Abstract
Nutritional fermentation aids [dried Aspergillus oryzae fermentation product (AO)] are used in livestock production to increase nutrient digestion and production efficiency. The objective was to determine AO impact on neutral detergent fiber (NDF) degradation of selected forage sources (FS). A series of in vitro fermentation experiments were conducted using rumen fluid (RF) from rumen fistulated dairy heifers or dairy goats evaluating AO at 0.0, 0.3, or 0.6 g/L inclusion rates. In experiment I, the optimum AO concentration using alfalfa hay (AH), Bermuda grass (BG) hay, and peanut skins (PS) was determined via 48-h in vitro neutral detergent fiber digestion (IVNDFd). In experiment II, 0.0 g/L and 0.3 g/L AO were used to determine in vitro dry matter digestion (IVDMD), in vitro organic matter digestion (IVOMD), IVNDFd, and NDF digestion kinetics. In experiment III, in vivo AO ruminal adaptation (AD) and withdrawal (WD) times were determined for both dairy heifers and goats on IVDMD, IVOMD, IVNDFd, and NDF digestion kinetics. In experiment I, IVNDFd was similar using RF from dairy heifers or goats with IVNDFd being increased 10%, 28%, and 23% for AH, BG, and PS, respectively, at 0.3 g/L of AO compared with 0.0 g/L AO, while adding 0.6 g/L AO reduced IVNDFd among all FS. In experiment II, IVNDFd was greater when adding 0.0 g/L AO compared with 0.3 g/L AO using dairy goat RF (26.7% and 37.6%, respectively) among all FS. The mean retention time and 50% digestion times were greater, while digestion rate was lower for PS compared to AH and BG. In vitro dry matter (DM) and organic matter (OM) digestibilities were greater with AO for AH and BG compared to PS but varied with RF donor source. In experiment III, in vitro DM digestibility increased then decreased with adaptation time, while AO withdrawal increased digestion of DM, OM, and NDF. The NDF digestion kinetics were similar across all FS (AH, BG, and PS), which resulted in no clear determination of AO adaptation and withdrawal times needed for AO efficacy. The optimal AO inclusion rate was determined to be 0.3 g/L for improving in vitro NDF digestion, but subsequent experiments could not confirm that inclusion rate. Inclusion rates greater than 0.3 g/L depressed NDF degradation, which should be avoided due to depression of NDF digestion. Sourcing ruminal fluid from dairy heifers or goats for conducting in vitro fermentations resulted in similar DM, OM, and NDF digestion and NDF degradation kinetics.
Keywords: Aspergillus oryzae, dairy goats, dairy cows
INTRODUCTION
Dairy cow and goat producers have experienced economic hardships several times during the past decades due to varying feed prices (i.e., corn $0.12–$0.28/kg; Macrotrends, 2021), resulting in small or negative profit margins. Additional hardships include, but are not limited to, resource utilization and environmental challenges. In addition, forage shortages due to drought or storm damages have influenced dairy producer’s profitability. Dairy producers are consistently looking for new and innovative technologies to utilize previously considered poor quality forages. Increasing feed efficiency with fermentation aides, such as dried Aspergillus oryzae fermentation product (AO) provide dairy producers an innovative technology to improve ruminal digestibility of forage sources (FS) having a high neutral detergent fiber (NDF) content. Dried AO is grown on cellulose via fermentation to produce a fungal AO product as a feed additive that enhances ruminal fermentation to utilize fibrous feeds more efficiently (Yoon and Stern, 1995; Chen et al., 2004). Ration AO supplementation has improved livestock performance via increasing feedlot cattle BW gains and for dairy cows reducing heat stress and increasing milk production (Hatfield and Hixon, 1975; Marcus et al.,1986; Yoon and Stern, 1995). Previous studies have not determined optimal AO inclusion rates, the required time for ruminal adaptation for maximum ruminal digestion and feed utilization (Arambel et al., 1987, Martin and Nisbet, 1990, Newbold el al., 1992, Denigan et al., 1992, Behark and Nagaraja 1993, Varel et al., 1993, Varel and Kreikemeier, 1994), and the time for carryover effects to persist as ruminal fermentation stabilizes to AO withdrawal.
The dairy goat industry continues to develop with increasing numbers of dairy goat operations and goat numbers, which has increased the necessity for evaluating goat-specific nutritional technologies. For example, a direct comparison between dairy cattle and dairy goats utilizing AO effects on cell wall digestion to our knowledge does not exist in the literature. Adapting dairy cow data to dairy goats could lead to erroneous feeding decisions due to differences in gastrointestinal tract retention times, ruminal turnover rates (Huston et al., 1986), and the longer ruminal forage retention times with increasing fibrous carbohydrate utilization by dairy cattle compared to dairy goats (Huston, 1978).
Few in vitro experiments exist evaluating AO impacts on cell wall digestibility, so the efforts here were directed to evaluating AO influencing the degradation of three typical FS being alfalfa hay (AH), Bermuda grass (BG), and peanut skins (PS), using rumen fluid (RF) from dairy heifers and dairy goats. Alfalfa hay and BG were chosen as common FS, while PS were chosen due to their low dry matter (DM) and NDF digestion (NRC, 2001; Hill, 2002). The hypothesis was that dairy heifer or dairy goat RF would result in similar digestion responses when fed without or with AO. The study objective(s) were to determine an optimal AO inclusion rate for in vitro NDF fermentation of AH, BG, and PS, determine the adaptation/withdrawal time periods required for ruminal AO digestion responses, and if RF sourced from dairy heifers and dairy goats impacted digestion estimates.
MATERIAL AND METHODS
The study was conducted at Tuskegee University (Tuskegee, AL), and all animal procedures were approved by the Animal Care and Use Committee by the School of Agriculture and Home Economics. Four ruminally cannulated dry nonpregnant ruminant animals were used consisting of two similar size 2-yr-old nonpregnant Holstein heifers (~500 kg) and two similar mature size (~19 kg) nonpregnant Nubian goat does. The dairy heifers had access to an exercise paddock having little available mixed grass, while the dairy goats had access to a similar exercise paddock with little mixed grass. The ingredient composition of the pelleted grain mix is given in Table 1 (Marion Junction Farmer CO-OP, Dallas, AL). The dairy heifers were fed 4.54 kg/animal once daily (grain ~0.90% of BW) and the dairy goats were confined in the evening and fed once daily 0.91 kg/animal (grain ~4.80% of BW) of the same pelleted grain mix [18.9% crude protein (CP) DM basis]. The dairy heifers and dairy goats were free choice supplemented with the same mature mixed BG hay supply as the paddocks for ad libitum intake.
Table 1.
Ingredient composition of pelleted grain mix
| Ingredient | % of Mix |
|---|---|
| Corn, ground | 47.8 |
| Alfalfa meal | 25.0 |
| Soybean hulls | 15.0 |
| Soybean meal | 6.0 |
| Molasses | 2.5 |
| Dicalcium phosphate | 0.5 |
| Mineral and vitamins | 0.5 |
| Salt | 0.2 |
| Masonexa | 2.5 |
Pelleted grain mix containing 84.69% dry matter with 18.9% crude protein dry matter basis.
a Synthetic hemicellulose.
Feed ingredients selected were AH, BG, and PS for evaluating AO impact on nutrient digestibility. Both AH and BG were sourced from local AL producers, while PS were donated by Universal Blanchers, Inc. (Blakley, GA). Aspergillus oryzae fermentation product (dry Amaferm product) was provided by Biozyme Inc. (St. Joseph, MO). All FS were dried for 48 h at 60 °C and ground through a Wiley mill (Thomas Scientific, Swedesboro, NJ) having a 1-mm screen. Analysis of forage nutrient composition (Table 2) was conducted using standard AOAC International (2019) laboratory procedures for DM (930.15), CP (990.03), NDF (2002.04), ADF (973.18), lignin (973.18), and ash (942.05). Hemicellulose and cellulose concentrations were determined by the equations of NDF–ADF and ADF-lignin, respectively.
Table 2.
Nutrient composition of AH, BG hay, and PS
| Ingredients used in experiments | |||
|---|---|---|---|
| Nutrient | AH | BG | PS |
| Dry matter % | 89.34 | 95.57 | 94.87 |
| ------------------- (% of DM) --------------------- | |||
| Crude protein, % | 13.80 | 6.10 | 14.07 |
| NDF, % | 55.86 | 76.46 | 55.93 |
| ADF, % | 43.94 | 36.80 | 33.75 |
| Hemicellulose, % | 11.92 | 39.66 | 22.22 |
| Cellulose % | 27.42 | 18.76 | 16.88 |
| Lignin, % | 9.09 | 6.35 | 6.86 |
| Ash, % | 7.16 | 4.75 | 2.42 |
RF Collections for In Vitro Fermentations
Dairy heifers were fed BG hay 3–4 h prior to RF collection and the grain mix was offered after RF collection, while the dairy goats were pen confined until RF was collected and then released to graze. Rumen fluid was collected via the rumen cannula from both animals per species and combined in an insulated prewarmed thermos and transported to the laboratory (Goering and Van Soest, 1970; Yáñex-Ruiz, et al., 2016). Rumen fluid was then strained through four layers of cheese cloth being flushed with CO2. For experiments I and II, the AO was added directly to a 236.6-mL glass baby bottle (Gerber, Florham Park, NJ) used as the in vitro fermentation bottle. The AO concentrations of 0.3 g/L (0.012 g/bottle) and 0.6 g/L (0.025 g/bottle) were calculated based on the assumption of 40 mL of buffer solution being added to each bottle. For experiment III, AO was encapsulated in a gelatin capsule (2.63 g/d and 0.26 g/d for dairy heifers and dairy goats, respectively) and inserted through the rumen cannula and placed just below the rumen mat 4 h prior to RF collection.
For experiment I, 10 mL of strained dairy heifer or dairy goat RF was added to 40 mL of micromineral and micromineral phosphate buffer (Goering and Van Soest, 1970) containing AO at 0.0, 0.3, or 0.6 g/L per serum bottle with each bottle containing 1 g of AH, BG, or PS (18 treatment combinations), which represents approximately 12 and 25 g/kg forage. Fermentations were stopped by immediately opening the bottles and cooling to room temperature for subsequent sample collections. Three replicated fermentations were conducted with samples in triplicate (nine total observations per treatment) for 48 h at 39 °C, and residues were analyzed for DM (930.1) and NDF (2002.04). Experiment II was replicated twice with AO added at 0.0 or 0.3 g/L per serum bottle in duplicate with 1 g of AH, BG, and PS incubated for 0, 6, 12, 24, 48, 72, and 96 h, and the residues were analyzed for DM (930.15), OM (Ash: 942.05), and NDF (2002.04) to calculate in vitro dry matter digestion (IVDMD), in vitro organic matter digestion (IVOMD), and in vitro neutral detergent fiber digestion (IVNDFd). For experiment III to assess ruminal AO adaptation (12 treatment combinations), feed samples were incubated in duplicate for 0, 6, 12, 24, 48, 72, and 96 h. Rumen fluid was collected every 2 wk for 8 wk, after which AO addition was discontinued and RF was collected twice at 2-wk intervals (4 wk time period).
Calculations and Statistical Analyses
To estimate the amount of potentially digestible NDF (PDNDF) at each incubation time point, the undigested NDF fraction at 96 h was subtracted from the NDF fraction remaining at each incubation time point. Each ingredient data set was subjected to an iterative nonlinear least-squares regression analysis using first-order kinetics regression model with a lag phase (Solaiman, 1984; Salaiman et al., 1990) using the PROC NLIN SAS (SAS Institute Inc., Cary, NC) procedure. The Salaiman et al. (1990) regression model was a modification of passage rate regression models (Weiss, 1979) that was adapted to digestion data by Solaiman (1984). The PDNDF model is: PDNDFi = PDNDFd0x e-k (t-l) + IDNDF96, where PDNDFi = potential digestible NDF at any given time point; NDFi = cell wall residue at any given time point; IDNDF96 = indigestible NDF (NDF remaining at 96 h); t = time point of in vitro ruminal digestion; l = lag time; and -k = rate of NDF digestion.
Prior to statistical analysis, all data were checked for normality and outliers using the Univariate procedure of SAS (version 9.4, SAS Institute Inc., Cary, NC). The box and whisker plots and Shapiro–Wilk Test were used to verify that data were normally distributed (P > 0.15). All data were then subjected to least-square analysis of variance (ANOVA) using SAS’s PROC MIXED (version 9.4, SAS Institute Inc., Cary, NC). For experiment I, a completely randomized design (CRD) having a factorial arrangement of treatments (Steele and Torrie, 1980) accordingly was used having the following statistical model: Yijk = µ + AOi + FSj + RFk + (AOi × FSj) + (AOi × RFk) + (FSj × RFk) + (AOi × FSj × RFk) + eijk, where Yijk = dependent variable; µ = overall mean; AOi = main effect of dried AO(j=0.0, 0.3 or 0.6 g/L); FSj = main effect of forage source(j = AH, BG, or PS); RFk = main effect of RF source(k =dairy heifer or dairy goat); AOi × FSj = interaction of AO by FS; AOi × RFk = interaction of AO by RF source; FSj × RFk = interaction of FS by RF source; AOi × FSj × RFk = interaction of AO by FS by RF source; and eijk = the residual error. The main effects of AO, FS, RF and all interactions were fixed. Polynomial contrasts were used to test the linear and quadratic effects of AO (i.e., AO inclusion rate). Significance was declared at P < 0.05 and trends at 0.05 < P ≤ 0.10.
Experiment II data were subjected to least-square ANOVA using SAS’s PROC MIXED (version 9.4, SAS Institute Inc., Cary, NC) using a CRD having a factorial arrangement of treatments (Steele and Torrie, 1980) according to the following statistical model: Yijk = µ + AOi + FSj + RFk + (AOi × FSj) + (AOi × RFk) + (FSj × RFk) + (AOi × FStj × RFk) + eijk, where Yhijk = dependent variable; µ = overall mean; AOi = main effect of AO (j=0.0 or 0.3); FSj = main effect of FS(j=AH, BG, or PS); RFk = main effect of RF source(k =dairy heifer or dairy goat); AOi × FSj = interaction of AO by FS; AOi × RFk = interaction of AO by RF source; FSj × RFk = interaction of FS by RF source; AOi × FSj × RFk = interaction of AO by FS by RF source; and eijk = the residual error. The main effects of AO, FS, RF and interactions were fixed. The -k values were determined by regressing the calculated amount of PDNDF on time points 0, 6, 12, 24, 48, 72, and 96 h of incubation. Significance was declared at P < 0.05 and trends at 0.05 < P ≤ 0.10.
Experiment III data were subjected to least-square ANOVA using SAS’s PROC MIXED (version 9.4, SAS Institute Inc., Cary, NC) using a CRD having a factorial arrangement of treatments (Steele and Torrie, 1980) according to the following statistical model: Yijkl = µ + AOi + FSj + RFk + Timel + (AOi × FSj) + (AOi × RFk) + (FSj × RFk) + (AOi × FSj × RFk) + eijkl, where Yijkl = dependent variable; µ = overall mean; AOi = AO main effect(j=0.0 or 0.3); FSj = main effect of FS(j=AH, BG, or PS); RFk = main effect of RF source(k =dairy heifer or dairy goat); Timel = Time period of adaptation or removal of AO(l = 2, 4, 6, and 8, 2, and 8); AOi × FSj = interaction of AO by FS; AOi × RFk = interaction of AO by RF source; FSj × RFk = interaction of FS by RF source; AOi × FSj × RFk = interaction of AO by FS by RF source; and ehijk = the residual error. The main effects of AO, FS, and RF source and interactions were considered fixed. Time (week of adaptation or removal) was considered a repeated measurement in time having an autoregressive covariance structure. The k values were determined by regressing the calculated amount of PDNDF on time points 0, 6, 12, 24,48, 72, and 96 h of incubation. Significance was declared at P < 0.05 and trends at 0.05 < P ≤ 0.10.
RESULTS AND DISCUSSION
Feed Analysis
The nutrient composition of the AH was considerably lower in CP and slightly lower in hemicellulose concentrations but much greater in NDF, ADF, lignin, cellulose, and ash concentrations compared to NRC (2001) tabular values for a mature legume hay having greater than 46% NDF. Thus, this AH would be a poor-quality and a poorly digestible FS given the nutrient concentrations. The BG hay was greater in CP, cellulose, and ash concentrations but lower in NDF, hemicellulose and lignin concentrations, while ADF and lignin concentrations were very similar to NRC (2001) tabular concentrations. The PS used in this study were lower in CP, cellulose, and lignin but dramatically higher in NDF, ADF, hemicellulose, lignin, and ash concentrations than tabular values published by Hill (2002). The NRC (2001) did not publish nutrient concentrations of PS, but given these nutrient concentrations, the PS used here would be considered more of an FS or nonforage fiber source that might be somewhat comparable to soy hulls.
Experiment I
The two-way and three-way interactions between AO inclusion rate, FS, and RF source were similar (P > 0.53; Table 3). The AO inclusion rate main effect resulted in a significant quadratic response (P < 0.01) with increasing AO inclusion demonstrating an increase (P < 0.05) in NDF digestibility (NDFd) at 0.3 g/L followed by a significant reduction in NDFd at the 0.6 g/L compared with 0.0 and 0.3 g/L AO. The NDFd was greater (P < 0.05) for AH compared with BG, which was greater (P < 0.05) than PS (Table 4). The RF source main effect was nonsignificant (P > 0.65), which indicates that NDFd was responding similarly among FS when using RF from both dairy heifers and dairy goats as an inoculum source for conducting in vitro fermentations.
Table 3.
Probably of significant F-test for main effects and interactions for NDFd for dried AO, FS, or RF for experiment I
| Main effects, P < a | Main effects and interactions, P < a | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | AOb | FSc | RFd | AO × FS | AO × RF | FS × RF | AO × FS × RF |
| NDFd, % | 0.01 | 0.01 | 0.65 | 0.73 | 0.53 | 0.75 | 0.75 |
a Probably of F-tests for main effects and interactions.
b AO levels were 0.0, 0.3, and 0.6 g/L.
c FS were AH, BG hay, and PS.
d RF source was from either dairy heifer or goat.
Table 4.
Main effects of dried AO, FS, and RF source for experiment 1
| a. Inclusion rate of Aspergillus oryzae at 0.0, 0.3, and 0.6 g/L on NDFd | ||||
| Aspergillus oryzae inclusion rate, g/L | ||||
| Measurementa,b | 0 | 0.3 | 0.6 | SEM |
| NDFd, % of NDF | 39.7b | 47.3a | 33.3c | 1.89 |
| b. NDFd of AH, BG hay, and PS | ||||
| FS | ||||
| Measurement | AH | BG | PS | SEM |
| NDFd, % of NDF | 49.4a | 37.5b | 33.3c | 1.89 |
| c. NDFd when sourcing RF from dairy heifers or goats | ||||
| RF source | ||||
| Measurement | Dairy heifer | Dairy goat | SEM | |
| NDFd, % of NDF | 40.5 | 39.7 | 1.28 |
a Linear contrast of dried AO inclusion rate, P < 0.01.
b Quadratic contrast of dried AO inclusion rate, P < 0.01.
a, b, cMeans within the same row with unlike superscripts differ, P < 0.05.
These data indicate that a certain amount of AO (0.3 g/L) included in the ration can be beneficial to enhancing NDFd but that feeding higher amounts may not improve NDFd and excessive inclusion rates could be detrimental to NDFd. However, these data support our hypothesis that feeding AO can improve feed or fiber digestibility across the three FS evaluated (i.e., AH, BG, and PS). These results are in agreement with Chen et al. (2004), who reported that the addition of 0.4 AO g/L of fermentation solution increased AH IVNDFd from 41.6% to 46.1%, but evaluation with peanut vine and Bermuda straw resulted in similar IVNDFd. Martin and Nisbet (1990) reported that AO supplementation at 0, 0.4, and 1.0 g/L of fermentation solution to BG decreased IVNDFd (59.9%, 59.1%, and 55.1%, respectively), indicating that higher AO concentrations decreases IVNDFd, in agreement with these results for AH, BG, and PS. Beharka and Nagaraja (1993) using rumen-cannulated Holstein steers evaluated increasing rates of AO (0, 0.8, and 1.2 g/L fermentation solution) demonstrated that 0.8 and 1.2 g/L of AO increased in vitro NDFd of alfalfa and bromegrass hay compared to 0.0 g/L. However, the in vitro NDFd and ADFd within corn silage, prairie hay, and wheat straw samples were similar (Beharka and Nagaraja, 1993) among increasing AO inclusion rates. The difference between the BG results in this experiment and previous studies (Martin and Nisbet,1990; Gomez-Alarcon et al., 1990; Beharka and Nagaraja, 1993; Chen et al., 2004) could be due to AH or BG varieties, forage maturity, and/or nutrient concentrations. Grass is typically more digestible than legumes, but the BG NDF concentration (Table 2) was very high, indicating that the BG was very mature, so that AH NDF was more digestible. In addition, the PS are known to be high in phenolic (lignin) compounds but the lignocellulose complex digestibility was improved by 0.3 g/L AO inclusion rate. However, these results could have potentially been further improved if the PS would not have been sticking to the sides of the fermentation bottles.
Experiment II
There was a significant AO × FS × RF interaction (P < 0.02) for DMD and OMD in experiment 2 (Table 5), but all main effect interactions were similar (P > 0.15) for NDFd. The combination of including 0.3 g/L AO with BG and dairy heifer’s RF demonstrated the greater (P < 0.05) DMD compared with all PS treatment combinations having the lowest DMD with the remaining treatments being intermediate (Fig. 1). Also, OMD was greater for all treatment combinations compared with all PS treatment combinations for dairy heifers (Fig. 2). Even within the PS treatment combinations, the responses to AO and RF source were inconsistent. Thus, why the DMD and OMD responses to AO inclusion rate were not consistent across FS and/or across RF source is not known. The optimal 0.3 g/L AO inclusion rate determined in experiment 1 for eliciting positive DMD or OMD responses was not consistently observed in experiment II, which may depend on FS and RF sources. Why the responses are not more consistent across experiments could be related to fiber composition, forage maturity, and/or other unidentified factors. Chen et al. (2004) reported similar IVDMD or IVOMD without or with AO inclusion with AH, peanut vines, or Bermuda straw. Gomez-Alarcon et al. (1990) reported IVDMD or IVOMD differences between milo, AH, or wheat straws substrates when using cow RF with or without 25 mg/L of AO in the in vitro buffer solution. Therefore, more work is warranted to elucidate factors affecting digestibility responses to AO or the lack thereof.
Table 5.
Probably of significant F-test for main effects and interactions for digestibility of dry matter (DMD), organic matter (OMD), and NDF (NDFd) for dried AO, FS, or RF for experiment II
| Main effects, P < a | Main effects and interactions, P < a | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | AOb | FSc | RFd | AO × FS | AO × RF | FS × RF | AO × FS × RF |
| DMD, % | 0.33 | 0.01 | 0.11 | 0.98 | 0.31 | 0.17 | 0.02 |
| OMD, % | 0.42 | 0.01 | 0.17 | 0.92 | 0.68 | 0.19 | 0.01 |
| NDFd, % | 0.07 | 0.01 | 0.01 | 0.21 | 0.15 | 0.90 | 0.57 |
a Probably of F-tests for main effects and interactions.
b AO levels were 0.0, 0.3, and 0.6 g/L.
c FS were AH, BG hay, and PS.
d RF source was from either dairy heifer or goat.
Figure 1.
Effect of Aspergillus oryzae (AO) inclusion rate on dry matter digestibility of AH, BG hay, and PS using RF from dairy heifers or goats for experiment II (AO × FS × RF source, P < 0.02).
Figure 2.
Effect of Aspergillus oryzae (AO) inclusion rate on organic matter digestibility of AH, BG hay, and PS using RF from dairy heifers or goats for experiment II (AO × FS × RF source, P < 0.01).
The AO, FS, and RF main effects were tending (P < 0.07) or significant (P < 0.01) for NDFd. The individual treatment means presented in Fig. 3 demonstrate that, in contrast to experiment 1, the inclusion of AO at 0.3 g/L was tending to reduce NDFd compared with 0.0 g/L of AO. Besides experimental variability, it is unknown why AO would reduce NDFd. This NDFd reduction was more evident for dairy goats compared with dairy heifers as RF donors, which could be related to the greater grain intake for dairy goats as a percentage of BW and/or dietary forage to concentrate ratio. The significant (P < 0.01) FS main effect was due to AH and BG hay containing greater concentrations of NDFd compared with PS, which had numerically the lowest NDFd. In contrast, Chen et al. (2004) reported similar IVNDFd or IVADFD differences for AH or BG comparing without to with AO inclusion, which agreed with Varel et al. (1993) using 0.067 mg/mL and 0.2 mg/mL of AO on effect on bromegrass or switch grass. In contrast, Gomez-Alarcon et al. (1990) reported IVNDFd differences between Control and AO supplemented at 25 mg/L using milo, AH, or wheat straw.
Figure 3.
Effect of Aspergillus oryzae (AO) inclusion rate on digestibility of neutral detergent fiber of AH, BG hay, and PS using RF from dairy heifers or goats for experiment II (AO × FS × RF source, P < 0.57).
There were no significant (P > 0.15) three-way or two-way interactions among main effects for NDF degradation kinetics parameters. Therefore, only the AO, FS, and RF main effects are presented in Table 6. Including AO at 0.0 and 0.3 g/L demonstrated no differences (P > 0.10) on NDF degradation parameters digestion rate, lag, and half-life of the PDNDF fraction.
Table 6.
Digestion rate, lag, and half-life of NDF for the main effects of dried AO fed at 0.0 and 0.3 g/L, or FS of AH, BG, or PS, or RF source from either dairy heifers or goats as RF donors for in vitro fermentations in experiment II
| AO | FS | RF source | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0.0 | 0.3 | SEM | AH | BG | PS | SEM | Heifer | Goat | SEM |
| Rate, %/h | 3.40 | 3.81 | 0.60 | 4.94a | 4.06a | 1.82b | 0.65 | 4.50 | 2.71 | 0.59 |
| Lag, h | 4.22 | 8.81 | 2.38 | 5.13 | 8.06 | 6.37 | 2.86 | 4.83 | 8.20 | 2.34 |
| Half life, h | 28.3 | 22.3 | 5.10 | 16.2a | 18.6a | 41.0b | 6.76 | 20.1 | 30.5 | 4.90 |
a,bMeans within main effects with unlike superscripts differ, P < 0.05.
The NDF degradation kinetics were greater (P < 0.05) for AH and BG compared with PS, while no differences were observed in the lag time. The PS NDF fraction is more slowly degraded. However, given the typical PS particle size, the expectation would be an earlier ruminal passage than predicted by NDF digestion kinetics. The PS half-life value may indicate that PS are resisting the absorption of ruminal fluid and floating for incorporation in the ruminal mat to achieve NDF digestion.
The use of RF from a dairy heifer resulted in higher (P < 0.05) predicted NDF digestion rates and mean ruminal retention times compared with dairy goat RF (Table 6), while lag times and half-life were similar (P > 0.10). Thus, future experiments evaluating NDFd of feeds and forages for feeding dairy goats should use ruminal fluid from dairy goats to obtain more accurate estimates or at least realize that data generated using dairy heifers or cows may have significant bias if results are being applied to feeding dairy goats. Yáñex-Ruiz et al. (2016) recommended sourcing RF from the same livestock species that results would be conducted for.
Experiment III
The adaptation (AD) and withdrawal (WD) time periods needed in order to detect significant digestibility responses to the addition or elimination of any feed additive in the ration is both a product efficacy and economic concern. The time needed for the appearance or disappearance of AO digestibility responses was evaluated by feeding the dairy heifers and goats AO (2.63 or 0.26 g/hd/d, respectively) and collecting RF over 16 wk to measure in vitro nutrient digestibilities. A significant (P < 0.01) three-way interaction of FS × RF × week for DMD, OMD, and NDFd (Table 7) demonstrated AO adaptation increased DMD at some time points but the trend over time appeared to be a digestibility depression, except for PS using inoculum from both dairy heifers and goats (Figs. 4–6). The adaptative response in DMD, OMD, and NDFd to the feeding of AO was not consistent across all FS and RF sources but identified responses occurred within 4 wk if a response was observed. The dairy heifer ruminal adaptation to AO inclusion demonstrated declining DMD for AH and BG with time even though DMD for all FS combined was similar (P > 0.10). Dairy goat ruminal adaptation to AO inclusion was increasing with time (Fig. 4). However, removal of feeding AO resulted in an improvement in DMD across time points, except that AO withdrawal decrease (P < 0.05) PS DMD. Why AO withdrawal would enhance DMD is not known; however, it may be related to inclusion rate and/or changes in ruminal bacteria. Weimer et al. (2010) demonstrated that ruminal bacteria are host specific. Therefore, most feed additives have to be continually fed. Gomez-Alaron et al. (1990) using dairy cow RF as inoculum evaluating the degradation rate of potentially digestible DM fraction in vitro was similar for control and AO treatments for milo and wheat straw, but the digestibility rate of potentially digestible DM fraction of AL hay was increased with AO with similar IVDMD extent. In the second trial, these same authors reported that regardless of the forage to concentrate ratio, the IVDMD was similar between control and AO treatments (Gomez-Alaron et al., 1990), in agreement with other studies (Fondevila et al., 1990; Newbold et al., 1992)
Table 7.
Probably of significant F-test for main effects and interactions for digestibility of dry matter (DMD), organic matter (OMD), and NDF (NDFd) for FS, RF, and week when dairy heifers and goats were fed dried AO during adaptation or withdrawal periods for experiment III
| Main effects, P < a | Main effects and interactions, P < a | ||||||
|---|---|---|---|---|---|---|---|
| Measurement | FSb | RFc | Week | FS × RF | FS × week | RF × week | FS × RF × week |
| DMD, % | 0.01 | 0.01 | 0.01 | 0.03 | 0.01 | 0.01 | 0.01 |
| OMD, % | 0.01 | 0.07 | 0.01 | 0.30 | 0.01 | 0.01 | 0.01 |
| NDFd, % | 0.01 | 0.85 | 0.43 | 0.04 | 0.34 | 0.99 | 0.01 |
aProbably of F-tests for main effects and interactions.
b FS were AH, BG hay, and PS.
c RF source was from either dairy heifer or goat.
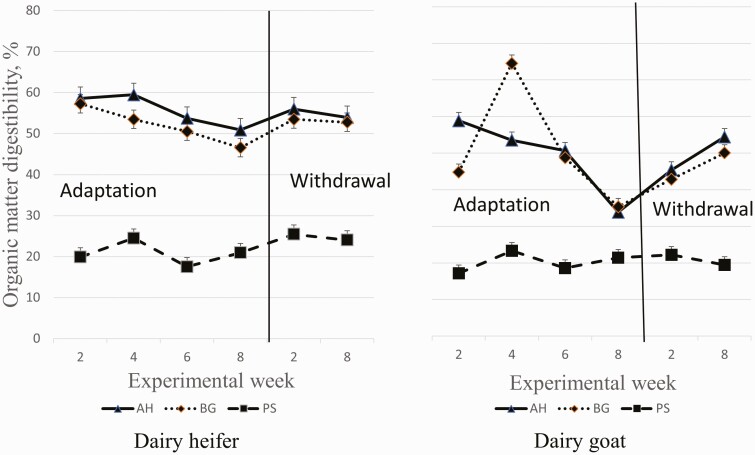
Figure 4.
Adaptation and withdrawal times for Aspergillus oryzae (AO) fed to dairy heifers or goats on in vitro dry matter digestibility of AH, BG hay, and PS using RF from dairy heifers or goats for experiment III (interaction of RF source by FS by week, P < 0.01, SEM 2.84).
Figure 6.
Adaptation and withdrawal times for Aspergillus oryzae (AO) fed to dairy heifers or goats on in vitro organic matter digestibility of AH, BG hay, and PS using RF from dairy heifers or goats for experiment III (interaction of RF source by FS by week, P < 0.01, SEM 4.01).
Ruminal AO adaptation resulted in slight increases (P < 0.05) in OMD for the three FS during the first 2–4 wk (Fig. 5), but then OMD declined by the sixth to eighth wk followed by increasing OMD when withdrawing AO (i.e., quit feeding) for all FS, except PS using RF from dairy goats (Fig. 5). The changes in NDFd following the removal of AO feeding did not follow consistent responses across the different FS and RF sources. Using field observations to determine inclusion or feeding rates can lead to overfeeding feed additives compared to standardized titration studies. Several ruminal AO studies confirmed our results. First, Denigan et al. (1992) reported that adding AO to a TMR containing CrO3 fed to lactating dairy cows resulted in similar milk production and composition when fed at 0, l.5, 3.0, 6.0 g/d of AO. Total tract DM, CP, and NDF digestion was similar among lactating dairy cows fed all AO inclusion rates. Frumholtz et al. (1989) reported similar DM degradation using the in vitro Rusitec procedure inoculated with sheep RF evaluating AO added at 0 or 250 mg. Arambel et al. (1987) reported no differences for DM degradation after 21 d when heifers were fed AO at 0 or 2.63 g/d to an ad libitum AH-based ration. In contrast, Weidmeier et al. (1987) reported that nonlactating Holstein cows fed AO at 2.63 g/d increase DM degradation 4.2% when feeding a base ration of 50% concentrate. Thus, the lack of consistent AO responses for enhancing DM and NDF degradation are consistently observed across both in vitro and in vivo research techniques.
Figure 5.
Adaptation and withdrawal times for Aspergillus oryzae (AO) fed to dairy heifers or goats on in vitro organic matter digestibility of AH, BG hay, and PS using RF from dairy heifers or goats for experiment III (interaction of RF source by FS by week, P < 0.01, SEM 2.80).
The main effects interactions were nonsignificant (P > 0.40) for NDF degradation kinetics. Therefore, data were summarized comparing feeding with (adaptation) and without (withdrawal) AO time periods (Table 8). The ruminal AO adaptation and withdraw was similar across weeks (Fig. 6). All NDF degradation kinetic parameters were similar (P > 0.29) except for the FS main effect, which had a trend (P < 0.08). The AH NDF digestion rate across both adaptation and withdrawal timer periods (6.78, 6.50, and 3.80%/h for AH, BG, and PS, respectively, SEM = 0.01) was greater (P < 0.05) than the PS NDF degradation rate with BG NDF degradation rate being intermediate and similar (P > 0.05). The NDF degradation kinetics were similar (P > 0.10) when using RF sourced from dairy heifers or dairy goats (Table 9). Varel and Kreikemeier (1994) conducted an in situ study with eight nonpregnant nonlactating beef cows fed AH or brome grass without or with 3 g/d AO in the grain mix plus 1 g/d of AO added directly through the rumen cannula, which demonstrated similar 0–72 h NDF, cellulose, and hemicellulose degradation during the 28-d trial.
Table 8.
Digestion rate, lag, and half-life of NDF when dairy heifers or goats (RF donors) were fed dried AO during adaptation or withdraw time periods for AH, BG hay, and PS for in vitro fermentations in experiment III
| Forage | Main effects and interaction, P < a | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | AH | BG | PS | SEM | Forage | Period | Forage × period |
| Rate, %/h | |||||||
| Adaptation | 6.75 | 6.38 | 3.63 | 0.01 | 0.08 | 0.81 | 0.99 |
| Withdraw | 6.81 | 6.63 | 3.98 | ||||
| Lag, h | |||||||
| Adaptation | 4.50 | 6.50 | 3.75 | 2.57 | 0.91 | 0.87 | 0.73 |
| Withdraw | 4.63 | 4.88 | 6.38 | ||||
| Half life, h | |||||||
| Adaptation | 10.8 | 12.1 | 21.3 | 4.21 | 0.29 | 0.66 | 0.72 |
| Withdraw | 12.0 | 11.7 | 15.7 |
a Probably of F-tests for main effects and interactions.
Table 9.
Digestion rate, lag, and half-life of NDF using RF sourced from dairy heifers or goats that were fed AO during an adaptation or withdrawal time period for in vitro fermentations in experiment III
| Rumen fluid source | |||
|---|---|---|---|
| Parameter | Heifer | Goat | SEM |
| Rate, %/h | 5.33 | 6.06 | 0.62 |
| Lag, h | 4.0 | 6.2 | 1.48 |
| Half life, h | 15.0 | 12.9 | 2.43 |
CONCLUSIONS
The optimal AO inclusion rate was determined to be 0.3 g/L for improving NDF digestion when conducting in vitro fermentations, but that inclusion rate was not confirmed in subsequent experiments. Inclusion rates of AO greater than 0.3 g/L depressed NDF degradation, which should be avoided in future feeding studies. The exact AO mechanism for enhancing ruminal NDF digestion is not fully understood, but the hemicellulose fraction is widely proposed as the targeted nutrient when AO is included as a feed additive. No clear AO adaptation and withdrawal times could be determined since NDF digestion kinetics were similar across all FS. Sourcing ruminal fluid from dairy heifers or dairy goats for conducting in vitro fermentations resulted in inconsistent predictions of DM, OM, and NDF digestion and NDF degradation kinetics across experiments, which confirmed and refuted the hypothesis. Therefore, RF should be sourced from the same livestock species that the results will be applied for to alleviate inconsistent results.
ACKNOWLEDGMENTS
The authors express their sincere appreciation to Biozyme Inc., (St. Joseph, MO) for providing the Amaferm used in this study and for partial financial support of the project. In addition, the authors extend their appreciation to Drs. S. G. Solaiman, W. Hill, and M. Maloney from the Department of Animal Science and D. Bukaka and J. Sparks from the School of Veterinary Medicine, Tuskegee University for their assistance.
Conflict of Interest:The authors declare no conflict of interest.
Footnotes
The mention of specific products is for informational purposes only and does not imply any warranty or endorsement over products not mentioned.
REFERENCES
- AOAC International . 2019. Official methods of analysis. 21st ed. AOAC International, Rockville, MD. [Google Scholar]
- Arambel, M. J., Wiedmeier R. D., and Walter J. L.. . 1987. Influence of donor animal adaptation to added yeast culture extract on in vitro rumen fermentation. Nutr. Rep. Inter. 35:433–436. [Google Scholar]
- Beharka, A. A., and Nagaraja T. G.. . 1993. Effect of Aspergillus oryzae fermentation extract (Amaferm) on in vitro fiber degradation. J. Dairy Sci. 76:812–818. doi: 10.3168/jds.s0022-0302(93)77405-6. [DOI] [PubMed] [Google Scholar]
- Chen, C. R., Yu B., and Chiou P. W. S.. . 2004. Roughage energy and degradability estimation with Aspergillus oryzae inclusion using daisy® in vitro fermentation. Asian-Aust. J. Anim. Sci. 17:53–62. doi: 10.5713/ajas.2004.53. [DOI] [Google Scholar]
- Denigan, M. E., Huber J. T., Alhadhram G., and AL-Dehneh A.. . 1992. Influence of feeding varying levels of Amaferm® on performance of lactating dairy cows. J. Dairy Sci. 75:1616-11621. doi: 10.3168/jds.S0022-0302(92)77918-1. [DOI] [PubMed] [Google Scholar]
- Fondevila, M., Newbold C. J., Hottern P. M., and Orskov E. R.. . 1990. A note on the effect of Aspergillus oryzae fermentation on sheep given straw. Br. Soc. Anim. Prod. 51:422–425. doi: 10.1017/S0003356100005584. [DOI] [Google Scholar]
- Frumholtz, P. P., Newbold C. T., and Wallace R.T.. . 1989. Influence of Aspergillus oryzae fermentation extract on the fermentation of a basal ration in the rumen simulation technique (Rusitic). J. Agric. Sci. 113:169–172. doi: 10.1017/S002185960008672X. [DOI] [Google Scholar]
- Gomez-Alarcon, R. A., Dudas C., and Huber J. T.. . 1990. Influence of cultures of Aspergillus oryzae on rumen and total tract digestibility of dietary components. J. Dairy Sci. 73:703–710. doi: 10.3168/jds.S0022-0302(90)78723-1. [DOI] [PubMed] [Google Scholar]
- Goering, H. K., and Van Soest P. J.. . 1970. Forage fiber analyses (apparatus, reagents, procedures, and some application). Agricultural Handbook 379. USDA, ARS, Washington, DC. [Google Scholar]
- Hatfield, E. E., and Hixon S.. . 1975. Dietary additive for new feeder cattle. Illinois Beef Day, AS-669h. University of Illinois, Champaign-Urbana. [Google Scholar]
- Hill, G. M. 2002. Peanut by-products fed to cattle. Vet. Clin. North Am. Food Anim. Pract. 18:295–315. doi: 10.1016/s0749-0720(02)00019-1. [DOI] [PubMed] [Google Scholar]
- Huston, J. E. 1978. Forage utilization and nutrient requirements of the goat. J. Dairy Sci. 61:988–993. doi: 10.3168/jds.S0022-0302(78)83679-0. [DOI] [Google Scholar]
- Huston, J.E., Rector B. S., Ellis W. C. and Allen M.L.. . 1986. Dynamics of digestion in cattle, sheep, goat and deer. J. Anim. Sci. 62:208–215. doi: 10.2527/jas1986.621208x. [DOI] [PubMed] [Google Scholar]
- Macrotrends . 2021. Corn prices—59 year historical chart. Available from: https://www.macrotrends.net/2532/corn-prices-historical-chart-data. Accessed March 5, 2021.
- Marcus, K. M., Huber J. T., and Cramen S.. . 1986. Influence of feeding Vitaferm during hot weather on performance of lactating cows in a large dairy herd. J. Dairy Sci. 69 (Suppl.1):188. [Google Scholar]
- Martin, S. A., and Nisbet D. J.. . 1990. Effects of Aspergillus oryzae fermentation extract on fermentation of amino acids, Bermuda grass and starch by mixed ruminal microorganisms in vitro. J. Anim. Sci. 68:2142–2149. doi: 10.2527/1990.6872142x. [DOI] [PubMed] [Google Scholar]
- NRC. 2001. Nutrient requirements of dairy cattle. National Academy Press, Washington, DC. [Google Scholar]
- Newbold, C. J., Frumholtz P. P., and Wallace R. J.. . 1992. Influence of Aspergillus oryzae fermentation extract on rumen fermentation and blood constituents in sheep given diets of grass hay and barley. J. Agric. Sci. 119:423–427. doi: 10.1017/S0021859600012272. [DOI] [Google Scholar]
- Solaiman, S. G. 1984. In situ cell wall digestion rate as influenced by particle size, digestibilities and markers passage studies in cattle fed orchard grass alone or supplemented, compared to alfalfa in Ph.D. Thesis University of Missouri at Columbia. University of Missouri Columbia. [Google Scholar]
- Solaiman, S. G., Martz F. A., Weiss M. F., and Belyea R. L.. . 1990. Effects of protein and energy supplementation of Guernsey cows on the kinetics of digestion and passage of orchardgrass versus alfalfa. J. Anim. Sci. 68:2119–2129. doi: 10.2527/1990.6872119x. [DOI] [PubMed] [Google Scholar]
- Steel, R. G. D., and Torrie J. H.. . 1980. Principles and procedures of statistics. 2nd ed. McGraw-Hill Book, Co., New York, NY. [Google Scholar]
- Varel, V. H., Kreikemeier K. K., Jung H. J., and Hatfield R. D.. . 1993. In vitro stimulation of forage fiber degradation by ruminal microorganisms with Aspergillus oryzae fermentation extract. Appl. Environ. Microbiol. 59:3171–3176. doi: 10.1128/AEM.59.10.3171-3176.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel, V. H., and Kreikemeier K. K.. . 1994. Influence of feeding aspergilus oryzae fermentation extract (Amaferm) on in situ fiber degradation, ruminal fermentation, and microbial protein synthesis in nonlactating cows fed alfalfa or bromegrass hay. J. Anim. Sci. 72:1814–1822. doi: 10.2527/1994.7271814x. [DOI] [PubMed] [Google Scholar]
- Weiss, M. F. 1979. Intake, digestibilities, fiber composition and marker passage of three tall fescue varieties fed ad libitum to sheep. M.S. Thesis. University of Missouri at Columbia. [Google Scholar]
- Wiedmeier, R. S., Arambel M. J., and Walters J. L.. . 1987. Effect of yeast culture and aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibilities. J. Dairy Sci. 70:2063–2068. doi: 10.3168/jds.S0022-0302(87)80254-0. [DOI] [PubMed] [Google Scholar]
- Weimer, P. J., Stevenson D. M., Mantovani H. C., and Man S. L. C.. . 2010. Host specificity of the ruminal bacteria community in the dairy cow following near-total exchange of ruminal contents. J. Dairy Sci. 93:5902–5912. doi: 10.3168/jds.2010-3500. [DOI] [PubMed] [Google Scholar]
- Yáñex-Ruiz, D. R., Bannink A., Dijkstra J., Kebreab E., Morgavi D. P., O’Kiely P., Reynolds C. K., Schwarm A., Shingfield K. J., Yu Z., . et al. 2016. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants-a review. Anim. Feed Sci. Tech. 216:1–18. doi: 10.1016/j.anifeedsci.2016.03.016. [DOI] [Google Scholar]
- Yoon, I. K., and Stern M. D.. . 1995. Influence of direct-fed microbials on ruminal microbial fermentation and performance of ruminants: a review. Asian-Australas J. Anim. Sci. 8:533–555. doi: 10.5713/ajas.1995.553. [DOI] [Google Scholar]