delayed-onset cutaneous reactions at the vaccine injection site are rare, such reactions to other vaccines have been reported and attributed to the presence of the preservatives thimerosal, 2-phenoxyethanol, and aluminum as vaccine excipients [8, 9]. However, none of these are constituents of mRNA-1273 or BNT162b2. However, both mRNA-1273 and BNT162b2 contain the vaccine excipient polyethylene glycol, which is a rare cause of hypersensitivity with many skin-care products, medications, and foods and has been suggested as a potential allergen in mRNA vaccines [10]. More study is needed to determine what vaccine component is driving these reactions, the immunologic mechanism, and a reason for the apparent differential incidence between these 2 mRNA SARS-CoV-2 vaccines. One hypothesis to explore in attempting to explain this difference in delayed-onset reactions between these 2 COVID019 mRNA vaccines is that the specific polyethylene glycol in mRNA-1273 (1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 [PEG2000-DMG]) may cause more inflammation or be more immunogenic than the compound in BNT162b2 (2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide). In support of this hypothesis, a recent registry-based report of various cutaneous reactions following COVID-19 mRNA vaccination mentions 175 injection site reactions that occurred 4 or more days after the first dose and 31 similarly delayed reactions after the second dose, of which 95% were associated with mRNA-1273 and 5% with BNT162b2 [11].
The exclusive occurrence that we observed in females is remarkable. In combining 2 previous case series [3, 4] with ours, 28 of 30 such cases (93%) were female (the registry-based report mentioned above does not mention the sex of those who had this reaction [11]). Previous research has reported that women are at greater risk than men for adverse drug reactions. Hypotheses such as decreased body mass, sex-based differences in pharmacokinetics and pharmacodynamics, and gender-based differences in health information–seeking behaviors and self-image have been proposed [12, 13].
In conclusion, our data suggest that potential recipients of mRNA-1273 can be reassured that delayed injection site reactions consistent with what we and others have observed are a common, benign, self-limited adverse event that should resolve within a week and do not appear to lead to serious sequela and that second mRNA-1273 doses are safe to administer to those who have the reaction after their first dose. In future phase 3 vaccine trials to prevent COVID-19, safety reporting systems that capture the specific timing of adverse event onset might lead to earlier recognition and characterization of such delayed-onset side effects. More research is needed to characterize the epidemiology, immunologic mechanism, prevention, and treatment of delayed injection site reactions to mRNA-based vaccines and why the delayed injection site reaction that we and others have observed has a predilection for females.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Karmen Louie, Patricia McCarthy, Joy Kang, Rochelle Santos, Emilia Patrick, Stella Landry, Jackie Tulsky, and all the Occupational Health Service COVID-19 Emergency Response program staff at San Francisco General Hospital whose efforts in responding to coronavirus disease 2019 vaccine hotline calls were so helpful to employees and made this study possible. We are also very grateful to the employees who consented to participate in this study.
Financial support. A. Z. is supported by a National Institutes of Health TL-1 grant (2020-2021, $34,800 Research Stipend to cover personal living expenses during research year between 3rd and 4th year of medical school).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. US Food and Drug Administration. Daily COVID-19 vaccine doses administered. Our World in Data. Published May 13, 2021. https://ourworldindata.org/grapher/daily-covid-19-vaccination-doses?tab=chart&stackMode=absolute&time=earliest..latestregion=World. Accessed 28 January 2021.
- 2. Our World in Data. Vaccines and Related Biological Products Advisory Committee. Published December 17, 2020. https://www.fda.gov/media/144434/download. Accessed 13 May 2021.
- 3. Blumenthal KG, Freeman EE, Saff RR, et al. . Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2 [published online ahead of print, 2021 Mar 3]. N Engl J Med 2021. doi:10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. “COVID arm”: a reaction to the Moderna vaccine. JAAD Case Rep 2021; 10:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howden LM, Meyer JA. United States Census Bureau; 2011. https://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed 4 March 2021.
- 6. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaiser Family Foundation. KFF COVID-19 vaccine monitor: December 2020. https://www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine-monitor-dashboard/. Accessed 4 March 2021.
- 8. McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol 2018; 141:463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stone CA Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol 2019; 85:2694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banerji A, Wickner PG, Saff R, et al. . mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract 2020:S2213-2198(20)31411–2. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMahon DE, Amerson E, Rosenbach M, et al. . Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 2021:S0190-9622(21)00658–7. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Vries ST, Denig P, Ekhart C, et al. . Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol 2019; 85:1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol 2001; 2:349–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.