Abstract
Local anesthetic agents play a key role in the treatment and prevention of pain in children. Although generally safe and effective, as with any pharmacologic agent, adverse effects may occur with the administration of these medications. Systemic absorption or inadvertent systemic injection during bolus dosing or continuous infusion can result in local anesthetic systemic toxicity with life-threatening neurological and cardiac complications. The following article reviews the pharmacology of local anesthetic agents, outlines previous reports of systemic toxicity during regional anesthesia, and discusses prevention and treatment algorithms.
Keywords: adverse drug effect, amide, ester, local anesthetic agent, local anesthetic systemic toxicity, pediatric, review
Introduction
Advances in the understanding of pharmacology, improvement in techniques, and refinement of the equipment, including the widespread use of ultrasonography, have led to increased applications of regional anesthesia in infants and children.1–3 In the pediatric population, regional anesthesia can be used alone, as an alternative to general anesthesia, or more commonly combined with general anesthesia to decrease intraoperative anesthetic requirements and to provide prolonged postoperative analgesia, frequently without the use of opioids. The initial interest in regional anesthesia instead of general anesthesia gained popularity years ago in the former pre-term infant (<60 weeks' postgestational age) as a means of avoiding the potential for postoperative apnea following the administration of volatile anesthetic agents.4,5 More recently, various other factors have pushed the expansion of regional anesthesia in children including concerns of the ongoing opioid epidemic and the implementation of enhanced recovery after surgery protocols, for which regional anesthesia is an integral component of the perioperative anesthetic plan. Additionally, concerns regarding the potential neurocognitive effects of general anesthetic agents have led to a resurgence of the use of regional anesthesia instead of general anesthesia in neonates and infants.6–9
Regional anesthesia for intraoperative and postoperative analgesia has numerous benefits over systemic opioids by providing effective pain control with fewer opioid-related adverse effects.10 Although generally safe and effective, complications may occur related to placement of the needle or catheter and the subsequent administration of local anesthetic agents. The incidence of complications related to regional anesthesia in infants and children remains extremely low, with a reported incidence of transient neurological deficits in 2.4 cases per 10,000 and local anesthetic systemic toxicity (LAST) in 0.76 cases per 10,000 procedures.11 Systemic absorption during bolus dosing of continuous infusions as well as inadvertent systemic injection can result in LAST with life-threatening neurological and cardiac complications with potentially devastating effects.12 LAST extends beyond the arena of anesthesiology as these agents are used during a variety of procedures in the field of medicine and surgery, outside of the operating room, in the emergency department as well as the inpatient and outpatient setting. The following article reviews the pharmacology of local anesthetic agents, outlines previous reports of systemic toxicity during regional anesthesia, and discusses prevention and treatment algorithms.
Pharmacology of Local Anesthetic Agents
The chemical structure of the local anesthetic agents that are currently used in clinical practice include 2 organic rings, a lipophilic and hydrophilic component, that are linked by either an amide or ester bond.13 The type of bond linking the 2 rings is used to classify these agents as either esters or amides (Table 1). Esters are the older class of local anesthetic agents, dating back to 1884, when cocaine was used clinically for the first time by the Viennese ophthalmologist, Carl Kolle, as a topical anesthetic for ophthalmologic surgery to treat glaucoma.14 In 1943, Lofgren developed the first amide local anesthetic agent, lidocaine, which was later introduced into clinical use in 1948.15 Currently, the amides of the local anesthetic group that are used clinically include lidocaine, mepivacaine, prilocaine, bupivacaine, levobupivacaine, and ropivacaine while the esters include procaine, chloroprocaine, and tetracaine.
Table 1.
Local Anesthetic Agents: Esters and Amides
| Esters |
| Procaine |
| Tetracaine |
| Chloroprocaine |
| Amides |
| Lidocaine |
| Mepivacaine |
| Bupivacaine |
| Ropivacaine |
| Levobupivacaine |
| Etidocaine |
| Prilocaine |
The amides and esters differ in their site of metabolism, plasma half-lives, adverse effect profile, and potency.16 Esters undergo metabolism in the plasma by plasma cholinesterases, while amides undergo hepatic metabolism. The metabolism of the esters by plasma chlolinesterases results in a short plasma half-life, measured in seconds, with a decreased potential for toxicity, especially in neonates and infants (see below).17,18 Recently, there has been resurgence in the interest in using the ester local anesthetic 2-chloroprocaine for continuous epidural infusions for postoperative analgesia especially in neonates and infants, given its rapid systemic metabolism and limited potential for toxicity even during prolonged infusions.19
The amide anesthetic agents (lidocaine, bupivacaine, and ropivacaine) are the agents that are used most in clinical practice. As such, the majority of clinical and investigational information regarding LAST surrounds these agents. Lidocaine is used most for subcutaneous infiltration to provide dermal analgesia for superficial procedures. Bupivacaine and ropivacaine are used most in the practice of regional anesthesia in children for neuraxial and peripheral nerve blockade, given their relatively longer duration of action, which make them suitable for providing a prolonged period of analgesia following a single injection.20 The amides are metabolized by the hepatic cytochrome P450 enzyme system. Bupivacaine is metabolized to an active metabolite, 2′,6′-pipecoloxylidide by cytochrome P450 (CYP3A4) while ropivacaine is predominantly metabolized to the inactive metabolite, hydroxyropivacaine, by CYP1A2.21,22 The local anesthetic agents of the amide class including bupivacaine and ropivacaine are highly protein bound to α1-acid glycoprotein (AAG) and to a lesser extent to albumin. The plasma concentration of AAG at birth is approximately 20% to 50% of adult values, increasing to those seen in adults by 1 year of age.20 As such, the potential for toxicity is greater in neonates and infants because the concentration of unbound local anesthetics is higher owing to low levels of AAG and immaturity of the hepatic microsomal enzymes. These factors reduce the clearance of local anesthetic agents of the amide class, resulting in a terminal half-life 3 to 8 times longer in neonates than in adults.23–25
Unlike the amide local anesthetic agents, 2-chloroprocaine is rapidly metabolized by plasma cholinesterase independently of hepatic metabolic activity. The concentration and activity of plasma cholinesterases at birth is similar to that in adults, thereby resulting in the rapid metabolism of local anesthetic agent of the ester class. Studies of 2-chloroprocaine clearance and metabolism have reported a plasma half-life of seconds to minutes, with limited age-dependent changes.26,27 Continuous caudal epidural infusions with 2-chloroprocaine at an average dose of 2.8 ± 1 mL/kg/hr (84 ± 30 mg/kg/hr) administered during elective inguinal hernia repair resulted in a plasma concentration of 0.5 pg/mL in 1 infant and undetectable concentrations in 4 others from their cohort of 11 infants, ranging in postconceptual age from 35 to 49.5 weeks.28
Local Anesthetic Systemic Toxicity
LAST affects the CNS and the cardiovascular (CV) system (Table 2). The potential for CNS versus cardiovascular effects varies with the specific agents. With most local anesthetic agents, CNS toxicity occurs at doses and plasma concentrations lower than those that produce CV toxicity. Data from adult volunteers and experimental animals have demonstrated that CNS toxicity from lidocaine occurs at plasma concentrations of 8 to 10 mg/mL, whereas CV toxicity occurs at 20 mg/mL.29–31 This differential effect in the CNS versus CV toxicity provides some degree of safety because the CNS symptoms (seizures) are generally more amenable to treatment than the CV effects (arrhythmias and conduction blockade).
Table 2.
Systemic Toxicity of Local Anesthetic Agents *
| System Impacted or Clinical Effect | Toxicity |
|---|---|
| Central nervous system | Lightheadedness and dizziness |
| Circumoral paresthesia and numbness | |
| Visual and auditory disturbances | |
| Muscle twitching and tremors | |
| Generalized convulsions | |
| Direct cardiac effects | Depresses rapid phase of depolarization of Purkinje fibers leading to cardiac dysrhythmias |
| Depresses spontaneous pacemaker activity in the sinus node | |
| Negative inotropic effect by affecting calcium influx and triggered release | |
| Direct peripheral vascular effects | Biphasic effect; low concentrations cause vasoconstriction, high concentrations cause vasodilation |
| Increased pulmonary vascular resistance (animal studies) | |
| Ventricular arrhythmogenic effects | Ventricular fibrillation |
| Re-entrant arrhythmias including torsades de pointes |
* Acidosis and hypoxia markedly increase the toxicity of local anesthetic agents.
Research with adult volunteers has shown that the threshold for CNS toxicity is approximately 2 to 3 mg/L for total and 0.1 to 0.2 mg/L for unbound bupivacaine, levobupivacaine, and ropivacaine. Infants are more susceptible to develop CNS toxicity by bupivacaine at lower concentrations than the adult values.32 In healthy adult volunteers receiving continuous infusions, ropivacaine had a higher tolerated dose and greater unbound plasma concentration for CNS symptoms and less pronounced CVS symptoms at doses producing CNS symptoms than bupivacaine.33,34 These data suggest that it may offer some degree of safety in respect to toxicity when compared with bupivacaine.
At the level of the CNS, local anesthetic agents express toxicity by 2 mechanisms including first excitatory (activation) and then inhibitory (suppression) pathways mediated by N-methyl-D-aspartate and gamma amino-butyric acid (GABA) receptors, respectively.35 These receptor interactions are responsible for the genesis of clinical manifestations ranging from excitatory effects with taste alterations, circumoral numbness, lightheadedness, dizziness, tinnitus, muscle twitching, tremors, and ultimately tonic-clonic seizures. Seizures result from the blockade of inhibitory GABA pathways in the cerebral cortex, yielding unopposed activity of facilitatory neurons. With larger doses, CNS depression (inhibitory effects) predominates with unconsciousness, coma, respiratory arrest, and eventual electrical silence in the electroencephalogram. The CNS excitatory signs may be masked when regional anesthesia is performed while children and adults are receiving general anesthesia or sedation. When a patient is sedated or anesthetized, they would be unable to relate or communicate specific excitatory effects (e.g., taste alterations, circumoral numbness, lightheadedness, dizziness, or tinnitus). Likewise, the inhibitory effects of sedative and general anesthetic agents on the CNS would inhibit or blunt other excitatory effects such as muscle twitching, tremors, and tonic-clonic seizures. The excitatory CNS manifestations including seizures due to an elevated plasma concentration of local anesthetic agents are readily treated by the administration of intravenous anesthetic/sedative agents including benzodiazepines (preferred agent) such as lorazepam or midazolam, barbiturates, or propofol. However, these agents do not reverse or prevent the CV effects (see below). Hypercarbia and acidosis decrease the convulsive threshold of local anesthetic agents as well as potentiating their cardiotoxicity.
Cardiotoxicity related to local anesthetic agents includes both arrhythmias and myocardial depression. The spectrum of arrhythmias is variable including bradyarrhythmias, reentry tachyarrhythmias, and wide-complex arrhythmias. Extremely large doses, as may occur with the rapid injection of a bolus directly into the systemic circulation, can produce immediate cardiac arrest.36 Although CNS signs and symptoms generally present first, infants can develop cardiotoxicity prior to neurological manifestations.37 This may occur because neonates, infants, and toddlers are unable to communicate changes in sensorium related to the initial CNS signs of local anesthetic toxicity including taste alterations, circumoral numbness, lightheadedness, dizziness, or tinnitus. Furthermore, as noted above, regional anesthesia may be performed in infants and children as an adjunct to general anesthesia. As such the general anesthetic or sedative agents may blunt the initial excitatory CNS signs of LAST.
At the cellular level, local anesthetic agents block sodium and potassium channels, leading to reentry arrhythmias due to disruption of the intrinsic conduction system.38 The degree of sodium channel block is dependent on the state of that channel because local anesthetic agents have a higher affinity for channels in the open or inactivated states and a lower affinity for channels in the rested or closed state. Nerves with more rapid firing rates have greater susceptibility to blockade than nerves with low firing rates. Therefore, the intensity of the block may be higher in neonates and infants because they have a higher heart rate (firing rate) than adults. Blockade of sodium channels develops during the upstroke and plateau of the action potential and dissipates during the diastolic interval. By prolonging the depolarization phase, the affinity for the channels is increased, thus prolonging the presence of the local anesthetic agent molecule in the myocardium and therefore potentiating its toxicity.38
Local anesthetic agents also block the voltage-dependent calcium channels because their structure closely resembles that of the sodium channels. Calcium channels mediate synaptic transmission within cardiac muscle cells and are involved in the coupling of electrical excitability with mechanical contraction.39 This leads to a decrease in intracellular calcium release from the sarcoplasmic reticulum and depressed myocardial contractility. Inactivation of β-adrenergic receptors and depressed adenylate cyclase activity results in decreased generation of cyclic adenosine monophosphate. Bupivacaine can also accumulate in the mitochondria of metabolically active tissue and uncouple oxidative phosphorylation thereby decreasing ATP synthesis. The reduction in ATP production at this level results from decreased fatty acid oxidation because local anesthetic agents block fatty acid transport into the mitochondria by inhibiting carnitine acylcarnitine translocase. These effects result in overall decreased ATP production, which further depresses myocardial function thereby decreasing cardiac output. This secondarily results in tissue hypoxia and metabolic acidosis, which augment the cellular effects of LAST and further depresses myocardial contractility. Additionally, the changes in intracellular pH lead to ion trapping of the local anesthetic agent. These effects further emphasize the need for effective CPR and resuscitative efforts during LAST to reverse inadequate cardiac output and tissue hypoxia.
Prevention of LAST
Various strategies can be implemented during the performance of regional anesthesia in infants and children to limit the incidence of LAST (Table 3). Careful selection of the patient, choice of local anesthetic agent, use of adjunctive agents, and proper technique are instrumental in preventing LAST.40 During the performance of regional blockade, adequate hemodynamic and respiratory function are crucial because low cardiac states impede the delivery of local anesthetic agents to the liver and their subsequent metabolism. Hypoxemia and hypercarbia significantly increase the risk of toxicity related to these medications. Likewise, younger chronologic ages (neonate and infants) or co-morbid conditions including prematurity, hepatic, renal, or cardiac dysfunction can affect metabolism and the presence of binding proteins, thereby increasing the free fraction of the drug. Local anesthetic agents are also taken up by the skeletal muscle, thus patients with a low muscle mass, usually those at the extremes of age, are at higher risk for LAST. Smaller doses for both single bolus and continuous infusions are suggested in these patient populations.
Table 3.
Strategies to Reduce Local Anesthetic Systemic Toxicity
| Maintain adequate hemodynamic and respiratory function by ensuring adequate oxygenation and ventilation. |
| Identify high-risk groups (i.e., neonates and infants) that may require dosage modification. |
| Identify patient populations (i.e., comorbid hepatic, renal, and cardiac states) and adjust dosage as needed. |
| Identify administration to high-risk sites (i.e., interpleural and fascial plane blocks) and decrease dose by 20%–30% of maximum dose. |
| Adhere to dosing guidelines for both single bolus and continuous infusions. |
| Use lowest effective product concentration and smallest volume of local anesthetic agent. Use ultrasonography as needed to limit the volume required. |
| Consider using local anesthetic agents with lower risk of toxicity (e.g., ropivacaine versus bupivacaine; chloroprocaine). |
| Decrease systemic absorption of local anesthetic agent by using epinephrine. |
| Careful incremental aspiration and injection. |
| Identify inadvertent systemic injection via a test dose with epinephrine and the use of ultrasonography. |
Careful dose selection and attention to the technique of injection are also important in preventing LAST. The practice of intermittent aspiration and injection is encouraged. Even if no response is noted to the test dose, the volume of local anesthetic should be injected incrementally and aspiration performed prior to each injection, not just prior to the first injection. The aspiration method may not be reliable because the vessels can collapse with negative pressure and thereby yield a negative aspiration response. Slow administration, with constant monitoring of the patient for clinical signs of inadvertent systemic administration, will help in early detection of the toxicity (see below for test dosing and use of ultrasonography).
Some of the initial anecdotal reports of toxicity were noted with excessively high and inappropriate dosing regimens, which resulted in the first publications of dosing guidelines for continuous infusions.24,25,37 Although these dosing guidelines provide maximum doses that should be used for both bolus dosing and infusions, in many cases, even lower concentrations and volumes may be effective. When adjunctive agents are not used, effective analgesia can be achieved with a bupivacaine concentration of 0.125% and a ropivacaine concentration of 0.175% to 0.2% when using volumes of 1 mL/kg.41–44 Likewise, lower volumes may also be feasible as the analgesia following inguinal herniorrhaphy was equivalent with volumes of 0.7, 1, and 1.3 mL/kg of 0.175% bupivacaine with epinephrine.45 Volumes should be critically evaluated from the dermatomes required because the amount needed will vary for coverage of umbilical, inguinal, and penoscrotal procedures.
While caudal epidural blockade is generally placed as an adjunctive to general anesthesia, awake caudal blockade has also been used instead of general anesthesia to avoid the risks associated with general anesthesia in high-risk, former preterm infants (<60 weeks' postgestational age).46 When used as the sole anesthetic for inguinal herniorrhaphy, doses at or above the recommended amount, up to 3.5 to 4 mg/kg, have been suggested. Alternatively, spinal anesthesia with lower amounts (1–1.2 mg/kg) or caudal epidural anesthesia using a continuous infusion of chloroprocaine are appropriate alternatives to limit the amount of local anesthetic required or the potential for toxicity.28,47 The safety advantage with the use of chloroprocaine is demonstrated by 2 anecdotal reports of toxicity with the inadvertent systemic administration of chloroprocaine. Although adverse systemic effects were noted (CNS toxicity with altered consciousness, tonic-clonic movements, and mild oxygen desaturation in one patient and CV toxicity with a wide complex bradycardia in the other), the duration was short-lived and resuscitation easily achieved.48,49
The development of ultrasound technology has allowed anesthesiologists the ability to directly visualize nerves, neural plexuses, and fascial planes, thereby permitting the accurate placement of local anesthetic agents in closer proximity to neural structures than was previously possible. Prior to the application of ultrasound technology, regional anesthesia techniques were reliant on surface landmarks, the development of a paresthesia when the nerve was contacted, or eliciting motor movement with use of a nerve stimulator. All of these techniques generally required the use of a larger volume of the local anesthetic agent because the direct visualization of the nerve was not feasible.
Adjunctive analgesic agents may be added to the local anesthetic solution to augment analgesia and thereby decrease the concentration of the local anesthetic agent that is required to achieve effective blockade (Table 4).50 In addition to potentially augmenting analgesia and prolonging the duration of blockade, depending on the site of the block and its vascularity, epinephrine has been shown to decrease the plasma concentration of local anesthetic agents following regional blockade.51–53
Table 4.
Reported Adjunctive Agents for Regional Anesthesia *
| Class | Agents |
|---|---|
| Nucleoside | Adenosine |
| Alpha2-adrenergic agonists | Clonidine; dexmedetomidine |
| Adrenergic agonist | Epinephrine |
| Anti-inflammatory agents | Parecoxib; lornoxicam |
| Opioids | Buprenorphine; butorphanol; fentanyl; hydromorphone; morphine; sufentanil; tramadol |
| Corticosteroids | Dexamethasone |
| Benzodiazepine | Midazolam |
| NMDA antagonists | Ketamine; magnesium |
| Cholinesterase inhibitor | Neostigmine |
NMDA, N-methyl-D-aspartate
* Many of these medications are not approved for use in regional anesthesia techniques and their long-term safety has not been fully studied.
Additional dosing restrictions should take place for continuous infusions because the risk of toxicity may be even greater during prolonged infusions especially in neonates and infants. LAST has been reported days after starting a local anesthetic infusion.54 Careful dose restriction is needed especially in neonates and infants when using continuous infusions of epidural bupivacaine or ropivacaine.55–57 With epidural infusion rates of bupivacaine at 0.2 mg/kg/hr, increasing plasma concentrations were noted at 48 hours, leading the authors to caution against infusions beyond that period. However, other investigators noted stable plasma concentrations with epidural infusions of ropivacaine at 0.2 to 0.4 mg/kg/hr.57 Concerns with the variable pharmacokinetics of amide local anesthetic agents during prolonged infusions in neonates and infants have led to the increased use of 2-chloroprocaine for postoperative epidural infusions.58–61
One must also recognize the impact of the site of the block on the resultant plasma concentration of the local anesthetic agent. Areas with high vascularity (intrapleural and intercostal) result in higher plasma concentrations than less vascular areas (caudal). With the advent of newer regional anesthetic techniques including fascial plane blocks and local infiltration anesthesia, the incidence of toxicity may be higher because the targeted tissue planes are very vascular and these blocks require a high volume of local anesthetic agent to ensure adequate spread in fascial planes.62 The potential for high plasm concentration is further increased by the higher cardiac output and local blood flow of infants compared with adults. Specific recommendations for these techniques include strict attention to dosing guidelines with dosing based on lean body weight, the use of dilute local anesthetic solutions to allow the needed volumes, the addition of epinephrine to limit systemic absorption, the use of less cardiotoxic local anesthetic agents, and monitoring the patient for 30 to 45 minutes after the block to allow for the peak plasma concentrations to be achieved.62
In addition to the other measures outlined above, avoidance of systemic injection is paramount especially with the initial bolus dose. Given the potential flaws with intermittent aspiration, other techniques are needed to prevent inadvertent systemic injection. In addition to prolonging the duration of the block, augmenting analgesia, and decreasing the peak plasma concentration of the local anesthetic agent, the addition of epinephrine may also be used as a marker or test dose to identify inadvertent systemic injection.63 This test dose generally entails the administration of 0.1 mL/kg of the 5-μg/mL epinephrine and local anesthetic agent solution, which provides an epinephrine dose of 0.5 μg/kg. When this dose of epinephrine is injected intravascularly, it can generally be detected by changes in heart rate, blood pressure, or the ST-T segment of the electrocardiogram. Recent work has demonstrated the efficacy of ultrasonography in potentially being able to provide early detection and therefore avoidance of inadvertent systemic injection.64,65 The LAST episodes were reduced by 65% when comparing ultrasonography with conventional landmark techniques.64
Treatment of LAST
The clinical signs and symptoms of LAST can vary significantly and are impacted by the use of sedative or general anesthetic agents. Although regional blockade is rarely if ever performed during general anesthesia in adults, this practice is common in children. In adults, it has been reported that CNS manifestations occur 43% of the time, cardiovascular and hemodynamic manifestations 24% of the time, and a combination of the two in 33% of cases.65 However, cardiovascular symptoms are the primary manifestations in most of the pediatric cases, as the patient may be under general anesthesia or sedation.
Treatment starts with early identification of the signs and symptoms of impending LAST, including subtle CNS changes followed by immediate cessation of the bolus dose or continuous infusion. Once signs or symptoms of LAST are noted, treatment algorithms then direct attention to the control of oxygenation and ventilation to prevent or reverse hypoxia, hypercarbia, and acidosis. Resuscitation follows standard Pediatric Advanced Life Support guidelines. Central nervous system and CV treatment algorithms are outlined in the Figure.
Figure.
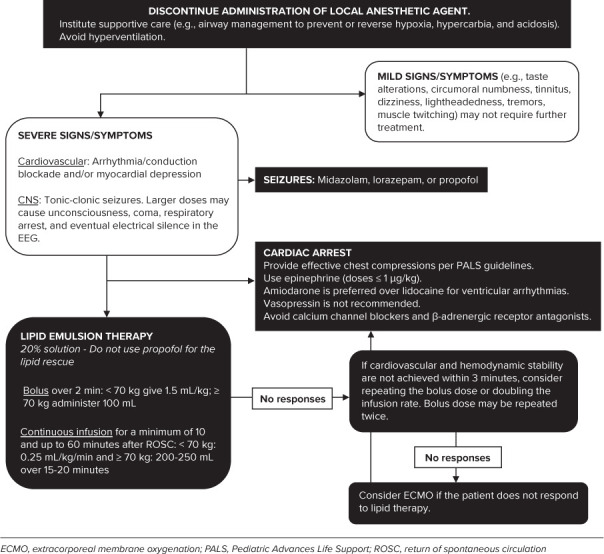
Treatment of Local Anesthetic Systemic Toxicity.
Lipid emulsion therapy was initially proposed for the management of LAST in 1998 and was accepted into clinical practice years later.66 The proposed mechanisms of action involve the hypothesis that the lipid emulsion creates an intravascular lipophilic sink into which lipid-soluble local anesthetic agents are partitioned and thereby removed from the active circulation and tissues. Further research has suggested other potential mechanisms of action for lipid emulsion therapy, including shuttling of the local anesthetic agents out of the heart and brain, cardiotonic or vasoactive effects, and postconditioning cardioprotective effects.67 The shuttling mechanisms suggest that the lipid molecules act as dynamic transporters of the local anesthetic molecules out of the highly perfused organs (brain and heart) with redistribution to organs that store and metabolize the drug. It is postulated that the positively charged, fat-soluble local anesthetic molecules bind to the negatively charged lipid particles. These pharmacokinetic attributes accelerate the redistribution of the local anesthetic agent, increase the α half-life in whole blood, while decreasing the concentration of the local anesthetic agent in the non-lipid fraction. The net effect is an acceleration of the elimination half-life.68–70 Lipid emulsions also increase cardiac contractility with an improvement of cardiac output and systemic blood flow, thereby enhancing the shuttling effect through augmentation of tissue perfusion. An increase in blood pressure through a poorly described effect on the peripheral vasculature has also reported.71 Recent animal studies have demonstrated that local anesthetic agents activate multiple cellular apoptotic pathways in cardiac cells, which are blocked or reversed by lipid emulsion therapy.72
Use of the checklist for treatment of LAST from the American Society of Regional Anesthesia has shown to be effective when used in simulations and helps the operator to follow the current recommendations in an appropriate manner.73 As such, these guidelines and appropriate doses of lipid emulsion should be readily available whenever local anesthetic agents are used. The practice advisory also recommends the prompt administration of lipid emulsion therapy at the first sign of arrhythmia, prolonged seizures, or rapid clinical deterioration of the patient during any suspected LAST event. Even though the maximum lipid emulsion dose approved by the US Food and Drug Administration has been increased to 12 mL/kg, the amount required for resuscitation is generally much less and dosing should be stopped as soon as it is considered safe because excessive dosing can have clinical consequences. Current dosing recommendations for lipid emulsion therapy are outlined in the Figure. Early initiation of effective CPR is important to ensure that coronary perfusion is preserved thus helping to reduce the myocardial concentration of the local anesthetic agent and attain maximum benefit from lipid emulsion therapy. Epinephrine at doses ≤1 μg/kg should be used to maintain blood pressure that does not respond to lipid emulsion therapy.
Summary
Local anesthetic agents play an integral role in the management in infants and children. Applications include superficial infiltration to provide cutaneous and dermal analgesia during minor invasive procedures as well as the performance of neuraxial and peripheral nerve blockade to provide surgical anesthesia and postoperative analgesia. By blocking sodium channels, these agents interrupt nocioception. Inadvertent high plasma concentrations related to bolus dosing or continuous infusions can lead to morbidity and even mortality. To ensure the safe and effective use of these agents, the practitioner should have a clear understanding of their mechanism of action, potential adverse effects, pharmacology, and dosing guidelines. Toxicity can generally be prevented by adherence to dosing guidelines as well as use of techniques to avoid inadvertent systemic administration. Should LAST occur, a thorough understanding of current guidelines for resuscitation, including the use of intralipid therapy, is suggested.
ABBREVIATIONS
- AAG
α1-acid glycoprotein
- ATP
adenosine triphosphate
- CNS
central nervous system
- CPR
cardiopulmonary resuscitation
- CV
cardiovascular
- GABA
gamma aminobutyric acid
- LAST
local anesthetic systemic toxicity
Footnotes
Disclosure. The authors declare no conflicts or financial interests in any products or services mentioned in the manuscript including grants, equipment, medications, employment, gifts, and honoraria.
Ethical Approval and Informed Consent. As this was a review article and did not involve human subjects research, institutional review board/ethics committee review was not required.
References
- 1.Lam DKM, Corry GN, Tsui BCH. Evidence for the use of ultrasound imaging in pediatric regional anesthesia: a systematic review. Reg Anesth Pain Med. 2016;41(2):229–241. doi: 10.1097/AAP.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 2.Suresh S, Wheeler M. Practical pediatric regional anesthesia. Anesthesiol Clin North Am. 2002;20(1):83–113. doi: 10.1016/s0889-8537(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 3.Goeller JK, Bhalla T, Tobias JD. Combined use of neur-axial and general anesthesia during major abdominal procedures in neonates and infants. Pediatr Anesth. 2014;24(6):553–560. doi: 10.1111/pan.12384. [DOI] [PubMed] [Google Scholar]
- 4.Welborn LG, Rice LJ, Hannallah RS et al. Postoperative apnea in former preterm infants: prospective comparison of spinal and general anesthesia. Anesthesiology. 1990;72(5):838–842. doi: 10.1097/00000542-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Harnik EV, Hoy GR, Potolicchio S et al. Spinal anesthesia in premature infants recovering from respiratory distress syndrome. Anesthesiology. 1986;64(1):95–99. doi: 10.1097/00000542-198601000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Disma N, O'Leary JD, Loepke AW et al. Anesthesia and the developing brain: a way forward for laboratory and clinical research. Paediatr Anaesth. 2018;28(9):758–763. doi: 10.1111/pan.13455. [DOI] [PubMed] [Google Scholar]
- 7.Davidson A. The effect of anaesthesia on the infant brain. Early Hum Dev. 2016;102(2):37–40. doi: 10.1016/j.earlhumdev.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Whitaker EE, Wiemann BZ, DaJusta DG et al. Spinal anesthesia for pediatric urological surgery: reducing the theoretic neurotoxic effects of general anesthesia. J Pediatr Urol. 2017;13(4):396–400. doi: 10.1016/j.jpurol.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Trifa M, Tumin D, Whitaker EE et al. Spinal anesthesia for surgery longer than 60 min in infants: experience from the first 2 years of a spinal anesthesia program. J Anesth. 2018;32(4):637–640. doi: 10.1007/s00540-018-2517-5. [DOI] [PubMed] [Google Scholar]
- 10.Bosenberg A. Benefits of regional anesthesia in children. Pediatr Anesth. 2012;22(1):10–18. doi: 10.1111/j.1460-9592.2011.03691.x. [DOI] [PubMed] [Google Scholar]
- 11.Walker BJ, Long JB, Sathyamoorthy M et al. Complications in pediatric regional anesthesia an analysis of more than 100,000 blocks from the pediatric regional anesthesia network. Anesthesiology. 2018;129(4):721–732. doi: 10.1097/ALN.0000000000002372. [DOI] [PubMed] [Google Scholar]
- 12.Neil JM, Barrington MJ, Fettiplace MR et al. The Third American Society of Regional Anesthesia and Pain Medicine practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2018;43(2):113–123. doi: 10.1097/AAP.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 13.Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012;59(2):90–101. doi: 10.2344/0003-3006-59.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink BR. Leaves and needles: the introduction of surgical local anesthesia. Anesthesiology. 1985;63(1):77–83. [PubMed] [Google Scholar]
- 15.Lazansky JP, Robinson L. A study of the effectiveness of xylocaine as a local anesthetic agent in dentistry. Oral Surg Oral Med Oral Pathol. 1949;10(2):1286–1297. doi: 10.1016/0030-4220(49)90400-4. [DOI] [PubMed] [Google Scholar]
- 16.Hassan HG, Renck H, Akerman B et al. On the relative potency of amino-amide local anaesthetics in vivo. Acta Anaesthesiol Scand. 1994;38(5):505–509. doi: 10.1111/j.1399-6576.1994.tb03937.x. [DOI] [PubMed] [Google Scholar]
- 17.Raj PP, Ohlweiler D, Hitt BA et al. Kinetics of local anesthetic esters and the effects of adjuvant drugs on 2-chloroprocaine hydrolysis. Anesthesiology. 1980;53(4):307–314. doi: 10.1097/00000542-198010000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Besunder JB, Reed MD, Blumer JL. Principles of drug biodisposition in the neonate: a critical evaluation of the pharmacokinetic–pharmacodynamic interface (part II) Clin Pharmacokinet. 1988;14(5):261–286. doi: 10.2165/00003088-198814050-00001. [DOI] [PubMed] [Google Scholar]
- 19.Veneziano G, Tobias JD. Chloroprocaine for epidural anesthesia in infants and children. Pediatr Anesth. 2017;27(6):581–590. doi: 10.1111/pan.13134. [DOI] [PubMed] [Google Scholar]
- 20.Mazoit JX, Denson DD, Samii K. Pharmacokinetics of bupivacaine following caudal anesthesia in infants. Anesthesiology. 1988;68(3):387–391. doi: 10.1097/00000542-198803000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Gantenbein M, Attolini L, Bruguerolle B et al. Oxidative metabolism of bupivacaine into pipecolylxylidine in humans is mainly catalyzed by CYP3A. Drug Metab Dispos. 2000;28(4):383–385. [PubMed] [Google Scholar]
- 22.Oda Y, Furuichi K, Tanaka K et al. Metabolism of a new local anesthetic, ropivacaine, by human hepatic cyto-chrome P450. Anesthesiology. 1995;82(1):214–220. doi: 10.1097/00000542-199501000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Hansen TG, Ilett KF, Reid C et al. Caudal ropivacaine in infants: population pharmacokinetics and plasma concentrations. Anesthesiology. 2001;94(4):579–584. doi: 10.1097/00000542-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Berde CB. Convulsions associated with pediatric regional anesthesia. Anesth Analg. 1992;75(2):164–166. doi: 10.1213/00000539-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Gutlove DP, Lockhart CH. Seizures occurring in pediatric patients receiving continuous infusion of bupivacaine. Anesth Analg. 1992;75(2):284–286. doi: 10.1213/00000539-199208000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Cox B, Durieux ME, Marcus MA. Toxicity of local anaesthetics. Best Pract Res Clin Anaesthesiol. 2003;17(1):111–116. doi: 10.1053/bean.2003.0275. [DOI] [PubMed] [Google Scholar]
- 27.Wilder RT. Local anesthetics for the pediatric patient. Pediatr Clin North Am. 2000;47(3):545–558. doi: 10.1016/s0031-3955(05)70225-x. [DOI] [PubMed] [Google Scholar]
- 28.Henderson K, Sethna NF, Berde CB. Continuous caudal anesthesia for inguinal hernia repair in former preterm infants. J Clin Anesth. 1993;26(5):129–133. doi: 10.1016/0952-8180(93)90140-a. [DOI] [PubMed] [Google Scholar]
- 29.Scott DB. Toxic effects of local anaesthetic agents on the central nervous system. Br J Anaesth. 1986;58(7):732–735. doi: 10.1093/bja/58.7.732. [DOI] [PubMed] [Google Scholar]
- 30.Wagman IH, DeJong RH, Prince DA. Effects of lidocaine on the central nervous system. Anesthesiology. 1967;28(1):155–172. doi: 10.1097/00000542-196701000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Moller RA, Covino BG. Cardiac electrophysiologic effects of lidocaine and bupivacaine. Anesth Analg. 1988;67(2):107–114. [PubMed] [Google Scholar]
- 32.Sugimoto M, Uchida I, Fukami S et al. The alpha and gamma subunit-dependent effects of local anesthetics on recombinant GABA(A) receptors. Eur J Pharmacol. 2000;401(3):329–337. doi: 10.1016/s0014-2999(00)00463-5. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen K, Beckman Suurkula M, Blomberg S et al. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78(5):507–514. doi: 10.1093/bja/78.5.507. [DOI] [PubMed] [Google Scholar]
- 34.Bardsley H, Gristwood R, Baker H et al. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1998;46(3):245–249. doi: 10.1046/j.1365-2125.1998.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luz G, Wieser C, Innerhofer P et al. Free and total bupivacaine plasma concentrations after continuous epidural anaesthesia in infants and children. Paediatr Anaesth. 1998;8(6):473–478. doi: 10.1046/j.1460-9592.1998.00285.x. [DOI] [PubMed] [Google Scholar]
- 36.Berde CB. Toxicity of local anesthetics in infants and children. J Pediatr. 1993;122(5):S14–S20. doi: 10.1016/s0022-3476(11)80004-1. [DOI] [PubMed] [Google Scholar]
- 37.Maxwell LG, Martin LD, Yaster M. Bupivacaine-induced cardiac toxicity in neonates: Successful treatment with intravenous phenytoin. Anesthesiology. 1994;80(3):682–686. doi: 10.1097/00000542-199403000-00028. [DOI] [PubMed] [Google Scholar]
- 38.Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89(1):52–61. doi: 10.1093/bja/aef163. [DOI] [PubMed] [Google Scholar]
- 39.Rossner KL, Freese KJ. Bupivacaine inhibition of l-type calcium current in ventricular cardiomyocytes of hamster. Anesthesiology. 1997;87(4):926–934. doi: 10.1097/00000542-199710000-00028. [DOI] [PubMed] [Google Scholar]
- 40.Ivani G, Suresh S, Ecoffey C et al. The European Society of Regional Anesthesia and Pain Medicine and the American Society of Regional Anesthesia and Pain Medicine joint committee practice advisory on controversial topics in pediatric regional anesthesia. Reg Anesth Pain Med. 2015;40(5):526–532. doi: 10.1097/AAP.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 41.Gunter JB, Dunn CM, Bennie JB et al. Optimum concentration of bupivacaine for combined caudal-general anesthesia in children. Anesthesiology. 1991;75(1):57–61. doi: 10.1097/00000542-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Ingelmo PM, Locatelli BG, Sonzogni V et al. Caudal 0.2% ropivacaine is less effective during surgery than 0.2% levobupivacaine and 0.2% bupivacaine: a double-blind, randomized, controlled trial. Paediatr Anaesth. 2006;16(9):955–961. doi: 10.1111/j.1460-9592.2006.01903.x. [DOI] [PubMed] [Google Scholar]
- 43.Karkera MM, Harrison DR, Aunspaugh JP, Martin TW. Assessing caudal block concentrations of bupivacaine with and without the addition of intravenous fentanyl on postoperative outcomes in pediatric patients: a retrospective review. Am J Ther. 2016;23(3):e792–e798. doi: 10.1097/MJT.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 44.Khalil S, Lingadevaru H, Bolos M et al. Caudal regional anesthesia, ropivacaine concentration, postoperative analgesia, and infants. Anesth Analg. 2006;102(2):395–399. doi: 10.1213/01.ane.0000194590.82645.4c. [DOI] [PubMed] [Google Scholar]
- 45.Schrock CR, Jones MB. The dose of caudal epidural analgesia and duration of postoperative analgesia. Paediatr Anaesth. 2003;13(5):403–408. doi: 10.1046/j.1460-9592.2003.01078.x. [DOI] [PubMed] [Google Scholar]
- 46.Spear RM, Deshpande JK, Maxwell LG. Caudal anesthesia in the awake, high-risk infant. Anesthesiology. 1988;69(3):407–409. doi: 10.1097/00000542-198809000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Williams RK, Adams DC, Aladjem EV et al. The safety and efficacy of spinal anesthesia for surgery in infants: the Vermont Infant Spinal Registry. Anesth Analg. 2006;102(1):67–71. doi: 10.1213/01.ANE.0000159162.86033.21. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez MA, Boretsky K. Chloroprocaine: local anesthetic systemic toxicity in a 9-month infant with paravertebral catheter. Pediatr Anesth. 2016;26(6):665–666. doi: 10.1111/pan.12912. [DOI] [PubMed] [Google Scholar]
- 49.Cladis FP, Litman RS. Transient cardiovascular toxicity with unintentional intravascular injection of 3% 2-chloroprocaine in a 2-month old infant. Anesthesiology. 2004;100(1):181–183. doi: 10.1097/00000542-200401000-00030. [DOI] [PubMed] [Google Scholar]
- 50.Swain A, Nag DB, Sahu S, Samaddar DP. Adjuvants to local anesthetics: current understanding and future trends. World J Clin Cases. 2017;5(8):307–322. doi: 10.12998/wjcc.v5.i8.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warner MA, Kunkel SE, Offord KO et al. The effects of age, epinephrine, and operative site on duration of caudal analgesia in pediatric patients. Anesth Analg. 1987;66(10):995–998. [PubMed] [Google Scholar]
- 52.Burm AG, van Kleef JW, Gladines MP et al. Epidural anesthesia with lidocaine and bupivacaine: effects of epinephrine on the plasma concentration profiles. Anesth Analg. 1986;65(12):1281–1284. [PubMed] [Google Scholar]
- 53.Miranda P, Corvetto MA, Altermatt FR et al. Levobupivacaine absorption pharmacokinetics with and without epinephrine during TAP block: analysis of doses based on the associated risk of local anaesthetic toxicity. Eur J Clin Pharmacol. 2016;72(10):1221–1227. doi: 10.1007/s00228-016-2086-1. [DOI] [PubMed] [Google Scholar]
- 54.McCloskey JJ, Haun SE, Deshpande JK. Bupivacaine toxicity secondary to continuous caudal epidural infusion in children. Anesth Analg. 1992;75(2):287–290. doi: 10.1213/00000539-199208000-00024. [DOI] [PubMed] [Google Scholar]
- 55.Peutrell JM, Holder K, Gregory M. Plasma bupivacaine concentrations associated with continuous extradural infusions in babies. Br J Anaesth. 1997;78(2):160–162. doi: 10.1093/bja/78.2.160. [DOI] [PubMed] [Google Scholar]
- 56.Larsson BA, Lönnqvist PA, Olsson GL. Plasma concentrations of bupivacaine in neonates after continuous epidural infusion. Anesth Analg. 1997;84(3):501–505. doi: 10.1097/00000539-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Bosenberg AT, Thomas J, Cronje L et al. Pharmacokinetics and efficacy of ropivacaine for continuous epidural infusion in neonates and infants. Pediatr Anesth. 2005;15(9):739–749. doi: 10.1111/j.1460-9592.2004.01550.x. [DOI] [PubMed] [Google Scholar]
- 58.Ross EL, Reiter PD, Murphy ME et al. Evaluation of prolonged epidural chloroprocaine for postoperative analgesia in infants. J Clin Anesth. 2015;27(6):463–469. doi: 10.1016/j.jclinane.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Veneziano G, Iliev P, Tripi J et al. Continuous chloroprocaine infusion for thoracic and caudal epidurals as a postoperative analgesia modality in neonates, infants, and children. Pediatr Anesth. 2015;26(1):84–91. doi: 10.1111/pan.12807. [DOI] [PubMed] [Google Scholar]
- 60.Muhly WT, Gurnaney HG, Kraemer FW et al. A retrospective comparison of ropivacaine and 2-chloroprocaine continuous thoracic epidural analgesia for management of post-thoracotomy pain infants. Pediatr Anesth. 2015;25(11):1162–1167. doi: 10.1111/pan.12745. [DOI] [PubMed] [Google Scholar]
- 61.Kamata M, Corridore M, Tobias JD. Thoracic epidural infusion with chloroprocaine for postoperative analgesia following epicardial pacemaker placement in an infant. J Pain Res. 2014;7:609–613. doi: 10.2147/JPR.S73309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chin KJ, McDonnell JG, Carvalho B et al. Essentials of our current understanding: abdominal wall blocks. Reg Anesth Pain Med. 2017;42(2):133–183. doi: 10.1097/AAP.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 63.Tobias JD. Caudal epidural block: test dosing and recognition of systemic injection. Anesth Analg. 2001;93(5):1156–1161. doi: 10.1097/00000539-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 64.Barrington MJ, Kluger R. Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve blockade. Reg Anesth Pain Med. 2013;38(4):289–299. doi: 10.1097/AAP.0b013e318292669b. [DOI] [PubMed] [Google Scholar]
- 65.Gitman M, Barrington MJ. Local anesthetic systemic toxicity: a review of recent case reports and registries. Reg Anesth Pain Med. 2018;43(2):124–130. doi: 10.1097/AAP.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 66.Weinberg GL, VadeBoncouer T, Ramaraju GA et al. Pre-treatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88(4):1071–1075. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 67.Fettiplace MR, Weinberg G. The mechanisms underlying lipid resuscitation therapy. Reg Anesth Pain Med. 2018;43(2):138–149. doi: 10.1097/AAP.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 68.Dureau P, Charbit B, Nicolas N et al. Effect of Intralipid® on the dose of ropivacaine or levobupivacaine tolerated by volunteers. Anesthesiology. 2016;125(3):474–483. doi: 10.1097/ALN.0000000000001230. [DOI] [PubMed] [Google Scholar]
- 69.Shi K, Xia Y, Wang Q et al. The effect of lipid emulsion on pharmacokinetics and tissue distribution of bupivacaine in rats. Anesth Analg. 2013;116(4):804–809. doi: 10.1213/ANE.0b013e318284123e. [DOI] [PubMed] [Google Scholar]
- 70.Heinonen JA, Litonius E, Salmi T et al. Intravenous lipid emulsion given to volunteers does not affect symptoms of lidocaine brain toxicity. Basic Clin Pharmacol Toxicol. 2015;116(4):378–383. doi: 10.1111/bcpt.12321. [DOI] [PubMed] [Google Scholar]
- 71.Fettiplace MR, Ripper R, Lis K et al. Rapid cardio-tonic effects of lipid emulsion infusion. Crit Care Med. 2013;41(8):e156–e162. doi: 10.1097/CCM.0b013e318287f874. [DOI] [PubMed] [Google Scholar]
- 72.Rahman S, Li J, Bopassa JCJ et al. Phosphorylation of GSK-3b mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology. 2011;115(2):242–253. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McEvoy MD, Hand WR, Stoll WD et al. Adherence to guidelines for the management of local anesthetic systemic toxicity is improved by an electronic decision support tool and designated “reader”. Reg Anesth Pain Med. 2014;39(4):299–305. doi: 10.1097/AAP.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]


