Abstract
Amphetamine is a potent psychostimulant also used to treat attention deficit/hyperactivity disorder and narcolepsy. In vivo and in vitro data have demonstrated that amphetamine increases the amount of extra synaptic dopamine by both inhibiting reuptake and promoting efflux of dopamine through the dopamine transporter. Previous studies showed that chronic use of amphetamine causes tolerance to the drug. Thus, since the molecular mechanisms underlying tolerance to amphetamine are still unknown, an animal model to identify the neurochemical mechanisms associated with drug tolerance, is greatly needed. Here we took advantage of a unique behavior caused by amphetamine in C. elegans to investigate whether this simple, but powerful genetic model, develops tolerance following repeated exposure to amphetamine. We found that at least three treatments with 0.5 mM amphetamine were necessary to see a reduction in the amphetamine induced behavior and, thus, to promote tolerance. Moreover, we found that after intervals of 60/90 minutes between treatments, animals were more likely to exhibit tolerance than animals that underwent 10-minute intervals between treatments. Taken together, our results show that C. elegans is a suitable system to study tolerance to drugs of abuse such as amphetamines.
Keywords: amphetamine, tolerance, C. elegans
Introduction
Psychostimulants, such as amphetamines and cocaine as well as all addictive drugs, significantly increase the amount of dopamine released by the dopaminergic neurons in the brain’s reward centers [Di Chiara and Imperato, 1988]. Several groups have shown that hyperactivity of the dopaminergic neurons, with consequent increase in dopamine levels, represents the first step that ultimately generates addiction [Volkow et al., 1999; Sulzer, 2011]. Specifically, psychostimulants directly bind to and alter the function of dopaminergic proteins such as the dopamine transporter [Mortensen and Amara, 2003; Zhu and Reith, 2008] and the vesicular monoamine transporter (VMAT) [Partilla et al., 2006; Freyberg et al., 2016]. In fact, in mice lacking expression of the dopamine transporter or expressing a cocaine-insensitive dopamine transporter, cocaine had reduced or lost completely its reinforcing effects, respectively [Thomsen et al., 2009a; Thomsen et al., 2009b].
Before and while drug addiction occurs, people develop tolerance to the drug. This means that the effects generated by these drugs are diminished after repeated use and, for this reason, higher doses of drug are needed to achieve the initial effects [Ambre et al., 1988; Calipari et al., 2014]. This process can rapidly escalate to drug overdose. Though few studies have investigated the neurobiological mechanisms of tolerance in relation to the dopaminergic response to psychostimulants [Barnett et al., 1987; Mateo et al., 2005], some studies reported that while mice with intermittent access to cocaine exhibited sensitization of the dopaminergic response, i.e. increased release and uptake of dopamine [Calipari et al., 2013], continuous access to cocaine for six hours generated tolerance as measured by a decrease in cocaine-induced locomotion [Ferris et al., 2013]. However, whether dopamine is involved in the mechanisms that ultimately generate drug tolerance has not been proved yet. One limiting factor has been the absence of a drug-induced phenotype that is exclusively mediated by dopamine. While murine models offer several advantages in studying the effects generated by psychostimulants [Müller, 2018; Golden et al., 2019], behaviors in mammals are often the result of the action of multiple proteins. For this reason, it is not an easy task to dissect a drug-induced behavior into its molecular components in mammals. On the other hand, invertebrate models, because of their higher degree of simplicity, have been instrumental in studying the cause-effect relation between genes/proteins and behaviors [Søvik and Barron, 2013; Engleman et al., 2016; van Staaden and Huber, 2019]. For example, the nematode Caenorhabditis elegans (C. elegans) is a broadly used model for genetic dissection of animal behaviors. With only 302 neurons, C. elegans is capable to perform a variety of behaviors including motor and learning behaviors [Hobert, 2003; Ardiel and Rankin, 2010]. Thus, we reasoned that C. elegans might help us identify the genes/proteins involved in the mechanisms that ultimately generate tolerance to amphetamine. The use of C. elegans is also justified by the fact that most of the proteins linked to the dopaminergic neurons, which are the main targets of psychostimulants, are highly conserved in mammals and C. elegans [Lai et al., 2000]. For example, proteins involved in the synthesis, storage, release, and reuptake of dopamine, as well as the D1- and D2-like dopamine receptors, share high degree of structural and functional similarities between C. elegans and humans [Chase and Koelle, 2007]. Moreover, we and other groups have shown that the C. elegans dopamine transporter (DAT-1), the vesicular monoamine transporter (CAT-1) and the D2-like dopamine receptor (DOP-3) exhibit pharmacological profiles that are similar to those observed in mammals [Nass et al., 2002; Carvelli et al., 2004; McDonald et al., 2007; Carvelli et al., 2008; Carvelli et al., 2010; Refai and Blakely, 2019] . More specifically, we demonstrated that, as in mammals, DAT-1 inhibitors inhibit dopamine uptake [Carvelli et al., 2004], and amphetamine induces dopamine efflux through DAT-1 in cultures of dopaminergic neurons isolated from C. elegans embryos [Safratowich et al., 2014; Hossain et al., 2014]. Importantly for this study, we have previously showed that C. elegans exhibits a unique behavior when treated with amphetamine. While C. elegans swims vigorously in water, in the presence of amphetamine they gradually stop moving and sink to the bottom of the well. We named this behavior Swimming Induced Paralysis or SWIP. By using both genetic and pharmacological approaches, we demonstrated that amphetamine-induced SWIP is caused by an overload of dopamine in the synapses which, in turn, overstimulates the D2 post-synaptically located receptors DOP-3 causing reversable paralysis in the worms [Carvelli et al., 2010]. Indeed, when the media is replaced with amphetamine-free solution, animals repristinate their ability to swim. The excess of extracellular dopamine can be achieved by treating wild type animals with dopamine releasers such as amphetamine [Carvelli et al., 2010] or by blocking the activity of the dopamine transporter either pharmacologically [Carvelli et al., 2008] or genetically [McDonald et al., 2007; Carvelli et al., 2010]. In both cases, we observe SWIP. Importantly, genetic ablation of tyrosine hydroxylase, the key enzyme for dopamine synthesis, prevents SWIP induced by lack of function of DAT-1 [McDonald et al., 2007] confirming, therefore, dopamine as the main culprit of the SWIP behavior.
Since the data mentioned above have already demonstrated that amphetamine causes a consistent and quantifiable behavior (SWIP) in C. elegans, and that SWIP is mainly mediated by overflow of extracellular dopamine [McDonald et al., 2007; Carvelli et al., 2010], we investigated whether C. elegans develops tolerance to amphetamine by using SWIP as a behavioral readout. By repeatedly exposing C. elegans to amphetamine and testing them for SWIP, we can not only see if the behavioral response to amphetamine changes over time, but also correlate such changes to the dopaminergic activity since SWIP is predominantly caused by overstimulation of the DOP-3 receptors through the endogenously released dopamine.
Materials and Methods
C. elegans husbandry and age synchronization
Both wild type (N2) and dat-1 knockout animals (dat-1(ok157)) were obtained from the Caenorhabditis Genetic Center (University of Minnesota) and grown at 20°C in agar plates seeded with NA22 bacteria. Animals underwent age synchronization to ensure that all animals tested were at the same age [Kudumala et al., 2019]. Briefly, gravid adult animals were collected from plates using autoclaved Milli-Q water, transferred into a 15-mL conical tube, and pelleted by centrifugation at 1200 rpm for 3 minutes. The supernatant was removed using a sterile tip connected to vacuum aspirator and Milli-Q water was added to the tube to resuspend the pellet. This step was repeated at least two times or until the supernatant was clear from bacteria. In order to release the embryos, worms were lysed with a mixture of 1.0 mL of 3% NaOCl, 0.25 mL 10M NaOH and 3.75 mL water for 5-10 minutes or until 70% animals were lysed. The tube was filled with egg buffer (in mM: 118 NaCl, 48 KCl, 2 CaCl-2H2O, 2 MgCl2-6H2O, 25 HEPES, pH 7.3 and 335-346 mOsml/L) and centrifuged at 1200 rpm for 3 minutes to form a pellet. Then, the supernatant was removed from the tube and the pellet was resuspended in egg buffer. This step was repeated three times to wash off any residual bleach. The pellet was then suspended in 30% sucrose solution (5 mL of water and 5 mL of 60% sucrose), and the tube was centrifuged at 1200 rpm for 6 minutes to separate the embryos from the debris in the sucrose gradient. Embryos were then collected at the solution meniscus using a sterile glass pipette and transferred into a 15-mL conical tube. The tube was filled to 10 mL with autoclaved water and centrifuged at 1200 rpm for 3 minutes. This step was repeated for a total of three times to remove sucrose from the samples. After the last centrifugation, the supernatant was removed and M9 buffer (for 1 liter of buffer: in g, 3 KH2PO4, 6 NaHPO4, 5 NaCl and 1 mL of 1 M MgSO4) was added to the tube, which was then centrifuged, and once again filled with M9 solution to 10 mL. The tube containing the embryos suspended in the M9 solution was kept for 14 hours at room temperature on a shaker. During this time, all embryos will mature into the first larval stage and will not grow beyond this stage because the M9 media does not contain food. This guarantees age synchronization of all animals. Larvae were washed with water, placed on agar plates containing food (NA22 bacteria) and kept for about 36 hours in a 20°C incubator or until the animals had reached the late-L4 larval stage.
Behavioral Assay (SWIP)
Swimming Induced Paralysis (SWIP) was performed as previously published [Kudumala et al., 2019]. Briefly, under a dissecting microscope, 8 to 12 late-L4 larvae were transferred with an eyelash pick from the plate to a glass spot plate well containing 40 μL of 0.5 mM amphetamine dissolved in 200 mM sucrose solution. Sucrose solution was used instead of milli-Q H2O to guarantee constant osmolarity (200 mOsmol/L). Animals were left in the amphetamine solution for 10 minutes and, each minute, the number of paralyzed animals was recorded to quantify the percent of animals exhibiting SWIP. After 10 minutes, animals were transferred into a well containing 40 μL of water for about 1 minute. Then, animals were transferred to a NA22-bacteria containing plate and allowed to recover for 30 minutes. Animals were transferred from the recovery plate into another glass well containing fresh amphetamine solution and were tested for 10 minutes again for SWIP, washed off in water, and allowed to recover for 60 minutes on the agar plate. This procedure was done one more time, but a 90-minute recovery time was allowed before the animals were tested again for amphetamine-induced SWIP. Parallel experiments were performed using 200 mM sucrose solution alone (control group). To prevent contamination between the amphetamine- and control-treated groups, we thoroughly washed the eyelash pick in ethanol and water while we transferred the animals from and to the wells.
Rigor and statistical analysis
Each SWIP assay was performed using 8-12 animals per group. For the experiments shown in Figure 1, a total of 35 trials were performed on 15 different days over a period of 5 weeks and a total of 263 or 115 animals were repeatedly exposed to amphetamine or control solutions, respectively.
Figure 1.
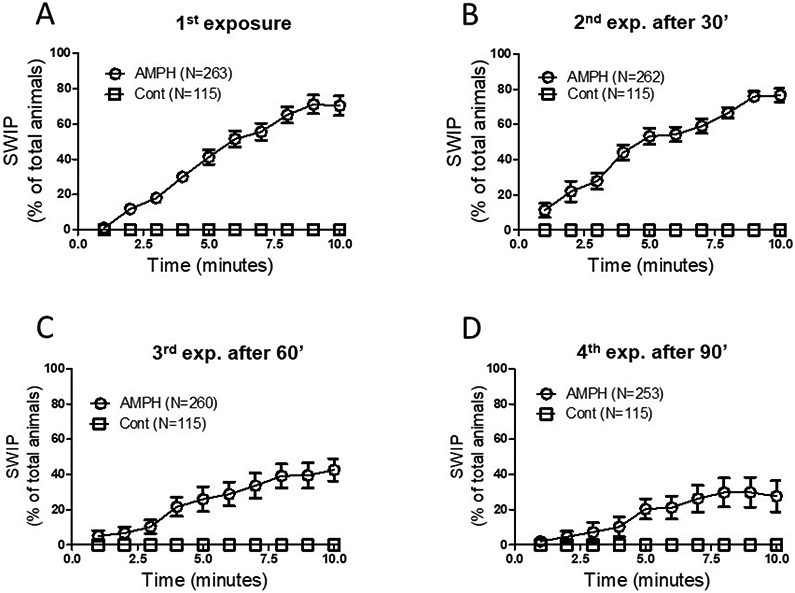
Amphetamine-induced behaviors decrease after multiple exposures to the drug. Animals were immerged in a solution containing control (open squares) or control and 0.5 mM amphetamine (AMPH, open circles) for 10 minutes. The percentage of animals exhibiting swimming induced paralysis (SWIP) are reported on the Y axis. While no change in the percentage of animals exhibiting SWIP was observed between the first and second exposures to AMPH (A-B), a strong reduction was observed during the third (p < 0.001) and fourth (p < 0.001) exposures (C-D) with respect to the first exposure (2-way ANOVA test). N represents the number of animals tested per each group.
Data shown in Figure 2A includes a total of 614 animals (246 treated three times with control and one time with amphetamine, 253 treated four times with amphetamine, 115 treated with control four times). Animals were tested in 22 separated trials in 11 days over a 5-month period.
Figure 2.
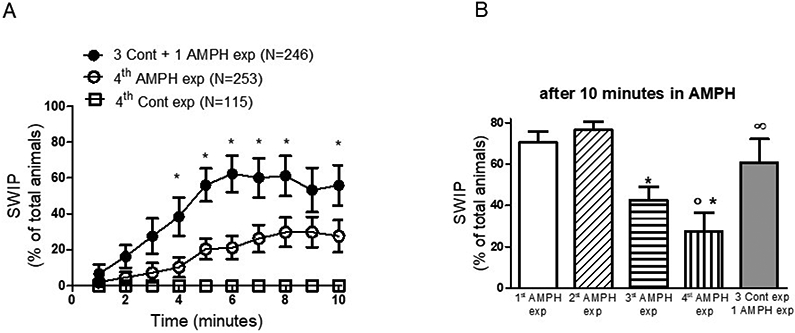
Aging does not account for the decreased behavioral effects seen after repeated exposures to amphetamine. A, the percentage of animals exhibiting SWIP over a 10-minute treatment with control (open squares) or AMPH (open circles) is reported during the fourth exposure. Animals that received three consecutive exposures in control solution followed by one AMPH exposure (filled circles) exhibit higher levels of SWIP with respect to animals that were exposed four times to AMPH (*p < 0.001, 2-way ANOVA test). N represent the number of animals tested per group. B, the percentage of animals exhibiting SWIP after 10-minute treatment with AMPH during the 1st, 2nd, 3rd and 4th exposures are presented as bar graph. Values collected after the third and fourth exposure with AMPH were statistically different than values collected during the first and second exposure. Animals that were treated three times in control solution and one time with AMPH (gray bar) exhibited statistically higher levels of SWIP then animals treated four times with AMPH (∞p < 0.05, 1-way ANOVA test). But no difference was measured between animals treated one time with AMPH and those treated three times with control and one-time AMPH (compare white and gray bars). The number of animals tested per each group are as in B.
Experiments shown in Figure 2B reflect the same numbers of animals presented in Figure 1 and 2A
Experiments shown in Figure 3A include the 22 trials shown in Figure 2A plus 6 extra trials for a total of 28 trials and 129 animals treated four times with control solution, 299 animals treated four times with amphetamine and 300 animals treated three times with control and one time with amphetamine solution.
Figure 3.
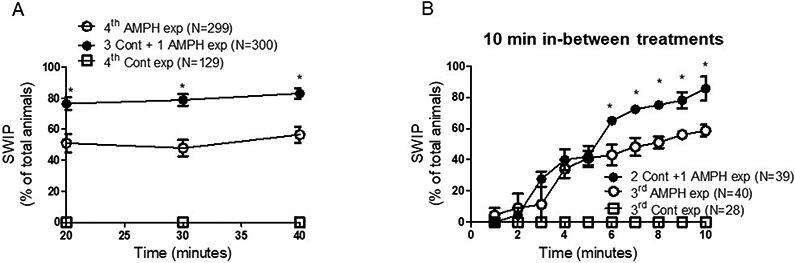
Tolerance to amphetamine is maintained over 40-minute treatments and with reduced intervals between exposures. A, animals were exposed to control (open squares), AMPH (open circles) or control followed by AMPH (filled circles) as described in Figure 2A. But, during the last exposure, the percentage of animals exhibiting SWIP was measured up to 40 minutes. The percentage of animals exhibiting SWIP in the group that received four AMPH exposures was statistically lower than the animals that received three control and one AMPH exposure throughout the 40-minute observation (*p < 0.001, 2-way ANOVA test). B, animals were exposed three times to control- (open squares) or AMPH-solution (open circles) and two times to control followed by one time to AMPH (filled circles). Between each exposure, animals were allowed to recover and feed in drug-free agar plates for 10 minutes. Statistical difference between animals that received three AMPH exposures and animals that received two control- followed by one AMPH-exposure was achieved after six minutes of the last exposure (*p < 0.05, 2-way ANOVA test). N represents the number of animals tested per each group.
Experiments shown in Figure 3B were performed in 4 trials and include 39 animals which received two control- and one amphetamine-exposure, 40 animals exposed to amphetamine three times and 28 animals exposed to control solution three times.
Statistical analysis was performed with GraphPad Prism software using 1-Way ANOVA (Figure 2B) or 2-Way ANOVA (Figure 1A-C, 1A-D, 2A, 3A, 3B) with Bonferroni’s Multiple Comparison post-test. The error bars in all figures represent ± SE.
Results
Repeated exposures to amphetamine reduce amphetamine-induced behaviors
To investigate whether C. elegans develops tolerance to amphetamine, we measured amphetamine induced SWIP in wild type animals during and after repeated exposures to the drug (Figure 1). Animals had four consecutive 10-minute exposures to amphetamine with in-between recoveries of 30, 60, and 90 minutes on agar plates seeded with food. Longer intervals between treatments were used to allow animals to feed and recover from swimming while undergoing treatments. During the first exposure, animals immerged in a solution containing 0.5 mM amphetamine exhibited a gradual increase in the number of paralyzed animals (SWIP) and reached a plateau at 10 minutes (Figure 1A, open circles). After 10 minutes, 70 ± 5% of animals exhibited SWIP (Figure 2B, white bar). Parallel experiments showed that animals exposed to control solution did not exhibit SWIP over the 10-minute timeframe (Figure 1A, open squares). These results confirm our previously published data showing that amphetamine causes SWIP in C. elegans [Carvelli et al., 2010; Safratowich et al., 2013; Lanzo et al., 2018]. Following the first exposure to amphetamine or control solutions, animals were reallocated in food-seeded plates after being briefly immerged (~1 minute) in wells containing water to ensure that no residue of amphetamine was left on the animals’ bodies. After 30 minutes on the plate, animals were subjected to a second treatment with amphetamine and, after 10-minute exposure, 76 ± 4% animals exhibited SWIP (Figure 1B, open circles and 2B, diagonal striped bar). Again, animals were briefly washed in water and transferred to the plate where they recovered and fed for 60 minutes before being exposed to amphetamine for the third time. During the third exposure, we measured a significant reduction in the number of animals exhibiting SWIP over the 10-minute exposure (Figure 1C, open circles). Specifically, after 10 minutes, 42 ± 6% animals exhibited SWIP (Figure 2A, horizontal striped bar). *p < 0.05 for 3rd AMPH exp vs 2nd AMPH exp). Finally, animals were exposed for the fourth time to amphetamine after a recovery period of 90 minutes in the plate (Figure 1D, open circles). We observed a statistically significant reduction in SWIP with respect to the first and second exposure (compare Figure 1D, 1A and 2B), with only 27 ± 9% animals exhibiting SWIP after 10 minutes (Figure 2B, vertical striped bar; °p< 0.005 and *p < 0.0005 for 4th AMPH exp vs 1st AMPH exp and vs 2nd AMPH, respectively). Similar to the first exposure, no animal exhibited SWIP during the second, third and fourth exposure to control solution (Figure 1B-D open squares). These results show that, while two amphetamine exposures cause no change in how the animals respond to the drug, three or more exposures cause a significant reduction in the behavioral outcomes induced by amphetamine.
Repeated treatments with amphetamine caused a reduction in the percentage of animals exhibiting SWIP (Figure 1C-D open circles), but no change in SWIP was observed in animals that were repeatedly treated with control solution (Figure 1C-D open squares). Thus, we could conclude that the recurring exposure to amphetamine is the sine-qua-non cause for the reduced SWIP observed in Figure 1C-D. We cannot exclude, however, that a reduced behavioral response to amphetamine may occur in C. elegans because of an additive effect of aging, i.e. animals could respond differently to amphetamine as they age. As a matter of fact, between the first and last exposure to amphetamine, there was an interval of four hours which, for an animal like C. elegans with a lifespan of 2-3 weeks, could represent a significant aging step. Moreover, repeated swimming assays could per se alter the response to amphetamine. To exclude these possibilities, we designed another set of experiments done in parallel to the experiments described above, where a group of animals, after been exposed three times to control solution, was treated with amphetamine for the first time (Figure 2A, filled circles). In this group a larger number of animals exhibited SWIP with respect to the group that was exposed to amphetamine four consecutive times (Figure 2A, open circles; *p < 0.0001; two-Way ANOVA). Importantly, after 10 minutes, the percentage of SWIP in the animals that received three control exposures followed by one amphetamine exposure was not statistically different than those exposed to amphetamine for the first time, 56 ± 11% and 70 ± 5%, respectively (Figure 2B, compare gray and white bars). But a significant difference was measured between this group and animals which received four amphetamine treatments (compare gray and vertical stripes bars in Figure 2B, ∞p < 0.05). These data demonstrate that aging or repeated swimming assays do not account for the reduced response to amphetamine observed after repeated treatments.
Next, we tested whether the reduced behavioral response between first and third treatment was maintained if the last amphetamine treatment was prolonged up to 40 minutes. Animals were treated with 0.5 mM amphetamine or control solution for 10 minutes during the first, second and third exposure. During the last exposure, animals underwent the fourth treatment with amphetamine or control solution for 40 minutes (Figure 3A, open circles and open squares, respectively). Likewise, the subgroup of animals which received three 10-minute treatments with control solution were then treated with amphetamine for 40 minutes (Figure 3A, filled circles). As shown in Figure 3A, the significant difference between the percentage of animals treated four times with amphetamine (open circles) and those treated three times with control followed by one amphetamine treatment (filled circles) was maintained up to 40 minutes (*p < 0.001, two-way ANOVA).
The data shown above were collected during treatments with amphetamine or control solutions at intervals spanning from 30 to 90 minutes. Since no difference was observed after the first interval of 30 minutes between the first and second exposure (Figures 1A-B), we tested whether shorter interval between treatments would change the outcomes seen in Figure 1. Thus, animals were treated with amphetamine three times with in-between intervals of 10 minutes (Figure 3B). Again, we measured a statistically significant reduction of SWIP in animals that received three amphetamine treatments (Figure 3B, open circles) with respect to the group that received two control- and one amphetamine treatment (Figure 3B, filled circles; *p < 0.05; 2-way ANOVA). However, while during longer periods of intervals (30-90 minutes) between treatments (Figure 2A), the statistical significance between the two groups was achieved after 3 minutes, using 10 minutes in-between treatments, statistical significance was achieved after 6 minutes. Moreover, the power of the analysis was stronger in trials with longer in-between intervals (60-90 minutes) than those with 10-minute intervals, p < 0.001 and p < 0.05, respectively. Taken together these results show that three or more amphetamine exposures reduce the ability of C. elegans to exhibit SWIP. Thus, they suggest that C. elegans develops tolerance to amphetamine. Moreover, they suggest that 60/90-minute intervals between the second/third and third/fourth treatments are more likely to generate tolerance to amphetamine than 10-minute intervals.
Discussion
Living organisms have developed self-regulating processes which are used to keep their biological functions to a set point with a relatively narrow range of operation. This self-regulating process is known as homeostasis. When external/internal perturbations occur, animals respond to the change by activating different mechanisms aimed to repristinate the original homeostatic equilibrium. Based on this concept, tolerance to a drug might be proposed as a process of adaptation to the perturbating effect of the drug [Pepper et al., 1987; Pepper et al., 1988]. As drug tolerance is revealed through the slow decline in the effect of the drug after repeated administrations, this might suggest that the body slowly learns how to counteract the effects produced by the drug such that the overall system can run within its operation set point.
Although tolerance is a phenomenon that can occur with any type of drug, it is particularly important to understand the mechanisms that generate tolerance to drugs of abuse because, due to their addictive nature, these drugs are remarkably dangerous. In fact, while the “well-being” effects (euphoria, relaxation, and arousal) dissipate as tolerance takes place, the toxic effects caused by addictive drugs after prolonged use at high concentrations build up alongside. Moreover, because drugs of abuse affect and alter the reward system, when tolerance to drugs of abuse occurs, people feel the urge for more drug. Escalation of drug use can ultimately lead to death.
Different types of addictive drugs exist. But one common feature among all is their ability to activate, either directly or indirectly, the dopaminergic system. Indeed, previous data suggest that overflow of extracellular dopamine is the first step leading to addiction [Sulzer, 2011]. However, it is unknown whether dopamine plays a role in the process that generates drug tolerance. Here we have investigated tolerance to amphetamine because this drug directly interacts with specific proteins of the dopaminergic system and because amphetamine is broadly used and abused as a psychostimulant. To be able to investigate the molecular mechanisms underlying the development of tolerance to amphetamine, it is necessary to use a model organism that can develop tolerance to this drug and, at the same time, allows straightforward assays at the molecular level. We decided to consider C. elegans as a model to study the development of tolerance to amphetamine mainly because I) the simplicity of the nervous system and the genetic tools this nematode offers allow us to directly correlate specific behaviors to genes/proteins. This same approach would be very tedious, if not impossible, in mammalian models. II) the dopaminergic synapsis is highly conserved between C. elegans and mammals. III) since C. elegans exhibits the amphetamine-induced behavior SWIP, we can use SWIP to test whether repeated exposures to amphetamine would diminish the behavioral response and, thus, we can test if amphetamine causes tolerance in C. elegans. IV) Finally, because SWIP requires mainly dopaminergic proteins, we can test whether dopamine plays a role in amphetamine tolerance.
Our results indicate that, when C. elegans is repetitively exposed to amphetamine, a reduction in amphetamine-induced behavioral response (SWIP) occurs. This suggests that C. elegans develops tolerance to amphetamine. The animals that were repeatedly exposed to control solution did not show SWIP, excluding therefore the possibility that worms paralyzed due to fatigue for being forced to swim. At least three exposures to the drugs were necessary to see tolerance to amphetamine (Figure 1) and the inclusion of age-matched control groups allowed us to rule out the possibility that the decreased amphetamine-induced SWIP was caused by aging (Figure 2). As a matter of fact, the animals exposed four times to control solution did not show SWIP whereas, animals exposed three times to control and the fourth time to amphetamine showed higher levels of SWIP than the group exposed to amphetamine four times.
Someone could argue that the reduction of a dopamine-dependent behavior (SWIP) after repeated amphetamine treatments is the result of dopamine depletion from vesicles. If this was true, then longer time intervals between treatments allow more time to replenish dopamine stores via tyrosine hydroxylase activity. Consequently, we should see a stronger reduction of SWIP during short intervals between amphetamine treatments but reduced or no effect during longer recover intervals. However, this scenario is not supported by our data. In fact, animals were more likely to develop tolerance when 60- or 90-minute intervals (~140 or 240 minutes total from the first exposure) were allowed between treatments with respect to 10-minute intervals (~100 minutes total from the first exposure). In addition, our experiments show that the behavioral effects caused by repeated exposure to amphetamine were maintained up to 40 minutes during the fourth exposure (280 minutes total from the first exposure), suggesting therefore that it is unlikely that diminished dopamine store levels are responsible for the observed tolerance to amphetamine. However, because no data are currently available about the turnover of C. elegans dopamine-vesicle, more experiments need to be done to establish the role of dopamine depletion in the amphetamine-induced tolerance seen in our behavior experiments. Notwithstanding, our results demonstrate that repeated exposures to amphetamine reduce an amphetamine induced behavior and suggest that C. elegans develops tolerance to amphetamine. Accordingly, C. elegans may be used as a model organism to study the underlying molecular mechanisms of tolerance caused by repeated exposure to psychostimulants.
Two main types of tolerance to drugs exist: acute and chronic tolerance. The acute or short-term tolerance occurs within one session of consecutive drug administrations (binges) during which the effectiveness of the drug decreases after the first dose. This is a transient effect that does not carry over other sessions performed in different days [Hatsukami et al., 1994]. For example, in humans the increased heart rate, elevated blood pressure and euphoric effects caused by the first dose of cocaine cannot be attained 40 minutes later, nor even after nearly doubling the concentration of cocaine in the blood [Ambre et al., 1988; Foltin and Haney, 2004]. In the chronic or long-term tolerance, instead, the decreased effectiveness of a drug persists over time, during different sessions performed in different days, and it is likely a form of neuronal adaptation to constant exposure to a drug for months or years [Kalant et al., 1971].
The acute tolerance caused by psychostimulants in pseudo drug-naïve subjects resolves by itself within a relative short period. Thus, we could assume that psychostimulants temporarily and reversibly alter the activity of specific protein targets and, if these proteins are responsible for the acute physiological/behavioral effects caused by psychostimulants, then consecutive exposures to the drugs would result in reduced physiological/behavioral outcomes. Alternatively, the proteins activated by psychostimulants and responsible for the acute effects induced by these drugs could be a separate set of proteins/mechanism than those generating acute tolerance. The two processes, however, must be somehow connected since reduced effectiveness of a drug (tolerance) implies dampened physiological/behavioral effects.
In the case of chronic tolerance, the reduced effectiveness of the drugs after recurrent and long-term use of psychostimulants could be due to permanent alteration of the proteins/mechanisms underlying the acute tolerance phenomena. Then again, different proteins/mechanisms, that were not affected during acute tolerance, might be recruited when chronic tolerance takes place. Regardless of whether acute and chronic tolerance share or don’t the same type of proteins/mechanisms, the first step to understand drug tolerance is identifying the proteins/mechanisms underlying this phenomenon. This is a challenging task because of the complexity of the mammalian nervous system. On the other hand, the neuronal circuits in C. elegans are small and well-defined, thus making the correlation between gene activity within single cells and behavior of a mutant phenotype a much less intimidating task than in mammals.
Here, we have shown that C. elegans after repeated treatments develop tolerance to amphetamine. As the life cycle of C. elegans is very short (3-4 days) compared to mammals and because in C. elegans, tolerance to amphetamine is maintained over time after the third and fourth exposure (Figure 3A), we speculate that the amphetamine-induced tolerance observed in our experimental paradigm is a type of chronic tolerance. In fact, as observed in humans treated with cocaine, C. elegans showed a dampened response to amphetamine only after multiple 10-minute treatments (Figure 1). As the SWIP assay has been previously characterized as a predominantly dopamine mediated phenotype [McDonald et al., 2007; Carvelli et al., 2010], we can speculate that the reduced response to amphetamine observed in our experiments involves the reduced activity of one of the proteins responsible of the SWIP phenotype, i.e. I) tyrosine hydroxylase, which is the key enzyme in the dopamine synthesis, II) the vesicular monoamine transporter (VMAT) which storages dopamine into the synaptic vesicles, III) the dopamine transporter which reuptakes dopamine into the neurons and mediates dopamine efflux, and IV) the D2 receptors. These four proteins are required by amphetamine to generate SWIP in C. elegans [Carvelli et al., 2010]. Future experiments targeting each of these proteins will identify which of these proteins is required to initiate tolerance.
In summary, data presented here demonstrate that C. elegans develop tolerance to amphetamine. Thus, C. elegans can be used as model to identify the molecular mechanisms underlying tolerance. Moreover, as genetic screens in C. elegans are well-established and commonly used to assess gene function in any biological process, the behavioral paradigm presented in this study can be used to identify the genes/proteins involved with amphetamine tolerance.
Acknowledgments
Funding Sources
This project was supported by the NIH R01 grant (DA042156) to LC
Footnotes
Statement of Ethics
This study did not include human subjects or vertebrate animals.
Conflict of Interest Statement
The authors have no conflict of interest to declare
Reference
- Ambre J, Belknap SM, Nelson J, Ruo TI, Shin SG, Atkinson AJ Jr (1988): Acute Tolerance to Cocaine in Humans. Clin Pharmacol Ther 44(1):1–8. [DOI] [PubMed] [Google Scholar]
- Ardiel E, Rankin CH (2010): An elegant mind: learning and memory in Caenorhabditis elegans. Learn Mem. 17(4):191–201. [DOI] [PubMed] [Google Scholar]
- Barnett J, Segal DS, Kuczenski R (1987): Repeated Amphetamine Pretreatment Alters the Responsiveness of Striatal Dopamine-Stimulated Adenylate Cyclase to Amphetamine-Induced Desensitization. J Pharmacol Exp Ther 242(1):40–7. [PubMed] [Google Scholar]
- Calipari E, Ferris MJ, Zimmer BA, Roberts DC, Jones SR (2013) : Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38(12):2385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari E, Ferris MJ, Jones SR (2014): Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128(2):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD, DeFelice LJ (2008): Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci USA 105(37):14192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Matthies DS, Galli A (2010): Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol Pharmacol. 78(1):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, McDonald PW, Blakely RD, DeFelice LJ (2004): Dopamine transporters depolarize neurons by a channel mechanism. Proc Natl Acad Sci USA 101(45):16046–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D, Koelle MR (2007): Biogenic amine neurotransmitters in C. elegans. WormBook 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988): Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad USA 85(14):5274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E, Katner SN, Neal-Beliveau BS (2016): Caenorhabditis Elegans as a Model to Study the Molecular and Genetic Mechanisms of Drug Addiction. Prog Mol Biol Transl Sci 137(229–52). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris M, Calipari ES, Melchior JR, Roberts DC, España RA, Jones SR (2013): Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci 38(4):2628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin R, Haney M (2004): Intranasal cocaine in humans: acute tolerance, cardiovascular and subjective effects. Pharmacol Biochem Behav 78(1):93–101. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, Pizzo AB, Zhang Y, Farino ZJ, Chen A, Martin CA, Kopajtic TA, Fei H, Hu G, Lin YY, Mosharov EV, McCabe BD, Freyberg R, Wimalasena K, Hsin LY, Sames D, Krantz DE, Katz JL, Sulzer D, Javitch JA (2016): Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nature Communications 7(10652). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S, Jin M, Shaham Y (2019): Animal Models of (or for) Aggression Reward, Addiction, and Relapse: Behavior and Circuits. J Neurosci 39(21):3996–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Pentel PR, Glass J, Nelson R, Brauer LH., Crosby R, Hanson K (1994): Methodological issues in the administration of multiple doses of smoked cocaine-base in humans. Pharmacol Biochem Behav 47(3):531–40. [DOI] [PubMed] [Google Scholar]
- Hobert O (2003): Behavioral plasticity in C. elegans: paradigms, circuits, genes. J Neurobiol 54(1):203–23. [DOI] [PubMed] [Google Scholar]
- Hossain M, Wickramasekara RN, Carvelli L (2014): β-Phenylethylamine requires the dopamine transporter to increase extracellular dopamine in Caenorhabditis elegans dopaminergic neurons. Neurochem Int 73:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ (1971): Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev 23:136–91. [PubMed] [Google Scholar]
- Kudumala S, Sossi S, Carvelli L (2019): Swimming Induced Paralysis to Assess Dopamine Signaling in Caenorhabditis Elegans. J Vis Exp 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Chou CY, Ch'ang LY, Liu CS, Lin W (2000): Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res 10(5):703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzo A, Safratowich BD, Kudumala SR, Gallotta I, Zampi G, Di Schiavi E, Carvelli L (2018): Silencing of Syntaxin 1A in the Dopaminergic Neurons Decreases the Activity of the Dopamine Transporter and Prevents Amphetamine-Induced Behaviors in C. elegans. Front Physiol 9:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR (2005): Reduced Dopamine Terminal Function and Insensitivity to Cocaine Following Cocaine Binge Self-Administration and Deprivation. Neuropsychopharmacology 30:455–63. [DOI] [PubMed] [Google Scholar]
- McDonald P, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD (2007): Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci 27(51):14216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG (2003): Dynamic regulation of the dopamine transporter. Eur J Pharmacol 479(1-3):159–70. [DOI] [PubMed] [Google Scholar]
- Müller C (2018): Animal Models of Psychoactive Drug Use and Addiction - Present Problems and Future Needs for Translational Approaches. Behav Brain Res 352:109–15. [DOI] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM 3rd, Blakely RD (2002): Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA 99(5):3264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partilla J, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB (2006): Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther 329(1):237–46. [DOI] [PubMed] [Google Scholar]
- Peper A, Grimbergen CA, Kraal JW, Engelbart JH (1987): An approach to the modelling of the tolerance mechanism in the drug effect. Part I: the drug effect as a disturbance of regulations. J Theor Biol 127:413–26. [DOI] [PubMed] [Google Scholar]
- Peper A, Grimbergen CA, Kraal JW, Engelbart JH (1988): An approach to the modelling of the tolerance mechanism in the drug effect. Part II: on the implications of compensatory regulations. J Theor Biol 132:29–41. [DOI] [PubMed] [Google Scholar]
- Refai O, Blakely RD (2019): Blockade and reversal of swimming-induced paralysis in C. elegans by the antipsychotic and D2-type dopamine receptor antagonist azaperone. Neurochem Int 123:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safratowich BD, Lor C, Bianchi L, Carvelli L (2013): Amphetamine activates an amine-gated chloride channel to generate behavioral effects in Caenorhabditis elegans. J Biol Chem 288:21630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safratowich B, Hossain M, Bianchi L, Carvelli L (2014): Amphetamine Potentiates the Effects of β-Phenylethylamine through Activation of an Amine-Gated Chloride Channel. J Neurosci 34(13):4686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søvik E, Barron AB (2013): Invertebrate Models in Addiction Research. Brain Behav Evol 82(3):153–65. [DOI] [PubMed] [Google Scholar]
- Sulzer D (2011): How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69:628–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB (2009): Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci 29(4):1087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB (2009): Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther 331(1):204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staaden M, Huber R (2019): Editorial: Invertebrate Models of Natural and Drug-Sensitive Reward. Front Physiol 10:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappa NR (1999): Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291(1):409–15. [PubMed] [Google Scholar]
- Zhu J, Reith ME (2008): Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets 7(5):393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]


