Abstract
Background
Tuberculosis and human immune deficiency virus co-infections remained the most common cause of child mortality for the last ten years. Globally, 1.2 million cases of tuberculosis occurred in patients living with HIV/AIDS, of which 1.0 million cases occurred in children. The public health impact of tuberculosis and human immune deficiency virus co-infection among children is high in developing countries and Sub-Saharan Africa accompanied three fourth of the global burden. However, there are limited studies that assess the incidence and predictors of mortality among tuberculosis and human immune deficiency virus co-infected children in Ethiopia.
Methods
A facility-based retrospective cohort study was conducted at Public hospitals in Southern Ethiopia with a total of 286 randomly selected records of ART enrolled children from 1st January 2009 to 31stDecember 2018. Data were entered into Epi Data version 3.1 and exported to STATA version 14 for analysis. Bivariate and multivariable Cox proportional hazards model was fitted to identify the predictors of mortality. Variables that had a p-value<0.05 at 95%CI in the multivariable cox proportional hazard model were considered as statistically significant.
Results
A total of 274 tuberculosis and human immunodeficiency virus co-infected children’s records were reviewed. The incidence of mortality among tuberculosis and human immunodeficiency virus co-infected children was 17.15 per 100 children. The overall incidence density rate of mortality was 2.97(95%CI: 2.2, 3.9) per 100 child year of observation and being anemic (AHR: 2.6; 95%CI: 1.28, 5.21), not initiating isoniazid prophylaxis therapy (AHR: 2.8; 95%CI: 1.44, 5.48), developing extrapulmonary tuberculosis (AHR: 5.7; 95%CI: 2.67, 12.56) and non-adherence (AHR: 5.2; 95%CI: 2.19, 12.39) were independent predictors of mortality.
Conclusion
Mortality rate was high among TB/HIV co-infected children at the public hospitals in Southern Ethiopia. Extra-pulmonary tuberculosis, anemia, non-adherence, and isoniazid preventive therapy use were statistically significant predictors of mortality among TB/HIV co-infected children. Therefore, extra pulmonary tuberculosis, and anemia should be closely monitored to increase their adherence as well as they should be provided with isoniazid preventive therapy.
Background
Human immunodeficiency virus (HIV) infection is the greatest risk factor for acquiring Tuberculosis (TB) infection and developing the disease [1, 2]. Tuberculosis (TB) enhances human immunodeficiency virus (HIV) replication by accelerating the natural evolution of HIV infection; it is the leading cause of sickness and death of people living with HIV [2]. Human immunodeficiency virus and Tuberculosis (TB/HIV) co-infection is “bidirectional and synergistic in which HIV promotes the progression of latent tuberculosis infection to disease, and tuberculosis accelerates the progression of HIV disease to its advanced stage [3–5].
Tuberculosis and human immune deficiency virus co-infections remained the most common cause of child mortality for the last ten years. Globally, 1.2 million cases of tuberculosis occurred in patients living with HIV/AIDS, of which 1.0 million cases occurred in children [6]. It mostly affects the lungs (pulmonary TB) but can affect other sites as well [7]. Relatively, a small proportion of people infected with Mycobacterium tuberculosis will develop TB disease but the probability of developing TB is much higher among people infected with HIV [8].
The double burden of tuberculosis (TB) and human immunodeficiency virus (HIV) is one of the major global health challenges of the 21st century [9]. TB is the leading immune-suppressing infection and the commonest cause of death among HIV-infected children [10]. The burden of tuberculosis and human immunodeficiency virus co-infection is particularly high in developing countries and approximately three-fourths of the global TB/HIV co-infection observed in Sub-Saharan Africa including Ethiopia [6]. TB and HIV work together to suppress the immunity of the patients and thereby, shorten the lifespan if early treatment is not initiated [11, 12].
The Ethiopian Federal HIV and AIDS Prevention and Control Office estimated that the single National HIV/AIDS is among the top ten high burden counties with an incidence rate of 341/100,000 of which 31% of TB patients are living with HIV [13]. Still now TB/HIV co-infection is the leading cause of death in people living with HIV/AIDS [14].
Ethiopia is one of the 30 high burden countries and has been classified as having high burdens of TB/HIV co-infection. The country is striving to reduce the magnitude of TB and HIV disease in line with the strategies to achieve the Sustainable Development Goals (SDG) [2]. However, the problem remains significant, particularly in HIV-positive children. The cause of mortality among TB/HIV co-infected children is multi-factorial which includes diagnosis age, nutritional status, immunity status, and hemoglobin levels at ART initiation [15]. Therefore; this study was aimed to determine the incidence and predictor’s of mortality among children co-infected with TB/HIV at public hospitals in Southern Ethiopia.
Methods and materials
Study design and setting
A facility-based retrospective cohort study was conducted in seven selected public hospitals in Southern Ethiopia from the records enrolled from1st January 2009 to 31st December 2018. Those hospitals are located in the southern part of Ethiopia. All TB/HIV co-infected children under 15 years of age who ever enrolled in pediatrics ART clinics were the source populations. The starting point is from entry to children who were TB/HIV co-infected service from 1st January 2009 to 31st December 2018 and the endpoint was death or loss to follow up or transferred to another.
Study population and sampling technique
The source population was all TB/HIV co-infected children under 15 years of age who enrolled in a treatment program in public hospitals in Southern Ethiopia. The sample size was calculated based on estimation for the assessment of survival time using Epi info version 7 in considering the following assumptions: 95% CI, power of 80%, the ratio of unexposed to exposed 1:1 and Parameters: P1: is the proportion of exposed with the outcome 11.81, P2: is the proportion of non-exposed with the outcome 2.21 and 5% marginal error. Finally, by using 10% for incompleteness the sample size was 286. The Sample was allocated proportionally for the seven selected facility and records were selected randomly.
Data collection procedure and data quality control
The sources of data for this study were the Pre-ART register, the ART register, and the patients’ ART follow-up and medical charts. In those registers and follow-up charts, clients’ socio-demographic, clinical, and laboratory information, treatments being provided, the follow-up status of each client were recorded. Data was collected from client charts using a structured checklist for records review developed from the registers and follow-up charts. Twenty-one data collectors who are health professionals and working in pediatric ward were recruited for data collection after getting training on the tool.
Study variables and data analysis
The outcome variable is time to death from enrolment to the CART program. The survival time is measured as the time period between the date of enrolment and date of death and it is dichotomized as death and censored. The censored cases include the alive patients, defaulters, and transferred-outs. Data were cleaned, coded, and entered into Epi Data version 3.1 and exported to STATA version 14 for analysis. Bivariate analysis was carried out to determine the association between the dependent variable and the explanatory variables. Both Crude hazard ratio (CHR) and adjusted hazard ratio(AHR) together with the corresponding 95% confidence interval and P-value were used to assess the strength of association and statistical significance. The Kaplan Meier survival curve together with the log-rank test was fitted to determine the survival time. Variables which had p-value <0.25 in bivariate analysis were considered as a candidate for multivariable analysis and variables which had p-value <0.05 in multivariable cox regression analysis were considered as statistically significant. The backward stepwise regression method was applied.
Ethical consideration
Ethical clearance was obtained from the institutional review board (IRB) of the Arba Minch University College of medicine and health sciences. In addition; a permission letter was obtained from the Arba Minch University and public hospital administrations. HIV care clinics’ focal persons were informed about the objective and significance of the study prior to the data collection. Appropriate measures were applied to ensure the confidentiality of the data. All data were fully anonymized before we accessed them and the ethical review board waived the requirement for informed consent.
Results
Socio-demographic characteristics
A total of 286 TB/HIV co-infected children’s charts were reviewed. Of these, 12(4.19%) were excluded from the analysis due to incomplete data. Therefore, 274 TB/HIV co-infected children were included in the analysis. The mean age of the study participants was 8.9(±3.5 SD) years. Eight (2.9%) of the children were under one year of age and more than half (66.8%) of children’s caregivers were between the age group of (25–34) years with a median age of 30 (IQR (27.72–34) years. Regarding the sex of the children, nearly half (50.7%) were males. More than three fourths (77.4%) of the respondent were urban residents and 254 (92.4%) children were lives together with their parents. Approximately, two-thirds (70.1%) of the children’s caregivers were HIV positive in their HIV status. Regarding the family size of the children, 39(14.8%), 196(74.5%) and 28(10.6%) of the children had less than two, 3–4 and more than four family size respectively (Table 1).
Table 1. Socio-demographic characteristics of TB/HIV co-infected children at public hospitals in Southern Ethiopia, 2020.
| Characteristics | Categories | Survival Status | ||||
|---|---|---|---|---|---|---|
| Total N (%) N = 274 | N PY | Death N (%) N = 47 | Censored N (%) N = 227 | IDR | ||
| Age (year) | <1 | 11(4.0) | 58.8 | 2(4.3) | 9(4.0) | 3.40 |
| 1–5 | 18(6.6) | 109 | 4(8.5) | 14(6.2) | 3.66 | |
| 6–10 | 113(41.2) | 739 | 18(38.3) | 95(41.9) | 2.43 | |
| 11–14 | 132(48.2) | 674.5 | 23(48.9) | 109(48.0) | 3.40 | |
| Sex | Male | 139(50.7) | 747.7 | 22(46.8) | 117(51.5) | 2.97 |
| Female | 135(49.3) | 833.6 | 25(53.2) | 110(48.5) | 2.99 | |
| Age of caregiver | 15–24 | 35(12.8) | 220 | 6(12.8) | 29(12.8) | 2.27 |
| 25–34 | 183(66.8) | 1050 | 32(68.1) | 151(66.5) | 3.06 | |
| 35–44 | 40(14.6) | 216.3 | 4(8.5) | 36(15.9) | 1.86 | |
| >44 | 16(5.8) | 95 | 5(10.6) | 11(4.8) | 5.26 | |
| Residence | Urban | 212(77.4) | 1232.5 | 32(68.1) | 180(79.3) | 2.61 |
| Rural | 62(22.6) | 348.8 | 15(31.9) | 47(20.7) | 4.30 | |
| Caregiver of the child | Mother | 217(79.2) | 1291.5 | 37(78.7) | 180(79.3) | 2.87 |
| Father | 28(10.2) | 159.3 | 2(4.3) | 26(11.5) | 1.25 | |
| Stepparents | 11(4.0) | 28.5 | 2(4.3) | 9(40) | 7.01 | |
| Sibling | 18(6.6) | 102 | 6(12.8) | 12(5.3) | 5.94 | |
| Caregiver HIV status | Positive | 192(70.1) | 1156.8 | 33(70.2) | 159(70) | 2.86 |
| Negative | 39(14.2) | 180.5 | 6(12.8) | 33(14.5) | 3.34 | |
| Unknown | 43(15.7) | 244 | 8(17.0) | 35(15.4) | 3.27 | |
PY: Person years of observation, IDR: Incidence density rate
Clinical characteristics
Among the total 274 children, 165(60.2%) of children had baseline HIV WHO clinical stage (III and IV). The eligibility criteria for initiation of HAART were both CD4+ cell count or percent and WHO clinical stage. More than one fifths (21.2%) of the children were initiated HAART based on both WHO clinical staging and CD4+. More than one fourths (28.1%) of children had experienced initial regiment change during their follow-up time.
More than one tenths (19.5%) of the children face treatment failure, among them 6.4% were dead. The median hemoglobin level of the respondents was 11 (IQR: 10, 12). Hence; 16.1% of the children were Anemic at the baseline. Regarding prophylaxis, more than three fourths (79.9) of the children were initiated cotrimoxazole and nearly three fourths (74.8%) of the children were initiated isoniazid preventive therapy. Among the nutritional problems, 2.6% and 2.2% of the children develop underweight and stunting respectively. More than half (52.2%) of the children develop TB at the pre-ART period (Table 2).
Table 2. Clinical characteristics of TB/HIV co-infected children at public hospitals Southern Ethiopia, 2020.
| Characteristics | Total N (%) N = 274 | N PY | Death N% N = 47 | Censored N (%) N = 227 | IDR | |
|---|---|---|---|---|---|---|
| Baseline WHO stage | Mild (I & II) | 109(39.8) | 710.5 | 18(38.3) | 91(40.1) | 2.55 |
| Advanced (III&IV) | 165(60.2) | 870.8 | 29(61.7) | 136(59.9) | 3.33 | |
| ART Eligibility criteria | CD4+ cell | 43(15.7) | 233.5 | 12(25.5) | 31(13.7) | 5.13 |
| WHO stage | 169(61.7) | 1038 | 26(55.3) | 143(63.0) | 2.52 | |
| Both | 58(21.2) | 303.8 | 8(17.0) | 50(22.0) | 2.60 | |
| Test and treat | 4(1.5) | 6 | 1(2.1) | 3(1.3) | 16.6 | |
| Initial ART regimen | 4a = D4T-3TC-NVP | 143(52.2) | 964.5 | 20(42.6) | 123(54.2) | 2.08 |
| 4b = d4T-3TC-EFV | 6(2.2) | 34.8 | 1(2.1) | 5(2.2) | 2.87 | |
| 4c = AZT-3TC-NVP | 26(9.5) | 157.6 | 7(14.9) | 19(8.4) | 4.44 | |
| 4e = TDF-3TC-EFV | 75(27.4) | 301.4 | 16(34.0) | 59(26.0) | 5.34 | |
| 4f = AZT+3TC+LPV | 18(6.6) | 104 | 2(4.3) | 16(7.0) | 1.92 | |
| 4g = ABC+3TC+LPV | 6(2.2) | 19 | 1(2.1) | 5(2.2) | 5.26 | |
| Initial regimen change | Yes | 77(28.1) | 520.2 | 14(29.8) | 63(27.8) | 2.70 |
| No | 197(71.9 | 1056.1 | 33(70.2) | 164(72.2) | 3.12 | |
| Reason for regimen change | Side effect | 38(49.4) | 221.9 | 5(10.6) | 33(14.5) | 2.27 |
| Treatment failure | 15(19.5) | 115.5 | 3(6.4) | 12(5.3) | 2.59 | |
| TB | 17(22.1) | 77 | 4(8.5) | 13(5.7) | 5.19 | |
| Stock out | 7(9.1) | 30 | 2(4.3) | 5(2.2) | 6.66 | |
| Not change regimen | 197(71.9) | 1136.9 | 33(70.2) | 164(72.2) | 2.91 | |
| Treatment failure | Yes | 15(5.5) | 92 | 3(6.4) | 12(5.3) | 3.26 |
| No | 259(94.5) | 1489.3 | 44(93.6) | 215(94.7) | 2.96 | |
Among the records of children, 3(1.1%) of them developed an immunological failure and among them, 1(2.1%) was dead. Among the records reviewed, 269(97.4%) did not face virologic failure. Among the records that face virologic failure, one (2.1%) was the record of a dead child. Nearly three-fourths (74.8%) of the records of children, were records of children who use Isoniazid preventive therapy and 219 (79.9%) CPT (Table 3).
Table 3. Medication and disease related characteristics of TB/HIV co-infected children at public hospitals Southern Ethiopia, 2020.
| Variables | Category | Total N (%) N = 274 | N PY | Death N% N = 47 | Censored N (%) N = 227 | IDR |
|---|---|---|---|---|---|---|
| Immunologic failure | Yes | 3(1.1) | 18 | 1(2.1) | 2(0.9) | 5.55 |
| No | 271(98.9) | 1563.3 | 46(97.9) | 225(99.1) | 2.95 | |
| Virologic failure | Yes | 5(1.5) | 30 | 1(2.1) | 4(1.8) | 3.33 |
| No | 269(97.4) | 1551.3 | 46(97.9) | 223(98.2) | 2.97 | |
| Clinical failure | Yes | 7(2.6) | 41 | 3(6.4) | 4(1.8) | 7.31 |
| No | 267(97.4) | 1540.3 | 44(93.6) | 223(98.2) | 2.86 | |
| Isoniazid | Yes | 205(74.8) | 1243 | 16(34.0) | 189(83.3) | 1.28 |
| No | 69(25.2) | 338.3 | 31(66.0) | 38(16.7) | 9.35 | |
| CPT | Yes | 219(79.9) | 1302.3 | 20(42.6) | 199(87.7) | 1.54 |
| No | 55(20.1) | 279 | 27(57.4) | 28(12.3) | 9.71 | |
| Weight for age | Normal | 267(97.4) | 1541.3 | 46(97.9) | 221(97.4) | 2.97 |
| Underweight | 7(2.6) | 30 | 1(2.1) | 6(2.6) | 3.33 | |
| Height for age | Normal | 268(97.8) | 1548.3 | 46(97.9) | 222(97.8) | 2.98 |
| Stunting | 6(2.2) | 33 | 1(2.1) | 5(2.2) | 3.03 | |
| Functional status | Working | 208(75.9) | 1271.5 | 34(72.3) | 174(76.7) | 2.68 |
| Ambulatory | 36(13.1) | 167.2 | 7(14.9) | 29(12.8) | 4.18 | |
| Bedridden | 30(10.9) | 142.6 | 6(12.8) | 24(10.6) | 4.23 | |
| Adherence | Adherence | 193(70.4) | 1186.5 | 9(19.1) | 184(81.1) | 0.75 |
| Non-adherence | 81(29.6) | 394.8 | 38(80.9) | 43(18.9) | 9.77 | |
| Type of TB | PTB | 187(68.2) | 1153.3 | 9(19.1) | 178(78.4) | 0.78 |
| EPTB | 87(31.8) | 428 | 38(80.9) | 49(21.6) | 8.92 | |
| Period of TB diagnoses | PRE ART | 143(52.2) | 793 | 27(57.4) | 116(51.1) | 3.43 |
| ART | 131(47.8) | 788.3 | 20(42.6) | 111(48.9) | 2.53 | |
| Hgb level at TB diagnose | <10 mg/dl | 44(16.1) | 217 | 27(57.4) | 17(7.5) | 12.5 |
| > = 10 mg/dl | 230(83.9) | 1364.3 | 20(42.6) | 210(92.5) | 1.47 |
PY: Person years of observation, IDR: Incidence density rate
Incidence of mortality
In this study, 274 children were followed for a total of 1581.3 child years of observation. The minimum and maximum follow-up periods were one and ten years respectively with the median follow-up period of six(IQR: 3–8) years. Therefore; the incidence of mortality among TB/HIV co-infected children was 17.15% and the incidence density rate was 2.97 per 100 child year observation (95%CI: 2.2, 3.9).
The survival status of TB/HIV co-infected children
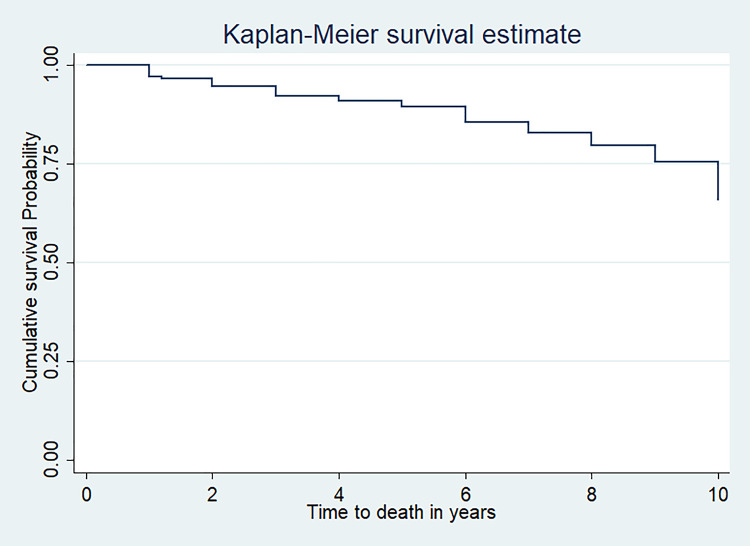
In this study, 3.28% of the children were dead at the end of the first year. In addition, 5.10% and 9.12% of the children were died at the end of the first two years and first five years respectively. The cumulative proportion of survival at the end of the first year, third years, fifth years, and the tenth year was 96 (95% CI: 93, 98), 92 (95% CI; 87, 94), 89 (95% CI; 84, 92) and 56 (95% CI; 39,70) respectively (Table 4).
Table 4. Life table showing the cumulative survival probability among TB/HIV co-infected children at public hospitals in Southern Ethiopia, 2020.
| Year | No of children at start | Withdrawn during years | At risk | Deaths | Prob. of death | Prob. of surviving year | Cumulative probability of surviving | 95% CI |
|---|---|---|---|---|---|---|---|---|
| 1 | 274 | 36 | 256 | 9 | 0.04 | 0.96 | 0.96 | 0.93,0.98 |
| 2 | 229 | 12 | 223 | 5 | 0.02 | 0.98 | 0.94 | 0.90,0.96 |
| 3 | 212 | 11 | 206.5 | 5 | 0.02 | 0.98 | 0.92 | 0.87,0.94 |
| 4 | 196 | 11 | 190.5 | 3 | 0.02 | 0.98 | 0.91 | 0.86,0.93 |
| 5 | 182 | 14 | 175 | 3 | 0.02 | 0.98 | 0.89 | 0.84,0.92 |
| 6 | 165 | 29 | 150.5 | 7 | 0.05 | 0.95 | 0.85 | 0.79,0.89 |
| 7 | 129 | 25 | 116.5 | 4 | 0.03 | 0.97 | 0.82 | 0.75,0.86 |
| 8 | 100 | 36 | 82 | 4 | 0.05 | 0.95 | 0.78 | 0.70,0.83 |
| 9 | 60 | 26 | 47 | 3 | 0.06 | 0.94 | 0.73 | 0.63,0.80 |
| 10 | 31 | 27 | 17 | 4 | 0.23 | 0.77 | 0.56 | 0.39,0.70 |
In this study, 197(71.9%) children were alive at the end of the follow-up period. In addition; 47(17.1%), 17(6.2%) and 13(4.7%) children were dead, lost to follow up, and transferred out respectively. The overall mean survival of TB/HIV co-infected children was 8.8 year (95%CI: 8.5, 9.19) (Fig 1).
Fig 1. Kaplan Meier curve of survival proportion for TB/HIV co-infected children at public hospitals in Southern Ethiopia, 2020.
The log-rank estimate of mortality among TB/HIV Co-infected children
The log-rank test estimate revealed that the survival pattern of mortality among TB/HIV Co-infected children was significantly varied among the covariates. The Kaplan Meier survival curve together with the log-rank test shows the effect of each variable on mortality of TB/HIV Co-infected children. Type of TB, Cotrimixazole initiation, Isoniazid preventive therapy initiation, and Hgb level at TB diagnosis were the variables that had a significant effect on mortality among TB/HIV Co-infected children (Table 5).
Table 5. Log rank estimate of variables among TB/HIV co-infected children at public hospitals in Southern Ethiopia, 2020.
| Variables | Log-rank test estimate |
|---|---|
| Age(year) | X2 = 1.13, p-value = 0.77 |
| Sex of respondents | X2 = 0.001 p-value = 0.995 |
| Mother HIV status | X2 = 2.30,p-value = 0.317 |
| Relationship to the child | X2 = 6.93, p-value = 0.74 |
| Residence | X2 = 3.54,p-vaue = 0.60 |
| Place of health service utilization | X2 = 1.17,p-value = 0.27 |
| Family size | X2 = 0.28,p-value = 0.86 |
| Age of caregiver | X2 = 2.44,p-value = 0.48 |
| WHO clinical stage | X2 = 1.03,p-value = 0.30 |
| HIV status of the caregiver | X2 = 0.61,p-value = 0.97 |
| Initial regimen | X2 = 10.42,p-value = 0.06 |
| Treatment failure | X2 = 0.037,p-value = 0.84 |
| Immunologic failure | X2 = 0.49,p-value = 0.48 |
| Virologic failure | X2 = 0.003,p-value = 0.95 |
| Clinical failure | X2 = 2.52,p-value = 0.112 |
| Weight for age | X2 = 0.086,p-value = 0.77 |
| Height for age | X2 = 0.015,p-value = 0.090 |
| Adherence to ART | X2 = 84.80,p-value = 0.0001 |
| Type of TB | X2 = 73.53, p-value = 0.0001 |
| Cotrimoxazole | X2 = 52.28, p-value = 0.0001 |
| Isoniazid preventive therapy | X2 = 56.32, p-value = 0.0001 |
| Functional status | X2 = 2.17,p-value = 0.337 |
| Hgb level at TB diagnose | X2 = 86.23, p-value = 0.0001 |
Comparison of survival probability among categories of variables
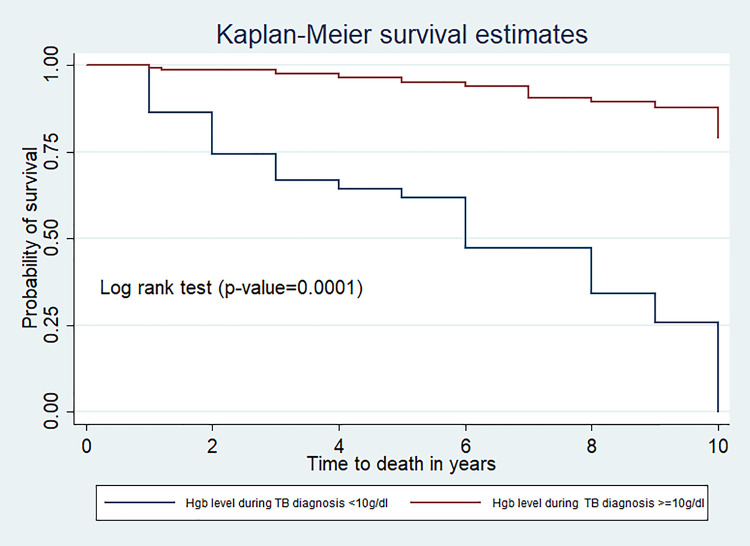
The mean survival time for those who had hemoglobin level less than 10 g/dl at the TB diagnosis period was 6.0(95%CI; 5.0,7.15) years and it was 9.48(95%CI: 9.28, 9.73) years for the children who had hemoglobin level greater than 10g/dl at the diagnosis of TB (p-value,<0.0001) (Fig 2).
Fig 2. Kaplan Meier survival estimates of hemoglobin level at TB diagnosis among TB/HIV co-infected children at public hospitals, Southern Ethiopia.
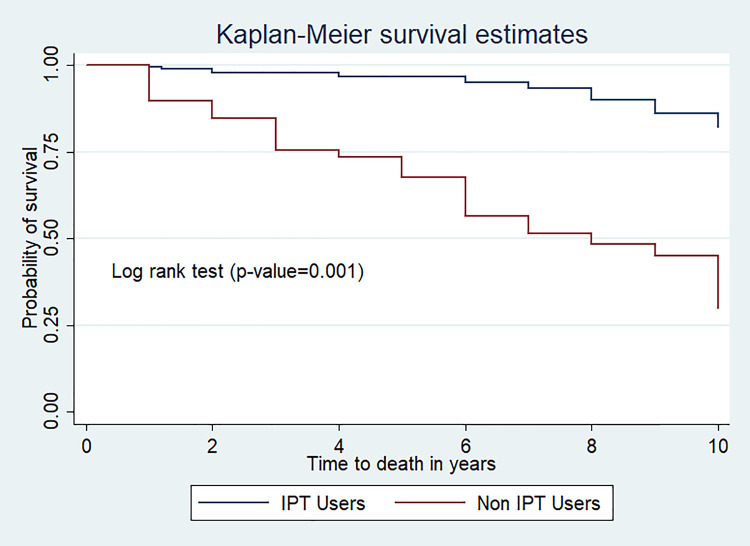
The mean survival time of those who use CPT was 9.52 (95%CI; 9.27, 9.77) year while it was 6.92(95%CI; 6.0, 7.81) year for those who did not use CPT (p-value<0.001) (Fig 3).
Fig 3. Kaplan Meier survival curve of IPT use among TB/HIV co-infected children at public hospitals, Southern Ethiopia, 2020.
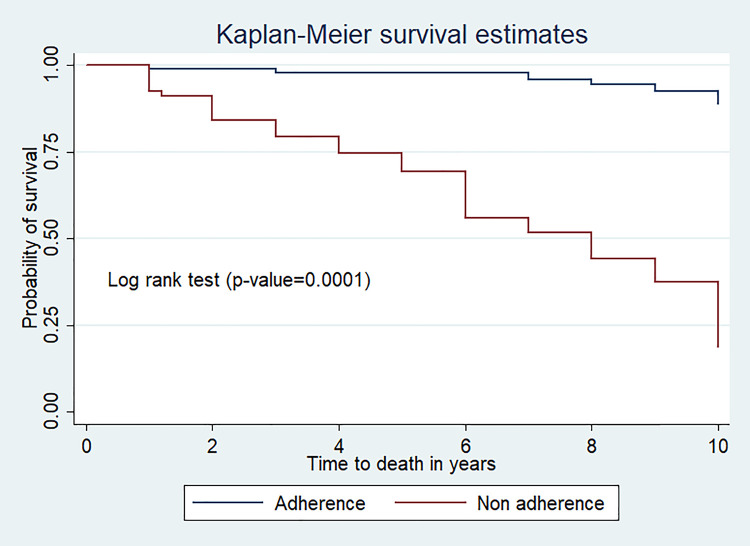
The mean survival time of children co-infected with TB/HIV who had adherence to ART drugs was high 9.71(95%CI; 9.50, 9.92) year as compared to non-adherence 6.8(95%CI; 6.09, 7.6) year (p-value<0.0001) (Fig 4).
Fig 4. Kaplan Meier survival curve of ART adherence level among TB/HIV co-infected children at seven public hospitals, Southern Ethiopia.
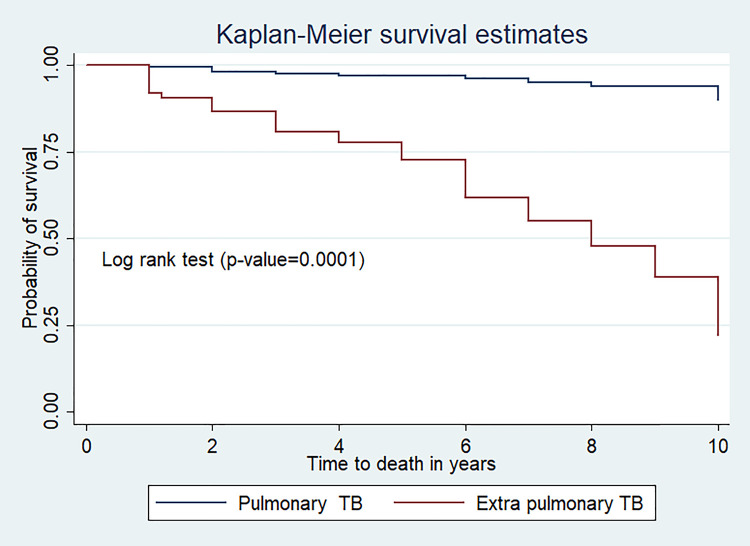
The mean survival time of children with pulmonary TB was 9.68(95%CI; 9.44, 9.91) year and it was 7.12 (95%CI; 6.38, 7.87) years for those with extrapulmonary TB (p-value<0.0001) (Fig 5).
Fig 5. Kaplan Meier survival curve of infection among TB/HIV co-infected children at public hospitals, Southern Ethiopia.
Predictors of mortality among TB/HIV co-infected children
The bivariate analysis result showed that hemoglobin level, initiation of cotrimoxazole preventive therapy (CPT), initiation of Isoniazid prophylaxis (IPT), type of tuberculosis (TB), and adherence to ART drugs were candidate variables for the time to death among TB/HIV co-infected children. On the other hand; initiation of IPT, type of TB, adherence to ART drugs and hemoglobin level were statistically significant in multivariable cox proportional hazard model.
Hemoglobin level was statistically significant in the multivariable Cox proportional hazard model. The risk of mortality among TB/HIV co-infected children who had hemoglobin levels less than 10g/dl was 2.6 times higher as compared with those children who had hemoglobin levels≥10g/dl (AHR: 2.6; 95%CI: 1.28, 5.21). Children who had not initiated Isoniazid prophylaxis (IPT) had a 2.8 times higher risk of mortality as compared with the counterparts who initiate IPT (AHR:2.8; 95%CI:1.44,5.48).
The risk of mortality among TB/HIV co-infected children who had developed extra pulmonary or/and disseminated tuberculosis was 5.7 times higher as compared with those who had developed pulmonary tuberculosis (AHR: 5.7; 95%CI: 2.67, 12.56). TB/HIV co-infected children who were not adhered to ART drugs had5.2 times higher risk of mortality as compared with the counterparts, who had adhered to the ART drugs (AHR: 5.2; 95%CI: 2.19, 12.39) (Table 6).
Table 6. Predictors of mortality among TB/HIV Co-infected children at public hospitals in Southern Ethiopia, 2020.
| Variables | Category | Survival status | 95% CI | P-value | ||
|---|---|---|---|---|---|---|
| Dead n = 47 | Censored n = 227 | CHR(95%CI) | AHR(95%CI) | |||
| Age (year) | <1 | 2 | 9 | 0.84(0.19,3.59) | ||
| 1–5 | 4 | 14 | 0.98(0.33,2.85) | 0.77 | ||
| 6–10 | 18 | 95 | 0.72(0.38,1.34) | |||
| 11–14 | 23 | 109 | 1 | |||
| Sex | Male | 22 | 117 | 0.99(0.56,1.77) | 0.99 | |
| Female | 25 | 110 | 1 | |||
| Health service utilization | Primary hospital | 6 | 43 | 0.62(0.26,1.48) | 0.29 | |
| General Hospital | 41 | 184 | 1 | |||
| Residence | Urban | 32 | 180 | 1 | ||
| Rural | 15 | 15 | 1.77(0.96,3.29) | 0.067 | ||
| Baseline WHO | Mild | 18 | 91 | 1 | ||
| Advanced | 29 | 136 | 1.34(0.74,2.43) | 0.31 | ||
| Hgb at TB diagnose | <10 mg/dl | 27 | 17 | 9.69(5.36,17.53) | 2.6(1.28,5.21) | 0.008 |
| > = 10 mg/dl | 20 | 210 | 1 | 1 | ||
| CPT | Yes | 20 | 199 | 1 | 1 | 0.7 |
| No | 27 | 28 | 6.27(3.5,11.89) | 1.14(0.57,2.27) | ||
| IPT | Yes | 16 | 189 | 1 | 1 | 0.002 |
| No | 31 | 38 | 7.11(3.88,13.04) | 2.8(1.44,5.48) | ||
| Type of TB | PTB | 9 | 178 | 1 | 1 | |
| EPTB | 38 | 49 | 11.86(5.72,24.57) | 5.7(2.67,12.56) | 0.0001 | |
| Adherence | Adherence | 9 | 184 | 1 | 1 | |
| Non-adherence | 38 | 43 | 13.43(6.48,27.83) | 5.2(2.19,12.39) | 0.0001 | |
Discussion
The multivariable Cox proportional hazard model revealed that anemia, isoniazid preventive therapy initiation, extra-pulmonary tuberculosis, and non-adherence to ART were independent predictors of mortality among TB/HIV co-infected children.
The incidence density of TB/HIV co-infected children in this study was 2.97(95%CI; 2.2, 3.9) per 100 Child-year of follow-up which was slightly higher than a study conducted in Nigeria which was 1.4per 100 Child-year follow-up) [1]. It was slightly consistent with the study conducted at Northwest Ethiopia, which was 3.27 per 100 child-year of follow-up [2]. Such incidence difference might be due to the difference in the follow-up period and study setting. The incidence rate in this study was 17.15% which was almost similar to the study conducted in South Africa and India which was 17.5% and 17% respectively [1, 16]. This might be explained by the similarity in WHO HIV/AIDS implementation strategy, treatment, care, and support strategy. This finding was slightly higher than the study conducted in Northwest Ethiopia, which was 14.02% [2]. This might be due to the variation in the type of care provision across institutions.
However, the incidence of mortality in this study was lower than a study conducted in Thailand and India which was 30% and 36.5 respectively [16, 17]. This discrepancy might be due to the difference in study period and setting. The cumulative survival rate at the end of the follow-up period was 56%. This finding was lower than the study conducted in Nigeria, which was 73%. This discrepancy in survival rate might be due to the difference in the follow-up period of the studies.
Consistent with the study conducted at Tanzania and Malawi [18, 19], the risk of mortality among TB/HIV co-infected children who had diagnosed anemia was two times higher as compared with the counterparts, who had no diagnosed anemia (AHR: 2.6; 95%CI: 1.28, 5.21). This is due to the effect of anemia on the oxygen intake capacity which had a synergistic effect with tuberculosis and HIV co-infections that increase the prognosis of the disease process which may end up with death [2]. In addition; it might be associated with the decrease in hemoglobin level (commonly lower than 10mg/dl) due to the occurrence of pediatric infections [20].
The initiation of isoniazid preventive therapy had a protective effect against death among TB/HIV co-infected children. In line with the study conducted at South Africa and Nigeria [21, 22], this study revealed that, children who did not initiate isoniazid preventive therapy had two times higher risk of mortality as compared with the counterparts who initiated isoniazid preventive therapy (AHR: 2.8; 95%CI: 1.44, 5.48). This is due to the fact, IPT decreases mycobacterium load and reduces progression of latent bacilli to active TB [2].
Consistent with the study conducted at Gondar Comprehensive Specialized hospital and the United States of America and Thailand [2, 17, 23], the risk of mortality among TB/HIV co-infected children who had extrapulmonary tuberculosis was five times higher as compared with those who had pulmonary tuberculosis (AHR: 5.7; 95%CI: 2.67, 12.56). Extra pulmonary TB especially the disseminated one was more severe than pulmonary TB because which resulted in delayed recognition and which had hematogenous dissemination, finally which increases the mortality rate [2, 24].
In line with the study conducted at Northwest Ethiopia, India, and Addis Ababa [2, 24, 25], the risk of mortality among TB/HIV co-infected children who had non-adherence to ART drugs were five times higher as compared with those who had adherence to ART drug (AHR: 5.2; 95%CI: 2.19, 12.39). This indicates the beneficiary effect of adherence to ART because ART improves the immune status of the children and reduces the risk of mortality. In addition; when the child adheres to ART, viral replication was suppressed. This suppression of viral replication results from an increase in CD4 cells and increases the survival of children with TB/HIV co-infection. On the other hand, children who were not adhered to ART became at risk for treatment failure, which finally leads to death [2, 26].
The limitations of this study was, since it was conducted through record review certain variables which were not recorded such as viral load, family monthly income and drug abuse status of the parents were not analyzed.
Conclusion
The mortality rate was high among TB/HIV co-infected children at public hospitals in southern Ethiopia. Extra-pulmonary tuberculosis, anemia, non-adherence to ART and initiation of isoniazid preventive therapy was an independent predictor of mortality among TB/HIV co-infected children. Therefore, children with extra pulmonary tuberculosis and anemia should be closely monitored to increase their adherence as well as they should initiate isoniazid preventive therapy.
Supporting information
(DTA)
Acknowledgments
We would like to thank the administrator of each hospital for their effort and permission to conduct the study.
Abbreviations
- ART
Antiretroviral therapy
- AOR
Adjusted odd ratio
- ART
antiretroviral therapy
- CI
Confidence interval
- HIV
Human immunodeficiency virus
- IDR
Incidence density rate
- OIs
Opportunistic infections: Tuberculosis
- TB/HIV
Human immunodeficiency virus and Tuberculosis co-infection
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Augostie AE, Stephen O, Oche OA, Atiene SS, Prosper IO, John AI, et al. Mortality among pulmonary tuberculosis and HIV-1 co-infected Nigerian children beingtreated for pulmonary tuberculosis and on antiretroviral therapy: a retrospective cohort study. Germs. 2016;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendalem AA, Nigusie BT, Daniale TE. Survival and predictors of mortality amongchildren co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia: A retrospective cohort study. PLoS One. 2018;13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Refera H, Wencheko E. Survival of HIV-TB co-infected adult patients under ART. Ethiopian journal of health development. 2013;27(2) [Google Scholar]
- 4.The CDC Division of Global HIV & TB activities are implemented as part of the U.S. President’s Emergency Planfor AIDS Relief (PEPFAR); non-HIV related TB activities are supported by non-PEPFAR funding. July 2019.
- 5.Zahra T. Virological and immunological impact of tuberclosis on human immuno dificency virus type 1. J Infect Dis. 2003: 188(8). [DOI] [PubMed] [Google Scholar]
- 6.WHO. Estimated WHO TB mortality statistics for HIV positive people. 2018.
- 7.TUberculosis(TB)/CDC. Center for Disease Control and Prevention, division of Tuberculosis Elimination. December 13,2017.
- 8.Tiemersma EW, Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJD. Natural history of tuberculosis duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011;6(4). doi: 10.1371/journal.pone.0017601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hailay A, Birtukan T, Desalegn M, Amanuel T, Hafte K. Survival Experience and its Predictors among TB/HIV Co-infected Patients. Epidemiology (sunnyvale). 2015;Volume 5(Issue 3). [Google Scholar]
- 10.Swaminathan S, Nagendran G. HIV and tuberculosis in India. Journal of biosciences. 2008;33(4). doi: 10.1007/s12038-008-0071-2 [DOI] [PubMed] [Google Scholar]
- 11.Zar HJ, Cotton MF, Strauss S, Karpakis J, Hussey G, Schaaf HS, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomized controlled trial. BMJ. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Global tuberculosis report. Geneva, Switzerland: World Health Organization. 2016. [Google Scholar]
- 13.Tesfaye B, Alebel A, Gebrie A, Zegeye A, Tesema C, Kassie B. Prevalence of TB/HIV coinfection and its associated factors. PLoS One. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aung ZZ, Saw YM, Saw TN, Oo N, Aye HN, Aung S, et al. Survival rate and mortality risk factors among TB–HIV co-infected patients. International Journal of Infectious Diseases 2019;80:10–5. doi: 10.1016/j.ijid.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 15.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients withand without antiretroviral therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;43(1) [DOI] [PubMed] [Google Scholar]
- 16.Pawlowski A, Jansson M, Skold M, Rottenberg ME, Kallenius G. Tuberculosis and HIV Co- Infection. PLos Pathog. 2012,8(2) doi: 10.1371/journal.ppat.1002464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvadori N, Ngo-Giang-Huong N, Duclercq C, Kanjanavanit S, Ngampiyaskul C, Techakunakorn P, et al. Incidence of Tuberculosis and Associated Mortality in a Cohort of Human Immunodeficiency Virus-Infected Children Initiating Antiretroviral Therapy. Journal of the Pediatric Infectious Diseases Society. 2017, 6(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck WC, Oslen D, Kabue MM, Ahmed S, Nchama LK, Munthali A, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis coinfection. The International Journal of Tuberculosis and Lung Disease. 2013;17(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mwiru RS, Spiegelman D, Duggan C, Seage GR, Semu Helen, Chalamilla G, et al. Nutritional status and other baseline predictors of mortality among HIV-infected children initiating antiretroviral therapy in Tanzania. Journal of the International Association of Providers of AIDS Care (JIAPAC). 2015: 14(2) doi: 10.1177/2325957413500852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adejumo OA, Daniel OJ, Adebayo BI, Adejumo EN, Jaiyesimi EO, Akang G, et al. Treatment Outcomes of Childhood TB in Lagos, Nigeria. Journal of tropical pediatrics. 2016;62(2). doi: 10.1093/tropej/fmv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamla DD, Asadu C, Davies A, de Wagt A, Ilesanmi O, Adeyinka D, et al. Patching the gaps towards the 90-90-90 targets: outcomes of Nigerian children receiving antiretroviral treatment who are co-infected with tuberculosis. Journal of the International AIDS Society. 2015;18(6). doi: 10.7448/IAS.18.7.20251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zar HJ, Cotton MF, Strauss S, Karpakis J, Hussey G, Schaaf HS, et al. Isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomized controlled trial. BMJ. 2007;334(7585). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwara A, Roahen-Harrison S, Prystowsky E, Kissinger m R, Adams R, Mathison J, et al. Manifestations and outcome of extra-pulmonary tuberculosis: impact of human immunodeficiency virus co-infection. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2015;9(5). [PubMed] [Google Scholar]
- 24.Ingela P, Berhanu G, Judith B, Lulu M, Johan G. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. The Pediatric infectious disease journal. 2002;21(11). [DOI] [PubMed] [Google Scholar]
- 25.Isaakidis P, Paryani R, Khan S, Mansoor H, Manglani M, Valiyakath A, et al. Poor outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrugresistant tuberculosis in Mumbai, India. PLoS One. 2013;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achappa B, Madi D, Bhaskaran U, Ramapuram JT, Rao S, Mahalingam S. Adherence to Antiretroviral Therapy Among People Living with HIV. N Am J Med Sci. 2013;5(3). doi: 10.4103/1947-2714.109196 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DTA)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.