Abstract
Adequate provision of calcium (Ca) and phosphorus (P) is essential for bone formation and high growth performance in pigs. Nevertheless, reliable serum biomarkers for pig’s Ca and P intake are still missing. Here, we used phytase supplementation to alter the dietary available P (aP) level in order to investigate the effect of differences in dietary aP levels on serum parameters related to the Ca and P homeostasis in pigs. Moreover, we assessed whether serum parameters can be used to predict the Ca, total P (tP), and aP intake in barrows and gilts throughout the fattening period. In total, 216 pigs (115 gilts and 101 barrows) were randomly allotted to one of the two diets in three replicate batches, each lasting 56 d (n = 108/diet). Pigs had free access to the diets without (Con) or with phytase (Phy; 650 phytase units/kg) via a transponder-based feeding system. Blood samples were collected on days 2, 23, and 52, and serum parameters were correlated with the daily Ca, tP, and aP intake. The intake of tP, aP, and Ca was overall 14.2%, 13.8%, and 14.2% higher in barrows compared with gilts, respectively (P < 0.001). Concurrently, phytase decreased the intake of tP and Ca by 8.4% and 6.7%, respectively, whereas it raised the intake of aP by 16.3% compared with the Con diet (P < 0.001). Serum levels of fibroblast growth factor 23, alkaline phosphatase (ALP), vitamin D (VitD), and osteocalcin (OCN) decreased with age (P < 0.05). The higher aP intake of pigs fed the Phy diet increased serum P on days 2 and 23 but decreased it on day 52 compared with the Con diet (P = 0.004). Pigs fed the Phy diet had higher serum ALP compared with pigs fed the Con diet on days 23 and 52 (P < 0.05). Correlation analysis between serum parameters and Ca, tP, and aP intake showed age- and sex-related associations. With 12 wk of age, serum P in both sexes, serum VitD in barrows, and serum OCN and ALP in gilts correlated with aP intake (|r| > 0.38), whereas serum OCN correlated with Ca in both sexes’ intake (r > 0.50). At 20 wk, serum Ca and ALP in gilts correlated with aP intake, whereas serum P, Ca, and VitD correlated with Ca intake in both sexes (|r| > 0.39). In conclusion, the present results showed that the daily Ca and aP intake could be most reliably estimated from serum parameters for an approximate age of 12 and 20 wk. Serum P and the Ca:P ratio at 12 wk of age and serum VitD at 20 wk of age may be used to predict pig’s daily aP intake in both sexes.
Keywords: growing pig, mineral intake, phosphorus homeostasis, phytase, serum parameters, sex
INTRODUCTION
Leg weakness is a major economic problem in today’s pig production, affecting the thriftiness of growing pigs and increasing the culling rate of breeding animals. Therefore, providing adequate amounts of minerals, especially calcium (Ca) and phosphorus (P), to pigs is critical to support adequate mineralization of the skeleton and hence optimal growth (Létourneau-Montminy et al., 2012; Li and Stahl, 2014). For the clinical diagnosis of leg weakness, physical examination of the living pig and certain serum parameters are assessed (Crenshaw et al., 2014). Histology and mechanical tests as well as bone ash measurements are more accurate than physical examination alone, but they require to kill the animal and hence cannot be used for herd screening in order to early detect nutritional inadequacies. Moreover, these analyses have partly long turn-around times and rapidity of diagnosis is an important issue in clinical diagnostics when dealing with acute problems in a pig herd (Crenshaw et al., 2014). In addition, it is not clear whether the measured serum parameters are reliably corresponding to abnormalities in pig’s Ca and P intake and are similar in barrows and gilts. Therefore, it is still difficult to assess whether pigs in a herd received adequate amounts of Ca and P via their diet by using little invasive methods. Currently, several serum parameters related to the Ca homeostasis, such as vitamin D (VitD), parathyroid hormone, and osteocalcin (OCN), are used as markers for bone health in practice (Sørensen et al., 2018; Lee et al., 2020). However, these regulatory factors do not sufficiently correspond to the dietary P intake (Oster et al., 2016; Amundson et al., 2017; Sørensen et al., 2018). As potential candidates, fibroblast growth factor 23 (FGF23), with its primary function to decrease systemic P and VitD levels (Erben, 2016), is used in the early detection of bone abnormalities in humans (Rupp et al., 2019), whereby evidence in pigs is still scarce. We could previously show that FGF23 has a certain role in the Ca homeostasis, but we could not find a clear association with the P metabolism (Vötterl et al., 2020). Further regulators for bone mineralization that are linked to the P homeostasis are alkaline phosphatase (ALP), which enhances the local P concentration, and OCN, which binds P in the bone (Penido and Alon, 2012; van Riet et al., 2013). In addition to FGF23, the hormone VitD increases Ca and P retention and absorption, in the case of Ca by the aid of parathormone (Lederer, 2014; Pu et al., 2016).
The requirement for total P (tP) to achieve optimal bone mineralization has been estimated to be 1 g/kg diet higher than to reach optimal body weight (BW) gain (O′Doherty et al., 2010; Varley et al., 2010). Due to the impact that the dietary ratio of Ca to tP has on Ca and P uptake and its systemic utilization (Veum, 2010; Vier et al., 2019), the optimal dietary Ca:tP ratio from 1:1 to 2.5:1 or Ca:aP ratio between 2:1 and 3:1 (NRC, 2012; Schneider et al., 2019) should guarantee optimal utilization of Ca and P for bone synthesis and mineralization. To render the otherwise nondigestible phytate-P more available to the pig, pig diets are typically supplemented with phytase (Dersjant-Li et al., 2015). Based on this, 500 phytase units (FTU) per kilogram diet should generate about 1.15 g aP/kg diet (Humer et al., 2015). Moreover, phytase supplementation may also improve dietary Ca availability (González-Vega and Stein, 2014; McGhee and Stein, 2019), which, in turn, may alter intestinal and systemic Ca availabilities. Because imbalances in the dietary supply and availability impact absorption and body utilization of Ca and P, the identification of appropriate serum markers that correspond to both, the dietary Ca and aP intake, may help to detect imbalances in mineral intake on farms before skeletal problems become apparent. We, therefore, hypothesized that changes in the dietary aP intake are traceable in serum levels of regulatory factors related to both Ca and P homeostasis. In the present study, we used phytase supplementation to alter the dietary aP level in order to investigate the effect of differences in dietary aP levels on serum parameters related to the Ca and P homeostasis in pigs during the fattening period. Moreover, we assessed whether serum parameters can be used to predict the Ca, tP, and aP intake in barrows and gilts throughout the fattening period.
MATERIALS AND METHODS
All procedures involving animal handling and treatment were approved by the institutional ethics committee of the University of Veterinary Medicine Vienna and the National authority according to the Law for Animal Experiments, Tierversuchsgesetz in Austria (BMWFW-68.205/01221-WF/V/3b/2017).
Animals and Housing
A total of 216 crossbred growing pigs (Large White × Piétrain; 115 gilts and 101 barrows) from 19 litters at 11 wk of age (average initial BW 38.5 ± 7.1 kg) from the research and teaching farm of the University of Veterinary Medicine Vienna were used in three replicate batches (n = 72/replicate batch). Pigs were acclimatized to the experimental conditions (i.e., housing and feeding stations) for 1 wk before the start of the experiment. Each replicate run lasted for 56 d (8 wk). Pigs were housed in an outdoor climate fattening unit comprising six pens (19.18 m2, 3.50 × 5.48 m per pen) with slatted flooring. In each replicate batch, pigs were penned in groups of 12 littermates per pen. Pens were randomly allocated to diets; in each replicate batch, three pens received one of the two diets, resulting in a total of nine pens per diet across all three replicate batches (n = 108 pigs/diet). Each pen was equipped with two nipple drinkers. Water was freely available throughout the experimental period. The health status of the pigs was monitored daily by visual inspection and checking the feed intake records.
Diets and Feeding
Two diets (Table 1) were formulated to meet or exceed the current recommendations for nutrient requirements (Flachowsky et al., 2006; NRC, 2012). The diets were either not supplemented (Con) or supplemented with a 6-phytase (Phy) derived from Escherichia coli (VM Phytase XP 897420, Garant-Tiernahrung GmbH, Pöchlarn, Austria), which was added at a concentration of 650 FTU/kg complete feed (Dersjant-Li et al., 2015). The dietary Ca:tP ratio of 1.3:1 for both diets and the dietary Ca:aP ratio of 2.5:1 and 2.0:1 for the Con and Phy diets, respectively, were in the recommended range (NRC, 2012). Feed samples were collected three times on days 1, 22, and 49. The pigs had free access to pelleted feed via transponder-based feeders (Feed Intake Recording Equipment feeder, SCHAUER Agrotonic GmbH, Prambachkirchen, Austria). On the day of the transfer to the fattening unit, each pig received an individual radio-frequency identification ear tag (CI Tiris AF-Ohrchip 25.5 mm, SCHAUER Agrotonic GmbH, Prambachkirchen, Austria). One pig at a time got access to the feeder by means of the radio-frequency identification ear tag. The amount of feed consumed by each pig was registered automatically by weighing the trough before and after the pig got access to the trough. Every visit of the pig at the feeder (amount of feed eaten and time) was registered. The data for each pig per day were summed up and expressed as consumed feed per day (g/d).
Table 1.
Ingredients and analyzed nutrient composition of experimental diets
| Dietary treatment | Con | Phy |
|---|---|---|
| Ingredient, % | ||
| Barley | 45.06 | 45.00 |
| Wheat, 11% CP | 35.51 | 35.46 |
| Soybean meal HP, 47% CP | 8.11 | 8.10 |
| Soybean meal, 42% CP | 7.01 | 7.00 |
| Calcium carbonate | 1.28 | 1.28 |
| Rapeseed oil | 1.00 | 1.00 |
| Monocalcium phosphate | 0.46 | 0.46 |
| Salt | 0.46 | 0.46 |
| Lysine-HCL 98 | 0.41 | 0.41 |
| Vitamin–mineral premixa,b | 0.39 | 0.39 |
| l-Threonine | 0.13 | 0.13 |
| Magnesium oxide | 0.10 | 0.10 |
| dl-Methionine | 0.08 | 0.08 |
| Phytase, FTU/kg dietc | — | 650 |
| Analyzed nutrient content, g/kg DM | ||
| DM, g/kg | 893 | 893 |
| Crude ash | 46.7 | 47.4 |
| Acid insoluble ash | 3.63 | 3.61 |
| Crude protein | 165 | 165 |
| Crude fiber | 33.9 | 35.7 |
| Neutral detergent fiber | 125 | 125 |
| Acid detergent fiber | 50.5 | 50.3 |
| Crude fat | 33.0 | 33.1 |
| Nitrogen-free extract | 618 | 617 |
| Total starch | 450 | 457 |
| Resistant starch | 3.07 | 3.07 |
| Nonresistant starch | 437 | 437 |
| Phosphorus | 5.68 | 5.59 |
| Available phosphorus | 2.95 | 3.68 |
| Calcium | 7.48 | 7.50 |
| Magnesium | 2.70 | 2.70 |
| Sodium | 1.86 | 1.82 |
| Phytase, FTU/kg diet | 257 | 596 |
| Calculated energy content | ||
| Metabolizable energy, MJ/kg | 15.2 | 15.1 |
CP, crude protein.
a The Vitamin–Mineral premix without phytase provided per kilogram of experimental diet (Garant-Tiernahrung GmbH, Pöchlarn, Austria): 6,510 IU of vitamin A, 2,003 IU of vitamin D3, 156 IU of vitamin E, 3.01 mg of vitamin K3, 1.50 mg of vitamin B1, 4.01 mg of vitamin B2, 20.03 mg of vitamin B3, 2.00 mg of vitamin B6, 0.02 mg vitamin of B12, 10.02 mg pantothenic acid, 0.50 mg of folic acid, 0.05 mg of biotin, 1,242.04 mg of choline, 132.17 mg of choline chloride, 160.46 mg of Fe, 21.57 mg of Cu, 122.06 mg of Zn, 67.36 mg of Mn, 0.86 mg of Mo, 1.72 mg of J, 0.10 mg of Co, and 0.57 mg of Se.
b The Vitamin–Mineral premix with phytase provided per kilogram of experimental diet (Garant-Tiernahrung GmbH, Pöchlarn, Austria): 6,502IU of vitamin A, 2,001 IU of vitamin D3, 156 IU of vitamin E, 3.00 mg of vitamin K3, 1.50 mg of vitamin B1, 4.00 mg of vitamin B2, 20.01 mg of vitamin B3, 2.00 mg of vitamin B6, 0.02 mg of vitamin B12, 10.00 mg of pantothenic acid, 0.50 mg of folic acid, 0.05 mg of biotin, 1,240.43 mg of choline, 132.00 mg of choline chloride, 160.25 mg of Fe, 21.54 mg of Cu, 121.90 mg of Zn, 67.27 mg of Mn, 0.86 mg of Mo, 1.72 mg of J, 0.10 mg of Co, and 0.57 mg of Se.
c 6-Phytase (VM Phytase XP 897420, Garant-Tiernahrung GmbH, Pöchlarn, Austria).
Body Weight Measurement and Calculations
The BW of each pig was measured individually on experimental days 1, 8, 22, 35, and 49 using a transportable animal scale (Agreto Einzeltierwaage, Agreto electronics GmbH, Raabs, Austria). The average daily gain (ADG), average daily feed intake (ADFI), and gain:feed (G:F) ratio as well as the Ca, tP, and aP intake were calculated for the whole experimental period and for the intervals days 1 to 8, days 9 to 22, days 23 to 35, and days 36 to 49.
Blood Sampling
Blood samples were collected from the jugular vein of each pig on one of the two consecutive sampling days by separating the pigs one by one and restraining them with a pig holder (36 pigs per day). Blood was collected on experimental days 2 and 3, 23 and 24, and 52 and 53 in serum tubes (S-Monovette 9 mL Z, Sarstedt AG & Co. KG, Nümbrecht, North Rhine-Westphalia, Germany). To simplify the presentation of the data, the sampling days for serum will be referred to as days 2, 23, and 52. During sampling, blood was kept on ice and centrifuged afterward at 2,700 × g for 10 min at 4 °C (Centrifuge 5804 R, Eppendorf AG, Hamburg, Germany). Serum aliquots were stored at −80 °C until analysis.
Chemical Analysis
Dried diet samples were ground to pass a 1-mm sieve (Ultra-Zentrifugalmühle ZM 200, Retsch GmbH, Haan, North Rhine-Westphalia, Germany). Dry matter (DM), crude ash, crude protein, crude fat, tP, Ca, magnesium, potassium, sodium, neutral detergent fiber, acid detergent fiber, total starch, resistant starch, and nonresistant starch were measured. To determine the DM (method 3.1) content, diet samples were oven-dried at 103 °C for 4 h, whereas crude ash (method 3.5) was determined by incinerating at 580 °C for 4 h (Naumann and Bassler, 2012). Crude fat (method 5.1.1/3.5.2) was analyzed by solvent extraction with petroleum ether (Naumann and Bassler, 2012). Calcium (method 10.3.1.), sodium (method 10.1.1), potassium (method 10.2.1), and magnesium (method 10.4.1) were measured with atomic absorption spectroscopy, whereas tP was measured photometrically (method 10.6.1; Naumann and Bassler, 2012). The content of crude protein was determined using the Kjeldahl method (method 4.1.1). The adapted methods of Van Soest et al. (1991) with FiberTherm FT 12 (C. Gerhardt GmbH & Co. KG, Königswinter, North Rhine-Westphalia, Germany) were used for the analyses of neutral and acid detergent fibers (methods 6.5.1 and 6.5.2; Naumann and Bassler, 2012). The nitrogen-free extract fraction was calculated by subtracting crude ash, crude protein, crude fiber, and crude fat from the DM (Naumann and Bassler, 2012; Kamphues et al., 2014). Commercial enzymatic assay kits were used to analyze total starch, resistant starch, and nonresistant starch (K-RSTAR, Megazyme International Ireland Ltd., Bray, County Wicklow, Ireland). All analyses were done in duplicates and results are provided on a DM basis. Analyses of phytase activity were conducted by LUFA Nord-West after the DIN EN ISO 30024 method (Institut für Futtermittel, LUFA Nord-West, Oldenburg, Lower Saxony, Germany). The amount of aP was calculated using data for aP contents in individual feed ingredients either without or with phytase addition to reduce inorganic P supply by 1 g per diet (Schneider et al., 2019).
Serum Parameter Analyses
An auto analyzer for clinical chemistry using enzymatic colorimetric assays (Cobas 6000/c501; Roche Diagnostics GmbH, Rotkirch, Canton of Zug, Switzerland) was used to determine serum content of P, Ca, ALP, cholesterol, nonesterified fatty acids (NEFA), triglycerides, and urea. The Ca:P ratio in serum was calculated to assess the systemic availability of Ca and P and their association with the regulatory hormones (Veum, 2010; Gerlinger et al., 2020; Vötterl et al., 2020). A porcine-specific enzyme-linked immunosorbent assay (ELISA) was used to determine FGF23 (Porcine FGF23 ELISA kit, Wuhan Fine Biotech Co., Ltd., Wuhan, Hubei, China; coefficient of variation [CV] < 10%). For calcitonin, a porcine-specific ELISA kit (Calcitonin ultrasensitive ELISA; DRG Instruments GmbH, Marburg, Hesse, Germany; CV < 8%) was used and the non-diluted samples were analyzed. Nevertheless, most of the samples contained calcitonin levels at the quantification limit of 0.7 pg/mL and were, therefore, not reported. Vitamin D (25-Hydroxy Vitamin Ds EIA, Immunodiagnostic Systems Holdings PLC, Tyne & Wear, NE35 9PD, UK; CV < 20%) and OCN (N-MID Osteocalcin ELISA, Immunodiagnostic Systems Holdings PLC, Tyne & Wear, NE35 9PD, UK; CV < 6%) were analyzed with commercial ELISA kits that were previously evaluated for pig serum (Kolp et al., 2017; Sørensen et al., 2018).
Statistical Analyses
After testing the data for normal distribution and outlier using the Shapiro–Wilk test in SAS (version 9.4, SAS Inst. Inc., Cary, NC, USA), data were subjected to ANOVA using the MIXED procedure. Data were first analyzed as repeated measures over time (sampling days). Since effects for phytase and sex differed during the experimental period, data were analyzed with a second random model, separately per day. This model accounted for the fixed effects of phytase, sex, and the phytase × sex interaction. To account for a potential effect of the starting BW, it was implemented in the model as a covariate. Replicate batch was considered as the random effect and the individual pig nested within litter (pen) as the experimental unit assuming a compound symmetry variance–covariance structure (type = cs). Degrees of freedom were approximated by the Kenward–Roger method. Data were expressed as least square means ± SEM. The pairwise comparisons between least square means were tested using the PDIFF option in SAS. Differences at P ≤ 0.05 and 0.05 < P ≤ 0.10 were defined as significance and trend, respectively. For the characterization of potential serum markers for Ca, tP, and aP intake, Pearson correlation coefficients (r) were calculated separately for gilts and barrows between daily Ca, tP, and aP intake and serum parameters separately for experimental days 1 to 8 (feed intake from day 1 to 7 and blood sample on days 2 and 3), days 22 to 28 (feed intake from day 22 to 28 and blood sample on day 23 or 24), and days 50 to 56 (feed intake from day 50 to 56 and blood sample day 52 or 53) individually using PROC CORR of SAS. A significant correlation was defined as P ≤ 0.05 and |r| ≥ 0.35.
RESULTS
Animals and Diets
In total, 16 pigs (i.e., 6 barrows and 10 gilts) were removed from the experiment mostly in the first 28 d of the experiment (replicate batch 1, n = 6; replicate batch 2, n = 3; and replicate batch 3, n = 7) due to tail biting or low feed intake, which was not related to dietary treatments. The Con and Phy diets provided 15.2 and 15.1 MJ metabolizable energy/kg diet, respectively, and had a crude protein content of 18.5% and 18.4% DM, respectively. The analyzed phytase activity was 257 and 596 FTU/kg complete feed for the Con and Phy diets, respectively, resulting in a 24.7% higher aP content in the Phy diet compared with the Con diet (Table 1).
Growth Performance, Feed Intake, and Daily Consumption of Ca and P
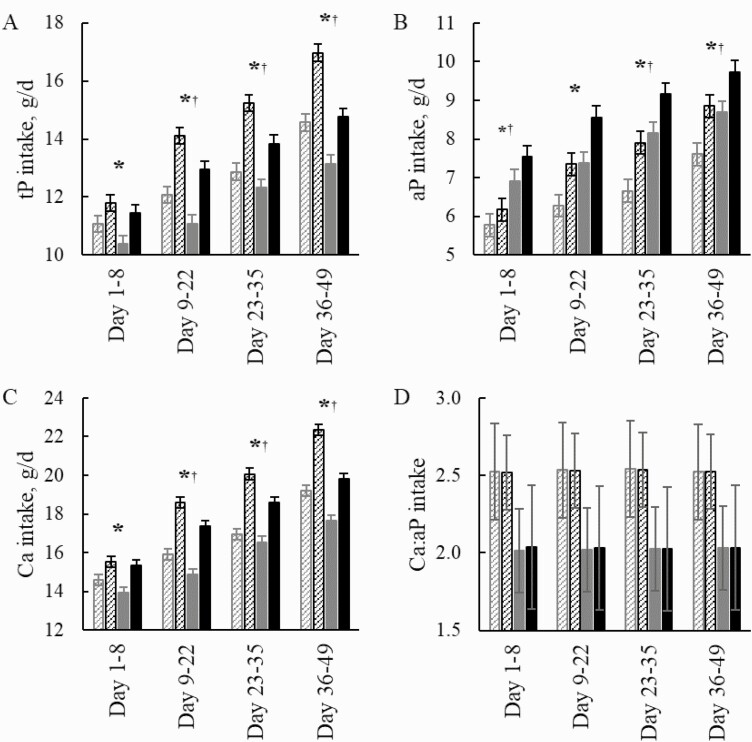
The BW at the beginning of the experiment differed between sexes; therefore, the initial BW was considered as a covariate for the performance parameters (Table 2 and Supplementary Table S1; Fig. 1). Barrows had a 5.6% higher BW at the start of the experiment and weighed on average 4.1% more on days 9 to 22, days 23 to 35, and days 36 to 49 compared with the gilts (P < 0.05). This resulted in an ADG that was 5.7% higher in barrows compared with gilts on days 1 to 49 (P = 0.014). The greatest sex-related difference was on days 9 to 22 when the ADG of barrows was 18.1% higher than that of gilts (P < 0.001). The ADG continued to increase in gilts from days 1–8 to days 36–49, whereas in barrows the highest growth rate was reached in the period of days 9 to 22 and declined afterward (P < 0.001). For the whole experimental period, the ADFI was 14.2% higher in barrows compared with gilts (P < 0.001). As a result, the G:F ratio was 7.8% lower in barrows compared with gilts (P < 0.001). Based on the ADFI, the intake of tP, aP, and Ca over the whole experimental period was 14.2%, 13.8%, and 14.2% higher in barrows compared with gilts, respectively (Supplementary Fig. S2; P < 0.001).
Table 2.
Effect of dietary phytase and sex on BW, BW gain, and G:F ratio in growing pigs during the fattening period
| Dietary treatmenta | Con | Phy | P-valueb | |||||
|---|---|---|---|---|---|---|---|---|
| Item | Gilts | Barrows | Gilts | Barrows | SEM | Phytase | Sex | Phytase × Sex |
| Body weight, kg | ||||||||
| Day 1 | 36.2 | 38.7 | 38.9 | 40.6 | 0.80 | 0.005 | 0.009 | 0.549 |
| Day 8 | 45.1 | 45.3 | 44.9 | 45.2 | 0.25 | 0.520 | 0.306 | 0.957 |
| Day 22 | 57.5 | 59.8 | 56.8 | 59.4 | 0.56 | 0.314 | <0.001 | 0.816 |
| Day 35 | 69.3 | 73.3 | 68.6 | 71.4 | 0.75 | 0.098 | <0.001 | 0.487 |
| Day 49 | 83.7 | 86.7 | 82.9 | 84.8 | 0.98 | 0.193 | 0.016 | 0.624 |
| Average daily gain, kg/d | ||||||||
| Day 1 to 49 | 0.94 | 1.00 | 0.92 | 0.96 | 0.021 | 0.185 | 0.014 | 0.602 |
| Day 1 to 8 | 0.96 | 0.99 | 0.93 | 0.98 | 0.036 | 0.554 | 0.278 | 0.935 |
| Day 9 to 22 | 0.89 | 1.04 | 0.86 | 1.03 | 0.031 | 0.384 | <0.001 | 0.748 |
| Day 23 to 35 | 0.87 | 1.00 | 0.87 | 0.89 | 0.056 | 0.348 | 0.215 | 0.379 |
| Day 36 to 49 | 1.00 | 0.92 | 0.99 | 0.93 | 0.059 | 0.969 | 0.262 | 0.942 |
| Average daily feed intake, kg/d | ||||||||
| Day 1 to 49 | 2.04 | 2.36 | 1.92 | 2.17 | 0.033 | <0.001 | <0.001 | 0.349 |
| Day 1 to 8 | 1.75 | 1.87 | 1.67 | 1.84 | 0.051 | 0.276 | 0.009 | 0.651 |
| Day 9 to 22 | 1.91 | 2.23 | 1.78 | 2.08 | 0.039 | 0.001 | <0.001 | 0.779 |
| Day 23 to 35 | 2.04 | 2.41 | 1.98 | 2.22 | 0.045 | 0.010 | <0.001 | 0.209 |
| Day 36 to 49 | 2.31 | 2.68 | 2.11 | 2.37 | 0.066 | <0.001 | <0.001 | 0.429 |
| G:F ratio | ||||||||
| Day 1 to 49 | 0.46 | 0.42 | 0.48 | 0.44 | 0.008 | 0.026 | <0.001 | 0.996 |
| Day 1 to 8 | 0.55 | 0.54 | 0.55 | 0.53 | 0.015 | 0.738 | 0.475 | 0.833 |
| Day 9 to 22 | 0.48 | 0.46 | 0.49 | 0.49 | 0.009 | 0.079 | 0.182 | 0.563 |
| Day 23 to 35 | 0.44 | 0.43 | 0.45 | 0.44 | 0.010 | 0.499 | 0.221 | 0.724 |
| Day 36 to 49 | 0.40 | 0.37 | 0.44 | 0.42 | 0.011 | <0.001 | 0.010 | 0.734 |
a Values are presented as least square means with the SEM.
b Fixed effect of days was significant (P < 0.001) for all parameters.
Figure 1.
Daily intake (g/d) of tP (A), aP (B), Ca (C), and the ratio of Ca:aP intake (D) in gilts (gray and cross-striped columns) and barrows (black and cross-striped columns) fed the Con diet and gilts (gray and solid columns) and barrows (black and solid columns) fed the diet with Phy. Significant effects (P ≤ 0.05) of sex are indicated by the symbol *; effects of phytase by the symbol †; and the interaction of sex and phytase by the symbol ‡.
When comparing the diet groups, pigs fed the Phy diet had a 6.1% higher BW compared with pigs fed the Con diet (P = 0.005). However, the phytase tended (P = 0.098) to lower pig’s BW by 1.8% on days 22 to 35 compared with the Con diet without phytase. Moreover, the phytase supplementation decreased the ADFI in the periods of days 9 to 22 and 23 to 35 by 6.1% (P < 0.01) and on days 36 to 49 by 10.1% (P < 0.001) compared with the Con diet. As result, phytase led to an overall improvement of the G:F ratio by 4.2% compared with the Con diet (P = 0.026). This was especially apparent on days 35 to 49, where the G:F ratio was 11.1% higher in pigs fed the Phy diet compared with those fed the Con diet (P < 0.001). For the whole experimental period, the intake of tP and Ca decreased in pigs fed the Phy diet on average by 8.4% and 6.7%, respectively (P < 0.001), whereas the intake of aP increased by 16.3% in the Phy group compared with the Con group (P < 0.001). When comparing the Ca and tP intake on the 14-d basis, phytase reduced the intake of tP by 8.2%, 6.9%, and 11.5% compared with the Con diet on days 9 to 22, 23 to 35, and 36 to 49, respectively (P ≤ 0.001). By contrast, the increase in the daily intake of aP with the phytase supplementation was greatest with 21.0% from days 1 to 8 and lower for the following days with an increase of 17.0%, 18.9%, and 12.0% in pigs fed the Phy diet on days 9 to 22, 23 to 35, and 36 to 49, respectively, compared with the Con group (P < 0.001). Similarly, the intake of Ca was 6.4%, 5.1%, and 9.8% lower in the Phy group compared with the Con group from days 9 to 22, 23 to 35, and 36 to 49, respectively (P < 0.05).
Serum Parameters
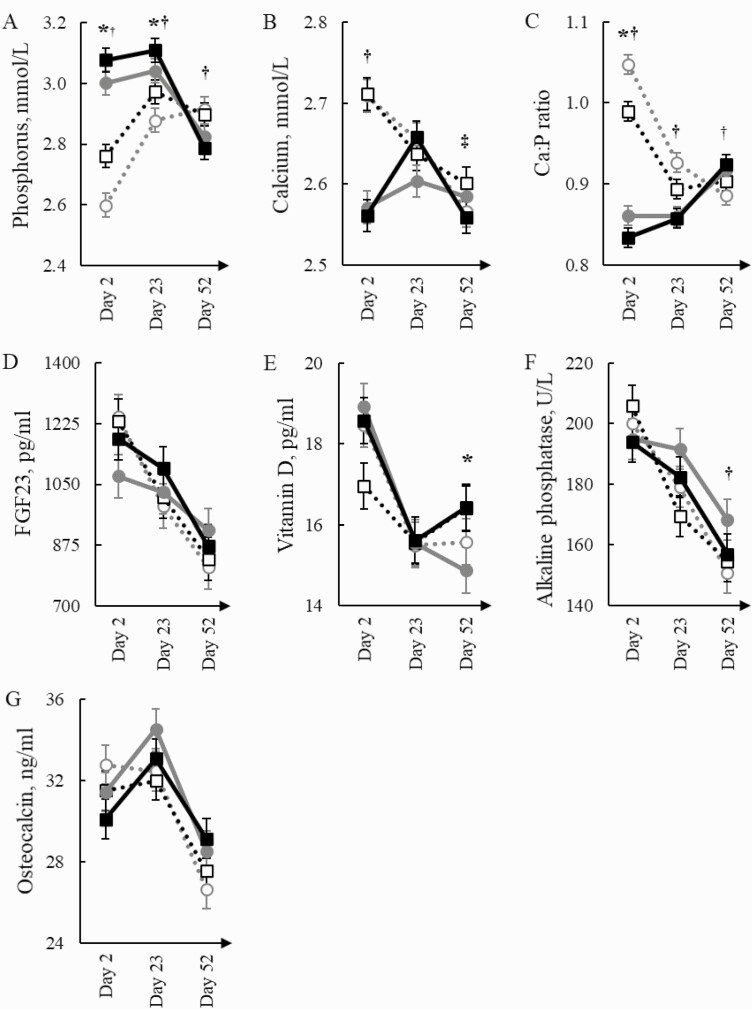
Except for FGF23, sex and phytase supplementation affected serum parameters. Additionally, age-related alterations occurred from day 1 to 49 (Supplementary Table S2, Fig. 2). Serum Ca was similar across sexes, whereas barrows had a 4.2% and 2.7% higher serum P content than gilts on days 2 and 23, respectively (P < 0.05), resulting in a 4.4% (P = 0.006) and 2.0% (P = 0.069) lower serum Ca:P ratio in barrows compared with gilts on days 2 and 23, respectively. Moreover, barrows had a 7.8% higher serum VitD content on day 23 and 9.9%, 25.7%, and 28.1% higher serum urea contents on days 2, 23, and 52 than gilts, respectively (P < 0.05). Moreover, barrows contained 4.0% more cholesterol in their serum than gilts on day 23 (P = 0.016).
Figure 2.
Serum content of phosphorus (A), calcium (B), Ca:P ratio (C), FGF23 (D), VitD (E), ALP (F), and OCN (G) in gilts (dotted gray line with blank circle items) and barrows (dotted black with blank square items) fed the Con diet and gilts (gray line with gray circle items) and barrows (black line with black square items) fed the diet with Phy. Significant effects (P ≤ 0.05) of sex are indicated by the symbol *; effects of phytase by the symbol †; and the interaction of sex and phytase by the symbol ‡.
Over time, P, Ca, and OCN in serum increased from day 2 to 23 but decreased toward day 52 compared with the previous measurement (P < 0.001). In contrast, the Ca:P ratio, VitD, and triglycerides decreased in serum toward day 23 but increased again on day 52 compared with the previous sampling days (P < 0.001). A continuous decrease over time was apparent for FGF23 and ALP in serum, while urea, cholesterol, and NEFA content in serum increased throughout the experiment (P < 0.001).
Phytase supplementation increased serum P by 13.4% and 5.1% on days 2 and 23, respectively (P < 0.001), but reduced it by 3.4% on day 52 (P = 0.004) compared with the Con group. On day 2, the serum Ca content was 5.3% lower in the Phy group compared with the Con group (P < 0.001). The phytase × sex interaction on day 52 for serum Ca (P = 0.029) indicated that the barrows of the Con group and gilts of the Phy group had higher serum Ca contents compared with the other two pig groups. The serum Ca:P ratio was 16.8% and 5.6% lower on days 2 and 23, respectively, and 2.9% higher on day 52 in the Phy group compared with the Con group (P < 0.05). The phytase supplementation increased (P = 0.028) the serum ALP by 7.3% on day 23 and tended (P = 0.063) to increase it by 6.4% on day 52 compared with the Con group. Likewise, phytase tended (P < 0.10) to increase serum OCN on days 23 and 52 compared with the Con group. Moreover, the serum urea content from pigs of the Phy group tended to be 6.9% higher on day 2 (P = 0.087) and was 8.2% higher on day 52 (P = 0.033) compared with pigs of the Con group. Serum cholesterol and triglycerides were 5.3% and 9.9% lower on day 2, whereas on day 52 triglycerides were 10.3% higher in the Phy group compared with the Con group (P < 0.05). Lastly, phytase supplementation increased serum NEFA by 71.6% on day 52 compared with the Con group (P < 0.001).
Relationships Between Daily Ca and P Intake on Days 1 to 8, 22 to 35, and 36 to 49 With Serum Parameters on Days 2 (Week 1), 23 (Week 4), and 52 (Week 8)
The sex-specific differences in serum parameters indicated that sex-related responses may interfere in the identification of potential serum markers for the dietary Ca and P intake. Therefore, Pearson correlations (Table 3) were calculated for each sex separately. In total, 47 correlations between serum parameters and Ca, tP, and aP intake were found in gilts but only 38 in barrows. Moreover, more correlations existed for days 1 to 7 (week 1) and days 50 to 56 (week 8) compared with days 22 to 28 (week 4). On days 1 to 7 (week 1), serum P content was positively correlated with aP intake, dietary Ca:tP ratio (0.58 < r < 0.76) but negatively with the dietary Ca:aP ratio (−0.76 < r < −0.61) in both sexes, whereas on days 22 to 28 (week 4) serum P only correlated with aP intake in barrows (r = 0.42). On days 50 to 56 (week 8), serum P correlated with tP and Ca intake in both sexes (0.39 < r < 0.41). Serum Ca levels in both sexes were negatively correlated with the dietary Ca:tP ratio (−0.61 < r < −0.58) but positively with the dietary Ca:aP ratio (0.58 < r < 0.61) on days 1 to 7 (week 1). Additionally, a positive correlation between serum Ca levels and tP and Ca intake existed in week 8 (0.45 < r < 0.58) for both sexes, whereas in gilts serum Ca correlated also with the aP intake (r = 0.50) on days 50 to 56 (week 8). Vitamin D positively correlated with aP intake on days 1 to 7 (week 1) in barrows (r = 0.38) and negatively with intake of tP, aP, and Ca on days 50 to 56 (week 8) in both sexes (−0.60 < r < −0.48). Only in gilts, ALP showed a negative correlation with tP, aP, and Ca intake on days 1 to 7 (week 1; r = −0.47), whereas a positive association between ALP and the aP intake existed on days 50 to 56 (week 8; r = 0.44). Serum OCN positively correlated with tP and Ca intake on days 1 to 7 (week 1) in both sexes (0.50 < r < 0.60) and additionally in gilts with the aP intake on days 1 to 7 (week 1; r = 0.44). Moreover, Pearson correlations were used to determine potential metabolic relationships between the dietary Ca and P intake and lipid and protein metabolism (Supplementary Table S3).
Table 3.
Pearson’s correlation between Ca, tP, and aP, daily Ca:tP ratio and daily Ca:aP ratio, and serum phosphorus, serum calcium, serum calcium to phosphorus ratio, FGF23, VitD, ALP, and OCN in gilts and barrows
| Gilts | Barrows | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Weeka | tP intake, g/d | aP intake, g/d | Ca intake, g/d | Daily Ca:tP ratio | Daily Ca:aP ratio | tP intake, g/d | aP intake, g/d | Ca intake, g/d | Daily Ca:tP ratio | Daily Ca:aP ratio |
| Serum P, mmol/L | 1 | 0.24 | 0.58* | 0.27 | 0.76* | −0.76* | 0.16 | 0.55* | 0.21 | 0.61* | −0.61* |
| 4 | 0.12 | 0.32 | 0.15 | 0.33 | −0.33 | 0.31 | 0.42* | 0.33 | 0.22 | −0.22 | |
| 8 | 0.41* | 0.2 | 0.39* | −0.25 | 0.25 | 0.39* | 0.33 | 0.39* | −0.23 | 0.23 | |
| Serum Ca, mmol/L | 1 | 0.09 | −0.22 | 0.06 | −0.58* | 0.58* | 0.31 | −0.19 | 0.27 | −0.61* | 0.61* |
| 4 | 0.06 | −0.09 | 0.05 | −0.17 | 0.17 | 0.27 | 0.28 | 0.28 | 0.06 | −0.06 | |
| 8 | 0.57* | 0.50* | 0.58* | 0.01 | −0.01 | 0.46* | 0.33 | 0.45* | −0.26 | 0.26 | |
| Serum Ca:P ratio | 1 | −0.17 | −0.57* | −0.21 | −0.86* | 0.86* | −0.01 | −0.52* | −0.06 | −0.74* | 0.74* |
| 4 | −0.12 | −0.41* | −0.16 | −0.46* | 0.46* | −0.19 | −0.35 | −0.21 | −0.27 | 0.27 | |
| 8 | −0.05 | 0.1 | −0.04 | 0.25 | −0.25 | −0.23 | −0.21 | −0.23 | 0.14 | −0.14 | |
| Serum FGF23, pg/mL | 1 | 0.11 | −0.03 | 0.1 | −0.22 | 0.22 | −0.27 | −0.2 | −0.28 | 0.04 | −0.04 |
| 4 | −0.23 | −0.13 | −0.22 | 0.02 | −0.02 | 0.13 | 0.19 | 0.14 | 0.1 | −0.1 | |
| 8 | −0.14 | 0.01 | −0.13 | 0.2 | −0.2 | −0.07 | −0.07 | −0.07 | 0.05 | −0.05 | |
| Serum VitD, ng/mL | 1 | −0.12 | −0.08 | −0.12 | 0.07 | −0.07 | 0.12 | 0.38* | 0.15 | 0.33 | −0.33 |
| 4 | 0.12 | 0.05 | 0.12 | −0.04 | 0.04 | 0.14 | 0.07 | 0.14 | −0.06 | 0.06 | |
| 8 | −0.48* | −0.50* | −0.49* | −0.11 | 0.11 | −0.51* | −0.60* | −0.52* | −0.1 | 0.1 | |
| Serum ALP, U/L | 1 | −0.47* | −0.47* | −0.47* | −0.16 | 0.16 | −0.12 | −0.14 | −0.12 | −0.1 | 0.1 |
| 4 | −0.06 | 0.06 | −0.05 | 0.16 | −0.16 | −0.02 | 0.15 | −0.01 | 0.24 | −0.24 | |
| 8 | 0.32 | 0.44* | 0.34 | 0.21 | −0.21 | 0.32 | 0.31 | 0.32 | 0 | 0 | |
| Serum OCN, ng/mL |
1 | 0.60* | 0.45* | 0.59* | −0.11 | 0.11 | 0.50* | 0.29 | 0.50* | −0.19 | 0.19 |
| 4 | 0.11 | 0.25 | 0.13 | 0.24 | −0.24 | 0.04 | 0.12 | 0.05 | 0.11 | −0.11 | |
| 8 | 0.15 | 0.2 | 0.15 | 0.18 | −0.18 | 0.15 | 0.27 | 0.16 | 0.2 | −0.2 |
a Week 1: correlation between Ca, tP, and aP intake from days 1 to 7 and serum parameters from days 2 + 3 (in week 1); week 4: correlation between Ca, tP, and aP intake from days 22 to 28 and serum parameters 23 + 24 (in week 4); week 8: correlation between Ca, tP, and aP intake from days 50 to 56 and serum parameters days 52 + 53 (in week 8).
*|r| > 0.35 and P < 0.05.
DISCUSSION
The importance of the dietary Ca and P supply and the impact on their systemic availability and skeletal mineralization in pigs are well known (van Riet et al., 2013; Schlegel and Gutzwiller, 2017). From the available literature data, it was not clear how reliably serum parameters that are related to the Ca and P homeostasis can be used to predict the dietary supply with Ca and aP. Moreover, it was not clear whether different serum parameters were necessary to predict dietary Ca and aP intake in gilts and barrows due to sex-related differences in ADFI and growth rate. The latter was, for instance, suggested by the generally greater ADG and higher growth rate between days 9 and 22 of the barrows, whereas the gilts reached their highest ADG at a later stage in the present study. We could identify several relationships between the dietary Ca, tP, and aP intake and serum Ca, P, VitD, ALP, and OCN levels but apparently not with FGF23 levels, whereby all serum parameters remained in their physiological range. However, from the present correlations, it is obvious that the predictability of pig’s intake of Ca, tP, and aP by serum parameters depends on pig’s age and sex, not allowing the recommendation of one marker for the whole growing period for both sexes. The predictability of the intake of aP by serum parameters showed a greater sex-specificity, whereby these relationships again mostly existed on days 2 and 52. Serum P on day 2 and VitD on day 52 were the best predictors for the dietary aP intake in both sexes. In contrast, on day 23, the majority of correlations with the serum parameters were too weak to allow the prediction of the daily intake of Ca, tP, and aP. These findings are in line with the decreasing Ca and P requirements with increasing age and hence related to regulatory mechanisms, reflecting the actual Ca and P requirements for skeletal growth and physiological functions (Horst et al., 1990; Pattanaungkul et al., 2000; Xu et al., 2002).
Barrows and gilts showed the expected sex-related ADFI and BW development, which were higher in barrows for the whole experimental period. Moreover, the present data supported the greater appetite of barrows throughout the fattening period despite similar growth, resulting in higher daily intakes of Ca, tP, and aP compared with the gilts. Accordingly, we would have expected that the higher supply of nutrients in barrows compared with gilts was mirrored in sex- and BW gain-related serum profiles, especially as we only fed one fattening diet throughout the experimental period, oversupplying the pigs with nutrients toward the end of the experimental period. The dietary Ca and P content met the requirements (0.66% Ca and 0.56% tP for 25 to 50 kg pigs; NRC, 2012) at the start of the experiment but were above them (0.52% Ca and 0.47% P for 75 to 100 kg pigs; NRC, 2012) toward the end of the experiment. Nevertheless, serum Ca and P did not reflect consistently the elevated dietary intake of Ca and P over time in barrows compared with gilts. From the investigated parameters, only serum urea, a marker for protein turnover, corresponded to the increased protein intake in barrows compared with the gilts. The increased serum urea also reflected the lower amino acid requirements with increasing age. These discrepancies demonstrate the challenge to link the dietary intakes of Ca, tP, and aP with serum profiles due to the regulatory action of the body to maintain serum levels in physiological ranges. Due to the many crucial functions in the body (e.g., enzyme activation and cell differentiation), serum Ca levels are tightly controlled by means of Ca-sensing receptors, which are evenly distributed in the body compared with serum P (Veum, 2010; Pu et al., 2016; Liu et al., 2018). In contrast, P-sensing receptors seem to be confined to the intestine and parathyroid gland, thereby allowing a less strict regulation of serum P levels (Berndt and Kumar, 2009; Pu et al., 2016; Chande and Bergwitz, 2018). These differences in the regulation of serum Ca and P were also suggested by the serum Ca:P ratio, which was below 1 throughout the experimental period across sexes and treatments despite dietary Ca:tP and Ca:aP ratios above 1. The present data support lower Ca and P requirements for bone formation when the animal reaches maturity (Clarke, 2008; Lederer, 2014). Against this background, serum FGF23, VitD, ALP, and OCN levels responded according to the maturational stage of the animal, reflecting skeletal growth and, except for FGF23, also reflected dietary Ca and aP provision on days 2, 23, and 52. Albeit ALP is rather a non-specific marker for tissue growth as all tissues and organs produce ALP during growth (Christenson, 1997; Penido and Alon, 2012; Gonzalo et al., 2018), it can be assumed that serum ALP and OCN as OCN is only synthesized by the bone, mirrored osteoblast activity and hence bone formation (Szulc et al., 2000; Allen, 2003; Owen and Reilly, 2018). Similarly, serum FGF23, a bone-derived hormone that suppresses renal phosphate reabsorption and VitD synthesis (Erben, 2018), was higher at the start of the experiment than at the end. However, the present data do not allow distinguishing whether the higher serum FGF23 was associated with more efficient P utilization for bone formation in the older pigs or with the adjustment of body VitD levels. Notably, serum ALP and FGF23 showed an age-specific decline, which emphasizes the importance of animal’s age when evaluating serum markers. These findings further indicate that the level of FGF23 in the blood might be depending on skeletal growth as it is the case for ALP in growing animals (Allen, 2003).
In contrast to previous observations (Vigors et al., 2014; Torres-Pitarch et al., 2017), phytase supplementation decreased the ADFI while not compromising the ADG in the present study. This resulted in the trend for the improved G:F ratio, indicating increased digestibility and conversion of nutrients of phytase-supplemented diets. Phytase supplementation increases the intestinal aP availability and absorption (Vötterl et al., 2020), which likely induced the higher serum P of pigs receiving the Phy diet until experimental day 23. Afterward, however, serum P was lower in pigs fed the Phy compared with the Con diet, suggesting a reduced intestinal absorption but increased renal excretion as regulatory response in these pigs. The increased serum levels of ALP and OCN from day 23 in pigs receiving the Phy diet would support that more Ca and P were used for bone formation (Carter et al., 1996; Golub and Boesze-Battaglia, 2007; Clarke, 2008), which may be indicative of a more balanced systemic Ca and P availability with the Phy diet. This assumption may be further supported by the greater serum Ca:P ratio on day 52. Both ALP and OCN are used in human osteomalacia diagnosis, but study results are inconsistent with respect to the interpretation of elevated values (Hlaing and Compston, 2014). Nevertheless, they may represent useful indicators for the dietary aP supply in growing pigs.
Pearson correlation analysis supported the importance to differentiate between sexes and stages of growth. The fact that none of the investigated serum parameters correlated with the daily intake of Ca, tP, and aP across the three sampling time points emphasizes the need to define the age stages for which the respective serum marker can reliably predict the dietary intake of Ca, tP, and aP. Moreover, the present correlations point out that sampling days 2 and 52 would be superior time points to indicate adequate supply with Ca and aP. These age stages may diverge when phase feeding is applied to fit the actual aP requirements over the fattening period, which need to be evaluated in further experiments. The pigs used in the present experiment came from one farrowing group with birth dates being 4 d apart at maximum. Accordingly, the days on which serum was collected corresponded to an age of 12, 16, and 20 wk. Hence, the first and third time points represented the early growing and early finishing period; time points at which often a dietary change occurs and, therefore, would be useful for adjustments in the dietary Ca and aP amounts. Nevertheless, the validation of the identified serum markers should include the characterization of “time slots” for their efficacy, using a tighter schedule of blood samplings. In fact, in practice, pigs of different ages are often mixed due to nonuniform growth or BW within age groups, and blood samplings may not be always possible at the exact age we sampled in this study. For experimental day 2 (12 wk of age), the present correlations suggested a marker profile of serum P and the serum Ca:P ratio for the daily aP intake for both sexes. In gilts serum OCN and in barrows VitD could be used as additional marker in this profile to predict the aP intake. Serum OCN, and ALP only in gilts, may be further used to predict the Ca intake on experimental day 2. On experimental day 23 (16 wk of age), only the serum Ca:P ratio for gilts and serum P for barrows may be used to predict aP intake. On experimental day 52 (20 wk of age), the present correlations suggested a serum profile that comprises serum Ca and ALP in gilts and VitD in both sexes to predict pig’s daily aP intake. To predict pig’s daily intake of Ca, serum P, Ca, and VitD could be used in both sexes on experimental day 52. Overall, the present correlations confirmed the applicability of certain serum parameters that are used in practice. However, the present findings also largely emphasize that their applicability differs with pig’s age and sex.
In conclusion, results demonstrated certain relationships between pig’s intake of Ca, tP, and aP and serum parameters. However, they also emphasized the dependencies of these relations on pig’s age and sex, not allowing the recommendation of one marker profile for the entire growing period and valid for both sexes. According to the present correlations, the daily Ca and aP intake could be most reliably estimated from serum parameters for an approximate age of 12 and 20 wk. Serum P and the Ca:P ratio at 12 wk of age and serum VitD at 20 wk of age may be reliable markers to predict pig’s daily aP intake in both sexes. The present marker profiles should be validated in further studies, narrowing the age ranges for which these markers are applicable and evaluated for their applicability with different feeding regimes.
Supplementary Material
ACKNOWLEDGMENTS
This research received funding from the Tandem Ph.D. Program of the University of Veterinary Medicine, Vienna, Austria. We thank Sharma Suchitra, Simone Koger, Melanie Wild, Arife Sener, Annegret Lucke, Manfred Hollmann and Thomas Enzinger (Institute of Animal Nutrition and Functional Plant Compounds), Lukas Schwarz (University Clinic of Swine), as well as Sylvia Posseth and Tamara Strini (vetFarm) for their excellent assistance with the animals, sampling, and laboratory analysis.
Conflict of interest statement. The authors declare that there is no conflict of interest.
LITERATURE CITED
- Allen, M. J. 2003. Biochemical markers of bone metabolism in animals: uses and limitations. Vet. Clin. Pathol. 32:101–113. doi: 10.1111/j.1939-165x.2003.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Amundson, L. A., Hernandez L. L., and Crenshaw T. D.. . 2017. Serum and tissue 25-OH Vitamin D3 concentrations do not predict bone abnormalities and molecular markers of Vitamin D metabolism in the hypovitaminosis D kyphotic pig model. Br. J. Nutr. 118:30–40. doi: 10.1017/S0007114517001751. [DOI] [PubMed] [Google Scholar]
- Berndt, T., and Kumar R.. . 2009. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology. 24:17–25. doi: 10.1152/physiol.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, S. D., Cromwell G. L., Combs T. R., Colombo G., and Fanti P.. . 1996. The determination of serum concentrations of osteocalcin in growing pigs and its relationship to end-measures of bone mineralization. J. Anim. Sci. 74:2719–2729. doi: 10.2527/1996.74112719x. [DOI] [PubMed] [Google Scholar]
- Chande, S., and Bergwitz C.. . 2018. Role of phosphate sensing in bone and mineral metabolism. Nat. Rev. Endocrinol. 14:637–655. doi: 10.1038/s41574-018-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson, R. H. 1997. Biochemical markers of bone metabolism: an overview. Clin. Biochem. 30:573–593. doi: 10.1016/s0009-9120(97)00113-6. [DOI] [PubMed] [Google Scholar]
- Clarke, B. 2008. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenshaw, T. D., Rortvedt-Amundson L. A., Cuarón J. A., Bergstrom J. R., and Litta G.. . 2014. Triennial Growth Symposium: Vitamin D – establishing the basics to dispel the hype. J. Anim. Sci. 92:883–886. doi: 10.2527/jas.2014-7626. [DOI] [PubMed] [Google Scholar]
- Dersjant-Li, Y., Awati A., Schulze H., and Partridge G.. . 2015. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 95:878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben, R. G. 2016. Update on FGF23 and Klotho signaling. Mol. Cell. Endocrinol. 432:56–65. doi: 10.1016/j.mce.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Erben, R. G. 2018. Physiological actions of fibroblast growth factor-23. Front. Endocrinol. (Lausanne). 9:267. doi: 10.3389/fendo.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachowsky, G., Pallauf J., Pfeffer E., Rodehutscord M., Schenkel H., Staudacher W., and Susenbeth A.. . 2006. Empfehlungen zur Energie- und Nährstoffversorgung von Schweinen. DLG-Verlag,Frankfurt am Main, Deutschland. [Google Scholar]
- Gerlinger, C., Oster M., Reyer H., Polley C., Vollmar B., Muráni E., Wimmers K., and Wolf P.. . 2020. Effects of excessive or restricted phosphorus and calcium intake during early life on markers of bone architecture and composition in pigs. J. Anim. Physiol. Anim. Nutr. (Berl). 1–11. doi: 10.1111/jpn.13286. [DOI] [PubMed] [Google Scholar]
- Golub, E. E., and Boesze-Battaglia K.. . 2007. The role of alkaline phosphatase in osteogenesis. Curr. Opin. Orthop. 18:444–448. doi: 10.1084/jem.93.5.415. [DOI] [Google Scholar]
- González-Vega, J. C., and Stein H. H.. . 2014. Calcium digestibility and metabolism in pigs. Asian-Australas. J. Anim. Sci. 27:1–9. doi: 10.5713/ajas.2014.r.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo, E., Létourneau-Montminy M. P., Narcy A., Bernier J. F., and Pomar C.. . 2018. Consequences of dietary calcium and phosphorus depletion and repletion feeding sequences on growth performance and body composition of growing pigs. Animal 12:1165–1173. doi: 10.1017/S1751731117002567. [DOI] [PubMed] [Google Scholar]
- Hlaing, T. T., and Compston J. E.. . 2014. Biochemical markers of bone turnover – uses and limitations. Ann. Clin. Biochem. 51:189–202. doi: 10.1177/0004563213515190. [DOI] [PubMed] [Google Scholar]
- Horst, R. L., Goff J. P., and Reinhardt T. A.. . 1990. Advancing age results in reduction of intestinal and bone 1, 25-dihydroxyvitamin D receptor. Endocrinology. 126:1053–1057. doi: 10.1210/endo-126-2-1053. [DOI] [PubMed] [Google Scholar]
- Humer, E., Schwarz C., and Schedle K.. . 2015. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. (Berl). 99:605–625. doi: 10.1111/jpn.12258. [DOI] [PubMed] [Google Scholar]
- Kamphues, J., Wolf P., Coenen M., Eder K., Iben C., Kienzle E., Liesegang A., Männer K., Zebeli Q., and Zentek J.. . 2014. Supplemente zur Tierernährung. 12. Auflag. M. & H. Scharper GmbH,Hannover, Deutschland. [Google Scholar]
- Kolp, E., Wilkens M. R., Pendl W., Eichenberger B., and Liesegang A.. . 2017. Vitamin D metabolism in growing pigs: influence of UVB irradiation and dietary vitamin D supply on calcium homeostasis, its regulation and bone metabolism. J. Anim. Physiol. Anim. Nutr. (Berl). 101:79–94. doi: 10.1111/jpn.12707. [DOI] [PubMed] [Google Scholar]
- Lederer, E. 2014. Regulation of serum phosphate. J. Physiol. 592:3985–3995. doi: 10.1113/jphysiol.2014.273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. A., Lagos L. V., Bedford M. R., and Stein H. H.. . 2020. Increasing calcium from deficient to adequate concentration in diets for gestating sows decreases digestibility of phosphorus and reduces serum concentration of a bone resorption biomarker. J. Anim. Sci. 98:1–8. doi: 10.1093/jas/skaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létourneau-Montminy, M. P., Jondreville C., Sauvant D., and Narcy A.. . 2012. Meta-analysis of phosphorus utilization by growing pigs: effect of dietary phosphorus, calcium and exogenous phytase. Animal. 6:1590–1600. doi: 10.1017/S1751731112000560. [DOI] [PubMed] [Google Scholar]
- Li, Y., and Stahl C. H.. . 2014. Dietary calcium deficiency and excess both impact bone development and mesenchymal stem cell lineage priming in neonatal piglets. J. Nutr. 1:1935–1942. doi: 10.3945/jn.114.194787.risk. [DOI] [PubMed] [Google Scholar]
- Liu, G., Cao W., Jia G., Zhao H., Chen X., and Wang J.. . 2018. Calcium-sensing receptor in nutrient sensing: an insight into the modulation of intestinal homoeostasis. Br. J. Nutr. 120:881–890. doi: 10.1017/s0007114518002088. [DOI] [PubMed] [Google Scholar]
- McGhee, M. L., and Stein H. H.. . 2019. Effects of microbial phytase on standardized total tract digestibility of phosphorus in hybrid rye, barley, wheat, corn, and sorghum fed to growing pigs. Transl. Anim. Sci. 3:1238–1245. doi: 10.1093/tas/txz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann, C., and Bassler R.. . 2012. Die chemische Untersuchung von Futtermitteln. VDLUFA-Verlag,Darmstadt. [Google Scholar]
- NRC . 2012. Nutrition requirement of swine. 11th ed. National Academy of Sciences,Washington, D.C. [Google Scholar]
- O′Doherty, J. V., Gahan D. A., O′Shea C., Callan J. J., and Pierce K. M.. . 2010. Effects of phytase and 25-hydroxyvitamin D3 inclusions on the performance, mineral balance and bone parameters of grower-finisher pigs fed low-phosphorus diets. Animal. 4:1634–1640. doi: 10.1017/S1751731110000807. [DOI] [PubMed] [Google Scholar]
- Oster, M., Just F., Büsing K., Wolf P., Polley C., Vollmar B., Muráni E., Ponsuksili S., and Wimmers K.. . 2016. Toward improved phosphorus efficiency in monogastrics—interplay of serum, minerals, bone, and immune system after divergent dietary phosphorus supply in swine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310:R917–R925. doi: 10.1152/ajpregu.00215.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, R., and Reilly G. C.. . 2018. In vitro models of bone remodelling and associated disorders. Front. Bioeng. Biotechnol. 6:1–22. doi: 10.3389/fbioe.2018.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanaungkul, S., Riggs B., Yergey L., Vieira N., O′Fallon W., and Khosla S.. . 2000. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women : evidence for age-related intestinal resistance to 1,25(OH)2D action. J. Clin. Endocrinol. Metab. 85:4023–4027. doi: 10.1210/jcem.85.11.6938. [DOI] [PubMed] [Google Scholar]
- Penido, M. G. M. G., and Alon U. S.. . 2012. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 27:2039–2048. doi: 10.1007/s00467-012-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu, F., Chen N., and Xue S.. . 2016. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Well. 5:8–16. doi: 10.1016/j.fshw.2016.01.001. [DOI] [Google Scholar]
- van Riet, M. M. J., Millet S., Aluwé M., and Janssens G. P. J.. . 2013. Impact of nutrition on lameness and claw health in sows. Livest. Sci. 156:24–35. doi: 10.1016/j.livsci.2013.06.005. [DOI] [Google Scholar]
- Rupp, T., Butscheidt S., Vettorazzi E., Oheim R., Barvencik F., Amling M., and Rolvien T.. . 2019. High FGF23 levels are associated with impaired trabecular bone microarchitecture in patients with osteoporosis. Osteoporos. Int. 30:1655–1662. doi: 10.1007/s00198-019-04996-7. [DOI] [PubMed] [Google Scholar]
- Schlegel, P., and Gutzwiller A.. . 2017. Effect of dietary calcium level and source on mineral utilisation by piglets fed diets containing exogenous phytase. J. Anim. Physiol. Anim. Nutr. (Berl). 101:e165–e174. doi: 10.1111/jpn.12582. [DOI] [PubMed] [Google Scholar]
- Schneider, S., Brunlehner E.-M., Schäffler M., Propstmeier G., Preißinger W., Harms K., Weiß J., and Jais C.. . 2019. Futterberechnung für Schweine. Auflag. 22nd ed. Freising-Weihenstephan: Bayerische Landesanstalt für Landwirtschaft (LfL). p. 22. [Google Scholar]
- Sørensen, K. U., Kruger M. C., Hansen-Møller J., and Poulsen H. D.. . 2018. Bone biochemical markers for assessment of bone responses to differentiated phosphorus supply in growing-finishing pigs. J. Anim. Sci. 96:4693–4703. doi: 10.1093/jas/sky311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc, P., Seeman E., and Delmas P. D.. . 2000. Biochemical measurements of bone turnover in children and adolescents. Osteoporosis Int. 11:281–294. doi: 10.1007/s001980070116. [DOI] [PubMed] [Google Scholar]
- Torres-Pitarch, A., Hermans D., Manzanilla E. G., Bindelle J., Everaert N., Beckers Y., Torrallardona D., Bruggeman G., Gardiner G. E., and Lawlor P. G.. . 2017. Effect of feed enzymes on digestibility and growth in weaned pigs: a systematic review and meta-analysis. Anim. Feed Sci. Technol. 233:145–159. doi: 10.1016/j.anifeedsci.2017.04.024. [DOI] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Varley, P. F., Callan J. J., and O′Doherty J. V.. . 2010. Effect of phosphorus level and phytase inclusion on the performance, bone mineral concentration, apparent nutrient digestibility, and on mineral and nitrogen utilization in finisher pigs. Irish J. Agric. Food Res. 49:141–152. doi: 10.2307/41219179. [DOI] [Google Scholar]
- Veum, T. L. 2010. Phosphorus and calcium nutrition and metabolism. In: Vitti D. M. S. S. and Kebreab E., editors, Phosphorus and calcium utilization and requirements in farm animals. CAB International,Wallingford, UK. [Google Scholar]
- Vier, C. M., Dritz S. S., Tokach M. D., DeRouchey J. M., Goodband R. D., Gonçalves M. A. D., Orlando U. A. D., Bergstrom J. R., and Woodworth J. C.. . 2019. Calcium to phosphorus ratio requirement of 26-to 127-kg pigs fed diets with or without phytase. J. Anim. Sci. 97:4041–4052. doi: 10.1093/jas/skz257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigors, S., Sweeney T., O′Shea C. J., Browne J. A., and O′Doherty J. V.. . 2014. Improvements in growth performance, bone mineral status and nutrient digestibility in pigs following the dietary inclusion of phytase are accompanied by modifications in intestinal nutrient transporter gene expression. Br. J. Nutr. 112:688–697. doi: 10.1017/S0007114514001494. [DOI] [PubMed] [Google Scholar]
- Vötterl, J. C., Klinsoda J., Zebeli Q., Hennig-Pauka I., Kandler W., and Metzler-Zebeli B. U.. . 2020. Dietary phytase and lactic acid-treated cereal grains differently affected calcium and phosphorus homeostasis from intestinal uptake to systemic metabolism in a pig model. Nutrients 12:1542. doi: 10.3390/nu12051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H., Bai L., Collins J. F., and Ghishan F. K.. . 2002. Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)2 vitamin D3. Am. J. Physiol. Cell Physiol. 282:487–493. doi: 10.1152/ajpcell.00412.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.