Abstract
Aedes albopictus (Skuse) (Diptera: Culicidae) is one of the most invasive species globally, and has led to rapid declines and local extirpations of resident mosquitoes where it becomes established. A potential mechanism behind these displacements is the superior competitive ability of Ae. albopictus in larval habitats. Research on the context-dependent nature of competitive displacement predicts that Ae. albopictus will not replace native Aedes triseriatus (Say) (Diptera: Culicidae) in treeholes but could do so in artificial container habitats. Aedes albopictus remains rare in temperate treeholes but less is known about how Ae. albopictus fares in artificial containers in forests. Tyson Research Center (TRC) is a field station composed of mostly oak-hickory forest located outside Saint Louis, MO. The container community has been studied regularly at TRC since 2007 with permanently established artificial containers on the property since 2013. Aedes albopictus was detected each year when these communities were sampled; however, its abundance remains low and it fails to numerically dominate other species in these communities. We present data that show Ae. albopictus numbers have not increased in the last decade. We compare egg counts from 2007 to 2016 and combine larval sample data from 2012 to 2017.We present average larval densities and prevalence of Ae. albopictus and two competitors, Ae. triseriatus and Aedes japonicus (Theobald) (Diptera: Culicidae), as well as monthly averages by year. These data highlight a circumstance in which Ae. albopictus fails to dominate the Aedes community despite it doing so in more human-impacted habitats. We present hypotheses for these patterns based upon abiotic and biotic environmental conditions.
Keywords: Aedes albopictus, Aedes triseriatus, Aedes japonicus, invasive species, context-dependence
Aedes albopictus (Skuse) (Diptera: Culicidae) has been designated one of the 100 most invasive species globally (Lowe et al. 2000). Its invasion success story is due, in part, to its negative impacts on resident species; it is often a superior competitor in the larval habitat and is capable of mating interference and satyrization of Aedes aegypti (L.) (Bargielowski and Lounibos 2016, Fader 2016). Following its introduction and rapid spread across the continental Unites States, local extirpations of resident species occurred, often within only a few years (e.g., O’Meara et al. 1993, 1995). These extirpations were not complete across the landscape however, and areas of coexistence with resident species, and later invaders (e.g., Aedes japonicus Theobald (Diptera: Culicidae)), persist. This spurred decades of theoretical and empirical research to determine the ecological factors that facilitate coexistence or lead to exclusion (Juliano 2009, Kaufman and Fonseca 2014, Fader 2016). One pattern that emerges from this body of work, especially for interactions with Ae. aegypti, is the context-dependent nature of larval competition. Microclimates (Lounibos et al. 2010), detritus resources (Murrell and Juliano 2008), parasitism (Aliabadi and Juliano 2002), and predation (Juliano et al. 2010) all have the potential to change the outcomes of competitive interactions, and likely explain the observed patterns of coexistence and exclusion in the United States. The relationship between Ae. albopictus and Aedes triseriatus (Say) (Diptera: Culicidae) is less clearly understood, however. While individual studies (Livdahl and Willey 1991, Novak et al. 1993, Teng and Apperson 2000, Aliabadi and Juliano 2002, Bevins 2007, Yee et al. 2007) and reviews (see Juliano 2009) suggest that Ae. albopictus is the superior competitor, a meta-analysis, including a subset of these studies, found the two species to be competitively equivalent (Juliano 2010).
One of the earliest papers investigating the role of larval competition in the potential extirpation of Ae. triseriatus pitted the species against each other in tire and treehole habitats. Combining models and experiments, the authors concluded that Ae. albopictus could exclude Ae. triseriatus in tires but that the two species could coexist in treeholes (Livdahl and Willey 1991). To some degree, their predictions have held in the 30 yr since their experiments were conducted. Although Ae. triseriatus can still be found in artificial containers, Ae. albopictus is much more abundant in such containers and remains rare in treeholes where Ae. triseriatus dominates (Fukuda et al. 1997, Kesavaraju et al. 2008, Bartlett-Healy et al. 2012, Yee et al. 2012). Much less is known, however, about how Ae. albopictus fares in artificial containers in sylvan habitats; these containers may be rare or rarely studied. One exception to this is Tyson Research Center (TRC). Located 38 km from Saint Louis, MO, TRC is a 2,000 acre, mostly oak-hickory forested field station where mosquitoes have been studied in artificial containers since 2007.
Here, we present data from multiple years of sampling Aedes eggs and larval communities (2007, and 2012–2017) in a temperate forest that demonstrate that despite the permanent establishment of artificial containers on the study site, Ae. albopictus has not excluded Ae. triseriatus or other species. Rather, our data show that Ae. albopictus larval and egg abundances are lower than those for Ae. japonicus for the most of the active season, and are lower than those for Ae. triseriatus for the entire season. Additionally, fewer Ae. albopictus eggs were collected in 2016 compared with 2007.
Methods
Egg Sampling: 2007 and 2016
Eggs were collected using identical protocols during 2007 and 2016 to compare the average number of Ae. albopictus eggs laid at TRC when these communities were first studied (2007) and a decade later (2016). Fifty 500-ml black plastic cups lined with seed germination paper (thus forth ‘egg papers’) were attached to trees 1–2 m from the ground and filled with 270-ml tap water and 30 ml of a 10%, by weight, hay infusion incubated for 7 d. Cups were placed along five transects, in approximately the same locations for both years. Per transect, five traps were placed along the forest edge on service roads (2-m wide, full canopy) 50 m apart, and five were placed 50 m into the forest. Egg papers were collected on three dates, at weekly intervals, within each of three collection periods; early June, mid-July, and late August after each paper had been in the field for 4 d (450 samples/year). Egg papers were incubated in an environmental chamber for 4–7 d before being placed in 0.35 g/liter nutrient broth solution (Difco) to stimulate egg hatching. Larvae were identified to species as third or fourth instars. No attempt was made to count total eggs laid or to identify unhatched eggs. Larvae identified as Ae. albopictus are reported as the number of Ae. albopictus eggs laid per day. We used a Generalized Linear Mixed Model (GLMM) with a zero inflated Poisson error distribution using eggs laid per day as the response variable, year and month as independent variables, and transect as a random effect (PROC GLIMMIX). The three samples within each month and the forest versus edge samples were collapsed into ‘month’. We also analyzed proportion of egg papers with Ae. albopictus present using a GLMM (PROC GLIMMIX) with a binary distribution (present vs absent) testing for effects of years, months, and interaction, with transect as a random variable. Both analyses were performed in SAS 9.4.
Larval Sampling: 2007 and 2012–2017
To compare the larval densities and frequency of collection of common species at TRC, we summarized the data collected from larval samples from 2012 to 2017. These data were collected from black plastic containers which always received an initial input of rainwater and oak leaf detritus. Each year of data originates from a different field experiment, with different manipulations, designed to answer research questions not directly related to this study and is repurposed here (Westby and Juliano 2017, Juliano et al. 2019, Westby et al. 2019). Details about the experimental manipulations and sampling schedule can be found in the supplemental file. Depending on the year, larval communities were either subsampled destructively or the entire community was identified and returned to its container. To standardize the data, accounting for differences in methods, we present the prevalence of each Aedes species in samples from containers by month with the years 2012–2017 combined, in addition to prevalence for the dominant predator Toxorhynchites rutilus (Coquillett). We also present mean densities (larvae/liter) from containers where the focal species was present. We present the average monthly Ae. albopictus larval density for every liter of water sampled (e.g., not excluding samples where they absent) and prevalence by month for all the years including larval samples collected in 2007 (not included in the prevalence and density data above). Larval samples were collected in 2007 but volumes were not recorded.
We limited our statistical treatment to the egg sampling data collected in the 2 yr in which we replicated field methods exactly (2007 vs 2016). For other years, the data were collected differently or there were not enough data points in each month for each manipulation in all years to include in a statistical model. We also present no data on Culex as they were rare in larval samples later in the season when we began to detect Ae. albopictus.
Results
Egg Sampling: 2007 and 2016
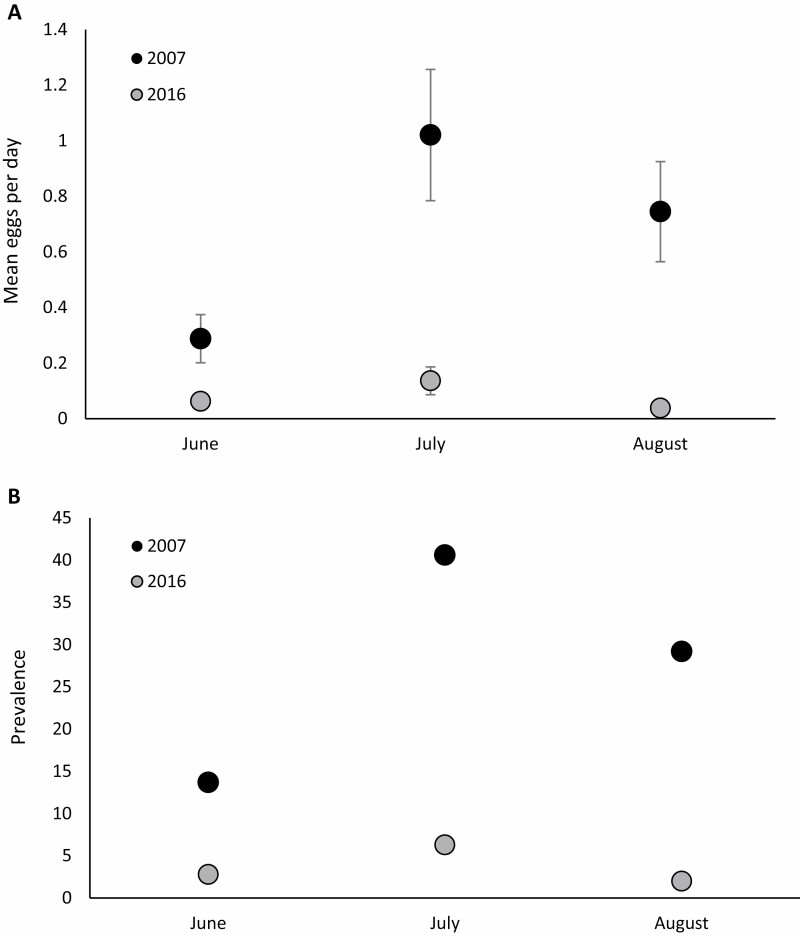
We detected significantly more Ae. albopictus eggs per day in 2007 (2,054 total eggs collected) compared with 2016 (117 eggs collected; F1,4 = 59.06 P = 0.0015) in addition to significant differences among months (F2,8 = 6.93 P = 0.018), but the interaction of year and month was not significant (F2,8 = 1.71 P = 0.2406) (Fig. 1A). The same pattern held for the proportion of ovicup samples in which Ae. albopictus was present. Aedes albopictus was significantly more likely to be present in a sample in 2007 (present in 26.1 ± 5.7% of samples, mean ± SE) compared with 2016 (present in 3.1 ± 1.2% if samples) (F1,881 = 65.90, P < 0.0001). Aedes albopictus presence was significantly influenced by month (F2,881 = 7.94, P = 0.0004), but the interaction with year was not significant (F2,881= 1.71, P = 0.1812). There was significant spatial variation among transects (c2 = 28.60, P = 0.0001). Least squares means for percent Ae. albopictus present are shown in Fig. 1B.
Fig. 1.
(A) Least squares means number of Aedes albopictus eggs laid per day in 500-ml black oviposition cups. The same sampling protocol, in approximately the same locations, was conducted in 2007 and 2016. (B) Least squares means prevalence (% occupancy) of Ae. albopictus in the same egg samples.
Larval Sampling: 2007 and 2012–2017
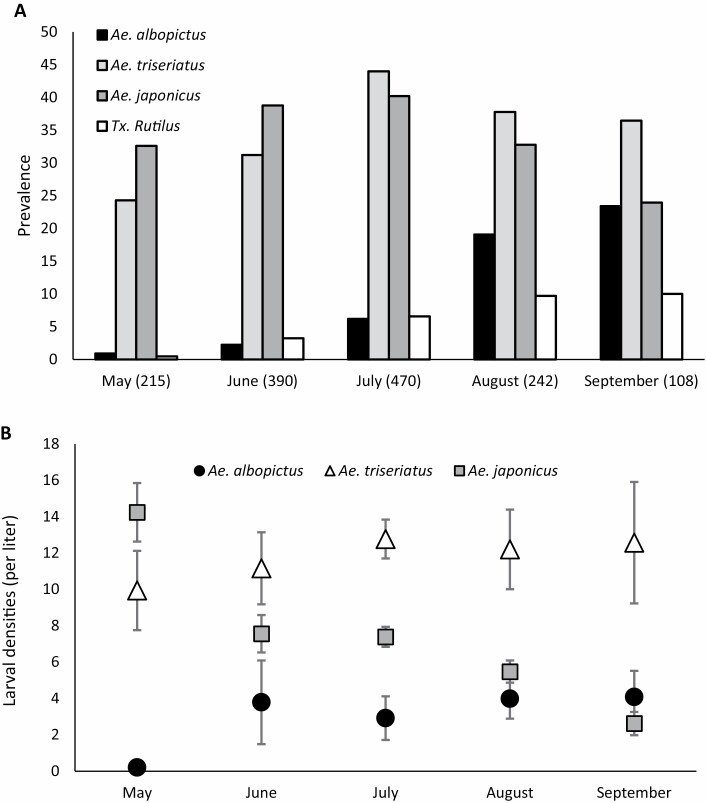
When the data from 2012 to 2017 were combined, Ae. albopictus was observed in less than 5% of all larval samples collected from May to July and reached its greatest prevalence in September when it was present in 25% of samples. The three Aedes species were relatively equal in prevalence in larval samples collected during August and September. The predatory species Tx. rutilus was not detected in more than 10% of larval samples from any month (Fig. 2A) . When calculated only from samples where they were present, average Ae. albopictus larval densities did not exceed 4 larvae/liter, which was lower than that of Ae. triseriatus (~12 larvae/liter) and closer to that of Ae. japonicus (~6 larvae/liter) though these average numbers changed slightly during the summer (Fig. 2B). When the samples in each month were broken down by year, Ae. albopictus larvae per liter of water sampled (including samples in which they were absent) were an order of magnitude lower in all samples from 2012 to 2017 than in the sample from August 2007, when the average density was 23.65 larvae/liter (Table 1). Prevalence of Ae. albopictus across containers was also consistently high (>40% of containers) in 2007, but considerably lower (≤25% of containers) in all samples from 2012 to 2016 (Table 1). Only the final two samples of 2017 attained prevalence of >40% of containers (Table 1).
Fig. 2.
(A) The prevalence (% occupancy) of Aedes albopictus, Aedes triseriatus, Aedes japonicus, and Toxorhynchites rutilus in larval samples taken in each month, combining the data from 2012 to 2017. Numbers in parentheses are the total number of samples collected. (B) Natural larval densities, in larvae per liter, of these species in artificial habitats at TRC, excluding samples where the focal species was absent.
Table 1.
Mean Aedes albopictus larvae collected per liter of water sampled in 2007 and from 2012 to 2017, the total number of samples taken in each month and year, and the number and percent of those samples that were occupied by Ae. albopictus na = larvae per liter is not available because water volume was not recorded. Aedes albopictus was collected in 2012 from egg samples; data not presented.
| Year | Month | Mean larval density (per liter) | Standard error | Total samples taken | Number of containers occupied | Percent of containers occupied |
|---|---|---|---|---|---|---|
| 2007 | July | na | na | 19 | 8 | 42.11 |
| 2007 | Aug. | 23.65 | 10 | 20 | 9 | 45 |
| 2007 | Oct. | na | na | 10 | 5 | 50 |
| 2012 | June | 0 | 0 | 36 | 0 | 0 |
| 2012 | July | 0 | 0 | 36 | 0 | 0 |
| 2012 | Aug. | 0 | 0 | 36 | 0 | 0 |
| 2013 | May | 0 | 0 | 123 | 0 | 0 |
| 2013 | June | 0.21 | 0.2 | 100 | 2 | 2 |
| 2013 | July | 2.6 | 2 | 132 | 19 | 14.39 |
| 2013 | Aug. | 5 | 3.9 | 98 | 13 | 13.27 |
| 2013 | Sept. | 2.7 | 2 | 78 | 9 | 11.54 |
| 2014 | June | 0 | 0 | 48 | 0 | 0 |
| 2014 | July | 0.07 | 0.05 | 96 | 3 | 3.13 |
| 2014 | Aug. | 0.33 | 0.11 | 48 | 10 | 20.83 |
| 2015 | June | 0 | 0 | 160 | 1 | 0.63 |
| 2015 | July | 0.01 | 0 | 160 | 3 | 1.88 |
| 2016 | May | 0.01 | 0.01 | 32 | 2 | 6.25 |
| 2016 | June | 0.62 | 0.58 | 16 | 4 | 25 |
| 2016 | July | 0.1 | 0.05 | 16 | 4 | 25 |
| 2017 | May | 0 | 0 | 60 | 0 | 0 |
| 2017 | June | 0.12 | 0.09 | 30 | 2 | 6.67 |
| 2017 | July | 0.12 | 0.09 | 30 | 2 | 6.67 |
| 2017 | Aug. | 1.56 | 0.32 | 60 | 33 | 55 |
| 2017 | Sept. | 2.32 | 0.62 | 30 | 24 | 80 |
Discussion
Aedes albopictus larvae were collected each year that mosquitoes were sampled under the forest canopy at this site (TRC) except for 2012. Despite the absence of Ae. albopictus larvae in 2012, this species was present in egg samples from that year (data not shown). Published data show that Ae. albopictus also present at TRC in 2009 (Murrell et al. 2015), 2010 (Murrell and Juliano 2013), and 2011 (Murrell et al. 2014), indicating that either TRC has an established population or is recolonized from urban and suburban locations each summer. Data sets from the United States (Lounibos et al. 2001, Shragai and Harrington 2019), Brazil (Carvalho et al. 2014), and Switzerland (Flacio et al. 2016) show that, within a shorter timespan than our data represent, Ae. albopictus prevalence increases after establishment. Using identical methods in 2016 as 2007, we detected a significant decline in egg abundance and prevalence for this species from 2007 to 2016. Acknowledging the limitations of inferring a trend from only two years of data, as interannual variation is well known to occur and is represented in the larval abundance data from TRC, our data suggest that there is no obvious long-term upward trend for Ae. albopictus populations at this site more than 10 yr after it established. Combining the data from 2012 to 2017, we see that Ae. albopictus abundance per liter remains below that of Ae. triseriatus and Ae. japonicus during the early summer and lower or equal in the later summer, and container prevalence remains low in most months and years (see Table 1). Our data on egg and larval abundances suggest that Ae. albopictus has not increased to dominate at this site and may have declined over the period we sampled. Further, if Ae. albopictus is overwintering at TRC, it takes several months from the time of first detection (May) until it reaches its highest container prevalence (August or September). This pattern is in stark contrast to the dominance that Ae. albopictus quickly achieves in urban and suburban Saint Louis (Westby and Medley, unpublished data, manuscript in preparation)
It is not entirely clear why Ae. albopictus does not dominate the mosquito community in artificial containers in this temperate forest. Aedes albopictus has been described as a forest edge species in its native range (Hawley 1988) and has been repeatedly documented utilizing forested areas of Florida (Kesavaraju et al. 2008) and Brazil (Lourenço-de-Oliveira et al. 2004, Ferreira-de-Lima et al. 2020). The most common larval mosquito habitat in temperate oak–hickory forests are likely treeholes (Westby et al., personal observations), though it is plausible that forested areas are used as dump sites elsewhere. Aedes albopictus is rarely found in treehole surveys in northern latitudes potentially limiting the potential for populations to grow large in these habitats (Livdahl and Willey 1991, Edgerly et al. 1999, Bartlett-Healy et al. 2012, Freed and Leisnham 2014). The reason that Ae. albopictus is rare or absent from treeholes may be intense competition with, or intraguild predation by (Edgerly et al. 1999), the native Ae. triseriatus (the eastern treehole mosquito), differences in resource availability (Livdahl and Willey 1991, Yee et al. 2012), high tannin concentrations (Sota 1993), or predation by Toxorhynchites (Griswold and Lounibos 2005, Murrell and Juliano 2013, Freed and Leisnham 2014). Toxorhynchites rutilus, which is an effective predator on Ae. albopictus, was detected in ≤10% of container samples throughout the course of this study (Fig 2B), a pattern observed in a field experiment manipulating size and drying in plastic containers at TRC (Westby and Juliano 2017). These previous studies suggest that natural densities of this predator in artificial containers at TRC are unlikely to account for the habitat-wide paucity of Ae. albopictus at TRC, despite evidence from manipulative experiments that Tx. rutilus can impact community composition within experimental containers (Juliano et al. 2019). No Ae. albopictus have been found in treehole surveys at TRC (Westby, unpublished data; Juliano, unpublished data), though most of the data collected at this site have been from artificial containers. Importantly, dozens of artificial containers of multiple sizes have been permanently established on the TRC property since 2013, which would presumably allow for successful overwintering of populations and re-emergence in the spring.
Aedes albopictus is often shown to be the superior competitor compared with Ae. triseriatus and Ae. japonicus (Novak et al. 1993, Aliabadi and Juliano 2002, Bevins 2007, Armistead et al. 2008, Freed and Leisnham 2014) or competing simultaneously against both of these species (Murrell et al. 2015) in controlled experiments. A meta-analysis, however, yielded no evidence of competitive advantage of one species over the other and suggested competitive equivalence (Juliano 2010). Controlled laboratory and field competition experiments, however, are likely a poor representation of the conditions larvae encounter in these containers at TRC. First, the natural densities of Ae. albopictus recorded at TRC are well below the numbers typically used in these experiments by as much as an order of magnitude (see Supp File [online only]). Second, these experiments always begin with synchronously hatched, 24-h-old larvae. With installment hatching and phenological differences, Aedes larvae in natural communities would interact with all instars for much of the summer. Importantly, Ae. triseriatus and Ae. japonicus hatch earlier in the spring than Ae. albopictus (Murrell et al. 2014) potentially leading to priority effects that may partially explain why Ae. albopictus has failed to dominate artificial containers in this forest. Additionally, work in Japan has indicated that Ae. albopictus is not competitively dominant in forest habitats and may only be able to dominate in areas with large numbers of ephemeral, artificial containers where other species are rare or absent (e.g., urban centers; Sunahara et al. 2002; Mogi et al. 2017, 2020). Predation and larval competition are not the only plausible explanations for the patterns observed at TRC.
In fact, Ae. albopictus is often considered a human adapted, synanthropic species that is more abundant in urban and suburban areas compared with rural and sylvan areas (Barker et al. 2003, Obenauer et al. 2009, Li et al. 2014). Females have been documented migrating toward human habitats from forested areas of Brazil, implying a preference for human habitats which could explain the results presented here (Maciel-de-Freitas et al. 2006). Additionally, the abiotic environment in forests may be a factor. Aedes albopictus may be more adapted to higher temperatures than the species that dominate forested areas giving Ae. albopictus an advantage in cities which experience a heat island effect (Alam and Tuno 2020), although lower survival has been documented in urban areas compared with suburban and rural areas of Georgia (Murdock et al. 2017). It is also plausible that a preference for human and domestic animal hosts is limiting the abundance of this species in forests (Faraji et al. 2014).
Further research is needed to test the different proposed hypotheses about why it appears that Ae. albopictus does not dominate in these sylvan artificial containers (e.g., priority effects, temperature, host preferences). It would be informative to locate other large forest plots with an abundance of artificial containers to validate the findings of this study. Finally, it is important to continue to monitor these populations in the long term to assess changes in abundance and community composition under climate change.
Supplementary Material
Acknowledgments
We thank Solny Adalsteinsson, Lexie Beckermann, Beth Biro, Peter Brabant, Tim Derton, Leslie Garcia, Omair Habib, Pete Jamerson, Tyler Malone, Pat McCormick, Kris McIntire, Travis Mohrman, Ebony Murrell, Geoff Ower, Jolena Pang, Hanna Peterman, Delilah Sayer, Bailey Saylor, Molly Schumacher, Kevin Smith, Leslie Sterling, Brenden Sweetman, Thomas Van Horn, Adam Wiggins, and Jake Williams for field, laboratory, or equipment assistance. This research was supported by NIAID grants R15AI075306 and R15AI094322 to S. A. J., and by Tyson Research Center.
Data Availability
Data from this study are available from the Dryad Digital Repository: doi:10.5061/dryad.3r2280gdr (Westby, 2020).
References Cited
- Alam, M S, and Tuno N. 2020. A study comparing the growth rates of two related species, Aedes albopictus and Aedes flavopictus (Diptera: Culicidae) at different temperature regimes. Med. Entomol. Zool. 71: 25–30. [Google Scholar]
- Aliabadi, B W, and Juliano S A. 2002. Escape from gregarine parasites affects the competitive interactions of an invasive mosquito. Biol. Invasions. 4: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead, J S, Arias J R, Nishimura N, and Lounibos L P. 2008. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera: Culicidae) in northern Virginia. J. Med. Entomol. 45: 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargielowski, I E, and Lounibos L P. 2016. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Insect Sci. 23: 162–174. [DOI] [PubMed] [Google Scholar]
- Barker, C M, Paulson S L, Cantrell S, and Davis B S. 2003. Habitat preferences and phenology of Ochlerotatus triseriatus and Aedes albopictus (Diptera: Culicidae) in southwestern Virginia. J. Med. Entomol. 40: 403–410. [DOI] [PubMed] [Google Scholar]
- Bartlett-Healy, K, Unlu I, Obenauer P, Hughes T, Healy S, Crepeau T, Farajollahi A, Kesavaraju B, Fonseca D, Schoeler G, et al. 2012. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J. Med. Entomol. 49: 813–824. [DOI] [PubMed] [Google Scholar]
- Bevins, S N. 2007. Timing of resource input and larval competition between invasive and native container-inhabiting mosquitoes (Diptera: Culicidae). J. Vector Ecol. 32: 252–262. [DOI] [PubMed] [Google Scholar]
- Carvalho, R G, Lourenço-de-Oliveira R, and Braga I A. 2014. Updating the geographical distribution and frequency of Aedes albopictus in Brazil with remarks regarding its range in the Americas. Mem. Inst. Oswaldo Cruz. 109: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerly, J S, Willey M S, and Livdahl T. 1999. Intraguild predation among larval treehole mosquitoes, Aedes albopictus, Ae. aegypti, and Ae. triseriatus (Diptera: Culicidae), in laboratory microcosms. J. Med. Entomol. 36: 394–399. [DOI] [PubMed] [Google Scholar]
- Fader, J E. 2016. The importance of interspecific interactions on the present range of the invasive mosquito Aedes albopictus (Diptera: Culicidae) and persistence of resident container species in the United States. J. Med. Entomol. 53: 992–1001. [DOI] [PubMed] [Google Scholar]
- Faraji, A, Egizi A, Fonseca D M, Unlu I, Crepeau T, Healy S P, and Gaugler R. 2014. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban Northeastern USA and implications for disease transmission. PLoS Negl. Trop. Dis. 8: e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-de-Lima, V H, Câmara D C P, Honório N A, and Lima-Camara T N. 2020. The Asian tiger mosquito in Brazil: observations on biology and ecological interactions since its first detection in 1986. Acta Trop. 205: 105386. [DOI] [PubMed] [Google Scholar]
- Flacio, E, Engeler L, Tonolla M, and Müller P. 2016. Spread and establishment of Aedes albopictus in southern Switzerland between 2003 and 2014: An analysis of oviposition data and weather conditions. Parasites Vectors. 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed, T Z, and Leisnham P T. 2014. Roles of spatial partitioning, competition, and predation in the North American invasion of an exotic mosquito. Oecologia. 175: 601–611. [DOI] [PubMed] [Google Scholar]
- Fukuda, T, Willis O R, and Barnard D R. 1997. Parasites of the Asian tiger mosquito and other container-inhabiting mosquitoes (Diptera:Culicidae) in northcentral Florida. J. Med. Entomol. 34: 226–233. [DOI] [PubMed] [Google Scholar]
- Griswold, M W, and Lounibos L P. 2005. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol. Entomol. 30: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley, W A. 1988. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1: 1–39. [PubMed] [Google Scholar]
- Juliano, S A. 2009. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu. Rev. Entomol. 54: 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, S A. 2010. coexistence, exclusion, or neutrality? A meta-analysis of competition between Aedes albopictus and resident mosquitoes. Isr. J. Ecol. Evol. 56: 325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, S A, Lounibos L P, Nishimura N, and Greene K. 2010. Your worst enemy could be your best friend: predator contributions to invasion resistance and persistence of natives. Oecologia. 162: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, S A, Westby K M, and Ower G D. 2019. Know your enemy: effects of a predator on native and invasive container mosquitoes. J. Med. Entomol. 56: 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, M G, and Fonseca D M. 2014. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu. Rev. Entomol. 59: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju, B, Damal K, and Juliano S A. 2008. Do natural container habitats impede invader dominance? Predator-mediated coexistence of invasive and native container-dwelling mosquitoes. Oecologia. 155: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y, Kamara F, Zhou G, Puthiyakunnon S, Li C, Liu Y, Zhou Y, Yao L, Yan G, and Chen X-G. 2014. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 8: e3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livdahl, T P, and Willey M S. 1991. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 253: 189–191. [DOI] [PubMed] [Google Scholar]
- Lounibos, L, O’Meara G, Escher R, Nishimura N, Cutwa M, Nelson T, Campos R, and Juliano S. 2001. Testing predictions of displacement of native Aedes by the invasive Asian Tiger Mosquito Aedes albopictus in Florida, USA. Biol. Conserv. 3: 151–156. [Google Scholar]
- Lounibos, L P, O’Meara G F, Juliano S A, Nishimura N, Escher R L, Reiskind M H, Cutwa M, and Greene K. 2010. Differential survivorship of invasive mosquito species in south Florida cemeteries: Do site-specific microclimates explain patterns of coexistence and exclusion? Ann. Entomol. Soc. Am. 103: 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço-de-Oliveira, R, Castro M G, Braks M A H, and Lounibos L P. 2004. The invasion of urban forest by dengue vectors in Rio de Janeiro. J. Vector Ecol. 29: 94–100. [PubMed] [Google Scholar]
- Lowe, S, Brown M, Boudjelas S, and De Porter M. 2000. 100 of the world’s most invasive species. Invasive Species Spec. Gr. 1–12. [Google Scholar]
- Maciel-de-Freitas, R, Neto R B, Gonçalves J M, Codeço C T, and Lourenço-de-Oliveira R. 2006. Movement of dengue vectors between the human modified environment and an urban forest in Rio de Janeiro. J. Med. Entomol. 43: 1112–1120. [DOI] [PubMed] [Google Scholar]
- Mogi, M, Armbruster P A, Tuno N, Aranda C, and Yong H S. 2017. The climate range expansion of Aedes albopictus (Diptera: Culicidae) in Asia Inferred from the distribution of Albopictus subgroup species of Aedes (Stegomyia). J. Med. Entomol. 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- Mogi, M, Armbruster P A, and Tuno N. 2020. Differences in responses to urbanization between Invasive mosquitoes, Aedes japonicus japonicus (Diptera: Culicidae) and Aedes albopictus, in their native range, Japan. J. Med. Entomol. 57: 104–112. [DOI] [PubMed] [Google Scholar]
- Murdock, C, Evans M, McClanahan T, Miazgowicz K, and Tesla B. 2017. Fine-scale variation in microclimate across and urban landscape changes the capacity of Aedes albopictus to vector arboviruses. PLoS Negl. Trop. Dis. 11: e0005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, E G, and Juliano S A. 2008. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 45: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, E G, and Juliano S A. 2013. Predation resistance does not trade off with competitive ability in early-colonizing mosquitoes. Oecologia. 173: 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, E G, Ives A R, and Juliano S A. 2014. Intrinsic and extrinsic drivers of succession: Effects of habitat age and season on an aquatic insect community. Ecol. Entomol. 39: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, E G, Noden B H, and Juliano S A. 2015. Contributions of temporal segregation, oviposition choice, and non-additive effects of competitors to invasion success of Aedes japonicus (Diptera: Culicidae) in North America. Biol. Invasions. 17: 1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak, M G, Higley L G, Christianssen C A, and Rowley W A. 1993. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera: Culicidae) through replacement series experiments. Environ. Entomol. 22: 311–318. [Google Scholar]
- O’Meara, G F, Gettman A D, L FEvans, Jr., and Curtis G A. 1993. The spread of Aedes albopictus in Florida. Am. Entomol. 39: 163–172. [Google Scholar]
- O’Meara, G F, L FEvans, Jr., Gettman A D, and Cuda J P. 1995. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 32: 554–562. [DOI] [PubMed] [Google Scholar]
- Obenauer, P J, Kaufman P E, Allan S A, and Kline D L. 2009. Host-seeking height preferences of Aedes albopictus (Diptera: Culicidae) in north central Florida suburban and sylvatic locales. J. Med. Entomol. 46: 900–908. [DOI] [PubMed] [Google Scholar]
- Shragai, T, and Harrington L C. 2019. Aedes albopictus (Diptera: Culicidae) on an invasive edge: abundance, spatial distribution, and habitat usage of larvae and pupae across urban and socioeconomic environmental gradients. J. Med. Entomol. 56: 472–482. [DOI] [PubMed] [Google Scholar]
- Sota, T. 1993. Performance of Aedes albopictus and A. riversi larvae (Diptera: Culicidae) in waters that contain tannic acid and decaying leaves: is the treehole species better adapted to treehole water? Ann. Entomol. Soc. Am. 86: 450–457. [Google Scholar]
- Sunahara, T, Ishizaka K, and Mogi M. 2002. Habitat size: a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. J. Vector Ecol. 27: 8–20. [PubMed] [Google Scholar]
- Teng, H J, and Apperson C S. 2000. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperature. J. Med. Entomol. 37: 40–52. [DOI] [PubMed] [Google Scholar]
- Westby, K M, and Juliano S A. 2017. No detectable role for predators mediating effects of aquatic habitat size and permanence on populations and communities of container-dwelling mosquitoes. Ecol. Entomol. 42: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby, K M, Sweetman B M, Van Horn T R, Biro E G, and Medley K A. 2019. Invasive species reduces parasite prevalence and neutralizes negative environmental effects on parasitism in a native mosquito. J. Anim. Ecol. 88: 1215–1225. [DOI] [PubMed] [Google Scholar]
- Westby, K M, Juliano S A, and Medley K A. 2020. Data from: Aedes albopictus has not become the dominant species in artificial container habitats in a temperate forest more than a decade after establishment. Dryad Digital Repository. doi: 10.5061/dryad.3r2280gdr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee, D A, Kaufman M G, and Juliano S A. 2007. The significance of ratios of detritus types and micro-organism productivity to competitive interactions between aquatic insect detritivores. J. Anim. Ecol. 76: 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee, D A, Allgood D, Kneitel J M, and Kuehn K A. 2012. Constitutive differences between natural and artificial container mosquito habitats: vector communities, resources, microorganisms, and habitat parameters. J. Med. Entomol. 49: 482–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available from the Dryad Digital Repository: doi:10.5061/dryad.3r2280gdr (Westby, 2020).