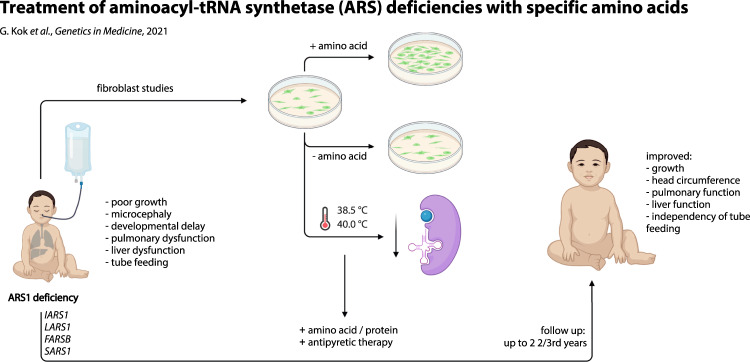
Abstract
Purpose
Recessive cytosolic aminoacyl-tRNA synthetase (ARS) deficiencies are severe multiorgan diseases, with limited treatment options. By loading transfer RNAs (tRNAs) with their cognate amino acids, ARS are essential for protein translation. However, it remains unknown why ARS deficiencies lead to specific symptoms, especially early life and during infections. We set out to increase pathophysiological insight and improve therapeutic possibilities.
Methods
In fibroblasts from patients with isoleucyl-RS (IARS), leucyl-RS (LARS), phenylalanyl-RS-beta-subunit (FARSB), and seryl-RS (SARS) deficiencies, we investigated aminoacylation activity, thermostability, and sensitivity to ARS-specific amino acid concentrations, and developed personalized treatments.
Results
Aminoacylation activity was reduced in all patients, and further diminished at 38.5/40 °C (PLARS and PFARSB), consistent with infectious deteriorations. With lower cognate amino acid concentrations, patient fibroblast growth was severely affected. To prevent local and/or temporal deficiencies, we treated patients with corresponding amino acids (follow-up: 1/2–2 2/3rd years), and intensified treatment during infections. All patients showed beneficial treatment effects, most strikingly in growth (without tube feeding), head circumference, development, coping with infections, and oxygen dependency.
Conclusion
For these four ARS deficiencies, we observed a common disease mechanism of episodic insufficient aminoacylation to meet translational demands and illustrate the power of amino acid supplementation for the expanding ARS patient group. Moreover, we provide a strategy for personalized preclinical functional evaluation.
INTRODUCTION
Aminoacyl-tRNA synthetases (ARS) facilitate loading of transfer RNAs (tRNAs) with their cognate amino acids [1], a pivotal process in translating messenger RNA (mRNA) to protein. ARS function in the cytosol (encoded by ARS1), mitochondria (encoded by ARS2), or both (encoded by GARS1, KARS1, QARS1). Autosomal recessive ARS1 variants cause severe symptoms in various organs, especially during the first year of life and infectious episodes, and may lead to premature death [2], putatively through loss of aminoacylation activity [3]. To improve care for the increasingly recognized group of ARS-deficient patients, we further investigated the disease mechanism for four different ARS deficiencies, and developed a personalized treatment strategy.
MATERIALS AND METHODS
In silico analyses
Protein structure visualizations were based on pdb entries 1ile, 1ffy, 3l4g, 4rge, and 6lfp, using molscript, Raster3D, and Bragi [4–6].
Fibroblast cultures
Fibroblasts were obtained via skin biopsy and cultured in F-12 with 10% FBS and 1% penicillin–streptomycin (PS). For amino acid sensitivity experiments, amino acid free DMEM/F-12 with HEPES and NaHCO3 was supplemented with 1% PS, 1% GlutaMAX, 10% dialyzed FBS, and all amino acids except the tested amino acid. Amino acid concentrations were related to average plasma concentrations (L-isoleucine: 57 µM; L-leucine: 100 µM; L-phenylalanine: 58 µM; and L-serine: 136 µM).
Aminoacylation activity
IARS, LARS, FARS, SARS, and GARS activities were measured in fibroblast lysates, incubated at 37 °C in reaction buffer (50 mM Tris buffer [pH 7.5], 12 mM MgCl, 25 mM KCl, 1 g/l bovine serum albumin, 0.5 mM spermine, 1 mM ATP, 0.2 mM yeast total tRNA, 1 mM dithiothreitol, 0.3 mM [13C6, 15N] isoleucine, 0.3 mM [13C2] leucine, 0.3 mM, [2H5] phenylalanine, 0.3 mM [13C3, 15N] serine, and 0.3 mM [2H2] glycine). Aminoacyl-tRNA was precipitated with trichloroacetic acid (TCA). Labeled amino acids were detached from tRNAs with ammonia. [13C, 15N] glycine was added as internal standards. Labeled amino acids were quantified by liquid chromatography–tandem mass spectrometry. Analyses were performed in triplicates. To determine thermolability, lysates were incubated at 37 °C, 38.5 °C, and 40 °C.
Amino acid sensitivity
Patient and age-matched control fibroblasts were seeded in triplicates (E-Plate 96 PET, 3,000 cells/well). After 24 hours, medium with the desired L-isoleucine, L-leucine, L-serine, or L-phenylalanine concentrations was applied. Proliferation of fibroblasts was evaluated by continuous impedance analysis over three days using a real-time cell analyzer (xCELLigence MP, ACEA Biosciences). Per donor, impedance at 72 hours was normalized against 100% amino acid concentration.
Medical treatment
Based on the genetic diagnoses and in vitro functional studies, we developed personalized intervention and monitoring protocols for objective safety and outcome parameters (Table S1) as n = 1 studies with parents as partners in care [7, 8].
We treated PIARS and PLARS with high doses of L-isoleucine (35–70 mg/kg/day in three doses), and L-leucine (35–100 mg/kg/day), respectively, and natural protein fortification (2.5 g/kg/day; during illness 3.5 g/kg/day). PFARSB and PSARS received L-phenylalanine (40–100 mg/kg/day), and L-serine (85.7–97.5 mg/kg/day), respectively (Table S2). Similar amino acid dosages were safely used for other disorders [8, 9].
RESULTS
Patient fibroblasts
We studied fibroblasts from the following:
PIARS (and PIARS-2 with similar phenotype): compound heterozygous IARS1-variants (NM_002161.5): c.1305G>C p.Trp435Cys and c.3377dup p.Asn1126fs (OMIM 600709) [2, 10–12], previously described as P2 and P1, respectively [2].
PLARS: compound heterozygous LARS1-variants (NM_020117.10): c.1503 + 3A>G p.? and c.1292T>A p.Val431Asp (OMIM 151350) [2].
PFARSB: compound heterozygous FARSB-variants (NM_005687.5): c.3G>T p.Met1? and c.1118G>C p.Gly373Ala (OMIM 609690) [13–15].
PSARS: homozygous SARS1-variant (NM_006513.3): c.638G>T p.Arg213Leu (OMIM 607529) [16].
In silico analyses
For all variants, we predicted pathogenicity using protein structure analyses (Supplementary Text, Figure S1).
Fibroblast studies
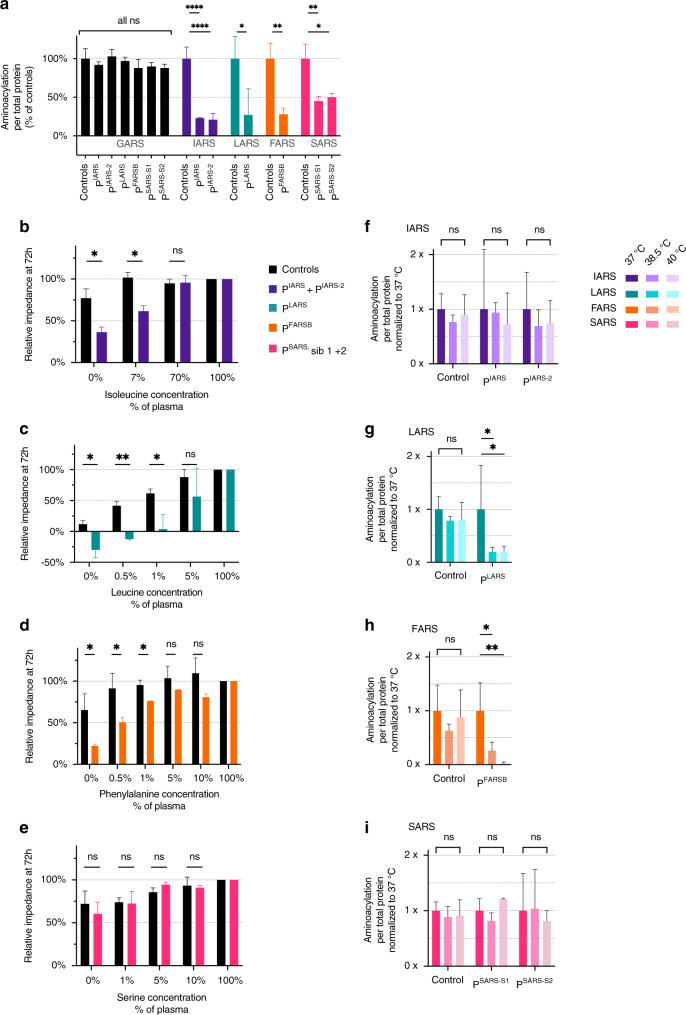
We confirmed pathogenicity of the variants with decreased aminoacylation activity in patient-derived fibroblasts to 23% and 21% IARS activity in PIARS and PIARS-2, respectively, 27% LARS activity in PLARS, 28% FARS activity in PFARSB, and 45% and 50% SARS activity in two siblings of PSARS with the same homozygous variants (Fig. 1a). Because patients deteriorate during infections [2], we investigated thermostability of the affected enzymes. At 38.5 °C and 40 °C, LARS activity of PLARS decreased to 5%, and FARS activity of PFARSB to 0% at 40 °C (Fig. 1g, h). Corresponding ARS activities in fibroblasts from PIARS, PIARS-2, PSARS, and controls, and GARS activity (internal control) were the same at 37 °C, 38.5 °C, and 40 °C (Fig. 1f, i, S2).
Fig. 1. Enzyme activity is decreased but not absent in all patients, and IARS, LARS, and FARSB patient fibroblasts are increased sensitive to isoleucine, leucine and phenylalanine deprivation, respectively.
(a) Enzyme activity is decreased but not absent in all patients. Aminoacylation activity in fibroblasts of PIARS, PIARS-2, PLARS, PFARSB, and two siblings with the same homozygous variant as PSARS, presented as percentage of age-matched controls. GARS activity was measured as an internal control. All measurements were performed in triplicate (n = 3), except IARS controls (n = 6) and GARS controls (n = 12). Error bars show standard deviation. Unpaired t-test: ns p ≥ 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (b–e) IARS, LARS, and FARSB patient fibroblasts are sensitive to isoleucine, leucine, and phenylalanine deprivation, respectively. Seventy-two hours proliferation of fibroblasts of PIARS, PIARS-2, PLARS, PFARSB, and two siblings with the same homozygous variant as PSARS, compared to age-matched control fibroblasts, exposed to decreasing concentrations of isoleucine (b: PIARS/PIARS-2), leucine (c: PLARS), phenylalanine (d: PFARSB), or serine (e: two siblings of PSARS), shown as normalized impedance, measured with an xCELLigence MP. Amino acid concentrations were compared to average plasma concentrations. Every donor was measured once (a; n = 1 each) or twice (b–d; n = 2 each). Two (a–c) or three (d) controls were measured once (n = 1 each). Error bars show standard deviation. Unpaired t-test: ns p ≥ 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. (f–i) LARS and FARS activity deteriorate in LARS and FARSB patient fibroblasts, respectively. Aminoacylation activity in fibroblasts of PIARS and PIARS-2 (f), PLARS (g), PFARSB (h), and two siblings with the same homozygous variant as PSARS (i) at 37 °C, 38.5 °C, and 40 °C. Data are presented compared to the enzymatic activity at 37 °C. All measurements were performed in triplicate (n = 3). Error bars show standard deviation. Unpaired t-test: ns p ≥ 0.05, *p < 0.05, **p < 0.01.
Based on severe symptoms at young age and during infections, reflecting periods of increased translation and decreased amino acid availability, we tested if patient fibroblasts were sensitive to ARS-specific amino acid concentrations. Indeed, patient fibroblast proliferation was normal at high concentrations, but decreased in a dose-dependent manner at lower concentrations of isoleucine for PIARS and PIARS-2, leucine for PLARS, and phenylalanine for PFARSB (Fig. 1b–d), when compared to controls. Fibroblasts from PIARS and PLARS died upon combined amino acid deprivation (data not shown). Serine deprivation did not affect fibroblasts from PSARS’ two siblings (Fig. 1e). As serine is nonessential, effects may have been compensated by biosynthesis from glycine and glucose in our culture media.
Patient treatment
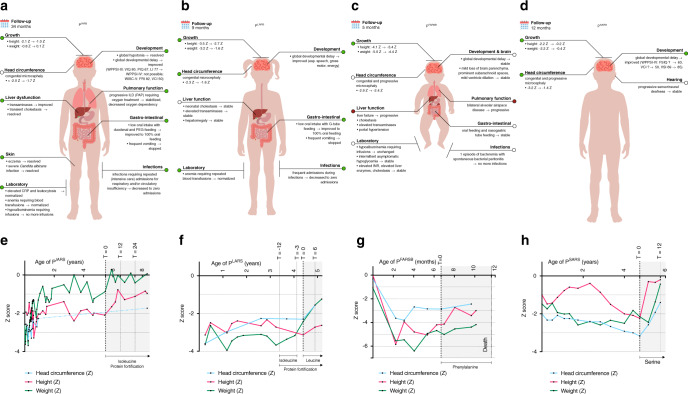
PIARS was an 8-year-old boy with a history of dysmaturity (Fig. 2e), failure to thrive requiring duodenal tube feeding, global developmental delay, autism spectrum disorder, interstitial lung disease (ILD, Figure S3) requiring oxygen treatment, and repeated (intensive care) admissions for respiratory and circulatory insufficiency during infectious episodes (Fig. 2a). Within weeks after treatment initiation, oral intake increased, vomiting decreased, and pulmonary function improved. After three weeks, isoleucine supplementation was stopped for two weeks due to pharmacy delivery problems. Vomiting, mucus production, and respiratory distress all increased. Upon reinitiation, symptoms improved. Similarly, during gastroenteritis and reduced amino acid intake, laboratory markers (albumin, coagulation) deteriorated (Table S3; T = 32 months). During treatment, PIARS experienced no infections requiring hospital admission, compared to five admissions two years prior. Growth improved (height: −2.1ZNL to −1.0ZNL; weight: −1.0ZNL to +0.1ZNL), and tube feeding could be stopped after 16 months (Fig. 2a, e). Periods without oxygen therapy increased in frequency and duration. Upon treatment, progression of ILD ceased as confirmed by computed tomography (CT) scans (Figure S3). PIARS became more energetic, interaction increased, and expressive speech and language skills improved according to professionals at school. Behavioral abnormalities became more apparent, and hindered repeat psychological testing. Although incomplete, Dutch Wechsler Intelligence Scale for Children, fifth edition (WISC-V-NL) [17] evaluation showed a disharmonic profile: fluid reasoning and processing speed were stronger (fluid reasoning index [FRI]: 82), compared to verbal understanding and working memory (verbal comprehension index [VCI]: 50).
Fig. 2. Summary of symptoms with treatment effects, and growth charts for all patients.
(a–d) Summary of symptoms and treatment effects. Summary of the symptoms of PIARS (top left), PLARS (top right), PFARSB (bottom left), and PSARS (bottom right). Colors indicate the generalized treatment effect on the symptom (green: improved; white: stable or stabilized; red: progressed). Standard deviation scores (Z) for height, weight, and head circumference were calculated using Netherlands (NL) reference charts (PIARS; head circumference of PLARS), World Health Organization (WHO) reference charts (PFARSB), Centers for Disease Control and Prevention (CDC) reference charts (height and weight of PLARS), and French (FR) reference charts (PSARS). CRP C-reactive protein, FSIQ full-scale intelligence quotient, G-tube gastrostomy-tube, ILD interstitial lung disease, FRI fluid reasoning index, INR international normalized ratio, LI language index, PAP pulmonary alveolar proteinosis, PEG percutaneous endoscopic gastrostomy, PIQ performance intelligence quotient, VIQ verbal intelligence quotient, VCI verbal comprehension index, VSI visual–spatial index, WISC-V Wechsler Intelligence Scale for Children, fifth edition, WPPSI-III/IV Wechsler Preschool and Primary Scale of Intelligence, third/fourth edition. (e–h) Growth charts: Z-scores of height, weight and head circumference for age. Growth charts of height, weight, and head circumference in standard deviation score (Z) of (a) PIARS (NL reference charts); (b) PLARS (CDC reference charts for height and weight, NL reference charts for head circumference); (c) PFARSB (WHO reference charts); and (d) PSARS (FR reference charts). T in months from start of treatment.
PLARS was a 5-year-old girl with a history of pre/dysmaturity (Fig. 2f), failure to thrive requiring tube feeding, global developmental delay, anemia requiring repeated transfusions, neonatal cholestasis, liver disease, and frequent hospital admissions during infections (Fig. 2b). During nine months of protein fortification and erroneous isoleucine supplementation, she was not admitted to hospital, and was more energetic. Her weight improved, but not height (further deviation), nor head circumference (stable). Within one month after switching to leucine, her oral intake increased. Tube feeding became unnecessary after six months. Height increased from −3.1ZCDC to −2.7ZCDC, weight further increased from −2.4ZCDC to −1.6ZCDC, and microcephaly resolved from −2.3ZNL to −1.6ZNL (Fig. 2b, f). Liver transaminases, bilirubin, immunoglobulins, and liver ultrasound normalized. Teachers reported more interaction and an accelerated speech/language development; on neurologic examination, gross motor skills normalized, fine motor skills improved and muscle mass, tonus, and strength increased. Recently, she suffered COVID-19 with flu-like symptoms, without derailing. Repeat neuropsychologic testing was delayed due to COVID-19 restrictions.
PFARSB was an 11-month-old boy. Pregnancy was complicated by intrauterine growth restriction and placental abruption. His phenotype was dominated by pre/dysmaturity (Fig. 2g), failure to thrive requiring nasogastric tube feeding and parenteral nutrition, hypoalbuminemia (with edema, ascites) requiring frequent albumin infusions, progressive liver failure, and lung disease (Fig. 2c). After starting treatment, liver function initially improved, as did growth in height (−4.5ZWHO to −3.2ZWHO), head circumference (−2.9ZWHO to −2.4ZWHO), and parental perception of psychomotor development (Fig. 2c, g). After three months, he developed esophageal variceal bleeding. Bleeding was stopped via octreotide and sclerotherapy, but hepatic encephalopathy and respiratory failure shortly ensued. His death was attributed to disease progression.
PSARS was a 5-year-old boy, fourth of five children born to consanguineous parents (first cousins). Symptoms involved dysmaturity (Fig. 2h), progressive sensorineural deafness, and moderate developmental delay (Fig. 2d). Three siblings with the same SARS1 variants died following infections. One sibling without SARS1 variants is clinically well. Before treatment, height, weight, and head circumference standard deviations of PSARS gradually decreased with age. After six months of treatment, height improved from −2.2ZFR to −0.2ZFR and microcephaly resolved from −3.2ZFR to −1.4ZFR (Fig. 2d, h). Development improved. Pretreatment, he had significant difficulties in receptive and expressive language, and none of the primary Wechsler Preschool and Primary Scale of Intelligence, fourth edition (WPPSI-IV) [18] subtests could be completed. After one year of treatment, the six primary and five additional subtests of the WPPSI-IV could be completed. While he scored below average on tests requiring sustained attention and working memory and exhibited attentional difficulties in everyday life (CBCL) [19], he scored within normal limits on tasks assessing spatial visualization and constructive skills, as well as logic and perceptual reasoning.
Figure 2a–d summarizes treatment effects. In all patients, treatment was well tolerated, and safety parameters remained stable (Tables S3–6).
DISCUSSION
We report how clinical observations led to targeted studies in patient-derived fibroblasts, providing insight in the disease mechanism and a personalized treatment strategy. Based on pronounced symptoms during the first year of life and episodes of intercurrent illness, we hypothesized that patient aminoacylation may suffice for cellular functions under normal conditions, but not during periods of increased translation (temporally and spatially controlled tissue formation, rapid growth in early life, illness) or decreased amino acid availability (starvation, vomiting), in particular when the cognate amino acid is essential. Indeed, patient fibroblast studies showed increased sensitivity to ARS-specific amino acid deprivation. Further contributing to deterioration during infections [2], we evidenced strongly decreased aminoacylation activity for LARS and FARSB at feverish temperatures (38.5–40 °C). Variants that decrease protein stability decrease the temperature at which the protein unfolds. It is not possible to predict whether these variants result in unfolding of the protein around body temperature (as for PLARS and PFARSB). The effect of temperature on proteins with variants affecting dimerization (PSARS) and/or specific protein domains (PIARS) is even less predictable.
Motivated by these studies and because protein folds typically stabilize when bound to substrates, we initiated supplementation of the ARS-specific amino acid for individual patients with IARS, LARS, FARSB, and SARS deficiencies, with protein fortification for PIARS and PLARS. To prevent the ARS proteins from irreversible processes of unfolding, aggregation and degradation, we intensified treatment during infections, and advised strict antipyretic treatment. Furthermore, we provided an emergency protocol for triggers such as fasting, fever, and infections. This approach is radically different from the “high glucose, no protein” emergency treatment to avoid metabolic decompensations for other inherited metabolic diseases.
Overall, we found strikingly beneficial effects and good tolerance and safety. Most consistent was the improvement in growth (including head circumference) quickly after initiation of treatment (Fig. 2), leading to independency from tube feeding, improved development, and coping with infections. In addition, for PIARS, oxygen dependency decreased and previously progressive pulmonary abnormalities stabilized, and for PFARSB, liver function improved. The instable FARS protein, as evidenced by severely reduced enzyme activity upon minimal increase over physiological temperature (Fig. 1h), may have contributed to the detrimental disease course in PFARSB.
Because ARS deficiencies were only recently discovered, it is difficult to relate treatment effects to the natural disease course. One FARSB-, two IARS-, and two SARS-deficient patients reached adulthood and retained severe growth retardation, moderate-to-severe intellectual disability (IARS/SARS), restrictive lung disease (FARSB), and liver dysfunction (IARS/FARSB) [10, 14, 16]. While protein fortification alone caused some improvements, the need for treatment with the corresponding amino acid was evidenced by improved effects after leucine instead of erroneous isoleucine supplementation in PLARS, and by transient clinical deterioration during two-week delivery failure of isoleucine in PIARS. Concordantly, total parenteral nutrition improved liver function of one MARS-deficient patient [20], as did protein fortification in LARS deficiency [21]. One IARS-deficient patient thrived better upon high-caloric feeding, and isoleucine supplementation decreased susceptibility to infections [10]. Recently, protein fortification with methionine supplementation resulted in improved growth, pulmonary function and neurodevelopment in two MARS-deficient patients [22]. These effects for different ARS deficiencies, and prompt onset after initiation suggest a true treatment effect.
In conclusion, we provide therapeutic recommendations for ARS deficiencies based on in vitro and in vivo evidence of beneficial effects of amino acid and protein supplementation (up to 2 2/3rd years) in single patients. This affordable, accessible, and safe strategy holds the potential to improve outcomes for the expanding group of severe, often progressive, multiorgan ARS deficiencies. Although further research and validation for other ARS deficiencies are necessary, our in vitro studies in patient-derived cells may guide personalized therapeutic strategies.
Supplementary information
Acknowledgements
We are grateful to the patients and their families for participation in our study, and to our clinical colleagues at the Utrecht and Amsterdam University Medical Centers (The Netherlands), the University of Alabama School of Medicine (USA), and Nancy Regional and University Hospital Center (France) for their contribution to diagnosis and management of the patients. The study was performed by members of United for Metabolic Diseases, funded by Stichting Metakids. The graphical abstract was created using Biorender.
Author contributions
G.K., L.T., C.D.M.v.K., and S.A.F. conceptualized and wrote the original draft of the manuscript. G.K., I.F.S., G.S., M.I.M., and D.E.C.S. did the laboratory investigation, validation, and analysis (G.K., I.F.S.: amino acid sensitivity experiments; G.S., M.I.M., D.E.C.S.: aminoacylation and thermolability experiments). G.K. and H.R. did visualization (G.K.: figures, tables; H.R.: protein structures). M.E.D., S.W.J.T.-L., C.v.K., E.A.F., A.W., M.C., F.F., M.B., J.D., and R.K. were involved in clinical investigation (M.E.D., S.W.J.T.-L., C.D.M.v.K.: treatment PIARS; C.D.M.v.K. and E.A.F.: treatment PLARS; A.W., M.C., F.F.: treatment PSARS; M.B., J.D., R.K.: treatment PFARSB). M.v.d.B. was involved in conceptualization of treatment design of PIARS. G.K., C.D.M.v.K., S.A.F., T.J.d.K., E.E.S.N., and H.R. reviewed and edited the final version of the manuscript. C.D.M.v.K. and S.A.F. contributed equally.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary files. All biological materials used in this manuscript are primary patient materials, and access is therefore restricted.
Ethics declaration
Patients’ parents provided written informed consent for our studies. Laboratory studies were conducted with local medical ethical approval (Institutional Review Board of the University Medical Center Utrecht [STEM: 10–402/K, TC Bio 190489]). All medical studies were performed as clinical care following local institutional guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Clara D. M. van Karnebeek, Sabine A. Fuchs.
Contributor Information
Clara D. M. van Karnebeek, Email: clara.vankarnebeek@radboudumc.nl
Sabine A. Fuchs, Email: s.fuchs@umcutrecht.nl
Supplementary information
The online version contains supplementary material available at 10.1038/s41436-021-01249-z.
References
- 1.Antonellis A, Green ED. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu Rev Genomics Hum Genet 2008;9:87–107. [DOI] [PubMed]
- 2.Fuchs SA, et al. Aminoacyl-tRNA synthetase deficiencies in search of common themes. Genet Med. 2019;21:319–30. doi: 10.1038/s41436-018-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer-Schuman R, Antonellis A. Emerging mechanisms of aminoacyl-tRNA synthetase mutations in recessive and dominant human disease. Hum Mol Genet. 2017;26:R114–27. [DOI] [PMC free article] [PubMed]
- 4.Kraulis PJ. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr. 1991;24:947–50. doi: 10.1107/S0021889891004399. [DOI] [Google Scholar]
- 5.Merritt EA, Murphy MEP. Raster3D version 2.0 A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr. 1994;50:869–73. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- 6.Schomburg D, Reichelt J. BRAGI: a comprehensive protein modeling program system. J Mol Graph. 1988;6:161–5. doi: 10.1016/0263-7855(88)80069-9. [DOI] [Google Scholar]
- 7.Tseng LA, Sowerbutt C, Lee JJY, van Karnebeek CDM. P4 medicine for epilepsy and intellectual disability: nutritional therapy for inherited metabolic disease. Emerg Top Life Sci. 2019;3:75–95. doi: 10.1042/ETLS20180180. [DOI] [PubMed] [Google Scholar]
- 8.Müller AR, et al. The power of 1: systematic review of N-of-1 studies in rare genetic neurodevelopmental disorders. Neurology. 2021;96:529–40. [DOI] [PMC free article] [PubMed]
- 9.de Koning TJ. Amino acid synthesis deficiencies. J Inherit Metab Dis. 2017;40:609–20. doi: 10.1007/s10545-017-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopajtich R, et al. Biallelic IARS mutations cause growth retardation with prenatal onset, intellectual disability, muscular hypotonia, and infantile hepatopathy. Am J Hum Genet. 2016;99:414–22. doi: 10.1016/j.ajhg.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orenstein N, et al. Bi-allelic IARS mutations in a child with intra-uterine growth retardation, neonatal cholestasis, and mild developmental delay. Clin Genet. 2017;91:913–7. doi: 10.1111/cge.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smigiel R, et al. New evidence for association of recessive IARS gene mutations with hepatopathy, hypotonia, intellectual disability and growth retardation. Clin Genet. 2017;92:671–3. doi: 10.1111/cge.13080. [DOI] [PubMed] [Google Scholar]
- 13.Zadjali F, et al. Homozygosity for FARSB mutation leads to Phe-tRNA synthetase-related disease of growth restriction, brain calcification, and interstitial lung disease. Hum Mutat. 2018;39:1355–9. doi: 10.1002/humu.23595. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, et al. Bi-allelic mutations in Phe-tRNA synthetase associated with a multi-system pulmonary disease support non-translational function. Am J Hum Genet. 2018;103:100–14. doi: 10.1016/j.ajhg.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonellis A, et al. Compound heterozygosity for loss-of-function FARSB variants in a patient with classic features of recessive aminoacyl-tRNA synthetase-related disease. Hum Mutat. 2018;39:834–40. doi: 10.1002/humu.23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musante L, et al. Mutations of the aminoacyl-tRNA-synthetases SARS and WARS2 are implicated in the etiology of autosomal recessive intellectual disability. Hum Mutat. 2017;38:621–36. doi: 10.1002/humu.23205. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman AS, Raiford SE, Coalson DL. Intelligent testing with the WISC-V. Hoboken, NJ, USA: John Wiley & Sons Inc; 2016. p. 683–702.
- 18.Wechsler D. Wechsler preschool and primary scale of intelligence. 4th edition. San Antonio, TX, USA: The Psychological Corporation; 2012.
- 19.Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: Aseba; 2001. [Google Scholar]
- 20.van Meel E, et al. Rare recessive loss-of-function methionyl-tRNA synthetase mutations presenting as a multiorgan phenotype. BMC Med Genet. 2013;14:106. doi: 10.1186/1471-2350-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey JP, et al. Clinical and genetic characterisation of infantile liver failure syndrome type 1, due to recessive mutations in LARS. J Inherit Metab Dis. 2015;38:1085–92. doi: 10.1007/s10545-015-9849-1. [DOI] [PubMed] [Google Scholar]
- 22.Lenz D, et al. Rescue of respiratory failure in pulmonary alveolar proteinosis due to pathogenic MARS1 variants. Pediatr Pulmonol. 2020;55:3057–66. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary files. All biological materials used in this manuscript are primary patient materials, and access is therefore restricted.