Abstract
Background
People with Down syndrome (DS) develop Alzheimer’s disease (AD) at an earlier age of onset than those with sporadic AD. AD neuropathology is typically present in DS by 40 years of age with an onset of dementia approximately 10 years later. This early onset is due to the overexpression of amyloid precursor protein from the third copy of chromosome 21. Cerebrovascular neuropathology is thought to contribute in 40–60% of cases sporadic AD. However, the vascular contribution to dementia in people with DS has been relatively unexplored. We hypothesised that vascular perfusion is compromised in older adults with DS relative to younger individuals and is further exacerbated in those with dementia.
Method
Cerebral blood flow (CBF) was measured using pulsed arterial spin labelling in 35 cognitively characterised adults with DS (26–65 years). DS participants were also compared with 15 control subjects without DS or dementia (26–65 years). Linear regression evaluated the difference in CBF across groups and diagnosis along with assessing the association between CBF and cognitive measures within the DS cohort.
Results
Cerebral blood flow was significantly lower among DS participants with probable AD compared with controls (P = 0.02) and DS participants with no dementia (P = 0.01). Within the DS cohort, CBF was significantly associated with the Severe Impairment Battery (SIB) measure and the Dementia Questionnaire for People with Learning Disabilities (DLD) rating (F3,25 = 5.13; P = 0.007). Both the SIB (β = 0.74; t = 2.71; P = 0.01) and DLD (β = −0.96; t = −3.87; P < 0.001) indicated greater impairment as global CBF decreased. Age was significantly associated with CBF among participants with DS. There was a non-linear effect of age, whereby CBF declined more rapidly after 45 years of age.
Conclusions
This preliminary study of CBF in DS indicates that cerebrovascular pathology may be a significant contributor to dementia in DS. CBF was associated with diagnosis, cognition and age. Notably, CBF decreases at a greater rate after age 45 and may represent a significant prodromal event in AD progression.
Keywords: aging, Alzheimer’s disease, cerebral blood flow, Down syndrome, neuroimaging
1. Background
Down syndrome (DS) occurs in 1 in 700 live births in the United States (Parker et al. 2010) with 95% of infants born with an extra copy of chromosome 21 (Mutton et al. 1996). The amyloid precursor protein is located on chromosome 21 (Tanzi et al. 1987). Triplication of chromosome 21 yields a 1.5-fold increase in amyloid-beta (Aβ) protein (Oyama et al. 1994; Prasher et al. 1998). Increased expression of amyloid precursor protein is thought to cause the accumulation of neurofibrillary tangles and neurotic plaques (Citron et al. 1992; Selkoe 1994), the characteristic neuropathology observed in Alzheimer’s disease (AD).
Due to improved life expectancy, AD is a concern for individuals with DS and their caregivers. Life expectancy for individuals with DS has increased dramatically over the last few decades (Yang et al. 2002; Glasson et al. 2003; Presson et al. 2013). Through the 1970s, the average age of death was 10 years, but over the past four decades, life expectancy for a person with DS had increased to 50 years (Presson et al. 2013). Now that individuals with DS are living longer, dementia and AD are a new concern.
Researchers have observed that by age 40 years individuals with DS have significant AD-related neuropathology (Wisniewski et al. 1985; Lemere et al. 1996). Despite significant AD pathology by age 40, most individuals with DS are not diagnosed with AD until the age of 55 (Sinai et al. 2018). Moreover, despite AD-related pathology not everyone with DS had a dementia diagnosis (Castro et al. 2017). Early identification of AD pathology in DS has the potential to elucidate possible targets for intervention and shorten the delay between pathology and clinical diagnosis. Furthermore, identifying factors associated with dementia in DS may help determine why some individuals do not develop dementia.
One such factor might be cerebrovascular disease. Cerebrovascular disease is a comorbidity that could accelerate disease progression and emergence of clinical symptoms (Snyder et al. 2015). In individuals without DS, 5.7–45% exhibited AD with cerebrovascular disease (Jellinger 2013). Additionally, decreased cerebral blood flow (CBF) has been observed prior to Aβ accumulation in mouse models (Koike et al. 2010; Garcia-Alloza et al. 2011). However, it is unclear if hypoperfusion is a cause or consequence of AD (Mazza et al. 2011).
Other health conditions are common risk factors for cerebrovascular disease and AD. Epidemiological studies show that risk factors for vascular diseases, including hypertension, diabetes, hypercholesterolemia, hyperhomocysteinemia and the apolipoprotein-003B54 genotype, are also risk factors for AD (de la Torre 2002). In an autopsy study of AD and non-AD cases, atherosclerosis-induced hypoperfusion was significantly associated with AD pathology (Roher et al. 2003). Shared risk factors could yield shared pathophysiological mechanisms.
Down syndrome is a unique population in which to study the relationship between CBF and AD. First, AD pathology in DS is age dependent. Thus, within cohorts of people with DS age is a strong surrogate indicator of disease progression. Second, individuals with DS are protected against many of the vascular risk factors that are associated with CBF and AD. Specifically, individuals with DS do not exhibit atheroma and have lower blood pressure than similarly aged adults without DS (Murdoch et al. 1977; Brattström et al. 1987; van de Louw et al. 2009). Thus, measuring CBF in individuals with fewer vascular risk factors may help elucidate the relationship between global CBF and AD.
The objective of the current study is to determine the association between age and CBF in individuals with DS. Age and CBF were of primary interest due to previously reported relationships in AD. To this end, the relationship between CBF and clinical diagnosis was first determined in people with and without DS. Next, we determined the degree of association between age, clinical scores and CBF within the DS cohort.
The first aim assessed the relationship between CBF and diagnosis in people with DS, with and without AD, and compared these results to those of typically developing controls without AD. We hypothesised that CBF will be lower overall among people with DS compared with controls and further decrease with AD diagnosis. Secondarily, we measured the degree of association between clinical scores and CBF within the DS cohort. We hypothesised that clinical scores will show increasing impairment with decreasing CBF, indicating greater cognitive dysfunction. The final aim addressed how CBF changes with age. Specifically, linear and non-linear models assessed whether CBF decreases constantly with increasing age.
2. Methods
2.1. Participants
Arterial spin labelling (ASL) measures were collected from a single visit during an ongoing longitudinal study of aging in DS (Powell et al. 2014). We recruited participants older than 25 years through local DS support groups and residential facilities primarily in Kentucky, Indiana and southern Ohio. Non-DS control participants were recruited from the general population through flyers and personal contacts. These controls were free of neurological and psychiatric medical conditions. Selection of these participants was first based on the age distribution of the participants with DS such that controls ranged in age from 25 to 59 and were ±3 years of age from a DS participant. After age frequency matching, they were then selected based on the gender distribution of the DS sample. The study was approved by a university Institutional Review Board.
Because thyroid dysfunction is common in individuals with DS, we included these participants if their thyroid hormone levels were medically controlled. People with DS who were demented and may have been prescribed AD medications, and these individuals were also included in the study. Participants were excluded from the current analysis if they had active and unstable medical conditions (e.g., cardiovascular complications).
Dementia diagnosis was determined through a consensus review of each participant. Medical records were also reviewed to identify medical conditions and level of intellectual disability. The expert panel consisted of two neurologists and two psychologists using NINCDS-ADRDA criteria for dementia (McKhann et al. 2011). DS participants were classified as having no dementia, indeterminate dementia or dementia. Those with indeterminate dementia were individuals who were demonstrating cognitive and/or functional declines that were not sufficiently severe enough reach the probable dementia level or had other contributing factors (e.g. life event and medical condition). The study cohort included 35 adults with DS with 7 people meeting criteria for dementia (DSAD+) (Table 1). Upon request, 13 (37%) participants were provided with lorazepam (1 or 2 mg) to manage claustrophobia during magnetic resonance imaging scanning. Because lorazepam has been shown to decrease global CBF (Matthew et al. 1995), we evaluated whether there was any difference in lorazepam use across DS groups or any association with age. There was no association between lorazepam usage with DS groups or age. Thus, lorazepam was not included as a covariate in the analyses.
Table 1.
Participant demographics
| Characteristic | Control (n = 15) | DS – No Dementia (n = 20) | DS – Indeterminate (n = 7) | DS – Probable AD (n = 7) | P value |
|---|---|---|---|---|---|
| Age | 50.48 (11.92) | 39.71 (10.15) | 44.23 (8.82) | 58.12 (4.75) | <0.001 |
| Female | 73.33% (11) | 40.00% (8) | 85.71% (6) | 57.14% (4) | 0.096 |
| Global CBF | 43.90 (5.66) | 46.50 (6.42) | 46.83 (9.72) | 31.69 (9.05) | <0.001 |
| RAS | |||||
| None | 26.67% (4) | 30.00% (6) | 28.57% (2) | 0.00% (0) | 0.126 |
| Mild | 46.67% (7) | 35.00% (7) | 42.86% (3) | 0.00% (0) | |
| Moderate | 20.00% (3) | 20.00% (4) | 28.57% (2) | 57.14% (4) | |
| Severe | 6.67% (1) | 15.00% (3) | 0.00% (0) | 42.86% (3) | |
| Total grey volume | 0.29 (0.01) | 0.31 (0.03) | 0.33 (0.06) | 0.25 (0.03) | <0.001 |
| Lorazepam used | – | 30.00% (6) | 42.85% (3) | 42.85% (3) | 0.691 |
| BPT | – | 72.65 (5.32) | 65.14 (11.89) | 69.00 (7.44) | 0.083 |
| SIB | – | 87.90 (9.24) | 80.43 (16.81) | 77.25 (11.35) | 0.142 |
| DLD | – | 7.42 (7.17) | 15.57 (7.66) | 42.29 (13.83) | <0.001 |
AD, Alzheimer’s disease; BPT, Brief Praxis Test; CBF, cerebral blood flow; DLD, Dementia Questionnaire for People with Learning Disabilities; DS, Down syndrome; RAS, residual arterial signal; SIB, Severe Impairment Battery.
Cognitive impairment was measured using informant and cognitive assessments. Dementia Questionnaire for People with Learning Disabilities (DLD) was collected as an informant rating of dementia (Evenhuis 1996). Two primary cognitive measures were also conducted during the study visit: the Severe Impairment Battery (SIB) (Panisset et al. 1994) and Brief Praxis Test (BPT) (Dalton 2008).
We also recruited 15 age-matched and gender-matched (by frequency matching) non-DS control participants (CTL). CTLs reported no history of significant neurologic, cardiovascular or psychiatric disorders and had no evidence of dementia. All participants completed informed written consent or assent with guardian consent for the DS participants. The study and research procedures were approved by the University of Kentucky Institutional Review Board.
2.2. Neuroimaging
Magnetic resonance imaging experiments were performed using a 3 T TIM Siemens MR scanner at the Magnetic Resonance Imaging and Spectroscopy Center at the University of Kentucky. A three-dimensional (3D) pulsed ASL (PASL) sequence with a gradient and spin echo readout was used for CBF measurement (Alsop et al. 2015). The parameters were as follows: TR/TE/TI (inflow time)/TS (clipping saturation time) = 4500/13.04/1900/500 ms, slab thickness = 154 mm, slice-selective labelling gradient = 10 mT/m, matrix = 64 × 64 × 44, field of view (FOV) = 224 × 224 × 154 mm. Nine tagged and untagged data sets were acquired. Five additional M0 images were acquired with no labelling for CBF quantification. DS participants were more likely to move while in the scanner than the control participants; therefore, a minimum number of non-motion corrupted data sets were set in order for the participant to be included in the analysis. To be included in the analysis, participants were required to have at least six tagged and untagged datasets and three M0 data sets not corrupted by motion artefact. A total of 10 participants did have at least one data set excluded; however, all participants met the minimum criteria to be included in the analysis.
Selected data sets were coregistered using an intensity-based registration algorithm in MATLAB (Mathworks, Natick, MA, USA) before being averaged to yield a tagged, an untagged and an M0 volume for each subject. Quantitative CBF maps (in mL/100 g/min) were calculated using in-house MATLAB software applying the equation (Alsop et al. 2015)
where λ is the blood–brain partition coefficient (assumed to be 0.9 mL/g) (Herscovitch and Raichle 1985), SIuntag and SItag are the average signal intensity of the untagged and tagged volumes, respectively, T1blood is the longitudinal relaxation time of the blood (assumed to be 1.65 s at 3.0 T) (Lu et al. 2004). SIM0 is the average signal intensity of the M0 volumes, and α is the labelling efficiency (assumed to be 0.98 for PASL) (Wong et al. 1998).
For CBF analysis, a single return on investment (ROI) encompassing the entire brain volume was drawn manually with the assistance of the in-house MATLAB software. This ROI was drawn using the M0 image for its higher contrast to noise ratio and included grey and white matter (WM) regions, ventricles and cerebellum. Global CBF was calculated as the average of all voxels in the ROI.
2.3. Residual arterial signal scoring
Preliminary observation of the CBF maps revealed that in some subjects a portion of the ASL signal is retained in the large arteries of the brain. The large ASL signal in the arteries gives the appearance of a low-resolution angiogram. An example of minimal, moderate and severe residual arterial signal (RAS) is shown in Fig. 1. To quantify the effect of this RAS, two expert reviewers who were blinded to participant information reviewed the same axial slice from each subject to determine if RAS was severe or not. A third blinded reviewer was consulted in the case of disagreement. The Cohen kappa statistic for inter-observer agreement was κ = 0.79 (P < 0.001).
Figure 1.
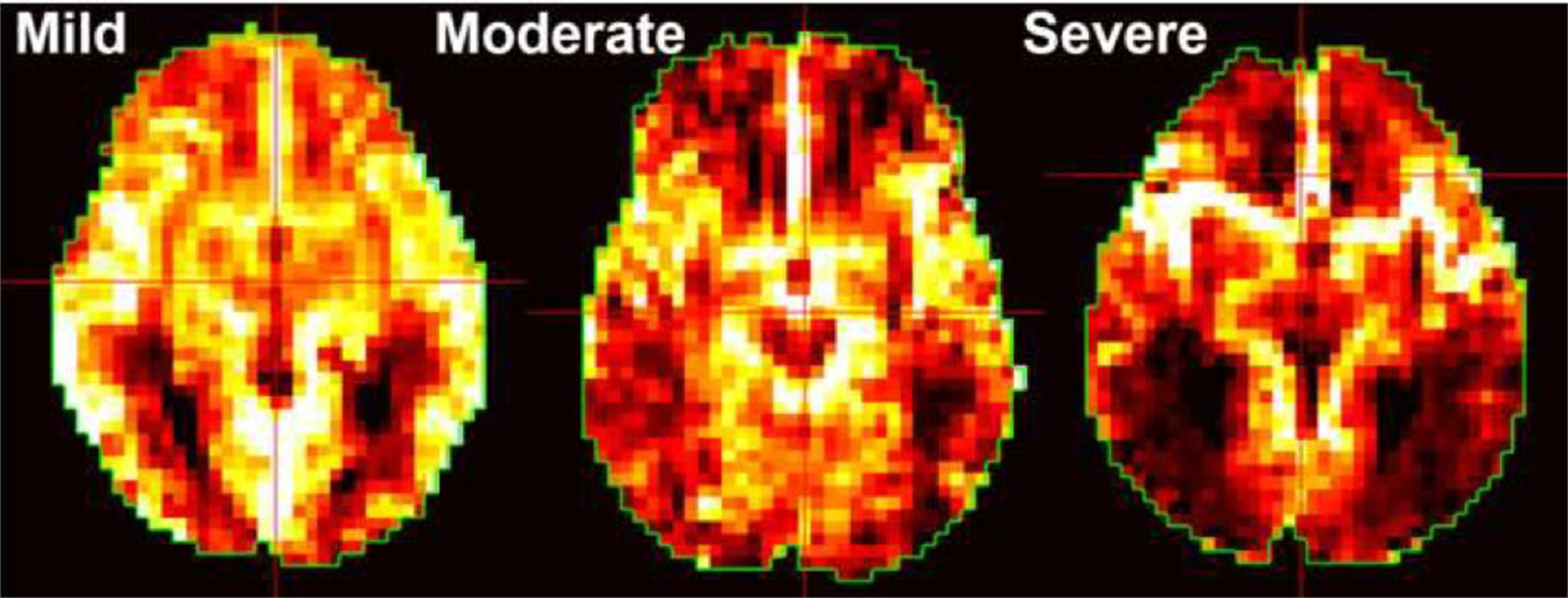
Examples of residual arterial signal scores. Examples of residual arterial sign severity when present.
2.4. Statistics
Cohen’s kappa (Cohen 1960) was used to determine the level of agreement between raters of RAS. The strength of agreement for the kappa statistic are as follows: <0 poor, 0–2 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial and 0.81–1.00 almost perfect agreement (Landis and Koch 1977). Chi-squared and one-way analysis of variance (ANOVA) tests evaluated differences in participant demographics. Post hoc t-tests with Tukey correction evaluated group differences if ANOVA was significant.
Data for all 49 participants, controls and DS, were analysed using linear regression models to evaluate the relationship between group and CBF, controlling for proportion of total grey volume. Only DS participants were used in the analyses of CBF with clinical measures. Of the 34 DS participants, four participants were excluded for missing SIB and BPT scores (n = 3) or DLD scores (n = 1). Thus, 30 DS participants were analysed. Multivariate ANOVA assessed whether CBF was significantly associated with clinical scores. Follow-up ANOVAs assessed specific relationships between CBF and cognitive measures, controlling for proportion of total grey volume.
Finally, linear regression also modelled the linear and polynomial effects of age on CBF. All 34 DS participants were included in the analyses. The linear and polynomial models were compared using Akaike information criterion (AIC) and Bayesian information criterion (BIC), the model with the lowest AIC and BIC was considered the model with superior fit. All statistical tests were two-tailed, and the alpha-level was set at 0.05. All analyses were completed in R 3.6.1.
3. Results
A total of 49 participants (n = 34 with DS) completed the neuroimaging protocol. Of the participants with DS, 20 were classified as no dementia (DSnon), 7 diagnosed with indeterminate dementia (DSi) and 7 with probable AD (DSAD). Full participant characteristics are provided in Table 1. Age was significantly different across groups (F3,45 = 7.05; P < 0.001). DSnon were significantly younger than controls (Mean Difference = −10.77; 95% Confidence Interval (CI): −19.94 to −1.61; P = 0.015) and DSAD (Mean Difference = −18.41; 95% CI: −30.20 to −6.63; P < 0.001). The proportion of male and female participants was not significantly different across groups (; P = 0.10). There was no significant effect of group on RAS (; P = 0.14) or lorazepam use within the DS cohort (; P = 0.69).
Total CBF (F3,45 = 8.09; P < 0.001) and total grey matter (F3,45 = 9.05; P < 0.001) were also significantly different across groups. When controlling for total grey matter, there was still a significant effect of group on CBF (F3,44 = 8.22; P < 0.001) (Fig. 2). DSAD had significantly lower global CBF compared with controls (t44 = −2.96; P = 0.025; Mean Difference = −10.41; 95% CI: −17.29 to −3.53) and DSnon (t44 = −3.20; P = 0.013; Mean Difference = −12.02; 95% CI: −19.39 to −6.65). There was a trend for DSAD to have lower global CBF than DSi (t44 = −2.59; P = 0.06; Mean Difference = −11.80; 95% CI: −20.73 to −2.86). There were no significant differences between controls, DSnon or DSi groups (all Ps > 0.05).
Figure 2.
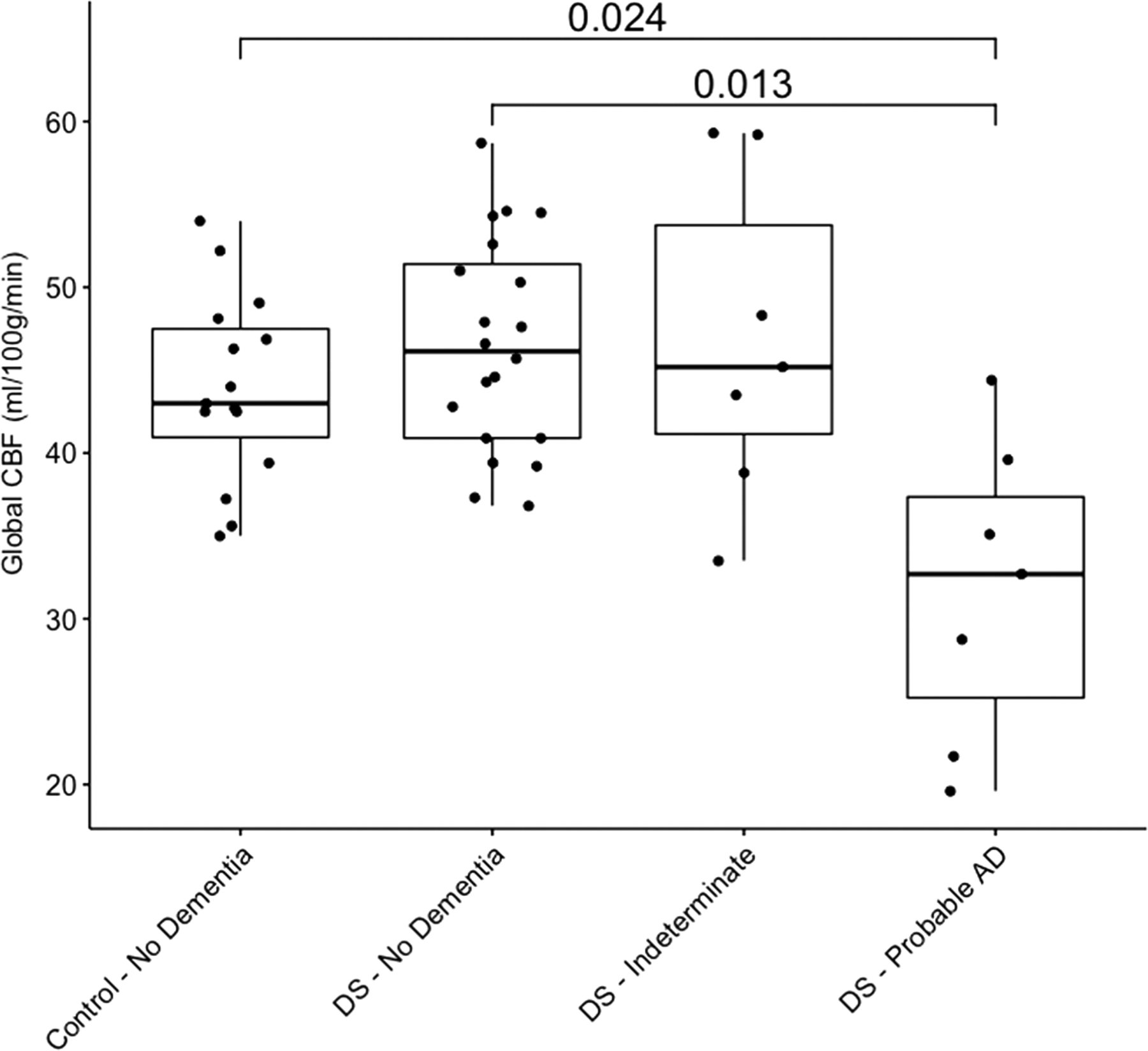
Effect of group global cerebral blood flow. Individual data points for cerebral blood flow by group are plotted along with summary boxplots and significant pairwise comparisons and associated P values. DS, Down syndrome.
There was a significant effect of group on DLD (F2,30 = 38.53; P < 0.001), but no significant effect on SIB or BPT (both Ps > 0.05). DSAD had significantly higher DLD scores compared with DSnon (Mean Difference = 34.84; 95% CI: 25.07 to 44.66; P < 0.001) and DSi (Mean Difference = 26.17; 95% CI: 14.87 to 38.56; P < 0.001). There was no significant difference between DSnon and DSi (P > 0.05).
Within the DS cohort, global CBF was associated with clinical scores (F3,25 = 5.13; P = 0.007) even when controlling for total grey matter volume. Specifically, there was a significant positive association between global CBF and total SIB (β = 0.74; t = 2.71; P = 0.01) and a significant negative association between global CBF and total DLD (β = −0.96; t = −3.87; P < 0.001). The significant associations between global CBF and clinical scores are depicted in Fig. 3. Thus, poorer cognitive test scores were associated with lower CBF.
Figure 3.
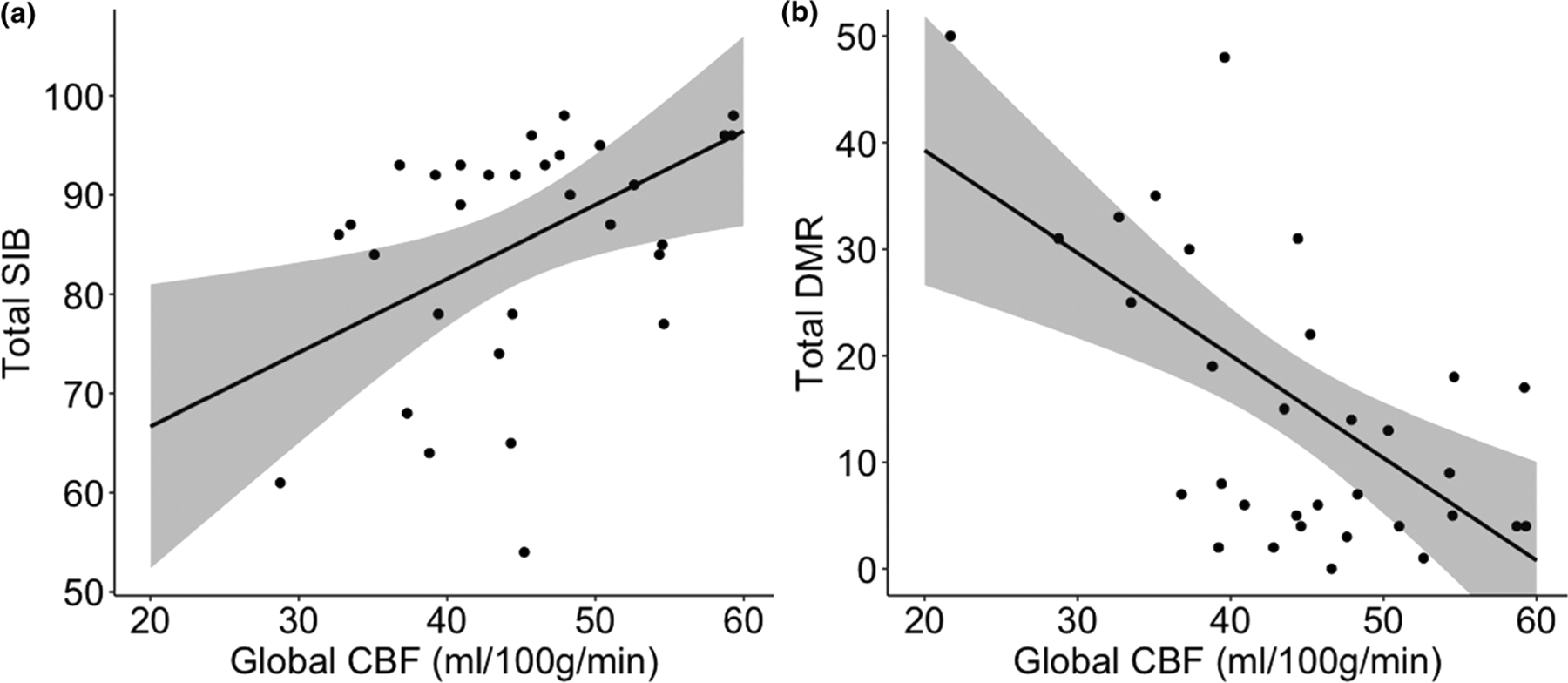
Association between global cerebral blood flow and clinical scores in Down syndrome. Model predicted results and 95% confidence interval for depicting relationship between (A) Severe Impairment Battery or (B) Dementia Questionnaire for People with Learning Disabilities total score and global cerebral blood flow. CBF, cerebral blood flow.
To evaluate the effect of age on CBF in DS, a linear and polynomial model was fit to the data to determine whether CBF changes at a continuous rate with age. Based on AIC and BIC values, the polynomial model (AIC = 240.46; BIC = 248.09) provided the best fit compared with the linear model (AIC 244.77; BIC = 250.87). The adjusted R2 for the polynomial model was 37.42%. Results for both models are provided in Table 2. Figure 4 depicts the rapid decline in CBF after age 45.
Table 2.
Linear and polynomial effects of age on global cerebral blood flow in Down syndrome: coefficients, 95% confidence intervals, T-statistic and P values
| Characteristic | Linear | Polynomial | Mediation |
|---|---|---|---|
| Age | β = −0.13 (95% CI: −0.41 to 0.16) | β = 2.26 (95% CI: 0.98 to 4.17) | β = 2.13 (95% CI: 0.26 to 4.01) |
| t = −0.88; P = 0.39 | t = 2.32; P = 0.03 | t = 2.23; P = 0.03 | |
| Age2 | β = −0.03 (95% CI: −0.05 to −0.01) | β = −0.02 (95% CI: −0.04 to −0.01) | |
| t = −2.47; P = 0.02 | t = −2.28; P = 0.03 | ||
| Total grey volume | β = 94.97 (95% CI: 26.84 to 163.09) | β = 89.77 (95% CI: 26.52 to 153.02) | β = 67.02 (95% CI: 6.57 to 127.47) |
| t = 2.73; P = 0.02 | t = 2.78; P = 0.01 | t = 2.17; P = 0.04 | |
| Residual arterial sign: severe vs. mild | – | – | β = −9.18 (95% CI: −16.89 to −1.46) |
| t = −2.33; P = 0.03 | |||
| Residual arterial sign: moderat vs. mild | – | – | β = −5.07 (95% CI: −11.40 to 1.26) |
| t = −1.57; P = 0.13 | |||
| Residual arterial sign: none vs. mild | – | – | β = 2.15 (95% CI: −5.15 to 9.45) |
| t = 0.58; P = 0.57 | |||
| Intercept | β = 20.33 (95% CI: −8.98 to 49.63) | β = −28.29 (95% CI: −75.43 to 18.84) | β = −18.77 (95% CI: −65.98 to 28.43) |
| t = 1.36; P = 0.19 | t = 1.18; P = 0.25 | t = −0.78; P = 0.44 | |
| Observations | 34 | 34 | 34 |
| R2 | 0.32 | 0.43 | 0.57 |
| Adjusted R2 | 0.27 | 0.37 | 0.47 |
| AIC | 244.77 | 240.46 | 237.17 |
| BIC | 250.87 | 248.09 | 249.38 |
| F statistic | 7.132,31; P = 0.003 | 7.583,30; P < 0.001 | 7.026,27; P < 0.001 |
AIC, Akaike information criterion; BIC, Bayesian information criterion; CI, confidence interval.
Figure 4.
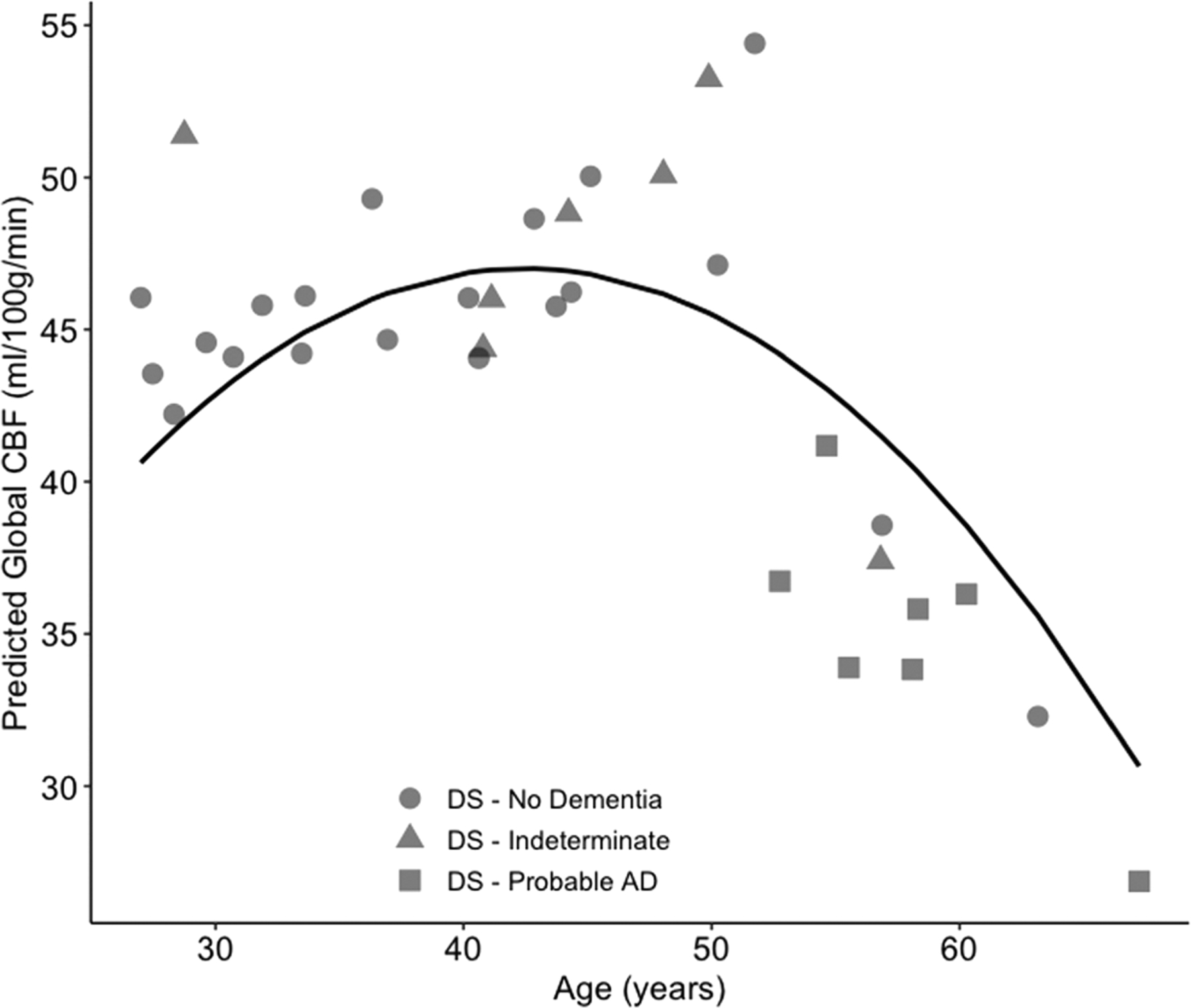
Rapid decline in global cerebral blood flow with increasing age in Down syndrome. Individual data points are plotted along with the model predicted result depicting the non-linear relationship between age and global cerebral blood flow. CBF, cerebral blood flow.
Because RAS could mediate or moderate the relationship between age and global CBF, we examined the relationship between RAS and global CBF. Based on Baron and Kenny’s mediation definition (Baron and Kenny 1986), in order for sufficient mediation to be present, three criteria must be met. First, the predictor (e.g. age) must be associated with the mediator (e.g. RAS). Second, the mediator (e.g. RAS) must be associated with the outcome (e.g. global CBF). Finally, the relationship strength between predictor (e.g. age) and outcome (e.g. global CBF) decreases when the mediator (e.g. RAS) is included in the model. To evaluate the relationship between age and RAS, a multinomial regression was used. Both linear and polynomial age were significantly associated with RAS (all Ps > 0.05). Overall, with increasing age, the probability of no RAS decreased while mild to severe RAS increased. Global CBF differed across levels of RAS (F3,29 = 3.46; P = 0.029), controlling for total grey volume. Pairwise comparisons with Tukey correction for multiple comparisons revealed severe RAS had had reduced global CBF compared with mild RAS (t = 2.84; P = 0.038; Mean Difference = −11.38; 95% CI: −19.22 to −3.54). While not significant, severe RAS had reduced global CBF compared with no RAS (t = −2.52; P = 0.078; Mean Difference = −11.24%; 95% CI: −19.98 to −2.49). Finally, we evaluated whether the strength of the relationship decreased between age and global CBF when RAS was included in the model. Adding RAS to the model decreased the strength of the relationship between age and CBF, suggesting a partial mediation effect. Results indicated a similar polynomial association between age and global CBF. However, with increasing RAS severity, global CBF was decreased (Table 2; Fig. 5).
Figure 5.
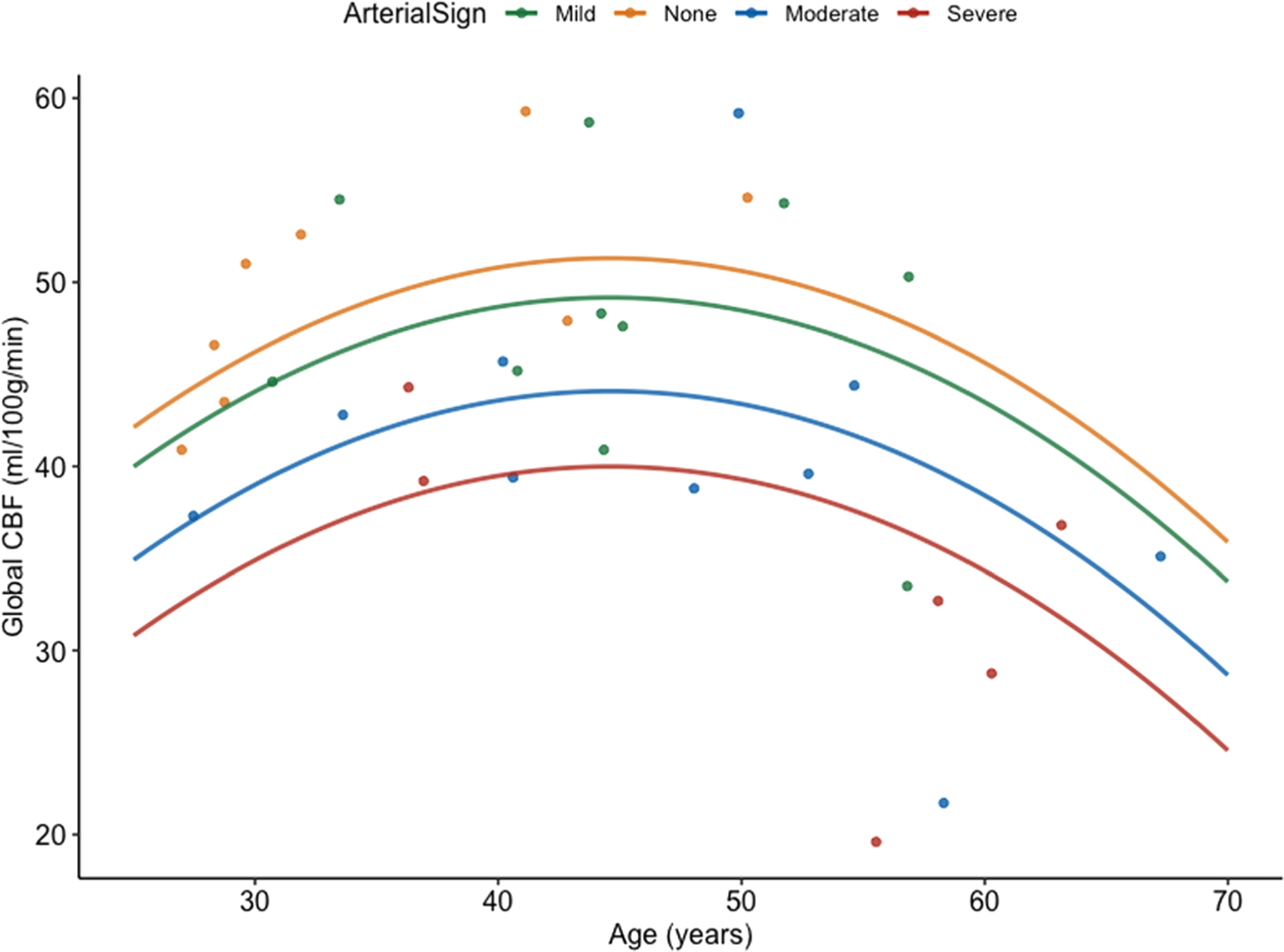
More severe residual arterial sign and increased age associated with decreased global cerebral blood flow. Individual data points are plotted along with the model predicted result depicting the effect of residual arterial sign severity on global cerebral blood flow across age. CBF, cerebral blood flow.
4. Conclusions
In this preliminary study, CBF was associated with an AD diagnosis, neuropsychological test scores and age in individuals with DS. The association between global CBF and AD diagnosis supports findings from the typically developing population. For example, a previous study in the typically developing population found lower CBF among those with MCI or AD compared with persons with subjective cognitive complaints (Binnewijzend et al. 2013). CBF has also been shown to be lower individuals with AD compared with cognitively normal controls (Schuff et al. 2009) (Roher et al. 2012). Furthermore, among typically developing individuals, decreased regional CBF was associated with worse clinically relevant outcome measures (Ones et al. 2012).
Secondly, the current study found that older individuals with DS demonstrate lower CBF values compared with younger individuals with DS. The age difference in CBF found in the current study supports and extends the existing literature. For example, Puri et al. (1994) described a marked decrease in CBF in a 52-year-old person with DS (Puri et al. 1994). Further, individuals with DS demonstrate 30% less carotid blood flow and 20% less vascular conductance when compared with individuals without DS (Wee et al. 2017). While these prior studies demonstrated reduced CBF in DS, it was unknown whether CBF changes with age in DS. Our findings add to the literature by identifying the non-linear change in CBF with age. Further, this accelerated decline in global CBF after age 45 may represent an early marker of impending dementia.
Previous studies detect region specific decreases in CBF within the precuneus and bilateral parietal cortices (Binnewijzend et al. 2013) when comparing individuals with MCI or AD to those with subjective cognitive complaints. Further regional differences were observed when comparing individuals with AD with cognitively normal individuals. The frontal lobe also displays marked decreases in CBF among those with AD compared with controls (Schuff et al. 2009). The current investigation found decreases in global CBF among individuals with DS and probable AD. These findings support what has been observed in the non-DS population. Lower CBF is associated with a faster rate of cognitive decline among individuals with AD (Benedictus et al. 2017). Importantly, the cross-sectional nature of this study limited our ability to evaluate the rate of cognitive decline. Future longitudinal studies within DS should also evaluate whether lower CBF is not only associated with current diagnosis but rate of conversion.
The current study also found global CBF significantly correlated with clinical measures. Determining the relationship between CBF and cognitive measures adds to the clinical relevance of CBF. Specifically, lower global CBF was associated with greater impairment on a neuropsychological battery (SIB) and informant’s report of dementia (DLD). This finding supports previous research in the non-DS population demonstrating associations between CBF and greater cognitive impairment (Roher et al. 2012; Okonkwo et al. 2014; Wolters et al. 2017). Furthermore, the significant association between CBF and clinical measures reinforces the clinical impact of reduced CBF. However, it remains unknown whether the rate of global CBF decrease is a prodromal marker of AD in DS.
Hypoperfusion produces cognitive decline as a result of neurovascular decoupling (Ogoh 2017) and WM compromise (Ota et al. 2009). Previous imaging studies demonstrate the effects of vascular compromise on WM health (Pantoni 2002; Wakita et al. 2002; Yin et al. 2018). Periventricular WM is particularly susceptible to compromised CBF (Pantoni 2002). Chronically reduced CBF generates changes in WM that are detectable via magnetic resonance imaging in the form of WM hyperintensities (Yata and Tomimoto 2014). Periventricular WM hyperintensities occur in sporadic AD (Holland et al. 2008) and predict the rate of progression of cognitive symptoms among individuals with AD (Brickman 2013). Thus, WM damage may contribute to impaired cognition in sporadic AD and AD in DS. For example, our previous work demonstrates that periventricular WM microstructure is compromised in individuals with DS with AD compared with individuals with DS without AD (Powell et al. 2014). Future longitudinal studies involving this cohort will be important to establish the sequence of perfusion and WM changes related to dementia evolution.
4.1. Limitations
The present study has some caveats that highlight areas that need further investigation. First, the PASL sequence used in this study may be sensitive to prolonged arterial transit times, which may manifest as RAS. For example, the 3D gradient and spin echo readout of our PASL sequence is known to suppress the signal intensity of faster moving arterial blood with the rapid and repeated application of gradients. This is similar to the signal suppression observed in diffusion weighted imaging (Petcharunpaisan et al. 2010). Thus, extreme delays in perfusion can lead to an apparent suppression of the measured signal intensity. Zaharchuk et al. (2009) observed a similarly effect in watershed regions, which are last to perfuse. However, to determine the role of RAS in our findings, we evaluated whether more severe RAS mediated the relationship between age and CBF. The mediation analysis indicated that RAS does partially mediate the relationship between age and CBF. Thus, more objective and direct measures of arterial transit time may better elucidate the relationships between age, CBF and arterial transit time. Differences in blood flow velocities could be a result of stenosis due to atherosclerosis. However, given that our DS volunteers typically do not have atherosclerosis or hypertension, it is unlikely that this is an underlying factor for DS. Another limitation is the frequency matching, ideally each DS participant would have had an age and sex matched control. Future studies should consider larger sample sizes and one to one matching. Finally, because cortical grey matter was significantly lower in the DSAD group and cortical grey matter is associated with perfusion, grey matter atrophy could cause a decrease in global CBF. However, because we controlled for total grey matter in our analyses, we believe that age and diagnosis provide added information and contribute to decreased global CBF above and beyond decreased grey matter.
The current investigation identified significant and clinically relevant relationships between CBF and diagnosis, cognitive measures and age. Global CBF is decreased among DS individuals with probable AD. Moreover, global CBF appears to decrease more rapidly after age 45. Identifying the significant “tipping point” for reduced CBF may help design future clinical trials and interventions.
Acknowledgements
The authors would like to thank Stacey Brothers, Katie McCarty and Roberta Davis for their assistance with data collection; we thank Anders Andersen and Alex Helman for their technical help, and Amelia Anderson-Mooney for conducting some of the clinical exams.
Source of funding
Funding for this project was supported by the following grants: Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD064993) and National Institute on Aging (T32 AG057461).
Footnotes
Conflict of Interest
No conflicts of interest have been declared.
References
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L et al. (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine 73, 102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM & Kenny DA (1986) The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology 51, 1173–82. [DOI] [PubMed] [Google Scholar]
- Benedictus MR, Leeuwis AE, Binnewijzend MA, Kuijer JP, Scheltens P, Barkhof F et al. (2017) Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. European Radiology 27, 1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewijzend MA, Kuijer JP, Benedictus MR, van der Flier WM, Wink AM, Wattjes MP et al. (2013) Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 267, 221–30. [DOI] [PubMed] [Google Scholar]
- Brattström L, Englund E & Brun A (1987) Does Down syndrome support homocysteine theory of arteriosclerosis? The Lancet 329, 391–2. [DOI] [PubMed] [Google Scholar]
- Brickman AM (2013) Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities. Current Neurology and Neuroscience Reports 13(12), 415–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro P, Zaman S & Holland A (2017) Alzheimer’s disease in people with Down’s syndrome: the prospects for and the challenges of developing preventative treatments. Journal of Neurology 264, 804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P et al. (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360, 672–4. [DOI] [PubMed] [Google Scholar]
- Cohen J (1960) A coefficient of agreement for nominal scales. Educational and Psychological Measurement 20, 37–46. [Google Scholar]
- Dalton AJ (2008) The dyspraxia scale for adults with Down syndrome. In: Neuropsychological Assessments of Dementia in Down Syndrome and Intellectual Disabilities (ed. Prasher V), pp. 67–89. Springer, London. [Google Scholar]
- Evenhuis HM (1996) Further evaluation of the Dementia Questionnaire for Persons with Mental Retardation (DLD). Journal of Intellectual Disability Research 40, 369–73. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Gregory J, Kuchibhotla KV, Fine S, Wei Y, Ayata C et al. (2011) Cerebrovascular lesions induce transient beta-amyloid deposition. Brain 134, 3697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD & Bittles AH (2003) Comparative survival advantage of males with Down syndrome. American Journal of Human Biology 15, 192–5. [DOI] [PubMed] [Google Scholar]
- Herscovitch P & Raichle ME (1985) What is the correct value for the brain – blood partition coefficient for water? Journal of Cerebral Blood Flow and Metabolism 5, 65–9. [DOI] [PubMed] [Google Scholar]
- Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ et al. (2008) Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke 39, 1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA (2013) Pathology and pathogenesis of vascular cognitive impairment – a critical update. Frontiers in Aging Neuroscience 5(17), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike MA, Green KN, Blurton-Jones M & Laferla FM (2010) Oligemic hypoperfusion differentially affects tau and amyloid-beta. The American Journal of Pathology 177, 300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR & Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33, 159–74. [PubMed] [Google Scholar]
- Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC & Selkoe DJ (1996) Sequence of deposition of heterogeneous amyloid beta-peptides and APOE in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiology of Disease 3, 16–32. [DOI] [PubMed] [Google Scholar]
- van de Louw J, Vorstenbosch R, Vinck L, Penning C & Evenhuis H (2009) Prevalence of hypertension in adults with intellectual disability in the Netherlands. Journal of Intellectual Disability Research 53, 78–84. [DOI] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X & van Zijl PC (2004) Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magnetic Resonance in Medicine 52, 679–82. [DOI] [PubMed] [Google Scholar]
- Matthew E, Andreason P, Pettigrew K, Carson RE, Herscovitch P, Cohen R et al. (1995) Benzodiazepine receptors mediate regional blood flow changes in the living human brain. Proceedings of the National Academy of Sciences of the United States of America 92, 2775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M, Marano G, Traversi G, Bria P & Mazza S (2011) Primary cerebral blood flow deficiency and Alzheimer’s disease: shadows and lights. Journal of Alzheimer’s Disease 23, 375–89. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JC, Rodger JC, Rao SS, Fletcher CD & Dunnigan MG (1977) Down’s syndrome: an atheroma-free model? British Medical Journal 2, 226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutton D, Alberman E & Hook EB (1996) Cytogenetic and epidemiological findings in Down syndrome, England and Wales 1989 to 1993. National Down Syndrome Cytogenetic Register and the Association of Clinical Cytogeneticists. J Med Genet 33, 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S (2017) Relationship between cognitive function and regulation of cerebral blood flow. The Journal of Physiological Sciences 67, 345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL et al. (2014) Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cerebral Cortex 24, 978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ones T, Midi I, Dede F, Tuncer N, Erdil TY, Onultan O et al. (2012) Initial mini-mental state and cerebral perfusion in Alzheimer’s disease. Clinical Neuroradiology 22, 219–26. [DOI] [PubMed] [Google Scholar]
- Ota M, Nemoto K, Sato N, Yamashita F & Asada T (2009) Relationship between white matter changes and cognition in healthy elders. International Journal of Geriatric Psychiatry 24, 1463–9. [DOI] [PubMed] [Google Scholar]
- Oyama F, Cairns NJ, Shimada H, Oyama R, Titani K & Ihara Y (1994) Down’s syndrome: up-regulation of beta-amyloid protein precursor and tau mRNAs and their defective coordination. Journal of Neurochemistry 62, 1062–6. [DOI] [PubMed] [Google Scholar]
- Panisset M, Roudier M, Saxton J & Boller F (1994) Severe impairment battery. A neuropsychological test for severely demented patients. Archives of Neurology 51, 41–5. [DOI] [PubMed] [Google Scholar]
- Pantoni L (2002) Pathophysiology of age-related cerebral white matter changes. Cerebrovascular Diseases 13, 7–10. [DOI] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE et al. (2010) Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Research. Part A, Clinical and Molecular Teratology 88, 1008–16. [DOI] [PubMed] [Google Scholar]
- Petcharunpaisan S, Ramalho J & Castillo M (2010) Arterial spin labeling in neuroimaging. World Journal of Radiology 2, 384–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D, Caban-Holt A, Jicha G, Robertson W, Davis R, Gold BT et al. (2014) Frontal white matter integrity in adults with Down syndrome with and without dementia. Neurobiology of Aging 35, 1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC et al. (1998) Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Annals of Neurology 43, 380–3. [DOI] [PubMed] [Google Scholar]
- Presson AP, Partyka G, Jensen KM, Devine OJ, Rasmussen SA, McCabe LL et al. (2013) Current estimate of Down syndrome population prevalence in the United States. The Journal of Pediatrics 163, 1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri BK, Zhang Z & Singh I (1994) SPECT in adult mosaic Down’s syndrome with early dementia. Clinical Nuclear Medicine 19, 989–91. [DOI] [PubMed] [Google Scholar]
- Roher AE, Debbins JP, Malek-Ahmadi M, Chen K, Pipe JG, Maze S et al. (2012) Cerebral blood flow in Alzheimer’s disease. Vascular Health and Risk Management 8, 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD et al. (2003) Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arteriosclerosis, Thrombosis, and Vascular Biology 23, 2055–62. [DOI] [PubMed] [Google Scholar]
- Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F et al. (2009) Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 5, 454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ (1994) Normal and abnormal biology of the beta-amyloid precursor protein. Annual Review of Neuroscience 17, 489–517. [DOI] [PubMed] [Google Scholar]
- Sinai A, Mokrysz C, Bernal J, Bohnen I, Bonell S, Courtenay K et al. (2018) Predictors of age of diagnosis and survival of Alzheimer’s disease in Down syndrome. Journal of Alzheimer’s Disease 61, 717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D et al. (2015) Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 11, 710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML et al. (1987) Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235, 880–4. [DOI] [PubMed] [Google Scholar]
- de la Torre JC (2002) Alzheimer disease as a vascular disorder: nosological evidence. Stroke 33, 1152–62. [DOI] [PubMed] [Google Scholar]
- Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M et al. (2002) Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Research 924, 63–70. [DOI] [PubMed] [Google Scholar]
- Wee S, Rosenberg A, Kanokwan B, Griffith G, Baynard T & Fernhall B (2017) Carotid vascular blood flow in individuals with Down syndrome following low body negative pressure challenge. The FASEB Journal 31, 840–921.27856557 [Google Scholar]
- Wisniewski K, Wisniewski H & Wen G (1985) Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Annals of Neurology 17, 278–82. [DOI] [PubMed] [Google Scholar]
- Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW et al. (2017) Cerebral perfusion and the risk of dementia: A population-based study. Circulation 136, 719–28. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB & Frank LR (1998) Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magnetic Resonance in Medicine 39, 702–8. [DOI] [PubMed] [Google Scholar]
- Yang Q, Rasmussen SA & Friedman JM (2002) Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet 359, 1019–25. [DOI] [PubMed] [Google Scholar]
- Yata K & Tomimoto H (2014) Chronic cerebral hypoperfusion and dementia. Neurology and Clinical Neuroscience 2, 129–34. [Google Scholar]
- Yin X, Zhou Y, Yan S & Lou M (2018) Effects of cerebral blood flow and white matter integrity on cognition in CADASIL patients. Frontiers in Psychiatry 9(741), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharchuk G, Bammer R, Straka M, Shankaranarayan A, Alsop DC, Fischbein NJ et al. (2009) Arterial spin-label imaging in patients with normal bolus perfusion-weighted MR imaging findings: pilot identification of the borderzone sign. Radiology 252, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]


