Abstract
Dual antiplatelet therapy has long been the standard of care in preventing coronary and cerebrovascular thrombotic events in patients with chronic coronary syndrome and acute coronary syndrome undergoing percutaneous coronary intervention, but choosing the optimal treatment duration and composition has become a major challenge. Numerous studies have shown that certain patients benefit from either shortened or extended treatment duration. Furthermore, trials evaluating novel antithrombotic strategies, such as P2Y12 inhibitor monotherapy, low-dose factor Xa inhibitors on top of antiplatelet therapy, and platelet function- or genotype-guided (de-)escalation of treatment, have shown promising results. Current guidelines recommend risk stratification for tailoring treatment duration and composition. Although several risk stratification methods evaluating ischaemic and bleeding risk are available to clinicians, such as the use of risk scores, platelet function testing , and genotyping, risk stratification has not been broadly adopted in clinical practice. Multiple risk scores have been developed to determine the optimal treatment duration, but external validation studies have yielded conflicting results in terms of calibration and discrimination and there is limited evidence that their adoption improves clinical outcomes. Likewise, platelet function testing and genotyping can provide useful prognostic insights, but trials evaluating treatment strategies guided by these stratification methods have produced mixed results. This review critically appraises the currently available antithrombotic strategies and provides a viewpoint on the use of different risk stratification methods alongside clinical judgement in current clinical practice.
Keywords: Dual antiplatelet therapy, Patient-tailored antithrombotic therapy, Risk stratification
Graphical Abstract
 Listen to the audio abstract of this contribution.
Listen to the audio abstract of this contribution.
Introduction
Dual antiplatelet therapy (DAPT), consisting of aspirin and a P2Y12 inhibitor, prevents stent-related and non-stent-related coronary and cerebrovascular thrombotic events and remains the standard of care following percutaneous coronary intervention (PCI) in patients with chronic coronary syndrome (CCS) and acute coronary syndrome (ACS).1–3 Inevitably, DAPT increases bleeding complications, which are associated with significant morbidity and mortality.4 Therefore, when determining the duration and composition of an antithrombotic regimen, physicians must carefully balance the advantages and drawbacks associated with this therapy. Historically, DAPT was recommended for at least 12 months after first-generation drug-eluting stent (DES) implantation because of concerns over late and very late stent thrombosis. However, the rates of late and very late stent thrombosis have decreased considerably with the advent of new generation DES.5,6 In addition, some, but not all, randomized controlled trials (RCTs) have shown a reduction in bleeding complications without a signal of increased ischaemic events with a short course DAPT (3–6 months) as compared to 12 months DAPT in patients at relatively low risk of thrombotic events.7–18 On the other hand, extending DAPT up to 3 years has been associated with a reduction in ischaemic events, with a similar increase in bleeding events, as compared to 12 months DAPT in patients treated with DES or in patients with a previous myocardial infarction (MI).17–25 Furthermore, the field of antithrombotic therapy is rapidly evolving with novel antithrombotic strategies emerging, such as P2Y12 inhibitor monotherapy, low-dose factor Xa inhibitors in addition to antiplatelet therapy, and platelet function- or genotype-guided de-escalation or escalation of P2Y12 inhibition.26–35 However, interpretation of results from RCTs investigating antithrombotic therapies is hampered by the use of composite (primary) endpoints, which combine ischaemic events [i.e. (cardiovascular) mortality, MI, and stroke] and major bleeding. The individual components of these combined endpoints can have markedly different impact on mortality, morbidity, and quality of life.36 For example, bleeding (even major bleeding) is rarely fatal or disabling, whereas ischaemic stroke frequently results in permanent disability. Taken together, clinical decision-making regarding the optimal duration and composition of antithrombotic therapy has become a major challenge.
Current guidelines highlight the importance of risk stratification to identify patients who benefit from either short or prolonged DAPT, or potent or less potent antithrombotic therapy as displayed in graphical abstract.1–3 There are numerous risk stratification methods available to clinicians, such as the use of risk scores, platelet function testing (PFT) and genotyping, each with their advantages and drawbacks. This review summarizes evidence on different antithrombotic strategies after PCI, provides an overview and critical appraisal of currently available risk stratification methods, and puts into perspective the clinical need for patient-tailored antithrombotic therapy.
Short dual antiplatelet therapy followed by aspirin monotherapy
To date, 12 RCTs (Supplementary material online, Table S1) have evaluated short DAPT.7–18 The vast majority of these trials demonstrated that short DAPT was non-inferior compared to standard DAPT in terms of the primary ischaemic endpoint, while some trials also showed a significant reduction in bleeding complications. In most studies, patients had a relatively low risk of recurrent ischaemia (mostly patients with CCS or low-risk ACS).7–12,15,18 The investigated short DAPT varied from 3 to 6 months and in the majority of studies clopidogrel was used. Importantly, the SMART-DATE trial, which only included ACS patients, did show a higher risk of MI with 6 months DAPT as compared to 12 months.13
Several limitations of these trials should be acknowledged. Most trials used an open-label design. The majority of trials randomized patients at the time of index PCI instead of DAPT cessation thereby including early events when both groups were still using DAPT.7–13,16–18 This may have diluted differences occurring after DAPT cessation. Some studies had lower-than-expected event rates or enrollment was prematurely discontinued, and were therefore underpowered.9,15,18 Many trials pre-specified a wide non-inferiority margin, and in some trials, there were a large number of cross-overs between study groups, which hampers interpretation of the results.37 Nonetheless, these studies collectively suggest that short DAPT might improve outcomes in patients with a relatively low thrombotic risk and/or high bleeding risk. Accordingly, current guidelines recommend that short DAPT should be considered in patients at high bleeding risk.1–3
Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy
In recent years, the status of aspirin as the mainstay of antithrombotic therapy has been challenged. Aspirin use is associated with an increased risk of bleeding (in particular gastrointestinal bleeding), especially in the elderly and those who concurrently use other antithrombotic agents.38 The advent of potent P2Y12 inhibitors, i.e. ticagrelor and prasugrel, has raised questions as to whether the additional antithrombotic benefit of aspirin outweighs the increase in bleeding complications. Especially since the antithrombotic potency of ticagrelor alone seems to be comparable to that of ticagrelor and aspirin with respect to ex vivo blood thrombogenicity.39 In addition, contemporary pharmacological therapies for cardiovascular risk factors, such as hypertension, dyslipidemia, and impaired glucose metabolism, have led to reductions in an individual’s cardiovascular risk.38 These therapies were not available at the time of the pivotal studies evaluating aspirin in the setting of secondary prevention. Therefore, relative benefits of adding aspirin might translate into smaller absolute risk reductions in current clinical practice as compared to previous clinical trials.38 Together, these observations have supported the hypothesis that P2Y12 inhibitor monotherapy (after a short course DAPT) might be superior to standard 12 months DAPT. In fact, even complete omission of aspirin after PCI is now a topic of investigation. Recently, the ASET pilot has shown that an aspirin-free prasugrel monotherapy strategy directly following PCI was feasible in CCS patients opening the door to RCTs investigating complete aspirin omission in coronary artery disease.40
To date, five RCTs have investigated the efficacy and safety of aspirin discontinuation (i.e. P2Y12 inhibitor monotherapy) after a short course of DAPT in patients undergoing PCI with new generation DES.26–30 These trials are summarized in Supplementary material online, Table S2. Importantly, three of these trials were underpowered to test non-inferiority of short DAPT compared to standard DAPT with regard to ischaemic events.28–30 Four trials applied an open-label design and randomized patients at the time of PCI (instead of at DAPT discontinuation).27–30 In the pivotal, placebo-controlled, double-blind TWILIGHT trial, 3 months DAPT followed by ticagrelor monotherapy up to 15 months was associated with a significant reduction in BARC types 2, 3, and 5 bleeding compared to 15 months DAPT (with ticagrelor).26 Three months DAPT followed by ticagrelor monotherapy was non-inferior to 15 months DAPT in terms of ischaemic events.26 Importantly, 29% of patients included in TWILIGHT had CCS, for whom 6 months DAPT with clopidogrel would be standard practice. The TWILIGHT trial included patients with at least one clinical and angiographic feature associated with high ischaemic or bleeding risk, but the rate of all-cause mortality, MI, or stroke between 3 and 15 months was relatively low compared with other trials investigating high-risk PCI (3.9% in both treatment arms), suggesting that the investigated study population actually consisted of more low-risk patients. Although TWILIGHT sub-studies in high-risk groups (e.g. diabetic patients or complex PCI) have been reassuring, whether DAPT for 3 months followed by ticagrelor monotherapy actually is non-inferior to 12 months DAPT with regard to ischaemic events in true high-risk patients remains to be investigated.41,42 In line with the results of TWILIGHT, P2Y12 inhibitor monotherapy preceded by short DAPT (1–3 months) was associated with a lower incidence of clinically relevant bleeding compared to standard DAPT treatment without an increase in cardiovascular events after 1 year in multiple recent meta-analyses.43–46
Based on the available evidence, P2Y12 inhibitor monotherapy after an initial short course DAPT should be considered as an alternative to standard DAPT in patients without high ischaemic risk undergoing PCI.3 Ticagrelor should be the agent of choice for ACS patients, due to its superiority to clopidogrel and its predominant use in trials evaluating P2Y12 inhibitor monotherapy.26,27,30 For CCS patients, clopidogrel might be the preferred option, although there are concerns of high on-treatment platelet reactivity. To address these concerns, physicians may consider PFT to assess treatment response, but this specific strategy remains to be investigated. Clopidogrel monotherapy has only been evaluated in East Asian patients, who have an unique risk profile.28,29 Therefore, caution should be taken when extrapolating these trials results to other ethnicities. Thus far, experience with prasugrel monotherapy in the setting of P2Y12 inhibitor monotherapy has been limited. Of note, there are currently no randomized studies available comparing P2Y12 inhibitor monotherapy to aspirin monotherapy after a short course of DAPT and experience with P2Y12 inhibitor monotherapy beyond 1 year after stent implantation is limited.
Extended dual antiplatelet therapy
Nine RCTs compared extended DAPT (18–48 months) with standard DAPT (6–12 months) (Supplementary material online, Table S3).17–25 Most trials did not demonstrate a benefit of extended DAPT. The majority of patients enrolled in these trials had CCS and clopidogrel was used almost exclusively. Two studies randomized patients at the time of PCI or shortly thereafter, potentially diluting differences in outcome between the two groups.17,18 Importantly, the adequately powered DAPT trial demonstrated that long DAPT (30 months) significantly reduced the risk of definite or probable stent thrombosis and major adverse cardiac events (MACE), but was also associated with an increased risk of bleeding.22 Overall, at 30 months after index PCI, there was a trend towards increased all-cause mortality (0.5% absolute increase) with extended DAPT, explained by a statistically significant increase in non-cardiovascular mortality, mainly attributed to increased cancer related mortality. The PEGASUS-TIMI 54 trial included patients who suffered an MI 1–3 years before enrollment and had at least one additional high-risk feature (age ≥65 years, diabetes mellitus requiring medication, multiple prior MI’s, multivessel disease or renal impairment). The study showed that extended DAPT with ticagrelor (median 33 months) compared to aspirin monotherapy reduced the risk of MI, stroke, and cardiovascular death combined but increased the risk of major bleeding and did not reduce all-cause mortality.24 The absolute decrease in the primary efficacy endpoint was similar in magnitude to the increase in the primary bleeding endpoint indicating no clear benefit for the study population as a whole.
In a pre-specified subgroup of CCS patients with diabetes and previous PCI of the THEMIS trial, long-term DAPT with ticagrelor (60 mg twice daily) on top of aspirin (for a median of 3.3 years) was associated with a 1.3% absolute reduction in cardiovascular death, MI, and stroke [number needed to treat (NNT) 77], coupled with an increase in major bleeding [0.9% absolute increase, number needed to harm (NNH) 111].47 The high NNT and similar NNH currently only support extended DAPT in diabetic patients having undergone PCI and at high ischaemic risk without high bleeding risk. Accordingly, ticagrelor has been approved by the FDA to reduce the risk of MI or stroke in high-risk patients with CCS. Importantly, in THEMIS, in patients without a previous intervention, long-term ticagrelor plus aspirin increased the rate of major bleeding (including intracranial haemorrhage) without a reduction in ischaemic events and should therefore be avoided.
A meta-analysis investigating extended DAPT in patients with prior MI showed a reduction in stent thrombosis, stroke, and MI, which translated into decreased cardiovascular mortality.48 However, other meta-analyses including lower risk patients did not show reduced cardiovascular mortality and extended DAPT was even associated with an increased risk of non-cardiovascular and all-cause mortality.49–51 Hence, current guidelines recommend that extended DAPT can be considered in patients with high thrombotic risk without high bleeding risk.1–3
Low-dose factor Xa inhibitor on top of antiplatelet therapy
Factor Xa inhibitors and other anticoagulants ultimately inhibit the formation or activation of thrombin, which plays a crucial role in both coagulation and platelet activation and may offer a synergistic benefit when added to antiplatelet therapy.52 A strong asset of dual-pathway inhibition is that anticoagulants, like factor Xa-inhibitors, modulate a number of inflammatory pathways which may reduce their contribution to atherogenesis.52 Oral anticoagulants have been shown to mitigate the risk of arterial thrombotic events, but because DAPT was superior to aspirin and warfarin in preventing stent thrombosis, the latter strategy was abandoned in favour of DAPT following PCI.53 However, recently reported studies investigating (low-dose) non-vitamin K antagonist oral anticoagulants (Supplementary material online, Table S4) have renewed interest in combining lower anticoagulant doses with antiplatelet therapy.
In the placebo-controlled ATLAS ACS 2-TIMI 51 trial, low-dose rivaroxaban (2.5 mg twice daily) reduced the incidence of cardiovascular death, MI, and stroke in ACS patients mostly treated with aspirin and clopidogrel, at the expense of increased bleeding.31 Subsequently, in the COMPASS trial, low-dose rivaroxaban (2.5 mg twice daily) on top of aspirin as compared to aspirin alone was associated with reduced risk of cardiovascular death, MI or stroke in patients with CCS and peripheral artery disease at moderate-high risk of ischaemic events.32 In high-risk subgroups (e.g. patients with diabetes, moderate chronic kidney disease, and current smokers), low-dose rivaroxaban was associated with even greater absolute risk reductions. Unfortunately, pre-specified significance thresholds for cardiovascular and all-cause mortality were not met and patients on low-dose rivaroxaban on top of aspirin had more major (though not-fatal) bleeding events.32 Interestingly, low-dose rivaroxaban and aspirin as compared to aspirin alone significantly reduced the rate of stroke by 42% (driven by a 49% relative reduction in ischaemic stroke partially offset by a numeric increase in haemorrhagic stroke), making low-dose rivaroxaban plus aspirin an important new option for stroke prevention in patients with atherosclerosis.54
A substantial fraction (approximately 50%) of coronary or peripheral artery disease patients encountered in daily practice seem eligible for this strategy based on an analysis in the REACH registry.55 However, exclusion criteria like high bleeding risk, an indication for therapeutic anticoagulation or DAPT, and a history of recent stroke are common, warranting careful patient selection for this novel approach. Current guidelines now highlight low-dose rivaroxaban on top of aspirin as an option for extended long-term secondary prevention in patients with high ischaemic risk and low bleeding risk.3 Of note, a head-to-head comparison between low-dose rivaroxaban in addition to aspirin vs. extended DAPT for long-term secondary prevention in high-risk patients is currently lacking.
Risk stratification and risk scores
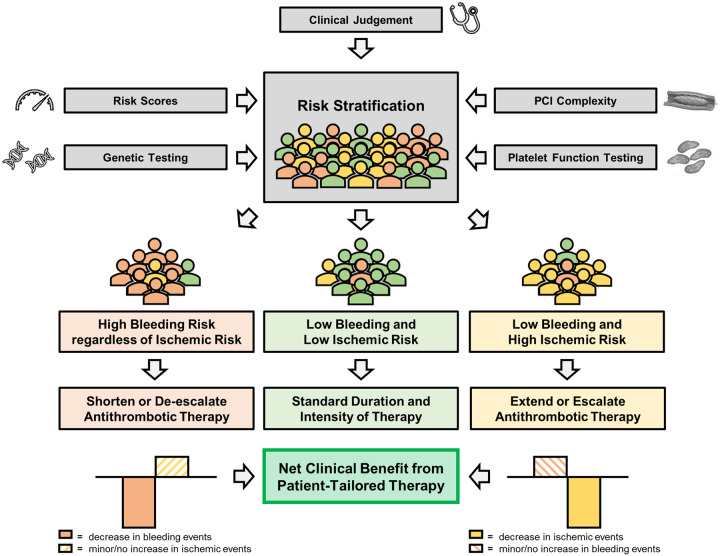
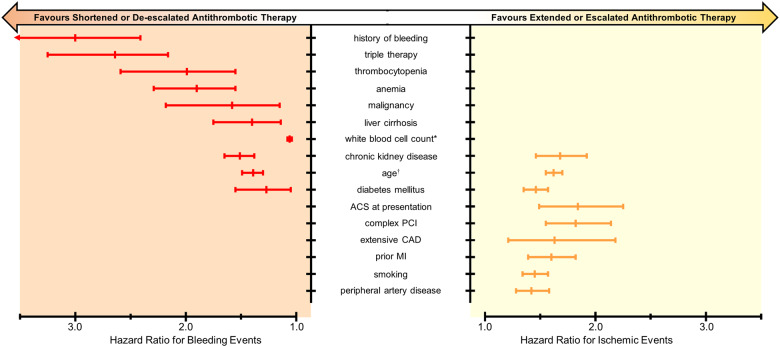
Given the benefits and risks of various DAPT durations and compositions, current guidelines recommend risk stratification to identify patients who benefit from either shortened or extended DAPT, or potent or less potent antithrombotic therapy.1–3 Risk stratification involves determining a patient’s risk of bleeding and thrombotic events, taking into account clinical, anatomical, and procedural characteristics. Figure 1 illustrates the impact of established risk factors on thrombotic and bleeding risk by showing pooled results of previously published hazards ratios for thrombotic and bleeding events (for methodology see Supplementary material online, Appendix S5).
Figure 1.
Clinical risk factors associated with increased risk of bleeding and/or ischaemic events. *Age per 10 years; †white blood cell count 103 cells/µL. ACS, acute coronary syndrome; CAD, coronary artery disease; MI, myocardial infarction.
In recent years, multiple risk scores have been developed aimed at maximizing ischaemic protection and minimizing bleeding risk for individual patients by tailoring treatment duration (net clinical benefit).56,57 Various studies, however, have questioned their calibration, predictive value, and generalizability in real-world patients. Therefore, their utility in routine clinical practice has been subject of debate.58 Currently available risk scores developed to determine DAPT duration are summarized in Table 1. An overview of the derivation cohorts is shown in Supplementary material online, Table S6. The PEGASUS-TIMI 54 investigators have developed a simple patient selection algorithm incorporating both bleeding and thrombotic risk to identify patients who may benefit from long-term secondary prevention with DAPT.59 By applying the tool in PEGASUS-TIMI 54, a patient subset at high risk of thrombotic events and low risk of bleeding was identified, who derived net clinical benefit (defined as a reduction in the composite of cardiovascular death, MI, stroke, intracranial haemorrhage, or fatal bleeding) and a reduction in all-cause mortality with extended DAPT. This promising tool, however, has not undergone the peer-review process; therefore, a detailed discussion of this tool is beyond the scope of this manuscript. The Academic Research Consortium recently proposed a consensus definition for high bleeding risk.60 Although these criteria adequately identified patients with high bleeding risk, the criteria were not developed to tailor DAPT duration and are—like other risk scores (e.g. the PARIS risk scores), which were not specifically designed to tailor DAPT duration—therefore beyond the scope of this review. So far, risk scores have focused on determining optimal antiplatelet treatment duration, not optimal composition.
Table 1.
Overview of risk scores developed to guide clinical decision-making surrounding optimal dual antiplatelet therapy duration
| PRECISE-DAPT score56 | DAPT score57 | |
|---|---|---|
| Clinical outcome | Out-of-hospital TIMI minor or major bleeding | Out-of-hospital MI, ST, and GUSTO moderate or severe bleeding |
| Predictors |
|
|
| Time of use | At time of PCI | After 12 months |
| Score range | 0 to 100 | −2 to 10 |
| Interpretation | ||
| Very low risk | ≤10 | |
| Low risk | 11 to 17 | <2 |
| Moderate risk | 18 to 24 | |
| High risk | ≥25 | ≥2 |
| Clinical implications | ≥25 net clinical benefit from shortened (3–6 months) DAPT | ≥2 net clinical benefit from extended (30 months) DAPT |
| Calculator | www.precisedaptscore.nl | www.tools.acc.org/DAPTriskapp |
CHF, congestive heart failure; DAPT, dual antiplatelet therapy; GUSTO, Global Use of Strategies to Open Occluded Coronary Arteries; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; ST, stent thrombosis; TIMI, thrombolysis in myocardial infarction.
Risk scores to be used at the time of percutaneous coronary intervention
The PRECISE-DAPT score
The PRECISE-DAPT score is a five-item risk score to predict out-of-hospital bleeding after PCI (Table 1).56 The c-statistic for out-of-hospital TIMI major or minor bleeding was 0.73 and 0.71 for TIMI major bleeding in the development cohort. The PRECISE-DAPT score was externally validated in PLATO and the BERN-PCI registry with good (c-statistic 0.70) and moderate (c-statistic 0.66) discrimination, respectively. In both study populations, calibration was good. Among patients with a high bleeding risk (PRECISE-DAPT score ≥25), standard DAPT was associated with no reduction in ischaemic events, but a strong increase in bleeding with an NNH of 38. By contrast, standard treatment in patients without high bleeding risk (PRECISE-DAPT score <25) was associated with significant reductions in the combined ischaemic endpoint, without an increase in bleeding, with an NNT of 65. Thus, by upfront deciding on a short course of DAPT in patients at high bleeding risk, a substantial proportion of excess bleeding events might be prevented, while patients without high bleeding risk benefit from standard or extended DAPT. Noteworthy, in five out of eight RCTs included in the PRECISE-DAPT derivation cohort, exclusion criteria such as thrombocytopenia, anaemia, or history of bleeding were applied.7–9,17,61 In the derivation cohort, the incidence of bleeding was relatively low (1.5% and 0.8% for major and minor and major bleeding events, respectively) suggesting that patients at high risk of bleeding were not included. Importantly, individual information regarding drug adherence was not available in the derivation cohort and was therefore only based on the pre-specified or randomized treatment duration at the time of PCI.56
External retrospective validation in other PCI cohorts showed overall moderate discrimination and adequate calibration for bleeding, but the PRECISE-DAPT score may be less suitable for elderly patients and those on concomitant oral anticoagulant therapy (Supplementary material online, Table S7). A 4-item PRECISE-DAPT score, without white blood cell count, has also been recently validated as a tool to identify patients who benefit from shortened DAPT.62
Risk scores to be used 12 months after percutaneous coronary intervention
The dual antiplatelet therapy score
The DAPT score was derived from 11 648 patients enrolled in the DAPT trial who tolerated DAPT during the first year without MACE or bleeding.57 It is a combined ischaemic and bleeding risk score designed to predict which patients benefit from DAPT extension (up to 30 months) (Table 1). Among patients with a high score (≥2) treatment with extended DAPT (12–30 months) resulted in reductions in ischaemic events (NNT 34) without an increase in bleeding and thus in net clinical benefit. Yet, this apparent benefit disappeared when patients having received paclitaxel-eluting stents were removed from the analysis.57 Among patients with a low score (<2) treatment with extended DAPT was associated with an increase in moderate or severe bleeding events (NNH 64), without reductions in ischaemic events. The DAPT score had good predictive value for ischaemic events (c-statistic 0.70) and moderate predictive value for bleeding events (c-statistic 0.68) in the development cohort.
The ability of the DAPT score to retrospectively identify patients who derive net clinical benefit from extended DAPT has been investigated in RCTs but none reached statistical significance in terms of absolute risk differences (Supplementary material online, Table S8). However, a pooled meta-analysis showed that extended DAPT reduced ischaemic events with no effect on bleeding in patients with a high DAPT score, and conversely increased bleeding without an ischaemic benefit was seen in patients with a low DAPT score who received extended DAPT.63
Due to the lack of randomization, identifying patients who derive net clinical benefit from extended DAPT in registry-based validation studies is not possible, but discrimination and calibration can be assessed. In the SWEDEHEART registry, a large Swedish nationwide cohort of patients with cardiovascular disease showed that the DAPT score poorly discriminated ischaemic risk and was unable to discriminate bleeding risk.64 In addition, the absolute risk rates for MACE followed a U-shaped pattern suggesting poor calibration of the DAPT score. However, the developers of the DAPT score argued that this might be related to the definition of MACE in SWEDEHEART, which was a composite of all-cause mortality, MI, and stroke. Older patients have a relatively low DAPT score (−1 or −2 points for those aged 65–74 or ≥75 years, respectively) and a high risk of mortality due to non-cardiovascular causes (which is not prevented by extended DAPT) explaining why MACE rates were also high in patients with a low DAPT score.65 Furthermore, the (non-fatal) bleeding rate might have been underestimated in this cohort since events were based on administrative codes.65 Other external validation studies of the DAPT score showed conflicting results in terms of calibration and discrimination (Supplementary material online, Table S9), but classic validation metrics may not be appropriate for this combined bleeding and ischaemic risk score.65 Possible explanations for the lack of calibration and discrimination of the DAPT score in external validation studies include the fact that (i) patients with a contraindication for extended DAPT were excluded from the trial; (ii) <50% of those screened were included in the trial population, (iii) common bleeding determinants, such as previous bleeding, creatinine clearance, and anaemia, are not included in the DAPT score, and (iv) older generation stents were used, which might have inflated the risk of stent thrombosis.
Platelet function- or genotype-guided P2Y12 inhibitor therapy
Clopidogrel and prasugrel require conversion to an active metabolite by the cytochrome P450 enzyme system. Genetic polymorphisms, especially loss-of-function mutations, have been shown to contribute to impaired conversion of clopidogrel to the active metabolite.66 Impaired drug conversion can lead to high (on-treatment) platelet reactivity (HPR), which is common among patients on clopidogrel (∼42%, reported range 7–75%), but relatively rare in prasugrel users.67,68 HPR has consistently been associated with an increased risk of stent thrombosis and MACE.67 Conversely, low platelet reactivity has been associated with an increased risk of bleeding.67 Thus, PFT and genotyping might be of utility in ischaemic or haemorrhagic risk stratification and tailoring of P2Y12 inhibitor therapy. For instance, clinicians can escalate (switch from a less potent agent, i.e. clopidogrel, to a potent agent, i.e. ticagrelor or prasugrel) or de-escalate treatment (switch from a potent agent to a less potent agent).69 In recent years, multiple rapid (bedside) assays have become available, enabling easy implementation in routine practice.70 Characteristics of RCTs investigating a platelet function-guided or genotype-guided escalation or de-escalation approach are given in Supplementary material online, Tables S10 and S11, respectively.
Unfortunately, none of the RCTs investigating platelet function-guided escalation, which mainly included CCS patients, met their respective primary endpoint. Therefore, in an updated expert consensus document, the routine use of PFT to escalate P2Y12 inhibitor therapy in patients with HPR on clopidogrel was not recommended.71 However, PFT-guided P2Y12 inhibitor therapy can be considered in selected patients without high bleeding risk, in whom adequate platelet inhibition is of the utmost importance (e.g. left main stenting, last patent vessel PCI, or previous stent thrombosis).71
To date, two RCTs have compared PFT-guided de-escalation or dose-adjustment of P2Y12 inhibitor therapy to standard treatment in patients with ACS.35,72 In TROPICAL-ACS, platelet function-guided de-escalation of prasugrel to clopidogrel was non-inferior (but not superior) to standard prasugrel in terms of the primary net clinical benefit endpoint, a composite of cardiovascular death, MI, stroke, or BARC type ≥2 bleeding.35 The rates of ischaemic events were similar in the guided vs. non-guided group (2.5% vs. 3.2%, P = 0.12) and there was a trend towards less bleeding in the platelet function-guided group (4.9 vs. 6.0%, P = 0.23), mainly driven by a reduction in minor bleedings. Of note, almost 4 out of 10 patients in the guided de-escalation group were switched back to prasugrel after 2 weeks because of HPR while on clopidogrel. Although the trial was not powered to test non-inferiority in terms of ischaemic events, the low MACE rate in the platelet function-guided group is reassuring. Therefore, PFT-guided de-escalation of P2Y12 inhibitor therapy may be considered in specific clinical scenarios, such as high bleeding risk or a recent bleeding event.
Genotype-guided escalation or de-escalation was associated with improved outcomes in small, single, or dual centre RCTs.73,74 In the large-scale TAILOR PCI trial, in a predominantly ACS population treated with PCI (n = 5302), a genotype-guided escalation strategy (ticagrelor instead of clopidogrel in carriers of CYP2C19 loss-of-function alleles) numerically, though not statistically significantly (5.9% vs. 4.0%, P = 0.06), reduced adverse cardiovascular events as compared to standard treatment with clopidogrel on top of aspirin in the subgroup of carriers of loss-of-function alleles (n = 1849).75 A pre-specified sensitivity analysis taking into account recurrent events (not only the time to first event) did reach statistical significance and a post hoc analysis showed an almost 80% reduced rate of adverse events in the first 3 months, suggesting that most gain is to be made in the early high-risk period following PCI.75 Of note, the vast majority of study population consisted of ACS patients (84%), for whom treatment with potent P2Y12 inhibitors and not clopidogrel is the current standard of care.1–3
In the POPular Genetics trial, 2488 patients undergoing primary PCI were randomized open-label to genotype-guided P2Y12 inhibition (de-escalation based on CYP2C19 genetic testing) or standard treatment with either ticagrelor or prasugrel for 12 months. Genotype-guided P2Y12 de-escalation was non-inferior to standard treatment in terms of the primary outcome net clinical benefit, and there was a significant reduction in the primary bleeding outcome (PLATO major or minor bleeding), driven by a reduction in minor bleeding. Although the trial was not powered to test non-inferiority with regard to ischaemic events, there was no signal of increased ischaemic events in the de-escalation group.
Taken together, there is some evidence supporting genotype-guided P2Y12 inhibition, but still insufficiently for its routine adoption in clinical practice. For now, genotype-guided P2Y12 inhibition may be considered in patients with a particular risk profile or for socioeconomic reasons. Interestingly, the recently proposed ABCD-GENE score integrates four clinical factors (age, body mass index, chronic kidney disease, and diabetes mellitus) and CYP2C19 genotype.76 The ABCD-GENE score identifies patients with HPR on clopidogrel and those who are subsequently at increased risk for death, MI, or stroke.76 Clinicians may consider escalating antithrombotic therapy in patients on clopidogrel with a high ABCD-GENE score, but prospective validation of this risk score is warranted.
Patient-tailored antiplatelet therapy in daily practice
Deciding for whom to shorten, extend, de-escalate, or escalate antithrombotic therapy is complex and requires collaboration between the interventional cardiologist and the treating cardiologist at the outpatient clinic. Physicians need to weigh clinical, anatomical, procedural, and laboratory aspects together with input from risk scores and in selected patients from PFT or genotyping, before choosing an antithrombotic strategy. In addition, a patient’s bleeding and ischaemic risk may change over time. Treatment duration or composition dictated by risk scores or other stratification methods should therefore not be considered static and should be reassessed periodically.
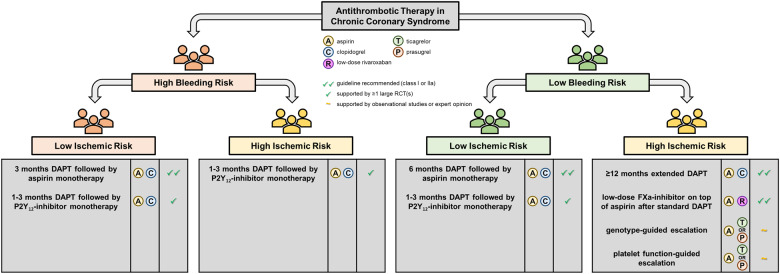
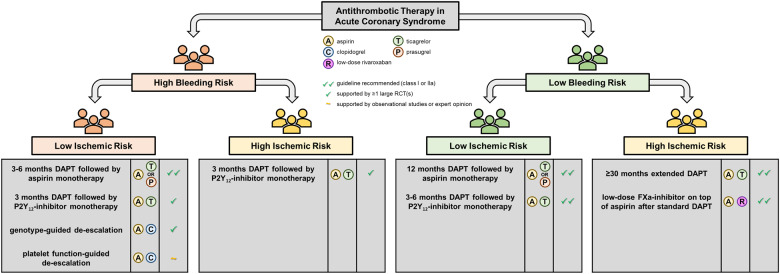
Graphical abstract shows the available risk stratification tools, while Figures 2 and 3 illustrate the subsequent treatment options for CCS and ACS patients with different risk profiles. Of the available tools, PFT and genetic testing have been most extensively investigated in RCTs. Although there is a clear biological rationale for the use of PFT or genotyping and the results of small proof-of-concept studies investigating a guided approach were promising, the robustness of the evidence, especially when considering the results of adequately powered RCTs, still does not support the routine use of PFT or genetic testing.71 Table 2 list advantages and drawbacks associated with the different risk stratification tools. Risk scores such as the PRECISE-DAPT and DAPT scores are easy to use and free of charge, thus facilitating broad adoption in clinical practice even among non-cardiologists. To date, there have been no RCTs comparing a risk score-based approach with standard care. Currently, the FORCE-ACS study, a prospective multicentre registry study, is comparing a risk score-guided approach to standard practice in ACS patients (ClinicalTrials.gov Identifier: NCT03823547).77 Interestingly, in this study, application of the PRECISE-DAPT and DAPT scores is combined, potentially improving the overall performance of the risk score-guided approach.
Figure 2.
Patient-tailored antithrombotic strategies for chronic coronary syndrome patients. Recommendations reflect the authors’ opinion. DAPT, dual antiplatelet therapy; FXa, factor Xa; RCT, randomized controlled trial.
Figure 3.
Patient-tailored antithrombotic strategies for acute coronary syndrome patients. Recommendations reflect the authors’ opinion. DAPT, dual antiplatelet therapy; FXa, factor Xa; RCT, randomized controlled trial.
Table 2.
Advantages and drawbacks of risk stratification methods
| Risk scores | PFT | Genotyping | |
|---|---|---|---|
| Easy to use, results rapidly available |


|

|

|
| Associated with ischaemic events |

|

|

|
| Associated with bleeding events |

|

|

|
| Provides an overall bleeding and ischaemic risk estimate |

|

|

|
| No need to be determined while on treatment |

|

|

|
| Direct measure of response to therapy |

|

|

|
| No additional healthcare costs |

|

|

|
| Benefits established in RCTs |

|

|
 a
a
|
 denotes the presents of a given feature linked to the respective risk stratification method, while
denotes the presents of a given feature linked to the respective risk stratification method, while
 denotes the absence of such a feature. PFT, platelet function testing; RCT, randomized controlled trial.
denotes the absence of such a feature. PFT, platelet function testing; RCT, randomized controlled trial.
Genotype-guided de-escalation has been shown to reduce minor bleeding events.
For patients with a high thrombotic risk and an acceptable bleeding risk, physicians can now choose between extending DAPT or adding low-dose rivaroxaban to aspirin. Comparing relative and absolute risk reduction from different RCTs is difficult due to variation in study populations and follow-up. Therefore, it remains to be investigated which of these two strategies is superior in terms of efficacy and safety. It is also still unknown if risk scores are able to identify patients who derive benefit from low-dose rivaroxaban in conjunction with aspirin.
Recently, two studies have underscored the importance of PCI complexity in determining DAPT duration.78,79 These studies showed that complex PCI was an independent predictor of ischaemic events in the first year, but not beyond 12 months after PCI. In the derivation cohort of the PRECISE-DAPT score, 12–24 months DAPT was associated with significant reductions in MACE compared to 3–6 months DAPT in patients with complex lesions.78 Conversely, in the DAPT trial, among patients without events in the first year, the benefits of extending DAPT beyond 1 year were similar regardless of PCI complexity.79 These findings suggest that patients who have undergone complex PCI may benefit from 12 months DAPT rather than 3–6 months DAPT. Extending DAPT beyond 1 year in these patients should be based on overall thrombotic risk and not on procedural characteristics alone.
A major challenge frequently facing physicians is concurrent high bleeding and high ischaemic risk (e.g. in the PARIS registry ∼40% of high bleeding risk patients also had high ischaemic risk).80 Findings of a recent post hoc analysis performed in the derivation cohort of the PRECISE-DAPT score suggest that in these patients bleeding risk rather than ischaemic risk should guide decision-making regarding treatment duration.81 This analysis found that high bleeding risk patients (i.e. PRECISE-DAPT score ≥25) with concordant high ischaemic risk (i.e. complex PCI and/or ACS at presentation) did not derive benefit from long DAPT (12–24 months) as compared to short DAPT (3–6 months) but did have excess bleeding complication.81
Conclusions
The future of antithrombotic therapy lies in an individualized duration and composition based on risk stratification. There are multiple risk stratification methods available to guide clinical decision-making, all with their own advantages and drawbacks. Future research will have to point out how to best stratify patients and subsequently provide them with patient-tailored therapy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: N.M.R.S., R.R., and D.R.P.P.C.P.Y. have no conflicts of interest to declare. M.V. has received research grants from Terumo and personal fees from AstraZeneca, Terumo, Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Bayer, CoreFLOW, Idorsia Pharmaceuticals Ltd, Universität Basel, Bristol Myers Squib SA, Medscape and Vesalio. S.W. has received research grants from Abbott, Amgen, BMS, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Guebert, Polares, Sanofi and Terumo. S.K.J. has received research grants from AstraZeneca, Jansen and Bayer. S.B. has no conflicts of interest to declare. J.M.B. has received research grants from ZonMw and AstraZeneca and personal fees from AstraZeneca, Accu-Metrics, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ferrer, Idorsia, Pfizer and The Medicines Company. J.P.S.H. has received research grants from AstraZeneca, Getinge, Infraredx and B. Braun. M.V. has no conflicts of interest to declare. W.J.K. has received research grants and personal fees from AstraZeneca.
Supplementary Material
References
- 1. Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann F-J, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN, Badimon L, Vranckx P, Agewall S, Andreotti F, Antman E, Barbato E, Bassand J-P, Bugiardini R, Cikirikcioglu M, Cuisset T, De Bonis M, Delgado V, Fitzsimons D, Gaemperli O, Galiè N, Gilard M, Hamm CW, Ibanez B, Iung B, James S, Knuuti J, Landmesser U, Leclercq C, Lettino M, Lip G, Piepoli MF, Pierard L, Schwerzmann M, Sechtem U, Simpson IA, Uva MS, Stabile E, Storey RF, Tendera M, Van de Werf F, Verheugt F, Aboyans V, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Roithinger FX, Aliyev F, Stelmashok V, Desmet W, Postadzhiyan A, Georghiou GP, Motovska Z, Grove EL, Marandi T, Kiviniemi T, Kedev S, Gilard M, Massberg S, Alexopoulos D, Kiss RG, Gudmundsdottir IJ, McFadden EP, Lev E, De Luca L, Sugraliyev A, Haliti E, Mirrakhimov E, Latkovskis G, Petrauskiene B, Huijnen S, Magri CJ, Cherradi R, Ten Berg JM, Eritsland J, Budaj A, Aguiar CT, Duplyakov D, Zavatta M, Antonijevic NM, Motovska Z, Fras Z, Montoliu AT, Varenhorst C, Tsakiris D, Addad F, Aydogdu S, Parkhomenko A, Kinnaird T; Group ESD, Guidelines ECfP, Societies ENC. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 2. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC. Jr. 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082–1115. [DOI] [PubMed] [Google Scholar]
- 3. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; Group ESD. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2020;doi: 10.1093/eurheartj/ehaa575. [Google Scholar]
- 4. Sharma A, Hagstrom E, Wojdyla DM, Neely ML, Harrington RA, Wallentin L, Alexander JH, Goodman SG, Lopes RD. Clinical consequences of bleeding among individuals with a recent acute coronary syndrome: insights from the APPRAISE-2 trial. Am Heart J 2019;215:106–113. [DOI] [PubMed] [Google Scholar]
- 5. Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 2013;6:1267–1274. [DOI] [PubMed] [Google Scholar]
- 6. Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, Wenaweser P, Daemen J, Meier B, Juni P, Serruys PW, Windecker S. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 2012;125:1110–1121. [DOI] [PubMed] [Google Scholar]
- 7. Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012;125:505–513. [DOI] [PubMed] [Google Scholar]
- 8. Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Park BE, Kang WC, Lee SH, Yoon JH, Hong BK, Kwon HM, Jang Y. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60:1340–1348. [DOI] [PubMed] [Google Scholar]
- 9. Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, Oteo Dominguez JF, Steffanon L, Tarantini G, Presbitero P, Menozzi A, Pucci E, Mauri J, Cesana BM, Giustino G, Sardella G. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014;64:2086–2097. [DOI] [PubMed] [Google Scholar]
- 10. Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB 3rd, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ Jr., Nicolela EL Jr., Perin MA, Devito FS, Labrunie A, Salvadori D Jr., Gusmao M, Staico R, Costa JR Jr., de Castro JP, Abizaid AS, Bhatt DL; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013;310:2510–2522. [DOI] [PubMed] [Google Scholar]
- 11. Han Y, Xu B, Xu K, Guan C, Jing Q, Zheng Q, Li X, Zhao X, Wang H, Zhao X, Li X, Yu P, Zang H, Wang Z, Cao X, Zhang J, Pang W, Li J, Yang Y, Dangas GD. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv 2016;9:e003145. [DOI] [PubMed] [Google Scholar]
- 12. Hong SJ, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Her AY, Kim YH, Jang Y, Hong MK. 6-month versus 12-month dual-antiplatelet therapy following long everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JACC Cardiovasc Interv 2016;9:1438–1446. [DOI] [PubMed] [Google Scholar]
- 13. Hahn J-Y, Song YB, Oh J-H, Cho D-K, Lee JB, Doh J-H, Kim S-H, Jeong J-O, Bae J-H, Kim B-O, Cho JH, Suh I-W, Kim D-I, Park H-K, Park J-S, Choi WG, Lee WS, Kim J, Choi KH, Park TK, Lee JM, Yang JH, Choi J-H, Choi S-H, Gwon H-C, Gwon H-C, Hahn J-Y, Song YB, Park TK, Lee JM, Yang JH, Choi J-H, Choi S-H, Lee J-Y, Choi WG, Bae J-H, Park HS, Hwang J-Y, Hur S-H, Rha S-W, Cho D-K, Cho SC, Kang WY, Lim S-H, Lee JB, Kim MH, Cha KS, Choi RK, Chae I-H, Oh J-H, Jang WJ, Park YH, Chun WJ, Kim S-H, Cho JH, Suh I-W, Park J-S, Choi JW, Kim B-O, Doh J-H, Kim D-I, Jeong MH, Kang SH, Lee WS, Park H-K, Jeong J-O, Ahn K-J. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet 2018;391:1274–1284. [DOI] [PubMed] [Google Scholar]
- 14. Kedhi E, Fabris E, van der Ent M, Buszman P, von Birgelen C, Roolvink V, Zurakowski A, Schotborgh CE, Hoorntje JCA, Eek CH, Cook S, Togni M, Meuwissen M, van Royen N, van Vliet R, Wedel H, Delewi R, Zijlstra F. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non-inferiority trial. BMJ 2018;363:k3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schulz-Schupke S, Byrne RA, Ten Berg JM, Neumann FJ, Han Y, Adriaenssens T, Tolg R, Seyfarth M, Maeng M, Zrenner B, Jacobshagen C, Mudra H, von Hodenberg E, Wohrle J, Angiolillo DJ, von Merzljak B, Rifatov N, Kufner S, Morath T, Feuchtenberger A, Ibrahim T, Janssen PW, Valina C, Li Y, Desmet W, Abdel-Wahab M, Tiroch K, Hengstenberg C, Bernlochner I, Fischer M, Schunkert H, Laugwitz KL, Schomig A, Mehilli J, Kastrati A; On behalf of the Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015;36:1252–1263. [DOI] [PubMed] [Google Scholar]
- 16. De Luca G, Damen SA, Camaro C, Benit E, Verdoia M, Rasoul S, Liew HB, Polad J, Ahmad WA, Zambahari R, Postma S, Kedhi E, Suryapranata H. Final results of the Randomised Evaluation of short-term DUal antiplatelet therapy in patients with acute Coronary syndromE treated with a new generation stent (REDUCE) trial. EuroIntervention 2019;15:e990–e998. [DOI] [PubMed] [Google Scholar]
- 17. Valgimigli M, Borghesi M, Tebaldi M, Vranckx P, Parrinello G, Ferrari R, Valgimigli M, Campo G, Percoco G, Ferrari R, Avigni N, Mazzucco R, Vranckx P, Curello S, Guardigli G, Monti M, Gambetti S, Bristot L, Parrinello G; for the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY) Investigators. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY). Eur Heart J 2013;34:909–919. [DOI] [PubMed] [Google Scholar]
- 18. Didier R, Morice MC, Barragan P, Noryani AAL, Noor HA, Majwal T, Hovasse T, Castellant P, Schneeberger M, Maillard L, Bressolette E, Wojcik J, Delarche N, Blanchard D, Jouve B, Ormezzano O, Paganelli F, Levy G, Sainsous J, Carrie D, Furber A, Berlan J, Darremont O, Le Breton H, Lyuycx-Bore A, Gommeaux A, Cassat C, Kermarrec A, Cazaux P, Druelles P, Dauphin R, Armengaud J, Dupouy P, Champagnac D, Ohlmann P, Ben Amer H, Kiss RG, Ungi I, Gilard M. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: final results of the ITALIC trial (Is There a Life for DES After Discontinuation of Clopidogrel). JACC Cardiovasc Interv 2017;10:1202–1210. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura M, Iijima R, Ako J, Shinke T, Okada H, Ito Y, Ando K, Anzai H, Tanaka H, Ueda Y, Takiuchi S, Nishida Y, Ohira H, Kawaguchi K, Kadotani M, Niinuma H, Omiya K, Morita T, Zen K, Yasaka Y, Inoue K, Ishiwata S, Ochiai M, Hamasaki T, Yokoi H, Okada H, Ito Y, Hara H, Ando K, Anzai H, Tanaka H, Ueda Y, Takiuchi S, Nishida Y, Ohira H, Kawaguchi K, Kadotani M, Niinuma H, Omiya K, Morita T, Zen K, Yaita Y, Inoue K, Ishiwata S, Ochiai M, Takamisawa I, Yajima J, Ishihara T, Nakamura S, Fujii K, Ashida K, Ota H, Okutsu M, Oshima M, Kongoji K, Jinno Y, Shutta R, Shiode N, Oumi T, Doijiri T, Yokoi Y, Ogawa T, Kimura K, Munemasa M, Mukawa H, Komiyama K, Suzuki T, Inoue T, Ueno T, Sugano T, Yamashita J, Yasumura Y, Kamiya H, Fujita H, Shinke T, Urasawa K, Ono S, Ajioka M, Ando J, Mizuno K, Hirayama H, Tojo T, Maekawa Y, Kawasaki T, Okamura T, Toyota F, Hikichi Y, Michishita I, Yagi T, Kamihata H, Shindo N, Ishizaka N, Ashikaga T, Ozaki Y, Hara H, Sakamoto H, Kada K, Doi N, Honye J, Yokoi H, Takano H, Kawata M, Houzawa H, Ozawa T, Kikuchi A, Kadota K, Kijima Y, Ikemoto T, Shimada Y, Yumoto K, Kawajiri K, Nozaki Y, Sakakibara M, Tosaka A, Noma S, Wakabayashi Y, Okada M, Hirose M, Takagi Y, Takagi T, Miyauchi K, Misu K, Yasuda S, Yoshikawa R, Inoue I, Yoshiyama M, Masuyama T, Tomobuchi Y, Yamazaki S, Tanabe K, Wagatsuma K, Kato M, Kawai K, Hamazaki Y, Yamagishi M, Shibata Y, Watanabe K, Tachibana K, Wada H, Ninomiya K, Suzuki H, Yoshioka J, Mori C, Sonoda M, Kataoka T, Terai H, Onishi Y, Toma M, Serikawa T, Otsuka Y, Yano S, Ebisawa S, Takashima H, Shimomura H, Kurumatani Y, Sonoda S, Uehara H. Dual antiplatelet therapy for 6 versus 18 months after biodegradable polymer drug-eluting stent implantation. JACC Cardiovasc Interv 2017;10:1189–1198. [DOI] [PubMed] [Google Scholar]
- 20. Collet JP, Silvain J, Barthelemy O, Range G, Cayla G, Van Belle E, Cuisset T, Elhadad S, Schiele F, Lhoest N, Ohlmann P, Carrie D, Rousseau H, Aubry P, Monsegu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Beygui F, Vicaut E, Montalescot G. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014;384:1577–1585. [DOI] [PubMed] [Google Scholar]
- 21. Lee CW, Ahn JM, Park DW, Kang SJ, Lee SW, Kim YH, Park SW, Han S, Lee SG, Seong IW, Rha SW, Jeong MH, Lim DS, Yoon JH, Hur SH, Choi YS, Yang JY, Lee NH, Kim HS, Lee BK, Kim KS, Lee SU, Chae JK, Cheong SS, Suh IW, Park HS, Nah DY, Jeon DS, Seung KB, Lee K, Jang JS, Park SJ. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014;129:304–312. [DOI] [PubMed] [Google Scholar]
- 22. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr., Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Helft G, Steg PG, Le Feuvre C, Georges JL, Carrie D, Dreyfus X, Furber A, Leclercq F, Eltchaninoff H, Falquier JF, Henry P, Cattan S, Sebagh L, Michel PL, Tuambilangana A, Hammoudi N, Boccara F, Cayla G, Douard H, Diallo A, Berman E, Komajda M, Metzger JP, Vicaut E; OPTImal DUAL Antiplatelet Therapy Trial Investigators. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 2016;37:365–374. [DOI] [PubMed] [Google Scholar]
- 24. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 25. Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, Held C, Andersson M, Himmelmann A, Ridderstråle W, Leonsson-Zachrisson M, Liu Y, Opolski G, Zateyshchikov D, Ge J, Nicolau JC, Corbalán R, Cornel JH, Widimský P, Leiter LA. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med 2019;381:1309–1320. [DOI] [PubMed] [Google Scholar]
- 26. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Dzavik V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S, Gibson CM. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med 2019;381:2032–2042. [DOI] [PubMed] [Google Scholar]
- 27. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, van Es GA, McFadden EP, Onuma Y, van Meijeren C, Chichareon P, Benit E, Möllmann H, Janssens L, Ferrario M, Moschovitis A, Zurakowski A, Dominici M, Van Geuns RJ, Huber K, Slagboom T, Serruys PW, Windecker S, Abdellaoui M, Adlam D, Akin I, Albarran Gonzalez-Trevilla A, Almeida M, Alves Lemos Neto P, Aminian A, Anderson R, Andreae R, Angioi M, Asano T, Barbato E, Barlis P, Barraud P, Benit E, Bertrand O, Beygui F, Bolognese L, Botelho R, Bouwman C, Bressers M, Brunel P, Buszman P, Buysschaert I, Canas da Silva P, Carrie D, Cequier A, Chichareon P, Chin Chang C, Chowdhary S, Collet C, Colombo A, Cotton J, Cruz Ferreira R, Curello S, Curzen N, de Bot J, de Vreede T, Delle Karth G, Dijksma L, Dominici M, Édes I, Eeckhout E, Eitel I, Faluközy J, Fath-Ordoubadi F, Ferrario M, Fontos G, Francisco Diaz J, Freitas Quintella E, Frey B, Friedrich G, Galasko G, Galuszka G, Gama Ribeiro V, Garg S, Gargiulo G, Geisler T, Gelev V, Ghandilyan A, Goicolea J, Gori T, Gragnano F, Guimarães A, Hamm C, Haude M, Heg D, Heijke P, Hernández Antolin RA, Hildick-Smith D, Hillen D, Hoekman I, Hofma S, Holmvang L, Hoole S, Horváth I, Huber K, Hugense A, Ibrahim K, Iñiguez A, Isaaz K, Jambrik Z, Janssens L, Jasionowicz P, Jonk J, Jung W, Jüni P, Katagiri Y, Kogame N, Koh TH, Koning R, Konteva M, Kőszegi Z, Krackhardt F, Kreuger Y, Kukreja N, Ladan B, Lantelme P, Leandro S, Leibundgut G, Liebetrau C, Lindeboom W, Macaya Miguel C, Mach F, Magro M, Maillard L, Manavifar N, Mauri L, McFadden E, Merkely B, Miyazaki Y, Młodziankowski A, Moccetti T, Modolo R, Möllman H, Morelle J-F, Moschovitis A, Munndt Ottesen M, Muurling M, Naber CK, Neumann F-J, Oldroyd K, Ong P, Onuma Y, Palsrok S, Petrov I, Plante S, Prokopczuk J, Rademaker-Havinga T, Raffel C, Rensing B, Roffi M, Royaards K-J, Sabate M, Schächinger V, Seidler T, Serra Peñaranda A, Serruys P, Sikarulidze L, Slagboom T, Soliman OI, Sousa A, Spitzer E, Stables R, Steg G, Steinwender C, Subkovas E, Suryapranata H, Takahashi K, Talwar S, Teiger E, ter Weele A, Teurlings E, Thury A, Tijssen J, Tonev G, Trendafilova-Lazarova D, Tumscitz C, Umans V, Ungi I, Valkov V, van der Harst P, van Geuns RJ, van Meijeren C, Vassilev D, Velchev V, Velthuizen E, Verheugt F, Vlcek N, Vom Dahl J, Vrolix M, Walsh S, Werner N, Windecker S, Witsenburg M, Zaman A, Żmudka K, Zrenner B, Zurakowski A, Zweiker R. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392:940–949. [DOI] [PubMed] [Google Scholar]
- 28. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, Hata Y, Yagi M, Suematsu N, Yokomatsu T, Takamisawa I, Doi M, Noda T, Okayama H, Seino Y, Tada T, Sakamoto H, Hibi K, Abe M, Kawai K, Nakao K, Ando K, Tanabe K, Ikari Y, Hanaoka KI, Morino Y, Kozuma K, Kadota K, Furukawa Y, Nakagawa Y, Kimura T; Investigators S. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA 2019;321:2414–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, Im ES, Jeong JO, Cho BR, Oh SK, Yun KH, Cho DK, Lee JY, Koh YY, Bae JW, Choi JW, Lee WS, Yoon HJ, Lee SU, Cho JH, Choi WG, Rha SW, Lee JM, Park TK, Yang JH, Choi JH, Choi SH, Lee SH, Gwon HC; for the SMART-CHOICE Investigators. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA 2019;321:2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim B-K, Hong S-J, Cho Y-H, Yun KH, Kim YH, Suh Y, Cho JY, Her A-Y, Cho S, Jeon DW, Yoo S-Y, Cho D-K, Hong B-K, Kwon H, Ahn C-M, Shin D-H, Nam C-M, Kim J-S, Ko Y-G, Choi D, Hong M-K, Jang Y; Investigators ftT. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA 2020;323:2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mega JL, Braunwald E, Wiviott SD, Bassand J-P, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KAA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FWA, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 32. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim J-H, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 33. Ohman EM, Roe MT, Steg PG, James SK, Povsic TJ, White J, Rockhold F, Plotnikov A, Mundl H, Strony J, Sun X, Husted S, Tendera M, Montalescot G, Bahit MC, Ardissino D, Bueno H, Claeys MJ, Nicolau JC, Cornel JH, Goto S, Kiss RG, Güray Ü, Park DW, Bode C, Welsh RC, Gibson CM. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trial. Lancet 2017;389:1799–1808. [DOI] [PubMed] [Google Scholar]
- 34. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ’t Hof AWJ, van der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, Asselbergs FW, Mosterd A, Herrman J-PR, Dewilde WJM, Janssen PWA, Kelder JC, Postma MJ, de Boer A, Boersma C, Deneer VHM, ten Berg JM. A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N Engl J Med 2019;381:1621–1631. [DOI] [PubMed] [Google Scholar]
- 35. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, Komócsi A, Dézsi CA, Holdt L, Felix SB, Parma R, Klopotowski M, Schwinger RHG, Rieber J, Huber K, Neumann F-J, Koltowski L, Mehilli J, Huczek Z, Massberg S, Parma R, Parma Z, Lesiak M, Komosa A, Huczek Z, Koltowski L, Kowara M, Rymuza B, Klopotowski M, Malek L, Aradi D, Veress G, Dézsi AD, Merkely B, Lux Á, Kiss RG, Papp J, Kovács A, Dézsi CA, Amer S, Ruzsa Z, Róna S, Komócsi A, Ili R, Ungi I, Nagy F, Zweiker R, Tóth-Gayor G, Huber K, Haller P, von Scheidt W, Blüthgen A, Neumann F-J, Trenk D, Leggewie S, Kreider-Stempfle HU, Remp T, Kara K, Mügge A, Wutzler A, Fichtlscherer S, Zeiher AM, Seeger F, Hinterseer M, König A, Lederle S, Jacobshagen C, Czepluch F, Maier L, Schillinger W, Sossalla S, Hummel A, Felix S, Karakas M, Sydow K, Rudolph T, Halbach M, Gori T, Münzel T, May A, Gerstenberg C-M, Pilecky D, Rieber J, Deichstetter M, Sibbing D, Mehilli J, Gross L, Kääb S, Löw A, Orban M, Orban M, Sattler S, Deuschl S, Teupser D, Holdt L, Mudra H, Räder T, Schütz T, Vahldiek F, Divchev D, Ince H, Nienaber CA, Radunski H, Boekstegers P, Horstkotte J, Mueller R, Geisler T, Müller K, Schwinger R, Rasp O. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet 2017;390:1747–1757. [DOI] [PubMed] [Google Scholar]
- 36. Steg PG, Bhatt DL. Is there really a benefit to net clinical benefit in testing antithrombotics? Circulation 2018;137:1429–1431. [DOI] [PubMed] [Google Scholar]
- 37. Steg PG, Simon T. Duration of antiplatelet therapy after DES implantation: can we trust non-inferiority open-label trials? Eur Heart J 2017;38:1044–1047. [DOI] [PubMed] [Google Scholar]
- 38. Capodanno D, Mehran R, Valgimigli M, Baber U, Windecker S, Vranckx P, Dangas G, Rollini F, Kimura T, Collet JP, Gibson CM, Steg PG, Lopes RD, Gwon HC, Storey RF, Franchi F, Bhatt DL, Serruys PW, Angiolillo DJ. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol 2018;15:480–496. [DOI] [PubMed] [Google Scholar]
- 39. Baber U, Zafar MU, Dangas G, Escolar G, Angiolillo DJ, Sharma SK, Kini AS, Sartori S, Joyce L, Vogel B, Farhan S, Gurbel P, Gibson CM, Fuster V, Mehran R, Badimon JJ. Ticagrelor with or without aspirin after PCI: the TWILIGHT platelet substudy. J Am Coll Cardiol 2020;75:578–586. [DOI] [PubMed] [Google Scholar]
- 40. Kogame N, Guimarães PO, Modolo R, De Martino F, Tinoco J, Ribeiro EE, Kawashima H, Ono M, Hara H, Wang R, Cavalcante R, Moulin B, Falcão BAA, Leite RS, de Almeida Sampaio FB, Morais GR, Meireles GC, Campos CM, Onuma Y, Serruys PW, Lemos PA. Aspirin-free prasugrel monotherapy following coronary artery stenting in patients with stable CAD: the ASET pilot study. JACC Cardiovasc Interv 2020;13:2251–2262. [DOI] [PubMed] [Google Scholar]
- 41. Angiolillo DJ, Baber U, Sartori S, Briguori C, Dangas G, Cohen DJ, Mehta SR, Gibson CM, Chandiramani R, Huber K, Kornowski R, Weisz G, Kunadian V, Oldroyd KG, Ya-Ling H, Kaul U, Witzenbichler B, Dudek D, Sardella G, Escaned J, Sharma S, Shlofmitz RA, Collier T, Pocock S, Mehran R. Ticagrelor with or without aspirin in high-risk patients with diabetes mellitus undergoing percutaneous coronary intervention. J Am Coll Cardiol 2020;75:2403–2413. [DOI] [PubMed] [Google Scholar]
- 42. Dangas G, Baber U, Sharma S, Giustino G, Mehta S, Cohen DJ, Angiolillo DJ, Sartori S, Chandiramani R, Briguori C, Dudek D, Escaned J, Huber K, Collier T, Kornowski R, Kunadian V, Kaul U, Oldroyd K, Sardella G, Shlofmitz R, Witzenbichler B, Ya-Ling H, Pocock S, Gibson CM, Mehran R. Ticagrelor with or without aspirin after complex PCI. J Am Coll Cardiol 2020;75:2414–2424. [DOI] [PubMed] [Google Scholar]
- 43. Bianco M, Careggio A, Destefanis P, Luciano A, Perrelli MG, Quadri G, Rossini R, Campo G, Vizzari G, D’Ascenzo F, Anselmino M, Biondi-Zoccai G, Ibáñez B, Montagna L, Varbella F, Cerrato E. P2Y12 inhibitors monotherapy after short course of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: a meta-analysis of randomized clinical trials including 29 089 patients. Eur Heart J Cardiovasc Pharmacother 2020;doi:10.1093/ehjcvp/pvaa038. [DOI] [PubMed] [Google Scholar]
- 44. McClure JD, Ramsay JC, Berry C. Pooled analysis of bleeding, major adverse cardiovascular events, and all-cause mortality in clinical trials of time-constrained dual-antiplatelet therapy after percutaneous coronary intervention. J Am Heart Assoc 2020;9:e017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O’Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y(12) inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation 2020;142:538–545. [DOI] [PubMed] [Google Scholar]
- 46. Khan SU, Singh M, Valavoor S, Khan MU, Lone AN, Khan MZ, Khan MS, Mani P, Kapadia SR, Michos ED, Stone GW, Kalra A, Bhatt DL. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation 2020;142:1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, Held C, Andersson M, Himmelmann A, Ridderstråle W, Chen J, Song Y, Diaz R, Goto S, James SK, Ray KK, Parkhomenko AN, Kosiborod MN, McGuire DK, Harrington RA. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet 2019;394:1169–1180. [DOI] [PubMed] [Google Scholar]
- 48. Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J 2016;37:390–399. [DOI] [PubMed] [Google Scholar]
- 49. Navarese EP, Andreotti F, Schulze V, Ko Odziejczak M, Buffon A, Brouwer M, Costa F, Kowalewski M, Parati G, Lip GYH, Kelm M, Valgimigli M. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ 2015;350:h1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palmerini T, Benedetto U, Bacchi-Reggiani L, Riva DD, Biondi-Zoccai G, Feres F, Abizaid A, Hong M-K, Kim B-K, Jang Y, Kim H-S, Park KW, Genereux P, Bhatt DL, Orlandi C, De Servi S, Petrou M, Rapezzi C, Stone GW. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015;385:2371–2382. [DOI] [PubMed] [Google Scholar]
- 51. Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1116–1139. [DOI] [PubMed] [Google Scholar]
- 52. Capodanno D, Bhatt DL, Eikelboom JW, Fox KAA, Geisler T, Michael Gibson C, Gonzalez-Juanatey JR, James S, Lopes RD, Mehran R, Montalescot G, Patel M, Steg PG, Storey RF, Vranckx P, Weitz JI, Welsh R, Zeymer U, Angiolillo DJ. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat Rev Cardiol 2020;17:242–257. [DOI] [PubMed] [Google Scholar]
- 53. Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KKL, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, Pocock SJ, Kuntz RE. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med 1998;339:1665–1671. [DOI] [PubMed] [Google Scholar]
- 54. Sharma M, Hart RG, Connolly SJ, Bosch J, Shestakovska O, Ng KKH, Catanese L, Keltai K, Aboyans V, Alings M, Ha J-W, Varigos J, Tonkin A, O’Donnell M, Bhatt DL, Fox K, Maggioni A, Berkowitz SD, Bruns NC, Yusuf S, Eikelboom JW. Stroke outcomes in the COMPASS trial. Circulation 2019;139:1134–1145. [DOI] [PubMed] [Google Scholar]
- 55. Darmon A, Bhatt DL, Elbez Y, Aboyans V, Anand S, Bosch J, Branch KR, Connolly SJ, Dyal L, Eikelboom JW, Fox KAA, Keltai K, Probstfield J, Yusuf S, Abtan J, Sorbets E, Eagle KA, Ducrocq G, Steg PG. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur Heart J 2018;39:750–757a. [DOI] [PubMed] [Google Scholar]
- 56. Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025–1034. [DOI] [PubMed] [Google Scholar]
- 57. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L; for the DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315:1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Capodanno D, Angiolillo DJ. Tailoring duration of DAPT with risk scores. Lancet 2017;389:987–989. [DOI] [PubMed] [Google Scholar]
- 59. Bonaca MP, Storey RF, Bhatt DL, Steg PG, Cohen M, Im KP, Johanson P, Braunwald EP, Sabatine MS. Abstract 16658: patient selection for long-term secondary prevention with ticagrelor: insights from PEGASUS-TIMI 54. Circulation 2018;138:A16658. [DOI] [PubMed] [Google Scholar]
- 60. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, Farb A, Gibson CM, Gregson J, Haude M, James SK, Kim HS, Kimura T, Konishi A, Laschinger J, Leon MB, Magee PFA, Mitsutake Y, Mylotte D, Pocock S, Price MJ, Rao SV, Spitzer E, Stockbridge N, Valgimigli M, Varenne O, Windhoevel U, Yeh RW, Krucoff MW, Morice MC. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J 2019;40:2632–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Räber L, Kelbæk H, Taniwaki M, Ostojic M, Heg D, Baumbach A, von Birgelen C, Roffi M, Tüller D, Engstrøm T, Moschovitis A, Pedrazzini G, Wenaweser P, Kornowski R, Weber K, Lüscher TF, Matter CM, Meier B, Jüni P, Windecker S. Biolimus-eluting stents with biodegradable polymer versus bare-metal stents in acute myocardial infarction: two-year clinical results of the COMFORTABLE AMI trial. Circ Cardiovasc Interv 2014;7:355–364. [DOI] [PubMed] [Google Scholar]
- 62. Costa F, van Klaveren D, Colombo A, Feres F, Raber L, Pilgrim T, Hong MK, Kim HS, Windecker S, Steyerberg EW, Valgimigli M. A 4-item PRECISE-DAPT score for dual antiplatelet therapy duration decision-making. Am Heart J 2020;223:44–47. [DOI] [PubMed] [Google Scholar]
- 63. Witberg G, Zusman O, Yahav D, Perl L, Vaknin-Assa H, Kornowski R. Meta-analysis of studies examining the external validity of the dual antiplatelet therapy score. Eur Heart J Cardiovasc Pharmacother 2020;6:285–291. [DOI] [PubMed] [Google Scholar]
- 64. Ueda P, Jernberg T, James S, Alfredsson J, Erlinge D, Omerovic E, Persson J, Ravn-Fischer A, Tornvall P, Svennblad B, Varenhorst C. External validation of the DAPT score in a nationwide population. J Am Coll Cardiol 2018;72:1069–1078. [DOI] [PubMed] [Google Scholar]
- 65. Yeh RW, Kereiakes DJ, Secemsky EA, Steg PG, Mauri L. The DAPT score in Sweden: successful validation, flawed interpretation. J Am Coll Cardiol 2019;73:113–114. [DOI] [PubMed] [Google Scholar]
- 66. Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 2010;304:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, Freynhofer MK, ten Berg J, Janssen P, Angiolillo DJ, Siller-Matula JM, Marcucci R, Patti G, Mangiacapra F, Valgimigli M, Morel O, Palmerini T, Price MJ, Cuisset T, Kastrati A, Stone GW, Sibbing D. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015;36:1762–1771. [DOI] [PubMed] [Google Scholar]
- 68. Winter M‐P, Schneeweiss T, Cremer R, Biesinger B, Hengstenberg C, Prüller F, Wallner M, Kolesnik E, Lewinski D, Lang IM, Siller‐Matula JM. Platelet reactivity patterns in patients treated with dual antiplatelet therapy. Eur J Clin Invest 2019;49:e13102–e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ, Sibbing D, So DYF, Trenk D, Alexopoulos D, Gurbel PA, Hochholzer W, De Luca L, Bonello L, Aradi D, Cuisset T, Tantry US, Wang TY, Valgimigli M, Waksman R, Mehran R, Montalescot G, Franchi F, Price MJ. International expert consensus on switching platelet P2Y(12) receptor-inhibiting therapies. Circulation 2017;136:1955–1975. [DOI] [PubMed] [Google Scholar]
- 70. Franchi F, Rollini F, Rivas J, Rivas A, Agarwal M, Briceno M, Wali M, Nawaz A, Silva G, Shaikh Z, Maailiki N, Been L, Pineda AM, Suryadevara S, Soffer D, Zenni MM, Bass TA, Angiolillo DJ. Prasugrel versus ticagrelor in patients with CYP2C19 loss-of-function genotypes: results of a randomized pharmacodynamic study in a feasibility investigation of rapid genetic testing. JACC Basic Transl Sci 2020;5:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, Gurbel P, Jeong YH, Mehran R, Moliterno DJ, Neumann FJ, Pereira NL, Price MJ, Sabatine MS, So DYF, Stone GW, Storey RF, Tantry U, Trenk D, Valgimigli M, Waksman R, Angiolillo DJ. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
- 72. Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, Delarche N, Bellemain-Appaix A, Range G, El Mahmoud R, Carrié D, Belle L, Souteyrand G, Aubry P, Sabouret P, Du Fretay XH, Beygui F, Bonnet JL, Lattuca B, Pouillot C, Varenne O, Boueri Z, Van Belle E, Henry P, Motreff P, Elhadad S, Salem JE, Abtan J, Rousseau H, Collet JP, Vicaut E, Montalescot G. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016;388:2015–2022. [DOI] [PubMed] [Google Scholar]
- 73. Notarangelo FM, Maglietta G, Bevilacqua P, Cereda M, Merlini PA, Villani GQ, Moruzzi P, Patrizi G, Malagoli Tagliazucchi G, Crocamo A, Guidorossi A, Pigazzani F, Nicosia E, Paoli G, Bianchessi M, Comelli MA, Caminiti C, Ardissino D. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLO trial. J Am Coll Cardiol 2018;71:1869–1877. [DOI] [PubMed] [Google Scholar]
- 74. Xie X, Ma YT, Yang YN, Li XM, Zheng YY, Ma X, Fu ZY, Ba B, Li Y, Yu ZX, Chen Y, Chen BD, Liu F, Huang Y, Liu C, Baituola G. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: a randomized control trial. Int J Cardiol 2013;168:3736–3740. [DOI] [PubMed] [Google Scholar]
- 75. Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, Bell M, Bae J-H, Jeong MH, Chavez I, Gordon P, Abbott JD, Cagin C, Baudhuin L, Fu Y-P, Goodman SG, Hasan A, Iturriaga E, Lerman A, Sidhu M, Tanguay J-F, Wang L, Weinshilboum R, Welsh R, Rosenberg Y, Bailey K, Rihal C. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA 2020;324:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Angiolillo DJ, Capodanno D, Danchin N, Simon T, Bergmeijer TO, ten Berg JM, Sibbing D, Price MJ. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: the ABCD-GENE score. JACC Cardiovasc Interv 2020;13:606–617. [DOI] [PubMed] [Google Scholar]
- 77. Chan Pin Yin D, Vos GA, van der Sangen NMR, Walhout R, Tjon Joe Gin RM, Nicastia DM, Langerveld J, Claassens DMF, Gimbel ME, Azzahhafi J, Bor WL, Oirbans T, Dekker J, Vlachojannis GJ, van Bommel RJ, Appelman Y, Henriques JPS, Kikkert WJ, Ten Berg JM. Rationale and design of the Future Optimal Research and Care Evaluation in Patients with Acute Coronary Syndrome (FORCE-ACS) Registry: towards "personalized medicine" in daily clinical practice. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, Costa RA, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Gilard M, Morice MC, Sawaya F, Sardella G, Genereux P, Redfors B, Leon MB, Bhatt DL, Stone GW, Colombo A. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol 2016;68:1851–1864. [DOI] [PubMed] [Google Scholar]
- 79. Yeh RW, Kereiakes DJ, Steg PG, Cutlip DE, Croce KJ, Massaro JM, Mauri L. Lesion complexity and outcomes of extended dual antiplatelet therapy after percutaneous coronary intervention. J Am Coll Cardiol 2017;70:2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
- 81. Costa F, Van Klaveren D, Feres F, James S, Räber L, Pilgrim T, Hong M-K, Kim H-S, Colombo A, Steg PG, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M. Dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J Am Coll Cardiol 2019;73:741–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.