Abstract
Sphingolipids and their synthetic enzymes have emerged as critical mediators in numerous diseases including inflammation, aging, and cancer. One enzyme in particular, sphingosine kinase (SK) and its product sphingosine-1-phosphate (S1P), has been extensively implicated in these processes. SK catalyzes the phosphorylation of sphingosine to S1P and exists as two isoforms, SK1 and SK2. In this review, we will discuss the contributions from the laboratory of Dr. Lina M. Obeid that have defined the roles for several bioactive sphingolipids in signaling and disease with an emphasis on her work defining SK1 in cellular fates and pathobiologies including proliferation, senescence, apoptosis, and inflammation.
1. Introduction
The concept of bioactive lipids has emerged and developed over the last three decades with the identification of several bioactive sphingolipids as key players in important biologic and pathophysiologic processes. Many of the advancements in the field have been driven by an ever-increasing appreciation of the pleotropic functions of this pathway and the potential for therapeutic targeting. Research in the laboratory of Dr. Lina M. Obeid has been critical in discovering and defining the role of bioactive sphingolipids, including roles of ceramide in apoptosis and senescence, and in particular the regulation and roles of sphingosine kinase 1 (SK1) and its lipid product sphingosine-1-phosphate (S1P) in numerous biologic processes. This review will discuss the significant contributions made by Dr. Obeid in these discoveries and advancements, especially those associated with the structure and function of SK1 as well as the role of this enzyme in cell signaling, inflammation and cancer.
1.1. Sphingolipid metabolism
Sphingolipid metabolism constitutes an elaborate network of interconnected metabolic pathways [1]. The de novo generation of sphingolipids begins with the condensation of palmitoyl Co-A and serine via serine palmitoyl transferase (SPT). The amino acid alanine, can also be utilized by SPT to form deoxysphinganine (dSA), a novel sphingoid base lacking the hydroxyl group at the first carbon [2]. Dihydrosphingosine is generated via the reduction of 3-ketosphinganine, which then can be acylated via ceramide synthases (CerS) to form dihydroceramide and desaturated to form ceramide. Ceramide can then be utilized to form sphingomyelin via sphingomyelin synthases (SMSs), ceramide-1-phosphate (C1P) via ceramide kinase (CerK), or glycosphingolipids via glucosylceramide synthase (GCS) and galactosylceramide synthase. Thus, ceramide sits at the hub of this intricate metabolic pathway with multiple forms of ceramide and many compartments in regulation of its metabolism [3]. The generation of S1P first requires the de-acylation of ceramide and the production of sphingosine by one of five ceramidases. Either SK1 or SK2 can then phosphorylate sphingosine generating S1P. S1P can be dephosphorylated by S1P phosphatases (SPP) to generate sphingosine which can then be recycled back to ceramide via CerS, or S1P can be irreversibly broken down by S1P lyase to ethanolamine phosphate and hexadecenal. The latter represents the only known metabolic ‘exit’ pathway for sphingolipids.
1.2. Sphingosine Kinase 1
SK1 has often been associated with “tipping the balance” from apoptosis and differentiation to proliferation by increasing levels of S1P and decreasing levels of ceramide and sphingosine (Fig. 1); therefore, it is not surprising that SK1 is highly regulated. SK1 expression is regulated at the transcriptional level by several transcription factors, many of which are involved in hypoxia and angiogenesis, including hypoxia inducible factor 2 alpha (HIF2α) [4,5], LIM-domain-only protein 2 (LM02) [6], and E2F transcription factors [7–9]. SK1 activity is regulated by numerous growth factors (TGFβ, VEGF, NGF, IGF) and cytokines (TNFα and IL1β) leading to proliferation, migration, inflammation and angiogenesis in several cell types [10–20]. SK1 protein levels are also downregulated by proteolysis following exposure to DNA damage agents [21] and TNFα [22]. These studies, together with the critical contributions made by Dr. Obeid’s laboratory, demonstrate the importance of SK1 regulation in cellular fates and biologies including proliferation, senescence, apoptosis and inflammation (Fig. 2 and 3).
Fig. 1.
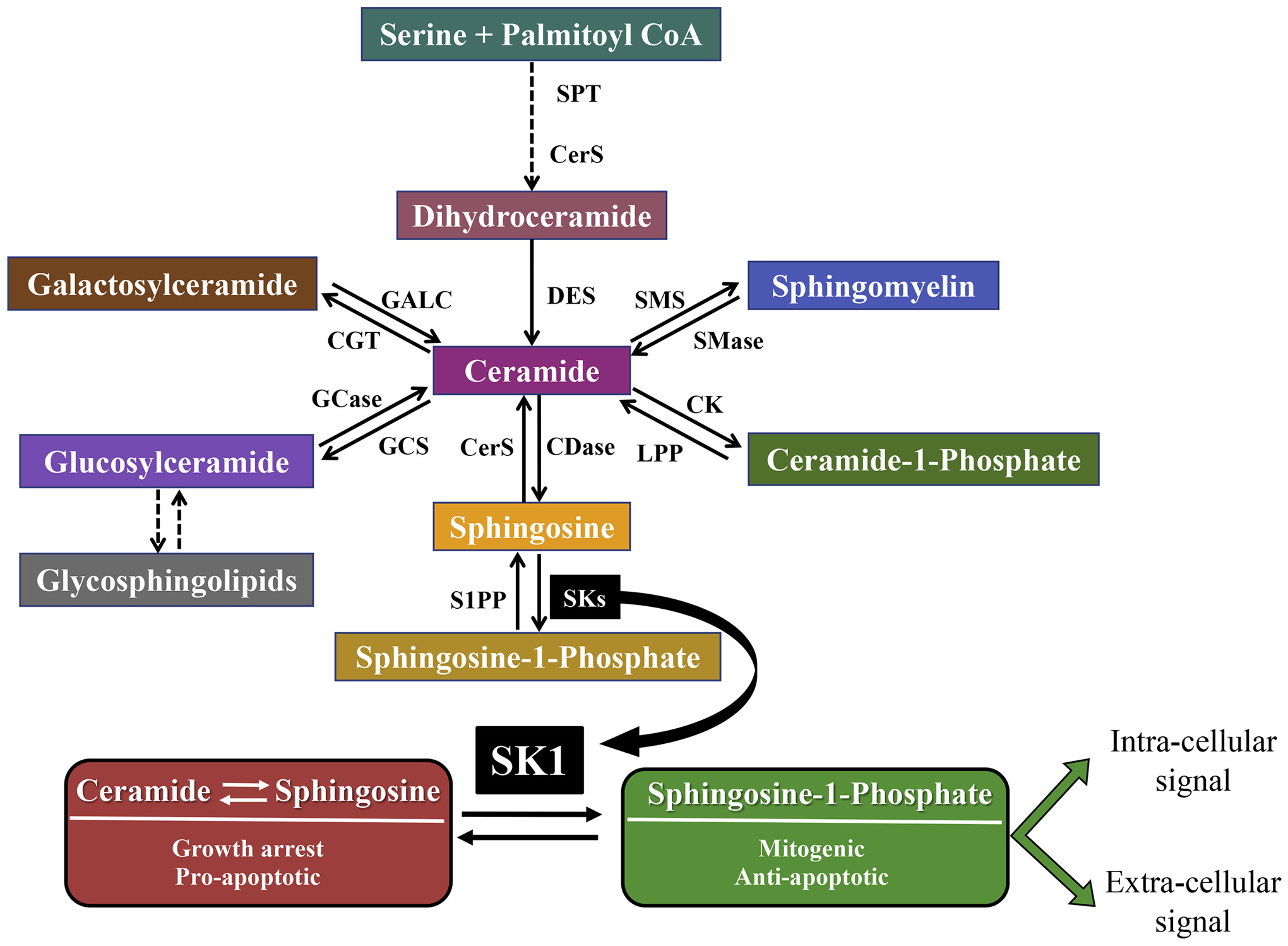
SK1: a critical point in sphingolipid metabolism. Sphingolipids are generated de novo via the condensation of serine and palmitoyl-CoA. Ceramide is formed after stepwise reduction, acylation and desaturation processes and can be used to generate many different sphingolipids. Ceramidases are required to generate sphingosine, which can then be phosphorylated by one of two sphingosine kinases (SK1 and SK2) to form S1P. SK1 activity is critical in regulating the balance between two groups of sphingolipids with opposite functions. Ceramide and sphingosine have been shown to cause growth arrest and to activate pro-apoptotic signaling, while S1P has been demonstrated to exert mitogenic and anti-apoptotic properties. S1P can act at the intracellular level, through the binding with different proteins, or upon export, can function as a ligand for one of 5 S1PRs triggering intracellular signaling cascades. SK1 participates in several processes such as cell survival, angiogenesis, lymphocyte trafficking, migration, inflammation and chemotherapeutic resistance, through its product S1P (or the alteration of ceramide and/or sphingosine levels). SPT, serine palmitoyltransferase; CerS, ceramide synthase; DES, desaturase; CK, ceramide kinase; LPP, lipid phosphate phosphatase, CDase, ceramidase, SMS, sphingomyelin synthase; SMase, sphingomyelinase; CGT, ceramide galactosyl transferase; GALC, galactosyl ceramidase; GCS, glucosylceramide synthase; GCase, glucocerebrosidase; S1PP, S1P phosphatase.
Fig. 2.
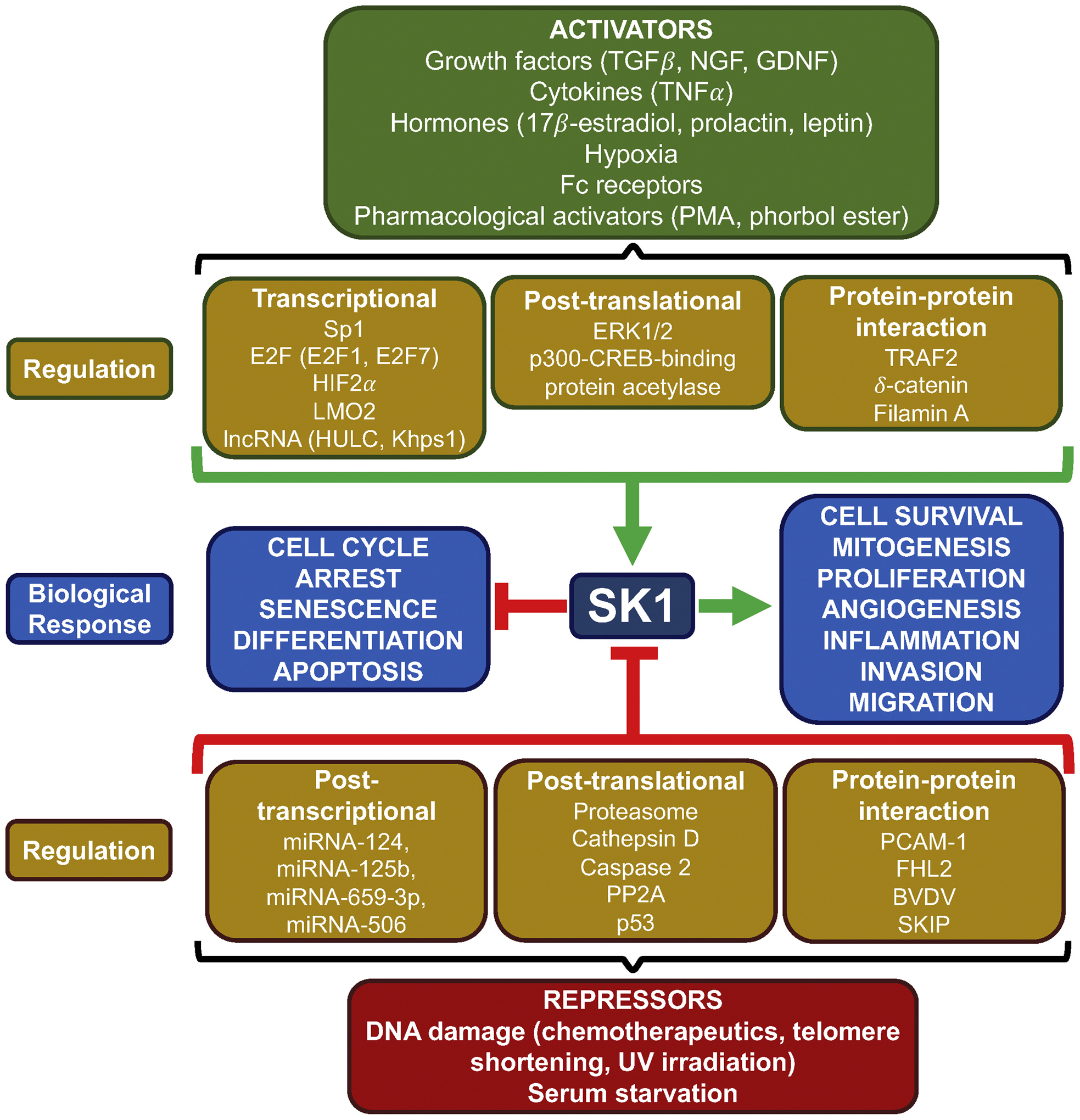
Regulation of SK1. SK1 is positively regulated by a wide range of signaling molecules including growth factors, cytokines and hormones as well as by pharmacological compounds, Fc receptors and hypoxia. These activators drive SK1 activity through various signaling pathways resulting in transcriptional upregulation, post-translational modifications including phosphorylation, or by protein-protein interactions. Increased SK1 activity is primarily associated with cell survival, mitogenesis, proliferation, angiogenesis, inflammation, invasion, or migration in a context-dependent manner. Alternatively, SK1 is negatively regulated by DNA damage induced by chemotherapeutics, UV irradiation, telomere shortening as well as serum starvation. Suppression of SK1 activity can be mediated through post-transcriptional repression by miRNA, post-translational proteasomal degradation, proteolysis via other proteases, dephosphorylation, or through interaction with proteins. Loss of SK1 activity is primarily associated with cell cycle arrest, senescence, apoptosis, and differentiation. PMA, phorbol 12-myristate 13-acetate; ERK, extracellular regulated kinase; TRAF2, TNF receptor-associated factor 2; PP2A, Protein phosphatase 2A; PECAM-1, platelet endothelial cell adhesion molecule 1; FHL2, four-and-a-half LIM domain 2; BVDV, bovine viral diarrhea virus.
Fig. 3.
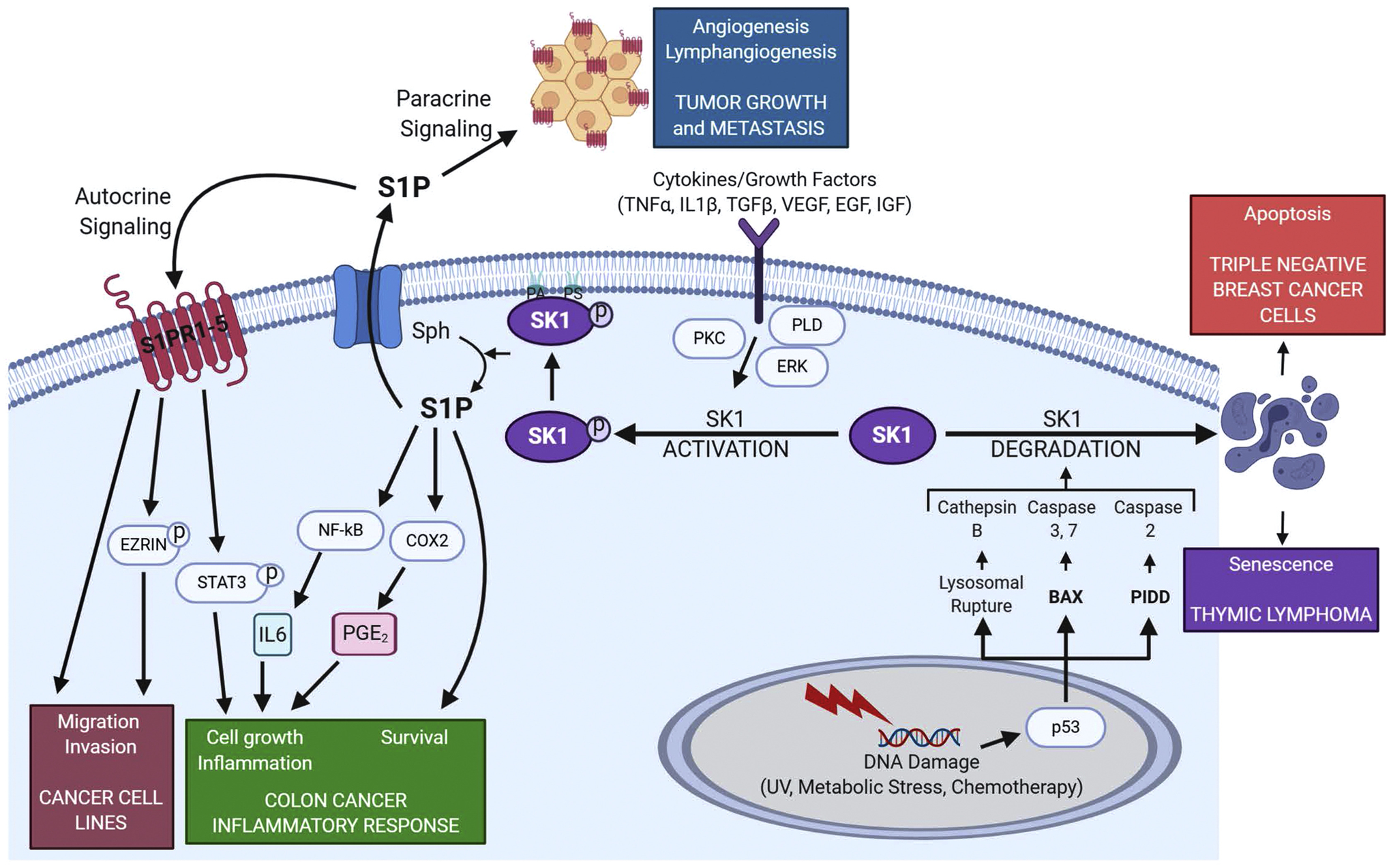
SK1 signaling and associated biological responses. SK1 is activated by a myriad of extra cellular signals, such as growth factors and cytokines. This in turn generates S1P which can be transported out of the cell to act in an autocrine manner where it can modulate many signaling pathways and responses, including increased migration and invasion of cancer cells through the phosphorylation of Ezrin and ERM (ezrin, radixin, moesin) proteins. S1P can also act in paracrine manner driving angiogenesis and lymphangiogenesis leading to tumor growth and metastasis. SK1 and S1P also activate inflammatory signaling via NF-kB, STAT3 and COX2, signals that are critical for colitis and colon cancer. Intracellular signaling via effectors such as PKC, PLD and ERK can result in the phosphorylation and membrane association of SK1, resulting in increased cell survival and proliferation. PLD also produces phosphatidic acid (PA) which helps anchor SK1 in the membrane. DNA damaging agents can also lead to the degradation of SK1 via proteases leading to apoptosis and senescent in cancer cells. *Figure created with BioRender.com.
2. SK1: Mechanisms and structure
SK1 is a highly regulated enzyme at multiple levels, most likely due to its central role in metabolism and interconversion of many important bioactive sphingolipids [23].
2.1. Membrane targeting of SK1
To generate S1P, SK1 most likely accesses sphingosine directly from membranes. Therefore, several studies have been conducted to better understand how SK1 associates with the membrane and how different types of membranes and membrane lipids affect the enzyme. Thus, much attention has been paid to translocation of SK1 to the plasma membrane [20,24–27]. Early work from the Obeid lab showed that SK1 translocates to the plasma membrane after stimulation with the protein kinase C (PKC) activator, phorbol 12-myristate 13-acetate (PMA) [24]. Furthermore, PMA-induced SK1 translocation was accompanied by increased SK1 activity and by secretion of S1P into the media, suggesting autocrine/paracrine signaling. Inhibition of PKC with known inhibitors bisindolylmaleimide, calphostin-C, or the indirect inhibitor C6-ceramide blunted PMA-induced translocation of SK1 to the plasma membrane. PMA also induced phosphorylation of green fluorescent protein (GFP)-tagged SK1, raising the possibility that SK1 is a direct substrate of PKC. However, subsequent studies suggested a key role for phospholipase D (PLD) [20], which is a major enzyme in the regulated production of phosphatidic acid (PA), and also a known target for PKC. Studies have shown that the translocation of SK1 to membrane compartments is dependent on the interaction of SK1 with PA, where specific and direct interactions of PA were studied at the C-terminus of recombinant SK1 [20,28]. In other studies, SK1 has been shown to be activated in vitro by anionic phospholipids, especially phosphatidylserine (PS) and PA [29]. PS and PA have been shown to enhance SK1 enzymatic activity in mixed micelle and liposome-based cell-free assays [25,27]. These studies raised the possibility that SK1 may be regulated in cells by interaction with membranes rich in anionic phospholipids (e.g. inner leaflet of the plasma membrane which is rich in PA).
Different molecular mechanisms have been proposed to explain SK1 translocation and interaction with membranes, where two specifically have identified certain residues that mediate membrane localization and have implicated these residues in mediating endocytosis and neurotransmission [25,30]. Stahelin et al. identified Thr54 and Asn89, as amino acid residues that interact with PS in membrane [31]. A biophysical cell study of SK1 and selected mutants was performed to understand the origin and structural determinant of the specific subcellular localization of SK1, where in vitro measurements showed that SK1 selectively bound PS over other anionic phospholipids and strongly preferred the plasma membrane-mimicking membrane to other cellular membrane mimetics. Specifically, mutational analysis indicated that conserved residues Thr54 and Asn89 were necessary for lipid selectivity and membrane targeting both in vitro and in the cell. Interestingly, phosphorylation of Ser225 enhances the membrane affinity and plasma membrane selectivity of human SK1, presumably by modulating the interaction of Thr54 and Asn89 with the membrane [25].
The ability to predict the potential binding interfaces of human SK1 was facilitated by the publication of the crystal structure [32]. The residues identified by Shen et al. are part of a small hydrophobic patch on the surface of SK1 [30]. This hydrophobic patch was shown to mediate SK1 membrane recruitment by directly interacting with the lipid bilayer. The patch corresponds to one of the two helices that were proposed to function as a gate to control the flux of the substrate sphingosine into the interior of the protein. Since some positively charged residues surround this hydrophobic patch, it may represent a lipid bilayer-binding interface with hydrophobic residues partially penetrating the membrane. This interpretation is supported by the diffuse cytosolic distribution of SK1 Leul94Gln and of a SK1 mutant that harbors two other mutations in the same hydrophobic patch, SK1 Phel97Ala/Leul98Gln. While both mutants folded correctly, they failed to bind to membranes, especially the tubular invaginations induced by cholesterol extraction, in vitro or in cell. The knockdown of SKs results in endocytic recycling defects, and a mutation that disrupts the hydrophobic patch of C. elegans SK fails to rescue the neurotransmission defects in loss-of-function mutants of this enzyme.
The Obeid lab subsequently identified a small highly positively charged three-residue motif, composed of the residues Lys27, Lys29, and Arg186, on the surface of SK1, adjacent to the substrate binding sites for both ATP and sphingosine (Fig. 4) [33]. This motif was identified using the Adaptive Poisson-Boltzman Solver in the Chimera software and studied for its electrostatic interactions with membranes, where hydrogen/deuterium exchange MS was used to study interactions of SK1 with membrane vesicles. This study demonstrated that SK1 interacts with membrane-associated anionic phospholipids using a single contiguous interface, containing both an electrostatic interaction site and a hydrophobic interaction site. Cellular studies demonstrated that mutation of the two sites causes decreased membrane association and SK1 activity. To disrupt the electrostatic site, Lys27 and Lys29 were mutated to glutamate residues and Arg186 to an aspartate residue, leaving the hydrophobic site intact. To disrupt the hydrophobic patch, Leul94 was mutated to a glutamine, leaving the electrostatic site intact, this resulted in the loss of SK1-dependent cell invasion and endocytosis. Therefore, both the hydrophobic patch and the electrostatic motif are essential for allowing SK1 binding to membranes in vitro and in cells. Altogether, these results from the Obeid lab defined a composite domain in SK1 that regulates its intrinsic ability to bind membranes and indicated that this binding is critical for proper SK1 function.
Fig. 4.
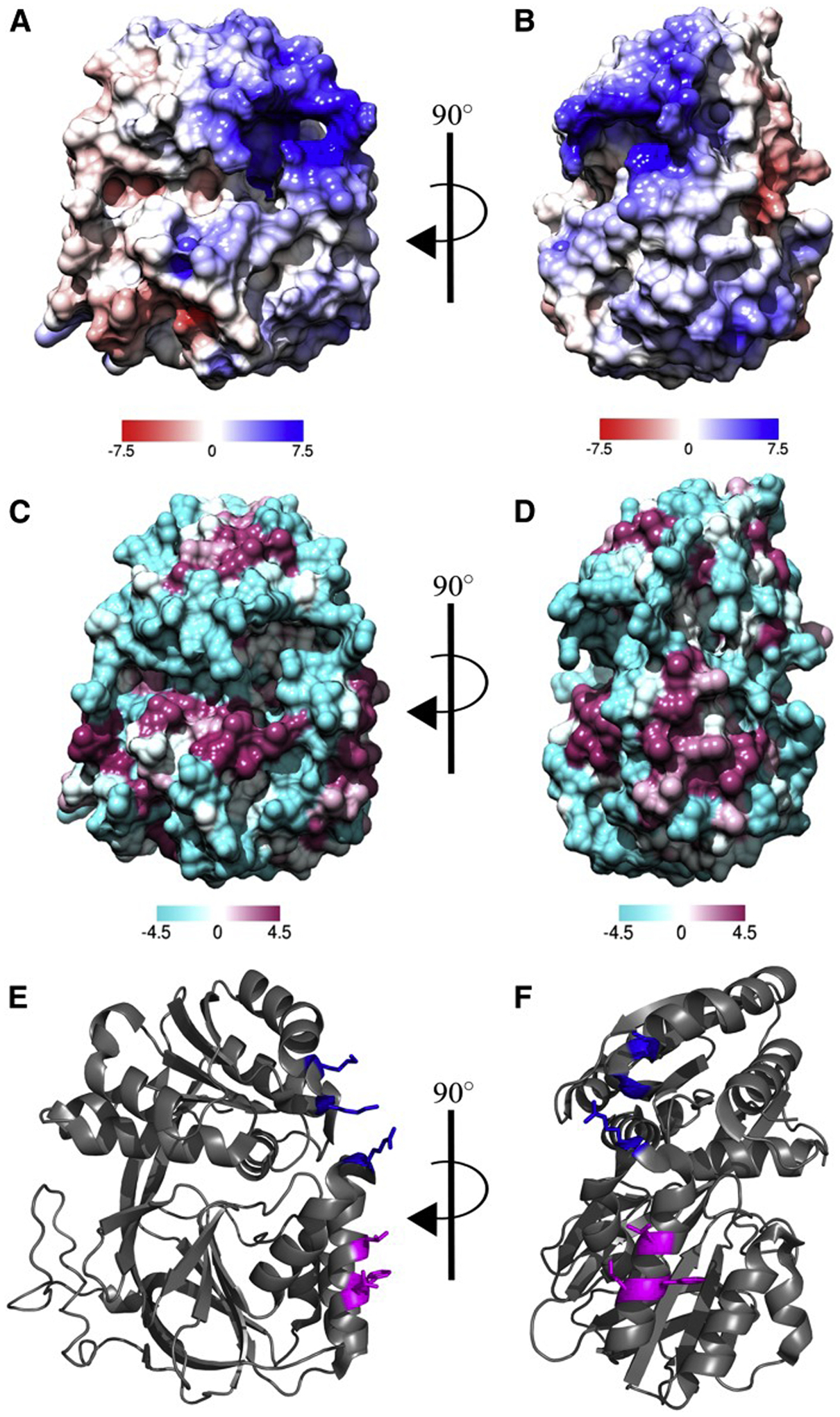
In silico surface binding analysis of SK1. A and B: Electrostatic potential maps were generated using Chimera software using the Adaptive Poisson-Boltzman Solver. The scale represents kcal·mol−1 where blue represents positively charged areas and red represents negatively charged areas. C and D: The Kyte-Doolittle scale of hydrophobicity was used to predict hydrophobicity and was mapped to the surface of SK1 where cyan represents hydrophilic areas and magenta represents hydrophobic areas. E and F: Cartoon representation of SK1 with residues of the hydrophobic patch and electrostatic patch represented as magenta and blue sticks, respectively. Protein Data Bank identification number 3VZB [32] used for all analyses. This figure was originally published in the Journal of Lipid Research. Pulkoski-Gross M. J., Jenkins, M.L., Truman, J.P., Salama, M.F., Clarke, C. J., Burke, J.E., Hannun, Y.A., Obeid L.M. An intrinsic lipid-binding interface controls sphingosine kinase 1 function. J Lipid Res. 2018; 59:462–474. © the American Society for Biochemistry and Molecular Biology.
2.2. Proteolysis of SK1
Several studies have been conducted to show degradation of SK1 can occur by ubiquitination and subsequent proteasomal degradation. Studies from the Obeid lab originally demonstrated that cells undergo a loss of the SK1 protein in response to DNA damage. Actinomycin treatment in MOLT4 cells resulted in the loss of SK1 protein. Interestingly, this loss of SK1 in response to DNA damage was prevented by transfection of cell with the E6 protein, which targets p53 for ubiquitination and subsequent proteasomal degradation, thus demonstrating the role of p53 in the loss of SK1. Furthermore, the knockdown of SK1 by small interfering RNA (siRNA) in MCF-7 cells resulted in a significant reduction in cell viability [21]. Subsequent studies focused on the mechanism of p53-mediated SK1 proteolysis, and it was shown that SK1 proteolysis occurred downstream of the tumor suppressor p53 in response to several DNA-damaging agents, by proteases including cathepsins. Furthermore, Dr. Obeid’s work showed that prolonged cytokine (TNFα) treatment of MCF7 breast cancer cells induced the loss of SK1 protein. Knock-down of cathepsin B by siRNA rescued the TNF-induced SK1 loss implying this process was dependent on cathepsin B [22]. Multiple cathepsin B cleavage sites in SK1 were identified by Obeid and colleagues [34]. Two cleavage sites of SK1 were identified, Hisl22 and Arg199. A Hisl22Tyr SK1 mutant showed significant reduction of cleavage at the first site but did not affect cleavage at the Arg199 site, while it maintained enzyme activity.
A subsequent study conducted by the Obeid lab concluded p53-mediated activation of caspase-2 was required for SK1 proteolysis [35]. Caspase-2 was not activated in triple-negative breast cancer (TNBC) cells harboring a mutation in p53, and interestingly SK1 was not degraded. Additionally, caspase-2 activity significantly altered the levels of endogenous sphingolipids (especially sphingosine, S1P, and ceramides). Further work in the Obeid Lab by Carroll et al. demonstrated that activation of the CHK1-suppressed pathway in TNBC resulted in the activation of caspase-2 and subsequent proteolysis of SK1 in response to doxorubicin. This work suggested that SK1 may be the first identified effector of the CHK1-suppressed pathway.
Development of SK1 inhibitors has been pursued as an attractive approach to inhibit tumor growth and metastasis formation. Many SK1 inhibitors, including the highly potent and selective inhibitor PF-543 [36], have dual mechanisms of action: 1) Sphingosine-competitive inhibition of kinase activity and 2) induction of proteolysis of SK1. It appears that substrate-based inhibitors of SK1 such as SKi, ABC294640, and FTY720 induce its ubiquitination and subsequent proteolysis [37,38]. The inhibitor SKI II was found to downregulate SK1 protein expression without affecting SK1 mRNA expression, and this degradation occurred through a lysosomal pathway and involved cathepsin B [39]. The Obeid lab collaborated on the study of LCL351 for its efficacy as a novel SK1 selective inhibitor in a murine model of inflammatory bowel disease (IBD). LCL351 was found to selectively inhibit SK1 both in vitro and in cells. LCL351, which accumulates in relevant tissues such as colon, did not have any adverse side effects in vivo [40].
3. S1P interaction with receptors: studies on the ERM family
The ERM (ezrin, radixin and moesin) proteins are involved in many important cellular events and link cortical actin to the plasma membrane and coordinate cellular events that require cytoskeletal rearrangement. Ceramide-induced activation of PP1α leads to dephosphorylation and inactivation of ERM proteins while S1P results in phosphorylation and activation of ERM proteins [41]. The Obeid lab has made significant contributions in elucidating the activities of S1P regulating phosphorylation of the ERM family of cytoskeletal proteins [42]. Most S1P-induced biologies are mediated by the interaction of S1P with high affinity G-protein-coupled receptors (GPCRs), namely, S1PR1–5 [43]. Intracellular S1P, derived from SK2 [44], was identified as an acute ERM activator in epidermal growth factor (EGF)-induced cancer cell invasion [44]. Specifically, S1P2R was shown to be necessary to induce phosphorylation of ERM proteins and subsequent filopodia formation [45]. EGF led to cell polarization in the form of lamellipodia, and it also induced cellular invasion. Both responses to EGF were shown to be dependent on SK2, S1P, and SlPR2-mediated phosphorylation of ezrin Thr567, and the S1PR2 antagonist, JTE-013, was specifically effective in preventing these responses. These contributions by the Obeid lab unveiled a novel mechanism of EGF-stimulated invasion, whereby S1P-mediated activation of S1PR2 and phosphorylation of ezrin Thr567 is required [46].
4. SK1: Senescence
Senescence is a state of stable cell cycle arrest triggered by diverse internal and/or external stress stimuli, including telomere shortening, hyper-proliferative signaling and epigenomic or genomic damage [47]. The senescent state is associated with increased expression of tumor suppressor proteins, dephosphorylation of the retinoblastoma protein (Rb), altered cellular morphology, increased senescence-associated ß-galactosidase activity (SA-ß-gal), and development of a secretory phenotype, termed senescence-associated secretory phenotype (SASP) [48]. Paradoxically, senescence is believed to be both a protective mechanism, preventing the proliferation of damaged cells [49], as well as a contributor to disease and dysfunction through accumulation with age and secretion of bioactive molecules into the local tissue microenvironment [50].
Work from Dr. Obeid first uncovered a link between sphingolipids and senescence [51]. Her laboratory observed elevated ceramide levels in high passage WI-38 human fibroblasts [52], which was subsequently observed by other groups [53] and in other human fibroblasts such as IMR-90 [54]. Her group was also the first to show that exogenous C6-ceramide induced senescence in WI-38 fibroblasts [51], via inhibition of cell cycle progression, increased SA-ß-gal, and dephosphorylation of Rb [55,56]. The mechanisms involved the activation of PP1 and PP2A, the induction of p21, and CDK2 inhibition, leading to cell cycle arrest and senescence [57]. Additional studies by Dr. Obeid’s group also identified a disruption in the PLD/PKCα pathway in senescence [52] and demonstrated that ceramide is an inhibitor of PLD [58] and of PKCα [59], implicating ceramide as a disruptor of this mitotic signaling pathway in senescence [60]. Furthermore, ceramide was identified as an upstream negative regulator of telomerase [61], a key enzyme driving immortalization of cancer cells and mediating escape from replicative senescence. Mechanistically, ceramide was demonstrated to reduce expression of telomerase by enhancing the degradation of the transcription factor c-Myc [62]. Several other groups demonstrated that exogenous ceramide induces senescence [63,64] in many cell lines [65,66], including cancer cells [67]. Ceramide was also shown to mediate senescence induced by chemotherapeutic drugs in cancer cells [68,69].
4.1. SK1 in aging and age-related diseases
Sphingolipids are also strongly implicated in aging and age-related diseases [70,71]. Genetic studies have buttressed the role of sphingolipids, especially ceramide, in aging. First, studies in Saccharomyces cerevisiae have shown that LAG1 (longevity assurance gene) regulates replicative capacity. This gene is the homolog of CerS2 gene in mammalian cells and functions as a CerS in yeast. The deletion of LAG1 induces an increase of yeast longevity, showing that decrease of ceramide synthesis slows down the aging process. Additional studies in Caenorhabditis elegans have revealed complex roles for ceramide in aging. In this system, 3 CerSs have been identified, HYL-1, HYL-2 and LAGR-1, synthesizing ceramide of variable chain length. Interestingly, these CerSs have distinct roles in aging. While loss of HYL-1 or LAGR-1 was shown to have no effect on lifespan [72] or decrease of some signs of aging [73], deletion of HYL-2 strongly decreased lifespan [72], suggesting that long-chain ceramide delays aging whereas longer-chain ceramide induces aging.
Clinical studies have also addressed potential roles and changes in sphingolipids, demonstrating that the levels of sphingolipids and their metabolic enzymes are altered during aging and age-related diseases. Very old subjects such as centenarians have higher serum levels of ceramide compared to younger subjects [74]. Positive correlation between the levels of ceramide in blood or in tissue was made in many diseases such as neurodegenerative, cardiovascular and metabolic diseases [75–78]. Patients with cardiovascular diseases have lower serum levels of S1P compared to heathy donors [79]. The challenge has been to evaluate if sphingolipids could be used as diagnostic or prognostic markers or therapeutic targets in those diseases. They may be particularly relevant in atherosclerosis and in Alzheimer’s disease.
Dr. Obeid’s laboratory was also the first to identify SK1 as a potential therapeutic target in p53-null cancers through the discovery that deleting SK1 induces senescence [80]. Genetic deletion of SK1 in a p53-deficient mouse model induced SA-ß-gal and p21 expression in the thymus, contributing to prevention of thymic lymphoma and extension of life span. These double KO mice exhibited significant increases in sphingosine levels in the thymus, suggesting that the biological response may be driven by this sphingolipid.
Expanding on this initial discovery, the genetic deletion of SK1 was reported to induce senescence in both primary liver cancer cells as well as MEFs, as determined by increased SA-ß-gal and p21 expression [81]. Molecular approaches targeting SK1 can also induce senescence in cancer cells. Pharmacological inhibition of SK activity with the inhibitors SKi or ABC294640, or combined siRNA knockdown of SK1 and dihydroceramide desaturase (Des1), increased p21 expression and induced cell cycle arrest in prostate cancer cells [38]. In vivo, SK1 deletion protects from chemically-induced liver tumorigenesis through inhibition of tumor growth and induction of tumor senescence along with apoptosis [81]. In this model, there was no difference in S1P levels. Rather, sphingosine levels were increased in the tumor. These studies demonstrate SK1 may be a powerful therapeutic target in multiple cancer types, through the induction of senescence.
There is also strong evidence that SK1 may be downregulated with aging and may contribute to age-related pathology by disrupting the balance between pro- and anti-proliferative sphingolipids. Dr. Obeid’s laboratory first determined that SK1 is negatively regulated by DNA damage and p53, key components of senescence activation [21,80]. In MEFs exposed to ultraviolet-B radiation, which induces senescence, SK1 was downregulated and associated with over 2-fold increase in Cl 6-ceramide level [80]. Also, treatment of MOLT-4 leukemia cells with the DNA damaging agent Actinomycin D, significantly reduced SK1 protein and increased sphingosine and ceramide [21]. In yeast, the expression of the SK homologs (LCB4 and LCB5) was significantly downregulated with age, contributing to an overall increase in sphingoid bases, which were also associated with reduced chronological lifespan [82]. Furthermore, the loss of SK1 was demonstrated to accelerate the deterioration of retinal function with age [83] and shortened lifespan, disrupted neuromuscular function, and locomotor behavior in Caenorhabditis elegans [73]. In human adipose-derived stromal cells, SK1 was reportedly downregulated in replicative senescence, and the downregulation of SK1 by shRNA induced a senescence phenotype and increased levels of C16-ceramide, sphingosine and dihydrosphingosine [84]. SK1 expression was also reported to be downregulated in neurons and in the frontal cortex and hippocampus of human patients with Alzheimer’s disease [85,86]. Based on these studies, the downregulation of SK1 disrupts the balance of pro- and anti-proliferative sphingolipids and accumulation of specific species may play a role in age-related diseases.
5. SK1: Inflammation
Inflammation is the body’s response to tissue injury and pathogens. This process involves increased cytokines and chemokines leading to the recruitment of leukocytes to the site of injury. Multiple studies have demonstrated that SK1 is regulated by a myriad of growth factors and cytokines, such as TNFα [18,87], IL-1β [18], lipopolysaccharide (LPS) [19] or VEGF [88]. Work in the laboratory of Dr. Obeid in cellular and in vivo models has revealed critical and potentially therapeutic roles for SK1 in the regulation of inflammation.
5.1. SK1 in inflammatory signaling
Several studies have demonstrated the importance of SK1 activity in the inflammatory response in cellular models. SK1 has been shown to be regulated by several cytokines, including TNFα and IL1β [89]. Conversely, SK1 has also been implicated in the regulation of specific cytokines and cell signaling pathways associated with inflammation [90,91]. SK1 has been shown to directly interact with, and be activated by, tumor necrosis associated factor 2 (TRAF2), stimulating NF-kB and JNK activity in a HEK-293T cell model [92]. Interestingly, interaction with SK1 is itself necessary to activate the E3 ubiquitin ligase activity of TRAF2, as this allows S1P to act as a cofactor to TRAF2, according to the same model [93]. Dr Obeid’s laboratory demonstrated that SK1 is also a key component in the interaction of p38 MAPK and TNFα signaling pathways, whereby SK1 has been shown to regulate this pathway by activating p38 MAPK. This leads to the suppression of the cytokine RANTES, generating a novel anti-inflammatory response [94]. In a collaborative publication from the laboratories of Drs. Obeid and Hannun, it was shown that TNFα is also implicated in the induction of cyclooxygenase 2 (COX2) and production of prostaglandin E2 (PGE2) in L929 fibroblasts [89] as well as in macrophages in colon cancer [95]. There is also evidence in the literature from Pitson and others demonstrating that SK1 activity is required for TNFα-induced activation of integrin, via intracellular signaling pathways [96]. In addition to TNFα, SK1 was activated by IL1β in astrocytes leading to interactions between SK1 and cellular inhibitor of apoptosis protein 2 (cIAP2) [97]. The activation and upregulation of SK1 via cytokines has been implicated in several pathways important in both healthy and diseased states; suggesting that future work aimed at determining the role of SK1 in inflammation is essential.
In addition to activation and upregulation by pro-inflammatory cytokines, SK1 has also been implicated in inflammatory signaling in response to growth factors, LPS, and fatty acids. TGFβ has also been shown to directly upregulate SK1 [98,99], with several downstream effects. In a collaboration involving Dr. Obeid’s laboratory, it was shown that TGFβ upregulated SK1, and this upregulation was required for subsequent induction of TIMP-1, and a more specific function for dhS1P as opposed to S1P [98,100]. In pulmonary arterial smooth muscle cells, Notch3 activation and increased proliferation of these cells also required TGFβ-induced SK1 expression [99]. Early work from Dr. Obeid demonstrated that SK1 provides macrophages with a pro-survival response through S1P production. First, in a collaborative effort using human U937 monocytic cells, it was shown that oxidized LDL-containing immune complexes caused translocation of SK1 to the plasma membrane and subsequent increased in S1P production. Additionally, treatment with these immune complexes increased cell survival over time, survival that was further increased with S1P co-treatment [101]. Subsequent work by Dr. Obeid’s group showed that LPS treatment induced SK1 expression and S1P production in RAW 264.7 mouse macrophages. The protective role of SK1 in this cell line was manifested when SK1 downregulation resulted in increased apoptosis after LPS or TNFα treatment [19]. Further studies delved into the role of SK1, not only in macrophages [95], but also in the function of other immune cells, such as neutrophils [102] and dendritic cells [103].
More recent collaborative work has demonstrated a role for SK1 in obesity and high fat diet (HFD). Co-treatment of macrophages with LPS and palmitate increased expression of SK1, and that SK1 was required for increased levels of IL6 [104]. Collaborative work with Dr. Cowart’s lab demonstrated that SK1−/− mice fed a HFD exhibit increased markers of adipogenesis and IL-10 and decreased levels of TNF and IL6 as compared to wildtype (WT) mice [105]. SK1-deficient mice also exhibit increased insulin sensitivity and glucose tolerance upon HFD challenge. In a cell culture model, palmitate treatment increased SK1 expression via PPARα leading to IL-6 production in in C2C12 myotubes [106]. Together these studies clearly indicate a critical role for SK1 in several inflammatory signaling pathways.
5.2. SK1 in inflammatory diseases
SK1 and S1P have been implicated in human inflammatory diseases and in several inflammatory diseases in mouse models. Inhibition of SK1 using pharmacologic inhibitors or genetic deletion of SK1 has been suggested to decrease disease in animal models of asthma and lung inflammation. These studies have implicated SK1 in the recruitment of immune cells in the lungs [107], as well as in the generation of inflammatory cytokines, and activation of NF-κB in the lungs [108]. SK1 and S1P have also been implicated extensively in multiple sclerosis (MS). The SK1 activator (compound 5) protected from experimental autoimmune encephalomyelitis (EAE) development; however, the mechanisms are not well established yet [109]. In addition, transplantation of mesenchymal stem cells overexpressing SK1 reduced the symptoms of EAE [110]. The sphingosine analog, FTY720/Fingolimod, acts as a substrate for SK2, and the phosphorylated product acts as a functional antagonist to S1PRs, resulting in sequestration of lymphocytes. This molecule is in clinical use in MS [111]. Together these studies implicate critical roles for SKs and S1P in inflammation and autoimmunity.
5.3. SK1 in arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory and destructive joint disease, characterized by elevated levels of inflammatory mediators such as TNFα and IL-1β, resulting in joint pain and disability [112]. S1P levels have been shown to be elevated in the synovial fluid of RA patients as compared to osteoarthritis (OA) [113]. In collaborative work, Dr. Obeid’s group demonstrated a role for SK1 in RA using the transgenic TNFα-mice (hTNFα). Signs of RA, such as joint swelling and deviation, were apparent in both hTNFα/SK1+/+ or hTNFα/SK1−/− mice at 4 months of age. However, articular/periarticular inflammation and erosive disease were significantly decreased in hTNFα/SK1−/− mice. These studies implicated SK1 and S1P in the development of Th17 T cells, and in the expression of SOCS3 (suppressor of cytokine signaling) in RA [114–116].
The pro-inflammatory role of SK1 in arthritis has also been supported by other cellular and in vivo models. B lymphoblastoid cell lines from peripheral blood of RA patients were resistant to apoptosis when compared to healthy controls [101]. SK1 siRNA reversed this resistance suggesting that SK1 inhibition could be a strategy to induce autoreactive B cell death in RA. Lai et al. demonstrated a pro-inflammatory role of SK1 in a collagen-induced arthritis (CIA) mouse model. The authors showed that N,N-dimethylsphingosine (DMS), an SK1 inhibitor, as well as SK1 siRNA suppressed pro-inflammatory cytokine expression (IL-6, TNFα) and reduced articular inflammation and joint destruction in a CIA model. This work also demonstrated elevations in synovial S1P from RA patients as compare to OA patients [113]. Most recently, Inoue et al. have unveiled the potential role the for S1PR3 in the CIA mouse model as SlPR3-deficient mice exhibited decreased clinical and histological severity of disease as well as significantly lower expression of IL6 in the synovial membrane [117]. Another group has demonstrated that S1P signaling via S1PR1 enhances inflammatory cytokine production in human fibroblast-like synoviocytes derived from RA patients [118]. Together, these studies clearly outline the importance of SK1 in in vivo arthritis models.
5.4. SK1 in inflammatory bowel disease
IBD includes ulcerative colitis (UC) and Crohn’s Disease (CD) and affects an estimated 3 million Americans. IBD is characterized by chronic inflammation of the gastrointestinal tract [119] and increases risk for the development of colorectal cancer [120]. Dr. Obeid’s group was the first to demonstrate increased expression of SK1 in the intestinal epithelium from colitis and colon cancer patients [121,122]. Snider et. al also uncovered a crucial role for SK1 in the development and progression of IBD in an in vivo model, namely dextran sulfate sodium (DSS)-induced colitis. SK1, but not SK2, expression and activity were elevated in wildtype (WT) mice treated with DSS. S1P levels were also elevated in the circulation of WT mice with DSS-induced colitis (also reported by Maines and colleagues [123]). Furthermore, SK1−/− mice were significantly protected from colitis as demonstrated by decreased intestinal damage, decreased recruitment of neutrophils, as well as decreased COX-2 expression as compared to WT mice [124]. These studies were bolstered by subsequent work from Liang et al. demonstrating that fingolimod (FTY720), an inhibitor of S1PR1, significantly protected from colorectal tumorigenesis associated with chronic colitis in a murine model [125].
Dr. Obeid’s group further demonstrated the importance of SK1 in colitis, indicating specific roles for SK1 in hematopoietic cells vs. non-hematopoietic cells in DSS-induced colitis. Bone-marrow chimeric mice were used to demonstrate the critical role for extra-hematopoietic SK1 in the induction of COX-2 in colon tissues in response to DSS-induced colitis [126]. These studies also highlighted the cross talk of the SK1/S1P pathway with that of STAT3, as loss of SK1 in either the hematopoietic or extra-hematopoietic compartments resulted in decreased phospho-STAT3 in DSS-induced colitis [126]. Similar links between SK1 and STAT3 have also been demonstrated in animal models of colitis-associated colon cancer (CAC) [125]. Use of the SK1 inhibitor LCL351 reduced the inflammatory responses (e.g. weight loss, splenomegaly, and loss of red blood cells) in acute DSS-induced colitis. Interestingly, in DSS-induced colitis, mice treated with LCL351 presented decreased levels of S1P in colon tissue as compared to vehicle treated mice. LCL351 also decreased expression of TNFα, CXCL1 and CXCL2, as well as neutrophil infiltration into colon tissues, suggesting that SK1 as a viable therapeutic target in IBD [40]. These findings were reinforced by work demonstrating that SK1 inhibition with PF543 also exerts a protective effect against colitis in mice treated with DSS [127]. Additional studies from Dr. Obeid and her collaborators have also defined the roles for ceramidases in IBD and colon cancer [128–131]. Together, these studies demonstrate the significant contributions by Dr. Obeid’s laboratory on the roles of SK1, and other sphingolipid enzymes, in intestinal inflammation.
6. SK1: Cancer
Cancer cells are characterized by uncontrolled division and the abilities to disseminate and avoid immune responses [132]. Bioactive sphingolipids are now understood to play essential roles in multiple aspects of cancer pathogenesis and therefore represent exciting and therapeutic targets. Whereas ceramide and sphingosine regulate cell death, senescence, and cell cycle arrest, S1P induces proliferation, inflammation and angiogenesis [133–135], and regulates immune responses [136,137]. Regulation of SK1 affects the levels and balance between ceramide, sphingosine, and S1P and has been demonstrated to impact cancer cell fate. Increasing lines of evidence, both in vitro cellular models as well as in vivo animal research, are unveiling the potential clinical significance of targeting SK1 in cancer [138].
Throughout her research career, Dr. Obeid’s studies and findings were crucial to the sphingolipid field and its implication in cancer cells. A large body of work in the Obeid laboratory shed light on the relevance of sphingolipids in cancer tumorigenicity and progression, angiogenesis, migration, inhibition of apoptosis, and chemotherapy resistance, as briefly reviewed below.
6.1. Ceramide and CerS in apoptosis and cancer
Dr. Obeid was the first to discover that ceramide induces programmed cell death. Her landmark study was published in Science in 1993 [139]. Her contribution to this topic, which has been extensively expanded on the sphingolipid field, continued for three decades. Many of her studies defined novel connections in apoptosis [140]; for example, work from her lab demonstrated that the anti-apoptotic Bcl2 interrupted death pathways downstream of initiating caspases [141], and her group defined regulation of release of apoptotic bodies and devised a simple assay to measure that process [142–144]. Her group also showed that Bcl2 acts downstream of ceramide in the apoptotic pathways [145], whereas Bcl-x acts upstream of ceramide generation. Other studies defined roles of ceramide in cytokine-induced apoptosis (and lack of role in activation of NF-kB) [146]. In related studies, her group implicated regulation of dihydroceramide desaturase (Des) in the regulation of the cell cycle [147].
Mechanistically, Obeid’s group defined compartmentalization of ceramide action and discovered a role for mitochondrial ceramide in induction of apoptosis [148–150] especially with activation of sphingomyelinases, and this led to the development of a mitochondrially-targeted ceramide analog that was sufficient to induce apoptosis [151]. Additional studies showed that long chain endogenous ceramides inhibit the mitochondrial permeability pore [152] and that neutral ceramidase may function in reverse to generate ceramide in mitochondria [153]. In a recent study, her group showed that dysregulation of mitochondrial ceramide metabolism contributes to pathogenesis of Charcot-Marie-Tooth type 2F, a neuropathic disorder [154].
The work in her lab also addressed the role of ceramide synthases in apoptosis and related biology and the mechanisms involved [155,156]. Her group showed that disruption of CerS2 induced autophagy and the unfolded protein response [157], whereas in vivo disruption of CerSl induced neurodegeneration [158]. On the other hand, CerSs modulated apoptosis downstream of the mitochondrial pathway [159], and were shown to be involved in regulation of membrane leakiness through regulation of focal adhesion kinases [160]. Biochemically, her group was the first to demonstrate that the LAG domain in CerS was required for activity of the enzymes [161]. Her laboratory established that the pro-apoptotic protein BAK was required for CerS activity [162]. Collaborative studies based on this finding also demonstrated the activation of BAK/BAX and mitochondrial out membrane permeabilization MOMP) required S1P and hexadecenal [163]. Work from Dr. Obeid’s lab published in 2017 in Cell Metabolism described the sequestration of ceramides as acylceramides via a novel interaction between DGAT2 and CerS that lead to protection of cells from apoptosis, thus defining a new pathway for clearance of pro-apoptotic ceramide. Together these studies defined CerS and specific ceramides as critical regulators in apoptosis and other regulator pathways in cancer signaling.
To advance ceramide-based therapeutics, her group collaborated with that of Dr. Sitharaman to promote graphene-based nanoparticles of ceramide [164,165].
These studies thus not only defined this critical function for ceramide but also advanced the cell biologic and biochemical understanding of the enzymes involved and the specific cellular contexts.
6.2. SK1 in carcinogenesis
Dr. Obeid’s laboratory was the first to demonstrate the elevation of SK1 in multiple cancers [166]. This has now been supported by several significant studies, and it ushered the specific focus on SK1 in carcinogenesis. Work in the Obeid laboratory demonstrated that SK1 is upregulated in the azoxymethane (AOM)-induced model of colon cancer [167]. These finding were then extended to a murine model for CAC where genetic loss of SK1 led to reduced formation of aberrant crypt foci and decreased cancer development [121]. The same study also found a much higher prevalence of SK1 in human tumor samples than in normal colon tissues, indicating its potential in human tumor formation. The roles of SK1 and S1P in colon cancer have been further developed by other studies [125,168].
Additional studies have supported roles for SK1 in tumorigenesis [169]. Overexpression of SK1 in MCF-7 breast cancer cells increased tumor growth in xenograft models [170]. Additionally, SK1 in tumor cells has been shown to stimulate metastasis, migration, invasion, angiogenesis, and chemoresistance [167,169,171,172]. SK1 and S1P receptor signaling are required for the invasiveness of glioblastoma cells through the urokinase plasminogen activator pathway [173]. Moreover, SK1 activity has recently been shown to be necessary for the invasion of triple negative breast cancer cells, through NF-kB activation and regulation of the protein fascin [174]. Furthermore, higher SK1 expression in colon cancer has been shown to increase invasiveness via Erkl/2 pathways, thus predicting metastasis and poor prognosis in patients [175,176]. Some work has also demonstrated that metastases of triple negative breast cancer are especially sensitive to SK1 /S1P signaling, showing that inhibition of SK1 leads to reduced growth, survival, and motility [177]. Deletion or inhibition of SK1 reduced both EGF and hormone-induced migration of breast cancer cells [178,179].
SK1 is also involved in angiogenesis and lymphangiogenesis, critical processes in both normal and cancer physiology. Knockdown of SK1 in glioblastoma (U87) as well as in triple negative breast cancer (MDA-MB-231) cells, significantly reduced vessel formation in vitro [180]. Interestingly, the reduction of tube formation was independent of the activity of VEGF, a cytokine commonly associated with angiogenesis. This is indicative of the importance of SK1/S1P signaling to angiogenesis independent of other pathways. Similar results have been shown in vivo, where SK1 inhibition reduced metastasis and angiogenesis in a syngeneic murine model of breast cancer metastasis using 4T1 cells [181]. Expression of SK1 and efficient signaling by S1P have been shown to stimulate angiogenesis in B-cell lymphoma [166]. Interestingly, endogenous genetic regulation of SK1 via micro-RNAs (miRs) has also been demonstrated to affect angiogenesis. miR-506 decreased SK1 expression in HepG2 cells and reduced angiogenesis in HUVEC cells [182]. SK1 expression was shown to negatively correlate with miR-506 expression in patient liver tissues. The emerging angiogenic properties of SK1 point to its importance for vascularization and tumors expansion.
6.3. SK1 as a prognostic marker
Much evidence has shown that SK1 is upregulated in a myriad of solid tumors, conferring resistance to chemotherapy and leading to poor prognosis to patients. Early research performed by the Obeid laboratory found that both gene and protein expression of SK1 is upregulated in lung cancer. SK1 expression was increased in non-small-cell lung cancer (NSCLC) tissue as compared with patient-matched normal tissue. This increase in expression also correlated with increased SK1 mRNA expression [183]. It was later demonstrated that overexpression of SK1 in NSCLC was associated with tumor progression, metastasis and low survival rate in patients [184]. Similar results have been published in several other human cancers, including breast, uterine, ovarian, colon and small intestine, where elevated expression of SK1 resulted in reduced patient survival [185–187]. Together, these findings positioned SK1 as a potential therapeutic target and its high expression being a negative prognosis marker.
6.4. SK1 in chemotherapy resistance
A growing body of work suggests that higher expression of SK1 also correlates with resistance to common chemotherapies. Manuscripts from 2004 and 2006 from Dr. Obeid’s group demonstrated that overexpression of SK1 in MCF-7 cells induced cell proliferation and protected from cell death. Conversely, downregulation of SK1 decreased cell viability and increased apoptosis in ER-positive MCF-7 breast cancer cells upstream of Bax oligomerization, cytochrome c release and caspase activation [188]. In addition, Watson and colleagues found that expression of SK1 was increased in tamoxifen-resistant MCF-7 cells as compared to sensitive cells [189]. Similarly, high expression of SK1 was shown to correlate with poor response to doxorubicin in NSCLC [184] and breast cancer cells [190], which was reversed upon co-treatment with an SK1 inhibitor (SK1–5C). Furthermore, the sensitivity of pancreatic cancer cells to gemcitabine was improved via either inhibition or knockdown of SK1, and the amount of S1P present in cells was shown to be key to resistance [191]. Song et al. demonstrated that silencing of SK1 by shRNAs or treatment with the inhibitor SK1-I decreased tumor growth in a xenograft model of NSCLC, both basally and in doxorubicin-treated mice, by increasing apoptosis. Thus, targeting SK1 sensitized NSCLC to chemotherapy in vitro and in vivo [184]. Most importantly, higher levels of SK1 mRNA were observed in patients who did not respond to doxorubicin and docetaxel treatment compared to partial or complete responders [190]. Activation of SK1 and S1P production has been demonstrated to occur downstream of the estrogen receptor- GPR30 axis [192]. Targeted therapies have also been shown to be less effective in instances of high SK1 expression. In chronic myeloid leukemia (CML), SK1 expression is correlated with resistance to imatinib, whereas inhibition or knockdown of SK1 restored imatinib-induced apoptosis [193,194]. The importance of SK1 in drug resistance highlights the need to better understand the regulatory mechanisms behind its expression.
6.5. Regulation of SK1 in cancer
SK1 is now recognized to be regulated at multiple levels and in response to many stimuli and mediators. The Obeid laboratory was one of the first to show the importance of the subcellular localization of SK1 for its kinase activity. As discussed above, in HEK293 cells, it was demonstrated that the PKC activator PMA induced SK1 translocation to the plasma membrane and a consequent increase in S1P secretion [24]. Other studies implicated PLD acting between PKC and SK1 through the generation of PA. The Obeid lab also showed that the oncogenic K-Ras regulates metabolism of bioactive sphingolipids in an SK1-dependent manner [195].
6.6. Role in p53-mediated biology
Also as discussed above, Dr. Obeid’s studies were the first to demonstrate that DNA damage resulted in the degradation of the SK1 protein in a p53-dependent manner. The significance of this interaction was then extended to an in vivo model where the combined genetic deletion of SK1 and p53 in mice prevented the development of thymic lymphoma and reduced the development of other tumors, resulting in increased survival [80]. Moreover, loss of SK1 in heterozygous p53 mice protected from the development of sarcomas, lymphoma and lung adenocarcinoma potentially via the induction of senescence and increased C16-ceramide [80]. Future studies on SK1 as a novel therapeutic target in tumors with loss or mutant p53 function will lend insight into the therapeutic potential for SK.
6.7. Regulation by hypoxia
Regulation of SK1 expression at the mRNA level has been also studied by the Obeid group. In glioma-derived U87MG cells, hypoxia increased SK1 mRNA levels, protein expression, and enzymatic activity. Under CoCI2 treatment (a known hypoxia mimetic model), the transcription factor HIF2α, but not HIFlα, increased SK1 expression, and the knock-down of HIF2α prevented SK1 expression under hypoxic conditions [196]. In clear cell renal cell carcinoma (ccRCC) cells, which lack the von Hippel-Lindau (VHL) protein, SK1 was upregulated due to increases in HIF2α. Furthermore, knock-down of HIF2α by siRNA caused a consequential decrease in SK1 at the mRNA and protein level [197]. Similar studies by others have reported that SK1 regulates the expression and activity of HIF2α in response to hypoxia in a number of lung and ccRCC cell lines [198], suggesting a potential feedback loop between SK1 and HIF2α in response to oxygen levels.
Several studies have shown SK1 expression to be regulated by different transcription factors in various cancers. In head and neck squamous cell carcinomas (HNSCCs), E2F7 directly increased the transcription of SK1 resulting in activation of AKT and subsequent drug resistance in these cells [9]. In hepatoma cells, E2F1 was found to directly bind the SK1 promoter and induced tumor angiogenesis in liver cancer via the action of the long non-coding RNA (IncRNA) HULC [7]. In HeLa cells, Khpsl, another IncRNA, was found to drive expression of the SK1 gene in an E2F1 dependent manner. Upregulation of SK1 counteracted the apoptotic function of E2F1 and promoted cell proliferation [8]. At the post-transcriptional level, a growing number of studies have implicated various microRNA, with critical roles in cancer initiation, progression and metastasis, in regulation of SK1 expression [199–201].
7. Conclusions
Dr. Obeid, and the contributions of her lab, have propelled sphingolipids and in particular SK1 to the forefront of biomedical research. Her studies have defined fundamental features for the structure and function of SK1, established SK1 and S1P as key mediators in cell signaling pathways, and established SK1 as a critical modulator in several disease states. Completion of ongoing studies from her lab will further define significant roles for SK1 in cancer and metabolism. These studies will highlight the roles of SK1 in serine deprivation of cancer cells, the emerging functions of dSa (deoxysphinganine) as a novel bioactive sphingolipid, the roles of SK1 in senescence, and roles of SK1 as a therapeutic target. These discoveries will fuel the next generation of research and promote SK1 as a valid therapeutic target in multiple human diseases.
Supplementary Material
Acknowledgements
The authors would like to thank the many students, trainees, and colleagues who have worked in or have collaborated with the Obeid lab over the years (Table S1). Dr. Obeid trained 18 graduate students in her laboratory (1 M.S. student, 10 Ph.D. students, and 7 M.D./Ph.D. students) served on the thesis committees for 34 additional graduate students, mentored 22 postdoctoral scientists, 4 visiting scientists on sabbatical, 25 junior faculty, and numerous undergraduate and medical students. This work was supported by multiple NIH grants and assistance programs spanning the career of Dr. Obeid.
Abbreviations:
- AOM
Azoxymethane
- cIAP2
Cellular inhibitor of apoptosis protein 2
- CerK
Ceramide kinase
- CerS
Ceramide synthase
- C1P
Ceramide-l-phosphate
- CML
Chronic myeloid leukemia
- ccRCC
Clear cell renal cell carcinoma
- CAC
Colitis-associated cancer
- CIA
Collagen-induced arthritis
- CD
Crohn’s Disease
- COX2
Cyclooxygenase 2
- dSA
Deoxysphinganine
- DGAT2
Diacylglycerol O-acyltransferase 2
- Des1
Dihydroceramide desaturas
- EGF
Epidermal growth factor
- EAE
Experimental autoimmune encephalomyelitis
- GCS
Glucosylceramide synthase
- GFP
Green fluorescent protein
- HNSCC
Head and neck squamous cell carcinoma
- HFD
High fat diet
- HIF2α
Hypoxia inducible factor 2 alpha
- IBD
Inflammatory bowel disease
- IGF
Insulin-like growth factor
- IL1β
Interleukin 1 beta
- IL6
Interleukin 6
- LM02
LIM-domain-only protein 2
- LPS
Lipopolysaccharide
- Lag
Longevity assurance gene
- LDL
Low density lipoprotein
- miR
microRNA
- MS
Multiple sclerosis
- DMS
N,N-dimethylsphingosine
- NGF
Nerve growth factor
- NSCLC
Non-small-cell lung cancer
- OA
Osteoarthritis
- PS
Phosphatidylserine
- PMA
Phorbol 12-myristate 13-acetate
- PA
Phosphatidic acid
- PLD
Phospholipase D
- PKC
Protein kinase C
- PP
Protein phosphatase
- RA
Rheumatoid arthritis
- S1PR
Sphingosine-1-phosphate receptor
- SASP
Senescence-associated secretory phenotype
- SPT
Serine palmitoyltransferase
- siRNA
Small interfering ribonucleic acid
- SMS
Sphingomyelin synthase
- SK
Sphingosine kinase
- S1P
Sphingosine-1-phosphate
- SOCS3
Suppressor of cytokine signaling 3
- TGFβ
Transforming growth factor beta
- TNBC
Triple-negative breast cancer
- TRAF2
Tumor necrosis associated factor 2
- TNFα
Tumor necrosis factor alpha
- UC
Ulcerative colitis
- VEGF
Vascular endothelial growth factor
- VHL
Von Hippel-Lindau
- WT
Wildtype
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellsig.2020.109875.
References
- [1].Hannun YA, Obeid LM, Principles of bioactive lipid signalling: lessons from sphingolipids, Nat. Rev. Mol. Cell Biol 9 (2008) 139–150. [DOI] [PubMed] [Google Scholar]
- [2].Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Gamier-Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, Merrill AH Jr., Riley RT, Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals, J. Biol. Chem 284 (2009) 4786–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hannun YA, Obeid LM, Many ceramides J. Biol. Chem 286 (2011) 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Salama MF, Carroll B, Adada M, Pulkoski-Gross M, Hannun YA, Obeid LM, A novel role of sphingosine kinase-1 in the invasion and angiogenesis of VHL mutant clear cell renal cell carcinoma, FASEB J 29 (2015) 2803–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anelli V, Gault CR, Cheng AB, Obeid LM, Sphingosine Kinase 1 Is Upregulated during Hypoxia in U87MG glioma cells: role of Hypoxia-inducible factors 1 and 2, J. Biol. Chem 283 (2008) 3365–3375. [DOI] [PubMed] [Google Scholar]
- [6].Matrone G, Meng S, Gu Q, Lv J, Fang L, Chen K, Cooke JP, Lmo2 (LIM-Domain-Only 2) Modulates Sphkl (Sphingosine Kinase) and promotes endothelial cell migration, Arterioscler. Thromb. Vase. Biol 37 (2017) 1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lu Z, Xiao Z, Liu F, Cui M, Li W, Yang Z, Li J, Ye L, Zhang X, Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1), Oncotarget 7 (2016) 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Postepska-Igielska A, Giwojna A, Gasri-Plotnitsky L, Schmitt N, Dold A, Ginsberg I Grummt, LncRNA Khpsl regulates expression of the proto-oncogene SPHK1 via triplex-mediated changes in chromatin structure, Mol. Cell 60 (2015) 626–636. [DOI] [PubMed] [Google Scholar]
- [9].Hazar-Rethinam M, de Long LM, Gannon OM, Topkas E, Boros S, Vargas AC, Dzienis M, Mukhopadhyay P, Simpson F, Endo-Munoz L, Saunders NA, A novel E2F/sphingosine kinase 1 axis regulates anthracycline response in squamous cell carcinoma, Clin. Cancer Res 21 (2015) 417–427. [DOI] [PubMed] [Google Scholar]
- [10].Olivera A, Spiegel S, Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens, Nature 365 (1993) 557–560. [DOI] [PubMed] [Google Scholar]
- [11].Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S, Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility, Sci. (New York, N.Y) 291 (2001) 1800–1803. [DOI] [PubMed] [Google Scholar]
- [12].Shu X, Wu W, Mosteller RD, Broek D, Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases, Mol. Cell. Biol 22 (2002) 7758–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Furukawa A, Kita K, Toyomoto M, Fujii S, Inoue S, Hayashi K, Ikeda K, Production of nerve growth factor enhanced in cultured mouse astrocytes by glycerophospholipids, sphingolipids, and their related compounds, Mol. Cell. Biochem 305 (2007) 27–34. [DOI] [PubMed] [Google Scholar]
- [14].El-Shewy HM, Johnson KR, Lee MH, Jaffa AA, Obeid LM, Luttrell LM, Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors, J. Biol. Chem 281 (2006) 31399–31407. [DOI] [PubMed] [Google Scholar]
- [15].Granata R, Trovato L, Lupia E, Sala G, Settanni F, Camussi G, Ghidoni R, Ghigo E, Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphKl-dependent mechanisms, J. Thromb. Haemost 5 (2007) 835–845. [DOI] [PubMed] [Google Scholar]
- [16].Melendez AJ, Ibrahim FB, Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis, J. Immunol 173 (2004) 1596–1603. [DOI] [PubMed] [Google Scholar]
- [17].Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, Khew-GoodaU Y, Bert AG, Barter PJ, Vadas MA, Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway, Proc. Natl. Acad. Sci. U. S. A 95 (1998) 14196–14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Billich A, Bomancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T, Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators, Cell. Signal 17 (2005) 1203–1217. [DOI] [PubMed] [Google Scholar]
- [19].Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM, Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages, Prostaglandins Other Lipid. Mediat 85 (2008) 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Delon C, Manifava M, Wood E, Thompson D, Krugmann S, Pyne S, Ktistakis NT, Sphingosine kinase 1 is an intracellular effector of phosphatidic acid, J. Biol. Chem 279 (2004) 44763–44774. [DOI] [PubMed] [Google Scholar]
- [21].Taha TA, Osta W, Kozhaya L, Bielawski J, Johnson KR, Gillanders WE, Dbaibo GS, Hannun YA, Obeid LM, Down-regulation of sphingosine kinase-1 by DNA damage: dependence on proteases and p53, J. Biol. Chem 279 (2004) 20546–20554. [DOI] [PubMed] [Google Scholar]
- [22].Taha TA, Kitatani K, Bielawski J, Cho W, Hannun YA, Obeid LM, Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism, J. Biol. Chem 280 (2005) 17196–17202. [DOI] [PubMed] [Google Scholar]
- [23].Pulkoski-Gross MJ, Obeid LM, Molecular mechanisms of regulation of sphingosine kinase 1, Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863 (2018) 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM, PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-l-phosphate induced by phorbol 12-myristate 13-acetate (PMA), J. Biol. Chem 277 (2002) 35257–35262. [DOI] [PubMed] [Google Scholar]
- [25].Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W, The mechanism of membrane targeting of human sphingosine kinase 1, J. Biol. Chem 280 (2005) 43030–43038. [DOI] [PubMed] [Google Scholar]
- [26].Siow D, Wattenberg B, The compartmentalization and translocation of the sphingosine kinases: mechanisms and functions in cell signaling and sphingolipid metabolism, Crit. Rev. Biochem. Mol. Biol 46 (2011) 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pitson SM, D’Andrea RJ, Vandeleur L, Moretti PA, Xia P, R Gamble J, Vadas MA, Wattenberg BW, Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes, Biochem. J 350 (Pt 2) (2000) 429–441. [PMC free article] [PubMed] [Google Scholar]
- [28].Wang X, Devaiah SP, Zhang W, Welti R, Signaling functions of phosphatidic acid, Prog. Lipid Res 45 (2006) 250–278. [DOI] [PubMed] [Google Scholar]
- [29].Olivera A, Rosenthal J, Spiegel S, Effect of acidic phospholipids on sphingosine kinase, J. Cell. Biochem 60 (1996) 529–537. [DOI] [PubMed] [Google Scholar]
- [30].Shen H, Giordano F, Wu Y, Chan J, Zhu C, Milosevic I, Wu X, Yao K, Chen B, Baumgart T, Sieburth D, De Camilli P, Coupling between endocytosis and sphingosine kinase 1 recruitment, Nat. Cell Biol 16 (2014) 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W, The mechanism of membrane targeting of human sphingosine kinase 1, J. Biol. Chem 280 (2005) 43030–43038. [DOI] [PubMed] [Google Scholar]
- [32].Wang Z, Min X, Xiao SH, Johnstone S, Romanow W, Meininger D, Xu H, Liu J, Dai J, An S, Thibault S, Walker N, Molecular basis of sphingosine kinase 1 substrate recognition and catalysis, Structure 21 (2013) 798–809. [DOI] [PubMed] [Google Scholar]
- [33].Pulkoski-Gross MJ, Jenkins ML, Truman JP, Salama MF, Clarke CJ, Burke J, Hannun YA, Obeid LM, An intrinsic lipid-binding interface controls sphingosine kinase 1 function, J. Lipid Res 59 (2018) 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Taha TA, El-Alwani M, Hannun YA, Obeid LM, Sphingosine kinase-1 is cleaved by cathepsin B in vitro: identification of the initial cleavage sites for the protease, FEBS Lett 580 (2006) 6047–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Carroll BL, Bonica J, Shamseddine AA, Hannun YA, Obeid LM, A role for caspase-2 in sphingosine kinase 1 proteolysis in response to doxorubicin in breast cancer cells - implications for the CHK1-suppressed pathway, FEBS Open Bio 8 (2018) 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schnute ME, McReynolds MD, Kasten T, Yates M, Jerome G, Rains JW, Hall T, Chrencik J, Kraus M, Cronin CN, Saabye M, Highkin MK, Broadus R, Ogawa S, Cukyne K, Zawadzke LE, Peterkin V, Iyanar K, Scholten JA, Wendling J, Fujiwara H, NemirovSK1y O, Wittwer AJ, Nagiec MM, Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1, Biochem. J 444 (2012) 79–88. [DOI] [PubMed] [Google Scholar]
- [37].Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ, FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary arteiy smooth muscle, breast cancer and androgen-independent prostate cancer cells, Cell. Signal 22 (2010) 1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McNaughton M, Pitman M, Pitson SM, Pyne NJ, Pyne S, Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SK1 or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells, Oncotarget 7 (2016) 16663–16675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ren S, Xin C, Pfeilschifter J, Huwiler A, A novel mode of action of the putative sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl) thiazole (SK1 II): induction of lysosomal sphingosine kinase 1 degradation, Cell. Physiol. Biochem 26 (2010) 97–104. [DOI] [PubMed] [Google Scholar]
- [40].Pulkoski-Gross MJ, Uys JD, Orr-Gandy KA, Coant N, Bialkowska AB, Szulc ZM, Bai A, Bielawska A, Townsend DM, Hannun YA, Obeid LM, Snider AJ, Novel sphingosine kinase-1 inhibitor, LCL351, reduces immune responses in murine DSS-induced colitis, Prostaglandins Other Lipid. Mediat 130 (2017) 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Canals D, Roddy P, Hannun YA, Protein phosphatase 1alpha mediates ceramide-induced ERM protein dephosphorylation: a novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase, J. Biol. Chem 287 (2012) 10145–10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Canals D, Jenkins RW, Roddy P, Hemandez-Corbacho MJ, Obeid LM, Hannun YA, Differential effects of ceramide and sphingosine 1-phosphate on ERM phosphorylation: probing sphingolipid signaling at the outer plasma membrane, J. Biol. Chem 285 (2010) 32476–32485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Adada M, Canals D, Hannun YA, Obeid LM, Sphingosine-1-phosphate receptor 2, FEBS J 280 (2013) 6354–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Adada MM, Canals D, Jeong N, Kelkar AD, Hemandez-Corbacho M, Pulkoski-Gross MJ, Donaldson JC, Hannun YA, Obeid LM, Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion, FASEB J 29 (2015) 4654–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gandy KA, Canals D, Adada M, Wada M, Roddy P, Snider AJ, Hannun YA, Obeid LM, Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins, Biochem. J 449 (2013) 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Orr Gandy KA, Adada M, Canals D, Carroll B, Roddy P, Hannun YA, Obeid LM, Epidermal growth factor-induced cellular invasion requires sphingosine-1-phosphate/sphingosine-l-phosphate 2 receptor-mediated ezrin activation, FASEB J 27 (2013) 3155–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Campisi J, Aging, cellular senescence, and cancer, Annu. Rev. Physiol 75 (2013) 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hemandez-Segura A, Nehme J, Demaria M, Hallmarks of cellular senescence, Trends Cell Biol 28 (2018) 436–453. [DOI] [PubMed] [Google Scholar]
- [49].Campisi J, Cellular senescence as a tumor-suppressor mechanism, Trends Cell Biol 11 (2001) S27–S31. [DOI] [PubMed] [Google Scholar]
- [50].van Deursen JM, The role of senescent cells in ageing, Nature 509 (2014) 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM, Role of ceramide in cellular senescence, J. Biol. Chem 270 (1995) 30701–30708. [DOI] [PubMed] [Google Scholar]
- [52].Venable ME, Blobe GC, Obeid LM, Identification of a defect in the phospholipase D/diacylglycerol pathway in cellular senescence, J. Biol. Chem 269 (1994) 26040–26044. [PubMed] [Google Scholar]
- [53].Miller CJ, Stein GH, Human diploid fibroblasts that undergo a senescent-like differentiation have elevated ceramide and diacylglycerol, J. Gerontol. A Biol. Sci. Med. Sci 56 (2001) B8–19. [DOI] [PubMed] [Google Scholar]
- [54].Meacci E, Vasta V, Neri S, Famararo M, Bruni P, Activation of phospholipase D in human fibroblasts by ceramide and sphingosine: evaluation of their modulatory role in bradykinin stimulation of phospholipase D, Biochem. Biophys. Res. Commun 225 (1996) 392–399. [DOI] [PubMed] [Google Scholar]
- [55].Lee JY, Leonhardt LG, Obeid LM, Cell-cycle-dependent changes in ceramide levels preceding retinoblastoma protein dephosphorylation in G2/M, Biochem. J 334 (Pt 2) (1998) 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mouton RE, Venable ME, Ceramide induces expression of the senescence histochemical marker, beta-galactosidase, in human fibroblasts, Mech. Ageing Dev 113 (2000) 169–181. [DOI] [PubMed] [Google Scholar]
- [57].Lee JY, Bielawska AE, Obeid LM, Regulation of cyclin-dependent kinase 2 activity by ceramide, Exp. Cell Res 261 (2000) 303–311. [DOI] [PubMed] [Google Scholar]
- [58].Venable ME, Bielawska A, Obeid LM, Ceramide inhibits phospholipase D in a cell-free system, J. Biol. Chem 271 (1996) 24800–24805. [DOI] [PubMed] [Google Scholar]
- [59].Lee JY, Hannun YA, Obeid LM, Ceramide inactivates cellular protein kinase Calpha, J. Biol. Chem 271 (1996) 13169–13174. [DOI] [PubMed] [Google Scholar]
- [60].Venable ME, Obeid LM, Phospholipase D in cellular senescence, Biochim. Biophys. Acta 1439 (1999) 291–298. [DOI] [PubMed] [Google Scholar]
- [61].Ogretmen B, Schady D, Usta J, Wood R, Kraveka JM, Luberto C, Birbes H, Hannun YA, Obeid LM, Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells, J. Biol. Chem 276 (2001) 24901–24910. [DOI] [PubMed] [Google Scholar]
- [62].Ogretmen B, Kraveka JM, Schady D, Usta J, Hannun YA, Obeid LM, Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line, J. Biol. Chem 276 (2001) 32506–32514. [DOI] [PubMed] [Google Scholar]
- [63].Matuoka K, Chen KY, Telomerase positive human diploid fibroblasts are resistant to replicative senescence but not premature senescence induced by chemical reagents, Biogerontology 3 (2002) 365–372. [DOI] [PubMed] [Google Scholar]
- [64].Castro ME, Ferrer I, Cascon A, Guijarro MV, Lleonart M, Ramon y Cajal S, Leal J, Robledo M, Camero A, PPP1CA contributes to the senescence program induced by oncogenic Ras, Carcinogenesis 29 (2008) 491–499. [DOI] [PubMed] [Google Scholar]
- [65].Deshpande SS, Qi B, Park YC, Irani K, Constitutive activation of racl results in mitochondrial oxidative stress and induces premature endothelial cell senescence, Arterioscler. Thromb. Vase. Biol 23 (2003) el–e6. [DOI] [PubMed] [Google Scholar]
- [66].Jadhav KS, Dungan CM, Williamson DL, Metformin limits ceramide-induced senescence in C2C12 myoblasts, Mech. Ageing Dev 134 (2013) 548–559. [DOI] [PubMed] [Google Scholar]
- [67].Chang WT, Wu CY, Lin YC, Wu MT, Su KL, Yuan SS, Wang HD, Fong Y, Lin YH, Chiu CC, C2-ceramide-induced Rb-dominant senescence-like phenotype leads to human breast cancer MCF-7 escape from p53-dependent cell death, Int. J. Mol. Sci (2019) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Modrak DE, Leon E, Goldenberg DM, Gold DV, Ceramide regulates gemcitabine-induced senescence and apoptosis in human pancreatic cancer cell lines, Mol. Cancer Res. MCR 7 (2009) 890–896. [DOI] [PubMed] [Google Scholar]
- [69].Chen JY, Hwang CC, Chen WY, Lee JC, Fu TF, Fang K, Chu YC, Huang YL, Lin JC, Tsai WH, Chang HW, Chen BH, Chiu CC, Additive effects of C(2)-ceramide on paclitaxel-induced premature senescence of human lung cancer cells, Life Sci 87 (2010) 350–357. [DOI] [PubMed] [Google Scholar]
- [70].Stith JL, Velazquez FN, Obeid LM, Advances in determining signaling mechanisms of ceramide and role in disease, J. Lipid Res 60 (2019) 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Trayssac M, Hannun YA, Obeid LM, Role of sphingolipids in senescence: implication in aging and age-related diseases, J. Clin. Invest 128 (2018) 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mosbech MB, Kruse R, Harvald EB, Olsen AS, Gallego SF, Hannibal-Bach HK, Ejsing CS, Faergeman NJ, Functional loss of two ceramide synthases elicits autophagy-dependent lifespan extension in C. elegans, PLoS One 8 (2013), e70087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chan JP, Brown J, Hark B, Nolan A, Servello D, Hrobuchak H, Staab TA, Loss of sphingosine kinase alters life history traits and locomotor function in caenorhabditis elegans, Front. Genet 8 (2017) 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Montoliu I, Scherer M, Beguelin F, DaSilva L, Mari D, Salvioli S, Martin FP, Capri M, Bucci L, Ostan R, Garagnani P, Monti D, Biagi E, Brigidi P, Kussmann M, Rezzi S, Franceschi C, Collino S, Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity, Aging (Albany NY) 6 (2014) 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mielke MM, Bandaru VV, Haughey NJ, Xia J, Fried LP, Yasar S, Albert M, Varma V, Harris G, Schneider EB, Rabins PV, Bandeen-Roche K, Lyketsos C, Carlson MC, Serum ceramides increase the risk of Alzheimer disease: the Women’s Health and Aging Study II, Neurology 79 (2012) 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yu J, Pan W, Shi R, Yang T, Li Y, Yu G, Bai Y, Schuchman EH, He X, Zhang, Ceramide is upregulated and associated with mortality in patients with chronic heart failure, Can. J. Cardiol 31 (2015) 357–363. [DOI] [PubMed] [Google Scholar]
- [77].Hughes JR, Deeley JM, Blanksby SJ, Leisch F, Ellis SR, Truscott RJ, Mitchell TW, Instability of the cellular lipidome with age, Age (Dordr.) 34 (2012) 935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Komfeld JW, Bluher M, Kronke M, Bruning JC, Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance, Cell Metab 20 (2014) 678–686. [DOI] [PubMed] [Google Scholar]
- [79].Soltau I, Mudersbach E, Geissen M, Schwedhelm E, Winkler MS, Geffken M, Peine S, Schoen G, Debus ES, Larena-Avellaneda A, Daum G, Serum-sphingosine-1-phosphate concentrations are inversely associated with atherosclerotic diseases in humans, PLoS One 11 (2016), e0168302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Heffeman-Stroud LA, Helke KI, Jenkins RW, De Costa AM, Hannun YA, Obeid LM, Defining a role for sphingosine kinase 1 in p53-dependent tumors, Oncogene 31 (2012) 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen J, Qi Y, Zhao Y, Kaczorowski D, Couttas TA, Coleman PR, Don AS, Bertolino P, Gamble JR, Vadas MA, Xia P, McCaughan GW, Deletion of sphingosine kinase 1 inhibits liver tumorigenesis in diethylnitrosamine-treated mice, Oncotarget 9 (2018) 15635–15649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yi JK, Xu R, Jeong E, Mileva I, Truman JP, Lin CL, Wang K, Snider J, Wen S, Obeid LM, A Hannun Y, Mao C, Aging-related elevation of sphingoid bases shortens yeast chronological life span by compromising mitochondrial function, Oncotarget 7 (2016) 21124–21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wilkerson JL, Stiles MA, Gurley JM, Grambergs RC, Gu X, Elliott MH, Proia RL, Mandal NA, Sphingosine kinase-1 Is essential for maintaining external/outer limiting membrane and associated adherens junctions in the aging retina, Mol. Neurobiol 56 (2019) 7188–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kim MK, Lee W, Yoon GH, Chang EJ, Choi SC, Kim SW, Links between accelerated replicative cellular senescence and down-regulation of SPHK1 transcription, BMB Rep 52 (2019) 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ceccom J, Loukh N, Lauwers-Cances V, Touriol C, Nicaise Y, Gentil C, Uro-Coste E, Pitson S, Maurage CA, Duyckaerts C, Cuvillier O, Delisle MB, Reduced sphingosine kinase-1 and enhanced sphingosine 1-phosphate lyase expression demonstrate deregulated sphingosine 1-phosphate signaling in Alzheimer’s disease, Acta Neuropathol. Commun 2 (2014) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lee JY, Han SH, Park MH, Baek B, Song IS, K Choi M, Takuwa Y, Ryu H, Kim S, He X, Schuchman EH, Bae JS, Jin HK, Neuronal SphKl acetylates COX2 and contributes to pathogenesis in a model of Alzheimer’s Disease, Nat. Commun 9 (2018) 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Xia P, Gamble JR, Rye KA, Wang L, Hii CS, CockeriU P, Khew-GoodaU Y, Bert A, Barter PJ, Vadas MA, Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway, Proc. Nad. Acad. Sci. U. S. A 95 (1998) 14196–14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shu X, Wu W, Mosteller RD, Broek D, Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases, Mol. Cell. Biol 22 (2002) 7758–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Pettus BJ, Bielawski J, Porcelli AM, Reames DL, R Johnson K, Morrow J, Chalfant C, Obeid LM, Hannun YA, The sphingosine kinase 1/sphingosine-l-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha, FASEB J 17 (2003) 1411–1421. [DOI] [PubMed] [Google Scholar]
- [90].Choi S, Snider JM, Cariello CP, Lambert JM, Anderson AK, Cowart LA, Snider AJ, Sphingosine kinase 1 is required for myristate-induced TNFalpha expression in intestinal epithelial cells, Prostaglandins Other Lipid. Mediat 149 (2020) 106423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ross JS, Hu W, Rosen B, Snider AJ, Obeid LM, Cowart LA, Sphingosine kinase 1 is regulated by peroxisome proliferator-activated receptor α in response to free fatty acids and is essential for skeletal muscle interleukin-6 production and signaling in diet-induced obesity, J. Biol. Chem 288 (2013) 22193–22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D’Andrea RJ, Gamble JR, Vadas MA, Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling, J. Biol. Chem 277 (2002) 7996–8003. [DOI] [PubMed] [Google Scholar]
- [93].Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S, Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2, Nature 465 1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Adada MM, Orr-Gandy KA, Snider AJ, Canals D, Hannun YA, Obeid LM, Clarke CJ, Sphingosine kinase 1 regulates tumor necrosis factor-mediated RANTES induction through p38 mitogen-activated protein kinase but independendy of nuclear factor kappaB activation, J. Biol. Chem 288 (2013) 27667–27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Furuya H, Tamashiro PM, Shimizu Y, lino K, Peres R, Chen R, Sun Y, Hannun YA, Obeid LM, Kawamori T, Sphingosine kinase 1 expression in peritoneal macrophages is required for colon carcinogenesis, Carcinogenesis 38 (2017) 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sun WY, Pitson SM, Bonder CS, Tumor necrosis factor-induced neutrophil adhesion occurs via sphingosine kinase-1-dependent activation of endothelial {alpha}5{beta}l integrin, Am. J. Pathol 177 (2010) 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Harikumar KB, Yester JW, Surace MJ, Oyeniran C, Price MM, Huang WC, Hait NC, Allegood JC, Yamada A, Kong X, Lazear HM, Bhardwaj R, Takabe K, Diamond MS, Luo C, Milstien S, Spiegel S, Kordula T, K63-linked polyubiquitination of transcription factor IRF1 is essential for IL-1-induced production of chemokines CXCL10 and CCL5, Nat. Immunol 15 (2014) 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yamanaka M, Shegogue D, Pei H, Bu S, Bielawska A, Bielawski J, Pettus B, Hannun YA, Obeid L, Trojanowska M, Sphingosine kinase 1 (SPHK1) is induced by transforming growth factor-beta and mediates TIMP-1 up-regulation, J. Biol. Chem 279 (2004) 53994–54001. [DOI] [PubMed] [Google Scholar]
- [99].Wang J, Feng W, Li F, Shi W, Zhai C, Li S, Zhu Y, Yan X, Wang Q, Liu L, Xie X, Li M, SphKl/S1P mediates TGF-betal-induced proliferation of pulmonary artery smooth muscle cells and its potential mechanisms, Pulm. Circ 9 (2019), 2045894018816977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bu S, Yamanaka M, Pei H, Bielawska A, Bielawski J, Hannun YA, Obeid L, Trojanowska M, Dihydrosphingosine 1-phosphate stimulates MMP1 gene expression via activation of ERKl/2-Etsl pathway in human fibroblasts, FASEB J 20 (2006) 184–186. [DOI] [PubMed] [Google Scholar]
- [101].Hammad SM, Taha TA, Nareika A, Johnson KR, Lopes-Virella MF, Obeid LM, Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells, Prostaglandins Other Lipid. Mediat 79 (2006) 126–140. [DOI] [PubMed] [Google Scholar]
- [102].Famoud AM, Bryan AM, Kechichian T, Luberto C, Del Poeta M, The granuloma response controlling cryptococcosis in mice depends on the sphingosine kinase 1-sphingosine 1-phosphate pathway, Infect. Immun 83 (2015) 2705–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Schwiebs A, Friesen O, Katzy E, Ferreiros N, Pfeilschifter JM, Radeke HH, Activation-induced cell death of dendritic cells is dependent on sphingosine kinase 1, Front. Pharmacol 7 (2016) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Jin J, Lu Z, Li Y, Ru JH, Lopes-Virella MF, Huang Y, LPS and palmitate synergistically stimulate sphingosine kinase 1 and increase sphingosine 1 phosphate in RAW264.7 macrophages, J. Leukoc. Biol 104 (2018) 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, Sammani S, Zhou G, Raj JU, Garcia JG, Berdyshev E, Yuan JX, Natarajan V, Machado RF, The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension, Am. J. Respir. Crit. Care Med 190 (2014) 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ross JS, Hu W, Rosen B, Snider AJ, Obeid LM, Cowart LA, Sphingosine kinase 1 is regulated by peroxisome proliferator-activated receptor alpha in response to free fatty acids and is essential for skeletal muscle interleukin-6 production and signaling in diet-induced obesity, J. Biol. Chem 288 (2013) 22193–22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lai WQ, Goh HH, Bao Z, Wong WS, Melendez AJ, Leung BP, The role of sphingosine kinase in a murine model of allergic asthma, J. Immunol 180 (2008) 4323–4329. [DOI] [PubMed] [Google Scholar]
- [108].Price MM, Oskeritzian CA, Falanga YT, Harikumar KB, Allegood JC, Alvarez SE, Conrad D, Ryan JJ, Milstien S, Spiegel S, A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma, J. Allergy Clin. Immunol 131 (2) (2013) 501–511 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Barbour M, McNaughton M, Boomkamp SD, MacRitchie N, Jiang HR, Pyne NJ, Pyne S, Effect of sphingosine kinase modulators on interleukin-lbeta release, sphingosine 1-phosphate receptor 1 expression and experimental autoimmune encephalomyelitis, Br. J. Pharmacol 174 (2017) 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wang YL, Xue P, Xu CY, Wang Z, Liu XS, Hua LL, Bai HY, Zeng ZL, Duan HF, Li JF, SPK1-transfected UCMSC has better therapeutic activity than UCMSC in the treatment of experimental autoimmune encephalomyelitis model of Multiple sclerosis, Sci. Rep 8 (2018) 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P, Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis, Nat. Rev. Drug Discov 9 (2010) 883–897. [DOI] [PubMed] [Google Scholar]
- [112].El Jamal A, Bougault C, Mebarek S, Magne D, Cuvillier O, Brizuela L, The role of sphingosine 1-phosphate metabolism in bone and joint pathologies and ectopic calcification, Bone 130 (115087) (2020). [DOI] [PubMed] [Google Scholar]
- [113].Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, Mclnnes IB, Melendez AJ, Leung BP, Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis, J. Immunol 181 (2008) 8010–8017. [DOI] [PubMed] [Google Scholar]
- [114].Baker DA, Eudaly J, Smith CD, Obeid LM, Gilkeson GS, Impact of sphingosine kinase 2 deficiency on the development of TNF-alpha-induced inflammatory arthritis, Rheumatol. Int 33 (2013) 2677–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Baker DA, Obeid LM, Gilkeson GS, Impact of sphingosine kinase on inflammatory pathways in fibroblast-like synoviocytes, Inflamm. Allergy Drug Targets 10 (2011) 464–471. [DOI] [PubMed] [Google Scholar]
- [116].Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS, Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis, J. Immunol 185 (2010) 2570–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Inoue T, Kohno M, Nagahara H, Murakami K, Sagawa T, Kasahara A, Kaneshita S, Kida T, Fujioka K, Wada M, Nakada H, Hla T, Kawahito Y, Upregulation of sphingosine-1-phosphate receptor 3 on fibroblast-like synoviocytes is associated with the development of collagen-induced arthritis via increased interleukin-6 production, PLoS One 14 (2019), e0218090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY, Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression, Arthritis Rheum 54 (2006) 742–753. [DOI] [PubMed] [Google Scholar]
- [119].Seyedian SS, Nokhostin F, Malamir MD, A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease, J. Med. Life 12 (2019) 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ullman TA, Itzkowitz SH, Intestinal inflammation and cancer, Gastroenterology 140 (2011) 1807–1816. [DOI] [PubMed] [Google Scholar]
- [121].Kawamori T, Kaneshiro T, Okumura M, Maalouf S, Uflacker A, Bielawski J, Hannun YA, Obeid LM, Role for sphingosine kinase 1 in colon carcinogenesis, FASEB J 23 (2009) 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM, A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis, FASEB J 23 (2009) 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, Upson JJ, Smith CD, Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase, Dig. Dis. Sci 53 (2008) 997–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Snider AJ, Kawamori T, Bradshaw SG, Orr KA, Gilkeson GS, Hannun YA, Obeid LM, A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis, FASEB J.: Off. Publ. Feder. Am. Soc. Exper. Biol 23 (2009) 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, Hait NC, Allegood JC, Price MM, Avni D, Takabe K, Kordula T, Milstien S, Spiegel S, Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer, Cancer Cell 23(2013)107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Snider AJ, Ali WH, Sticca JA, Coant N, Ghaleb AM, Kawamori T, Yang VW, Hannun YA, Obeid LM, Distinct roles for hematopoietic and extra-hematopoietic sphingosine kinase-1 in inflammatory bowel disease, PLoS One 9 (2014), e113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sun M, Zhou Y, Shi Y, Liu B, Effect of the sphingosine kinase 1 selective inhibitor, PF543 on dextran sodium sulfate-induced colitis in mice, DNA Cell Biol 38 (2019) 1338–1345. [DOI] [PubMed] [Google Scholar]
- [128].Espaillat MP, Snider AJ, Qiu Z, Channer B, Coant N, Schuchman EH, Kew RR, Sheridan BS, Hannun YA, Obeid LM, Loss of acid ceramidase in myeloid cells suppresses intestinal neutrophil recruitment, FASEB J 32 (2018) 2339–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wang K, Xu R, Snider AJ, Schrandt J, Li Y, Bialkowska AB, Li M, Zhou J, Hannun YA, Obeid LM, Yang VW, Mao C, Alkaline ceramidase 3 deficiency aggravates colitis and colitis-associated tumorigenesis in mice by hyperactivating the innate immune system, Cell Death Dis 7 (2016), e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Garcia-Barros M, Coant N, Kawamori T, Wada M, Snider AJ, Truman JP, Wu BX, Furuya H, Clarke CJ, Bialkowska AB, Ghaleb A, Yang VW, Obeid LM, Hannun YA, Role of neutral ceramidase in colon cancer, FASEB J 30 (2016) 4159–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Snider AJ, Wu BX, Jenkins RW, Sticca JA, Kawamori T, Hannun YA, Obeid LM, Loss of neutral ceramidase increases inflammation in a mouse model of inflammatory bowel disease, Prostaglandins Other Lipid. Mediat 99 (2012) 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Green DR, Evan GI, A matter of life and death, Cancer Cell 1 (2002) 19–30. [DOI] [PubMed] [Google Scholar]
- [133].Ogretmen B, Hannun YA, Biologically active sphingolipids in cancer pathogenesis and treatment, Nat. Rev. Cancer 4 (2004) 604–616. [DOI] [PubMed] [Google Scholar]
- [134].Saddoughi SA, Song P, Ogretmen B, Roles of bioactive sphingolipids in cancer biology and therapeutics, Subcell. Biochem 49 (2008) 413–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].A Hannun Y, Obeid LM, Sphingolipids and their metabolism in physiology and disease, Nat. Rev. Mol. Cell Biol 19 (2018) 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Camerer E, Regard JB, Comelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR, Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice, J. Clin. Invest 119 (2009) 1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Liu G, Yang K, Burns S, Shrestha S, Chi H, The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells, Nat. Immunol 11 (2010) 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gault CR, Obeid LM, Still benched on its way to the bedside: sphingosine kinase 1 as an emerging target in cancer chemotherapy, Crit. Rev. Biochem. Mol. Biol 46 (2011) 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Obeid LM, Linardic CM, Karolak LA, Hannun YA, Programmed cell death induced by ceramide, Sci. (New York, N.Y) 259 (1993) 1769–1771. [DOI] [PubMed] [Google Scholar]
- [140].Mullen TD, Obeid LM, Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death, Anti Cancer Agents Med. Chem 12 (4) (2012) 340–363. [DOI] [PubMed] [Google Scholar]
- [141].Smyth MJ, Perry DK, Zhang J, Poirier GG, Hannun YA, Obeid LM, prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of Bcl-2, Biochem. J 316 (Pt 1) (1996) 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zhang J, Driscoll TA, Hannun YA, Obeid LM, Regulation of membrane release in apoptosis, Biochem. J 334 (Pt 2) (1998) 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang J, Reedy MC, Hannun YA, Obeid LM, Inhibition of caspases inhibits the release of apoptotic bodies: Bcl-2 inhibits the initiation of formation of apoptotic bodies in chemotherapeutic agent-induced apoptosis, J. Cell Biol 145 (1999) 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Zhang J, Hannun YA, Obeid LM, A novel assay for apoptotic body formation and membrane release during apoptosis, Cell Death Differ 6 (1999) 596–598. [DOI] [PubMed] [Google Scholar]
- [145].Zhang J, Alter N, Reed JC, Bomer C, Obeid LM, Hannun YA, Bcl-2 interrupts the ceramide-mediated pathway of cell death, Proc. Natl. Acad. Sci. U. S. A 93 (1996) 5325–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Gamard CJ, Dbaibo GS, Liu B, Obeid LM, Hannun YA, Selective involvement of ceramide in cytokine-induced apoptosis. Ceramide inhibits phorbol ester activation of nuclear factor kappaB, J. Biol. Chem 272 (1997) 16474–16481. [DOI] [PubMed] [Google Scholar]
- [147].Kraveka JM, Li L, Szulc ZM, Bielawski J, Ogretmen B, Hannun YA, Obeid LM, Bielawska A, Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells, J. Biol. Chem 282 (2007) 16718–16728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhang P, Liu B, Jenkins GM, Hannun YA, Obeid LM, Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis, J. Biol. Chem 272 (1997) 9609–9612. [DOI] [PubMed] [Google Scholar]
- [149].Birbes H, El Bawab S, Obeid LM, Hannun YA, Mitochondria and ceramide: intertwined roles in regulation of apoptosis, Adv. Enzym. Regul 42 (2002) 113–129. [DOI] [PubMed] [Google Scholar]
- [150].Birbes H, El Bawab S, Hannun YA, Obeid LM, Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis, FASEB J 15 (2001) 2669–2679. [DOI] [PubMed] [Google Scholar]
- [151].Novgorodov SA, Szulc ZM, Luberto C, Jones JA, Bielawski J, Bielawska A, Hannun YA, Obeid LM, Positively charged ceramide is a potent inducer of mitochondrial permeabilization, J. Biol. Chem 280 (2005) 16096–16105. [DOI] [PubMed] [Google Scholar]
- [152].Novgorodov SA, Gudz TI, Obeid LM, Long-chain ceramide is a potent inhibitor of the mitochondrial permeability transition pore, J. Biol. Chem 283 (2008) 24707–24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Novgorodov SA, Wu BX, Gudz TI, Bielawski J, Ovchinnikova TV, Hannun YA, Obeid LM, Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA, J. Biol. Chem 286 (2011) 25352–25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Schwartz NU, Linzer RW, Truman JP, Gurevich M, Hannun YA, Senkal CE, Obeid LM, Decreased ceramide underlies mitochondrial dysfunction in Charcot-Marie-Tooth 2F, FASEB J 32 (2018) 1716–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Mullen TD, Spassieva S, Jenkins RW, Kitatani K, Bielawski J, Hannun YA, Obeid LM, Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism, J. Lipid Res 52 (2011) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Mullen TD, Hannun YA, Obeid LM, Ceramide synthases at the centre of sphingolipid metabolism and biology, Biochem. J 441 (2012) 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Spassieva SD, Mullen TD, Townsend DM, Obeid LM, Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response, Biochem. J 424 (2009) 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhao L, Spassieva SD, Jucius TJ, Shultz LD, Shick HE, Macklin WB, Hannun YA, Obeid LM, Ackerman SL, A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation, PLoS Genet 7 (2011), e1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Mullen TD, Jenkins RW, Clarke CJ, Bielawski J, Hannun YA, Obeid LM, Ceramide synthase-dependent ceramide generation and programmed cell death: involvement of salvage pathway in regulating postmitochondrial events, J. Biol. Chem 286 (2011) 15929–15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Hemandez-Corbacho MJ, Canals D, Adada MM, Liu M, Senkal CE, Yi JK, Mao C, Luberto C, Hannun YA, Obeid LM, Tumor necrosis factor-alpha (TNFalpha)-induced ceramide generation via ceramide synthases regulates loss of focal adhesion kinase (FAK) and programmed cell death, J. Biol. Chem 290 (2015) 25356–25373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Spassieva S, Seo JG, Jiang JC, Bielawski J, Alvarez-Vasquez F, Jazwinski SM, Hannun YA, Obeid LM, Necessary role for the Laglp motif in (dihydro)ceramide synthase activity, J. Biol. Chem 281 (2006) 33931–33938. [DOI] [PubMed] [Google Scholar]
- [162].Siskind LJ, Mullen TD, Romero Rosales K, Clarke CJ, Hemandez-Corbacho MJ, Edinger AL, Obeid LM, The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis, J. Biol. Chem 285 (2010) 11818–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR, Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis, Cell 148 (2012) 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Suhrland C, Truman JP, Obeid LM, Sitharaman B, Delivery of long chain C16 and C24 ceramide in HeLa cells using oxidized graphene nanoribbons, J Biomed Mater Res B Appl Biomater 108 (2020) 1141–1156. [DOI] [PubMed] [Google Scholar]
- [165].Suhrland C, Truman JP, Obeid LM, Sitharaman B, Oxidized graphene nanoparticles as a delivery system for the pro-apoptotic sphingolipid C6 ceramide, J. Biomed. Mater. Res. A 107 (2019) 25–37. [DOI] [PubMed] [Google Scholar]
- [166].Lupino L, Perry T, Margielewska S, Hollows R, Ibrahim M, Care M, Allegood J, Tooze R, Sabbadini R, Reynolds G, Bicknell R, Rudzki Z, Lin Hock Y, Zanetto U, Wei W, Simmons W, Spiegel S, Woodman CBJ, Rowe M, Vrzalikova K, Murray PG, Sphingosine-1-phosphate signalling drives an angiogenic transcriptional programme in diffuse large B cell lymphoma, Leukemia 33 (2019) 2884–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Shirai K, Kaneshiro T, Wada M, Furuya H, Bielawski J, Hannun YA, Obeid LM, Ogretmen B, Kawamori T, A role of sphingosine kinase 1 in head and neck carcinogenesis, Cancer Preven. Res. (Philadelphia, Pa.) 4 (2011) 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Degagne E, Saba JD, S1pping fire: Sphingosine-1-phosphate signaling as an emerging target in inflammatory bowel disease and colitis-associated cancer, Clin. Exp. Gastroenterol 7 (2014) 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, A Vadas M, An oncogenic role of sphingosine kinase, Curr. Biol 10 (2000) 1527–1530. [DOI] [PubMed] [Google Scholar]
- [170].Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S, Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells, Exp. Cell Res 281 (2002) 115–127. [DOI] [PubMed] [Google Scholar]
- [171].Ruckhaberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, Grosch S, Geisslinger G, Holtrich U, Kam T, Kaufmann M, Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer, Breast Cancer Res. Treat 112 (2008) 41–52. [DOI] [PubMed] [Google Scholar]
- [172].Li J, Guan HY, Gong LY, Song LB, Zhang N, Wu J, Yuan J, Zheng YJ, Huang ZS, Li M, Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival, Clin. Cancer Res 14 (2008) 6996–7003. [DOI] [PubMed] [Google Scholar]
- [173].Young N, Pearl DK, Van Brocklyn JR, Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61, Mol. Cancer Res. MCR 7 (2009) 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Acharya S, Yao J, Li P, Zhang C, Lowery FJ, Zhang Q, Guo H, Qu J, Yang F, Wistuba II, Piwnica-Worms H, Sahin AA, Yu D, Sphingosine kinase 1 signaling promotes metastasis of triple-negative breast cancer, Cancer Res 79 (2019) 4211–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Liu SQ, Huang JA, Qin MB, Su YJ, Lai MY, Jiang HX, Tang GD, Sphingosine kinase 1 enhances colon cancer cell proliferation and invasion by upregulating the production of MMP-2/9 and uPA via MAPK pathways, Int. J. Color. Dis 27 (2012) 1569–1578. [DOI] [PubMed] [Google Scholar]
- [176].Bae GE, Do SI, Kim K, Park JH, Cho S, Kim HS, Increased sphingosine kinase 1 expression predicts distant metastasis and poor outcome in patients with colorectal cancer, Anticancer Res 39 (2019) 663–670. [DOI] [PubMed] [Google Scholar]
- [177].Maiti A, Takabe K, Hait NC, Metastatic triple-negative breast cancer is dependent on SphKs/S1P signaling for growth and survival, Cell. Signal 32 (2017) 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Doll F, Pfeilschifter J, Huwiler A, Prolactin upregulates sphingosine kinase-1 expression and activity in the human breast cancer cell line MCF7 and triggers enhanced proliferation and migration, Endocr. Relat. Cancer 14 (2007) 325–335. [DOI] [PubMed] [Google Scholar]
- [179].Doll F, Pfeilschifter J, Huwiler A, The epidermal growth factor stimulates sphingosine kinase-1 expression and activity in the human mammary carcinoma cell line MCF7, Biochim. Biophys. Acta 1738 (2005) 72–81. [DOI] [PubMed] [Google Scholar]
- [180].Anelli V, Gault CR, Snider AJ, Obeid LM, Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro, FASEB J 24 (2010) 2727–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, Zhao R, Milstien S, Zhou H, Spiegel S, Takabe K, Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis, Cancer Res 72 (2012) 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Lu Z, Zhang W, Gao S, Jiang Q, Xiao Z, Ye L, Zhang X, MiR-506 suppresses liver cancer angiogenesis through targeting sphingosine kinase 1 (SPHK1) mRNA, Biochem. Biophys. Res. Commun 468 (2015) 8–13. [DOI] [PubMed] [Google Scholar]
- [183].Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM, Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue, J. Histochem. Cytochem 53 (2005) 1159–1166. [DOI] [PubMed] [Google Scholar]
- [184].Song L, Xiong H, Li J, Liao W, Wang L, Wu J, Li M, Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-kappaB pathway in human non-small cell lung cancer, Clin. Cancer Res 17 (2011) 1839–1849. [DOI] [PubMed] [Google Scholar]
- [185].Long JS, Edwards J, Watson C, Tovey S, Mair KM, Schiff R, Natarajan V, Pyne NJ, Pyne S, Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells, Mol. Cell. Biol 30 (2010) 3827–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Pyne NJ, Pyne S, Sphingosine 1-phosphate and cancer, Nat. Rev. Cancer 10 (2010) 489–503. [DOI] [PubMed] [Google Scholar]
- [187].Zhang Y, Wang Y, Wan Z, Liu S, Cao Y, Zeng Z, Sphingosine kinase 1 and cancer: a systematic review and meta-analysis, PLoS One 9 (2014), e90362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM, Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis, FASEB J.: Off. Publ. Feder. Am. Soc. Exper. Biol 20 (2006) 482–484. [DOI] [PubMed] [Google Scholar]
- [189].Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, Pyne S, Pyne NJ, Edwards J, High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients, Am. J. Pathol 177 (2010) 2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Datta A, Loo SY, Huang B, Wong L, Tan SS, Tan TZ, Lee SC, Thiery JP, Lim YC, Yong WP, Lam Y, Kumar AP, Yap CT, SPHK1 regulates proliferation and survival responses in triple-negative breast cancer, Oncotarget 5 (2014) 5920–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, Delisle MB, Cuvillier O, Susini C, Bousquet C, Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug, Mol. Cancer Ther 8 (2009) 809–820. [DOI] [PubMed] [Google Scholar]
- [192].Maczis M, Milstien S, Spiegel S, Sphingosine-1-phosphate and estrogen signaling in breast cancer, Adv. Biol. Regul 60 (2016) 160–165. [DOI] [PubMed] [Google Scholar]
- [193].Marfe G, Di Stefano C, Gambacurta A, Ottone T, Martini V, Abruzzese E, Mologni L, Sinibaldi-Salimei P, de Fabritis P, Gambacorti-Passerini C, Amadori S, Birge RB, Sphingosine kinase 1 overexpression is regulated by signaling through PI3K, AKT2, and mTOR in imatinib-resistant chronic myeloid leukemia cells, Exp. Hematol 39 (653–665) (2011), e656. [DOI] [PubMed] [Google Scholar]
- [194].Bonhoure E, Lauret A, Barnes DJ, Martin C, Malavaud B, Kohama T, Melo JV, Cuvillier O, Sphingosine kinase-1 is a downstream regulator of imatinib-induced apoptosis in chronic myeloid leukemia cells, Leukemia 22 (2008) 971–979. [DOI] [PubMed] [Google Scholar]
- [195].Gault CR, Eblen ST, Neumann CA, Hannun YA, Obeid LM, Oncogenic K-Ras regulates bioactive sphingolipids in a sphingosine kinase 1-dependent manner, J. Biol. Chem 287 (2012) 31794–31803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Anelli V, Gault CR, Cheng AB, Obeid LM, Sphingosine kinase 1 is upregulated during hypoxia in U87MG glioma cells. Role of hypoxia-inducible factors 1 and 2, J. Biol. Chem 283 (2008) 3365–3375. [DOI] [PubMed] [Google Scholar]
- [197].Salama MF, Carroll B, Adada M, Pulkoski-Gross M, Hannun YA, Obeid LM, A novel role of sphingosine kinase-1 in the invasion and angiogenesis of VHL mutant clear cell renal cell carcinoma, FASEB J.: Off. Publ. Feder. Am. Soc. Exper. Biol 29 (2015) 2803–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Bouquerel P, Gstalder C, Muller D, Laurent J, Brizuela L, Sabbadini RA, Malavaud B, Pyronnet S, Martineau Y, Ader I, Cuvillier O, Essential role for SphKl/S1P signaling to regulate hypoxia-inducible factor 2alpha expression and activity in cancer, Oncogenesis 5 (2016), e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Li J, Wu H, Li W, Yin L, Guo S, Xu X, Ouyang Y, Zhao Z, Liu S, Tian Y, Tian Z, Ju J, Ni B, Wang H, Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHKl/Akt/NF-kappaB signaling, Oncogene 35 (2016) 5501–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Chen MB, Yang L, Lu PH, Fu XL, Zhang Y, Zhu YQ, Tian Y, MicroRNA-101 down-regulates sphingosine kinase 1 in colorectal cancer cells, Biochem. Biophys. Res. Commun 463 (2015) 954–960. [DOI] [PubMed] [Google Scholar]
- [201].Zhao X, He W, Li J, Huang S, Wan X, Luo H, Wu D, MiRNA-125b inhibits proliferation and migration by targeting SphKl in bladder cancer, Am. J. Transl. Res 7 (2015) 2346–2354. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


