Abstract
Background and Aims:
Polysubstance use is common and contributes to morbidity and mortality of hospitalized patients, and yet little is known about patterns of substance use among hospitalized patients, or how an addiction consult service (ACS) might impact polysubstance use after discharge. The objective of this study was to identify patterns of substance use at admission and after discharge among hospitalized patients with substance use disorders who saw an ACS.
Design:
Prospective cohort study. We used latent transition analysis of substance use scores at the time of hospital admission and 30 to 90 days posthospitalization.
Setting:
Single, academic health center with an ACS in Portland, Oregon, from 2015 to 2018
Participants/Cases:
Patients were eligible if they received a consult to the inpatient ACS.
Measurements:
We used Addiction Severity Index-Lite scores to capture self-reported substance use at baseline and follow-up for heroin, other opioid, alcohol, amphetamine, and cocaine.
Findings:
From 2015 to 2018, 486 individuals consented to participate. More than half of patients used more than one substance at baseline. We identified three patterns of substance use at baseline: 1) alcohol use dominant, 2) polysubstance use dominant, and 3) heroin and other opioid use dominant. Patients transitioned along five trajectories to three different follow-up profiles that showed lower endorsement of all substances used. Slightly more than 40% (40.1%) of patients newly endorsed abstinence of at least one substance at follow-up.
Conclusions:
Polysubstance use is common in hospitalized patients with substance use disorders and identifying patterns of polysubstance use can guide clinical management. Hospital providers should prepare to manage polysubstance use during hospitalization and hospitals should broaden care beyond interventions for opioid use disorder.
Keywords: Inpatients, Opioid-related disorders, Amphetamine, Alcohol, Addictive behavior, Hospitalization
1. Introduction
Polysubstance use is increasingly common: over the last decade, opioid-related deaths have nearly tripled, but deaths among people using benzodiazepines, stimulants, and synthetic opioids have matched or outpaced this trend (CDC NCfHS, 2018). Polysubstance is the use of substances in different classes, but also within-classes; for example, people who use both illicit methadone and heroin may have polysubstance use. In the past decade, on the West Coast, in particular, there is increasing co-use of opioids and methamphetamine (Winkelman, Admon, Jennings, et al., 2018), although recent data suggests that methamphetamine use is emerging in the northeast (National Drug Threat Assessment, 2018). Hospitalizations from polysubstance use are also rising nationally, and polysubstance use is known to contribute to the morbidity and mortality of hospitalized patients (Barocas, Wang, Marshal, et al., 2019).
Despite this, little is known about the care of people with polysubstance use during hospitalization, and most hospital-based interventions for substance use focus on a single substance (Priest & McCarty, 2019). Hospitalization is an important care delivery environment warranting study, as patients are at higher risk for overdose after hospital discharge (Mudumbai, Lewis, Oliva, et al., 2019); substance use influences other disease outcomes (e.g., effects of methamphetamine on cardiomyopathy, effects of alcohol on liver disease); hospitalization is a reachable moment to engage people in substance use disorder (SUD) treatment (Englander, Dobbertin, Lind, et al., 2019); and hospitals can be stigmatizing and traumatizing spaces for people with SUDs (McNeil, Small, Wood & Kerr, 2014). It is unclear how hospitalization might affect polysubstance use, particularly in the context of an effective hospital-based intervention.
Addiction consult services (ACS) are emergent hospital-based organizational interventions (Priest & McCarty, 2019) that reduce substance use severity (Wakeman, Metlay, Chang, Herman, & Rigotti, 2017) and increase patient engagement in treatment (Englander, Dobbertin, Lind, et al., 2019) during and posthospitalization. While research has shown decreases in singular substance with use of ACS (Wakeman, Metlay, Chang, Herman, & Rigotti, 2017) research to date has not explored if or how ACS influence patterns of polysubstance use.
Latent transition analysis (LTA) allows researchers to identify subgroups of people with similar patterns of variables within their study data (Lanza & Collins, 2008; Lanza, 2010). LTA provides information about baseline behavior, follow-up behavior, and the probability of transitioning or changing over a period of time (Lanza, 2010). LTA has been used to explore changes in singular substance use, particularly among patients with alcohol use disorder (Choi, Elmquist, Shorey, et al., 2017; Shin, Lee, Lu, & Hecht, 2016; Cleveland, Mallett, Turrisi, et al., 2018; La Flair, Reboussin, Stor, et al., 2013; Halonen, Stenholm, Pulakk, et al., 2017; Hoyland & Latendresse, 2018; McBride, Adamson, Cheng, Slad, 2014; Jackson & Schulenberg, 2013; Lee, Chassin, & Villalta, 2013; Staudt, Freyer-Adam, & Meyer, 2018), and among adolescents and college students (Choi, Elmquist, Shorey, et al., 2017; La Flair, Reboussin, Stor, et al., 2013; Choi, Lu, Schulte, & Temple, 2018; Tomczyk, Pedersen, Hanewinkel, et al., 2016), but has been used widely in other areas of change research as well (Valente, Cogo-Moreira, Swardfager, & Sanchez, 2018; Wilkinson, El-Hayek, Fairley, & Rot, et al., 2017; Valmaggia, Stahl, Yung, Nelson, et al., 2013; Tisminetzky, Bray, Miozzo, Aupont, & McLaughlin, 2011; Lanza & Bray, 2010; Kenzik, Martin, Fouad, & Pisu, 2015; Guo, Aveyard, Fielding, & Sutton, 2009). LTA may be of particular use in modeling changes in substance use (Lanza, Patrick, & Maggs, 2010). The objective of this study was to identify patterns of substance use at admission and after discharge among hospitalized patients with SUDs who saw an ACS.
2. Methods
2.1. Study setting and design
The Improving Addiction Care Team (IMPACT) is a hospital-based ACS at an academic health center in Portland, Oregon. IMPACT provides interprofessional addiction-related care from addiction providers (physicians, nurse practitioner, physician assistant), social workers, and recovery peers with lived experience (Englander, Dobbertin, Lind, Nicolaidis, et al., 2019). IMPACT patients are hospitalized for acute medical and surgical conditions (e.g., endocarditis, acute alcohol withdrawal, abscess), and generally are not seeking addiction treatment at time of hospitalization. Any hospital provider or social worker can refer a patient to IMPACT. Patients are eligible for referral if they have known or suspected SUD, other than exclusively tobacco use disorder. Interest in SUD treatment or reducing substance use is not required for IMPACT participation. IMPACT performs substance use assessments; initiates pharmacotherapy and behavioral SUD treatment when appropriate; provides rapid-access to posthospital SUD care; and provides bridging peer support, in hospital and after discharge. IMPACT also provides pharmacotherapy, including buprenorphine, methadone, and extended-release naltrexone for opioid use disorder; acamprosate, naltrexone (extended-release and oral), and other medications for alcohol use disorder; buproprion and varencline for tobacco use disorder; and occasionally bupropion or mirtazapine for people with alternate indications who also have a stimulant use disorder (Chan, Freeman, Kondo, Ayers, et al., 2019). Behavioral treatments include but are not limited to motivational interviewing, brief intervention, and contingency management. IMPACT offers harm reduction and embraces principles of trauma-informed care. Earlier studies describe IMPACT in detail (Englander, Dobbertin, Lind, Nicolaidis, et al., 2019; Englander H, Weimer M, Solotaroff R, Nicolaidis, et al., 2017; Englander H, Collins D, Perry SP, Rabinowitz, 2018). The Oregon Health & Science University’s Institutional Review Board approved this study.
2.2. Participants
Between September 2015 and August 2018, IMPACT researchers consented and enrolled interested patients in an IMPACT evaluation study. All patients that IMPACT staff contacted were eligible to enroll. Patients readmitted at least six months after enrolling in this study were able to reenroll and repeat study surveys and interviews. This manuscript only includes data from participants’ first hospital admission in which they were enrolled in IMPACT research.
2.3. Data collection and measures
A research assistant who was not part of the clinical team administered the baseline survey to patients early in their hospitalization, and administered a follow-up survey 30 to 90 days after hospital discharge. If patients returned to the hospital within the 30 to 90 days after discharge, they were contacted during their hospital stay. This 20-minute survey asked questions related to demographics (e.g., income, partner status), substance use (e.g., days of use), and patient experience (e.g., care transition measure). Surveys included drug use items adapted from the Addiction Severity Index Lite (ASI-Lite), a tool used to monitor substance use over time. ASI-Lite items included measures of substance misuse of prescribed or illicit opioids, including heroin, amphetamines, cocaine, methadone, cannabis, other opioids, and alcohol in the previous 30 days (Cacciola, Alterman, McLellan, Lin, & Lynch, 2007; McLellan, Kushner, Metzger, Peters, et al., 1992). The other opioids category includes all nonprescribed opioids other than heroin and methadone, including fentanyl (Cacciola, Alterman, McLellan, Lin, & Lynch, 2007; McLellan, Kushner, Metzger, Peters, et al., 1992). Versus other parts of the United States, overdose deaths attributable to fentanyl occur at lower rates in Oregon (Wilson, Kariisa, Seth, Smith, & Davis, 2020). We stored all survey data in REDCap (Harris, Taylor, Thielke, Payne, et al., 2009).
2.4. Data analysis
2.4.1. Analyses
We used LTA to identify subgroups of patients by their ASI-Lite substance use at baseline and follow-up, and estimated distributions of our population across these profiles. Latent transition analysis provides three key outputs: 1) estimated subgroup membership probabilities at baseline and follow-up, 2) transition probabilities across subgroups from baseline to follow-up, and 3) ASI-Lite response probabilities at each time for each subgroup (Lanza & Collins, 2008; Lanza, 2010).
We first identified latent classes using participant ASI-Lite responses for five variables: alcohol, heroin, other opioids (e.g., fentanyl), cocaine, and amphetamines (mostly methamphetamine). We excluded patients whose only substance use at baseline was methadone (n=9), cannabis (n=11), or both (n=6) because we were primarily interested in nonmethadone opioids, alcohol, and stimulants. We reclassified the ASI-Lite scores to reflect the number of times per week of use for each substance. We hypothesized that our data would have a zero-inflated Poisson distribution for each substance, and so included the following categorical classifications: 0 days of use per week (0 days of use reported), 1 day of use per week (1–4 days per month), 2 to 5 days of use per week (5–20 days per month), and more than 5 days of use per week (21–30 days per month).
Because we believed, a priori, our population to be heterogeneous based on clinical observations, we considered models that identified between two and six subgroups of patients. We used latent class analysis to examine best fit models at baseline, and then tested if imposing measurement invariance over time (to allow subgroups to maintain the same meaning at baseline and follow-up) was appropriate using a likelihood-ratio test (nested G2 test) (Ryoo, Wang, Swearer, Hull, & Shi, 2018).
2.4.2. Model selection
2.4.2.1. Latent class analysis
We calculated Log-likelihood, G-squared, Akaike information criterion (AIC), Bayesian information criterion (BIC), Bozdogan’s Criterion, adjusted Bayesian information criterion, and entropy values from our models, as well as their degrees of freedom (Appendix 1). Following the work of Lanza & Bray (2010), we generated 1000 random sets of starting values for both our baseline and follow-up analyses in PROC LCA and also report the percentage of time that we found the optimal solution among the seeds specified. Collins & Lanza (2010) suggest that higher solution stability can provide further reassurance that the maximum likelihood solution has been identified. Separately, Nyland and Muthen (2007) found that the bootstrapped likelihood-ratio and, second to it, BIC value, were best in a simulation study at specifying the model, though BIC values tend to bias toward over-simplified models (Weakliem, 1999). While we initially planned to carry out the likelihood-ratio test to compare across models, we were only confident in our three-class models at baseline and follow-up because of the large drop off in solution stability of all other baseline models, and thus chose to proceed with three classes in our LTA. We provide our final code and model selection tables in Appendix 1.
2.4.2.2. Latent transition analysis
We calculated model fit statistics including log-likelihood, G-squared, AIC, and BIC for our latent transition model. We planned to consider invoking measurement invariance across time, which would have allowed our model’s subgroups to retain the same meanings over time. However, our likelihood ratio (nested G2 test) was significant (p<0.001), and so we did not invoke measurement invariance (Ryoo, Wang, Swearer, Hull, & Shi, 2018).
2.4.2.3. Case examples
To illustrate patients’ transitions, we selected patients with high posterior probabilities (probability of belonging in each trajectory) of baseline and follow-up profile membership among each trajectory identified; and describe the patient’s presentation, interventions received during hospitalization from IMPACT, and qualitative excerpts, if the patient completed a follow-up interview.
2.4.2.4. Missing data
Before analyses, we dropped participants missing all baseline ASI-Lite data. For participants who listed number of days of use of at least one drug (for example, heroin: 19 days), we replaced all other missing baseline values as 0 days (i.e., alcohol replaced as 0 days, cocaine replaced as 0 days, if missing at baseline), because we hypothesized that it was more likely that the person did not use the substance at baseline. We confirmed the rationale for this approach with the research assistant who collected the surveys. If the value was also missing at follow-up and the baseline value was imputed as zero, we imputed the follow-up value as zero.
LTA assumes that missing data are missing at random, and identifies the subgroup for a patient even if an outcome measure is missing. Thus, we included participants in our analysis who were missing follow-up data because we agreed that data could be missing at random (Mack, 2018).
We used Stata 15 to clean our data, and SAS PROC LCA & LTA (Lanza, Huang, Wagner, & Collins, 2015) and SAS 9.4 to analyze data. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
3. Results
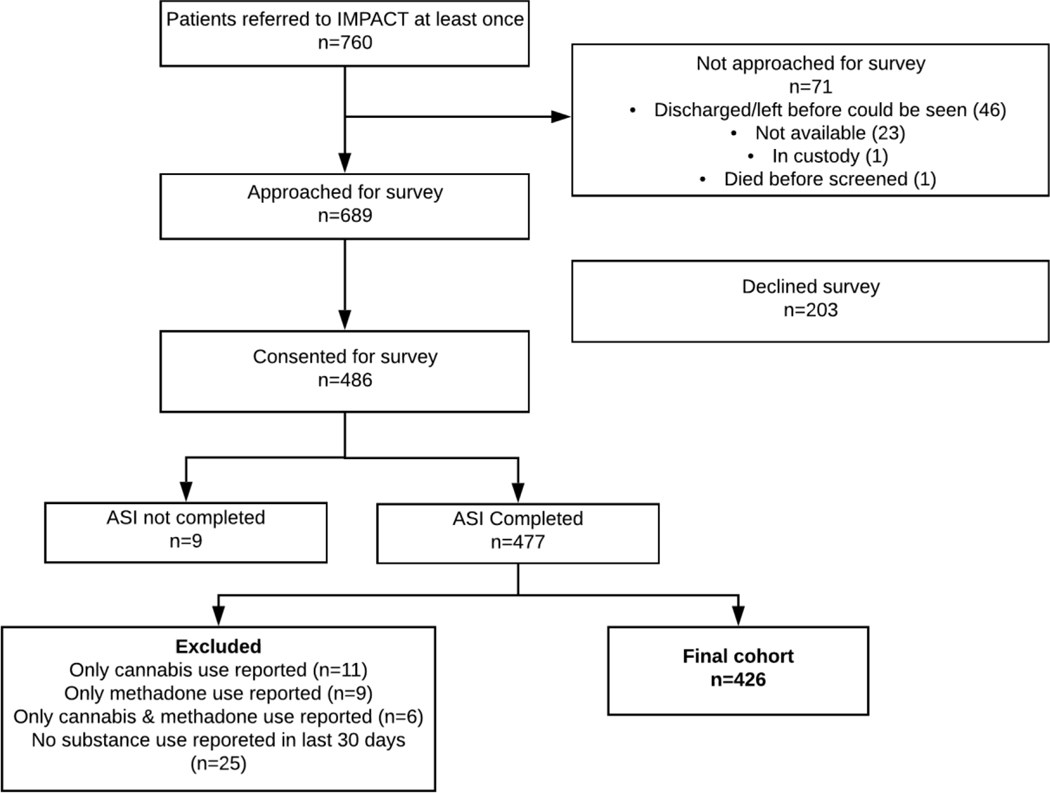
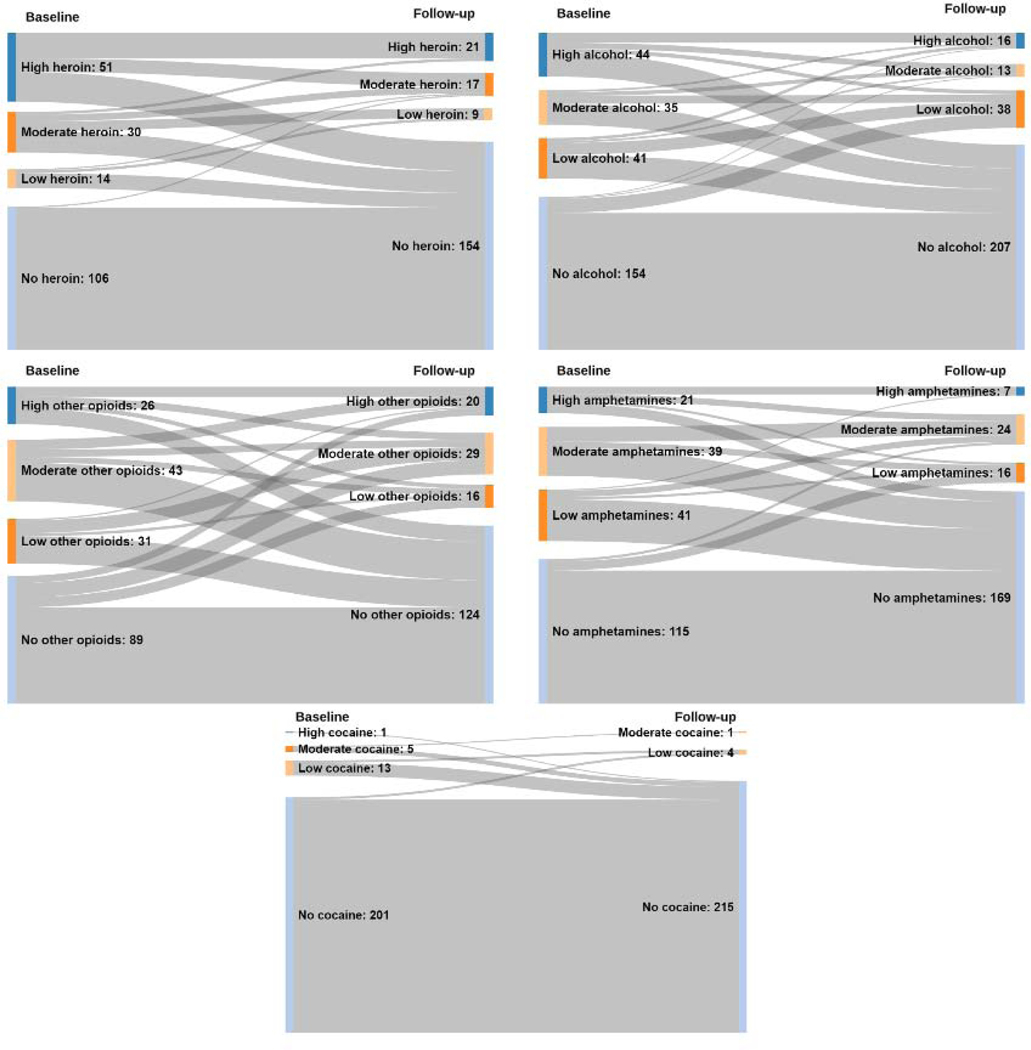
From 2015 to 2018, 486 individuals consented to participate. Nine participants had no baseline data, and we dropped the 51 participants who used methadone or cannabis exclusively from the analysis (Figure 1). Among our final participant cohort, most were male (64.1%), white (77.5%), with health insurance (94.8%), and access to a usual source of primary care (65.3%). Over half (52.8%) of participants used at least two substances. Two-hundred and one (201) participants used only one substance at baseline. Of those, 50.7% used only alcohol, 21.9% used only other opioids, 16.9% used only amphetamines, and 10.4% used only heroin. Follow-up survey rates for people with any alcohol, heroin, other opioid, cocaine, or methamphetamine use at baseline ranged from 50.3% to 63.3% (59.1%, 50.3%, 58.8%, 63.3%, 52.8%, respectively). Most patients completed the follow-up survey while out of the hospital; less than 10% completed the follow-up survey during a readmission. The median length of hospital admission was 8 days (range=[1,118]). In Figure 2, we show baseline and follow-up ASI-Lite past 30-day drug use prevalence by individual substance, not accounting for polysubstance use.
Figure 1.
Participant flowchart.
Figure 2.
Sankey flowcharts of baseline and follow-up ASI-Lite scores, 2015–2018.* [Print in Color]
*No use included 0 days of use per week (0 days of use reported); low use included 1 day of use per week (1–4 days per month); moderate use included 2 to 5 days of use per week (5–20 days per month); high use included >5 days of use per week (21–30 days per month).
3.1. Subgroup descriptions at baseline and follow-up
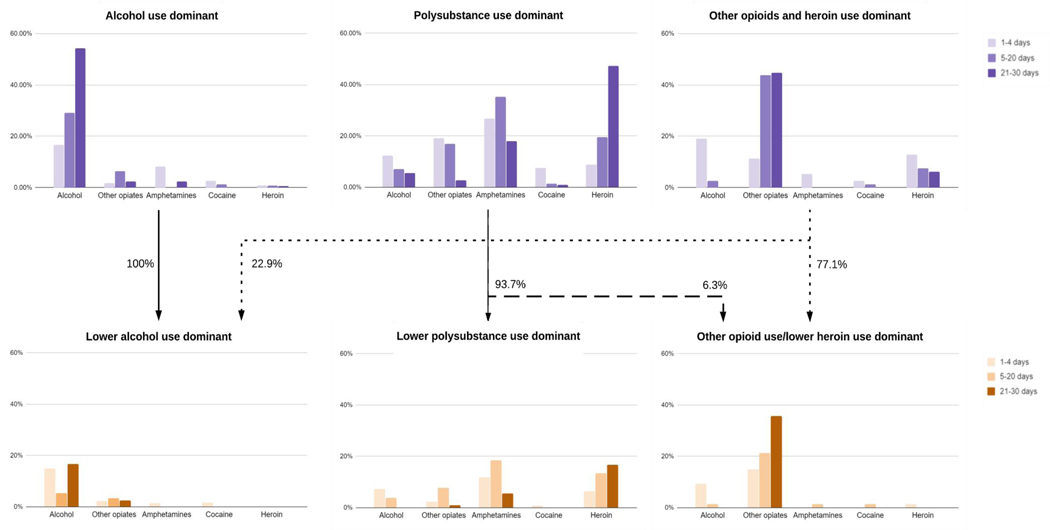
We identified three subgroups at baseline and three subgroups at follow-up (Figure 3). The three baseline subgroups were: 1) alcohol use dominant; 2) polysubstance use dominant; and 3) other opioids and heroin use dominant. In group 1, “alcohol use dominant”, 29.2% endorsed alcohol use 5 to 20 days per month, and 54.2% endorsed alcohol use more than 21 days per month. Additionally, nearly everyone abstained from using heroin in this group, and only 10.5% of participants used other opioids and amphetamines at least once a month. In group 2, “polysubstance use dominant”, 35.2% of participants used amphetamines 5 to 20 days per month, and 18.0% used amphetamines more than 21 days per month. Additionally, 19.6% used heroin 5 to 20 days per month and 47.5% used heroin more than 21 days per month. This population had varying levels of alcohol use (24.8% endorsed drinking at least one in the previous month) and other opioid use (38.9% endorsed other opioid use at least once in the previous month). Group 3, “other opioid and heroin use dominant”, had low probabilities of endorsing alcohol, amphetamines or cocaine use greater than 5 days in the previous month (all less than 5%). This group was more likely to endorse other opioid use over 21 days (44.8%) and heroin use over 21 days (6.1%), as well as other opioids and heroin at least 5 to 20 days (43.9% and 7.6%, respectively).
Figure 3.
Probabilities of item endorsement by subgroups at baseline and follow-up. [Print in Color]
We estimated that at baseline, 31.1% of the population belonged to the alcohol use dominant group, 52.0% belonged to the polysubstance use dominant group, and 16.9% belonged to the heroin and other opioid use dominant group.
The three follow-up subgroups were: 1) lower alcohol use dominant; 2) lower polysubstance use dominant; 3) other opioid use/lower heroin use dominant. In the first follow-up subgroup, “lower alcohol use dominant”, participants had some other opioid use over 5 days per month (5.7%), with 16.6% endorsing alcohol use over 21 days per month. This group was unlikely to have reported amphetamine, cocaine, or heroin use over 5 days per month, and less than 2% were likely to have used amphetamine, heroin, or cocaine at all during the month. Just more than 63 percent (63.1%) of this group had no alcohol use, and no one in this group was likely to have used heroin. The second group, “lower polysubstance use dominant”, had 16.7% of participants reporting at least 21 days of heroin use, with 23.9% likely to have endorsed using amphetamines between 5 and 30 days of the previous month. The final subgroup, “other opioid use/lower heroin use dominant”, shows less than 2% of participants were likely to endorse 5 days or more of alcohol, amphetamine, cocaine, or heroin use; however, 43.4% were likely to endorse using other opioids.
3.2. Transition probabilities
From baseline to follow-up, 100% of participants were likely to have transitioned from the alcohol use dominant to lower alcohol use dominant subgroup (Figure 3). For the same time period, 93.7% of participants transitioned from the polysubstance use dominant group to lower polysubstance use dominant group, and the remaining 6.3% transitioned to the other opioid use/lower heroin use dominant group. Among those in the other opioids and heroin use dominant group, 22.9% transitioned to the lower alcohol use dominant group, and 77.1% transitioned to the other opioid use/lower heroin use dominant group. In Table 3, we provide cases that illustrate the transition paths of patient subgroups using cases of patients probabilistically most likely to fall into each trajectory.
3.3. Participants abstaining at follow-up
In addition to our LTA, we report the number of participants who transitioned from any use to abstinence at follow-up. Two hundred and three (47.7%) of our 426 participants reported any alcohol use at baseline; 57 (28.1%) of those patients transitioned to 0 days of use at follow-up. One hundred and eighty-nine (44.4%) participants used heroin at least once at baseline, and 36 (19.0%) of those patients reported 0 days of use at follow-up. One hundred and seventy (39.9%) reported other opioid use at baseline, and 36 (21.2%) of those participants reported no other opioid use at follow-up. Thirty (7.0%) endorsed cocaine use at baseline, and 14 then reported no use at follow-up (46.7%). Finally, 195 (45.8%) participants used amphetamines at least once at baseline, and 55 (28.2%) of those participants reported no use at follow-up.
4. Discussion
Among patients that saw an ACS, we found three patterns of substance use at baseline and three distinct patterns at follow-up; patients moved through five trajectories among these patterns. Polysubstance use was common in patients that were seen in IMPACT. LTA classified 52.0% of patients in the polysubstance use dominant group, 16.9% in the other opioids and heroin use dominant group, and 31.1% in the alcohol use dominant profile at baseline. Follow-up profiles show lower levels of substance use than baseline profiles. While many participants continued to use some combination of substances, 40.1% patients reported abstinence at follow-up of at least one substance that they had been using prehospitalization.
Our results build on a growing body of literature related to polysubstance use. Earlier work found methamphetamine-related emergency department visits (Liu & Vivolo-Kantor, 2019), hospitalizations, and healthcare costs (Winkelman, Admon, Jennings, et al., 2018) are rising, particularly in the western United States, and that rates of methamphetamine use among people who use heroin are also rising (Strickland, Havens, & Stoops, 2019). Our study, which uses patient reported data compared to administrative data, mirrors these findings. In our study at baseline, 45.8% of patients endorsed using amphetamines at least once in the previous 30 days, and more than half reported polysubstance use. Additionally, of the 269 patients who reported any opioid use at baseline, nearly three-quarters had polysubstance use, including use of alcohol (n=80, 29.7%), cocaine (n=25, 9.3%), or amphetamines (n=142, 52.8%) in the previous 30 days (n=187, 69.5%). The high prevalence of polysubstance use among hospitalized patients is important given earlier work that shows strong associations between polysubstance use and opioid-related overdose deaths, and recent work demonstrating the methamphetamine use is increasing among people who co-use opioids (Jones, Underwood, & Compton, 2019). One Massachusetts study found that 83% of people who died of opioid-related overdose deaths had other substances in their bloodstream, stimulants being the most common (Barocas, Wang, Marshall, LaRochelle, et al., 2019).
Other studies have identified patterns of polysubstance use at single timepoints. A study in Shanghai, China, identified three patterns of polysubstance use (alcohol and heroin use, polysubstance use, and heroin and methamphetamine use) among treatment-seeking heroin-dependent adults and compared these patterns with treatment outcomes (Chen, Zhong, Du, Li, Zhao, et al., 2019). Our groups are similar in identifying polysubstance use, but our alcohol use dominant group endorsed lower levels of heroin use. Researchers in Australia examined patterns of polysubstance use among people who inject drugs, using a sample of national data and information about eighteen substances. They similarly identified patterns of polysubstance use (Betts, Chan, McIlwraith, Dietze, et al., 2016). Our study is the first to explore transitions in polysubstance use after hospitalization and a hospital ACS intervention. Understanding polysubstance use patterns can guide clinicians’ understanding of presentations of SUD among hospitalized patients. Further, studying these subgroups can help clinicians to understand the implications for engagement in care, treatment, and other outcomes among patients with SUD.
Our study had several important limitations. First, our study lacked a control group of patients who did not see an ACS. Thus, while it is likely that IMPACT services contributed to reduced substance use after discharge, our methods do not allow us to draw causal inference, and it is possible that other factors related to hospitalization contributed to changes in substance use. Second, our findings relied on patient self-reported substance use (Cacciola, Alterman, McLellan, Lin, & Lynch, 2007; McLellan, Kushner, Metzger, Peters, et al., 1992), which may not always be accurate. Third, we had a limited sample size, preventing us from testing covariate associations with transition probabilities and potentially limiting our power to detect additional substance use patterns. Fourth, this is a single site study, and substance use patterns may represent regional trends not reflected in other settings. Fifth, we asked participants 30 to 90 days after hospital discharge to recall information about substance use in the 30 days after hospital discharge. It is possible that recall bias impacted participant responses, and they may have underestimated substance use after hospital discharge. Sixth, LTA assumes that data are missing at random. It is possible that some data are also missing completely at random or missing not at random. Finally, we adapted the ASI-Lite survey instrument as part of our baseline survey. To avoid respondent survey burden, we did not ask about some substance use, including inhalants, hallucinogens, and benzodiazepines, which limited our understanding of how our study population used these substances.
Additionally, we are unsure why other opioid use remained high in our other opioid/lower heroin use group at follow-up. It is possible that patients included prescribed opioids in their survey response counts at follow-up, even though those opioids were not obtained illicitly or misused. Higher rates of other opioid use at follow-up may not reflect misuse or illicit use, but instead reflect prescribed opioids related to care received during a medical or surgical hospitalization. Furthermore, the ASI-Lite may not fully capture the severity of SUDs. Other instruments may have provided a more comprehensive look at not only substance use, but also, importantly, its impact on patients’ lives.
Our study has implications for clinical care, health systems, and future research. Because polysubstance use was common among our participants, hospital providers should be prepared to ask about, and care for, polysubstance use during hospitalization. Health systems should look to develop tools for patients, providers, hospitals, and communities at-large to help reduce potential harms from polysubstance use, regardless of patient plans to change use. Additionally, while first-line medications to treat OUD are an important, evidence-based pillar of care, addiction-related services in the inpatient setting should broaden beyond focused interventions for OUD. Future research should explore individual and population-level factors for emerging trends in polysubstance use, and identify additional ways to mitigate harm. Finally, recovery settings are predominately single-substance focused. Future research should explore how to best support patients who are in recovery for polysubstance use.
Our study found that following ACS consult, substance use decreased from baseline levels. These findings build on and support earlier work, which shows decreases in single substance use (Wakeman, Metlay, Chang, Herman, & Rigotti, 2017) in a setting with an ACS. That subgroups of patients reduced substance use after care with an ACS is important, and this finding adds to the growing literature (Priest & McCarty, 2019; Englander, Dobbertin, Lind, Nicolaidis, et al., 2019; Wakeman, Metlay, Chang, Herman, & Rigotti, 2017) that supports the importance of hospital-based addiction care as an emerging standard of care (Englander, Priest, Snyder, Martin, et al., 2019). It is also important to highlight that even with intensive hospital addiction consultation, many patients continue using substances. These findings underscore the need to integrate harm reduction into hospital care (Heller, McCoy, & Cunningham, 2004; Hyshka, Anderson-Baron, Karekezi, Belle-Isle, et al., 2017), including supporting safer use practices, providing access to clean syringes, considering pre-exposure HIV prophylaxis, and providing naloxone and other strategies for overdose prevention.
Finally, the use of LTA to identify changes in substance use provide insights not achievable with traditional statistical methods. Wider use of subgroup analyses, including other machine learning techniques, may help to guide the field toward a better understanding of heterogeneity among populations. Future work should explore differences in baseline patterns of substance use upon admission by region in the United States, as well as by hospital type and other patient and system level demographics. Hospital systems and communities should look to integrate and provide first-line, evidence-based addiction care during hospitalization, regardless of the presence of an ACS, and shift toward a polysubstance use frame in caring for patients with SUD in the United States.
Table 1.
Baseline demographics of IMPACT participants, 2015–2018.
| Total (n=426) | Alcohol use dominant (n=135)* | Polysubstance use dominant (n=218)* | Other opioids and heroin use dominant (n=73)* | |
|---|---|---|---|---|
| Age, years (SD) | 43.9 (12.5) | 47.5 (11.7) | 40.9 (12.0) | 46.3 (13.4) |
| Gender Male | 273 (64.1%) | 90 (66.7%) | 141 (64.7%) | 42 (57.5%) |
| Female | 149 (35.0%) | 45 (33.3%) | 73 (33.5%) | 31 (42.5%) |
| Race Caucasian (n=422) | 330 (77.5%) | 104 (77.7%) | 166 (76.1%) | 60 (82.2%) |
| American Indian/Alaska Native | 22 (5.2%) | 8 (5.9%) | 12 (5.5%) | 2 (2.7%) |
| Asian | 2 (0.5%) | 1 (0.7%) | 1 (0.5%) | 0 |
| Black or African American | 13 (3.1%) | 5 (3.7%) | 6 (2.8%) | 2 (2.7%) |
| Native Hawaiian or Other Pacific Islander | 5 (1.2%) | 1 (0.7%) | 4 (1.8%) | 0 |
| More than one race | 24 (5.6%) | 7 (5.2%) | 13 (6.0%) | 4 (5.5%) |
| Other/Refused | 26 (6.1%) | 9 (6.7%) | 14 (6.4%) | 3 (4.1%) |
| Current Homelessness (n=421) | 153 (35.9%) | 46 (34.1%) | 93 (42.7%) | 14 (19.2%) |
| Partner with substance use (n=421) | 120 (28.2%) | 46 (34.1%) | 63 (28.9%) | 11 (15.1%) |
| Insured (n=418) | 404 (94.8%) | 127 (94.1%) | 204 (93.6%) | 73 (100.0%) |
| Usual source of primary care (n=417) | 278 (65.3%) | 98 (72.6%) | 127 (58.3%) | 53 (72.6%) |
| Alcohol use, 30 days (n=411) | 7.9 (11.5) | 19.6 (10.7) | 2.6 (7.1) | 0.6 (2.5) |
| Heroin use, 30 days (n=327) | 11.8 (13.0) | 0.3 (2.0) | 18.4 (12.1) | 2.6 (6.8) |
| Other opioid use, 30 days (n=301) | 6.7 (9.8) | 3.2 (7.1) | 3.1 (5.2) | 18.0 (11.1) |
| Cocaine use, 30 days (n=338) | 0.5 (2.6) | 0.4 (1.9) | 0.6 (3.1) | 0.3 (2.0) |
| Amphetamine use, 30 days (n=344) | 6.5 (9.7) | 1.9 (6.5) | 9.8 (10.3) | 0.1 (0.7) |
We used posterior probability values to assign patients to a subgroup for the purposes of displaying demographic information in Table 1.
Table 2.
Latent transition patient examples.
| Baseline ASI Lite Score | Follow-up ASI Lite Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Followup | Patient description | In-hospital interventions provided by IMPACT | Alc | Her | Amph | OthOpi | Coc | Alc | Her | Amph | OthOpi | Coc |
| Alcohol use dominant | Lower alcohol use dominant | 27-year-old man with major depression and post-traumatic stress disorder admitted with suicidal ideation in the setting of acute alcohol intoxication | • Diagnosed with severe AUD • Provided SUD education • Administered extended release naltrexone • Coordinated naltrexone transition to primary care • Supported linkage to outpatient mental health care |
24 | 0 | . | 0 | 0 | 20 | 0 | 0 | 0 | 0 |
| Polysubs tance use dominant | Other opioid use/lower heroin use dominant | 35-year-old man with a history of MRSA abscess admitted to MICU for acute renal failure (newly hemodialysis dependent), critical anemia, pericardial effusion, acute chest pain, and opioid/benzodiazepine. Reported starting using prescription opioids after a broken leg about 11 years ago, and moved to intravenous heroin for the last 10 years. Had previously been in residential and outpatient treatment, and methadone maintenance (210 mg/day) for 18 months, stopping 1 year before admission. | • Diagnosed with severe OUD and benzodiazepine use disorder • Convened family meeting about treatment options post-discharge • Started and administered methadone • Managed benzodiazepine withdrawal • Provided peer support • Transitioned from methadone to buprenorphine-naloxone • Supported linkage to outpatient buprenorphine provider and primary care. |
0 | 30 | 4 | 2 | 2 | 4 | 0 | 0 | 3 | 0 |
| Polysubs tance use dominant | Less polysubs tance use dominant | 31-year-old man with a history of OUD and MUD and homelessness admitted with cellulitis and bacteremia. Patient was largely unwilling to engage. Reported seven years of intravenous heroin use and occasional methamphetamine use. Initially ambivalent, but eventually interested in trying methadone. | • Started and up titrated methadone • Supported establishment with OTP • Helped obtain a photo ID and coordinated transportation (both barriers to engagement) • Connected patient with a shelter. • Provided naloxone • Provided peer support |
0 | 30 | 7 | 7 | 0 | 0 | 30 | 0 | 0 | 0 |
| Other opioids and heroin use dominant | Other opioid use/lower heroin use dominant | 51-year-old woman with hypertension, depression, endometriosis, and Hepatitis C admitted for spinal epidural abscess with adjacent soft tissue infection following decompression and drainage extending from cervical to lumbar vertebrae, MRSA bacteremia, stress cardiomyopathy and possible endocarditis. Five-year history of OUD; started with pills and transitioned to heroin. Last methamphetamine use two months prior to admission. | • Diagnosed with severe OUD and moderate MUD • Started methadone • Provided pain management with hydromorphone • Started SSRI for depression • Received social work SUD treatment • Coordinated methadone at SNF • Provided harm reduction education about safer use practices, naloxone, and family and housing interventions. • Discharged to SNF to complete intravenous antibiotics with hydromorphone taper |
0 | 5 | 0 | 10 | . | 0 | 0 | 0 | 30 | 0 |
| Other opioids and heroin use dominant | Lower alcohol use dominant | 62-year-old man with HIV/AIDS, COPD, Hepatitis C, opioid use disorder, chronic pain, and hypertension admitted for soft tissue abscesses and pain control. Last opioid use one month prior to admission though persistent occasional methamphetamine use until admission. | • Started buprenorphinenaloxone • Connected to primary care; HIV care and community resources • Provided peer support |
1 | 12 | 0 | 7 | 0 | 1 | 0 | 0 | 0 | 0 |
Alc=Alcohol use, Her= Heroin use, Amph= Amphetamine use, Oth Opi= Other Opioid use, Coc= Cocaine use, AUD= Alcohol use disorder, OUD=Opioid use disorder, MUD=methamphetamine use disorder, SNF= Skilled nursing facility, SUD= Substance use disorder; a period (.) indicates missing data
Highlights.
We identified 3 patterns of polysubstance use at hospital admission and 3 post-discharge
Patterns included 1) alcohol use, 2) polysubstance use, or 3) heroin/opioid use, dominance
Patterns after hospitalization with an addiction consult service (ACS) reflect lower overall use
After ACS consult, 40.1% of patients newly abstain from at least one substance
Polysubstance use is common; identifying patterns of use can guide clinical management
Acknowledgements
We would like to thank the IMPACT patients for participating in this research.
Funding
IMPACT is funded by Oregon Health & Science University and CareOregon.
CK, HE, and PTK were supported by grants from the National Institutes of Health, National Institute on Drug Abuse (UG1DA015815 / R01DA037441). KCP was supported by a grant from the National Institute on Drug Abuse (F30 DA044700UG).
This publication was also made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1TR002369 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Role of funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
This study was approved by Oregon Health & Science University’s Institutional Review Board (IRB #IRB00010846).
APPENDIX 1
Table 1.
Model fit statistics for latent classes at baseline and follow-up with 1000 random starts.
| Baseline models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of subgroups | Log-likelihood | G-squared | AIC | BIC | CAIC | ABIC | Entropy | Degrees of Freedom | Solution % |
| 2 | −1965.43 | 483.41 | 545.41 | 671.09 | 702.09 | 572.72 | 0.87 | 992 | 8.0% |
| 3 | −1910.90 | 374.34 | 468.34 | 658.90 | 705.90 | 509.75 | 0.88 | 976 | 94.9% |
| 4 | −1889.47 | 331.49 | 457.49 | 712.92 | 775.92 | 513.00 | 0.94 | 960 | 5.0% |
| 5 | −1872.16 | 296.86 | 454.86 | 775.16 | 854.16 | 524.46 | 0.95 | 944 | 0.4% |
| 6 | −1855.19 | 262.92 | 452.92 | 838.09 | 933.09 | 536.62 | 0.90 | 928 | 2.2% |
| Follow-up models | |||||||||
| Number of subgroups | Log-likelihood | G-squared | AIC | BIC | CAIC | ABIC | Entropy | Degrees of Freedom | Solution % |
| 2 | −819.59 | 120.66 | 182.66 | 308.28 | 339.28 | 209.91 | 0.69 | 992 | 92.4% |
| 3 | −807.18 | 95.83 | 189.83 | 380.28 | 427.28 | 231.13 | 0.83 | 976 | 22.0% |
| 4 | −798.23 | 77.94 | 203.94 | 459.23 | 522.23 | 259.30 | 0.55 | 960 | 23.5% |
| 5 | −791.86 | 65.21 | 223.21 | 543.32 | 622.32 | 292.63 | 0.83 | 944 | 0.7% |
| 6 | −785.86 | 53.19 | 243.19 | 628.14 | 723.14 | 326.67 | 0.68 | 928 | 0.2% |
Table 2.
Model fit statistics for three group latent transition analysis, with and without measurement invariance.
| Model type | Log-likelihood | G-squared | AIC | BIC | Degrees of Freedom |
|---|---|---|---|---|---|
| With measurement invariance | −2757.11 | 1550.52 | 1656.52 | 1871.40 | 1048522 |
| Without measurement invariance | −2650.89 | 1338.10 | 1534.10 | 1931.43 | 1048477 |
Table 3.
Subgroup item response probabilities and membership probabilities at baseline.
| Alcohol use dominant | Polysubstance use dominant | Other opioids and heroin use dominant | ||
|---|---|---|---|---|
| Membership probability at baseline | 31.1% | 52.0% | 16.9% | |
| 0 days | Alcohol | 0 | 75.2% | 78.3% |
| Other opioids | 89.5% | 62.1% | 0 | |
| Amphetamines | 89.5% | 20.0% | 94.7% | |
| Cocaine | 96.1% | 90.1% | 96.0% | |
| Heroin | 98.6% | 24.2% | 73.5% | |
| 1–4 days | Alcohol | 16.7% | 12.3% | 19.0% |
| Other opioids | 1.8% | 19.2% | 11.2% | |
| Amphetamines | 8.2% | 26.8% | 5.3% | |
| Cocaine | 2.6% | 7.5% | 2.7% | |
| Heroin | 0.9% | 8.9% | 12.8% | |
| 5–20 days | Alcohol | 29.2% | 7.0% | 2.7% |
| Other opioids | 6.5% | 17.0% | 43.9% | |
| Amphetamines | 0 | 35.2% | 0 | |
| Cocaine | 1.3% | 1.5% | 1.3% | |
| Heroin | 0.9% | 19.6% | 7.6% | |
| 21–30 days | Alcohol | 54.2% | 5.5% | 0 |
| Other opioids | 2.3% | 2.7% | 44.8% | |
| Amphetamines | 2.3% | 18.0% | 0 | |
| Cocaine | 0 | 0.9% | 0 | |
| Heroin | 0.6% | 47.3% | 6.1% |
Table 4.
Subgroup item response probabilities and membership probabilities at follow-up.
| Lower alcohol use dominant | Lower polysubstance use dominate | Other opioid use/lower heroin use dominant | ||
|---|---|---|---|---|
| Membership probability at baseline | 35.0% | 48.7% | 16.3% | |
| 0 days | Alcohol | 63.1% | 88.8% | 89.2% |
| Other opioids | 92.0% | 89.1% | 28.0% | |
| Amphetamines | 98.6% | 63.9% | 98.5% | |
| Cocaine | 98.3% | 99.3% | 98.6% | |
| Heroin | 100% | 63.3% | 98.6% | |
| 1–4 days | Alcohol | 15.0% | 7.4% | 9.3% |
| Other opioids | 2.3% | 2.2% | 14.9% | |
| Amphetamines | 1.4% | 12.0% | 0 | |
| Cocaine | 1.7% | 0.8% | 0 | |
| Heroin | 0 | 6.5% | 1.4% | |
| 5–20 days | Alcohol | 5.4% | 3.8% | 1.5% |
| Other opioids | 3.3% | 7.8% | 21.3% | |
| Amphetamines | 0 | 18.5% | 1.5% | |
| Cocaine | 0 | 0 | 1.5% | |
| Heroin | 0 | 13.5% | 0 | |
| 21–30 days | Alcohol | 16.6% | 0 | 0 |
| Other opioids | 2.4% | 0.9% | 35.8% | |
| Amphetamines | 0 | 5.6% | 0 | |
| Cocaine | 0 | 0 | 0 | |
| Heroin | 0 | 16.7% | 0 |
Table 5.
Transition probabilities among subgroups from baseline to follow-up.
| Follow-up | ||||
|---|---|---|---|---|
| Lower alcohol use dominant | Lower polysubstance use dominant | Other opioid use/lower heroin use dominant | ||
| Baseline | Alcohol use dominant | 100.0% | 0 | 0 |
| Polysubstance use dominant | 0 | 93.7% | 6.3% | |
| Other opioids and heroin use dominant | 22.9% | 0 | 77.1% | |
SAS CODE
Final 3 class model using Latent Transition Analysis
PROC LTA DATA = WORK.LTA_FIN OUTPOST=post_prob;
NSTATUS 3;
NTIMES 2;
ITEMS alc0 opi0 meth0 coc0 her0 alc1 opi1 meth1 coc1 her1;
CATEGORIES 4 4 4 4 4;
SEED 975469647;
RUN;
Latent Class Analysis for baseline model
PROC LCA DATA = WORK.LTA_FIN;
NCLASS 3;
ITEMS alc0 opi0 meth0 coc0 her0;
CATEGORIES 4 4 4 4 4;
*MEASUREMENT time1 time2;
SEED 5489;
NSTARTS 1000;
RUN;
Latent Class Analysis for follow-up model
PROC LCA DATA = WORK.LTA_FIN;
NCLASS 3;
ITEMS alc1 opi1 meth1 coc1 her1;
CATEGORIES 4 4 4 4 4;
*MEASUREMENT time1 time2;
SEED 5489;
NSTARTS 1000;
RUN;
Footnotes
Conflict of Interest
The authors have no competing interest to report. Dr. Korthuis serves as principal investigator on NIH-funded grants that receive donated study medication from Indivior (buprenorphine) and Alkermes (extended-release naltrexone).
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barocas JA, Wang J, Marshall BDL, LaRochelle MR, Bettano A, Bernson D, et al. (2019). Sociodemographic factors and social determinants associated with toxicology confirmed polysubstance opioid-related deaths. Drug & Alcohol Dependence, 200, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts KS, Chan G, McIlwraith F, Dietze P, Whittaker E, Burns L, et al. (2016). Differences in polysubstance use patterns and drug-related outcomes between people who inject drugs receiving and not receiving opioid substitution therapies. Addiction, 111(7), 1214–1223. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. (2007). Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug & Alcohol Dependence, 87(2–3), 297–302. [DOI] [PubMed] [Google Scholar]
- CDC NCfHS. (2018). Multiple Cause of Death 1999–2017 on CDC Wide-ranging Online Data for Epidemiologic Research (CDC WONDER; ). [Google Scholar]
- Chan B, Freeman M, Kondo K, Ayers C, Montgomery J, Paynter R, et al. (2019). Pharmacotherapy for methamphetamine/amphetamine use disorder-a systematic review and meta-analysis. Addiction, 114(12), 2122–2136. [DOI] [PubMed] [Google Scholar]
- Chen T, Zhong N, Du J, Li Z, Zhao Y, Sun H, et al. (2019). Polydrug use patterns and their impact on relapse among heroin-dependent patients in Shanghai, China. Addiction, 114(2), 259–267. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Elmquist J, Shorey RC, Rothman EF, Stuart GL, Temple JR. (2017). Stability of alcohol use and teen dating violence for female youth: A latent transition analysis. Drug & Alcohol Review, 36(1), 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Lu Y, Schulte M, Temple JR. (2018). Adolescent substance use: Latent class and transition analysis. Addictive Behaviors, 77, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland MJ, Mallett KA, Turrisi R, Sell NM, Reavy R, Trager B. (2018). Using latent transition analysis to compare effects of residency status on alcohol-related consequences during the first two years of college. Addictive Behaviors, 87, 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. (2018). “We’ve learned it’s a medical illness, not a moral choice”: Qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. Journal of Hospital Medicine, 13(11), 752–8. [DOI] [PubMed] [Google Scholar]
- Englander H, Dobbertin K, Lind BK, Nicolaidis C, Graven P, Dorfman C, et al. (2019). Inpatient Addiction medicine consultation and post-hospital substance use disorder treatment engagement: A propensity-matched analysis. Journal of General Internal Medicine, 34(12), 2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. (2019). A call to action: Hospitalists’ role in addressing substance use disorder. Journal of Hospital Medicine, 14, E1–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander H, Weimer M, Solotaroff R, Nicolaidis C, Chan B, Velez C, et al. (2017). Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. Journal of Hospital Medicine, 12(5), 339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Aveyard P, Fielding A, Sutton S. (2009). Using latent class and latent transition analysis to examine the transtheoretical model staging algorithm and sequential stage transition in adolescent smoking. Substance Use & Misuse, 44(14), 2028–2042. [DOI] [PubMed] [Google Scholar]
- Halonen JI, Stenholm S, Pulakka A, Kawachi I, Aalto V, Pentti J, et al. (2017). Trajectories of risky drinking around the time of statutory retirement: A longitudinal latent class analysis. Addiction, 112(7), 1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. (2009). Research electronic data capture (REDCap)--A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D, McCoy K, Cunningham C. (2004). An invisible barrier to integrating HIV primary care with harm reduction services: Philosophical clashes between the harm reduction and medical models. Public Health Reports, 119(1), 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyland MA, Latendresse SJ. (2018). Stressful life events influence transitions among latent classes of alcohol use. Psychology of Addictive Behaviors, 32(7), 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyshka E, Anderson-Baron J, Karekezi K, Belle-Isle L, Elliott R, Pauly B, et al. (2017). Harm reduction in name, but not substance: a comparative analysis of current Canadian provincial and territorial policy frameworks. Harm Reduction Journal, 14(1), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Schulenberg JE. (2013). Alcohol use during the transition from middle school to high school: National panel data on prevalence and moderators. Developmental Psychology, 11, 2147–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Underwood N, Compton WM. (2019). Increases in methamphetamine use among heroin treatment admissions in the United States, 2008–17. Addiction, 115(2), 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzik KM, Martin MY, Fouad MN, Pisu M. (2015). Health-related quality of life in lung cancer survivors: Latent class and latent transition analysis. Cancer, 121(9), 1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Flair LN, Reboussin BA, Storr CL, Letourneau E, Green KM, Mojtabai R, et al. (2013). Childhood abuse and neglect and transitions in stages of alcohol involvement among women: A latent transition analysis approach. Drug & Alcohol Dependence, 132(3), 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza LCaS. (2010). Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Lanza ST, Bray BC. (2010). Transitions in drug use among high-risk women: An application of latent class and latent transition analysis. Advances and Applications in Statistical Sciences, 3(2), 203–35. [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Collins LM. (20080. A new SAS procedure for latent transition analysis: Transitions in dating and sexual risk behavior. Developmental Psychology, 44(2), 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza S DJ, Huang L, Wagner A, Collins LM. (2015). PROC LCA & PROC LTA Users’ Guide Version 1.3.2. The Methodology Center, Penn State University. [Google Scholar]
- Lanza ST, Patrick ME, Maggs JL. (2010). Latent transition analysis: Benefits of a latent variable approach to modeling transitions in substance use. Journal of Drug Issues, 40(1), 93–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Chassin L, Villalta IK. (2013). Maturing out of alcohol involvement: Transitions in latent drinking statuses from late adolescence to adulthood. Developmental Psychopathology, 25(4 Pt 1), 1137–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Vivolo-Kantor A. (2019). A latent class analysis of drug and substance use patterns among patients treated in emergency departments for suspected drug overdose. Addictive Behaviors, 101, 106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack C SZ, Westerich D. (2018). Managing missing data in patient registries: Addendum to registries for evaluating patient outcomes: A user’s guide. 3rd. Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- McBride O, Adamson G, Cheng HG, Slade T. (2014). Changes in drinking patterns in the first years after onset: A latent transition analysis of National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) data. Psychology of Addictive Behaviors, 28(3), 696–709. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. (1992). The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9(3), 199–213. [DOI] [PubMed] [Google Scholar]
- McNeil R, Small W, Wood E, Kerr T. (2014). Hospitals as a “risk environment”: An ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Social Science & Medicine, 105, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudumbai SC, Lewis ET, Oliva EM, Chung PD, Harris B, Trafton J, et al. (2019). Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Medicine, 20(5), 1020–31. [DOI] [PubMed] [Google Scholar]
- National Drug Threat Assessment [press release]. (2018). [Google Scholar]
- Nylund KLAT, & Muthen BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo Simulation Study. Structural Equation Modeling: A Multidisciplinary Journal, 14(4), 535–569. [Google Scholar]
- Priest KC, McCarty D. (2019). Role of the hospital in the 21st century opioid overdose epidemic: The Addiction Medicine Consult Service. Journal of Addiction Medicine, 13(2), 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo JH, Wang C, Swearer SM, Hull M, Shi D. (2018). Longitudinal model building using latent transition analysis: An example using school bullying data. Frontiers in Psychology, 9, 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y, Lee JK, Lu Y, Hecht ML. (2016). Exploring parental influence on the progression of alcohol use in Mexican-heritage youth: A latent transition analysis. Prevention Science, 17(2), 188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt A, Freyer-Adam J, Meyer C, John U, Baumann S. (2018). Short-term stability of different drinking patterns over the course of four weeks among adults. A latent transition analysis. Drug & Alcohol Dependence, 191, 181–186. [DOI] [PubMed] [Google Scholar]
- Strickland JC, Havens JR, Stoops WW. (2019). A nationally representative analysis of “twin epidemics”: Rising rates of methamphetamine use among persons who use opioids. Drug & Alcohol Dependence, 204, 107592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisminetzky M, Bray BC, Miozzo R, Aupont O, McLaughlin TJ. (2011). Identifying symptom profiles of depression and anxiety in patients with an acute coronary syndrome using latent class and latent transition analysis. International Journal of Psychiatry in Medicine, 42(2), 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczyk S, Pedersen A, Hanewinkel R, Isensee B, Morgenstern M. (2016). Polysubstance use patterns and trajectories in vocational students--A latent transition analysis. Addictive Behaviors, 58, 136–41. [DOI] [PubMed] [Google Scholar]
- Valente JY, Cogo-Moreira H, Swardfager W, Sanchez ZM. (2018) A latent transition analysis of a cluster randomized controlled trial for drug use prevention. Journal of Consulting and Clinical Psycology, 86(8), 657–65. [DOI] [PubMed] [Google Scholar]
- Valmaggia LR, Stahl D, Yung AR, Nelson B, Fusar-Poli P, McGorry PD, et al. (2013). Negative psychotic symptoms and impaired role functioning predict transition outcomes in the at-risk mental state: A latent class cluster analysis study. Psychological Medicine, 43(11): 2311–2325. [DOI] [PubMed] [Google Scholar]
- Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. (2017). Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. Journal of General Internal Medicine, 32(8), 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaklien D. (1999). A critique of the Bayesian information criterion for model selection. Sociological Methods & Research, 27(3), 359–397. [Google Scholar]
- Wilkinson AL, El-Hayek C, Fairley CK, Roth N, Tee BK, McBryde E, et al. (2017). Measuring transitions in sexual risk among men who have sex with men: The novel use of latent class and latent transition analysis in HIV sentinel surveillance. American Journal of Epidemiology, 185(8), 627–35. [DOI] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith Ht, Davis NL. (2020). Drug and opioid-involved overdose deaths - United States, 2017–2018. Morbidity and Mortal Weekly Report, 69 (11), 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman TNA, Admon LK, Jennings L, Shippee ND, Richardson CR, & Bart G. (2018). Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open, 1(6), e183758. [DOI] [PMC free article] [PubMed] [Google Scholar]