Abstract
We report a coronavirus disease 2019 case with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persisting beyond 333 days in an immunocompromised patient with chronic lymphocytic leukemia, asymptomatically carrying infectious SARS-CoV-2 at day 197 postdiagnosis. In addition, viral sequencing indicates major changes in the spike protein over time, temporally associated with convalescent plasma treatment.
Keywords: CLL, persistent infection, SARS-CoV-2, viral evolution
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with a robust antibody response and development of T-cell immunity [1–3]. Control of the pandemic has been based on testing, isolation, and contact tracing. Accordingly, health authorities advice self-isolation until 24–48 hours after recovery [4]. However, viable virus may persist for prolonged periods in certain severely immunocompromised individuals [5–7]. The immunological prerequisites for complete clearance of SARS-CoV-2 infection are thus unclear. In addition, concern is growing regarding the emergence of new SARS-CoV-2 variants, including B.1.1.7 and B.1.351, and their implications for future control of the coronavirus disease 2019 (COVID-19) pandemic. Increasing numbers of mutational changes in the spike protein genome pose a risk of reinfection, viral escape from present treatment modalities, and lowering vaccine efficacy [7–9].
In this study, we report a case of persistent SARS-CoV-2 infection in an immunocompromised patient with chronic lymphocytic leukemia (CLL), asymptomatically carrying infectious SARS-CoV-2 at day 197 postdiagnosis. Viral sequencing showed accumulation of mutations over time and indicated major changes in the spike protein, temporally associated with convalescent plasma treatment.
METHODS AND RESULTS
Clinical Report
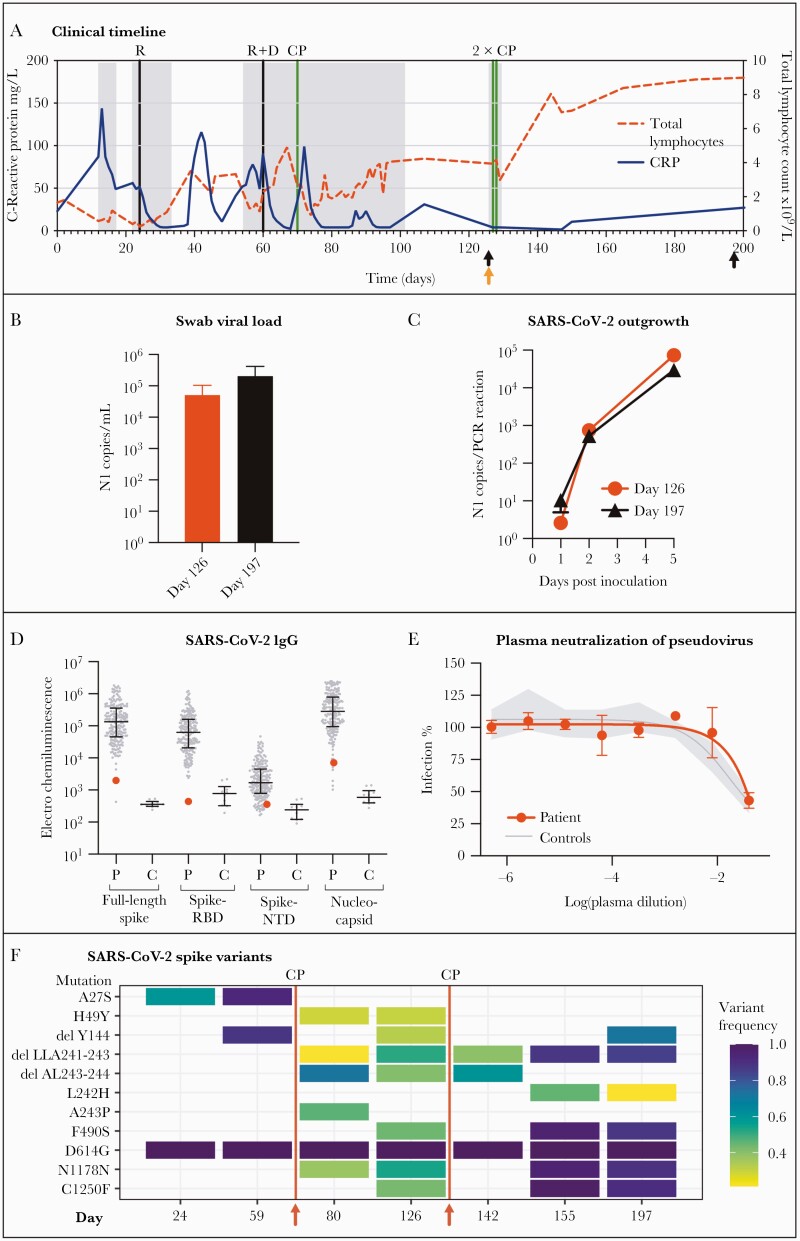
In May 2020, an asymptomatic 75-year-old Caucasian male with a history of CLL since 2011 tested SARS-CoV-2 positive by reverse-transcriptase polymerase chain reaction (RT-PCR) analysis of a pharyngeal swab specimen (day 0) (Figure 1A) as part of a routine screening. A timeline of the clinical history is depicted in Figure 1A, indicating timepoints for sampling of pharyngeal swabs for viral outgrowth (Figure 1A, black arrows) and blood samples for immunological analysis (Figure 1A, orange arrows).
Figure 1.
(A) Timeline depicting the clinical parameters from time of diagnosis (day 0) until day 200. Fluctuations in total lymphocyte counts (×109/L) and C-reactive protein (mg/L) are shown as dotted red and blue lines, respectively. Hospital admissions are shown with gray shaded areas. Initiation of treatment administration is indicated by vertical lines in black for remdesivir (R) and dexamethasone (D) and in green for convalescent plasma (CP). Pharyngeal swabs for viral outgrowth was sampled on day 126 and 197 postdiagnosis (black arrows), whereas blood samples for immunologic and serological assessments were sampled on day 126 (orange arrow). (B) Pharyngeal swab samples taken on day 126 and 197 postdiagnosis was used for ribonucleic acid (RNA) extraction and subsequent droplet digital polymerase chain reaction (ddPCR) for quantification of viral load. (C) Vero E6/TMPRSS2 cells were inoculated with swab transport media and subsequently assessed for viral outgrowth. Input swab material was washed away after 24 hours of inoculation, after which cell supernatant containing viral outgrowth was collected and used for RNA extraction and subsequent ddPCR, with viral copies increasing over a 5-day time period. (D) Meso Scale analysis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific immunoglobulin G (IgG) towards full-length spike, the spike receptor-binding domain (RBD), N-terminal domain (NTD), and nucleocapsid. “P”: The case patient antibody titers (large red dot) in the context of a cohort of 203 recovered SARS-CoV-2-infected individuals. “C”: Antibody levels of 10 prepandemic controls for comparison. (E) Plasma antibody SARS-CoV-2 neutralizing capacity was analyzed by a SARS-CoV-2 spike pseudovirus neutralization assay. Case patient plasma (red) is shown in the context of the 95% confidence interval (gray shaded area) of 10 prepandemic controls (Controls). (F) Illumina sequencing of SARS-CoV-2 RNA from clinical samples taken at day 24 (tracheal secretion), day 59 (bronchoalveolar lavage), day 80 (pharyngeal swab), day 126 (pharyngeal swab), day 142 (pharyngeal swab), day 155 (pharyngeal swab), and day 197 (pharyngeal swab). Red lines indicate timepoints between sampling days with administration of convalescent plasma (CP) (day 70, 127, and 128).
The patient previously received rituximab (5 doses administered in 2015, 3 doses administered in 2017), fludarabine and cyclophosphamide (3 doses administered in 2017), bendamustine (4 doses administered in 2015), and most recently Ibrutinib (administered daily from January 2018 to June 2020). He developed fever and shortness of breath and was admitted to hospital on day 12 with chest x-ray showing bilateral infiltrates, but he was discharged on day 17 after clinical and biochemical improvement. Recurrence of fever and respiratory symptoms resulted in readmission on day 22. Chest computed tomography scan showed progression of ground-glass opacities, and the patient was placed on ventilation in the intensive care unit (ICU). A 10-day remdesivir treatment was initiated on day 24 while ibrutinib was paused, after which he was discharged in recovery on day 33. After recurrence of respiratory symptoms, the patient was readmitted on day 54 and subsequently transferred to the ICU on day 60 for high-flow oxygen, dexamethasone, and a 5-day remdesivir treatment. Despite initial improvement in clinical parameters and inflammatory biomarkers, the patient received convalescent plasma (CP) (2 × 300 mL, both with an immunoglobulin [Ig]G ratio of 4.46 measured by semiquantitative enzyme-linked immunosorbent assay from EUROIMMUN [10]) on day 70, due to relapse in fever. After recovery to an asymptomatic state, the patient was discharged for home isolation on day 101, despite being continuously RT-PCR positive. In an attempt to eradicate this asymptomatic, viral carrier-state, the patient was readmitted for a double dose of CP on days 127–128 (Day 127: 2 × 300 mL, with an IgG ratio of 8.24; Day 128: 1 × 300 mL with an IgG ratio of 4.72 and 1 × 300 mL with an IgG ratio of 5.44). Virus eradication is thus far unsuccessful, because weekly performed RT-PCR tests remain positive for SARS-CoV-2, with the last sample acquired on day 333 just before submission of this report.
Additional antibiotic treatment is depicted in Supplementary Figure S1. Data were obtained from electronic patient records.
Severe Acute Respiratory Syndrome Coronavirus 2 Outgrowth from Pharyngeal Samples for Assessment of In Vitro Infectivity
Pharyngeal swab samples were taken on day 126 and 197, during asymptomatic home isolation, and collected in transport media optimized for viral outgrowth. Ribonucleic acid (RNA) was extracted and viral load was quantified using droplet digital PCR (ddPCR). Furthermore, Vero E6/TMPRSS2 cells were inoculated with swab transport media and subsequently assessed for viral outgrowth. Input swab material was washed away after 24 hours of inoculation, after which cell supernatant containing viral outgrowth was collected and used for RNA extraction and subsequent ddPCR against the nucleocapsid (N1) gene. Both sampling timepoints for viral outgrowth showed high SARS-CoV-2 RNA titers with viral N1 copies/mL above 5 × 104 and 2 × 105 for day 126 and 197, respectively (Figure 1B). Consequently, SARS-CoV-2 outgrowth was evident with viral loads in cell supernatant rapidly increasing between day 1 and 5 postinoculation, indicating presence of SARS-CoV-2 replication (Figure 1C).
Severe Acute Respiratory Syndrome Coronavirus 2-Specific Serological Response
Serological response was analyzed in serum and plasma samples taken on day 126 postdiagnosis. The SARS-CoV-2-specific IgG antibodies towards full-length spike, spike receptor-binding domain (RBD), N-terminal domain (NTD), and nucleocapsid were measured in serum with a multiplex Meso Scale Diagnostics (MSD) assay, detailed in Supplementary Methods and Results. Serum was diluted 1:4630 and incubated for 2 hours for antibody capture by SARS-CoV-2 antigens before detection with a SULFO-TAG Anti-Human IgG antibody.
Patient’s antibody titers (red) are shown in the context of a cohort comprising 203 convalescent COVID-19 patients (Figure 1D) representing a broad disease severity spectrum [11]. The patient’s SARS-CoV-2-specific antibody response was more equivalent to that of 10 healthy prepandemic controls collected in 2015, compared with convalescent COVID-19 patients [12].
Presence of SARS-CoV-2 neutralizing antibodies in patient plasma taken on day 126 was further assessed through SARS-CoV-2 spike pseudovirus neutralization. Five-fold dilutions of patient plasma were incubated with SARS-CoV-2 spike expressing pseudovirus, carrying an eGFP reporter gene. Infection of Vero E6/TMPRSS2 cells was determined by flow cytometry as eGFP expression. The patient’s plasma-neutralizing capacity was within the 95% confidence interval of 10 healthy prepandemic controls and thus unable to neutralize SARS-CoV-2 spike (Figure 1E).
Flow Cytometry Assessment of Cellular Immunodeficiency
Flow cytometry analyses of lymphocyte populations were performed on day 126, as described in Supplementary Methods and Results. The patient had a total CD3+ lymphocyte count within the standard reference range (Supplementary Figure S2) and an essentially normal T-cell subset distribution (Supplementary Table S1), albeit with few naive and slightly elevated numbers of memory T cells. Total natural killer cell counts were also within the standard reference range. The absolute CD19+ B lymphocyte count was approximately 10-fold above upper normal limit, dominated by B cells of a marginal-zone-like phenotype (IgD+CD27+CD38−) consistent with his known B cell malignancy.
Longitudinal Assessment of Viral Variants by Severe Acute Respiratory Syndrome Coronavirus 2 Sequencing
Clinical samples from day 24 (tracheal secretion), day 59 (bronchoalveolar lavage), day 80 (pharyngeal swab), day 126 (pharyngeal swab), day 142 (pharyngeal swab), day 155 (pharyngeal swab), and day 197 (pharyngeal swab) found RT-PCR positive for SARS-CoV-2, was used for RNA extraction and Illumina sequencing, and analyzed as detailed in Supplementary Methods and Results.
Observed mutations of the spike protein coding RNA with an allele frequency above 0.2 is shown in Figure 1F. Mutations accumulated increasingly after first administration of CP on day 70 and generally increased in frequency hereafter. The allele frequency of D614G was 1.0 in all samples, indicating this as a parent mutation. Analysis of the RNA-dependent RNA polymerase (RdRp) gene is shown in Supplementary Figure S3. Minor changes were observed over the sampling timeline, with exception of a single high-frequency variant (ORF1b:D31Y) appearing after the second remdesivir administration, which remained present throughout the subsequent samplings. All pan genome changes are showed in Supplementary Figure S4.
Patient Consent Statement
Verbal and written consent was obtained from the patient allowing analyses of samples and publication of the report. This study has been approved by The Central Denmark Region Committees on Health Research Ethics.
DISCUSSION
In this study, we report a case of SARS-CoV-2 infection persisting for 333 days in an immunocompromised individual. Infectious virus was readily detected at both 126 and 197 days after diagnosis during asymptomatic recovery from COVID-19-related symptoms. Sequencing analysis revealed accumulation of spike mutations over the course of 197 days of infection resulting in appearance of new SARS-CoV-2 spike variants within this individual.
The serological profile showed limited to no antibody response towards SARS-CoV-2, thus partly attributing the inadequate viral clearance to a defective humoral response as a consequence of the patient’s CLL, probably caused by a dynamic combination of disease- and treatment-related immune suppression and dysregulation after treatment with chemotherapy, tyrosine kinase inhibitor, and rituximab [13–15].
The high viral titer combined with the observed outgrowth profile from pharyngeal samples taken up to day 197 indicates that a severely immunocompromised person continuously testing positive for SARS-CoV-2, although asymptomatic, should be considered at risk of continuously carrying infectious virus, rendering isolation necessary.
With increasing risk of accumulating mutations in the viral genome over prolonged periods of infection, SARS-CoV-2 variants are likely to appear during this carrier state. The majority of the mutations appeared after the first CP treatment, probably selected from the pool of low-frequency variants generated during this long-standing infection, favoring their subsequent frequency increase. The mutational pattern selected in this case (deletion of amino acids Y144 and LLA241-243 in the NTD, and a F490S mutation in the RBD) resembles the patterns in variants like B.1.1.7 and B.1.351, which display enhanced transmission properties and increased resistance towards convalescent plasma and vaccinee sera [8, 16–18]. Specifically, the deletion of amino acid Y144 in the NTD and mutations of F490 within the RBD have previously been associated with increased resistance to neutralizing monoclonal antibodies [8,17–19]. The observed high-frequency RdRp variant (ORF1b:D31Y) shown in Supplementary Figure S3 is not currently well described in the literature. It is important to note that beneficial effects of CP therapy on SARS-CoV-2 infections have been reported in patients with hematological disorders and impaired humoral immunity [20].
CONCLUSIONS
The findings of our study add evidence to the growing body of literature supporting a test-based strategy for discontinuation of isolation for immunocompromised individuals. Our results highlight the importance of further investigations into both the nature of the long-term carrier state and the viral variants resulting from persistent infections with viable SARS-CoV-2 in immunocompromised individuals and, more importantly, the development of successful strategies to enable viral eradication.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the case patient for kindly donating sample material and participating in this study. The VSV-eGFP pseudovirus reporter system and the Vero E6/TMPRSS2 cells was kindly obtained through Dr. Stefan Pöhlmann, Head of the Infection Biology Unit, German Primate Center. Göttingen, Germany
Author contributions. I. M. and S. R. S. contributed equally to this manuscript. I. M., M. S., M. T., and L. K. V. contributed to the conception and design of the study. S. R. S., K. L. M., M. S., I. M., and L. K. V. contributed to patient inclusion and collection of patient data. I. M., S. S. F. N., L. Ø. P., M. S. P., C. M. K., I. H. T., M. H. S., M. T., and L. K. V. contributed to performance of experiments, data analysis, and interpretation. I. M. drafted the article with contributions from S. R. S. and L. K. V. All authors performed critical revision and approval of the manuscript.
Financial support. This work was funded by a research grant from RegionMidt/Aarhus University Hospital and Aarhus University (to M. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020; 584:115–9. [DOI] [PubMed] [Google Scholar]
- 2. Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584:457–62. [DOI] [PubMed] [Google Scholar]
- 3. Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. ; Karolinska COVID-19 Study Group . Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020; 183:158–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Discontinuation of isolation for persons with COVID-19 not in healthcare settings. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html. Accessed 4 February 2021.
- 5. Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020; 183:1901–12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383:2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kemp SA, Collier DA, Datir RP, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021; 592:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021; 593:130–5. [DOI] [PubMed] [Google Scholar]
- 9. Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021; 27:622–5. [DOI] [PubMed] [Google Scholar]
- 10. Beavis KG, Matushek SM, Abeleda APF, et al. Evaluation of the EUROIMMUN anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J Clin Virol 2020; 129:104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vibholm LK, Nielsen SSF, Pahus MH, et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine 2021; 64:103230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielsen SS, Vibholm LK, Monrad I, et al. SARS-CoV-2 elicits robust adaptive immune responses regardless of disease severity. EBioMedicine 2021; 68:103410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arruga F, Gyau BB, Iannello A, et al. Immune response dysfunction in chronic lymphocytic leukemia: dissecting molecular mechanisms and microenvironmental conditions. Int J Mol Sci 2020; 21:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood 2015; 126:573–81. [DOI] [PubMed] [Google Scholar]
- 15. Riches JC, Ramsay AG, Gribben JG. Immune reconstitution in chronic lymphocytic leukemia. Curr Hematol Malig Rep 2012; 7:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meng B, Kemp SA, Papa G, et al. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the variant of concern lineage B.1.1.7. Cell Rep 2021; 109292. doi: 10.1016/j.celrep.2021.109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mccallum M, De Marco A, Lempp FA, et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 2021; 184:2332–47.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCarthy KR, Rennick LJ, Nambulli S, et al. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 2021; 371:1139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies 1 by SARS-CoV-2 spike protein variants. Elife 2020; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 2020; 136:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.