Abstract
Introduction:
Intramedullary spinal cord tumors (IMSCT) account for about 2%–4% of all central nervous system tumors. Surgical resection is the main treatment step, but might cause damage to functional tissues. Intraoperative neuromonitoring (IONM) is an adopted measure to decrease surgical complications. Below, we describe the results of IMSCT submitted to surgery under IONM at a tertiary institution.
Methods:
The sample consisted of consecutive patients with IMSCT admitted to the Neurological Institute of Curitiba from January 2007 to November 2016. A total of 47 patients were surgically treated. Twenty-three were male (48.9%) and 24 were female (51.1%). The mean age was 42.77 years. The mean follow-up time was 42.7 months.
Results:
Neurological status improved in 29 patients (62%), stable in 6 (13%), and worse in 12 (25%). Patients who presented with motor symptoms at initial diagnosis had a worse outcome compared to patients with sensory impairment and pain (P = 0.026). Patients with a change in electromyography had worse neurological outcomes compared to patients who did not show changes in monitoring (P = 0.017).
Discussion and Conclusion:
No prospective randomized high evidence study has been performed to date to compare clinical evolution after surgery with or without monitoring. In our sample, surgical resection was well succeeded mainly in oligosymptomatic patients with low preoperative McCormick classification and no worsening of IONM during surgery. We believe that microsurgical resection of IMSCT with simultaneous IONM is the gold standard treatment and achieved with good results.
Keywords: Intraoperative neurophysiology, outcome, spinal cord tumors, surgery
Introduction
Intramedullary spinal cord tumors (IMSCT) comprise the least common types of spinal neoplasms. They account for about 2%–4% of all central nervous system tumors and 20%–30% of all spinal cord tumors.[1,2,3,4,5,6,7,8,9,10] The most common type of IMSCT is ependymoma, followed by astrocytoma.[11,12,13,14,15,16,17,18,19,20,21,22] Pain manifested as back pain, radicular pain, or neuropathic pain has been reported to be the most common presenting symptom in patients with an IMSCT. Other common symptoms include motor disturbances and sensory symptoms (dermatomal, saddle, or segmental). Sphincter disturbances may occur as an early symptom.[11,12,13,14,15,16,17,18,19,20,21,22]
Without treatment, IMSCT can lead to severe neurologic deterioration with serious motor deficits, including paraplegia or even quadriplegia. The most important part of the treatment is surgical resection. Radical resection has been associated with increased long-term overall survival.[23,24,25,26,27,28] However, surgery might cause damage to functional tissues, which leads to neurologic complications.[25,26]
To reduce the risk of iatrogenic complications, intraoperative neuromonitoring (IONM) is a worldwide adopted measure.[18,19,20,21,22,23,24,25,26,27,28] Several different monitoring modalities are currently in use.[8,9,10] Although IONM is widely used, and many single-center studies have been conducted, no high evidence study has clearly defined the added value, in terms of overall sensitivity and specificity to detect or prevent neuronal injury of the different monitoring techniques used in IMSCT surgery.[16,17,18,19,20,21,22,23,24,25,26,27,28]
Below, we describe the results of IMSCT submitted to surgery under IONM at a tertiary institution. Clinical and radiological data, lesion features, timing of symptom onset, and IONM findings were recorded. The IONM included continuous needle electromyography. We evaluated the outcome according to the modified McCormick scale.
Methods
The study sample consisted of consecutive patients with IMSCT admitted to the Neurological Institute of Curitiba (INC) Neurosurgical Ward from January 2007 to November 2016. Patients enrolled in the study were then followed during treatment. We evaluated age, gender, symptoms, histology, surgical data (IONM), and postoperative outcome.
Inclusion criteria
All patients undergoing microsurgery as a treatment strategy for IMSCT were eligible for inclusion.
Criteria for surgery
Criteria used to indicate surgery were as follows: patients with intramedullary spinal cord lesions, incidental or symptomatic. Patients in palliative care and/or with very low functional status (Karnofsky score <40) were not considered candidates for surgery.
Signs and symptoms were described according to neurological impairments: pain, sensorial disturbances, reflexes, motor strength, gait and balance disturbances, and sphincterian changes. Patients were classified according to McCormick classification (ranging from I to IV). Postoperatively, patients were then compared and considered with neurological improvement, stability, or neurological impairment.
Sample
A total of 47 patients were surgically treated. Twenty-three were male (48.9%) and 24 were female (51.1%). The mean age was 42.77 years, ranging from 7 to 80 years. The mean follow-up time was 42.7 months (ranging from 8 to 120 months). Survival analysis was extracted from the INC registry of patient data.
Surgical approach
In all cases, the institutional routine is to perform laminotomy of target levels and midline myelotomy with microsurgical technique. The surgical aim is to perform maximal safe resection without neurological impairment.
Neuromonitoring evaluation
IOM was recorded continuously, from before patient positioning, at which point baseline signals were obtained, until waking up from anesthesia. Our evaluation was limited to continuous electromyography. We did not apply somatosensory evoked potential (SSEPs), motor evoked potentials (MEPs), D-waves, or dorsal column mapping. Signals were obtained from all four extremities.
Statistical analysis
Statistical analyses were performed using SPSS Statistics, IBM, Armonk, New York, USA. We applied the Chi-square test when applicable. We considered statistically significant data when P < 0.05.
Ethics
The study protocol was approved by the Institutional Ethics in Research Board.
Results
Location of tumors
Cervical tumors occurred in 23 patients (40.4%), while cervicothoracic in 3 (6.4%) patients and thoracic in 25 (53.2%) patients. The extension of tumor was correspondent to 2 levels or less in 37 (78%) subjects, while 3 or more in 10 subjects (22%).
Gross total resection was achieved in 40 patients (85%), while partial resection was obtained in 7 patients (15%).
Histology
As described in Table 1, the most common tumor was ependymoma, with 23 cases (48.9%), followed by hemangioblastomas (6 cases, 12.8%) and astrocytomas (5 cases, 10.6%). We had also atypical pathological specimens, such as chordoma and lymphoma. There were two cases of metastases, being both of melanomas. We illustrate pre- and postoperative images of typical cases in Figure 1.
Table 1.
Histological types
| n (%) | |
|---|---|
| Cavernoma | 4 (8.5) |
| Astrocytoma | 5 (10.6) |
| Arachnoid cyst | 2 (4.3) |
| Chordoma | 1 (2.1) |
| Ependymoma | 23 (48.9) |
| Ganglioglioma | 1 (2.1) |
| Hemangioblastoma | 6 (12.8) |
| Lymphoma | 1 (2.1) |
| Arteriovenous malformation | 1 (2.1) |
| Neurocysticercosis | 1 (2.1) |
| Melanoma metastasis | 2 (4.3) |
| Total | 47 (100) |
Figure 1.
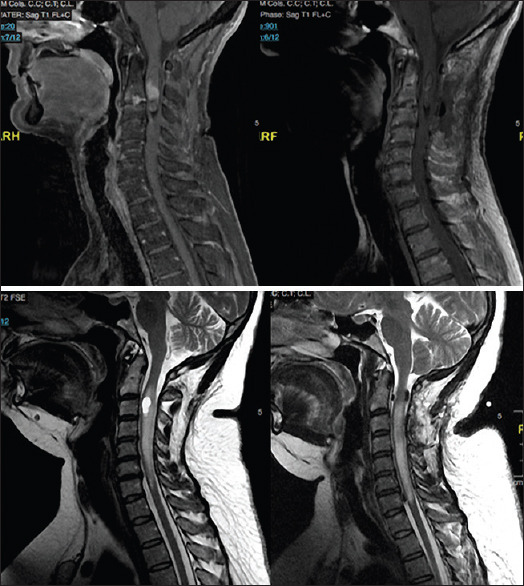
Pre- and postoperative images of typical cases. Above, pre- and postoperative magnetic resonance of an ependymoma. Below, pre- and postoperative magnetic resonance of a hemangioblastoma
Clinical presentation
The initial clinical presentation is presented in Table 2. The main symptoms were sensorial (29 cases, 62%), followed by pain (28%–60%) and motor deficits (15 cases, 32%). Hyperreflexia, esfincterian, and gait symptoms completed the main symptoms. Two patients were symptom free at the diagnosis.
Table 2.
Initial symptoms in evaluated patients
| Symptom | n (%) |
|---|---|
| Sensorial | 29 (62) |
| Pain | 28 (60) |
| Motor | 15 (32) |
| Hyperreflexia | 5 (11) |
| Sphincter | 5 (11) |
| Gait | 6 (13) |
| Asymptomatic | 2 (4) |
Preoperative McCormick
Preoperative McCormick was I in 8 subjects (17%), II in 22 subjects (46.8%), III in 15 patients (32%), and IV in 2 other patients (4.2%).
Follow-up
The mean follow-up time was 42.7 months (ranging from 8 to 120 months). In the late follow-up (1 year), neurological status was improved in 29 patients (62%), stable in 6 (13%) patients, and worse in 12 (25%). Overall survival rate free of disease was 90%, with three cases of postoperative complications (6%), being one case of pulmonary thromboembolic event, and two cases of cerebrospinal fluid fistula. At the end of the follow-up, we diagnosed one case of melanoma central nervous system dissemination and one case of tumoral progression instead of surgery.
Intraoperative neuromonitoring parameters and correlation to outcome
We divided IONM parameters into three groups: a group without decrease in intraoperative electromyography, a second group with decrease and recovery during surgery, and the last group with decrease without recovery.
In the late follow-up (1 year), neurological status was improved in 29 patients (62%), stable in 6 (13%) patients, and worse in 12 (25%). Among 47 patients, 24 (51%) did not present intraoperative signal decrease, while 23 (49%) presented decrease, being 12 patients (25.5%) with recovery and 11 (23.5%) without recovery.
Among 29 patients with clinical improvement, 19 had no decrease in IONM (65%), while 10 had decrease (35%), being 9 with recovery and 1 without. Among 6 patients clinically stable, 3 had no decrease in IONM (50%), while 3 had decrease (50%), being 0 with recovery and 3 without. Among 12 patients clinically worse, 2 had no decrease in IONM (8%), while 10 had decrease (92%), being 3 with recovery and 7 without [Table 3].
Table 3.
Correlation of intraoperative neuromonitoring parameters and clinical outcome
| Total, n (%) | Improvement, n (%) | Stable, n (%) | Worse, n (%) | |
|---|---|---|---|---|
| IONM | 47 | 29 (61.7) | 6 (12.7) | 12 (25.5) |
| No decrease | 24 (51.1) | 19 (65) | 3 (50) | 2 (17) |
| Decrease | 23 (48.9) | 10 (35) | 3 (50) | 10 (83) |
| Decrease with recovery | 12 (25.5) | 9 (31) | 0 | 3 (25) |
| Decrease without recovery | 11 (23.4) | 1 (4) | 3 (50) | 7 (63) |
IONM-Intraoperative neuromonitoring
Table 4 illustrates parameters evaluated and postoperative functional outcome. Gender, mean age, histology, anatomical localization, extension, preoperative McCormick, and resection rate did not change the outcome. Factors involved in outcome included initial motor symptoms presentation and intraoperative change in IONM. The worse outcome occurred in male patients, above 40-year-old, with partial resection and McCormick III or IV.
Table 4.
Factors related to postoperative outcome
| Factor | Total, n (%) | Improvement, n (%) | Stable, n (%) | Worsening, n (%) | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 23 (48.9) | 13 (56.5) | 2 (8.7) | 8 (34.8) | 0.318 |
| Female | 24 (51.1) | 16 (66.7) | 4 (16.7) | 4 (16.7) | |
| Age | |||||
| <40 | 20 (42.6) | 13 (65.0) | 3 (15.0) | 4 (20) | 0.735 |
| >40 | 27 (57.4) | 16 (59.3) | 3 (11.1) | 8 (29.6) | |
| Histology | |||||
| Neuroepithelial | 29 (61.7) | 18 (62.1) | 4 (13.8) | 7 (24.1) | 0.941 |
| Not neuroepithelial | 18 (38.3) | 11 (61.1) | 2 (11.1) | 5 (27.8) | |
| Location | |||||
| Cervical | 19 (40.4) | 12 (63.2) | 2 (10.5) | 5 (26.3) | 0.747 |
| Cervicothoracic | 3 (6.4) | 2 (66.7) | 1 (33.3) | 0 (0) | |
| Thoracic | 25 (53.2) | 15 (60) | 3 (12.0) | 7 (28.0) | |
| Extension (levels) | |||||
| <3 | 37 (78.7) | 20 (54.1) | 5 (13.5) | 12 (32.4) | 0.083 |
| ≥3 | 10 (21.3) | 9 (90) | 1 (10) | 0 (0) | |
| IONM change | |||||
| Yes | 23 (48.9) | 10 (43.5) | 3 (13.0) | 10 (43.5) | 0.017 |
| No | 24 (51.1) | 19 (79.2) | 3 (12.5) | 2 (8.3) | |
| IONM | |||||
| Improvement | 12 (52.2) | 9 (75.0) | 0 (0) | 3 (25.0) | 0.001 |
| No improvement | 11 (47.8) | 1 (9.1) | 3 (27.3) | 7 (63.6) | |
| McCormick | |||||
| I and II | 30 (63.8) | 19 (63.3) | 5 (16.7) | 6 (20) | 0.365 |
| III and IV | 17 (36.2) | 10 (58.8) | 1 (5.9) | 6 (35.3) | |
| Extent of resection | |||||
| Total | 40 (85.1) | 27 (67.5) | 4 (10) | 9 (22.5) | 0.133 |
| Partial | 7 (14.9) | 2 (28.6) | 2 (28.6) | 3 (42.9) | |
| Symptoms | |||||
| Pain | 28 (59.6) | 20 (71.4) | 4 (14.3) | 4 (14.3) | 0.026 |
| Motor | 15 (31.9) | 9 (60) | 2 (13.3) | 4 (26.7) | |
| Sensorial | 29 (61.7) | 19 (65.5) | 4 (13.8) | 6 (20.7) |
IONM-Intraoperative neuromonitoring
Patients who presented with motor symptoms at initial diagnosis had a worse outcome compared to patients with sensory impairment and pain (P = 0.026). Patients with a change in electromyography (reduction in peak amplitude >50%, increase in peak latency >10%, and total loss of waveform) had worse neurological outcomes compared to patients who did not show changes in monitoring (P = 0.017). Patients who showed changes in monitoring and returned to baseline potential during surgery had better neurological outcomes compared to patients who did not return to baseline wave pattern (P = 0.001) [Table 4].
Discussion
Intramedullary tumors account for 2%–4% of central nervous system tumors and 10% of spinal tumors, with ependymomas and astrocytomas being the most frequent histological types.[1,2,3,4,5,6,7,8,9,10] These tumors are usually benign, slow growing and may extend to various segments of the spinal cord and have few specific symptoms because they usually evolve insidiously.[11,12,13,14,15,16] Even with the progress in surgical technique and intraoperative monitoring, postoperative prognostic factors are conflicting in the literature.
Ependymomas are common in adults, while astrocytomas are far more common in children.[10] Most ependymomas have relatively demarcated borders, while astrocytomas are more infiltrative and need to be resected until a white matter “interphase” appears.[20]
Microsurgical resection of IMSCTs is currently considered the primary treatment modality, while radiotherapy and/or chemotherapy are reserved for recurrent or malignant tumors.[3,9] The observation that the majority of IMSCTs are benign and consequently gross-total removal might result in long-term survival further supports the need for safe resection.[10] The extent of resection has been correlated with progression-free survival and lower recurrence rates.[21] Advances in microsurgery have contributed to safer resection ability. The most common surgical complication is postoperative cerebrospinal fluid (CSF) leak which occurs in about 5%–10% of surgeries.[12,14]
However, despite all advances, surgery for IMSCTs is still very challenging and may carry significant morbidity. The most significant risk associated with surgery for IMSCTs is spinal cord injury and a resulting neurological deficit. Neurophysiological monitoring is an important tool intended to eliminate or reduce this surgical risk.[16,17,18,19,20,21,22,23,24,25,26,27,28] To date, both SSEP and transcranial MEP monitoring techniques have been used. Continuous electromyography and D-wave are also recommended, increasing the sensitivity and specificity of intraoperative findings.[16,17,18,19,20,21,22,23,24,25,26,27,28]
No prospective randomized high evidence study has been performed to date to compare clinical evolution after surgery with or without monitoring.[6,28] A recent meta-analysis failed to find a strong support for IONM use in spinal surgery.[23] On the other hand, there was a slight tendency to support IONM in IMSCT surgery.[23] Thus, the use of IONM is still only supported series of low scientific evidence. In addition, there are no studies comparing different modalities of IONM in IMSCT and thus, there is no established superiority of one modality over the other.[16,17,18,19,20,21,22,23,24,25,26,27,28]
Even though, the routine neurosurgical practice has incorporated IONM and the advocated advantages include detecting positioning disturbances, intraoperative disturbances due to anesthetic and surgical intervention, and it adds information to a neurosurgeon when deciding to continue resection or stopping it, avoiding further tissue damage.[6,7,8,9,10,11,12,13,14] Factors associated with improved outcomes for IMSCT include preoperative neurological status, presence of a tumor dissection plane, tumor size, the use of neuromonitoring, and postoperative radiation therapy.[6,11,12]
In our sample of 47 patients, histology was similar to most series in the literature, prevailing ependymomas, astrocytomas, and hemangioblastomas. Symptoms were mainly sensorial, pain, and motor symptoms. Surgical resection was the best treatment mainly in oligosymptomatic patients with low preoperative McCormick classification (I and II) and no worsening of IONM during surgery. Worst results were observed in patients with preoperative motor symptoms, high McCormick classification (III and IV), and with decrease in IONM sign and persistent decrease in the postoperative period.
There are some limitations of the study. At first, the small sample size does not allow us to perform an individualized analysis concerning each histological type. Then, we did not apply routinely SSEPs and MEPs and D-wave monitoring. We applied exclusively continuous electromyography which might decrease the final power to identify monitoring changes and thus interfere with optimal results. However, once there is not still a consensus on the ideal monitoring setup, we believe that this fact may interfere but not invalidate our results.
We believe that microsurgical resection of IMSCT is the gold standard treatment and achieved with acceptable complications. Simultaneous use of IONM is for us mandatory, mitigating positioning and manipulation problems and taken into account when deciding surgical and resection extent goals. The ideal setup of which monitoring modalities in each case is yet to be delimitated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hyun SJ, Rhim SC. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: Correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg. 2009;23:393–400. doi: 10.1080/02688690902964744. [DOI] [PubMed] [Google Scholar]
- 2.Quiñones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56:982–93. [PubMed] [Google Scholar]
- 3.Korn A, Halevi D, Lidar Z, Biron T, Ekstein P, Constantini S. Intraoperative neurophysiological monitoring during resection of intradural extramedullary spinal cord tumors: Experience with 100 cases. Acta Neurochir (Wien) 2015;157:819–30. doi: 10.1007/s00701-014-2307-2. [DOI] [PubMed] [Google Scholar]
- 4.Ghadirpour R, Nasi D, Iaccarino C, Giraldi D, Sabadini R, Motti L, et al. Intraoperative neurophysiological monitoring for intradural extramedullary tumors: Why not? Clin Neurol Neurosurg. 2015;130:140–9. doi: 10.1016/j.clineuro.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Rijs K, Klimek M, Scheltens-de Boer M, Biesheuvel K, Harhangi BS. In reply to the letter to the editor regarding “Intraoperative neuromonitoring in patients with intramedullary spinal cord tumor: A systematic review, meta-analysis, and case series”. World Neurosurg. 2019;127:664. doi: 10.1016/j.wneu.2019.03.277. [DOI] [PubMed] [Google Scholar]
- 6.Cannizzaro D, Mancarella C, Nasi D, Tropeano MP, Anania CD, Cataletti G, et al. Intramedullary spinal cord tumors: The value of intraoperative neurophysiological monitoring in a series of 57 cases from two Italian centres? J Neurosurg Sci Sep. 2019 Sep 23; doi: 10.23736/S0390-5616.19.04758-1. doi: 10.23736/S0390-5616.19.04758-1. [DOI] [PubMed] [Google Scholar]
- 7.Nagoshi N, Tsuji O, Nakashima D, Takeuchi A, Kameyama K, Okada E, et al. Clinical outcomes and prognostic factors for cavernous hemangiomas of the spinal cord: A retrospective cohort study. J Neurosurg Spine. 2019;31:271–8. doi: 10.3171/2019.1.SPINE18854. [DOI] [PubMed] [Google Scholar]
- 8.Persson O, Fletcher-Sandersjöö A, Burström G, Edström E, Elmi-Terander A. Surgical treatment of intra- and juxtamedullary spinal cord tumors: A population based observational cohort study. Front Neurol. 2019;10:814. doi: 10.3389/fneur.2019.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun XY, Wang W, Zhang TT, Kong C, Sun SY, Guo MC, et al. Factors associated with postoperative outcomes in patients with intramedullary Grade II ependymomas: A Systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e16185. doi: 10.1097/MD.0000000000016185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalid S, Kelly R, Carlton A, Wu R, Peta A, Melville P, et al. Adult intradural intramedullary astrocytomas: A multicenter analysis. J Spine Surg. 2019;5:19–30. doi: 10.21037/jss.2018.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin CG, Frempong-Boadu A, Hoch M, Bruno M, Shepherd T, Pacione D. Combined use of diffusion tractography and advanced intraoperative imaging for resection of cervical intramedullary spinal cord neoplasms: A case series and technical note. Oper Neurosurg (Hagerstown) 2019;17:525–30. doi: 10.1093/ons/opz039. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton KR, Lee SS, Urquhart JC, Jonker BP. A systematic review of outcome in intramedullary ependymoma and astrocytoma. J Clin Neurosci. 2019;63:168–75. doi: 10.1016/j.jocn.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Rijs K, Klimek M, Scheltens-De Boer M, Biesheuvel K, Harhangi BS. Intraoperative neuromonitoring in patients with intramedullary spinal cord tumor: A systematic review, meta-analysis, and case series. World Neurosurg. 2019;125:498–510.e2. doi: 10.1016/j.wneu.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Goyal A, Yolcu Y, Kerezoudis P, Alvi MA, Krauss WE, Bydon M. Intramedullary spinal cord metastases: An institutional review of survival and outcomes. J Neurooncol. 2019;142:347–54. doi: 10.1007/s11060-019-03105-2. [DOI] [PubMed] [Google Scholar]
- 15.Scibilia A, Terranova C, Rizzo V, Raffa G, Morelli A, Esposito F, et al. Intraoperative neurophysiological mapping and monitoring in spinal tumor surgery: Sirens or indispensable tools? Neurosurg Focus. 2016;41:E18. doi: 10.3171/2016.5.FOCUS16141. [DOI] [PubMed] [Google Scholar]
- 16.Verla T, Fridley JS, Khan AB, Mayer RR, Omeis I. Neuromonitoring for intramedullary spinal cord tumor surgery. World Neurosurg. 2016;95:108–16. doi: 10.1016/j.wneu.2016.07.066. [DOI] [PubMed] [Google Scholar]
- 17.Cheng JS, Ivan ME, Stapleton CJ, Quinones-Hinojosa A, Gupta N, Auguste KI. Intraoperative changes in transcranial motor evoked potentials and somatosensory evoked potentials predicting outcome in children with intramedullary spinal cord tumors. J Neurosurg Pediatr. 2014;13:591–9. doi: 10.3171/2014.2.PEDS1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.André-Obadia N, Mauguière F. Electrophysiological testing in spinal cord tumors. Neurochirurgie. 2017;63:356–65. doi: 10.1016/j.neuchi.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Siller S, Szelényi A, Herlitz L, Tonn JC, Zausinger S. Spinal cord hemangioblastomas: Significance of intraoperative neurophysiological monitoring for resection and long-term outcome. J Neurosurg Spine. 2017;26:483–93. doi: 10.3171/2016.8.SPINE16595. [DOI] [PubMed] [Google Scholar]
- 20.Ghadirpour R, Nasi D, Iaccarino C, Romano A, Motti L, Sabadini R, et al. Intraoperative neurophysiological monitoring for intradural extramedullary spinal tumors: Predictive value and relevance of D-wave amplitude on surgical outcome during a 10-year experience. J Neurosurg Spine. 2018;30:259–67. doi: 10.3171/2018.7.SPINE18278. [DOI] [PubMed] [Google Scholar]
- 21.Barzilai O, Lidar Z, Constantini S, Salame K, Bitan-Talmor Y, Korn A. Continuous mapping of the corticospinal tracts in intramedullary spinal cord tumor surgery using an electrified ultrasonic aspirator. J Neurosurg Spine. 2017;27:161–8. doi: 10.3171/2016.12.SPINE16985. [DOI] [PubMed] [Google Scholar]
- 22.Lakomkin N, Mistry AM, Zuckerman SL, Ladner T, Kothari P, Lee NJ, et al. Utility of Intraoperative Monitoring in the Resection of Spinal Cord Tumors: An Analysis by Tumor Location and Anatomical Region. Spine (Phila Pa 1976) 2018;43:287–94. doi: 10.1097/BRS.0000000000002300. [DOI] [PubMed] [Google Scholar]
- 23.Daniel JW, Botelho RV, Milano JB, Dantas FR, Onishi FJ, Neto ER, et al. Intraoperative Neurophysiological Monitoring in Spine Surgery: A Systematic Review and Meta-Analysis. Spine (Phila Pa 1976) 2018;43:1154–60. doi: 10.1097/BRS.0000000000002575. [DOI] [PubMed] [Google Scholar]
- 24.Kumar N, G V, Ravikumar N, Ding Y, Yin ML, Patel RS, et al. Intraoperative Neuromonitoring (IONM): Is There a Role in Metastatic Spine Tumor Surgery? Spine (Phila Pa 1976) 2019;44:E219–24. doi: 10.1097/BRS.0000000000002808. [DOI] [PubMed] [Google Scholar]
- 25.Taricco MA, Guirado VM, Fontes RB, Plese JP. Surgical treatment of primary intramedullary spinal cord tumors in adult patients. Arq Neuropsiquiatr. 2008;66:59–63. doi: 10.1590/s0004-282x2008000100014. [DOI] [PubMed] [Google Scholar]
- 26.Guirado VM, Taricco MA, Nobre MR, Couto EB, Jr, Ribas ES, Meluzzi A, et al. Quality of life in adult intradural primary spinal tumors: 36-Item Short Form Health Survey correlation with McCormick and Aminoff-Logue scales. J Neurosurg Spine. 2013;19:721–35. doi: 10.3171/2013.8.SPINE12706. [DOI] [PubMed] [Google Scholar]
- 27.Novak K, Widhalm G, de Camargo AB, Perin N, Jallo G, Knosp E, et al. The value of intraoperative motor evoked potential monitoring during surgical intervention for thoracic idiopathic spinal cord herniation. J Neurosurg Spine. 2012;16:114–26. doi: 10.3171/2011.10.SPINE11109. [DOI] [PubMed] [Google Scholar]
- 28.Deletis V, Bueno De Camargo A. Interventional neurophysiological mapping during spinal cord procedures. Stereotact Funct Neurosurg. 2001;77:25–8. doi: 10.1159/000064585. [DOI] [PubMed] [Google Scholar]


