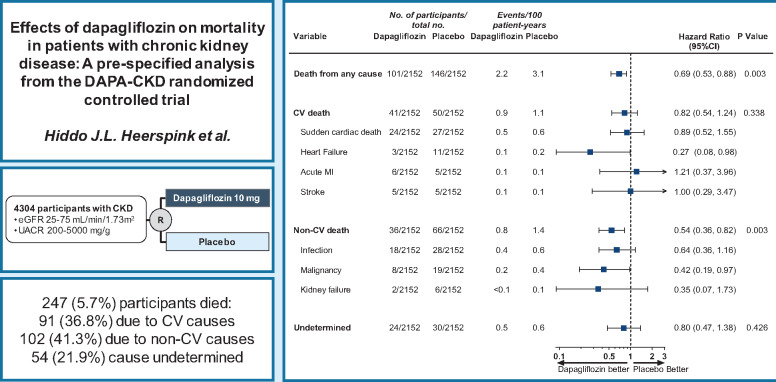
Abstract
Aims
Mortality rates from chronic kidney disease (CKD) have increased in the last decade. In this pre-specified analysis of the DAPA-CKD trial, we determined the effects of dapagliflozin on cardiovascular and non-cardiovascular causes of death.
Methods and results
DAPA-CKD was an international, randomized, placebo-controlled trial with a median of 2.4 years of follow-up. Eligible participants were adult patients with CKD, defined as a urinary albumin-to-creatinine ratio (UACR) 200–5000 mg/g and an estimated glomerular filtration rate (eGFR) 25–75 mL/min/1.73 m2. All-cause mortality was a key secondary endpoint. Cardiovascular and non-cardiovascular death was adjudicated by an independent clinical events committee. The DAPA-CKD trial randomized participants to dapagliflozin 10 mg/day (n = 2152) or placebo (n = 2152). The mean age was 62 years, 33% were women, the mean eGFR was 43.1 mL/min/1.73 m2, and the median UACR was 949 mg/g. During follow-up, 247 (5.7%) patients died, of whom 91 (36.8%) died due to cardiovascular causes, 102 (41.3%) due to non-cardiovascular causes, and in 54 (21.9%) patients, the cause of death was undetermined. The relative risk reduction for all-cause mortality with dapagliflozin (31%, hazard ratio [HR] [95% confidence interval (CI)] 0.69 [0.53, 0.88]; P = 0.003) was consistent across pre-specified subgroups. The effect on all-cause mortality was driven largely by a 46% relative risk reduction of non-cardiovascular death (HR [95% CI] 0.54 [0.36, 0.82]). Deaths due to infections and malignancies were the most frequently occurring causes of non-cardiovascular deaths and were reduced with dapagliflozin vs. placebo.
Conclusion
In patients with CKD, dapagliflozin prolonged survival irrespective of baseline patient characteristics. The benefits were driven largely by reductions in non-cardiovascular death.
Keywords: Dapagliflozin, SGLT2 inhibitor, Chronic kidney disease
Graphical Abstract
Dapagliflozin prolonged survival irrespective of baseline patient characteristics, largely driven by reductions in non-CV death.
See page 1228 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab092)
Introduction
The prevalence of chronic kidney disease (CKD) has increased in the last decade. Global estimates indicate that ∼700 million people are affected by CKD. According to the 2017 Global Burden of Disease study, the number of deaths that could be attributed to CKD increased by 41.5% from 1990 to 2017.1 While death due to cardiovascular diseases is an important contributor to all-cause mortality in patients with CKD, several studies have shown that other causes of death including infections and malignancies frequently occur in patients with CKD.2–4
Sodium-glucose co-transporter 2 (SGLT2) inhibitors reduce the risk of heart failure and delay progression to kidney failure in patients with Type 2 diabetes, both at the early and more advanced stages of CKD.5–7 These benefits appear independent of the improvements in glycemic control and are likely mediated by other mechanisms including favourable effects on glomerular hemodynamics.8,9 These findings have led to the hypothesis that SGLT2 inhibitors may also preserve kidney function in patients with CKD without Type 2 diabetes. The DAPA-CKD trial therefore enrolled patients with CKD with and without Type 2 diabetes and demonstrated that dapagliflozin significantly reduced the risk of kidney events, hospitalizations for heart failure or cardiovascular death, and prolonged survival irrespective of Type 2 diabetes status.10,11 In this pre-specified analysis from the DAPA-CKD trial, we investigated the causes of death in DAPA-CKD participants and assessed the effects of dapagliflozin on cardiovascular and non-cardiovascular causes of death.
Methods
Trial design and study participants
DAPA-CKD was a multicentre, double-blind, placebo-controlled, randomized trial conducted at 386 study sites in 21 countries. The trial was designed to assess the effects of dapagliflozin on kidney and cardiovascular outcomes in patients with CKD, with or without Type 2 diabetes. DAPA-CKD was registered with ClinicalTrials.gov as NCT03036150. The trial was approved by the ethics committees at each participating centre. All participants provided written informed consent before commencement of any study-specific procedure. An independent Data Monitoring Committee provided trial oversight. The study protocol, including a detailed description of the trial design, statistical analysis plan, and patient eligibility criteria, has been published previously.10,11
Eligible participants had CKD defined as estimated glomerular filtration rate (eGFR) between 25 and 75 mL/min/1.73 m2 and urinary albumin-to-creatinine ratio (UACR) between 200 and 5000 mg/g (22.6–565.6 mg/mmol). All participants were required to be receiving a stable dose of an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin II receptor blocker (ARB) for at least 4 weeks before trial enrolment, unless contraindicated. The main exclusion criteria included diagnosis of Type 1 diabetes, polycystic kidney disease, lupus nephritis, or anti-neutrophil cytoplasmic antibody-associated vasculitis. Participants receiving immunotherapy for primary or secondary kidney disease within the 6 months prior to enrolment were also excluded.
Participants were randomized in a 1:1 ratio to dapagliflozin 10 mg/day or matched placebo and followed for a median of 2.4 years (25th–75th percentile, 2.0–2.7 years). Study personnel (except the Independent Data Monitoring Committee) and participants were blinded to treatment allocation. In-person study visits occurred approximately every 4 months during follow-up to collect information about study endpoints, adverse events, and concomitant treatments, record vital signs, and take blood and urine samples for clinical chemistry assessments. The trial was stopped early for overwhelming efficacy based on a recommendation of the Independent Data Monitoring Committee following a regular review meeting.11
Outcomes
The primary composite endpoint was the time to first occurrence of a sustained decline in eGFR of at least 50%, onset of end-stage kidney disease, or death from kidney or cardiovascular causes. Kidney death was defined as death due to end-stage kidney disease where dialysis treatment was deliberately withheld (dialysis was not started or discontinued) for any reason. Secondary outcomes were the time to: a kidney specific composite outcome, which included the same components as the primary outcome except cardiovascular death; a composite cardiovascular endpoint defined as hospitalization for heart failure or cardiovascular death; and death from any cause (all-cause mortality).
The outcome of this pre-specified analysis was mortality. All deaths were adjudicated by an independent clinical events committee using rigorous outcome definitions. All mortality outcomes were sub-classified as cardiovascular, non-cardiovascular, or undetermined primary cause of death. Categories and definitions of cardiovascular death and non-cardiovascular death are reported in the Supplementary material online, Appendix. Undetermined cause of death refers to a death not attributable to a cardiovascular or non-cardiovascular cause due to the lack of information or insufficient supporting information to assign the cause of death. For the purpose of the primary efficacy assessment, undetermined causes of death were classified as cardiovascular death.11
Statistical analysis
We pre-specified an analysis of the effects of dapagliflozin on all-cause mortality and causes of death (Supplementary material online, Appendix). The efficacy analyses included all randomized participants and were conducted according to the intention-to-treat principle. Baseline characteristics were summarized using means (standard deviations), medians (25th–75th percentile range), or proportions.
We employed Cox proportional hazards regression models, stratified by the factors used at randomization (Type 2 diabetes and UACR), and adjusted for baseline eGFR. We graphically displayed the time to death in patients with and without Type 2 diabetes using the Kaplan–Meier product limit estimate. We calculated annualized incidence rates and expressed them as the number of events per 100 patient-years of follow-up. We calculated the absolute risk reductions by subtracting the annualized incidence rate in the dapagliflozin group from the placebo group. We performed the subgroup analyses to assess the consistency of the effect of dapagliflozin vs. placebo in pre-specified subgroups. We determined P-values for interaction by adding interaction terms between the subgroup and randomized treatment to the relevant Cox model. We conducted all statistical analyses with SAS version 9.4 (SAS Institute, Cary, NC, USA) or R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
The DAPA-CKD trial randomized 4304 participants with CKD. The mean age of participants was 62 years, 33% were women, the mean eGFR was 43.1 mL/min/1.73 m2, and the median UACR was 949 mg/g. Baseline characteristics of participants assigned to dapagliflozin and placebo were similar (Table 1).
Table 1.
Baseline characteristics
| Characteristic | Dapagliflozin (N = 2152) | Placebo (N = 2152) | Total (N = 4304) |
|---|---|---|---|
|
Age (years), mean (SD) ≤65 years, n (%) >65 years, n (%) |
61.8 (12.1) 1247 (57.9) 905 (42.1) |
61.9 (12.1) 1239 (57.6) 913 (42.4) |
61.8 (12.1) 2486 (57.8) 1818 (42.2) |
| Female sex, n (%) | 709 (32.9) | 716 (33.3) | 1425 (33.1) |
|
Region, n (%) Europe North America Latin America Asia |
610 (28.3) 401 (18.6) 449 (20.9) 692 (32.2) |
623 (28.9) 412 (19.1) 463 (21.5) 654 (30.4) |
1233 (28.6) 813 (18.9) 912 (21.2) 1346 (31.3) |
| Weight (kg), mean (SD) | 81.5 (20.1) | 82.0 (20.9) | 81.7 (20.5) |
| Current smoker, n (%) | 283 (13.2) | 301 (14.0) | 584 (13.6) |
|
Blood pressure (mmHg), mean (SD) Systolic Diastolic |
136.7 (17.5) 77.5 (10.7) |
137.4 (17.3) 77.5 (10.3) |
137.1 (17.4) 77.5 (10.5) |
| HbA1c (%), mean (SD) | 7.1 (1.7) | 7.0 (1.7) | 7.1 (1.7) |
| eGFR (mL/min/1.73 m2), mean (SD) | 43.2 (12.3) | 43.0 (12.4) | 43.1 (12.4) |
| Urinary albumin-to-creatinine ratio (mg/g), median (Q1–Q3) | 965 (472–1903) | 934 (482–1868) | 949 (477–1885) |
| Type 2 diabetes diagnosis, n (%) | 1455 (67.6) | 1451 (67.4) | 2906 (67.5) |
|
CKD aetiologya, n (%) Diabetic nephropathy Ischaemic/hypertensive nephropathy Chronic glomerulonephritis Other/unknown |
1271 (59.1) 324 (15.1) 343 (15.9) 214 (9.9) |
1239 (57.6) 363 (16.9) 352 (16.4) 198 (9.2) |
2510 (58.3) 687 (16.0) 695 (16.1) 412 (9.6) |
| History of cardiovascular disease, n (%) | 813 (37.8) | 797 (37.0) | 1610 (37.4) |
| History of heart failure, n (%) | 235 (10.9) | 233 (10.8) | 468 (10.9) |
| History of myocardial infarction, n (%) | 185 (8.6) | 207 (9.6) | 392 (9.1) |
| History of stroke, n (%) | 125 (5.8) | 140 (6.5) | 265 (6.2) |
|
Baseline medication, n (%) ACE inhibitor ARB Diuretic Statin |
673 (31.3) 1444 (67.1) 928 (43.1) 1395 (64.8) |
681 (31.6) 1426 (66.3) 954 (44.3) 1399 (65.0) |
1354 (31.5) 2870 (66.7) 1882 (43.7) 2794 (64.9) |
Most likely aetiology as reported by investigators.
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; SD, standard deviation.
Causes of death in participants with and without Type 2 diabetes
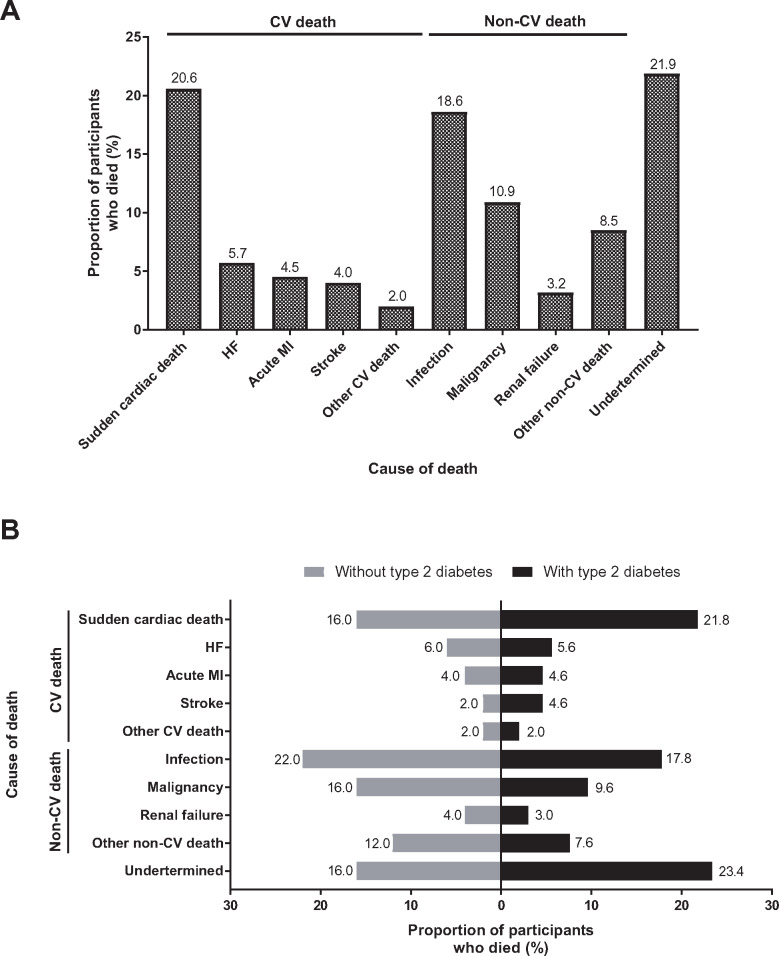
During follow-up, 247 (5.7%) participants died, of whom 91 (36.8%) died due to cardiovascular causes, and 102 (41.3%) due to non-cardiovascular causes, while in 54 (21.9%) patients the cause of death was undetermined (Figure 1A). Among participants with Type 2 diabetes 197 died; these deaths were attributed to a cardiovascular cause for 76 (38.6% of all deaths) participants, a non-cardiovascular cause for 75 (38.1%) participants and undetermined for 46 (23.4%) participants (Figure 1B). Overall, 50 patients without Type 2 diabetes died during the trial; of these, 15 (30.0%) died due to a cardiovascular cause, 27 (54.0%) due to a non-cardiovascular cause, and the cause of death for 8 (16.0%) participants remained undetermined (Figure 1B). The two most frequently occurring types of non-cardiovascular deaths in participants with and without Type 2 diabetes were deaths due to infections and deaths due to malignancies (Figure 1B). A summary of all causes of death is reported in Supplementary material online, Table S1.
Figure 1.
Cause of death in (A) the overall population and (B) patients with or without Type 2 diabetes. CV, cardiovascular; HF, heart failure; MI, myocardial infarction.
Causes of death with or without dialysis
During follow-up, dialysis was initiated in 167 participants, of whom 37 participants died (event rate 11.4 participants per 100 patient-years), 12 in the dapagliflozin group and 25 in the placebo group (Table 2). Deaths were recorded in 210 participants (2.2 participants per 100 patient-years) who did not require dialysis: 89 in the dapagliflozin group and 121 in the placebo group (Table 2). The non-cardiovascular death event rate was higher than the cardiovascular death rate in participants who received dialysis; the distribution of event rates (non-cardiovascular vs. cardiovascular) was similar in participants who did not require dialysis during follow-up (Table 2).
Table 2.
Causes of death in participants who did and did not reach chronic dialysis
| Dapagliflozin |
Placebo |
Total |
||||
|---|---|---|---|---|---|---|
| n (%) | Event rate (100 patient-years) | n (%) | Event rate (100 patient-years) | n (%) | Event rate (100 patient-years) | |
| Overall mortality | 101/2152 (4.7) | 2.2 | 146/2152 (6.8) | 3.1 | 247/4304 (5.7) | 2.6 |
| Without chronic dialysis, n | 2084 | 2053 | 4137 | |||
| All-cause mortality | 89 (4.3) | 1.9 | 121 (5.9) | 2.6 | 210 (5.1) | 2.2 |
| Cardiovascular death | 35 (1.7) | 0.7 | 44 (2.1) | 0.9 | 79 (1.9) | 0.8 |
| Non-cardiovascular death | 31 (1.5) | 0.7 | 48 (2.3) | 1.0 | 79 (1.9) | 0.8 |
| Undetermined cause of death | 23 (1.1) | 0.5 | 29 (1.4) | 0.6 | 52 (1.3) | 0.5 |
| With chronic dialysis, n | 68 | 99 | 167 | |||
| All-cause mortality | 12 (17.6) | 8.6 | 25 (25.3) | 13.4 | 37 (22.2) | 11.4 |
| Cardiovascular death | 6 (8.8) | 3.9 | 6 (6.1) | 2.6 | 12 (7.2) | 3.1 |
| Non-cardiovascular death | 5 (7.4) | 3.2 | 18 (18.2) | 9.0 | 23 (13.8) | 6.5 |
| Undetermined cause of death | 1 (1.5) | 0.6 | 1 (1.0) | 0.4 | 2 (1.2) | 0.5 |
Effect of dapagliflozin on mortality
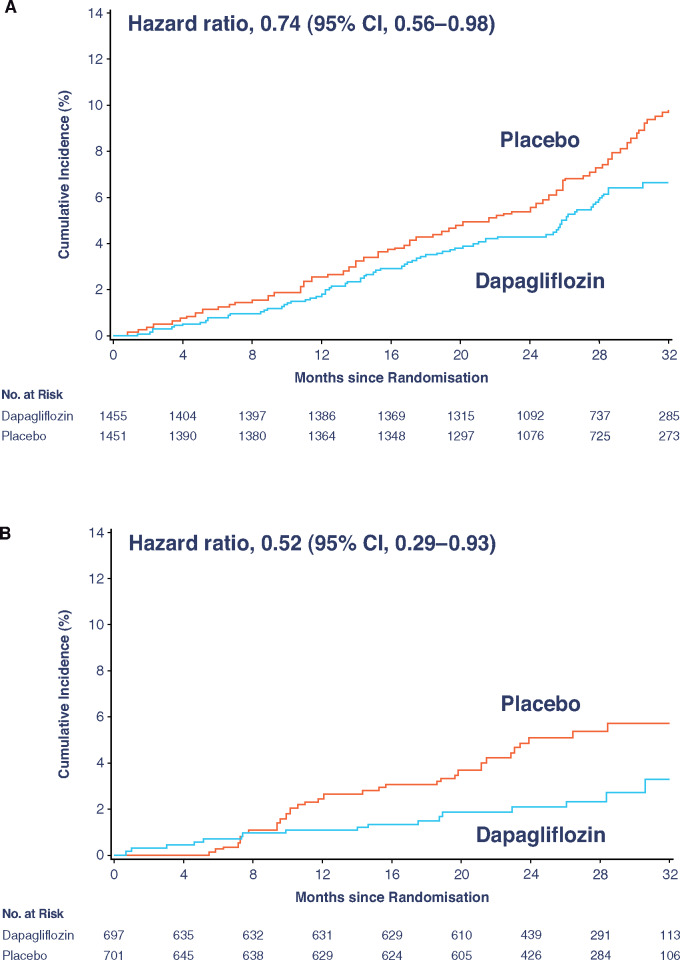
As previously reported, 101 (4.7%) participants died from any cause in the dapagliflozin group and 146 (6.8%) in the placebo group (hazard ratio [HR] 0.69; 95% confidence interval [CI] 0.53, 0.88; P = 0.003). In participants with Type 2 diabetes, the mortality rate was lower in the dapagliflozin group (2.6 events per 100 patient-years) compared with the placebo group (3.5 events per 100 patient-years; HR [95% CI] 0.74 [0.56, 0.98]; Figure 2A). Among participants without diabetes, mortality rates were lower in the dapagliflozin group (1.2 events per 100 patient-years) compared with the placebo group (2.3 events per 100 patient-years; HR [95% CI] 0.52 [0.29, 0.93]; Figure 2B). The interaction P-value for participants with diabetes vs. without diabetes was 0.25.
Figure 2.
Kaplan–Meier curve for all-cause mortality in participants (A) with Type 2 diabetes and (B) without Type 2 diabetes. CI, confidence interval.
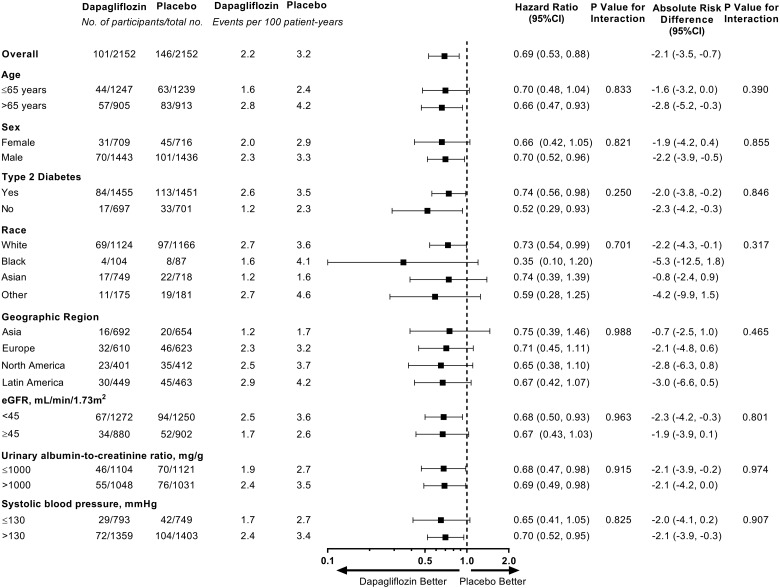
Mortality rates in the placebo arm were higher in older participants, in participants with Type 2 diabetes, and in those with a lower eGFR, a higher UACR, and higher systolic blood pressure (Figure 3). The effect of dapagliflozin in reducing the relative and absolute risks for all-cause mortality was consistent across all pre-specified subgroups (Figure 3; all P-values for interaction >0.25).
Figure 3.
All-cause mortality outcome by pre-specified subgroups at baseline. CI, confidence interval; eGFR, estimated glomerular filtration rate.
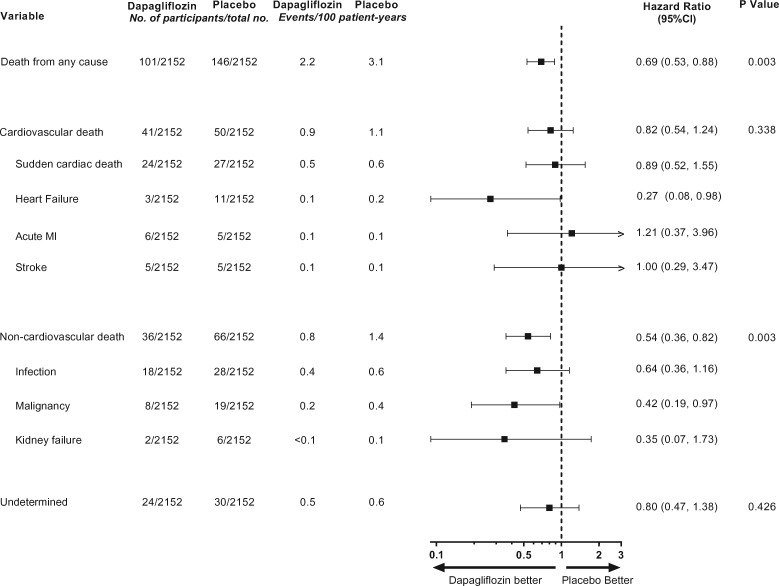
Cardiovascular death occurred in 41 (1.9%) participants in the dapagliflozin group and 50 (2.3%) participants in the placebo group (HR [95% CI] 0.82 [0.54, 1.24]; P = 0.338) (Graphical abstract). Sudden cardiac death was the most frequently reported cause of cardiovascular death, which occurred in 24 (1.1%) participants in the dapagliflozin group and 27 (1.3%) participants in the placebo group (HR [95% CI] 0.89 [0.52, 1.55]; Figure 4). Death due to heart failure was the second most frequently reported type of cardiovascular death and occurred in 3 (0.1%) participants in the dapagliflozin group and 11 (0.5%) participants in the placebo group (HR [95% CI] 0.27 [0.08, 0.98]; Figure 4).
Figure 4.
Effect of dapagliflozin on cardiovascular, non-cardiovascular and undetermined causes of deaths. Main causes of cardiovascular and non-cardiovascular death are shown. Event numbers for other causes of cardiovascular or non-cardiovascular deaths were below 5 and are not reported. CI, confidence interval; MI, myocardial infarction.
Death due to non-cardiovascular causes occurred in 36 (1.7%) participants in the dapagliflozin group and 66 (3.1%) participants in the placebo group (HR [95% CI] 0.54 [0.36, 0.82]; P = 0.003). Death due to infections was the most frequently reported cause of non-cardiovascular death and occurred in 18 (0.8%) participants in the dapagliflozin group and 28 (1.3%) participants in the placebo group (HR [95% CI] 0.64 [0.36, 1.16]; Figure 4). Death due to malignancies was reported in 27 patients in the overall population: 8 (0.4%) in the dapagliflozin group and 19 (0.9%) in the placebo group (HR [95% CI] 0.42 [0.19, 0.97]; Figure 4).
The cause of death was undetermined in 24 (1.1%) participants in the dapagliflozin group and 30 (1.4%) participants in the placebo group (HR [95% CI] 0.80 [0.47, 1.38]; P = 0.426; Figure 4).
Mortality after infections and malignancies
During follow-up, serious adverse events of infections were reported in 193 (9.0%) participants in the dapagliflozin group and 207 (9.6%) participants in the placebo group (difference in incidence rate, 0.4%; P = 0.49). In a post hoc analysis, we found that among these patients, 15 (7.8%) in the dapagliflozin group and 31 (15.0%) in the placebo group died (HR [95% CI] 0.53 [0.29, 0.99]). Serious adverse events of malignancies were reported in 59 (2.7%) participants in the dapagliflozin group and 71 (3.3%) participants in the placebo group (difference in incidence rate, 0.2; P = 0.29). In a post hoc analysis of patients with a reported serious adverse event of malignancy, 9 (15.3%) from the dapagliflozin group and 17 (23.9%) from the placebo group died (HR [95% CI] 0.69 [0.30, 1.56]).
Discussion
The DAPA-CKD trial showed that dapagliflozin significantly prolonged survival in patients with CKD with and without Type 2 diabetes. In this pre-specified analysis, we showed that survival benefits were consistent across a range of patient subgroups and were mainly driven by reductions in non-cardiovascular causes of death which accounted for 41% of all deaths. Deaths due to infections and malignancies were the most frequently occurring causes of non-cardiovascular death and each of these occurred less frequently in participants treated with dapagliflozin compared with placebo. Cardiovascular death accounted for 37% of all deaths with sudden cardiac death and deaths due to heart failure being the most frequently occurring causes of death in this category. The numerically lower event rate of cardiovascular death with dapagliflozin vs. placebo seemed to be mainly driven by fewer deaths due to heart failure. The effect of dapagliflozin on undetermined causes of deaths was similar to cardiovascular deaths.
Prior observational studies describing the causes of death in patients with CKD reported that the highest proportion of death was from cardiovascular death.2,3 These studies also highlighted the strong association between lower eGFR and higher albuminuria with cardiovascular death. Most observational studies use ICD coding or death certificates to classify causes of death. The quality of ascertaining the causes of death using these methods has been shown to be suboptimal.12,13 In contrast, causes of death were adjudicated by an independent clinical events committee in DAPA-CKD. Event adjudication has been employed in other large randomized controlled trials and should reduce the chance of misclassifications and bias. An analysis from the randomized controlled Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT) reported that in patients with Type 2 diabetes and CKD, >50% of all deaths were attributed to cardiovascular cause, of which heart failure and sudden cardiac death were the most frequently reported causes.14 In the same trial, as in DAPA-CKD, non-cardiovascular causes of deaths were mainly driven by deaths due to infections and malignancies.14 In contrast, in patients with CKD without diabetes, the Study of Heart And Renal Protection (SHARP) trial reported that non-vascular death occurred nearly twice as often as vascular deaths, with deaths due to malignancies, pulmonary causes and renal causes accounting for the majority of deaths.15 Thus, in patients with CKD with and without Type 2 diabetes recruited in the DAPA-CKD trial, the main causes of death being cardiovascular death and deaths due to infections and malignancies are in keeping with previous findings from other randomized controlled trials.
The reduction in all-cause mortality with dapagliflozin in our trial was achieved on top of guideline-recommended standard of care including optimal blood pressure management with ACEi or ARBs, which have not been shown to prolong survival in patients with CKD.16 It is possible that the observed effects of dapagliflozin on non-cardiovascular death may be related to its benefits on cardiovascular and kidney protection, with preserved cardiorenal function providing better resilience under strain from severe illness and intercurrent events, and thereby leading to improved survival. Furthermore, dapagliflozin reduced the risks of end-stage kidney disease, a condition which is known to be associated with higher cardiovascular and non-cardiovascular mortality rates.
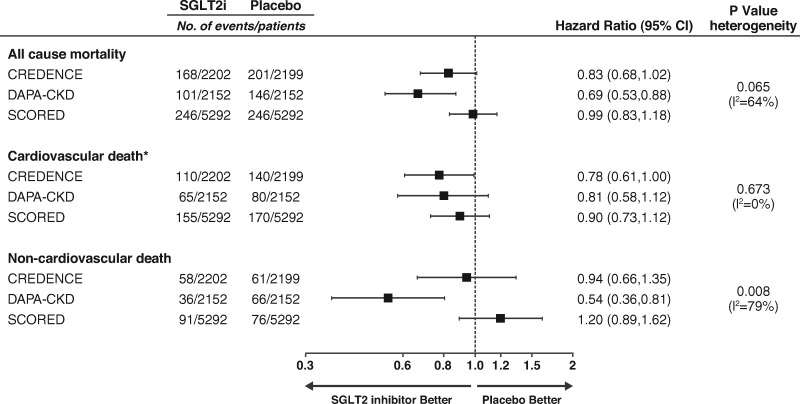
The effect size of dapagliflozin on cardiovascular death in the DAPA-CKD trial was generally similar to that observed in two other clinical trials of SGLT2 inhibitors in patients with Type 2 diabetes and CKD (CREDENCE and SCORED).7,17 Neither of these trials were designed and powered to assess effects of SGLT2 inhibition on cardiovascular death, and the relative risk reductions were not statistically significant in either trial. However, when meta-analysed, SGLT2 inhibition reduces the risk of cardiovascular death by 16% (HR [95% CI] 0.84 [0.73, 0.97]) with no evidence of heterogeneity (P = 0.67; I2 for heterogeneity 0.0%; Figure 5). This finding suggests that SGLT2 inhibitors prevent cardiovascular death in patients with CKD. Although there was no effect of canagliflozin or sotagliflozin on non-cardiovascular deaths, in the CREDENCE trial, canagliflozin also reduced the incidence of death due to infections, which is consistent with our findings.18 Reasons for contrasting effects on non-cardiovascular death across the three trials are unknown but may relate to differences in patient population since we included patients without diabetes, who are more likely to die of non-cardiovascular causes. Furthermore, we enrolled patients with lower eGFR compared to CREDENCE (25–75 vs. 30–90 mL/min/1.73 m2).7 SCORED exclusively enrolled patients with cardiovascular risk factors who are more likely to die of cardiovascular causes.17 A history of cardiovascular disease was not an inclusion criterion in DAPA-CKD. Indeed, while cardiovascular death event rates were comparable between DAPA-CKD and CREDENCE, the cardiovascular death rate was higher in SCORED while the non-cardiovascular death rate was somewhat higher in DAPA-CKD.19 There were also differences in the design of the trials, such as the possibility to continue study medication when dialysis was initiated in DAPA-CKD.
Figure 5.
Effect of sodium-glucose co-transporter 2 inhibitors on mortality in three clinical trials in patients with chronic kidney disease. Cardiovascular death was a component of the primary outcome in all trials. Because of loss of funding in the SCORED trial (sotagliflozin) not all endpoints were adjudicated and only investigator-reported endpoints were published. Data extracted from the CREDENCE (canagliflozin) and SCORED publications.7,17,19 Relative risk ratios are presented for SCORED since hazard ratios could not be extracted. *In all trials undetermined causes of death were assumed to be of cardiovascular cause and were combined with cardiovascular causes of death. Quantitative assessment of effect on all-cause mortality and each cause of death was made by random-effects meta-analysis and calculating P-value for heterogeneity of the individual trial results. Summary statistics across trials are not provided because of heterogeneity for all-cause mortality and non-cardiovascular death.
Focusing on the effect of dapagliflozin on death due to infections, dapagliflozin did not significantly reduce the actual risk of serious infections since the number of serious adverse events due to infections was similar between the dapagliflozin and placebo group. However, a post hoc analysis suggested that mortality was reduced with dapagliflozin among patients with severe infections, supporting the notion that dapagliflozin may provide organ protection and reduce the risk of complications and disease progression due to infections. These findings should be interpreted with caution due to the low number of events but are supported by experimental data showing beneficial effects of SGLT2 inhibition in animal models of infection. In a sepsis model, empagliflozin suppressed systemic inflammation and reduced mortality.20 Another study demonstrated that dapagliflozin reduced pulmonary infections with Pseudomonas aeruginosa by improving airway glucose homeostasis.21 Furthermore, in patients with Type 2 diabetes without CKD or established cardiovascular disease, SGLT2 inhibition appears to have a favourable effect on a number of detrimental processes that are triggered in a setting of severe infections. SGLT2 inhibitors decrease glucose and insulin levels and shift energy metabolism to an increased reliance on lipid oxidation, with a reduced reliance on glucose, and inhibition of glycolysis; this may lead to lipolysis, reduce reactive oxygen species and oxidative stress, and ultimately result in cellular and organ protection.22 What role such mechanisms would play in a patient with insulin resistance and potential catabolism induced by acute illness is currently unknown. Overall, these data highlight a potential role of SGLT2 inhibitors in reducing deaths due to infections through organ protection, a concept being further explored in ongoing clinical trials such as the DARE-19 (NCT04350593), which is evaluating the potential role of dapagliflozin in reducing mortality and kidney, cardiovascular, and pulmonary complications in patients hospitalized with COVID-19.
In DAPA-CKD, there were also fewer deaths due to malignancy in participants randomized to dapagliflozin compared to placebo. This finding should also be interpreted cautiously since relatively few patients died due to malignancies, and we did not observe a clear pattern in malignancy-related deaths. Nevertheless, increased expression and functional activity of SGLT2 transporters have been described in various tumours including prostate and lung adenocarcinomas, renal and hepatocellular carcinomas, and cervical and breast cancer cells.23–26 In addition, SGLT2 inhibition with dapagliflozin and canagliflozin reduced tumour growth and prolonged survival in a mouse model and patient-derived xenografts of lung adenocarcinomas.24 Dapagliflozin also inhibited cell growth in renal and hepatocellular carcinoma cell lines by inducing cell cycle arrest and enhancing apoptosis,23 while canagliflozin inhibited the progression of non-alcoholic steatohepatitis to hepatocarcinogenesis partly due to induction of apoptosis.27
This analysis has limitations. First, the DAPA-CKD study was terminated early for overwhelming efficacy based on a recommendation of the Independent Data Monitoring Committee; this has limited the precision of effect estimates for some of the secondary and exploratory endpoints including all-cause mortality. Second, while all mortality endpoints were adjudicated by the independent event adjudication committee, the cause of death could not be determined in 54 (1.3%) participants. However, this proportion is similar to that in other clinical trials with SGLT2 inhibitors or other agents. The comparison of cardiovascular and non-cardiovascular deaths in patients with or without dialysis may be biased and should be carefully interpreted as patients with cardiovascular instability due to underlying cardiovascular disease might not be expected to start dialysis. The duration that patients were followed while they were receiving dialysis was short and, therefore, comparisons of death rates with other studies that recruited patients with prevalent dialysis are likely to be inaccurate. Finally, we analysed data from a clinical trial that enrolled patients with CKD who were selected based on the inclusion and exclusion criteria and other factors that determined the trial participation, which may limit the generalizability of the results.
In conclusion, in this pre-specified analysis of the DAPA-CKD trial, we demonstrated that dapagliflozin consistently prolonged survival regardless of various patient characteristics. The benefits of dapagliflozin on overall mortality were driven largely by reductions in non-cardiovascular death, in particular deaths due to infections and malignancies.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors thank all investigators, trial teams, and patients for their participation in the trial. The authors would also like to acknowledge Parita Sheth and Nicola Truss, inScience Communications, London, UK, for assistance in editing and the preparation of figures, funded by AstraZeneca.
Funding
This work was supported by AstraZeneca.
Author contributions
H.J.L.H. was involved in the study design, conduct of the study, data analysis, and interpretation of the data, wrote the first draft of the manuscript, and participated in critical revision of all drafts of the manuscript. D.C.W., G.M.C., J.J.V.M., F.F.H., R.C-R., P.R., and R.D.T. are members of the study’s executive committee and were involved in the study design, data collection, and analysis or interpretation of the data. N.J. performed the data analyses. A.M.L., C.D.S., and B.V.S. were involved in the study design, conduct of the study, and interpretation of data. R.K. was involved in the conduct of the study and interpretation of data. M.K. was involved in the interpretation of the data. All authors reviewed the manuscript drafts, provided approval of the final version for submission, and took responsibility for the accuracy and integrity of the data.
Data sharing
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Conflict of interest: H.J.L.H. has received support from AstraZeneca to his institution for the DAPA-CKD trial; fees to his institution for his participation in advisory boards for Merck, Mitsubishi Tanabe, Janssen, and Mundipharma; as a consultant for AbbVie, Retrophin, Boehringer Ingelheim, and Novo Nordisk; for participation in steering committees for Janssen, Gilead, Bayer, Chinook, and CSL Pharma; and research support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen. N.J. has no conflicts of interest to declare. G.M.C. has received fees from AstraZeneca for the DAPA-CKD trial steering committee and research grants from NIDDK and Amgen; he is on the board of directors for Satellite Healthcare, has received fees for advisory boards for Ardelyx, Baxter, Cloud Cath, Cricket, Dia Medica, Durect, DxNow, Outset, and Reata, holds stock options for Ardelyx, CloudCath, Durect, DxNow, and Outset, has received fees from Akebia, Gilead, Sanifit, and Vertex for trial steering committees, and has received fees for DSMB service from Angion, Bayer, and ReCor. F.F.H. has received honoraria from AstraZeneca as a member of the executive member of the DAPA-CKD study and received honoraria from AbbVie for participation in a steering committee. J.J.V.M. has received support to his institution, Glasgow University, for work on clinical trials, consulting, and other activities: Abbvie, Alnylam, Amgen, AstraZeneca, Bayer, BMS, Cardurion, Cyclerion, Cytokinetics, DalCor, GSK, Kidney Research UK, Merck, Novartis, Pfizer, Servier, Theracos, and Vifor Fresenius. He has received personal lecture fees: Abbott, Hickman, Sun Pharmaceuticals, and Servier. R.C-R. has received fees from AstraZeneca for the DAPA-CKD trial steering committee; speaker fees from Boehringer Ingelheim, Amgen, and Janssen; research support from GlaxoSmithKline and Novo Nordisk; and honoraria for advisory boards from Boehringer Ingelheim, Novo Nordisk, and Medtronic. M.K. has served as consultant for Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novo Nordisk, Sanofi, and Vifor Pharma. He received research grants from AstraZeneca and Boehringer Ingelheim. P.R. has received fees to his institution for research support from AstraZeneca and Novo Nordisk; for steering group participation from AstraZeneca, Gilead, Novo Nordisk, and Bayer; for lectures from Bayer, Eli Lilly, and Novo Nordisk; and for advisory boards from Sanofi and Boehringer Ingelheim. R.D.T. has received support from AstraZeneca as a member of the executive committee for DAPA-CKD; is a consultant for Boehringer Ingelheim; has participated on advisory boards for Bayer and Relypsa; and served on data monitoring committees for Akebia and Reata Pharmaceuticals, executive committee for Amgen, and as a faculty associate for Quest Diagnostics. R.K., B.V.S., C.D.S., and A.M.L. are employees and stockholders of AstraZeneca. D.C.W. provides ongoing consultancy services to AstraZeneca and has received honoraria and/or consultancy fees from Amgen, Astellas, Boehringer Ingelheim, Bayer, GlaxoSmithKline, Janssen, Napp, Mundipharma, Merck Sharp and Dohme, Reata, Tricida, and Vifor Fresenius.
Contributor Information
for the DAPA-CKD Trial Committees and Investigators:
Hiddo J L Heerspink, David C Wheeler, Glenn Chertow, Ricardo Correa-Rotter, Tom Greene, Fan Fan Hou, John McMurray, Peter Rossing, Robert Toto, Bergur Stefansson, Anna Maria Langkilde., L E Maffei, P Raffaele, S E Solis, C A Arias, D Aizenberg, C Luquez, C Zaidman, N Cluigt, M Mayer, A Alvarisqueta, A Wassermann, R Maldonado, J Bittar, M Maurich, L E Gaite, N Garcia, L Sivak, P O Ramallo, J C Santos, R Garcia Duran, J A Oddino, A Maranon, L N Maia, D D Avila, E J G Barros, M H Vidotti, D Panarotto, I D L Noronha, L A A Turatti, L Deboni, M E Canziani, M C Riella, M R Bacci, R P Paschoalin, R J Franco, J C Goldani, E St-Amour, A W Steele, R Goldenberg, S Pandeya, H Bajaj, D Cherney, S M Kaiser, J R Conway, S S Chow, G Bailey, J Lafrance, J Winterstein, S Cournoyer, D Gaudet, F Madore, R L Houlden, A Dowell, M Langlois, N Muirhead, H Khandwala, A Levin, F Hou, Y Xue, L Zuo, C Hao, Z Ni, C Xing, N Chen, Y Dong, R Zhou, X Xiao, Y Zou, C Wang, B Liu, Q Chen, M Lin, Q Luo, D Zhang, J Wang, M Chen, X Wang, A Zhong, J Dong, C Zhu, T Yan, P Luo, Y Ren, P Pai, D Li, R Zhang, J Zhang, M Xu, Y Zhuang, Y Kong, X Yao, X Peng, F I Persson, T K Hansen, R Borg, U Pedersen Bjergaard, D Hansen, M Hornum, H Haller, G Klausmann, D Tschope, T Kruger, P Gross, C Hugo, N Obermuller, L Rose, P Mertens, H Zeller-Stefan, A Fritsche, L Renders, J Muller, K Budde, B Schroppel, I Wittmann, P Voros, M Dudas, G A Tabak, R Kirschner, A Letoha, I Balku, Z Hermanyi, G Zakar, I Mezei, G G Nagy, J Lippai, A Nemeth, D Khullar, P K Gowdaiah, E Fernando Mervin, V A Rao, D Dewan, V S K Maddi, M S Vyawahare, R K Pulichikkat, S K Sonkar, V K Gupta, S Agarwal, A J Asirvatham, A Ignatius, S Chaubey, S Melemadathil, H Alva, Y Kadam, H Shimizu, A Sueyoshi, H Takeoka, Y Abe, T Imai, Y Onishi, Y Fujita, Y Tokita, Y Makita, A Idogaki, R Koyama, H Kikuchi, N Kashihara, T Hayashi, Y Ando, T Tanaka, M Shimizu, S Hidaka, T Gohda, K Tamura, M Abe, Y Kamijo, T Imasawa, Y Takahashi, M Nakayama, M Tomita, F Hirano, M Nakayama, Y Fukushima, A Kiyosue, S Kurioka, E Imai, K Kitagawa, M Waki, J Wada, K Uehara, H Iwatani, K Ota, S Shibazaki, K Tamura, K Katayama, I Narita, M Iinuma, S Matsueda, S Sasaki, A Yokochi, T Tsukamoto, T Yoshimura, S Kang, S Lee, C S Lim, H Chin, K W Joo, S Y Han, T I Chang, S Park, H Park, C W Park, B G Han, D R Cha, S A Yoon, W Kim, S W Kim, D Ryu, R Correa Rotter, S S Irizar Santana, G Hernandez Llamas, R Valdez Ortiz, N C Secchi Nicolas, G Gonzalez Galvez, J R Lazcano Soto, T Bochicchio Riccardelli, E A Bayram Llamas, D R Ramos Ibarra, M G S Melo, J G Gonzalez Gonzalez, J H Sanchez Mijangos, M Madero Robalo, A Garcia Castillo, H A Manrique, J C Farfan, R Vargas, A Valdivia, A Dextre, E Escudero, J R Calderon Ticona, L Gonzales, J Villena, L Leon, G Molina, A Saavedra, E Garrido, H Arbanil, S Vargas Marquez, J Rodriguez, R Isidto, A J Villaflor, M A Gumba, L Tirador, R S Comia, R A Sy, M L V V Guanzon, G Aquitania, N C De Asis, A A Silva, M E Lim, R A Danguilan, M Nowicki, H Rudzki, K Landa, I Kucharczyk-Bauman, B Gogola-Migdal, M Golski, A Olech-Cudzik, T Stompor, T Szczepanik, B Miklaszewicz, R Sciborski, M Kuzniewski, K Ciechanowski, D Wronska, W Klatko, S Mazur, G Popenda, M Myslicki, L Z Bolieva, S Berns, A Galyavich, T Abissova, I Karpova, D Platonov, N Koziolova, L Kvitkova, R Nilk, T Medina, A Rebrov, M Rossovskaya, I Sinitsina, E Vishneva, N Zagidullin, T Novikova, N Krasnopeeva, O Magnitskaya, N Antropenko, M Batiushin, V Escudero Quesada, C Barrios Barrea, E Espinel Garauz, J M Cruzado Garrit, C Morales Portillo, J L Gorriz Teruel, S Cigarran Guldris, M Praga Terente, N R Robles Perez-Monteoliva, H Infanta Cristina, F J Tinahones Madueno, A Soto Gonzalez, C Diaz Rodriguez, H Furuland, A Saeed, K Dreja, J Spaak, A Bruchfeld, M Kolesnyk, O Levchenko, N Pyvovarova, V Stus, V Doretskyy, N Korobova, O Horoshko, I Katerenchuk, Y M Mostovoy, M Orynchak, O Legun, I Dudar, O Bilchenko, S Andreychyn, A Levchenko, L Zub, N Tereshchenko, I Topchii, T Ostapenko, S Bezuglova, M Kopytsya, O Turenko, P Mark, J Barratt, S Bhandari, D Fraser, P Kalra, S P Kon, K Mccafferty, A Mikhail, S P Kon, O P Alvarado, R Anderson, N S Andrawis, A Arif, S A Benjamin, G Bueso, R S Busch, K W Carr, Kenneth W Carr, P Crawford, N Daboul, G M De La Calle, B Delgado, J Earl, M A El-Shahawy, R J Graf, G Greenwood, A Guevara, E M Wendland, R K Mayfield, M Montero, D J Morin, P Narayan, V Numrungroad, A C Reddy, R Reddy, M B Samson, R Trejo, M B Butcher, J K Wise, L R Zemel, M Raikhel, D Weinstein, P Hernandez, A Wynne, B V Khan, G A Sterba, A Jamal, D Ross, S F Rovner, A Tan, F Ovalle, R J Patel, J Talano, D R Patel, A Burgner, N Aslam, M Elliott, S Goral, A Jovanovich, K Umanath, D Waguespack, D Weiner, M Yu, L Schneider, T Le, T D, N Nguyen, H Nguyen, D Nguyen, V Nguyen, T Do, P Chu, D Ta, N Tran, D Nguyen, Marc A Pfeffer, Stuart Pocock, Karl Swedberg, Jean L Rouleau, Nishi Chaturvedi, Peter Ivanovich, Andrew S Levey, Claes Held, Christina Christersson, Johannes Mann, and Christoph Varenhorst
References
- 1.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M; Alberta Kidney Disease Network. Cause of death in patients with reduced kidney function. J Am Soc Nephrol 2015;26:2504–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mok Y, Matsushita K, Sang Y, Ballew SH, Grams M, Shin SY, Jee SH, Coresh J.. Association of kidney disease measures with cause-specific mortality: the Korean heart study. PLoS One 2016;11:e0153429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang HE, Gamboa C, Warnock DG, Muntner P.. Chronic kidney disease and risk of death from infection. Am J Nephrol 2011;34:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I.. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606–617. [DOI] [PubMed] [Google Scholar]
- 6. Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, Hantel S, Woerle H-J, Broedl UC, von Eynatten M, Groop PH; EMPA-REG OUTCOME Investigators. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol 2018;29:2755–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 8. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI.. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 2018;94:26–39. [DOI] [PubMed] [Google Scholar]
- 9. Heerspink HJL, Desai M, Jardine M, Balis D, Meininger G, Perkovic V.. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017;28:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou F-F, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC; t; DAPA-CKD Investigators. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020;35:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjostrom CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 12. Falci L, Lee Argov EJ, Van Wye G, Plitt M, Soto A, Huynh M.. Examination of cause-of-death data quality among New York city deaths due to cancer, pneumonia, or diabetes from 2010 to 2014. Am J Epidemiol 2018;187:144–152. [DOI] [PubMed] [Google Scholar]
- 13. Smith Sehdev AE, Hutchins GM.. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med 2001;161:277–284. [DOI] [PubMed] [Google Scholar]
- 14. Charytan DM, Lewis EF, Desai AS, Weinrauch LA, Ivanovich P, Toto RD, Claggett B, Liu J, Hartley LH, Finn P, Singh AK, Levey AS, Pfeffer MA, McMurray JJ, Solomon SD.. Cause of death in patients with diabetic CKD enrolled in the trial to reduce cardiovascular events with aranesp therapy (TREAT). Am J Kidney Dis 2015;66:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, Investigators S.. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, Wiebe N, Ruospo M, Wheeler DC, Strippoli GF.. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015;385:2047–2056. [DOI] [PubMed] [Google Scholar]
- 17. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129–139. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Center for Drug Evaluation and Research: CREDENCE Integrated Review; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/204042Orig1s032IntegratedR.pdf. Date accessed 4 February 2021.
- 19. Yu J, Zhou Z, Mahaffey KW, Matthews DR, Neuen BL, Heerspink HJL, Jardine MJ, Li J, Perkovic V, Neal B, Arnott C.. An exploration of the heterogeneity in effects of SGLT2 inhibition on cardiovascular and all-cause mortality in the EMPA-REG OUTCOME, CANVAS Program, DECLARE-TIMI 58, and CREDENCE trials. Int J Cardiol 2021;324:165–172. [DOI] [PubMed] [Google Scholar]
- 20. Maayah ZH, Ferdaoussi M, Takahara S, Soni S, Dyck JRB.. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology 2020. doi:10.1007/s10787-020-00732-4. [DOI] [PubMed] [Google Scholar]
- 21. Astrand A, Wingren C, Benjamin A, Tregoning JS, Garnett JP, Groves H, Gill S, Orogo-Wenn M, Lundqvist AJ, Walters D, Smith DM, Taylor JD, Baker EH, Baines DL.. Dapagliflozin-lowered blood glucose reduces respiratory Pseudomonas aeruginosa infection in diabetic mice. Br J Pharmacol 2017;174:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daniele G, Xiong J, Solis-Herrera C, Merovci A, Eldor R, Tripathy D, DeFronzo RA, Norton L, Abdul-Ghani M.. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care 2016;39:2036–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuang H, Liao L, Chen H, Kang Q, Shu X, Wang Y.. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit 2017;23:3737–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scafoglio CR, Villegas B, Abdelhady G, Bailey ST, Liu J, Shirali AS, Wallace WD, Magyar CE, Grogan TR, Elashoff D, Walser T, Yanagawa J, Aberle DR, Barrio JR, Dubinett SM, Shackelford DB.. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Transl Med 2018;10:eaat5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K, Furukawa M, Kawaratani H, Kitade M, Moriya K, Namisaki T, Yoshiji H.. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer 2018;142:1712–1722. [DOI] [PubMed] [Google Scholar]
- 26. Komatsu S, Nomiyama T, Numata T, Kawanami T, Hamaguchi Y, Iwaya C, Horikawa T, Fujimura-Tanaka Y, Hamanoue N, Motonaga R, Tanabe M, Inoue R, Yanase T, Kawanami D.. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J 2020;67:99–106. [DOI] [PubMed] [Google Scholar]
- 27. Jojima T, Wakamatsu S, Kase M, Iijima T, Maejima Y, Shimomura K, Kogai T, Tomaru T, Usui I, Aso Y.. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci 2019;20:5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.