Abstract
Evidence links the liver to development of colorectal cancer (CRC). However, it remains unknown how liver function may influence CRC risk in the general population. We conducted a prospective cohort study in the UK Biobank of 375 693 participants who provided blood samples in 2006 to 2010. Circulating levels of liver function markers (alanine transaminase [ALT], aspartate transaminase [AST], total bilirubin [TBIL], gamma glutamyltransferase [GGT], alkaline phosphatase [ALP], total protein [TP] and albumin [ALB]) were measured. Incident cancer cases were identified through linkage to the national cancer registry up to 2019. Repeated biomarker measurements were available from a subset of 11 320 participants who were re-assessed in 2012 to 2013. After a median follow-up of 10.0 years, we documented 2662 cases of CRC. Circulating levels of ALT, AST, TBIL, GGT, TP and ALB at baseline were inversely associated with CRC risk (P < .01), with multivariable hazard ratio (95% confidence interval) comparing decile 10 vs 1 of 0.62 (0.51-0.75), 0.63 (0.53-0.75), 0.85 (0.72-1.02), 0.74 (0.61-0.89), 0.70 (0.59-0.84) and 0.66 (0.55-0.79), respectively. Strengthened associations were found after recalibration for repeated measurements. The associations appeared stronger for proximal colon cancer than distal colon cancer and rectal cancer, but consistent for early-, mid- and late-onset CRC. In a large cohort of general population, the UK Biobank, higher circulating levels of ALT, AST, TBIL, GGT, TP and ALB, largely within the normal range, were associated with a lower risk of CRC. The findings support a link between liver function and CRC, and may spur future research on the gut-microbiota-liver axis.
Keywords: gut microbiota, liver metastasis, liver panel, metabolic capacity
1 |. INTRODUCTION
Globally, colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death.1 Several lines of evidence have indicated a role of liver function in the development of CRC. First, a majority of CRC deaths are related to distant spread into the liver.2 Second, several large-scale studies have linked chronic liver diseases, such as nonalcoholic fatty liver disease, to higher risk of CRC.3 Third, recent studies have shown a potential relationship between liver-derived metabolites (eg, bile acids, bilirubin, and glutamate) and CRC risk, potentially mediated through the gut bacterial actions.4–8 However, despite these data, it remains unknown how liver function may influence CRC risk in the general population, and direct evidence regarding the relationship of liver function markers with CRC risk remains limited and inconclusive.
Routine circulating liver function assays include alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL), gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), total protein (TP) and albumin (ALB). These markers have been known to reflect the liver metabolism, biosynthesis and detoxification capability, as well as the immune and nutritional status of the body.9 However, systematic evaluation of liver function markers in relation to CRC risk remains lacking. Discrepant findings have been reported in prior studies that examined a few selected liver function markers only in relation to CRC risk.10–19 Moreover, these studies are limited by the relatively small sample size (most with <500 CRC cases) and inadequate control for confounding by general and visceral adiposity and lifestyle factors. In addition, none of these studies have yet examined the associations according to tumor subsites, despite the evidence that the gut-microbiota-liver axis may be of particular importance to proximal colon cancers compared to distal CRCs.20,21
Therefore, to extend our knowledge, we comprehensively examined the relationship with CRC risk of circulating liver function markers, including ALT, AST, TBIL, GGT, ALP, TP, and ALB, in the United Kingdom (UK) Biobank, a large prospective cohort. In addition to overall CRC, we performed separate analyses for proximal colon, distal colon and rectal cancers.
2 |. METHODS
2.1 |. Study participants
The UK Biobank is a prospective cohort study aiming to investigate the genetic, lifestyle and environmental causes of a range of diseases.22 Between 2006 and 2010, 502 536 adults aged between 37 and 73 years were recruited in 22 assessment centers throughout the United Kingdom. All participants were registered with the UK National Health Service (NHS). At the baseline recruitment visit, participants completed a self-administered touchscreen questionnaire on sociodemographics, lifestyle exposures, medical history and medication use, and underwent physical measurements, including body weight, height, waist and hip circumference. Blood samples were collected from all participants at recruitment and from a subset of ~18 000 participants with a repeat visit to the assessment center between 2012 and 2013. The current study was conducted under the UK Biobank application number 46466.
We excluded participants with missing data on any of the seven liver function markers (n = 75 359) and with prevalent cancer at recruitment (n = 38 998); those who withdrew informed consents (n = 18); and those who were indicated as outliers (n = 12 468) based on the extreme Studentized Deviate Many-Outlier procedure.23 Therefore, a total of 375 693 participants were included in our analysis (see the flow chart in Supplementary Figure 1).
2.2 |. Blood collection and laboratory methods
As part of the UK Biobank Biomarker Project,24 circulating levels of ALT, AST, GGT and ALP were determined using the enzymatic rate method (all Beckman Coulter AU5800); circulating levels of TBIL, TP and ALB using the colorimetric method; and high sensitivity C-reactive protein (CRP) levels using the immunoturbidimetric method. Full details of the assay performance have been published.24 In summary, the average within-laboratory coefficient of variation (CV) in quality-control samples ranged between 1.2%-2.9% for ALT, 1.3%-2.1% for AST, 1.5%-1.9% for TBIL, 1.4%-2.8% for GGT, 2.8%-3.1% for ALP, 1.1%-1.2% for TP, 2.1%-2.2% for ALB and 1.7%-2.3% for CRP.
2.3 |. Assessment of outcome
Incident cancer cases and deaths within the UK Biobank were identified through linkage to national cancer and national death registries. Cancer incidence data were coded using the 10th Revision of the International Classification of Diseases (ICD-10). Proximal colon cancers included those found in the cecum, appendix, ascending colon, hepatic flexure, transverse colon and splenic flexure (C18.0-18.5); distal colon cancers in the descending (C18.6) and sigmoid (C18.7) colon; and rectal cancer in the rectosigmoid junction (C19) and rectum (C20).
2.4 |. Statistical analysis
All participants were followed from the date of recruitment until that of CRC diagnosis, death, loss to follow-up or the end of the study period (28 February 2019), whichever occurred first. Nine hundred sixty-four participants were lost to follow-up due to emigration or indication by records of NHS and UK Biobank, and thus 99.7% completed the study. We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazards regression, with age as the timescale. Model 1 was adjusted for age at recruitment, sex, ethnicity and fasting status. Model 2 was further adjusted for a set of a priori-determined CRC risk factors, namely socioeconomic status (Townsend deprivation score), education level, total physical activity, body mass index (BMI), waist/hip ratio, height, smoking status and intensity, alcohol status and consumption frequency, frequency of red and processed meat consumption, frequency of oily fish consumption, family history of cancer, regular aspirin use, bowel cancer screen, overall health status, and serum levels of CRP. The proportional hazards assumption was tested using Schoenfeld residuals and no evidence of nonproportionality was detected.
We calculated intraclass correlation coefficients (ICCs) to assess the reproducibility in circulating levels of liver function markers among participants with repeated measurements after excluding those who developed cancer between the two repeated assessments (n = 11 320). We then used the ICCs to recalibrate the multivariable HR estimates for regression dilution.25,26 To assess the linearity in the associations of circulating liver function markers with CRC risk, we performed restricted cubic spline analysis with four knots and calculated the likelihood ratio test by comparing the model with only the linear term of these markers to the model with both the linear and the cubic spline terms.
We performed subgroup analyses according to anatomical subsite (proximal colon, distal colon and rectal cancer) and age of onset (early- [<50 years], mid- [50-60], and late- [≥60] onset CRC). To test whether the exposure-disease association differs among CRC subtypes, we calculated the P for heterogeneity across subtypes using the contrast test method based on a fully unconstrained approach in which the covariates’ effects are allowed to vary according to subtypes. The method was developed in the competing risks framework using cause-specific proportional hazards model.27
We also conducted stratified analyses according to age at recruitment, sex, BMI, smoking status and alcohol consumption. We calculated the P for interaction using the likelihood ratio test for the product terms between the stratified variables (categorical) and circulating levels of liver function markers (continuous).
Sensitivity analyses were performed after excluding the first 2 years of follow-up, excluding participants with abnormally low or high biomarker levels, and excluding participants with certain comorbidities at recruitment, including inflammatory bowel disease (IBD), hepatitis and other liver disease and hepatobiliary disease, cardiovascular disease and diabetes. To assess the influence of residual confounding, we conducted a sensitivity analysis by adjusting for additional lifestyle factors, including raw and cooked vegetable intake, fresh and dried fruit intake, frequency of poultry consumption, coffee intake, vitamin supplements, and mineral and other dietary supplements.
SAS 9.4 was used for all analyses (SAS Institute, Cary, NC). All statistical tests were two-sided, and P < .05 was defined as statistically significant. All authors had access to the study data, reviewed and approved the final manuscript.
3 |. RESULTS
After a median follow-up of 10.0 years, we documented 2662 cases of CRC (1535 in men and 1127 in women) with a mean age of 61 (SD, 7) years (Table 1). Compared to those without incident CRC, participants with incident CRC were older and taller; had higher BMI and less physical activity; were more likely to smoke and consume red meat; and were less likely to have college / university education and CRC screening and use aspirin (Table 1). Circulating levels of liver function markers were largely within the normal range for participants with and without CRC, and the percentage of participants with normal levels was higher than 85% for all biomarkers (Supplementary Table 1). The biomarkers were weakly correlated with each other, except for ALT and AST (r = 0.69) and ALT and GGT (r = 0.58) (Table 2).
TABLE 1.
Age-standardized characteristics of study participants at baselinea
| Total cohort (n = 375 693) | Participants without colorectal cancer (n = 373 031) | Participants with incident colorectal cancer (n = 2662) | |
|---|---|---|---|
| Age at recruitment (years) | 56.3 (8.1) | 56.2 (8.1) | 60.8 (6.5) |
| Male sex (%) | 46.5 | 46.5 | 46.5 |
| Socioeconomic status (Townsend deprivation index) | −1.3 (3.1) | −1.3 (3.1) | −1.3 (3.2) |
| Height (cm) | 168.6 (9.3) | 168.6 (9.3) | 168.7 (9.1) |
| Body mass index (kg/m2) | 27.4 (4.8) | 27.4 (4.8) | 27.6 (4.7) |
| Waist/hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) |
| Total physical activity (MET hours per week) | 44.5 (45.4) | 44.5 (45.4) | 43.5 (44.8) |
| White race (%) | 93.9 | 93.9 | 94.7 |
| College/university education (%) | 32.7 | 32.8 | 30.1 |
| Smoking status (%) | |||
| Never | 55.0 | 55.0 | 51.8 |
| Previous | 34.0 | 34.0 | 37.0 |
| Current | 10.5 | 10.5 | 10.6 |
| Unknown | 0.5 | 0.5 | 0.6 |
| Alcohol consumption frequency (%) | |||
| None | 8.0 | 8.0 | 8.1 |
| Special occasions only | 11.4 | 11.4 | 10.8 |
| One to three times a month | 11.2 | 11.2 | 10.8 |
| Once or twice a week | 26.0 | 26.0 | 25.6 |
| Three or four times a week | 23.2 | 23.2 | 22.5 |
| Daily or almost daily | 20.0 | 20.0 | 22.8 |
| Unknown | 0.2 | 0.2 | 0.2 |
| Family history of cancer (%) | 34.5 | 34.5 | 38.1 |
| Red and processed meat (%) | |||
| Never | 9.3 | 9.3 | 7.7 |
| <1 occasion per week | 30.2 | 30.2 | 29.0 |
| =1 occasion per week | 29.0 | 29.0 | 28.4 |
| 2–4 occasions per week | 27.1 | 27.1 | 30.4 |
| 5–6 occasions per week | 3.2 | 3.2 | 3.0 |
| ≥7 occasions per week | 0.8 | 0.8 | 1.3 |
| Unknown | 0.4 | 0.4 | 0.2 |
| Regular aspirin use (%) | 13.7 | 13.7 | 13.1 |
| Colorectal cancer screening (%) | 29.9 | 29.9 | 26.3 |
| Fasting status (%) | |||
| <8 hours | 95.8 | 95.8 | 95.6 |
| ≥8 hours | 4.2 | 4.2 | 4.4 |
| Alanine transaminase (ALT; U/L) | 22.6 (10.7) | 22.6 (10.7) | 21.9 (10.7) |
| Aspartate transaminase (AST; U/L) | 25.4 (6.7) | 25.4 (6.7) | 24.9 (6.8) |
| Total bilirubin (TBIL; μmol/L) | 9.0 (3.9) | 9.0 (3.9) | 8.9 (4.0) |
| Gamma glutamyltransferase (GGT; U/L) | 33.3 (22.8) | 33.3 (22.8) | 33.6 (24.1) |
| Alkaline phosphatase (ALP; U/L) | 82.3 (22.3) | 82.3 (22.3) | 83.6 (22.9) |
| Total protein (TP; g/L) | 72.5 (4.1) | 72.5 (4.1) | 72.1 (4.0) |
| Albumin (ALB; g/L) | 45.2 (2.6) | 45.2 (2.6) | 45.0 (2.6) |
| C-reactive protein (CRP; mg/L) | 2.5 (4.2) | 2.5 (4.2) | 2.7 (4.3) |
Abbreviations: MET, metabolic equivalents.
Mean (SD) values and percentages are reported for continuous and categorical variables, respectively. All variables are age-standardized except age itself.
TABLE 2.
Age-adjusted Spearman correlation coefficientsa
| ALT | AST | TBIL | GGT | ALP | TP | ALB | BMI | Waist/hip ratio | CRP | Age | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALT | 1 | 0.69b | 0.12b | 0.58b | 0.16b | 0.13b | 0.15b | 0.33b | 0.43b | 0.14b | 0.02b |
| AST | 1 | 0.16b | 0.40b | 0.12b | 0.19b | 0.17b | 0.12b | 0.24b | 0.03b | 0.12b | |
| TBIL | 1 | 0.09b | −0.12b | 0.08b | 0.21b | −0.06b | 0.13b | −0.18b | 0.01c | ||
| GGT | 1 | 0.19b | 0.16b | 0.13b | 0.32b | 0.45b | 0.26b | 0.10b | |||
| ALP | 1 | 0.12b | −0.01b | 0.16b | 0.07b | 0.27b | 0.18b | ||||
| TP | 1 | 0.47b | 0.03b | 0.05b | 0.09b | −0.07b | |||||
| ALB | 1 | −0.12b | 0.04b | −0.19b | −0.14b | ||||||
| BMI | 1 | 0.47b | 0.43b | 0.07b | |||||||
| Waist/hip ratio | 1 | 0.21b | 0.15b | ||||||||
| CRP | 1 | 0.12b | |||||||||
| Age | 1 | ||||||||||
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein; GGT, gamma glutamyltransferase; TBIL, total bilirubin; TP, total protein.
All correlation coefficients are age adjusted except for those with age itself.
P < .001.
P < .01.
Circulating levels of ALT, AST, TBIL, GGT, TP and ALB were inversely associated with CRC risk (P < .01 for all markers). The multivariable HR (95% CI) comparing participants in decile 10 vs 1 was 0.62 (0.51-0.75) for ALT, 0.63 (0.53-0.75) for AST, 0.85 (0.72-1.02) for TBIL, 0.74 (0.61-0.89) for GGT, 0.70 (0.59-0.84) for TP and 0.66 (0.55-0.79) for ALB (Table 3). The restricted cubic spline analysis showed a statistically significant nonlinearity for the relationship of ALT, AST, TBIL and GGT with CRC risk (P for nonlinearity<.05) (Figure 1). The HR decreased at a lower rate at approximately 20 U/L for ALT and reached a plateau at approximately 30 U/L for AST, 10 μmol/L for TBIL and 30 U/L for GGT (Figure 1).
TABLE 3.
Hazard ratios (95% confidence intervals) of colorectal cancer associated with circulating levels of liver function markers in the UK Biobank
| Decile 1 | Decile 2 | Decile 3 | Decile 4 | Decile 5 | Decile 6 | Decile 7 | Decile 8 | Decile 9 | Decile 10 | P for overall significance | P for nonlinear relation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alanine transaminase (ALT) | ||||||||||||
| Median, range | 11 (3-12) | 13 (12-14) | 15 (14-16) | 17 (16-18) | 19 (18-20) | 21 (20-22) | 24 (22-25) | 27 (25-29) | 32 (29-36) | 44 (36-82) | ||
| CRC cases | 238 | 280 | 265 | 285 | 262 | 237 | 310 | 301 | 257 | 227 | ||
| Person-years | 369 535 | 369 684 | 372 999 | 369 511 | 371 519 | 371 265 | 371 230 | 370 421 | 372 275 | 372 948 | ||
| HR (95% CI)a | 1 (referent) | 1.04 (0.87-1.23) | 0.88 (0.74-1.05) | 0.90 (0.76-1.07) | 0.79 (0.66-0.94) | 0.70 (0.58-0.83) | 0.89 (0.75-1.06) | 0.86 (0.72-1.02) | 0.76 (0.64-0.91) | 0.73 (0.60-0.88) | <.001 | .01 |
| HR (95% CI)b | 1 (referent) | 1.03 (0.87-1.23) | 0.87 (0.73-1.03) | 0.87 (0.73-1.04) | 0.75 (0.63-0.90) | 0.65 (0.54-0.78) | 0.82 (0.69-0.98) | 0.77 (0.65-0.92) | 0.67 (0.56-0.81) | 0.62 (0.51-0.75) | <.001 | .005 |
| HR (95% CI)c | 1 (referent) | 1.06 (0.76-1.49) | 0.75 (0.53-1.06) | 0.76 (0.54-1.07) | 0.57 (0.40-0.81) | 0.43 (0.30-0.62) | 0.68 (0.48-0.95) | 0.60 (0.43-0.85) | 0.46 (0.32-0.65) | 0.39 (0.27-0.57) | ||
| Aspartate transaminase (AST) | ||||||||||||
| Median, range | 17 (3-18) | 19 (18-20) | 21 (20-22) | 22 (22-23) | 24 (23-24) | 25 (24-26) | 27 (26-27) | 29 (28-30) | 31 (30-34) | 38 (34-62) | ||
| CRC cases | 252 | 262 | 243 | 292 | 282 | 222 | 317 | 293 | 251 | 248 | ||
| Person-years | 375 686 | 372 399 | 362 181 | 389 996 | 370 511 | 352 723 | 384 094 | 367 621 | 364 257 | 371 919 | ||
| HR (95% CI)a | 1 (referent) | 0.87 (0.74-1.04) | 0.77 (0.65-0.92) | 0.81 (0.69-0.96) | 0.79 (0.66-0.93) | 0.62 (0.52-0.75) | 0.81 (0.68-0.95) | 0.77 (0.65-0.91) | 0.66 (0.55-0.79) | 0.64 (0.54-0.77) | <.001 | .001 |
| HR (95% CI)b | 1 (referent) | 0.88 (0.74-1.05) | 0.78 (0.65-0.93) | 0.82 (0.69-0.98) | 0.80 (0.67-0.95) | 0.63 (0.53-0.76) | 0.82 (0.69-0.96) | 0.77 (0.65-0.92) | 0.66 (0.55-0.79) | 0.63 (0.53-0.75) | <.001 | .006 |
| HR (95% CI)c | 1 (referent) | 0.78 (0.54-1.11) | 0.60 (0.42-0.86) | 0.67 (0.47-0.95) | 0.63 (0.45-0.90) | 0.39 (0.27-0.57) | 0.66 (0.47-0.93) | 0.59 (0.41-0.83) | 0.43 (0.30-0.61) | 0.39 (0.27-0.56) | ||
| Total bilirubin (TBIL) | ||||||||||||
| Median, range | 5 (1-5) | 6 (5-6) | 6 (6-7) | 7 (7-7) | 8 (7-8) | 8 (8-9) | 9 (9-10) | 10 (10-11) | 12 (11-14) | 17 (14-29) | ||
| CRC cases | 262 | 271 | 260 | 277 | 252 | 249 | 244 | 292 | 275 | 280 | ||
| Person-years | 373 312 | 369 845 | 370 116 | 372 260 | 371 937 | 370 067 | 372 012 | 370 986 | 371 003 | 369 849 | ||
| HR (95% CI)a | 1 (referent) | 0.94 (0.80-1.12) | 0.87 (0.73-1.03) | 0.88 (0.75-1.05) | 0.78 (0.66-0.93) | 0.76 (0.64-0.90) | 0.72 (0.60-0.86) | 0.84 (0.71-1.00) | 0.78 (0.66-0.93) | 0.81 (0.68-0.96) | <.001 | <.001 |
| HR (95% CI)b | 1 (referent) | 0.95 (0.80-1.13) | 0.88 (0.74-1.05) | 0.90 (0.76-1.07) | 0.80 (0.67-0.96) | 0.78 (0.66-0.93) | 0.74 (0.62-0.89) | 0.88 (0.74-1.04) | 0.82 (0.68-0.97) | 0.85 (0.72-1.02) | .009 | .005 |
| HR (95% CI)c | 1 (referent) | 0.94 (0.74-1.18) | 0.84 (0.67-1.06) | 0.87 (0.69-1.10) | 0.74 (0.58-0.94) | 0.72 (0.56-0.91) | 0.67 (0.52-0.85) | 0.83 (0.66-1.06) | 0.76 (0.60-0.96) | 0.81 (0.64-1.02) | ||
| Gamma glutamyltransferase (GGT) | ||||||||||||
| Median, range | 13 (5-14) | 16 (15-17) | 18 (17-20) | 21 (20-23) | 24 (23-26) | 28 (26-30) | 33 (30-36) | 40 (36-45) | 51 (45-61) | 81 (62-156) | ||
| CRC cases | 210 | 230 | 237 | 265 | 263 | 283 | 270 | 276 | 311 | 317 | ||
| Person-years | 373 437 | 371 351 | 367 445 | 376 377 | 375 704 | 370 005 | 365 199 | 372 628 | 369 916 | 369 324 | ||
| HR (95% CI)a | 1 (referent) | 0.90 (0.75-1.09) | 0.83 (0.69-1.00) | 0.84 (0.70-1.01) | 0.80 (0.66-0.96) | 0.84 (0.70-1.01) | 0.80 (0.66-0.96) | 0.78 (0.65-0.94) | 0.89 (0.74-1.07) | 0.92 (0.77-1.10) | .02 | .01 |
| HR (95% CI)b | 1 (referent) | 0.88 (0.73-1.06) | 0.79 (0.65-0.95) | 0.77 (0.64-0.93) | 0.72 (0.60-0.86) | 0.73 (0.61-0.88) | 0.68 (0.57-0.83) | 0.66 (0.54-0.80) | 0.73 (0.61-0.88) | 0.74 (0.61-0.89) | <.001 | <.001 |
| HR (95% CI)c | 1 (referent) | 0.81 (0.60-1.10) | 0.68 (0.50-0.92) | 0.66 (0.49-0.89) | 0.58 (0.43-0.79) | 0.60 (0.45-0.82) | 0.54 (0.40-0.73) | 0.51 (0.37-0.69) | 0.60 (0.44-0.82) | 0.61 (0.45-0.83) | ||
| Alkaline phosphatase (ALP) | ||||||||||||
| Median, range | 51 (8-57) | 61 (57-64) | 67 (64-70) | 72 (70-75) | 77 (75-80) | 82 (80-85) | 88 (85-91) | 95 (91-99) | 104 (99-111) | 123 (111-203) | ||
| CRC cases | 229 | 229 | 245 | 267 | 273 | 279 | 244 | 284 | 279 | 333 | ||
| Person-years | 374 450 | 369 322 | 377 155 | 370 160 | 372 390 | 373 161 | 368 137 | 371 365 | 367 922 | 367 326 | ||
| HR (95% CI)a | 1 (referent) | 0.88 (0.73-1.06) | 0.86 (0.72-1.03) | 0.93 (0.78-1.11) | 0.91 (0.77-1.09) | 0.92 (0.77-1.10) | 0.81 (0.68-0.97) | 0.92 (0.78-1.10) | 0.92 (0.77-1.09) | 1.09 (0.92-1.29) | .13 | .24 |
| HR (95% CI)b | 1 (referent) | 0.88 (0.73-1.06) | 0.85 (0.71-1.02) | 0.92 (0.77-1.10) | 0.89 (0.75-1.07) | 0.90 (0.76-1.08) | 0.79 (0.66-0.94) | 0.89 (0.75-1.07) | 0.88 (0.74-1.05) | 1.03 (0.87-1.23) | .32 | .21 |
| HR (95% CI)c | 1 (referent) | 0.84 (0.66-1.08) | 0.81 (0.64-1.03) | 0.89 (0.71-1.13) | 0.86 (0.68-1.09) | 0.87 (0.69-1.10) | 0.73 (0.57-0.93) | 0.86 (0.68-1.09) | 0.84 (0.66-1.07) | 1.04 (0.83-1.31) | ||
| Total protein (TP) | ||||||||||||
| Median, range | 66 (52-68) | 68 (68-69) | 70 (69-70) | 71 (70-71) | 72 (71-72) | 73 (72-73) | 74 (73-74) | 75 (74-76) | 77 (76-78) | 79 (78-94) | ||
| CRC cases | 330 | 316 | 265 | 293 | 265 | 264 | 242 | 230 | 249 | 208 | ||
| Person-years | 372 915 | 373 768 | 375 479 | 372 382 | 371 427 | 372 497 | 370 049 | 369 884 | 367 311 | 365 674 | ||
| HR (95% CI)a | 1 (referent) | 0.97 (0.83-1.13) | 0.82 (0.70-0.97) | 0.93 (0.80-1.09) | 0.85 (0.72-1.00) | 0.86 (0.73-1.01) | 0.80 (0.68-0.94) | 0.77 (0.65-0.91) | 0.85 (0.72-1.00) | 0.72 (0.61-0.86) | <.001 | .56 |
| HR (95% CI)b | 1 (referent) | 0.96 (0.82-1.12) | 0.82 (0.69-0.96) | 0.92 (0.79-1.08) | 0.84 (0.71-0.99) | 0.84 (0.72-0.99) | 0.78 (0.66-0.92) | 0.75 (0.64-0.89) | 0.83 (0.70-0.98) | 0.70 (0.59-0.84) | <.001 | 0.52 |
| HR (95% CI) c | 1 (referent) | 0.92 (0.68-1.26) | 0.67 (0.48-0.92) | 0.85 (0.62-1.16) | 0.70 (0.51-0.97) | 0.71 (0.51-0.98) | 0.61 (0.44-0.85) | 0.57 (0.40-0.79) | 0.69 (0.49-0.95) | 0.50 (0.35-0.70) | ||
| Albumin (ALB) | ||||||||||||
| Median, range | 41 (32-42) | 43 (42-43) | 44 (43-44) | 44 (44-45) | 45 (45-45) | 46 (45-46) | 46 (46-47) | 47 (47-47) | 48 (47-49) | 50 (49-59) | ||
| CRC cases | 358 | 293 | 302 | 288 | 264 | 293 | 236 | 214 | 229 | 185 | ||
| Person-years | 368 321 | 372 990 | 370 112 | 374 749 | 367 819 | 373 629 | 375 104 | 368 838 | 371 268 | 368 556 | ||
| HR (95% CI)a | 1 (referent) | 0.82 (0.70-0.95) | 0.85 (0.73-0.99) | 0.82 (0.71-0.96) | 0.78 (0.67-0.92) | 0.87 (0.75-1.02) | 0.70 (0.60-0.83) | 0.67 (0.56-0.79) | 0.73 (0.62-0.86) | 0.63 (0.53-0.76) | <.001 | .87 |
| HR (95% CI)b | 1 (referent) | 0.83 (0.71-0.97) | 0.86 (0.74-1.01) | 0.84 (0.72-0.99) | 0.80 (0.68-0.94) | 0.90 (0.77-1.05) | 0.72 (0.61-0.86) | 0.69 (0.58-0.82) | 0.76 (0.64-0.89) | 0.66 (0.55-0.79) | <.001 | .84 |
| HR (95% CI)c | 1 (referent) | 0.67 (0.49-0.93) | 0.74 (0.53-1.02) | 0.70 (0.51-0.97) | 0.63 (0.45-0.88) | 0.79 (0.57-1.10) | 0.51 (0.36-0.72) | 0.46 (0.32-0.66) | 0.56 (0.39-0.79) | 0.42 (0.28-0.61) | ||
Abbreviations: CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio.
Model 1: Estimated from the Cox regression model with age as the underlying time scale and adjusted for sex, race (white, non-white, unknown), fasting status and age at recruitment.
Model 2: Further adjusted for Townsend deprivation index (continuous), waist circumference/hip circumference (continuous), height (continuous), BMI (continuous), C-reactive protein (continuous), total physical activity (quintile), alcohol status and consumption frequency (never, former, current—special occasions only, current—1-3 times per month, current—1-2 times per week, current—3-4 times per week, current—daily/almost daily, unknown), smoking status and intensity (never, former, current—<15 per day, current—≥15 per day, current—intensity unknown, unknown), frequency of red and processed meat consumption (never, <1, =1, 2-4, 5-6, ≥7 occasions per week, unknown), frequency of oily fish consumption (never, <1, =1, 2-4, 5-6, ≥7 occasions per week, unknown), family history of cancer (no, yes, unknown), educational level (college/university degree, non-college/university degree, unknown), regular aspirin use (no, yes, unknown), bowel cancer screening (no, yes, unknown) and overall health ranking (excellent, good, fair, poor, unknown).
Recalibrated multivariable estimates accounting for dilution bias using the intraclass correlation coefficients calculated in the subsample of participants with repeat measurements of circulating liver function markers.
FIGURE 1.
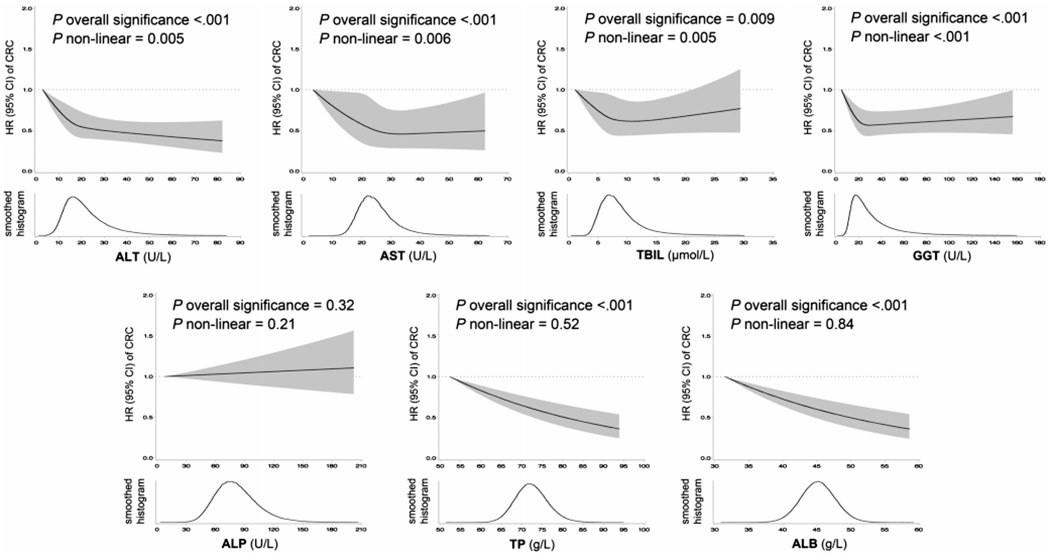
Hazard ratios (95% confidence intervals) of colorectal cancer associated with circulating levels of liver function markers and the smoothed histograms of liver function markers in the UK Biobank. The cloud represents the 95% confidence intervals of the hazard ratio. The distribution curve beneath represents the smoothed histograms of circulating liver function markers. Hazard ratios were estimated from multivariable Cox regression model adjusted for sex, race (white, non-white, unknown), fasting status, age at recruitment, Townsend deprivation index (continuous), waist circumference/hip circumference (continuous), height (continuous), body mass index (continuous), C-reactive protein (continuous), total physical activity (quintile), alcohol status and consumption frequency (never, former, current—special occasions only, current—1-3 times per month, current—1-2 times per week, current—3-4 times per week, current—daily/almost daily, unknown), smoking status and intensity (never, former, current—<15 per day, current—≥15 per day, current—intensity unknown, unknown), frequency of red and processed meat consumption (never, <1, =1, 2-4, 5-6, ≥7 occasions per week, unknown), frequency of oily fish consumption (never, <1, =1, 2-4, 5-6, ≥7 occasions per week, unknown), family history of cancer (no, yes, unknown), educational level (college/university degree, non-college/university degree, unknown), regular aspirin use (no, yes, unknown), bowel cancer screening (no, yes, unknown) and overall health ranking (excellent, good, fair, poor, unknown). Nonlinearity was modeled by cubic regression splines with 4 knots. ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CI, confidence interval; CRC, colorectal cancer; GGT, gamma glutamyltransferase; HR, hazard ratio; TBIL, total bilirubin; TP, total protein
The reproducibility (ICCs) of circulating liver function markers measured over a median of 4.4 years apart ranged between 0.48 and 0.75 (Supplementary Table 2). Recalibration for ICCs led to a stronger multivariable association between these biomarkers and CRC risk (Table 3).
When CRC cases were classified by anatomical subsites, we observed a stronger association between circulating liver function markers and risk of proximal colon cancer than distal colon cancer and rectal cancer. The HRs for ALT, AST, TBIL, GGT, TP and ALB were the lowest for proximal colon cancer in most cases (Table 4).
TABLE 4.
Hazard ratios (95% confidence intervals) of colorectal cancer according to anatomic subsites associated with circulating levels of liver function markers a
| Biomarker | Proximal colon cancer (n = 907) | Distal colon cancer (n = 791) | Rectal cancer (n = 912) | P for heterogeneity b |
|---|---|---|---|---|
| Alanine transaminase (ALT) | ||||
| HR (95% CI), decile 10 vs 1 | 0.55 (0.39-0.76) | 0.64 (0.46-0.90) | 0.65 (0.47-0.91) | .73 |
| HR (95% CI), per 1-SD increment | 0.84 (0.77-0.91) | 0.91 (0.84-0.99) | 0.87 (0.81-0.95) | .37 |
| Aspartate transaminase (AST) | ||||
| HR (95% CI), decile 10 vs 1 | 0.58 (0.42-0.80) | 0.65 (0.47-0.89) | 0.65 (0.48-0.87) | .86 |
| HR (95% CI), per 1-SD increment | 0.90 (0.83-0.96) | 0.93 (0.86-1.00) | 0.91 (0.85-0.98) | 0.84 |
| Total bilirubin (TBIL) | ||||
| HR (95% CI), decile 10 vs 1 | 0.76 (0.56-1.02) | 1.00 (0.72-1.39) | 0.89 (0.66-1.21) | .46 |
| HR (95% CI), per 1-SD increment | 0.97 (0.90-1.05) | 1.00 (0.93-1.08) | 0.98 (0.91-1.05) | .85 |
| Gamma glutamyltransferase (GGT) | ||||
| HR (95% CI), decile 10 vs 1 | 0.62 (0.45-0.87) | 0.61 (0.43-0.86) | 0.88 (0.64-1.21) | .21 |
| HR (95% CI), per 1-SD increment | 0.88 (0.81-0.94) | 0.99 (0.92-1.07) | 1.04 (0.98-1.11) | .004 |
| Alkaline phosphatase (ALP) | ||||
| HR (95% CI), decile 10 vs 1 | 1.19 (0.87-1.63) | 0.90 (0.66-1.23) | 1.05 (0.78-1.41) | .46 |
| HR (95% CI), per 1-SD increment | 1.05 (0.99-1.13) | 0.96 (0.89-1.04) | 1.02 (0.95-1.09) | .21 |
| Total protein (TP) | ||||
| HR (95% CI), decile 10 vs 1 | 0.68 (0.51-0.91) | 0.63 (0.46-0.88) | 0.82 (0.60-1.11) | .51 |
| HR (95% CI), per 1-SD increment | 0.89 (0.84-0.96) | 0.90 (0.84-0.97) | 0.93 (0.87-1.00) | .68 |
| Albumin (ALB) | ||||
| HR (95% CI), decile 10 vs 1 | 0.56 (0.40-0.78) | 0.68 (0.49-0.95) | 0.76 (0.56-1.02) | .41 |
| HR (95% CI), per 1-SD increment | 0.88 (0.82-0.94) | 0.92 (0.85-0.99) | 0.93 (0.87-0.99) | .54 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Cases with cancers in more than one site were counted multiple times. Multivariable Cox regression model with age as the underlying timescale was used and adjusted for sex, race (white, non-white, unknown), fasting status, age at recruitment, Townsend deprivation index (continuous), waist circumference/hip circumference (continuous), height (continuous), BMI (continuous), C-reactive protein (continuous), total physical activity (quintile), alcohol status and consumption frequency (never, former, current—special occasions only, current—1-3 times per month, current—1-2 times per week, current—3-4 times per week, current—daily/almost daily, unknown), smoking status and intensity (never, former, current—<15 per day, current—≥15 per day, current—intensity unknown, unknown), frequency of red and processed meat consumption (never, <1, =1, 2-4, 5-6, ≥7 occasions per week, unknown), frequency of oily fish consumption (never, <1, =1, 2-4, 5-6, ≥7 occasions per week, unknown), family history of cancer (no, yes, unknown), educational level (college/university degree, non-college/university degree, unknown), regular aspirin use (no, yes, unknown), bowel cancer screening (no, yes, unknown) and overall health ranking (excellent, good, fair, poor, unknown).
P heterogeneity for proximal colon vs distal colon vs rectal cancer was calculated using the contrast method based on a fully unconstrained approach.
No difference was found for CRC diagnosed at different age (P heterogeneity for early-, mid- and late-onset CRC >0.05) (Supplementary Table 3). The associations also appeared consistent across strata of demographic and lifestyle factors, except that ALB was inversely associated with CRC risk among never/former smokers only (P for interaction = .01) (Supplementary Table 4).
Sensitivity analyses showed robust relationships after excluding the first 2 years of follow-up, except that the inverse association between TBIL and CRC risk was attenuated to null. The results did not essentially change after excluding participants with abnormally low or high biomarker levels or participants with baseline IBD, hepatitis and other liver/hepatobiliary disease, cardiovascular disease or diabetes. The study findings were robust to adjustment for additional lifestyle factors (Supplementary Table 5).
4 |. DISCUSSION
In a large prospective cohort of the general population, circulating liver function markers (ALT, AST, GGT, TP and ALB) demonstrated an inverse association with CRC risk, with persistent associations observed after adjustment for conventional CRC risk factors and CRP. In secondary analyses, the inverse associations appeared to be stronger for proximal colon cancer than distal colon and rectal cancers. To the best of our knowledge, the current study represents the first comprehensive effort to assess liver function in relation to CRC risk. Our findings provide novel evidence that higher circulating levels of liver function markers within the normal ranges could be associated with a lower risk of CRC.
No clinical significance has yet been recognized for individuals with relatively low levels of circulating liver function markers within the normal range. Specifically, clinical practice guidelines recommend further workup when patients have elevated liver enzymes, elevated TBIL or reduced ALB, but there are no recommendations for individuals with low levels of liver enzymes and TBIL within or below the normal ranges or those with normal or abnormally high ALB or TP.9 In generally healthy individuals, lower levels of circulating liver function markers may reflect reduced functionality of the liver,28,29 as indicated by reduced liver size and cell turnover, leading to reduced synthetic and metabolic capacity and greater susceptibility to toxins.30 Our findings indicate that such reduction in the functionality of the liver, associated with lower levels of circulating liver function markers, could be associated with higher CRC risk, providing further evidence for the link between the liver and gut health.
We demonstrated that the liver function and CRC link might be particularly relevant to proximal colon cancer, consistent with previous evidence that the gut-microbiota-liver axis may be of particular importance to cancer development in the proximal colon.20,21 This may be explained by the higher concentrations of liver-derived metabolites (eg, bile acids, bilirubin) in the proximal colon due to enterohepatic cycling as well as the higher microbial abundance in the proximal colon.31,32 Bile acids play a role in the gut homeostasis and host defense by affecting the abundance and composition of the gut microbiota,33 through direct or indirect antimicrobial effects via activation of the innate immunity against enterotoxigenic and potentially carcinogenic bacteria (eg, Fusobacterium nucleatum, Escherichia coli).4,34,35 Oxidative stress and inflammation are considered as major contributing factors to carcinogenesis. Bilirubin is deconjugated by bacterial and mucosal actions in the ileum and may be antioxidative and anti-inflammatory.36,37 Another link between the liver and gut is the molecules (eg, proteins, liver function enzymes and amino acids) excreted from the liver into the systemic circulation. Among them, ALB is an antioxidant, protects the gastrointestinal mucosa,19,38 and has immunomodulatory and anti-inflammatory capacity by binding bacterial products.39 ALT, AST and GGT have antioxidative properties,40–42 contribute to amino acids balance,6,7 and ALT may have bacteriocidal activity for lipopolysaccharide-containing bacteria such as Escherichia coli.43 Inadequate liver functionality could lead to disturbances in the gut homeostasis and thus increase CRC risk.
For the first time, we demonstrated that lower levels of ALT or AST within or below the normal range were associated with higher CRC risk. An inverse association of ALT with risk of overall cancer10 or mortality has been reported in prior studies.30,44,45 The EPIC-Heidelberg case-cohort study, the only existing study that examined AST in relation to CRC risk, reported no associations.11 However, the EPIC-Heidelberg study had a small number of CRC (n = 256) and the participants had much higher levels of liver enzymes than ours, which may have explained the null findings, based on our observation that the inverse association between liver function markers and CRC weakened at the higher end of the biomarker levels.
As for GGT, previous studies reported inconsistent but mostly positive associations with overall cancer risk,11–14,46,47 and some also reported a positive association with CRC risk in men,12 in current alcohol drinkers13 and for colon cancer.14 However, participants in those studies had much higher levels of GGT and most observed a higher disease risk in the highest category only. Abnormally elevated liver enzymes primarily reflect liver injury due to viral hepatitis, other liver diseases, hepatobiliary diseases, alcohol consumption and metabolic disorders.9,48–50 Alcohol consumption and obesity are known CRC risk factors, and liver or hepatobiliary diseases had also been linked to higher CRC risk.3,21 In our study, the findings could be considered as robust to adjustment for known CRC risk factors and exclusion of participants with liver, hepatobiliary or metabolic disorders.
For TBIL, its inverse association with CRC risk was substantially attenuated after excluding the first 2 years of follow-up. This finding was in line with the evidence that CRC and other cancers may cause hyperbilirubinemia as a result of liver metastasis, biliary obstruction, and chemotherapy-induced hepatotoxicity.51 The inverse association of ALB with CRC risk was generally consistent with prior findings of its inverse association with cancer or colon cancer risk,17,19 borderline association with CRC risk,18 as well as the beneficial association with cancer mortality.52 As for circulating TP, it is composed of ALB and globulin. Both our and previous studies indicated that the inverse association between TP and CRC risk was largely due to ALB, while globin was not associated with CRC risk.19
The major strengths of our study include the prospective design and large sample size in the UK Biobank that enabled detailed examination of the dose-response relationship of various liver function markers with risk of overall and subsite-specific CRC. Moreover, biomarkers were all measured in the same laboratory using the same protocol, minimizing any potential measurement error. In addition, the repeated measurements in a subsample allowed us to assess the reproducibility and correct for dilution bias using the method developed to address “regression dilution” that generally tends to underestimate the real associations of disease rates with the “usual” levels of risk factors by using a single baseline measurement.26 Finally, we rigorously adjusted for a variety of conventional CRC risk factors and CRP levels. Our study also has some limitations. First, we lacked data on direct measurements of liver size/volume to assess their role in the relationship of liver function markers with CRC. Second, repeated biomarker assessments were limited to a small subset of participants, thereby precluding evaluation of the longitudinal change in biomarkers in relation to CRC. Third, as an observational study, residual confounding cannot be ruled out. Fourth, blood samples were collected from all participants at recruitment, minimizing the selection bias; however, 25% of the participants were excluded, among whom 15% were excluded due to missing assay results on liver function markers and the other 10% were excluded due to prevalent cancer at recruitment, withdrawal of consent, or outliers in biomarker measurements. Finally, the study participants were derived from the general population and had largely normal levels of liver function markers. Therefore, we were unable to assess the associations of much abnormal levels of markers with CRC risk.
In conclusion, higher levels of circulating liver function markers (ALT, AST, GGT, TP and ALB) were associated with a lower risk of CRC in a large cohort of the general population, the UK Biobank, with the greatest associations observed in the proximal colon. Whether lower levels of circulating liver function markers are causal or contributory to CRC risk, the findings support a link between liver function and CRC, and may spur future research on the gut-microbiota-liver axis contributing to liver function-CRC risk.
Supplementary Material
What’s new?
Recent studies have reported a possible relationship between circulating liver metabolites and colorectal cancer (CRC) risk, but nothing has been proven conclusively. Here, the authors examined the relationship between various circulating liver function markers and CRC risk, using data from the UK Biobank. Over 10 years of follow up, they documented 2,662 cases of CRC. They tested levels of 7 different circulating liver function markers. For 6 of the markers, higher circulating levels corresponded to a lower risk of CRC. The work may prompt future investigations into the gut-microbiota-liver axis.
ACKNOWLEDGEMENTS
We express our gratitude to the participants and those involved in building the UK Biobank resource. This work was supported by the American Cancer Society (MRSG-17-220-01-NEC to MS), the U.S. National Institutes of Health (R00 CA215314 to MS), the National Natural Science Foundation of China (81973127 to DH), the Natural Science Foundation of Jiangsu Province (BK20190083 to DH) and the German Research Foundation (DFG 426308975 to GP). UK Biobank was primarily funded by the Wellcome Trust and the Medical Research Council. Other areas of funding include the United Kingdom Department of Health, the Scottish Government, the Welsh Assembly Government, the British Heart Foundation and Diabetes UK. The funders had no role in design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Funding information
American Cancer Society, Grant/Award Number: MRSG-17-220-01-NEC; Deutsche Forschungsgemeinschaft, Grant/Award Number: DFG 426308975; National Institutes of Health, Grant/Award Number: R00 CA215314; National Natural Science Foundation of China, Grant/Award Number: 81973127; Natural Science Foundation of Jiangsu Province, Grant/Award Number: BK20190083
Abbreviations:
- ALB
albumin
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AST
aspartate transaminase
- CI
confidence interval
- CRC
colorectal cancer
- GGT
gamma glutamyltransferase
- HR
hazard ratio
- NHS
National Health Service
- TBIL
total bilirubin
- TP
total protein
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The UK Biobank received ethical approval from the NHS National Research Ethics Service North West (11/NW/0382; 16/NW/0274). Besides, it was also approved by the National Information Governance Board for Health and Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland. In addition, an independent Ethics and Governance Council was formed in 2004 to oversee UK Biobank’s continuous adherence to the Ethics and Governance Framework that was developed for the study (http://www.ukbiobank.ac.uk/ethics/). All participants provided written informed consent.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
This work has been conducted using the UK Biobank Resource under Application Number 46466. The UK Biobank is an open access resource and bona fide researchers can apply to use the UK Biobank dataset by registering and applying at http://ukbiobank.ac.uk/register-apply/. Further information is available from the corresponding author upon request.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases—a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JM, Park YM, Yun JS, et al. The association between nonalcoholic fatty liver disease and esophageal, stomach, or colorectal cancer: national population-based cohort study. PLoS One. 2020;15: e0226351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamoud AR, Weaver L, Stec DE, Hinds TD Jr. Bilirubin in the liver-gut signaling axis. Trends Endocrinol Metab. 2018;29:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttrose M, McKellar D, Welbourne TC. Gut-liver interaction in glutamine homeostasis: portal ammonia role in uptake and metabolism. Am J Physiol. 1987;252:E746–E750. [DOI] [PubMed] [Google Scholar]
- 7.Massafra V, Milona A, Vos HR, et al. Farnesoid X receptor activation promotes hepatic amino acid catabolism and ammonium clearance in mice. Gastroenterology. 2017;152:1462–76 e10. [DOI] [PubMed] [Google Scholar]
- 8.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen MD, Lauritzen T, Vilstrup H, Jepsen P. Alanine aminotransferase and 20-year risk of major chronic diseases and death in a healthy cohort aged 30 to 49 years. Clin Epidemiol. 2020;12:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzke V, Johnson T, Sookthai D, Husing A, Kuhn T, Kaaks R. Circulating liver enzymes and risks of chronic diseases and mortality in the prospective EPIC-Heidelberg case-cohort study. BMJ Open. 2020;10:e033532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mok Y, Son DK, Yun YD, Jee SH. Samet JM. Gamma-glutamyltransferase and cancer risk: the Korean cancer prevention study. Int J Cancer. 2016;138:311–319. [DOI] [PubMed] [Google Scholar]
- 13.Tsuboya T, Kuriyama S, Nagai M, et al. Gamma-glutamyltransferase and cancer incidence: the Ohsaki cohort study. J Epidemiol. 2012;22:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Hemelrijck M, Jassem W, Walldius G, et al. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. Eur J Cancer. 2011;47:2033–2041. [DOI] [PubMed] [Google Scholar]
- 15.Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827–835. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn T, Sookthai D, Graf ME, et al. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. 2017;117:1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319:1047–1052. [DOI] [PubMed] [Google Scholar]
- 18.Ghuman S, Van Hemelrijck M, Garmo H, et al. Serum inflammatory markers and colorectal cancer risk and survival. Br J Cancer. 2017;116:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko WF, Helzlsouer KJ, Comstock GW. Serum albumin, bilirubin, and uric acid and the anatomic site-specific incidence of colon cancer. J Natl Cancer Inst. 1994;86:1874–1875. [DOI] [PubMed] [Google Scholar]
- 20.Soreide K Gallstone disease and cancer risk: finding the bug in the system. Gastroenterology. 2017;152:1825–1828. [DOI] [PubMed] [Google Scholar]
- 21.Shabanzadeh DM, Sorensen LT, Jorgensen T. Association between screen-detected gallstone disease and cancer in a cohort study. Gastroenterology. 2017;152:1965–1974. e1. [DOI] [PubMed] [Google Scholar]
- 22.UK-Biobank. Protocol for a large-scale prospective epidemiological resources; https://www.ukbiobank.ac.uk/resources/: UK Biobank; 2010. [Google Scholar]
- 23.Rosner B Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- 24.UK-Biobank. UK Biobank Biomarker Project-Companion Document to Accompany Serum Biomarker Data. Prepared for: UK Biobank Showcase. Version 1.0 2019.
- 25.Murphy N, Carreras-Torres R, Song M, et al. Circulating levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 associate with risk of colorectal cancer based on serologic and Mendelian randomization analyses. Gastroenterology. 2020;158:1300 e20–12 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunutsor SK, Apekey TA, Seddoh D, Walley J. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:187–201. [DOI] [PubMed] [Google Scholar]
- 29.Pyzik M, Rath T, Kuo TT, et al. Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury. Proc Natl Acad Sci U S A. 2017;114:E2862–E2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford I, Mooijaart SP, Lloyd S, et al. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. Int J Epidemiol. 2011;40: 1530–1538. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann AF. Bile acids, cholesterol, gallstone calcification, and the enterohepatic circulation of bilirubin. Gastroenterology. 1999;116: 1276–1277. [DOI] [PubMed] [Google Scholar]
- 32.Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111:18321–18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urdaneta V, Casadesus J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front Med (Lausanne). 2017;4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt RA, Cochrane K. Tumor potentiating mechanisms of Fusobacterium nucleatum, a multifaceted microbe. Gastroenterology. 2017;152:694–696. [DOI] [PubMed] [Google Scholar]
- 35.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature. 2020;580:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. [DOI] [PubMed] [Google Scholar]
- 37.Horsfall LJ, Rait G, Walters K, et al. Serum bilirubin and risk of respiratory disease and death. JAMA. 2011;305:691–697. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B Albumin—an important extracellular antioxidant? Biochem Pharmacol. 1988;37:569–571. [DOI] [PubMed] [Google Scholar]
- 39.Ferrer R, Mateu X, Maseda E, et al. Non-oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert Rev Clin Pharmacol. 2018;11:125–137. [DOI] [PubMed] [Google Scholar]
- 40.Sookoian S, Castano GO, Scian R, et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am J Clin Nutr. 2016;103:422–434. [DOI] [PubMed] [Google Scholar]
- 41.Priolo C, Khabibullin D, Reznik E, et al. Impairment of gamma-glutamyl transferase 1 activity in the metabolic pathogenesis of chromophobe renal cell carcinoma. Proc Natl Acad Sci U S A. 2018;115:E6274–E6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajpert-De Meyts E, Shi M, Chang M, et al. Transfection with gamma-glutamyl transpeptidase enhances recovery from glutathione depletion using extracellular glutathione. Toxicol Appl Pharmacol. 1992;114:56–62. [DOI] [PubMed] [Google Scholar]
- 43.Jing X, Zhang S. An ancient molecule with novel function: alanine aminotransferase as a lipopolysaccharide binding protein with bacteriocidal activity. Dev Comp Immunol. 2011;35:94–104. [DOI] [PubMed] [Google Scholar]
- 44.Ruhl CE, Everhart JE. The association of low serum alanine aminotransferase activity with mortality in the US population. Am J Epidemiol. 2013;178:1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh CM, Won YJ, Cho H, et al. Alanine aminotransferase and gamma-glutamyl transferase have different dose-response relationships with risk of mortality by age. Liver Int. 2016;36:126–135. [DOI] [PubMed] [Google Scholar]
- 46.Strasak AM, Rapp K, Brant LJ, et al. Association of gamma-glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68:3970–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strasak AM, Pfeiffer RM, Klenk J, et al. Prospective study of the association of gamma-glutamyltransferase with cancer incidence in women. Int J Cancer. 2008;123:1902–1906. [DOI] [PubMed] [Google Scholar]
- 48.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. [DOI] [PubMed] [Google Scholar]
- 49.Ruhl CE, Everhart JE. Trunk fat is associated with increased serum levels of alanine aminotransferase in the United States. Gastroenterology. 2010;138:1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung GE, Kim D, Kwark MS, et al. Visceral adipose tissue area as an independent risk factor for elevated liver enzyme in nonalcoholic fatty liver disease. Medicine (Baltimore). 2015;94:e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subbiah V, West HJ. Jaundice (Hyperbilirubinemia) in Cancer. JAMA Oncol. 2016;2:1103. [DOI] [PubMed] [Google Scholar]
- 52.Phillips A, Shaper AG, Whincup PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. 1989;2:1434–1436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This work has been conducted using the UK Biobank Resource under Application Number 46466. The UK Biobank is an open access resource and bona fide researchers can apply to use the UK Biobank dataset by registering and applying at http://ukbiobank.ac.uk/register-apply/. Further information is available from the corresponding author upon request.


