Abstract
Background
Fear of recurrence (FoR) is a prevalent concern among breast cancer survivors (BCS), yet few accessible interventions exist. This study evaluated a targeted eHealth intervention, “FoRtitude,” to reduce FoR using cognitive behavioral skills training and telecoaching.
Methods
BCS (N = 196) were recruited from an academic medical center and 3 National Cancer Institute Community Oncology Research Program community sites, had stage 0-III breast cancer, were 1-10 years postprimary treatment, with moderate to high FoR and familiarity with the internet. Using the Multiphase Optimization Strategy, participants were independently randomly assigned to 3 cognitive behavioral skills (relaxation, cognitive restructuring, worry practice) vs an attention control condition (health management content [HMC]) and to telecoaching (motivational interviewing) vs no telecoaching. Website content was released across 4 weeks and included didactic lessons, interactive tools, and a text-messaging feature. BCS completed the Fear of Cancer Recurrence Inventory at baseline and at 4 and 8 weeks. Fear of Cancer Recurrence Inventory scores over time were compared using mixed-effects models. All statistical tests were 2-sided.
Results
FCRI scores [SD] decreased statistically significantly from baseline to postintervention (T0 = 53.1 [17.4], T2 = 41.9 [16.2], P < .001). The magnitude of reduction in FCRI scores was comparable across cognitive behavior therapy (CBT) and attention control HMC conditions and was predicted by increased self-efficacy. Telecoaching was associated with lower attrition and greater website use (mean adherence score [SD] = 26.6 [7.2] vs 21.0 [10.5], P < .001).
Conclusions
BCS experienced statistically significant reductions in FoR postintervention, but improvements were comparable between CBT and attention controls. Telecoaching improved adherence and retention. Future research is needed on optimal integration of CBT and HMC, dose, and features of eHealth delivery that contributed to reducing FoR. In the COVID-19 era, remote delivery has become even more essential for reaching survivors struggling with FoR.
Fear of recurrence (FoR) (1) is a common, distressing experience among cancer survivors. Among breast cancer survivors (BCS), 48% reported FoR-related intrusive thoughts and 24%-56% reported moderate-severe FoR (2-7). FoR is associated with increased anxiety and lower quality of life and persists for years following treatment even among survivors at low risk (2,5,6,8-12). Help managing FoR is the most pressing unmet need among long-term survivors (13,14). FoR costs resources, with higher health-care use among BCS with elevated FoR (15,16).
The need for evidence-based, targeted FoR interventions has been widely acknowledged (1,15,17,18). FoR interventions to date report small-to-moderate effects, suggesting further intervention development is needed (19,20). Among the most promising, cognitive behavior therapy (CBT) teaches active, adaptive strategies to cope with distress (21-24). CBT has been studied in combination with mind-body interventions (25) but not with techniques to bolster adherence, such as motivational interviewing. Moreover, lack of CBT providers, cost, insurance, and logistical challenges (26) limit access. eHealth interventions overcome these obstacles (27) and have demonstrated efficacy for distress (28-30).
“FoRtitude” (31) is a targeted eHealth CBT intervention grounded in a theoretical model of FoR (32) designed to teach strategies to manage FoR. To our knowledge, this is the first CBT-based eHealth intervention specifically targeting FoR. FoRtitude was developed by tailoring evidence-based CBT strategies for anxiety to the management of FoR and adapting these strategies for eHealth delivery based on user-centered design (31).
This study applied the Multiphase Optimization Strategy (MOST) (33,34) innovative design to evaluate 3 CBT strategies (relaxation, cognitive restructuring, worry practice) and telephone-based motivational interviewing (35) to increase adherence (telecoaching) to build an optimized eHealth intervention (36). We hypothesized that each CBT strategy would be more effective than an attention control (health management content [HMC]) in reducing FoR. We also hypothesized that BCS randomly assigned to telecoaching would demonstrate greater FoRtitude site use and a greater reduction in FoR. Based on our theoretical model (32), we further reasoned that increased self-efficacy to manage breast cancer would be associated with reduced FoR.
Methods
Participants
Eligibility criteria included stage 0-III breast cancer at diagnosis, completion of primary breast cancer treatment 1-10 years before consent (current hormonal treatment allowed), disease free, 18 years and older, score 13 and over on the Fear of Cancer Recurrence Inventory (FCRI) severity subscale (validated FoR screening) (37), internet familiarity, mobile telephone with text-messaging capabilities, proficiency in English, and ability to provide informed consent.
Recruitment
From December 2014 to September 2015, BCS were recruited from the Robert H. Lurie Comprehensive Cancer Center of Northwestern University and 3 National Cancer Institute Community Oncology Research Program (NCORP) Community Sites (Aurora, Colorado Cancer Research, and Metro Minnesota NCORPs). The study was approved by the institutional review boards at participating sites. Clinic staff introduced the study to potentially eligible BCS and provided an institutional review board–approved study brochure. BCS accessed the study website to provide informed consent and demographic and medical characteristics and to complete eligibility screening . Eligible BCS received a hyperlink directing them to the baseline assessment.
Design
The FoRtitude trial (clinicaltrials.gov #NCT03384992) used the MOST framework (33,34) to individually evaluate 4 intervention components (3 CBT-based strategies vs an attention control as well as telecoaching vs no telecoaching for a total of 23*2 = 16 unique groups) using a randomized, full factorial trial (Supplementary Table 1, available online). Attention control components included HMC in the same eHealth format as CBT-based treatment components. Telecoaching included 4 weekly telephone-based motivational interviews to promote FoRtitude site use adherence delivered by 2 coaches following a manualized protocol (38,39). New FoRtitude site content was released 3 times per week to maximize site engagement. Assessments were at baseline (T0) and at 4 (T1) and 8 (T2) weeks after the first FoRtitude site log-in.
Intervention: Description of FoRtitude eHealth Site
The FoRtitude eHealth site included didactic content, interactive tools, and an interactive text messaging feature. Participants were encouraged to use the FoRtitude site several times per week for 4 weeks. The website, intervention components, attention control components, and functionality are described in the Supplementary Methods (available online). FoRtitude CBT components have been previously described (31).
Treatment Adherence
FoRtitude eHealth Website Use. Objective data on FoRtitude website use were obtained by extracting the number of logins and website pages accessed to quantify lessons completed, times tools were used, and text messages sent. Adherence from random assignment to T2 was summarized using an index-based approach. Each adherence component (number of logins, lessons read, tool use, texts sent) was assigned a score from 0 (not adherent) to 10 (full adherence, ie, ≥6 logins, ≥6 lessons completed, ≥6 tools used, ≥3 text messages sent). Cronbach’s alpha (.74) was acceptable.
Telecoaching. Participants randomly assigned to telecoaching (n = 97) received up to 4 weekly telecoaching sessions (38,39) approximately 15 minutes in duration focused on FoRtitude site use adherence using motivational interviewing.
Measures
Patient-reported outcome (PRO) measures were administered online using Assessment Center (40) at T0 (baseline, before random assignment), T1 (4 weeks after the first FoRtitude login, immediately postintervention), and T2 (8 weeks after login, 4 weeks postintervention). Participants received incentives for completing PROs, described below (clinicaltrials.gov #NCT03384992).
Primary Outcome.
FoR was measured using FCRI total score calculated using the sum of FCRI subscales Distress, Triggers, Function, Insight, and Severity (41). We determined that a priori FCRI total score would not include the FCRI Coping subscale because coping was identified as an intervention target. The Reassurance subscale was not included in the FCRI total score because of low internal consistency, similar to previous reports (42). The FCRI total score ranged from 0 to 120, with a higher score reflecting higher FoR.
Secondary Outcomes.
The Concerns about Recurrence Scale 4-item subscale (3) was administered as a secondary measure of FoR. Secondary outcomes included cancer-specific distress (Impact of Events Scale–Revised) (43,44), anxiety, depression, fatigue, sleep disturbances, and cognitive problems (PROMIS computer adpative tests), health-related quality of life (PROMIS Global Health-10 item), and self-efficacy to manage breast cancer (Breast Cancer Self-Efficacy Scale [BCSE]) (45), which was defined a priori as an intermediary intervention target.
Sample Size
Based on previous research (47) and our pilot work (46), the sample size was chosen to detect a medium effect size (Cohen d = 0.50) (46,47) comparing each of the 3 CBT components with the attention control components regarding FoR scores 4 weeks postintervention (T2; week 8), with a 2-sided type I error probability of 0.10 and 80% power (36), requiring 112 patients total (56 per treatment vs control arm) to test main effects. With 20% anticipated attrition (29), we planned to accrue 144 patients. After enrolling 25% of participants, we observed slightly higher than anticipated attrition at 8 weeks and increased accrual to 196 BCS.
Random Assignment and Blinding
On baseline assessment completion, the study coordinator randomly assigned BCS to an experimental group (of 16) using a pregenerated random assignment table provided by the statistician using blocked random assignment to ensure balanced sample sizes in each condition. BCS were blinded to random assignment, with the exception of telecoaching, which required disclosure to coordinate telecoaching sessions. BCS in all 16 experimental groups received access to the FoRtitude site, and site content was tailored to experimental group. BCS received CBT or attention control content (HMC) each week. With 196 enrolled, we expected n = 16 BCS to be randomly assigned to each of the 16 experimental groups. Intervention content was delivered independently from the study team via web-based FoRtitude site. Outcomes were independently assessed via web-based PRO administration. The study coordinator played no role in intervention delivery or assessment of outcomes. Telecoaches were blinded to participant random assignment.
Statistical Analysis
All analyses were carried out in SAS (version 9.4, Cary, NC). A 2-sided alpha level of .05 was used to determine statistical significance. Descriptive statistics were computed to describe the study population, website use, and summarize outcome measures from T0 to T2. χ2 tests and independent samples t tests compared eligible BCS (vs noneligible) and BCS completing all 3 assessments (vs not) on demographic and other variables. Paired t tests assessed changes over time in bivariate analyses for our primary outcome of total FCRI score as well as our secondary PRO of interest.
Intervention effects on FCRI total score were estimated in a mixed model with a random participant effect , and fixed effects of time (T0, T1, T2), treatment assignment (yes/no), all possible interactions by treatment, and first-order interactions between each treatment assignment and time in an intent-to-treat analysis. Linear contrasts estimated average within-person changes by time by treatment group. An initial model included the covariates stage and age at diagnosis, time since diagnosis, time since treatment completion, and current hormonal therapy (yes/no) per the planned protocol analysis. Finally, we repeated the above modeling with time-varying change in BCSE score from baseline included as a covariate.
To obtain estimates of effect size, we divided average estimated FCRI total change scores by the baseline SD of the score. At the individual level, a 0.5-SD change from the participant’s own baseline score (48) was used to estimate the proportion of BCS with improved, stable, or worsened FoR from T0 to T2 and, similarly, improved, stable, or worsened self-efficacy from T0 to T1.
Results
Sample Characteristics
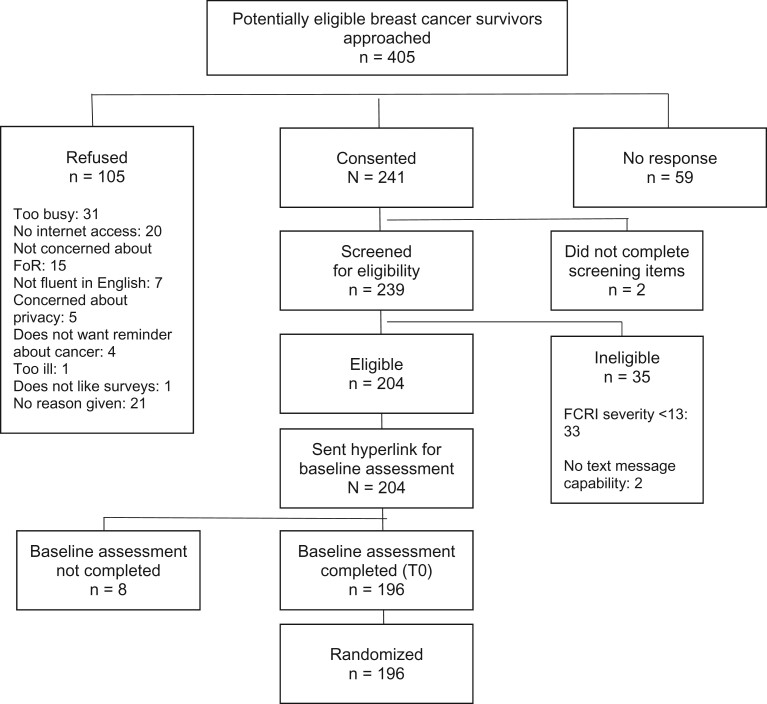
A total of 405 BCS were approached; 241 (59.5%) completed online consent and eligibility screening, and 204 (84.6%) were eligible (Figure 1, CONSORT). Of 204 eligible BCS, 196 (96.1%) completed baseline assessment and were randomly assigned. Among those randomly assigned, 186 (94.9%) logged onto the FoRtitude site at least once, 151 completed T1, and 153 completed T2. BCS randomly assigned to telecoaching were statistically significantly more likely to complete the 4-week assessment compared with those randomly assigned to no telecoaching (Table 1; P = .03). Attrition was comparable across other group assignments (Table 1).
Figure 1.
CONSORT diagram. FCRI = Fear of Cancer Recurrence Inventory; T0 = baseline; FoR = fear of recurrence.
Table 1.
FoRtitude site use and retention by intervention component
| Site use and retention | Total sample | Factors (N = 196 randomized) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
||||||||||
| Relaxation |
Cognitive restructuring |
Worry practice |
Telecoaching |
||||||||||
| On/yes | Off/HMCa | On/yes | Off/HMCa | On/yes | Off/HMCa | Yes | No | ||||||
| Allocated to intervention | 196 | 98 | 98 | 98 | 98 | 98 | 98 | 97 | 99 | ||||
| Site use, No. (%) | |||||||||||||
| No log-in | 10 (5.1) | 4 (4.1) | 6 (6.1) | 8 (8.2) | 2 (2.0) | 6 (6.1) | 4 (4.1) | 6 (6.2) | 4 (4.0) | ||||
| Low | 82 (46.8) | 35 (35.7) | 47 (48.0) | 41 (41.8) | 41 (41.8) | 42 (42.4) | 40 (41.2) | 29 (29.9) | 53 (53.5) | ||||
| Medium | 55 (28.1) | 31 (31.6) | 24 (24.5) | 30 (30.6) | 25 (25.5) | 32 (32.3) | 23 (23.9) | 33 (34.0) | 22 (22.2) | ||||
| High | 49 (25.0) | 28 (28.6) | 21 (21.4) | 19 (19.4) | 30 (30.6) | 19 (19.2) | 30 (30.9) | 29 (28.9) | 20 (20.2) | ||||
| Lost to follow-up, No. (%) | |||||||||||||
| Withdrew | |||||||||||||
| 0 to 4 wk | 6 (3.1) | 3 (3.1) | 3 (3.1) | 5 (5.1) | 1 (1.0) | 2 (2.0) | 4 (4.1) | 3 (3.1) | 3 (3.0) | ||||
| >4 to 8 wk | 6 (3.1) | 2 (2.0) | 4 (4.1) | 3 (3.1) | 3 (3.1) | 4 (4.0) | 2 (2.1) | 1 (1.0) | 5 (5.1) | ||||
| Completed 4-week assessment (T1), No. (%) | 151 (77.8) | 77 (78.6) | 74 (74.8) | 68 (69.4) | 83 (84.9) | 73 (73.7) | 78 (80.4) | 81 (83.5) | 70 (70.7) | ||||
| Completed 8-week assessment (T2), No. (%) | 153 (78.1) | 78 (79.6) | 75 (76.5) | 69 (70.4) | 84 (85.7) | 74 (74.8) | 79 (81.4) | 80 (82.5) | 73 (73.7) | ||||
HMC = health management content.
Demographic, medical characteristics, and baseline FCRI severity scores were well balanced by random assignment (Table 2) and were comparable between BCS who completed all assessments and those with missing data. Eligible BCS were comparable with noneligible BCS on demographic and disease characteristics.
Table 2.
Demographic and clinical characteristics of study sample (N = 196) overall and by randomization factor
| Demographic and clinical characteristics | Total sample | Sample characteristics by each randomization factora |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Relaxation |
Worry practice |
Cognitive restructuring |
Telecoaching |
||||||
| Yes | No | Yes | No | Yes | No | Yes | No | ||
| Sample size, No. | 196 | 98 | 98 | 98 | 98 | 98 | 98 | 97 | 99 |
| Demographics | |||||||||
| Mean age at screening (SD), y | 54.7 (9.8) | 54.6 (9.3) | 54.8 (10.2) | 55.0 (9.4) | 54.4 (10.2) | 53.7 (10.0) | 55.6 (9.5) | 54.1 (10.1) | 55.3 (9.4) |
| Range | 26.0-76.0 | 30.0-74.0 | 26.0-76.0 | 30.0-76.0 | 26.0-74.0 | 26.0-74.0 | 30.0-76.0 | 26.0-76.0 | 33.0-76.0 |
| Race, No. (%) | |||||||||
| Non-Hispanic White | 174 (88.8) | 90 (91.8) | 84 (85.7) | 87 (87.9) | 87 (89.7) | 84 (85.7) | 90 (91.8) | 89 (91.8) | 85 (85.9) |
| Hispanic White | 9 (4.6) | 4 (4.1) | 5 (5.1) | 6 (6.1) | 3 (3.1) | 6 (6.1) | 3 (3.1) | 4 (4.1) | 5 (5.1) |
| Non-Hispanic African American | 6 (3.1) | 1 (1.0) | 5 (5.1) | 3 (3.0) | 3 (3.1) | 2 (2.0) | 4 (4.1) | 3 (3.1) | 3 (3.0) |
| Non-Hispanic Asian | 4 (2.0) | 2 (2.0) | 2 (2.0) | 1 (1.0) | 3 (3.1) | 4 (4.1) | 0 (0.0) | 1 (1.0) | 3 (3.0) |
| Other | 1 (0.5) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Missing | 2 (1.0) | 0 (0.0) | 2 (2.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | 0 (0.0) | 2 (2.0) |
| Latinx, No. (%) | |||||||||
| No | 184 (93.9) | 93 (94.9) | 91 (92.9) | 91 (91.9) | 93 (95.9) | 90 (91.8) | 94 (95.9) | 93 (95.9) | 91 (91.9) |
| Yes | 11 (5.6) | 4 (4.1) | 7 (7.1) | 7 (7.1) | 4 (4.1) | 7 (7.1) | 4 (4.1) | 4 (4.1) | 7 (7.1) |
| Missing | 1 (0.5) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Education, No. (%) | |||||||||
| Some high school | 1 (0.5) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) |
| High school graduate | 7 (3.6) | 4 (4.1) | 3 (3.1) | 4 (4.0) | 3 (3.1) | 2 (2.0) | 5 (5.1) | 2 (2.1) | 5 (5.1) |
| Vocational or technical school | 15 (7.7) | 4 (4.1) | 11 (11.2) | 6 (6.1) | 9 (9.3) | 3 (3.1) | 12 (12.2) | 6 (6.2) | 9 (9.1) |
| Some college/associate’s | 40 (20.4) | 15 (15.3) | 25 (25.5) | 21 (21.2) | 19 (19.6) | 23 (23.5) | 17 (17.4) | 25 (25.8) | 15 (15.2) |
| College graduate | 71 (36.2) | 35 (35.7) | 36 (36.7) | 34 (34.3) | 37 (38.1) | 41 (41.8) | 30 (30.6) | 26 (26.8) | 45 (45.5) |
| Graduate or professional school | 53 (27.0) | 33 (33.7) | 20 (20.4) | 27 (27.3) | 26 (26.8) | 24 (24.5) | 29 (29.6) | 33 (34.0) | 20 (20.2) |
| Other | 2 (1.0) | 2 (2.0) | 0 (0.0) | 2 (2.0) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 1 (1.0) | 1 (1.0) |
| Missing | 7 (3.6) | 4 (4.1) | 3 (3.1) | 4 (4.0) | 3 (3.1) | 4 (4.1) | 3 (3.1) | 3 (3.1) | 4 (4.0) |
| Marital status, No. (%) | |||||||||
| Single | 19 (9.7) | 13 (13.3) | 6 (6.1) | 9 (9.1) | 10 (10.3) | 8 (8.2) | 11 (11.2) | 15 (15.5) | 4 (4.0) |
| Married/domestic partnership | 153 (78.1) | 73 (74.5) | 80 (81.6) | 76 (76.8) | 77 (79.4) | 77 (78.6) | 76 (77.6) | 73 (75.3) | 80 (80.8) |
| Divorced | 17 (8.7) | 9 (9.2) | 8 (8.2) | 10 (10.1) | 7 (7.2) | 6 (6.1) | 11 (11.2) | 7 (7.2) | 10 (10.1) |
| Separated | 2 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | 1 (1.0) | 2 (2.0) | 0 (0.0) | 0 (0.0) | 2 (2.0) |
| Widowed | 5 (2.6) | 2 (2.0) | 3 (3.1) | 3 (3.0) | 2 (2.1) | 5 (5.1) | 0 (0.0) | 2 (2.1) | 3 (3.0) |
| Employment status, No. (%) | |||||||||
| Employed ≥32 h/wk | 123 (62.8) | 61 (62.2) | 62 (63.3) | 58 (58.6) | 65 (67.0) | 60 (61.2) | 63 (64.3) | 68 (70.1) | 55 (55.6) |
| Unemployed | 6 (3.1) | 1 (1.0) | 5 (5.1) | 3 (3.0) | 3 (3.1) | 3 (3.1) | 3 (3.1) | 3 (3.1) | 3 (3.0) |
| Homemaker | 18 (9.2) | 11 (11.2) | 7 (7.1) | 9 (9.1) | 9 (9.3) | 11 (11.2) | 7 (7.1) | 5 (5.2) | 13 (13.1) |
| Retired | 34 (17.4) | 18 (18.4) | 16 (16.3) | 18 (18.2) | 16 (16.5) | 18 (18.4) | 16 (16.3) | 15 (15.5) | 19 (19.2) |
| Disabled/medical leave | 5 (2.6) | 3 (3.1) | 2 (2.0) | 4 (4.0) | 1 (1.0) | 1 (1.0) | 4 (4.1) | 0 (0.0) | 5 (5.1) |
| Student | 3 (1.5) | 1 (1.0) | 2 (2.0) | 2 (2.0) | 1 (1.0) | 1 (1.0) | 2 (2.0) | 3 (3.1) | 0 (0.0) |
| Other | 2 (1.0) | 1 (1.0) | 1 (1.0) | 2 (2.0) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 2 (2.1) | 0 (0.0) |
| Missing | 5 (2.6) | 2 (2.0) | 3 (3.1) | 3 (3.0) | 2 (2.1) | 3 (3.1) | 2 (2.0) | 1 (1.0) | 4 (4.0) |
| Diagnostic history | |||||||||
| Stage at diagnosis, No. (%) | |||||||||
| Stage 0 | 5 (2.6) | 1 (1.0) | 4 (4.1) | 5 (5.1) | 0 (0.0) | 2 (2.0) | 3 (3.1) | 2 (2.1) | 3 (3.0) |
| Stage I | 87 (44.4) | 48 (49.0) | 39 (39.8) | 40 (40.4) | 47 (48.5) | 42 (42.9) | 45 (45.9) | 47 (48.5) | 40 (40.4) |
| Stage II | 78 (39.8) | 35 (35.7) | 43 (43.9) | 42 (42.4) | 36 (37.1) | 42 (42.9) | 36 (36.7) | 39 (40.2) | 39 (39.4) |
| Stage III | 26 (13.3) | 14 (14.3) | 12 (12.2) | 12 (12.1) | 14 (14.4) | 12 (12.2) | 14 (14.3) | 9 (9.3) | 17 (17.2) |
| Mean age at diagnosis (SD), y | 51.8 (9.4) | 51.7 (8.8) | 51.8 (9.9) | 52.1 (8.9) | 51.4 (9.8) | 50.5 (9.3) | 53.0 (9.2) | 51.0 (9.8) | 52.5 (8.9) |
| Range | 24.8-73.6 | 28.6-68.6 | 24.8-73.6 | 28.6-73.6 | 24.8-68.9 | 24.8-68.9 | 28.6-73.6 | 24.8-73.6 | 30.6-71.3 |
| Mean time since diagnosis (SD), y | 3.5 (3.0) | 3.4 (3.1) | 3.6 (2.9) | 3.4 (2.8) | 3.5 (3.2) | 3.7 (3.4) | 3.2 (2.5) | 3.6 (3.2) | 3.3 (2.7) |
| Range | 0.2-19.1 | 0.3-19.1 | 0.2-16.9 | 0.2-15.2 | 0.4-19.1 | 0.2-19.1 | 0.4-15.2 | 0.4-19.1 | 0.2-15.2 |
| Treatment history | |||||||||
| Mean time since treatment (SD), y | 2.8 (2.5) | 2.6 (2.4) | 3.0 (2.6) | 3.0 (2.8) | 2.6 (2.0) | 2.9 (2.4) | 2.7 (2.6) | 2.7 (2.1) | 2.9 (2.8) |
| Range | 0.1-15.0 | 0.1-15.0 | 0.1-13.3 | 0.1-15.0 | 0.1-12.0 | 0.1-13.3 | 0.1-15.0 | 0.1-13.3 | 0.1-15.0 |
| Treatment received, No. (%) | |||||||||
| Surgery only | 21 (10.7) | 8 (8.2) | 13 (13.3) | 11 (11.1) | 10 (10.3) | 13 (13.3) | 8 (8.2) | 8 (8.3) | 13 (13.1) |
| Chemotherapy only | 1 (0.5) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.0) |
| Surgery + chemotherapy | 37 (18.9) | 20 (20.4) | 17 (17.4) | 21 (21.2) | 16 (16.5) | 18 (18.4) | 19 (19.4) | 18 (18.6) | 19 (19.2) |
| Surgery + RT | 37 (18.9) | 20 (20.4) | 17 (17.4) | 19 (19.2) | 18 (18.6) | 14 (14.3) | 23 (23.5) | 21 (21.7) | 16 (16.2) |
| Surgery + chemotherapy + RT | 99 (50.5) | 50 (51.0) | 49 (50.0) | 46 (46.5) | 53 (54.6) | 53 (54.1) | 46 (46.9) | 49 (50.5) | 50 (50.5) |
| Missing | 1 (0.5) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 0 (0.0) |
| Targeted therapy, No. (%) | |||||||||
| None | 148 (75.5) | 77 (78.6) | 71 (72.5) | 76 (76.8) | 72 (74.2) | 77 (78.6) | 71 (72.5) | 76 (78.4) | 72 (72.7) |
| Received | 43 (21.9) | 17 (17.4) | 26 (26.5) | 22 (22.2) | 21 (21.7) | 18 (18.4) | 25 (25.5) | 19 (19.6) | 24 (24.2) |
| Missing | 5 (2.6) | 4 (4.1) | 1 (1.0) | 1 (1.0) | 4 (4.1) | 3 (3.1) | 2 (2.0) | 2 (2.1) | 3 (3.0) |
| Hormonal therapy, No. (%) | |||||||||
| No | 38 (19.4) | 14 (14.3) | 24 (24.5) | 21 (21.2) | 17 (17.5) | 22 (22.5) | 16 (16.3) | 17 (17.5) | 21 (21.2) |
| Current | 137 (69.9) | 73 (74.5) | 64 (65.3) | 66 (66.7) | 71 (73.2) | 70 (71.4) | 67 (68.4) | 65 (67.0) | 72 (72.7) |
| Past | 20 (10.2) | 10 (10.2) | 10 (10.2) | 12 (12.1) | 8 (8.3) | 6 (6.1) | 14 (14.3) | 14 (14.4) | 6 (6.1) |
| Missing | 1 (0.5) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 0 (0.0) |
Participants were randomly assigned independently to each factor. Data were provided for full sample and separately by factor to demonstrate balanced randomization regarding demographics, clinical characteristics, and FoR at baseline. Sample size for each factor totaled 196; therefore, sample size summed across rows exceed 196. RT = radiation therapy.
Primary Outcome: FCRI Change Pre- to Postintervention by Intervention Component
FCRI total score, our primary endpoint, decreased statistically significantly from T0 to T2 for all conditions, including attention control (T0 = 53.1 [SD = 17.4], T2 = 41.9 [SD = 16.2], P < .001; Table 3). A statistically significantly decreased FCRI total score was also observed for all groups from T0 to T1 (T0 = 53.1 [SD = 17.4], T1 = 41.9 [SD = 16.2], P < .001). Similarly, FCRI subscale scores decreased from T0 to T1 (Supplementary Table 2, available online).
Table 3.
Unadjusted means and change scores for primary outcome measure by randomization (N = 196)
| Measure | Total sample (N = 196)c | Sample characteristics by each randomization factora, mean (SD) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Relaxation |
Worry practice |
Cognitive restructuring |
Telecoachingb |
||||||
| Yes (n = 98)c | No (n = 98)c | Yes (n = 98)c | No (n = 98)c | Yes (n = 98)c | No (n = 98)c | Yes (n = 97)c | No (n = 99)c | ||
| FCRI: totald | |||||||||
| T0 (baseline) | 53.1 (17.4) | 54.5 (17.9) | 51.7 (16.9) | 53.3 (15.4) | 52.9 (19.3) | 53.3 (17.4) | 52.8 (17.5) | 52.8 (17.5) | 53.4 (17.3) |
| T1 (wk 4) | 46.3 (16.5) | 48.3 (16.9) | 44.2 (16.0) | 48.2 (16.1) | 44.5 (16.8) | 47.9 (15.9) | 45.0 (17.0) | 45.4 (16.7) | 47.4 (16.3) |
| T2 (wk 8) | 41.9 (16.2) | 42.4 (16.0) | 41.4 (16.5) | 44.0 (15.2) | 39.9 (16.9) | 43.8 (16.4) | 40.3 (15.9) | 40.8 (16.3) | 43.1 (16.0) |
| T0-T2 change score | −10.9 (13.9) | −11.7 (13.5) | −10.2 (14.3) | −9.9 (14.1) | −11.9 (13.7) | −9.9 (12.4) | −11.8 (15.1) | −11.7 (14.4) | −10.1 (13.4) |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
Participants were randomly assigned independently to each factor. Data were provided for full sample and separately by factor. Sample size for each factor totaled 196; therefore, sample size summed across rows exceed 196. FCRI = Fear of Cancer Recurrence Inventory; T0 = baseline; T1 = 4 weeks; T2 = 8 weeks.
Participants randomly assigned to telecoaching had a higher rate of completed week 4 and week 8 assessments.
These numbers indicate sample sizes at randomization, which are lower at T1 and T2 because of attrition.
FCRI total score does not include Coping and Reassurance subscale items. FCRI total score range = 0-120, with a higher score reflecting a higher level of fear of recurrence.
Table 4 shows the main model results. For this model, we removed all covariates mentioned above (eg, stage, age at diagnosis) because a likelihood ratio test comparing the full model with these covariates to a reduced model was not statistically significant (P > .30). Additionally, inclusion of these covariates did not alter any conclusions about effects of treatment vs control conditions on outcome, and our random assignment led to groups well-balanced on these and other covariates. We found no evidence in model comparisons using likelihood ratio tests that any interaction term between treatments and time—first-order or higher-order—were statistically significant. However, we included first-order interactions between treatment and time in our final model despite their lack of individual and overall statistical significance, because of their relevance to our main hypothesis. We eliminated from our final model all other interactions.
Table 4.
Intent-to-treat analysis of main effects: FCRI total score at T2 (week 8)
| Component | No. | Estimated least squares mean |
Estimateda T2-T0 difference (95% CI) | Difference of estimated differences (95% CI) | Effect size T2-T0 difference (95% CI) | Difference of estimated effect sizes | P b | ||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | |||||||
| Relaxation | |||||||||
| Yes | 98 | 54.5 | 48.8 | 42.8 | −11.62 (−14.22 to −9.02) | −1.69 (−5.39 to 2.01) | −0.67 (−0.82 to −0.52) | −0.10 (−0.31 to 0.12) | .37 |
| No | 98 | 51.7 | 45.1 | 41.8 | −9.93 (−12.57 to −7.29) | −0.57 (−0.72 to −0.42) | |||
| Worry practice | |||||||||
| Yes | 99 | 53.3 | 48.0 | 43.8 | −9.51 (−12.18 to −6.85) | 2.52 (−1.18 to 6.22) | −0.55 (−0.70 to −0.39) | 0.14 (−0.07 to 0.36) | .18 |
| No | 97 | 52.9 | 45.9 | 40.8 | −12.03 (−14.61 to −9.45) | −0.69 (−0.84 to −0.54) | |||
| Cognitive restructuring | |||||||||
| Yes | 98 | 53.3 | 48.1 | 43.7 | −9.69 (−12.43 to −6.95) | 2.17 (−1.55 to 5.89) | −0.56 (−0.72 to −0.40) | 0.12 (−0.09 to 0.34) | .25 |
| No | 98 | 52.8 | 45.8 | 41.0 | −11.86 (−14.36 to −9.35) | −0.68 (−0.83 to −0.54) | |||
| Telecoaching | |||||||||
| Yes | 97 | 52.8 | 45.7 | 41.2 | −11.55 (−14.12 to −8.98) | −1.56 (−5.26 to 2.14) | −0.66 (−0.81 to −0.52) | −0.09 (−0.30 to 0.12) | .41 |
| No | 99 | 53.4 | 48.1 | 43.4 | −9.99 (−12.66 to −7.32) | −0.58 (−0.73 to −0.42) | |||
Estimated as least squares means from mixed model containing time (T0, T1, T2) and treatment condition (relaxation, worry practice, cognitive restructuring, telecoaching) and first-order time × treatment condition interaction terms. CI = confidence interval; FCRI = Fear of Cancer Recurrence Inventory; T0 = baseline; T1 = 4 weeks; T2 = 8 weeks.
P value from 2-sided t test of linear contrast of least squares means.
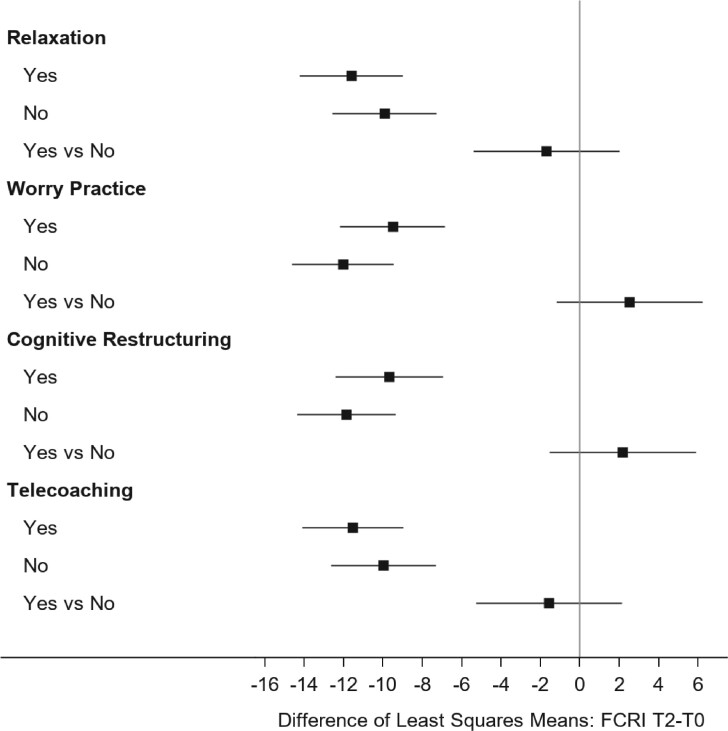
Our final model (Table 4; Figure 2) indicates statistically significant decreases in FCRI score at T2 within all groups, independent of CBT or HMC content. Thus, we found no statistically significant “treatment effect” because of the comparable magnitude of decrease for each CBT vs HMC comparison. The P values in the final column of Table 4 derive from a model-based estimated contrast of the difference in change scores for the 2 conditions (yes/no) of each component. For instance, P value (.37) pertains to the comparison of the estimated differences of –11.62 (relaxation “yes”) and −9.93 (relaxation “no” = HMC), resulting in the estimated “difference of differences” between conditions (relaxation vs HMC) of −1.69. The P value (.37) pertains to the null hypothesis that this estimated difference of differences = 0. Effect sizes for the decline T0-T2 ranged from −0.55 to −0.69. Differences in effect sizes (CBT components vs attention control) were small (−0.09 to 0.14). Change in FCRI total score T0 (SD = 17.8) to T2 of at least 8.9 points represented a minimal clinically important difference. Using this threshold, 53.0% of BCS across CBT and HMC conditions improved, 41.8% remained stable, and 5.2% reported worsened FoR from T0 to T2.
Figure 2.
Intent-to-treat analysis of main effects: Fear of Cancer Recurrence Inventory (FCRI) change score T0-T2 (baseline to week 8) by component. Participants were randomly assigned independently to each factor. Data were provided separately by factor. The sample size for each factor totaled 196. The estimated difference of least squares means was calculated using a mixed model containing time (T0, T1, T2, corresponding to 0, 4, 8 weeks) and treatment condition (relaxation, worry practice, cognitive restructuring, telecoaching) and first-order time × treatment condition interaction terms. The error bars represent a 95% confidence interval.
When time-varying change in BCSE from T0 to the relevant time point was included as a predictor, the magnitude of the T0-T2 decline in FCRI total score was attenuated by, on average, 23%, with effect sizes ranging from −0.39 to −0.51 (Supplementary Table 3, available online). Increase in BCSE was statistically significantly associated with decline in FCRI total score from T0 to T2 (P < .001). The P values in Supplementary Table 3 (available online) pertain to the same null hypothesis test as the corresponding P values in Table 4.
Secondary Outcomes
The Concerns about Recurrence Scale severity score decreased statistically significantly from baseline to 4 and 8 weeks in all groups, replicating our finding of reduced FoR postintervention using an alternative measure. PROs assessing secondary psychosocial outcomes (general and health-related anxiety, depression), symptoms (fatigue, sleep impairments, cognitive impairments), and overall health improved statistically significantly from baseline to 8 weeks (Supplementary Table 2, available online). Psychometric properties for PROs were acceptable to excellent (Supplementary Table 4, available online).
Intervention Targets
The BCSE total score increased statistically significantly from baseline to 4 and 8 weeks (Supplementary Table 2, available online) in all groups (CBT and HMC), indicating increased confidence in managing breast cancer recurrence from pre- to postintervention. As shown in Supplementary Figure 1, A (available online), BCS with BCSE scores that exceeded the minimal clinically important difference (≥3.9 points) had a greater magnitude of change on the FCRI, indicating greater FoR improvement compared with BCS with stable BCSE scores. The FCRI Coping subscale was identified as an intervention target, and plans to analyze this subscale separately were stated a priori. FCRI Coping subscale scores did not change from pre- to postintervention (Supplementary Table 2, available online).
FoRtitude eHealth Use
BCS averaged 8.1 log-ins (SD = 8.27, range = 0-57). Forty participants (20.4%) accessed the site 1-2 times and 10 (5.1%) never logged in. Site use was highest during week 1 (76.0%) and decreased at weeks 2 (57.1%), 3 (53.1%), and 4 (43.9%). Objective website use metrics indicate 51.0%-91.8% read some didactic content, 46.9%-75.0% read all didactic content, and 40.8%-74.0% read didactic content and used the tool (Table 5). Among all participants, 51.0% read all 6 lessons, 62.2% read 4 and more lessons, and 75.0% used at least 1 tool. Among those who completed week 8 assessment, adherence was higher, with 64.1% reading all 6 and 71.9% reading 4 and more lessons. Highest adherence with CBT-based didactic content and corresponding tool was with relaxation (65.3%), followed by cognitive restructuring (49.5%). Adherence was slightly lower with worry practice (39.4%). Mobile texting use was low (4.1%-14.3%; Table 5). BCS with high FoRtitude site use adherence had a more robust improvement in FoR than those with low site use (Supplementary Figure 1, B, available online). BCS randomly assigned to telecoaching used the FoRtitude site more frequently (mean adherence score [SD] = 26.6 [7.2] vs 21.0 [10.5], P < .001).
Table 5.
Number of participants who read assigned lessons and used assigned tools (N = 196)
| Component | No. assigned | Completed at least 1 lesson, No. (%) | Completed both lessons, No. (%) | Completed at least 1 lesson and used tool, No. (%) | Completed at both lessons and used tool, No. (%) | Requested at least 1 text, No. (%) |
|---|---|---|---|---|---|---|
| Relaxation | 98 | 90 (91.8) | 74 (75.1) | 72 (73.5) | 64 (65.3) | 14 (14.3) |
| Cognitive restructuring | 99 | 68 (68.7) | 57 (57.6) | 57 (57.6) | 49 (49.5) | 11 (11.1) |
| Worry practice | 99 | 65 (65.7) | 54 (54.5) | 44 (44.4) | 39 (39.4) | 13 (13.1) |
| General nutrition | 24 | 18 (75.0) | 17 (70.8) | 12 (50.0) | 12 (50.0) | 1 (4.2) |
| Nutrition for BCS | 24 | 15 (62.5) | 14 (58.3) | 10 (41.7) | 10 (41.7) | 9 (12.3) |
| General nutrition + Nutrition for BCS | 73 | 46 (63.0) | 45 (61.6) | —a | —a | 2 (8.3) |
| General health | 97 | 73 (75.3) | 69 (71.1) | 66 (68.0) | 63 (65.3) | 10 (10.3) |
| General health + general nutrition + nutrition for BCS | 74 | 38 (51.4) | 35 (47.3) | —a | —a | 3 (4.1) |
During analysis of FoRtitude website use data, it was discovered that BCS randomly assigned to this experimental group did not receive access to the food diary tool, which per the study design should have been released during week 3. Therefore, data on use of the didactic content and tool are not available. BCS = breast cancer survivors.
Discussion
On average, BCS reported statistically significant reductions in FoR following a 4-week eHealth intervention aimed at teaching skills to manage FoR. However, we found no evidence of differences between CBT or attention control, HMC, in the magnitude of FoR reduction. Improvement was observed postintervention and 1 month later. More than one-half of all BCS reported clinically significant improvement in FoR. Improvements were similar in magnitude for relaxation, cognitive restructuring, and worry practice compared with HMC. BCS randomly assigned to telecoaching had higher FoRtitude site use and retention compared with no telecoaching. Across treatment arms, increased self-efficacy postintervention corresponded with FoR reductions at 1 month follow-up. BCS reported improvements in anxiety, depression, fatigue, sleep, cognitive functioning, and health-related quality of life independent of content received (CBT vs HMC), survivors’ stage and age at diagnosis, time since diagnosis and treatment completion, and current hormonal therapy.
Though contrary to our hypothesis, the lack of main effects between CBT and HMC is an important result in its own right. Recent meta-analyses of FoR interventions demonstrated CBT is effective in reducing FoR, though traditional CBT had small effect sizes with more robust effects reported with contemporary CBT (20) and mindful meditation (19). Our negative results with traditional CBT (relaxation, cognitive restructuring) vs HMC may be due to our inability to detect a small effect. We anticipated a robust effect with worry practice, a contemporary CBT technique (24,49), which was not observed. Neither did we find an interaction effect for traditional CBT combined with contemporary CBT. Previously, CBT demonstrated superiority to relaxation in reducing FoR (24), which is consistent with our negative finding with relaxation vs HMC. We included relaxation because we previously found relaxation promotes eHealth intervention engagement (46). Given the potential for nonadherence and known decay in eHealth use (50), we sought to maximize engagement and adherence through including relaxation. Although our results indicate relaxation has limited efficacy over HMC for reducing FoR, relaxation did keep BCS engaged supporting its value in eHealth interventions.
eHealth delivery may be responsible for our negative findings. Therapist-delivered interventions demonstrated higher effect sizes than other platforms (eg, telephone), though only 3 of 23 trials used alternative means (20). Our eHealth platform may have diluted intervention effects. However, few targeted FoR interventions have used modalities that are scalable and overcome barriers to psychosocial care (26), such as eHealth programs (27), highlighting the unique contribution of this study. Research translating FoR interventions into scalable, remote delivery platforms improving accessibility to efficacious treatment is a priority (51).
In the COVID-19 pandemic era, rapid uptake of technology-enabled health care highlights the need for eHealth interventions that deliver evidence-based treatments remotely. Given the known decay in eHealth use over time (50), an appealing feature of FoRtitude’s targeted approach was its brief duration. However, longer intervention exposure, more intensive training on CBT-based strategies, and greater inclusion of contemporary CBT may be required for treatment effects (19). In contrast to CBT, teaching HMC is relatively didactic and straightforward. Future research should examine more intensive eHealth treatments with longer follow-up to achieve larger effects observed in therapist-delivered interventions (24). Our findings provide evidence that telecoaching increases eHealth adherence and engagement. Overall, higher use of the FoRtitude site was associated with greater reduction in FoR, though variability in FoRtitude use suggests the “dose” needed to derive benefit varies by individual. Future research should use innovative adaptive designs, including MOST (36), to advance personalized psychosocial eHealth approaches .
HMC, intended to be inert, may have taught BCS strategies that bolstered self-efficacy, improving FoR. Associations between health behaviors, including diet, and FoR severity (52) support this interpretation. Our findings are consistent with previous trials reporting comparable improvement between coping interventions and health content (53), including a healthy-eating attention control (54) resembling our HMC. Weight gain is a salient concern among BCS posttreatment (55) associated with health risks, including recurrence (56), which may be why FoRtitude HMC content may have lowered FoR. This presents a methodological dilemma for eHealth trials when an attention control is intended to hold website use constant to isolate potential benefit from treatment content. Alternative attention control designs are needed (57).
We required elevated FoR for eligibility, which few FoR intervention trials have done (20). Although BCS had elevated FoR at screening, FoR levels may fluctuate around the time of annual surveillance (58), and we cannot rule out regression to the mean as an explanation for the reduction from baseline to postintervention.
Our findings must be interpreted while considering the following limitations. We individually evaluated 3 CBT components comparing each with an attention control because we wanted to control for eHealth site use while only varying content (CBT vs HMC). Our design did not include an observation-only control, which is a limitation. We did not assess treatment expectancy to control for the potential nonspecific therapeutic effects of participating in an FoR intervention trial, explicitly described in recruitment materials and the consent as aimed at reducing FoR. Most adults in the United States use the internet for health-related information (59), so it is possible that study participation led BCS to seek additional information to decrease anxiety; we did not, however, measure this potential confound.
To our knowledge, this is the first trial to evaluate an eHealth intervention for managing FoR using CBT and telecoaching. Our findings support the value of telephone-based motivational interviewing to promote eHealth adherence. We report an initial step toward the development of a model for intervention delivery that is highly scalable and widely disseminable at low cost, while pointing to priorities for future targeted eHealth interventions for the management of this pervasive and distressing concern.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (CA173193, 1UG1CA189828) and by the ECOG-ACRIN Medical Research Foundation.
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: Lynne Wagner reported consulting fees from Celgene, Inc. and Athenex Inc. outside of the submitted work. David C. Mohr, PhD has accepted honoraria and consulting fees from Apple, Inc., Otsuka Pharmaceuticals, Pear Therapeutics, and the One Mind Foundation, and has an ownership interest in Adaptive Health, Inc. All authors have confirmed no conflicts of interest.
Author contributions: Wagner: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, supervision, visualization, writing—original draft and review and editing. Tooze: data curation, formal analysis, validation, visualization, writing—original draft and review and editing. Hall: writing—review and editing. Levine: data curation, formal analysis, validation, visualization, writing—original draft and review and editing. Beaumont: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, software, validation, visualization, writing—review and editing. Duffecy: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, writing—review and editing. Victorson: conceptualization, methodology, writing—review and editing. Gradishar: resources, supervision, writing—review and editing. Leach: funding acquisition, resources, supervision, writing—review and editing. Saphner: funding acquisition, resources, supervision, writing—review and editing. Stertz: funding acquisition, resources, supervision, writing—review and editing. Smith: funding acquisition, investigation, methodology, writing—review and editing. Penedo: conceptualization, methodology, resources, supervision, writing—review and editing. Mohr: conceptualization, funding acquisition, methodology, resources, software, supervision, writing—review and editing. Cella: conceptualization, funding acquisition, methodology, resources, software, supervision, writing—review and editing.
Acknowledgements: We thank the breast cancer survivors who participated in this study. We thank study investigators, nurses, clinicians and study personnel at Aurora, Metro Minnesota, and Colorado Cancer Research Program NCORP Sites and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University for support with trial recruitment.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Supplementary Material
References
- 1. Lebel S, Ozakinci G, Humphris G, et al. ; University of Ottawa Fear of Cancer Recurrence Colloquium attendees. From normal response to clinical problem: definition and clinical features of fear of cancer recurrence. Support Care Cancer. 2016;24(8):3265–3268. [DOI] [PubMed] [Google Scholar]
- 2. Johnson Vickberg SM. Fears about breast cancer recurrence. Cancer Pract. 2001;9(5):237–243. [DOI] [PubMed] [Google Scholar]
- 3. Vickberg S. The concerns about recurrence scale (CARS): a systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25(1):16–24. [DOI] [PubMed] [Google Scholar]
- 4. Simard S, Savard J, Ivers H.. Fear of cancer recurrence: specific profiles and nature of intrusive thoughts. J Cancer Surviv. 2010;4(4):361–371. [DOI] [PubMed] [Google Scholar]
- 5. Mehnert A, Berg P, Henrich G, Herschbach P.. Fear of cancer progression and cancer-related intrusive cognitions in breast cancer survivors. Psychooncology. 2009;18(12):1273–1280. [DOI] [PubMed] [Google Scholar]
- 6. van den Beuken-van Everdingen MHJ, Peters ML, de Rijke JM, Schouten HC, van Kleef M, Patijn J.. Concerns of former breast cancer patients about disease recurrence: a validation and prevalence study. Psychooncology. 2008;17(11):1137–1145. [DOI] [PubMed] [Google Scholar]
- 7. Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B.. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology. 2006;15(4):306–320. [DOI] [PubMed] [Google Scholar]
- 8. Gil K, Mishel M, Belyea M, et al. Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncol Nurs Forum. 2004;31(3):633–639. [DOI] [PubMed] [Google Scholar]
- 9. Helgeson VS, Tomich PL.. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psycho-Oncology. 2005;14(4):307–317. [DOI] [PubMed] [Google Scholar]
- 10. Hill J, Holcombe C, Clark L, et al. Predictors of onset of depression and anxiety in the year after diagnosis of breast cancer. Psychol Med. 2011;41(7):1429–1436. [DOI] [PubMed] [Google Scholar]
- 11. Northouse LL. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs. 1981;4(3):213–220. [PubMed] [Google Scholar]
- 12. Taylor C, Richardson A, Cowley S.. Surviving cancer treatment: an investigation of the experience of fear about, and monitoring for, recurrence in patients following treatment for colorectal cancer. Eur J Oncol Nurs. 2011;15(3):243–249. [DOI] [PubMed] [Google Scholar]
- 13. Armes J, Crowe M, Colbourne L, et al. Patients' supportive care needs beyond the end of cancer treatment: a prospective, longitudinal survey. J Clin Oncol. 2009;27(36):6172–6179. [DOI] [PubMed] [Google Scholar]
- 14. Harrison SE, Watson EK, Ward AM, et al. Primary health and supportive care needs of long-term cancer survivors: a questionnaire survey. J Clin Oncol. 2011;29(15):2091–2098. [DOI] [PubMed] [Google Scholar]
- 15. Thewes B, Husson O, Poort H, et al. Fear of cancer recurrence in an era of personalized medicine. J Clin Oncol. 2017;35(29):3275–3278. [DOI] [PubMed] [Google Scholar]
- 16. Otto AK, Soriano EC, Siegel SD, LoSavio ST, Laurenceau J-P.. Assessing the relationship between fear of cancer recurrence and health care utilization in early-stage breast cancer survivors. J Cancer Surviv. 2018;12(6):775–785. [DOI] [PubMed] [Google Scholar]
- 17. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300–322. [DOI] [PubMed] [Google Scholar]
- 18. Lebel S, Ozakinci G, Humphris G, et al. Current state and future prospects of research on fear of cancer recurrence. Psychooncology. 2017;26(4):424–427. [DOI] [PubMed] [Google Scholar]
- 19. Hall DL, Luberto CM, Philpotts LL, Song R, Park ER, Yeh GY.. Mind‐body interventions for fear of cancer recurrence: a systematic review and meta‐analysis. Psychooncology. 2018;27(11):2546–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tauber NM, O’Toole MS, Dinkel A, et al. Effect of psychological intervention on fear of cancer recurrence: a systematic review and meta-analysis. J Clin Oncol. 2019;37(31):2899–2915., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Wal M, Thewes B, Gielissen M, Speckens A, Prins J.. Efficacy of blended cognitive behavior therapy for high fear of recurrence in breast, prostate, and colorectal cancer survivors: the SWORD study, a randomized controlled trial. J Clin Oncol. 2017;35(19):2173–2183. [DOI] [PubMed] [Google Scholar]
- 22. Dieng M, Butow PN, Costa DS, et al. Psychoeducational intervention to reduce fear of cancer recurrence in people at high risk of developing another primary melanoma: results of a randomized controlled trial. J Clin Oncol. 2016;34(36):4405–4414. [DOI] [PubMed] [Google Scholar]
- 23. Lebel S, Maheu C, Lefebvre M, et al. Addressing fear of cancer recurrence among women with cancer: a feasibility and preliminary outcome study. J Cancer Surviv. 2014;8(3):485–496. [DOI] [PubMed] [Google Scholar]
- 24. Butow PN, Turner J, Gilchrist J, et al. Randomized trial of conquerfear: a novel, theoretically based psychosocial intervention for fear of cancer recurrence. J Clin Oncol. 2017;35(36):4066–4077. [DOI] [PubMed] [Google Scholar]
- 25. Hall DL, Park ER, Cheung T, Davis RB, Yeh GY.. A pilot mind-body resiliency intervention targeting fear of recurrence among cancer survivors. J Psychosom Res. 2020;137:110215. doi: 10.1016/j.jpsychores.2020.110215. Online ahead of print.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. The National Academies Press. Published 2008. http://www.iom.edu/CMS/3809/34252/47228.aspx. Accessed January 15, 2012. [PubMed] [Google Scholar]
- 27. Leykin Y, Thekdi SM, Shumay DM, Muñoz RF, Riba M, Dunn LB.. Internet interventions for improving psychological well-being in psycho-oncology: review and recommendations. Psychooncology. 2012;21(9):1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clarke G, Eubanks D, Reid E, et al. Overcoming Depression on the Internet (ODIN) (2): a randomized trial of a self-help depression skills program with reminders. J Med Internet Res. 2005;7(2):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cuijpers P, Straten A, Andersson G.. Internet-administered cognitive behavior therapy for health problems: a systematic review. J Behav Med. 2008;31(2):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carnahan LF, Ritterband LM, Bailey E, Thorndike FP, Lord HR, Baum LD.. Results from a study examining the feasibility and preliminary efficacy of a self-hypnosis intervention available on the web for cancer survivors with insomnia. EJAP. 2010;6(2):10–23. [Google Scholar]
- 31. Wagner LI, Duffecy J, Penedo F, Mohr DC, Cella D.. Coping strategies tailored to the management of fear of recurrence and adaptation for E-health delivery: the FoRtitude intervention. Cancer. 2017;123(6):906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee-Jones C, Humphris G, Dixon R, Bebbington Hatcher M.. Fear of cancer recurrence — a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. Psychooncology. 1997;6(2):95–105. [DOI] [PubMed] [Google Scholar]
- 33. Collins L, Murphy S, Nair V, Strecher V.. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med. 2005;30(1):65–73. ): [DOI] [PubMed] [Google Scholar]
- 34. Collins LM, Dziak JJ, Kugler KC, Trail JB.. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. 2014;47(4):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hershman DL, Unger JM, Hillyer GC, et al. Randomized trial of text messaging to reduce early discontinuation of adjuvant aromatase inhibitor therapy in women with early-stage breast cancer: SWOG S1105. J Clin Oncol. 2020;38(19):2122–2129., J Clin Oncol. 19.02699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collins LM, Murphy SA, Strecher V.. The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(Suppl 5):S112–S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simard S, Savard J.. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv. 2015;9(3):481–491. [DOI] [PubMed] [Google Scholar]
- 38. Mohr DC, Duffecy J, Ho J, et al. A randomized controlled trial evaluating a manualized telecoaching protocol for improving adherence to a web-based intervention for the treatment of depression. PLoS One. 2013;8(8):e70086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duffecy J, Kinsinger S, Ludman E, Mohr D, Brief telephone support program to enhance patient adherence to Technology Assisted Behavioral Interventions (TABIs): Therapist manual.
- 40. Gershon R, Rothrock NE, Hanrahan RT, Jansky LJ, Harniss M, Riley W.. The development of a clinical outcomes survey research application: assessment CenterSM. Qual Life Res. 2010;19(5):677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simard S, Savard J.. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Support Care Cancer. 2009;17(3):241–251. [DOI] [PubMed] [Google Scholar]
- 42. Costa DS, Dieng M, Cust AE, Butow PN, Kasparian NA.. Psychometric properties of the Fear of Cancer Recurrence Inventory: an item response theory approach. Psychooncology. 2016;25(7):832–838. [DOI] [PubMed] [Google Scholar]
- 43. Horowitz M, Wilner N, Alvarez W.. Impact of event scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–218. [DOI] [PubMed] [Google Scholar]
- 44. Weiss D, Marmar C.. The impact of events scale-revised. In: Wilson J, Keane T, eds. Assessing Psychological Trauma and PTSD. 2nd ed. New York: Guilford Press; 2004:168–189. [Google Scholar]
- 45. Champion VL, Ziner KW, Monahan PO, et al. Development and psychometric testing of a breast cancer survivor self-efficacy scale. Oncol Nurs Forum. 2013;40(6):E403–E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duffecy J, Sanford S, Wagner L, Begale M, Nawacki E, Mohr DC.. Project onward: an innovative e-health intervention for cancer survivors. Psychooncology. 2013;22(4):947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Faller H, Schuler M, Richard M, Heckl U, Weis J, Küffner R.. Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol. 2013;31(6):782–793. [DOI] [PubMed] [Google Scholar]
- 48. Yost KJ, Eton DT.. Combining distribution-and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28(2):172–191. [DOI] [PubMed] [Google Scholar]
- 49. Smith AB, Thewes B, Turner J, et al. Pilot of a theoretically grounded psychologist-delivered intervention for fear of cancer recurrence (Conquer Fear). Psychooncology. 2015;24(8):967–970. [DOI] [PubMed] [Google Scholar]
- 50. Christensen H, Griffiths KM, Farrer L.. Adherence in internet interventions for anxiety and depression: systematic review. J Med Internet Res. 2009;11(2):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shaw J, Kamphuis H, Sharpe L, et al. Setting an international research agenda for fear of cancer recurrence: an online Delphi consensus study. Front Psychol. 2021;12(241):596682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hall DL, Jimenez RB, Perez GK, et al. Fear of cancer recurrence: a model examination of physical symptoms, emotional distress, and health behavior change. JOP. 2019;15(9):e787–e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bartolo A, Pacheco E, Rodrigues F, Pereira A, Monteiro S, Santos IM.. Effectiveness of psycho-educational interventions with telecommunication technologies on emotional distress and quality of life of adult cancer patients: a systematic review. Disabil Rehabil. 2019;41(8):870–878. [DOI] [PubMed] [Google Scholar]
- 54. Jimenez DE, Begley A, Bartels SJ, et al. Improving health-related quality of life in older African American and non-Latino White patients. Am J Geriatr Psychiatry. 2015;23(6):548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gross AL, May BJ, Axilbund JE, Armstrong DK, Roden RBS, Visvanathan K. Weight Change in Breast Cancer Survivors Compared to Cancer-Free Women: A Prospective Study in Women at Familial Risk of Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(3):1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kroenke CH, Chen WY, Rosner B, Holmes MD.. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. [DOI] [PubMed] [Google Scholar]
- 57. Mohr DC, Spring B, Freedland KE, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78(5):275–284. [DOI] [PubMed] [Google Scholar]
- 58. McGinty HL, Small BJ, Laronga C, Jacobsen PB.. Predictors and patterns of fear of cancer recurrence in breast cancer survivors. Health Psychol. 2016;35(1):1–9. [DOI] [PubMed] [Google Scholar]
- 59.Pew Internet and American Life Project. Who's online: internet user demographics. Pew Internet and American Life Project. Published 2011. Updated January 20, 2012. http://pewinternet.org/Static-Pages/Trend-Data/Whos-Online.aspx. Accessed January 20, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.