Abstract
Background
Obesity has been linked to severe clinical outcomes among people who are hospitalized with coronavirus disease 2019 (COVID-19). We tested the hypothesis that visceral adipose tissue (VAT) is associated with severe outcomes in patients hospitalized with COVID-19, independent of body mass index (BMI).
Methods
We analyzed data from the Massachusetts General Hospital COVID-19 Data Registry, which included patients admitted with polymerase chain reaction–confirmed severe acute respiratory syndrome coronavirus 2 infection from March 11 to May 4, 2020. We used a validated, fully automated artificial intelligence (AI) algorithm to quantify VAT from computed tomography (CT) scans during or before the hospital admission. VAT quantification took an average of 2 ± 0.5 seconds per patient. We dichotomized VAT as high and low at a threshold of ≥100 cm2 and used Kaplan-Meier curves and Cox proportional hazards regression to assess the relationship between VAT and death or intubation over 28 days, adjusting for age, sex, race, BMI, and diabetes status.
Results
A total of 378 participants had CT imaging. Kaplan-Meier curves showed that participants with high VAT had a greater risk of the outcome compared with those with low VAT (P < .005), especially in those with BMI <30 kg/m2 (P < .005). In multivariable models, the adjusted hazard ratio (aHR) for high vs low VAT was unchanged (aHR, 1.97; 95% CI, 1.24–3.09), whereas BMI was no longer significant (aHR for obese vs normal BMI, 1.14; 95% CI, 0.71–1.82).
Conclusions
High VAT is associated with a greater risk of severe disease or death in COVID-19 and can offer more precise information to risk-stratify individuals beyond BMI. AI offers a promising approach to routinely ascertain VAT and improve clinical risk prediction in COVID-19.
Keywords: artificial intelligence, COVID-19 disease, visceral adiposity
People with obesity who become infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have a greater risk of severe clinical outcomes [1–3]. In the United States, over 160 million Americans are overweight or obese and over 500 000 individuals have died of coronavirus disease 2019 (COVID-19) since the start of the pandemic. However, clinical outcomes due to this infection are not uniformly worse among those who have obesity, and the mechanisms that link body habitus and clinical outcomes in people with COVID-19 remain poorly understood.
While body mass index is a convenient measure to obtain in clinical practice, it is widely recognized as a remarkably heterogeneous parameter for assessing metabolic health [4]. As such, people with similar body mass index (BMI) measurements have shown meaningfully different levels of health risk, in part due to the fact that BMI is not a reliable measure of total body or central abdominal fat mass and does not well capture wide variation in visceral adipose tissue (VAT) distribution between individuals [4]. There is a growing body of evidence that VAT may be an important conduit for the health risk associated with obesity. Macrophages have been shown to infiltrate the hypertrophied adipocytes that are characteristic of excess VAT; this is believed to result in increased inflammatory cytokines including both tumor necrosis factor–α and interleukin-6 in this tissue [4]. Given this, VAT has been proposed as 1 factor that may help to elucidate the relationship between body weight and COVID-19 disease severity [5, 6]. While VAT measurement is usually manually assessed by a radiologist and thus time-consuming to perform, artificial intelligence (AI) algorithms offer a novel approach to measuring VAT quickly and accurately in patients who have recently had computed tomography (CT) imaging performed, regardless of indication [7]. Moreover, segmentation of the tissue compartments from a single cross-sectional slice at 1 lumbar vertebra can well approximate VAT and subcutaneous adipose tissue (SAT), both of which can be ascertained in seconds using AI algorithms [8, 9].
In this study, we tested the hypotheses that VAT is associated with severe outcomes in patients who are hospitalized with COVID-19 and is a stronger predictor of such outcomes than BMI in adjusted models including both indicators. We assessed VAT using a fully automated end-to-end AI algorithm that provides this measure from the CT scans of patients who were hospitalized at Massachusetts General Hospital (MGH) with COVID-19 disease during the first surge of the 2020 pandemic. We then used the VAT measure and survival analysis to test hypotheses linking high VAT and poor clinical outcomes over 28 days from hospitalization for COVID-19.
METHODS
Data Source
This study used data from the MGH COVID-19 Data Registry [10, 11]. The registry included all patients who presented to care, defined as the first contact with the health care system for evaluation of COVID-19 symptoms, and were subsequently hospitalized at MGH between March 11 and May 4, 2020. All participants in the registry had PCR-confirmed SARS-CoV-2 infection. The data in the registry were collected in 2 ways. First, a manual chart review was performed to assess key aspects of the patient’s medical history and details of the hospitalization including the main outcomes of interest at 28 days after presentation to care [11]. This manual chart review also identified comorbidities of interest in this study including history of coronary artery disease or myocardial infarction (CAD or MI), history of congestive heart failure (CHF), history of diabetes, history of renal disease, and history of chronic obstructive pulmonary disease (COPD). This chart review was undertaken by physicians, research nurses, and a team of research assistants trained in a standard operating procedure for data extraction. In addition, height, weight, and BMI, as well as key laboratory values that were measured and recorded during the index hospitalization, were obtained electronically through the Enterprise Data Warehouse (EDW), a repository that was derived from the Epic electronic medical records system. There were no missing height or weight values in this sample. Imaging data of CT exams performed during or before the hospitalization for any indication that were used to ascertain the VAT measures were also obtained from hospital picture archive and communication system (PACS). This research was approved by the Massachusetts General Brigham Institutional Review Board protocol 2020P000829.
BMI was calculated as the weight in kilograms divided by the square of height in meters. BMI categories were defined using standard thresholds of <18.5 kg/m2 for underweight, 18.5–24.9 kg/m2 for normal weight, 25.0–29.9 kg/m2 for overweight, and ≥30.0 kg/m2 for obese. Diabetes was defined by meeting at least 1 of the following criteria: (1) medical history of diabetes documented in the medical record and manually retrieved on chart review, (2) HbA1c ≥6.5% during the index hospitalization, or (3) random blood glucose ≥200 mg/dL at admission to the hospital and supportive history by chart review. For those cases in which only the third diagnostic criterion was met, a detailed chart review was performed by 2 board-certified endocrinologists; this procedure has been described in detail previously. Of note, registry participants with active malignancy were excluded from this study. Demographic and clinical characteristics were defined as previously described [10, 11].
Ascertaining VAT and SAT Using an AI-Based Body Composition Detector
Body composition measures such as VAT can be ascertained from a single axial CT or magnetic resonance imaging (MRI) slice [8]. We used a previously validated, fully automated AI algorithm to quantify VAT from CT scans [9]. In brief, this application is written in Tensorflow 1.13 and uses an end-to-end 2-stage artificial neural network that first localizes a single axial slice at the L1 vertebral body level and then quantifies VAT in that specific slice in cm2. We chose the L1 vertebral body as it is routinely included in both CT scans of the chest and CT exams of the abdomen/pelvis and has an excellent correlation with overall VAT in prior studies (0.986) [8, 12]. Moreover, the validation of the AI algorithm itself showed excellent agreement with manual measurement by a radiologist. We used this AI algorithm to measure VAT in all patients with either a CT scan of the chest or a CT scan of the abdomen/pelvis that had been performed during the index hospitalization within a median (interquartile range) of 17 (4–25) months before the index hospitalization date. If both exam types were available, we chose the one that was temporally closest to the index hospitalization.
Exposures, Outcomes, and Statistical Analysis
Our primary exposure of interest was cross-sectional VAT area, which we dichotomized as high and low at a threshold of ≥100 cm2 based on prior literature demonstrating a meaningful increased risk of metabolic derangements above this threshold [13–15]. The primary outcome of interest in the study was need for intubation or death within 28 days after presentation to care. We first compared the demographic and health characteristics of those patients in the registry who had a relevant clinical imaging study to those for whom no applicable imaging study was conducted during the period of interest to assess for selection bias. Next, we compared differences in the demographic and clinical characteristics of the analytic sample among those with high vs low visceral fat. Then, we depicted differences in time to death or intubation over 28 days among those with high vs low visceral fat using Kaplan-Meier curves and log-rank testing. We performed this analysis first in the full sample and then stratified for those who were in the normal or overweight BMI category and separately for those who were in the obese BMI category. We conducted a score test for proportional hazards assumptions. Then, we fit adjusted Cox proportional hazards models to estimate the hazard of 28-day death or intubation including VAT, adjusted for age, sex, race, and diabetes diagnosis in the models. We provide these models with and without adjustment for BMI. We also provided a separate model with adjustment for BMI but without VAT included. A P value <.05 in the BMI-adjusted test of the association of visceral fat with COVID-19 outcomes indicated statistical significance. In the supplementary analyses, we examined these same relationships in registry participants with and without imaging and separately conducted a stratified analysis among those who had imaging performed before vs during the hospitalization. Finally, in the Supplementary Data, we also provided an analysis in which VAT was categorized in quintiles and displayed the adjusted hazard ratio of VAT over a range of alternative thresholds with respect to the outcome of interest, as a further empirical assessment of the chosen threshold. Confidence intervals for the latter analysis were obtained via bootstrapping (1000×).
RESULTS
The MGH COVID-19 Data Registry included 866 individuals, among whom 410 (47.3%) had an abdominal or chest CT imaging study available during or before the hospitalization. Among these, 32 (7.8%) were excluded due to the presence of active malignancy, leaving a final sample of 378 registry participants for this analysis. A total of 268 of 378 (70.9%) people had a CT of the abdomen and pelvis, while 110 (29.1%) people had a CT scan of the chest available. One hundred ninety-eight studies (52%) were performed during the hospitalization, and 180 studies (48%) were performed before the hospitalization. The total time to execute the analysis of an individual CT scan using the algorithm was 2 ± 0.5 seconds on a standard CPU desktop computer within our hospital system.
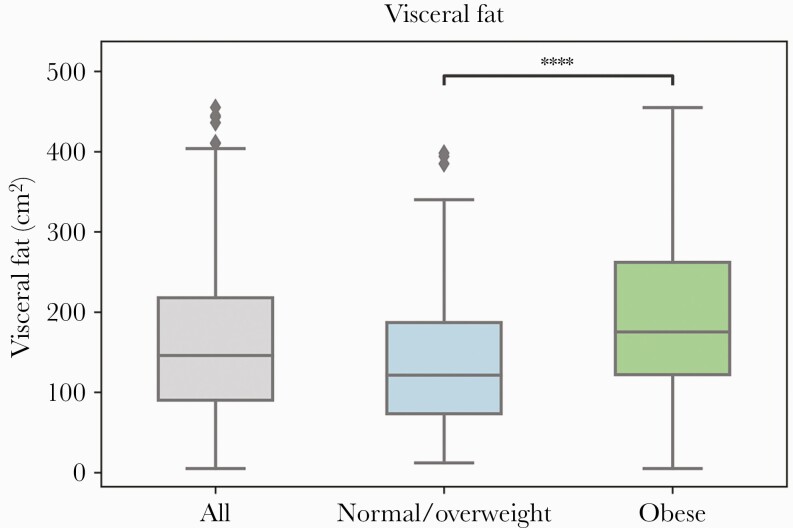
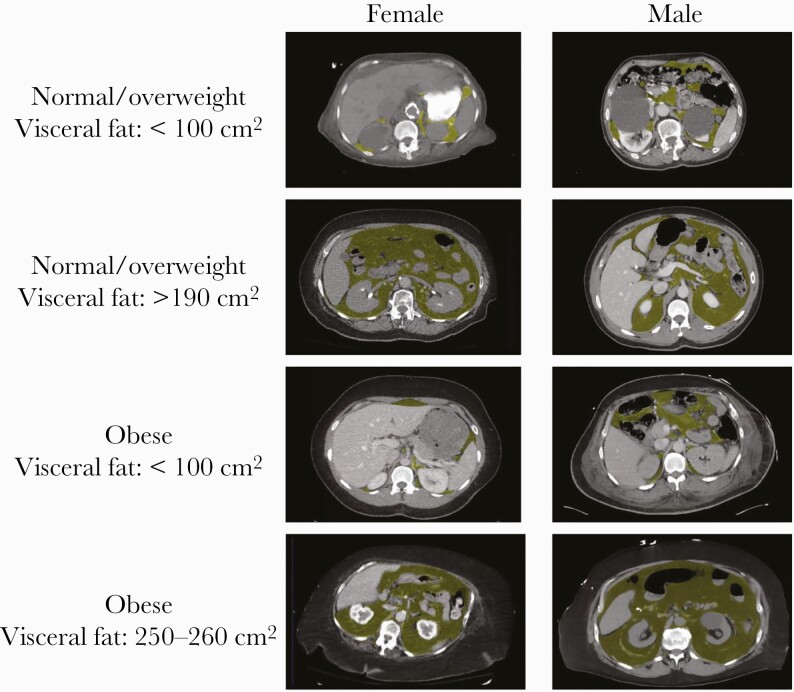
There were several differences in demographic and health characteristics of those participants for whom imaging data was available compared with those participants who did not have imaging data. Individuals with available imaging were older, more likely to be male, and had a higher number of comorbidities, including diabetes and a history of CAD or MI, but the distribution of BMI and other demographic and health characteristics did not differ between these groups (Supplementary Table 1). Participants who had a VAT ≥100 cm2 had higher rates of diabetes and renal disease and a higher C-reactive protein (CRP) on admission compared with those with a VAT ≤100 cm2. (Table 1) There were no significant differences in the rates of other key comorbidities, including CAD or MI and COPD, stratified by this VAT threshold. We found that the distribution of VAT differed significantly between those with a normal or overweight BMI compared with those with obesity (P < .0001) (Figure 1). Specifically, the median VAT was greater among those in the BMI group with obesity compared with those in the normal or overweight BMI group (Figure 1). Exemplary VAT on body composition imaging by BMI status and gender is shown in Figure 3. In Supplementary Figure 1, we also display the differences in the relationship between BMI and VAT by sex [16].
Table 1.
Demographic and Health Characteristics of 378 COVID-19 Registry Participants, Overall and by VAT Group
| Overall (n = 378) | VAT <100 cm2 (n = 115) | VAT ≥100 cm2 (n = 263) | P Value (Between 2 Subgroups) | |
|---|---|---|---|---|
| VAT, mean ± SD | 195 ± 107 | 63 ± 26 | 204 ± 80 | <.01 |
| Age, mean ± SD, y | 63.3 ± 17.8 | 62.2 ± 18.5 | 63.8 ± 16.0 | .41 |
| Male, % | 61.7 | 37.2 | 72.2 | <.01 |
| Race or ethnicity, % | .04 | |||
| White | 33.7 | 28.7 | 35.9 | |
| Hispanic | 24.1 | 21.7 | 25.2 | |
| Black | 9.0 | 15.7 | 9.0 | |
| Other | 33.2 | 33.9 | 29.9 | |
| BMI, % | <.01 | |||
| Normal | 23.7 | 44.4 | 15.6 | |
| Overweight | 33.2 | 32.2 | 33.2 | |
| Obese | 43.1 | 23.4 | 51.2 | |
| Diabetes | 43.0 | 33.9 | 47.0 | .02 |
| CAD or MI | 21.8 | 20.9 | 22.1 | .78 |
| COPD/asthma | 26.8 | 28.7 | 26.0 | .58 |
| CHF | 14.6 | 17.4 | 13.4 | .30 |
| Renal disease | 23.7 | 18.4 | 26.0 | .11 |
| ESR, mean ± SD | 40.9 ± 27.1 | 37.5 ± 24.3 | 42.4 ± 28.1 | .13 |
| CRP, mean ± SD | 89.1 ± 80.8 | 70.3 ± 70.8 | 97.5 ± 83.7 | <.01 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; MI, myocardial infarction; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MI, myocardial infarction; VAT, visceral adipose tissue.
Figure 1.
Visceral fat distribution, overall and by BMI category. ****P < .0001. Abbreviation: BMI, body mass index.
Figure 3.
Exemplary visceral fat body compositons by BMI status and gender. Abbreviation: BMI, body mass index.
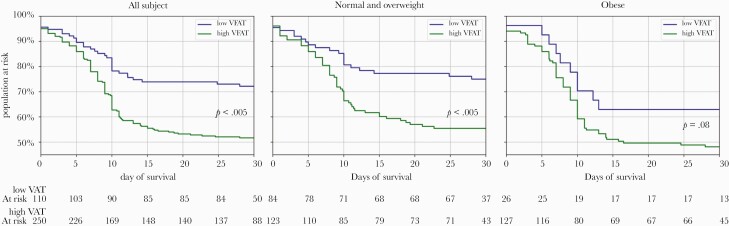
There were 114 (38%) intubations and 54 (18%) deaths among 249 people by 28 days in the high-VAT group, compared with 15 (19%) intubations and 7 (9%) deaths among 129 people by 28 days in the low-VAT group. Kaplan-Meier curves from the total study sample showed statistically significant differences in the risk of death or intubation over 28 days by VAT group (Figure 2). Those with high VAT had a greater risk of death or intubation over 28 days compared with those with low VAT (P < .001). When stratifying the analysis into 2 groups defined by BMI (normal or overweight compared with obese), this same relationship was preserved among those who were normal or overweight (P < .005). The differences were similar in magnitude but did not reach statistical significance in the group with obesity (P = .08). In Cox proportional hazards regression analyses, individuals with high VAT had an adjusted hazard ratio of 2.00 (95% CI, 1.32–3.02) of death or intubation at 28 days when adjusting for age, sex, race, and diabetes. Following additional adjustment for BMI, the adjusted hazard ratio for high VAT was unchanged at 1.97 (95% CI, 1.24–3.09) (Table 2). In a model with BMI but without VAT, the adjusted hazard ratio for obese vs normal BMI category was 1.57 (95% CI, 1.02–2.40); once VAT was included in the model, this declined to an adjusted hazard ratio of 1.14 (95% CI, 0.71–1.82). Our supplementary analyses revealed no clear dose–response effect in the relationship between quintile of VAT and death or intubation within 30 days (Supplementary Figure 2). Furthermore, a consideration of alternative dichotomous thresholds empirically reinforced the choice to use 100 cm2, as depicted in Supplementary Figure 3.
Figure 2.
Kaplan-Meier curves for intubation or death within 28 days. Abbreviation: VAT, visceral adipose tissue.
Table 2.
Multivariate Adjusted Hazard Ratio for Death or Intubation Within 28 Days From Hospitalization
| VAT Only | BMI + VAT | BMI Only | |
|---|---|---|---|
| aHR + 95% CI | aHR + 95% CI | aHR + 95% CI | |
| VAT ≥100 cm2 | 2.00 (1.32–3.02) | 1.97 (1.24–3.09) | — |
| Age, y | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) |
| Male | 1.21 (0.85–1.72) | 1.22 (0.85–1.76) | 1.51 (1.07–2.13) |
| Diabetes | 1.27 (0.93–1.74) | 1.20 (0.87–1.66) | 1.21 (0.88–1.67) |
| BMI | |||
| Normal | — | Reference | Reference |
| Overweight | — | 0.76 (0.47–1.21) | 0.95 (0.61–1.49) |
| Obese | — | 1.14 (0.71–1.82) | 1.57 (1.02–2.40) |
| Race | |||
| White | Reference | Reference | Reference |
| Hispanic | 1.05 (0.67–1.63) | 1.07 (0.69–1.68) | 1.09 (0.70–1.70) |
| Black | 1.88 (1.08–3.27) | 1.95 (1.11–3.40) | 1.67 (0.97–2.90) |
| Other | 1.05 (0.71–1.54) | 1.03 (0.70–1.52) | 1.01 (0.68–1.49) |
Abbreviations: aHR, adjusted hazard ratio; BMI, body mass index; VAT, visceral adipose tissue.
DISCUSSION
We found that patients hospitalized with COVID-19 who had high VAT (≥100 cm2) as ascertained by an AI algorithm from chest or abdominal CT scans had twice the risk of dying or being intubated within 28 days of admission than those with low VAT. This risk persisted after adjusting models for BMI, suggesting that VAT may have a stronger and more precise relationship with severe COVID-19. This finding reflects the hypothesized biological significance of VAT as a more precise measure of differences in adipose tissue distribution and the health risk associated with obesity than BMI. These data support the possible use of VAT to risk-stratify hospitalized individuals with COVID-19 for severe clinical outcomes. Moreover, the AI algorithm used could be used by clinical teams to ascertain this measure quickly and automatically from imaging studies performed for other indications.
These findings are important for several reasons. First, there is an ample body of literature regarding risk prediction for severe COVID-19 outcomes that has not regularly included VAT as a consideration, though it may offer a more precise approximation of the metabolic risk associated with obesity when compared with BMI [11, 17]. This study provides evidence that measurement of VAT in hospitalized patients could be used to improve COVID-19 risk prediction. Second, as has been suggested previously, VAT may serve as a distinct driver of poor outcomes in COVID-19. The underlying mechanism to explain this relationship is not clear but may include the angiotensin-converting enzyme 2 (ACE-2) receptor as a possible link. This receptor facilitates cellular entry of SARS-CoV-2 and has been shown to have high expression in VAT [18, 19]. Additionally, as detailed preciously, VAT is metabolically active and secretes a variety of adipokines and pro-inflammatory cytokines that are hypothesized to play a role in severe COVID-19 [4]. As such, VAT may serve as a pro-inflammatory reservoir that could contribute to increased severity of COVID-19 among individuals with high VAT [20].
One fundamental innovation of this study is the AI algorithm that was applied to ascertain VAT in a fully automated fashion and with a precise and well-validated 2-dimensional measure of this value. This is particularly unique, as many studies use a 1-dimensional “VAT thickness” that is measured manually by a radiologist and lacks validation in the body composition literature. This AI algorithm has been applied and validated in several independent data sets and can facilitate opportunistic collection of both VAT and SAT from routine clinical imaging studies. Given that many hospitalized patients with severe COVID-19 have a CT scan of the chest or abdomen performed as part of their clinical workup, it would be possible to adapt this technology and automate collection of this measure using this publicly available algorithm. If performed in this way, the role of VAT in driving outcomes could be better understood and used to enhance prediction of risk for severe outcomes in real time.
This finding is largely consistent with 3 smaller studies from China and Europe that have suggested that adipose tissue distribution may be associated with outcomes in COVID-19 disease. The first study to explore this relationship consisted of a single-center cohort of 143 patients with confirmed COVID-19 who were hospitalized in Wuhan, China, between January and March 2020. These individuals all had abdominal CT scans from which radiologists manually measured VAT and several other measures of adipose tissue distribution [15]. The rate of critical illness was almost double in people with higher VAT in this context, and in multivariate logistic regression models high VAT was associated with 2 times the odds of their severe disease end point. However, the sample represented a very small and select fraction of the total patients hospitalized with COVID-19 at this institution during this period, and models did not adjust for BMI. These findings were reinforced by a second study of 144 patients who were consecutively admitted to the emergency department (ED) of a public hospital in Bufalini, Cesena, Italy, between February and April of 2020. All of these patients were found to have PCR-confirmed SARS-CoV-2 infection [5]. Upper abdominal VAT was assessed on sagittal images from chest CTs in all study participants. The primary outcome of interest in this study was admission to the ICU. Those who were admitted to the ICU had a 30% higher VAT (P < .001) and a 30% lower SAT (P = .011), independent of age and sex. The latter findings were confirmed in similar studies in Rome, Italy, and a cohort of 30 patients in Berlin, Germany [20, 21].
Our study has several important limitations. First, the study utilized “opportunistic” imaging studies from people hospitalized with COVID-19 to estimate adipose distribution, and thus these parameters were only available in a subset of those hospitalized with COVID-19 during the study period. This design introduces important questions about how the inclusion of imaging may introduce additional selection bias in the sample of interest in this manuscript. As described, those individuals who had a CT scan available during or within 2 years before their hospitalization for COVID-19 were older, more likely to be male, and had a higher prevalence of several important comorbidities, namely diabetes, though the distribution of BMI was similar in the 2 groups. In a supplementary analysis, we explore the relationship between BMI and diabetes among those with and without imaging. In these stratified Cox proportional hazards models (Supplementary Table 2), we find that the relationships between diabetes and “obese” BMI and the outcome of interest were slightly attenuated in those with imaging compared with those for whom imaging was not available, but overall these relationships did not differ substantially. Given the lack of imaging in 1 group, differences in the relationship between VAT and the outcomes could not be explored in this secondary analysis. This selection of higher-risk patients into the study likely limited power to detect differences in outcomes according to comorbidities known to associate with COVID risk, including those we previously identified. Second, the timing of imaging collection was a second source of heterogeneity that could also introduce selection effects. In a supplemental analysis stratified by those with an imaging study and corresponding VAT measurement acquired during the index hospitalization and separately, those without a study and corresponding measurement that preceded the hospitalization, we found that in both groups VAT was associated with severe disease, though the magnitude of the effect was greater among those who had the imaging study performed before admission. This difference in magnitude may indicate potential unmeasured confounding, for instance related to the health condition that prompted the imaging study preceding the index hospitalization, but the relationship between VAT and the outcomes was preserved in both groups and the small sample in each of the 2 groups after stratification makes it difficult to determine with certainty the importance of this potential limitation. Future research with larger cohorts should further interrogate these differences.
Beyond the potential limitations associated with selection bias, as detailed above, it is important to also state that these data were derived from a single center and thus may not be widely generalizable to other populations of individuals with COVID-19. Moreover, while we standardized data collection as much as possible through training of those performing chart review, the assignment of comorbid diagnoses other than diabetes and high BMI may have been subject to some variability across chart reviewers. Finally, the utility of this parameter is inherently dependent on the availability of a recent imaging study from which VAT may be measured and thus may be less widely used in people who do not routinely undergo imaging at presentation with COVID-19, for instance younger people.
In conclusion, in this study we present robust evidence that VAT can be used to stratify patients hospitalized with COVID-19 regarding their risk of severe disease or death and may be more precise and closely linked to poor outcomes than BMI. We have done this in the largest cohort and first US-based study of this relationship to date. We utilize an AI algorithm for ascertainment of adipose tissue distribution that automates collection of these data from routine clinical imaging studies. This approach is promising because it is potentially scalable for use in real-world clinical settings and could improve prediction of poor outcomes among people who require hospitalization for COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank the COVID-19 Data Registry Team at MGH for their leadership in generating this resource, including the manual chart reviewers and experienced data managers, for their time and effort.
Financial support . Support for the MGH COVID-19 Data Registry was provided by the MGH Division of Clinical Research. J.S. was supported by Grant Number T32DK007028 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). V.A.T. is supported by National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), grant R01HL132786 and National Institute of Allergy and Infectious Diseases, NIH, grant R01AG062393. I.V.B. is supported by National Institute of Allergy and Infectious Diseases grant K24AI141036. A.S.F. is supported by National Institute of General Medical Sciences, NIH, grant R01GM127862. J.H. is supported by National Institute on Aging grant R01AG062282 and NIDDK grant R01DK085070. The contents of this research are solely the responsibility of the authors.
Potential conflicts of interest. D.J.W. reports serving on a data monitoring committee for Novo Nordisk. J.B.M. is an Academic Associate for Quest Diagnostics. J.H. has consulted for several health care systems. No other conflicts of interest relevant to this article were reported. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gao F, Zheng KI, Wang XB, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 2020; 43:e72–4. [DOI] [PubMed] [Google Scholar]
- 2. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020; 108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 2020; 43:1392–8. [DOI] [PubMed] [Google Scholar]
- 4. Neeland IJ, Ross R, Després JP, et al. ; International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity . Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019; 7:715–25. [DOI] [PubMed] [Google Scholar]
- 5. Battisti S, Pedone C, Napoli N, et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care 2020; 43:e129–30. [DOI] [PubMed] [Google Scholar]
- 6. Kuk JL, Katzmarzyk PT, Nichaman MZ, et al. Visceral fat is an independent predictor of all-cause mortality in men. Obesity 2006; 14:336–41. [DOI] [PubMed] [Google Scholar]
- 7. Choy G, Khalilzadeh O, Michalski M, et al. Current applications and future impact of machine learning in radiology. Radiology 2018; 288:318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schweitzer L, Geisler C, Pourhassan M, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr 2015; 102:58–65. [DOI] [PubMed] [Google Scholar]
- 9. Hsu TMH, Schawkat K, Berkowitz SJ, Wei JL, Makoyeva A, Legare K, DeCicco C, Paez SN, Wu JSH, Szolovits P, Kikinis R, Moser AJ, Goehler A. Artificial intelligence to assess body composition on routine abdominal CT scans and predict mortality in pancreatic cancer– a recipe for your local application. European Journal of Radiology. Forthcoming. 2021. [DOI] [PubMed]
- 10. Bassett IV, Triant VA, Bunda BA, et al. Massachusetts General Hospital Covid-19 registry reveals two distinct populations of hospitalized patients by race and ethnicity. PLoS One 2020; 15:e0244270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seiglie J, Platt J, Cromer SJ, et al. Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19. Diabetes Care 2020; 43:2938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schweitzer L, Geisler C, Pourhassan M, et al. Estimation of skeletal muscle mass and visceral adipose tissue volume by a single magnetic resonance imaging slice in healthy elderly adults. J Nutr 2016; 146:2143–8. [DOI] [PubMed] [Google Scholar]
- 13. Nicklas BJ, Penninx BW, Ryan AS, et al. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care 2003; 26:1413–20. [DOI] [PubMed] [Google Scholar]
- 14. Pickhardt PJ, Jee Y, O’Connor SD, del Rio AM. Visceral adiposity and hepatic steatosis at abdominal CT: association with the metabolic syndrome. AJR Am J Roentgenol 2012; 198:1100–7. [DOI] [PubMed] [Google Scholar]
- 15. Yang Y, Ding L, Zou X, et al. Visceral adiposity and high intramuscular fat deposition independently predict critical illness in patients with SARS-CoV-2. Obesity 2020; 28:2040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bredella MA. Sex differences in body composition. Adv Exp Med Biol 2017; 1043:9–27. [DOI] [PubMed] [Google Scholar]
- 17. Longmore DK, Miller JE, Bekkering S, Saner C, Mifsud E, Zhu Y, Saffery R, Nichol A, Colditz G, Short KR, Burgner DP; International BMI-COVID consortium; *International BMI-COVID consortium. Diabetes and Overweight/Obesity Are Independent, Nonadditive Risk Factors for In-Hospital Severity of COVID-19: An International, Multicenter Retrospective Meta-analysis. Diabetes Care. 2021 Apr 15:dc202676. doi: 10.2337/dc20-2676. Epub ahead of print. PMID: 33858854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Somers KR, Becari C, et al. Comparative expression of renin-angiotensin pathway proteins in visceral versus subcutaneous fat. Front Physiol 2018; 9:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes Med 2020; 19:100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petersen A, Bressem K, Albrecht J, et al. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 2020; 110:154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe M, Caruso D, Tuccinardi D, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020; 111:154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.