Cardiovascular disease (CVD) is increasingly common among new cancer patients, with a reported prevalence near 30% at diagnosis.1,2 Cancer patients with concurrent CVD have poorer outcomes than those without CVD.1–3 Despite the high prevalence of CVD and its prognostic significance, there is limited evidence to guide the care of this increasing population. One possible explanation may be the exclusion of CVD patients from clinical trials.3 In the US, pivotal clinical trials of anticancer drugs are reviewed by the Food and Drug Administration (FDA) for adequate representation and safety prior to approval and release.4 However, whether patients with CVD are well-represented in these important clinical trials is unknown.
Using the Drugs@FDA database, we manually identified all anticancer drugs and biologics given new-drug applications in adults by the FDA from January 1, 1998, to June 30, 2018.4 Non-cancer treatment therapies were excluded; IRB approval was not required. Latter-phase (II and III) clinical trials tied to drug-approvals were exhaustively identified through MEDLINE, clinicaltrial.gov, published abstracts, trial supplements, and publically available FDA drug-reviews. We did not included phase I trials due to their general focus on more healthy volunteers. Trial characteristics were derived from published manuscripts and summary FDA reviews. All trial data were extracted and reviewed by two independent reviewers, to determine whether patients with CVD were excluded. Corresponding authors were contacted in cases of ambiguity regarding exclusion. CVD was defined as hypertension, coronary artery disease (CAD), myocardial infarction, heart failure, cardiomyopathy, arrhythmia, valvular disease, thromboembolic disease, stroke, abnormal electrocardiogram, or any mention of CVD. Multivariable stepwise logistic regression was used to assess for trial characteristics associated with CVD exclusion. Two-sided P-values under .05 were considered statistically significant.
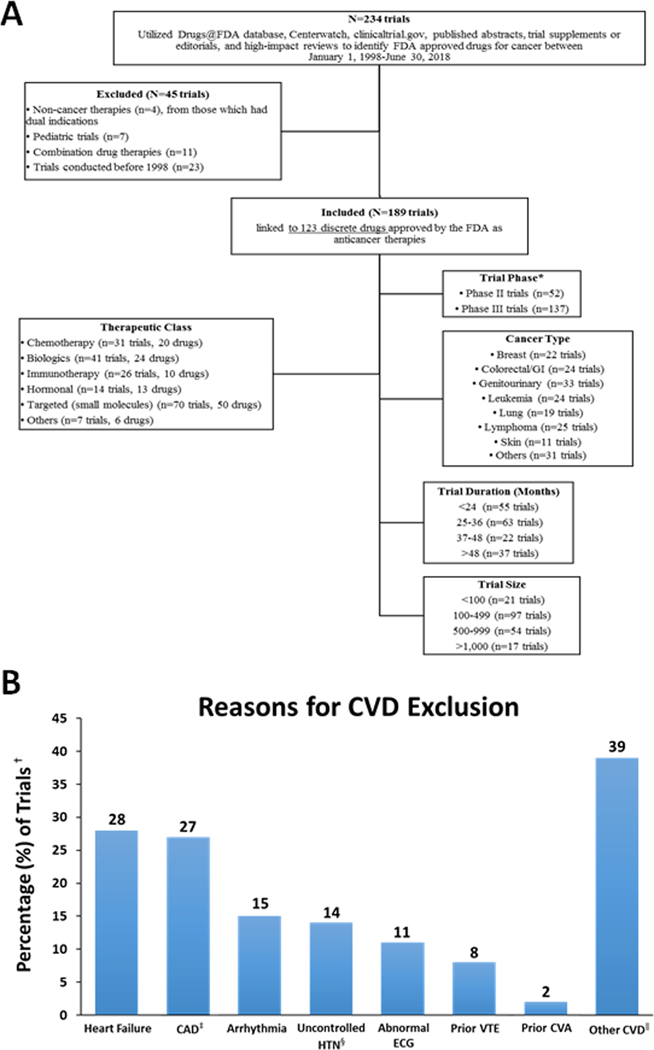
In total, 189 clinical trials, evaluating 97,556 participants, supporting 123 FDA-approved anticancer therapies were identified. Overall, 34% excluded patients with CVD (figure 1). Trials evaluating therapies within classes with prior cardiotoxicity reports (ex. immune checkpoint inhibitors), were more likely to exclude CVD [odds ratio: 3.09 (1.64–5.81)]. In multivariable analysis, no other measured trial characteristics, including the presence or absence of early-phase (safety) reports of excess-risk, were associated with CVD exclusion.
Figure 1.
Study consort diagram (A), and specific reasons for cardiovascular disease exclusion within pivotal cancer trials (B).
Abbreviations: BP, blood pressure; CAD, coronary artery disease; CVA, cerebrovascular accident; CVD, cardiovascular disease; ECG, electrocardiogram; HTN, hypertension; GI, gastrointestinal; VTE, venous thromboembolic disease.
*Several therapies were approved on the basis of breakthrough phase II data, with ongoing or as yet to be initiated phase III trials.
†The percentages reported reflect a denominator of 64 (the total number of trials with exclusion of cardiovascular disease patients).
‡CAD, includes myocardial infarction.
§Usually defined as >150/90 mmHg, consistent with mild (ie. stage 1) hypertension by the available contemporary guidelines.
∥Other CVD included valvular disease, myocarditis, or other mention of cardiovascular disease.
Heart failure was the most common exclusion criterion (28%), followed by prior coronary disease and arrhythmias (27 and 23%, respectively). Few trials used left ventricular ejection fraction (LVEF) thresholds for exclusion (8%). Recent myocardial infarction (<12 months) was a reason for exclusion in 20% of trials excluding CVD. Multiple exclusion criteria pertaining to CVD were reported in 37 trials (58%).
Among those trials using LVEF to define heart failure, all used thresholds of 50%, consistent with mild systolic dysfunction (8% of trials excluding CVD). For trials using blood pressure (BP) as an exclusion, thresholds were usually >150/90 mmHg (8%). Severe BP thresholds (BP >180/100 mmHg) were infrequently used (2%). Other threshold definitions of CVD exclusion also varied between trials (ex. use of a prolonged-QTc threshold of 440ms versus 480ms, or defining CAD as “reported angina” versus “documented acute myocardial infarction within 12-months”).
In this evaluation of clinical trials linked to FDA-approved anticancer therapies, over 1 in 3 trials excluded patients with underlying CVD, including >50% of breast cancer trials. These exclusion patterns were noted even in the absence of preceding reports of excess CVD risk. This is troublesome, particularly given the increasing prevalence of CVD among patients presenting with new cancer diagnoses, and the growing influx of novel cancer therapies. Furthermore, these exclusions may contribute to the discordance between cancer trial CVD event rates and those observed among real-world populations.1,2
Clinical trials primarily focused on the efficacy of anticancer interventions on disease control may elect to exclude CVD patients due to safety concerns, especially when interventions within therapeutic-classes known to associate with worsened clinical CVD are studied.3 However, the majority of anticancer therapies do not share this designation, and frequently within this review, CVD patients were excluded from interventions (e.g. immunotherapies) where no pharmacologic basis for CVD exclusion has been reported.3,5
Moreover, among trials evaluating therapies within classes with known potential cardiotoxicity, the measures for CVD exclusion were often inconsistent. Similarly, many trials employed non-discrete or ill-defined reasons for the exclusion of CVD. Yet, with the availability of more objective and reliable CVD measures, inconsistent application of discrete criteria is inadequate. The incorporation of standardized CVD definitions, including heart failure (ex. LVEF <50%) and hypertension (ex. BP >180/100 mmHg), may allow for more relevant and practical interpretations of potential drug safety.3 Study limitations include the focus on pivotal clinical trials, some trials may not have clearly noted CVD exclusion, and that trials did not report outcomes by CVD-status. Also, the number of patients with CVD excluded could not be determined, as these data were not reported.
In summary, patients with CVD were commonly excluded from clinical trials supporting FDA-approved contemporary cancer therapies. Given the increasing prevalence of CVD among patients presenting with cancer, judicious broadening of trial eligibility is needed.
Acknowledgements:
The manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The data, methods used in the analysis, and materials used that support the findings of this study are available from the corresponding author upon reasonable request.
Funding: This work was supported in part by an NIH P50-CA140158 grant. Dr. Awan was supported by NIH grant number R35-CA197734. Dr. Woyach was supported by NIH grant numbers K23-CA178183 and R01-CA197870. Dr. Addison was supported by NIH grant number K12-CA133250.
Footnotes
Disclosures: Dr. Awan has received research funding from Innate Pharma and provided consulting services to Gilead Sciences, Pharmacyclics, Inc, Janssen, Abbvie, and Novartis Oncology. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. [DOI] [PubMed] [Google Scholar]
- 2.Janssen-Heijnen ML, Szerencsi K, van de Schans SA, Maas HA, Widdershoven JW, Coebergh JW. Cancer patients with CVD have survival rates comparable to cancer patients within the age-cohort of 10 years older without cardiovascular morbidity. Crit Rev Oncol Hematol. 2010;76:196–207. [DOI] [PubMed] [Google Scholar]
- 3.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Drugs@FDA. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed September 11, 2018.
- 5.Sheng CC, Amiri-Kordestani L, Palmby T, Force T, Hong CC, Wu JC, Croce K, Kim G, Moslehi J. 21st Century Cardio-Oncology: identifying cardiac safety signals in the era of personalized medicine. JACC: Basic Transl Sci. 2016;1:386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]