Abstract
Objective.
To define biopsychosocial mechanisms of pain that go above and beyond disease activity and organ damage in systemic lupus erythematosus (SLE).
Methods.
We conducted a cross-sectional analysis of patient-reported data in a population-based registry of 766 people with SLE. Predictors of pain intensity and interference were examined using hierarchical linear regression. We built two main hierarchical regression models: pain intensity regressed on disease activity and organ damage; and pain interference regressed on disease activity and organ damage. For each model, we sought to establish the relationship between pain outcomes and the primary exposures using sequential steps comprising the inclusion of each construct in six stages: demographic, socioeconomic, physical, psychological, behavioral and social factors. We also conducted sensivity analyses eliminating all overt aspects of pain in the disease activity measure and reestimated the models.
Results.
Disease activity and organ damage explained 32–33% of the variance in pain intensity and interference. Sociodemographic factors accounted for an additional 4–9% of variance in pain outcomes, while psychosocial/behavioral factors accounted for the final 4% of variance. In the sensitivity analyses, we found that disease activity and organ damage explained 25% of the variance in pain outcomes.
Conclusion.
Disease activity only explained 33% of the variance of pain outcomes. However, there was an attenuation in these associations after accounting for psychosocial/behavioral factors, highlighting their roles in modifying the relationship between disease activity and pain. These findings suggest that multilevel interventions may be needed to tackle the negative impact of pain in SLE.
Keywords: Pain, biopsychosocial, lupus, social determinants of health, disease activity
Introduction
Roughly 50–100 million Americans are living with ongoing pain and costs $635 billion annually (1). An estimated 20 million live with high-impact chronic pain with substantially restricted work, social, and self-care activities(1). Pain is the most frequently reported symptom in rheumatology, with the etiology ascribed to inflammation, joint degeneration and central sensitization(2). Patients with systemic lupus erythematosus (SLE) rank pain as the most distressing symptom above other symptoms such as fatigue, depression, sleep disturbance, weight gain, rashes, and forgetfulness (3). A recent survey revealed that 32% of patients with SLE listed joint and muscle pain and/or swelling as the symptom associated the most negative impact in their lives(3). Despite treatment advances, pain remains the most prominent, unaddressed patient complaint. In a study of persistently frequent (≥3 visits per year) emergency department visits among patients with SLE, pain was coded as the chief concern for 50% of these visits(4).
Chronic pain has lasting personal costs to patients including poor quality of life, disability, social isolation, stress and other psychosocial problems. The prevalence of chronic pain (defined as persistent pain that occurs on at least half the days for ≥6 months) and increased pain intensity (defined as the magnitude of experienced pain) vary by age, race/ethnicity, gender, educational attainment, and income(1,5,6). Pain interference (defined as pain that hinders major life activities) is also patterned by sociodemographic determinants(1,5). Lifestyle related factors like smoking and obesity, as well as comorbidities are implicated in more severe pain manifestations (1,5). Despite the substantial impact of pain and extensive explorations of the determinants of health in SLE, the mechanisms of pain intensity and interference in SLE are not completely understood. We present a biopsychosocial approach (see Supplementary Figure 1) to explain pain intensity and interference as multidimensional, dynamic integration among disease-related, demographic, socioeconomic, physical, psychological, behavioral and social constructs that reciprocally influence one another (7,8). We hypothesized that disease activity and organ damage will be associated with increasing pain intensity and interference in a cross-sectional sample of predominantly black patients with SLE, along with other determinants. Quantifying the potential impact of modifiable behavioral, psychosocial and SLE-related factors is important for the development of appropriate interventions to address the problem of pain in SLE.
Methods
Study setting
The Georgians Organized Against Lupus (GOAL) Cohort is a population-based cohort of individuals with validated diagnosis of SLE supported by the Centers for Disease Control and Prevention (CDC). The overall aim is to examine the impact of sociodemographic and health care factors on outcomes that are relevant to patients, health care providers, and policy makers. Recruitment and data collection methods have been previously described(9). Consecutive annual sets of surveys have been administered to the GOAL Cohort participants since 2012. All participants completed a self-report questionnaire to return via mail or completed via Internet or phone.
The Emory University Institutional Review Board, Grady Health System Research Oversight Committee, and Georgia Department of Public Health Institutional Review Board approved the GOAL study protocol. All participants provided informed consent.
Main exposures
Patient-reported responses from October 2015 through August 2017 surveys were analyzed. The primary exposures of interest were disease activity and organ damage. Disease activity was measured using the Systemic Lupus Activity Questionnaire (SLAQ), a validated tool designed to be used in population-based studies outside the clinical setting, when physician assessment is not feasible(10,11). The SLAQ includes 24 questions to assess disease activity symptoms and signs (e.g. fatigue, fever, skin rashes, arthritis, …) in the past 3 months. Items are endorsed as “no problem”, “mild”, “moderate”, or “severe” and scored from 0 to 3. SLAQ scoring ranges from 0 to 44 with higher scores indicating greater SLE-related disease activity.
Organ damage accrual was measured using a validated self-administered version of the Brief Index of Lupus Damage (BILD)(12). The tool measures cumulative organ damage in 12 organ systems since the onset of SLE and present for at least 6 months. Items are coded as present or absent, with scores ranging from 0 to 30 and higher scores indicating greater organ damage. The tool has been used in epidemiological studies and has shown to predict or correlate with important outcomes such as death, work loss, and depression(13–15).
Main outcomes
The primary outcomes of interest were pain interference and pain intensity as reported at baseline and measured by the Patient Reported Outcomes Measurement System (PROMIS) adult short forms (SF)(16).
Pain Intensity was measured using the following question from the PROMIS Global-10 v.1.0: “In the past 7 days, how would you rate your pain on average?” An 11-item ordinal scale from 0 to 10 is provided to answer the question, with a higher score indicating more pain intensity. Pain Interference was measured using the PROMIS Pain Interference SF 4a. This 4-item questionnaire uses a 5-item Likert scale rated from 1 (not at all) to 5 (very much) to quantify the impact of pain on daily activities, working around the house, participation in social activities and household chores in the past 7 days. Raw scores were calculated by the PROMIS HealthMeasures Scoring Service (HMSS) and converted to T-scores. A T-score of 50 represents the reference population mean 50; standard deviation [SD]10. A higher PROMIS T-score represents more of the concept being measured.
Covariates
We broadly defined six main constructs, as shown in Supplementary Figure 1.
Demographic and socioeconomic factors included age at baseline, gender (female versus male), race (black versus non-black), marital status (single versus married/living with a partner), annual income in $10,000 increments, and educational attainment (high school or less, some college, and college and above).
Physical factors included quality of sleep, which was assessed with the PROMIS Sleep Disturbance SF 8a. This is an 8-item measure of self-reported perceptions of sleep quality, depth, and restoration within the past seven days. Patient reported data were collected to measure body mass index (BMI) in kg/m2. Physical health was measured using a 5-point Likert scale question from the PROMIS Global-10 v.1.0: “In the past 7 days, how would you rate your physical health?” Answers were scored from 1= Poor to 5=Excellent.
Psychological factors included anxiety, depression and anger. These domains were measured using PROMIS short forms (Depression SF 8a, Anxiety SF 4a, and Anger SF 5a). These three measures have demonstrated clinical validity across a range of chronic health conditions(17).
Behavioral factors included smoking, which was categorized as “current” versus “not current”. In addition, we used three scales from the Brief COPE tool to measure negative mechanism of coping (substance use and alcohol, self-blame, and denial) along with one scale to measure coping with religion. The tool has good psychometric properties to measure coping strategies used in everyday life or in distressful situations(18,19).
Social factors included emotional support and social isolation, which were measured with PROMIS short forms. Social Isolation SF 6a assessed perceptions of being avoided, excluded, detached, disconnected from, or unknown by others, and Emotional Support SF 4a measured feelings of being cared for and valued as a person and having confidant relationships(20). In addition, a modified version of the Everyday Discrimination Scale was used to measure various forms of interpersonal mistreatment in participants’ day-to-day lives over the previous 12 months. Examples include being “treated with less respect than other people” and “treated as if you are not smart”. The 10 items on the scale are framed in the context of general mistreatment, without reference to race/ethnicity and other demographics. Responses were assessed with a 4-point scale (1=never, 2=rarely, 3=sometimes, 4=often), which was summed and averaged for a final score. The everyday discrimination scale has been widely used across samples of African-American, Caucasian, and Chinese participants(21–24), and has shown high levels of internal consistency and convergent and divergent validity(21,25). We also measured unmet financial needs using the analogous 4-item scale included in the Conger Financial Strain measure(26). The scale assesses specific needs that cannot be met due to financial hardship (e.g., not enough money to buy the [home/clothing/food/medical] we need).
Statistical analysis
The data were analyzed using SAS version 9.4. Baseline characteristics obtained using summary statistics. Continuous variables were summarized using means, standard deviations, and medians. The unadjusted associations between covariates and the exposures and outcomes were estimated using linear regression. We used G*Power to conduct a post-hoc calculation of required sample size for a linear multiple regression to test increase in R-squared with a power of 95% and a model with 23 predictors, with small to medium effect size (0.05) (27). The required sample size was 664. We used all of the available data of 766 patients, making this study more than adequately powered.
Predictors of pain intensity and interference were examined using hierarchical linear regression. We built two main regression models: pain intensity regressed on disease activity and organ damage, and pain interference regressed on disease activity and organ damage. Each regression model sought to establish the relationship between pain outcomes and the primary exposures using hierarchical steps comprising the inclusion of each construct in six stages: demographic (gender, age, marital status, race); socioeconomic (annual household income, educational attainment); physical (sleep disturbance, body mass index, and global health – physical); psychological (anxiety, depression, anger); behavioral (smoking, coping with religion, coping with substance abuse and alcohol, and coping with denial) and social (emotional support, social isolation, financial strain, and discrimination). In advance of the analyses, we specified an a priori framework (see Supplementary Figure 1) and a logically determined priority of each construct based on clinical expertise on how pain would be evaluated in a clinical scenario. Thus, entered into the first stage were biological or disease related constructs: disease activity and organ damage. At stage two, demographic characteristics were entered, followed by socioeconomic factors at stage three, physical factors at stage four, psychological factors at stage five, behavioral factors at stage six and social factors at stage seven. Our goal was to determine whether newly added constructs show significant improvement in the proportion of explained variance in pain intensity and interference by the models (R2) over disease-related constructs.
Sensitivity analyses
To account for possible collinearity between disease activity and pain outcomes, we examined the effect of a modified SLAQ score without six pain related measures: stomach pain, chest pain, muscle pain, headache, joint swelling, and joint pain. We modeled the two pain outcomes on the modified SLAQ score with the other variables to determine if there were changes in the inferences from the original SLAQ score. We also examined the individual contributions of constitutional, mucocutaneous, organ system and musculoskeletal symptoms in SLAQ on pain outcomes. We replaced the full SLAQ with the scores for each of the individual contributions in Models 1 and 7.
Results
Baseline characteristics
There were 766 participants, of which 93% were female (Table 1). The mean age was 48 years. A majority were black (82%), non-smokers (87%), single (62%) and reported annual income <$40,000 (67%). The mean disease activity score was 16 and the mean organ damage score was 3. The mean and standard deviations were 5.3 ± 2.9 for pain intensity and 58 ± 10 for pain interference. Means and standard deviations of the following PROMIS measures were 56 ± 10 for sleep disturbance, 51 ± 9 for emotional support, 52 ± 10 for depression, 55 ± 10 for anxiety, 49 ± 11 for social isolation, 2.5 ± 1 for physical health, and 53 ± 12 for anger. Other means and standard deviations were 1.8 ± 0.6 for everyday discrimination and 9 ± 3 for financial strain.
Table 1.
Baseline characteristics of patients in the Georgians Organized Against Lupus cohort
| Baseline Characteristic | Category | Overall (n=766) |
|---|---|---|
| Pain outcomes | ||
| Pain intensity (one item score) | Mean ± SD | 5.3 ± 2.9 |
| Median(IQR) | 6.0 (3.0–8.0) | |
| Range | 0–10 | |
| PROMIS pain interference (t score) | Mean ± SD | 58.3 ± 9.7 |
| Median(IQR) | 60.3 (53.9–65.5) | |
| Range | 41.6–75.6 | |
| Disease related factors | ||
| Disease activity | Mean ± SD | 16.2 ± 9.1 |
| (SLAQ score) | Median(IQR) | 15.0 (9–22) |
| Range | 0–44 | |
| Organ damage | Mean ± SD | 2.5 ± 2.4 |
| (BILD score) | Median(IQR) | 2.0 (1–4) |
| Range | 0–15 | |
| Mild to severe pain manifestations in past 3 months, n (%) | Chest pain | 367 (47.9) |
| Stomach pain | 421 (55.0) | |
| Headache | 452 (58.9) | |
| Joint pain | 658 (85.9) | |
| Muscle pain | 573 (74.8) | |
| Current medications, n (%) | Steroids | 395 (51.6) |
| Hydroxychloroquine | 539 (70.4) | |
| Immunosuppressive drugs | 280 (36.6) | |
| Biologics | 44 (5.7) | |
| Cyclophosphamide | 15 (2.1) | |
| Renal damage, n (%)a | Yes | 53 (6.9) |
| No | 713 (93.1) | |
| Demographic factors | ||
| Age (years) | Mean ± SD | 48.4 ± 13.6 |
| Median(IQR) | 48.6 (38.3–58.4) | |
| Range | 17.1–89.5 | |
| Gender, n (%) | Male | 52 (6.8) |
| Female | 714 (93.2) | |
| Marital statusb, n (%) | Single | 478 (62.4) |
| Not single | 288 (37.6) | |
| Racec, n (%) | Non-black | 138 (18.0) |
| Black | 628 (82.0) | |
| Socioeconomic factors | ||
| Annual income level, n (%) | <$40,000 | 511 (66.7) |
| $40,000–69,000 | 120 (15.7) | |
| ≥$70,000 | 135 (17.6) | |
| Education level, n (%) | 1) High school or less | 251 (32.8) |
| 2) Some college | 253 (33.0) | |
| 3) College or above | 262 (34.2) | |
| Physical factors | ||
| PROMIS sleep disturbance (t score) | Mean ± SD | 55.7 ± 9.9 |
| Median(IQR) | 56.1 (51–63) | |
| Range | 32–73 | |
| Body mass index (kg/m2), n (%) | Mean ± SD | 29.4 ± 8.0 |
| Median(IQR) | 28.3 (24–34) | |
| Range | 13–66 | |
| PROMIS physical health (one item score) | Mean ± SD | 2.5 ± 0.9 |
| Median(IQR) | 2.0 (2–3) | |
| Range | 1–5 | |
| Psychological factors | ||
| PROMIS anxiety (T-score) | Mean ± SD | 54.8 ± 10.2 |
| Median(IQR) | 55.6 (47.9–61.7) | |
| Range | 40.3–81.4 | |
| PROMIS depression (T-score) | Mean ± SD | 51.8 ± 10.4 |
| Median(IQR) | 52.3 (44.4–58.9) | |
| Range | 38.0–82.4 | |
| PROMIS anger (T-score) | Mean ± SD | 52.8 ± 12.3 |
| Median(IQR) | 52.8 (44–61) | |
| Range | 33–83 | |
| Behavioral health | ||
| Cigarette smokingd, n (%) | Current | 100 (13.1) |
| Not current | 663(86.9) | |
| Coping with spirituality and religion | Mean ± SD | 6.2 ± 2.0 |
| Median(IQR) | 7.0 (5.0–8.0) | |
| Range | 2–8 | |
| Coping with substance use and alcohol | Mean ± SD | 2.4 ± 1.1 |
| Median(IQR) | 2.0 (2.0–2.0) | |
| Range | 2.0–8.0 | |
| Coping with self-blame | Mean ± SD | 3.5 ± 1.8 |
| Median(IQR) | 3.0 (2.0–5.0) | |
| Range | 2–8 | |
| Coping with denial | Mean ± SD | 3.1 ±1.6 |
| Median(IQR) | 2.0 (2.0–4.0) | |
| Range | 2–8 | |
| Social factors | ||
| PROMIS emotional support | Mean ± SD | 51.3 ± 9.3 |
| Median(IQR) | 49.9 (43.8–62.0) | |
| Range | 25.8–62.0 | |
| PROMIS social isolation (T-score) | Mean ± SD | 49.0 ± 10.9 |
| Median(IQR) | 49.0 (40.0–57.4) | |
| Range | 34.4–76.2 | |
| Financial strain, unmet needs | Mean ± SD | 9.2 ± 3.3 |
| Median(IQR) | 9.0 (8.0–11.0) | |
| Range | 4–16 | |
| Everyday discrimination scale | Mean ± SD | 1.8 ± 0.6 |
| Median(IQR) | 1.7 (1.2–2.1) | |
| Range | 1.0–4.0 |
Renal disease was defined as either receiving a kidney transplant or dialysis for more than six months
Marital status was defined as single = never married, divorced or widowed and not single = married or living with a partner
This analysis was limited to non-black and black individuals. The original GOAL data set is multiethnic but the numbers of non-white and non-black individuals were few.
Smoking status is defined as current = smokes everyday or some days and not current = does not smoke
Bivariate analyses
The unadjusted associations between predictors, main exposures, and outcomes are shown in Table 3. The following characteristics were significantly associated with higher pain intensity: single marital status, black race, lower household income, and lower educational attainment. Age and gender did not have a significant association with pain intensity. Higher levels of sleep disturbance and BMI were associated with increasing pain intensity. Better self-reported physical health was associated with lower pain intensity. Participants that reported higher levels of anxiety, depression and anger were also more likely to report higher pain intensity. Smokers were more likely to report higher pain intensity in comparison to non-smokers. Increasing levels of reported coping with religion or spirituality were associated with increasing pain intensity. Increasing negative coping characteristics (substance use/alcohol, self-blame and denial) were associated with higher pain intensity. However, the association between coping and substance use/alcohol was not significant. Higher levels of emotional support were associated with decreased pain intensity; however, higher levels of social isolation, financial strain and discrimination were associated with increased pain intensity. These bivariate associations were similar for pain interference, disease activity (except for gender) and organ damage (except for gender, marital status, race and educational attainment).
Table 3.
Factors associated with pain outcomes and disease related measures in individuals with SLE in the unadjusted linear regression
| Characteristic | Pain intensity | Pain interference | Disease activity | Organ damage |
|---|---|---|---|---|
| Demographic factors | ||||
| Age at survey | 0.006 (−0.009 to 0.022) | 0.024 (−0.027 to 0.075) | −0.072 (−0.119 to −0.025) | 0.034 (0.021 to 0.046) |
| Gender | ||||
| Male | Ref | Ref | Ref | Ref |
| Female | 0.396 (−0.433 to 1.226) | −0.483 (−3.232 to 2.265) | −2.382 (−4.965 to 0.201) | 0.316 (−0.368 to 1.000) |
| Marital status | ||||
| Not single | Ref | Ref | Ref | Ref |
| Single | 0.770 (0.342 to 1.199) | 2.059 (0.635 to 3.483) | 2.496 (1.169 to 3.823) | 0.261 (−0.094 to 0.617) |
| Race | ||||
| Non-black | Ref | Ref | Ref | Ref |
| Black | 1.650 (1.117 to 2.182) | 3.035 (1.243 to 4.827) | 1.863 (0.172 to 3.553) | 0.151 (−0.299 to 0.600) |
| Socioeconomic factors | ||||
| Household income | −0.439 (−0.514 to −0.365) | −1.262 (−1.515 to −1.008) | −1.139 (−1.376 to −0.902) | −0.147 (−0.213 to −0.082) |
| Education | ||||
| High school or less | Ref | Ref | Ref | Ref |
| Some college | −0.465 (−0.956 to 0.026) | −0.776 (−2.422 to 0.870) | 1.441 (−0.120 to 3.002) | 0.134 (−0.293 to 0.560) |
| College or above | −2.133 (−2.619 to −1.647) | −6.128 (−7.755 to −4.501) | −3.593 (−5.132 to −2.054) | −0.087 (−0.508 to 0.335) |
| Physical factors | ||||
| PROMIS sleep disturbance | 0.142 (0.123 to 0.160) | 0.518 (0.459 to 0.579) | 0.455 (0.397 to 0.512) | 0.036 (0.018 to 0.053) |
| Body mass index | 0.083 (0.057 to 0.108) | 0.237 (0.152 to 0.322) | 0.112 (0.031 to 0.193) | 0.010 (−0.011 to 0.033) |
| PROMIS physical health | −1.476 (−1.677 to −1.275) | −5.602 (−6.237 to −4.967) | −4.494 (−5.116 to −3.871) | −0.625 (−0.806 to −0.444) |
| Psychological factors | ||||
| PROMIS anxiety | 0.089 (0.069 to 0.108) | 0.379 (0.316 to 0.441) | 0.388 (0.331 to 0.446) | 0.018 (0.001 to 0.035) |
| PROMIS depression | 0.089 (0.070 to 0.108) | 0.348 (0.287 to 0.410) | 0.451 (0.398 to 0.505) | 0.027 (0.0100 to 0.043) |
| PROMIS anger | 0.092 (0.076 to 0.108) | 0.360 (0.310 to 0.410) | 0.338 (0.291 to 0.385) | 0.011 (−0.000 to 0.024) |
| Behavioral factors | ||||
| Smoking | ||||
| Not current | Ref | Ref | Ref | Ref |
| Current | 1.091 (0.476 to 1.707) | 3.469 (1.431 to 5.507) | 4.241 (2.340 to 6.142) | 0.331 (−0.179 to 0.842) |
| Coping with religion | 0.133 (0.028 to 0.237) | 0.443 (0.096 to 0.790) | 0.182 (−0.145 to 0.510) | 0.109 (0.027 to 0.196) |
| Coping with substance use and alcohol | 0.177 (−0.015 to 0.369) | 0.634 (−0.001 to 1.270) | 1.027 (0.434 to 1.620) | 0.009 (−0.149 to 0.167) |
| Coping with self-blame | 0.256 (0.138 to 0.374) | 1.281 (0.895 to 1.667) | 1.475 (1.123 to 1.828) | 0.109 (0.011 to 0.208) |
| Coping with denial | 0.474 (0.251 to 0.597) | 1.387 (0.970 to 1.803) | 1.467 (1.080 to 1.854) | 0.083 (−0.024 to 0.190) |
| Social factors | ||||
| PROMIS emotional support | −0.044 (−0.067 to −0.022) | −0.231 (−0.304 to −0.156) | −0.242 (−0.310 to −0.175) | −0.018 (−0.037 to 0.000) |
| PROMIS social isolation | 0.079 (0.061 to 0.097) | 0.342 (0.283 to 0.401) | 0.354 (0.300 to 0.408) | 0.031 (0.015 to 0.047) |
| Financial strain | 0.354 (0.294 to 0.415) | 1.139 (0.934 to 1.344) | 1.114 (0.925 to 1.304) | 0.102 (0.047 to 0.157) |
| Discrimination score | 0.640 (0.311 to 0.970) | 2.401 (1.312 to 3.490) | 3.658 (2.663 to 4.653) | 0.249 (−0.025 to 0.524) |
Bolded values are statistically significant
Values indicate beta coefficients (95% confidence intervals).
Hierarchical regression modeling
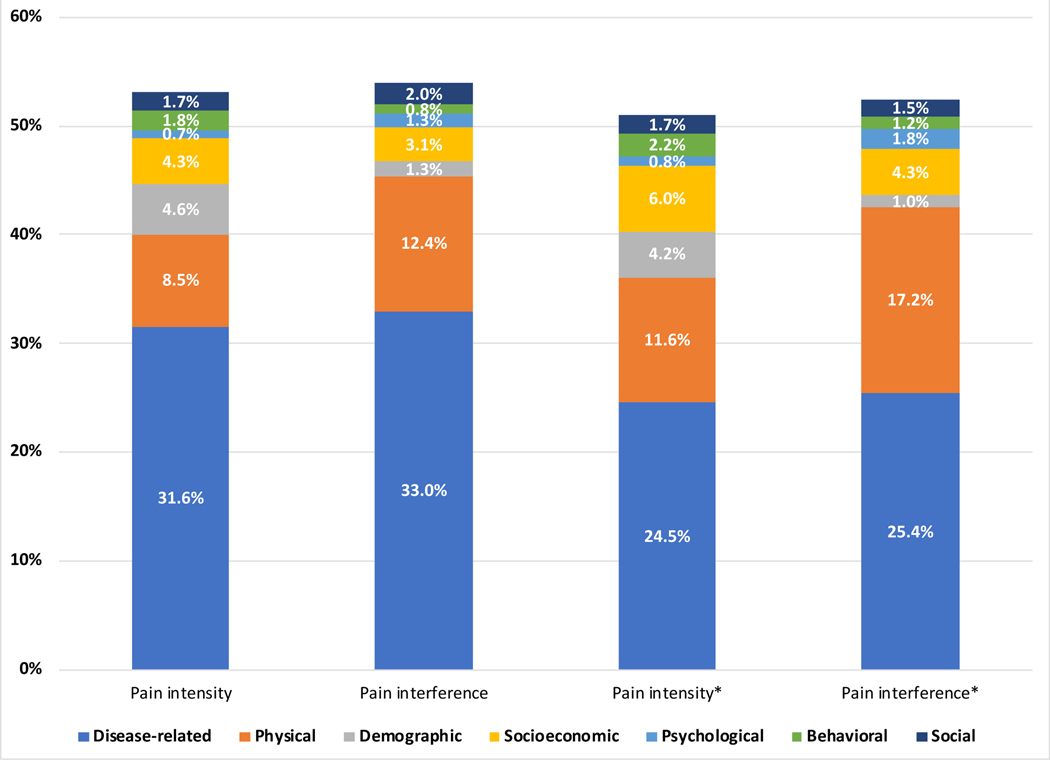
The association between pain intensity and disease activity and organ damage adjusted for all the constructs in the full model explained up to 53% of the variance in pain intensity; see Table 2, Table 4 and Figure 1. Disease activity and organ damage explained 32% of the variance in pain intensity, with increasing disease activity correlating with increased pain intensity. However, the association between organ damage and pain intensity was not significant in the seven models. The magnitude of the association between disease activity and pain intensity was attenuated going from the unadjusted model (β = 0.179, 95% CI: 0.159 to 0.199) to the full adjusted model (β = 0.106, 95% CI: 0.081 to 0.13). Demographic and socioeconomic factors accounted for an additional 9% of variance in pain intensity, with increasing age, male gender, black race, lower income and lower educational attainment showing significant association with increased pain intensity in the fully adjusted model. Sleep disturbance, BMI and physical health explained an additional 9% of the variance in pain intensity; all three measures remained significant in the fully adjusted model. Increasing sleep disturbance and increasing BMI were associated with increasing pain intensity, while better self-reported physical health was associate with lower pain intensity. Psychological, behavioral and social factors accounted for the final 4% of the variance in pain intensity. Only anger and coping with denial remained significant in the fully adjusted model.
Table 2.
Summary of the hierarchical linear regression models
| Outcome | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| Pain intensity | |||||||
| R2 | 0.312 | 0.362 | 0.405 | 0.489 | 0.496 | 0.514 | 0.531 |
| ΔR2 | -- | 0.046 | 0.043 | 0.085 | 0.007 | 0.018 | 0.017 |
| Pain interference | |||||||
| R2 | 0.330 | 0.343 | 0.374 | 0.498 | 0.512 | 0.520 | 0.540 |
| ΔR2 | -- | 0.013 | 0.031 | 0.124 | 0.013 | 0.008 | 0.020 |
| Pain intensity* | |||||||
| R2 | 0.245 | 0.288 | 0.348 | 0.463 | 0.471 | 0.493 | 0.510 |
| ΔR2 | -- | 0.042 | 0.060 | 0.116 | 0.008 | 0.022 | 0.017 |
| Pain interference* | |||||||
| R2 | 0.254 | 0.264 | 0.307 | 0.479 | 0.500 | 0.509 | 0.524 |
| ΔR2 | -- | 0.010 | 0.043 | 0.172 | 0.018 | 0.012 | 0.015 |
Model 1: Disease-related factors; Model 2: Model 1 + demographic factors; Model 3: Model 2 + socioeconomic factors; Model 4: Model 3 + physical factors; Model 5: Model 4 + psychological factors; Model 6: Model 5 + behavioral factors; Model 7: Model 6 + social factors.
These models comprise the sensitivity analyses that include the modified Systemic Lupus Activity Questionnaire (SLAQ) which removes overt mentions of pain including stomach pain, chest pain, headache, joint swelling, joint pain, muscle pain
Table 4.
Hierarchical linear regression analyses of seven constructs with pain intensity as outcome
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| R2 | 0.312 | 0.362 | 0.405 | 0.489 | 0.496 | 0.514 | 0.531 |
| ΔR2 | -- | 0.046 | 0.043 | 0.085 | 0.007 | 0.018 | 0.017 |
| Disease related factors | |||||||
| Disease activity | 0.179 (0.159 to 0.199) | 0.182 (0.163 to 0.202) | 0.165 (0.144 to 0.185) | 0.114 (0.092 to 0.136) | 0.114 (0.091 to 0.137) | 0.112 (0.088 to 0.135) | 0.106 (0.081 to 0.130) |
| Organ damage | 0.018 (−0.057 to 0.092) | −0.021 (−0.096 to 0.053) | −0.027 (−0.101 to 0.046) | −0.044 (−0.114 to 0.025) | −0.042 (−0.111 to 0.028) | −0.043 (−0.114 to 0.028) | −0.026 (−0.098 to 0.046) |
| Demographic factors | |||||||
| Age at survey | 0.024 (0.011 to 0.036) | 0.024 (0.011 to 0.036) | 0.021 (0.009 to 0.033) | 0.023 (0.011 to 0.035) | 0.021 (0.009 to 0.034) | 0.021 (0.008 to 0.034) | |
| Gender | |||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref | |
| Female | −0.851 (−1.528 to −0.174) | −0.676 (−1.333 to −0.019) | −0.809 (−1.443 to −0.175) | −0.817 (−1.449 to −0.185) | −0.841 (−1.502 to −0.181) | −0.932 (−1.607 to −0.257) | |
| Marital status | |||||||
| Not single | Ref | Ref | Ref | Ref | Ref | Ref | |
| Single | 0.072 (−0.293 to 0.437) | −0.387 (−0.777 to 0.003) | −0.310 (−0.679 to 0.06) | −0.248 (−0.619 to 0.123) | −0.208 (−0.591 to 0.174) | −0.186 (−0.577 to 0.205) | |
| Race | |||||||
| Non-black | Ref | Ref | |||||
| Black | 1.334 (0.874 to 1.794) | 0.905 (0.44 to 1.37) | 0.792 (0.353 to 1.232) | 0.766 (0.325 to 1.207) | 0.668 (0.212 to 1.125) | 0.726 (0.251 to 1.201) | |
| Socioeconomic factors | |||||||
| Household income | −0.181 (−0.274 to −0.088) | −0.139 (−0.227 to −0.052) | −0.143 (−0.231 to −0.056) | −0.148 (−0.238 to −0.058) | −0.121 (−0.218 to −0.024) | ||
| Education | |||||||
| High school or less | Ref | Ref | Ref | Ref | Ref | ||
| Some college | −0.457 (−0.876 to −0.038) | −0.472 (−0.866 to −0.078) | −0.452 (−0.848 to −0.056) | −0.337 (−0.747 to 0.074) | −0.356 (−0.786 to 0.073) | ||
| College or above | −0.853 (−1.338 to −0.369) | −0.829 (−1.284 to −0.374) | −0.792 (−1.248 to −0.336) | −0.574 (−1.048 to −0.101) | −0.630 (−1.119 to −0.142) | ||
| Physical factors | |||||||
| PROMIS sleep disturbance | 0.060 (0.041 to 0.079) | 0.056 (0.037 to 0.076) | 0.057 (0.037 to 0.077) | 0.051 (0.030 to 0.071) | |||
| Body mass index | 0.041 (0.021 to 0.061) | 0.04 (0.020 to 0.060) | 0.037 (0.017 to 0.058) | 0.041 (0.019 to 0.063) | |||
| PROMIS physical health | −0.455 (−0.655 to −0.256) | −0.456 (−0.659 to −0.253) | −0.538 (−0.748 to −0.329) | −0.637 (−0.855 to −0.419) | |||
| Psychological factors | |||||||
| PROMIS anxiety | −0.015 (−0.038 to 0.008) | −0.01 (−0.034 to 0.015) | −0.006 (−0.032 to 0.019) | ||||
| PROMIS depression | −0.016 (−0.036 to 0.005) | −0.012 (−0.034 to 0.009) | −0.010 (−0.032 to 0.012) | ||||
| PROMIS anger | 0.028 (0.010 to 0.047) | 0.028 (0.009 to 0.047) | 0.026 (0.005 to 0.046) | ||||
| Behavioral factors | |||||||
| Smoking | |||||||
| Not current | Ref | Ref | |||||
| Current | −0.024 (−0.516 to 0.469) | 0.041 (−0.474 to 0.557) | |||||
| Coping with religion | 0.015 (−0.068 to 0.098) | 0.021 (−0.065 to 0.108) | |||||
| Coping with substance use and alcohol | 0.002 (−0.151 to 0.155) | 0.004 (−0.15 to 0.158) | |||||
| Coping with self-blame | −0.178 (−0.294 to −0.063) | −0.197 (−0.317 to −0.076) | |||||
| Coping with denial | 0.152 (0.040 to 0.264) | 0.159 (0.045 to 0.274) | |||||
| Social factors | |||||||
| PROMIS emotional support | 0.007 (−0.014 to 0.029) | ||||||
| PROMIS social isolation | 0.001 (−0.022 to 0.025) | ||||||
| Financial strain | 0.030 (−0.034 to 0.094) | ||||||
| Discrimination score | −0.196 (−0.494 to 0.102) | ||||||
Model 1: Disease-related factors; Model 2: Model 1 + demographic factors; Model 3: Model 2 + socioeconomic factors; Model 4: Model 3 + physical factors; Model 5: Model 4 + psychological factors; Model 6: Model 5 + behavioral factors; Model 7: Model 6 + social factors.
Statistically significant variables in the final model are bolded
Figure 1.
A representation of the proportion of variance in pain intensity and pain interference explained by seven constructs in the biopsychosocial framework
*Corresponds to results of the sensitivity analysis that tested the model using the modified SLAQ. The modified Systemic Lupus Activity Questionnaire (SLAQ) score removes stomach pain, chest pain, headache, joint swelling, joint pain, muscle pain
The findings of the hierarchical regression analyses of pain interference on disease activity and organ damage adjusted for the other constructs are shown in Table 5. Approximately 54% of the variance in pain interference were explained by the constructs. Disease activity and organ damage explained 33% of the variance in pain interference, however, only disease activity remained significantly predictive of pain interference in the fully adjusted model (Table 2 and Figure 1). The effect size of the association between disease activity and pain interference was attenuated going from the unadjusted model (β = 0.593, 95% CI: 0.528 to 0.659) to the full adjusted (β = 0.261, 95% CI: 0.180 to 0.341). Demographic and socioeconomic factors explained an additional 4% of the variance in pain interference. Only Increasing age and lower educational attainment remained significant in the final model. BMI, sleep disturbance, and physical health explained a further 12% of the variance in pain interference, with all three measures remaining significant in the final model. Psychological, behavioral and social factors accounted for the final 4% of the variance in pain interference, however, only anger and coping with self-blame remained significant in the fully adjusted model.
Table 5.
Hierarchical linear regression analyses of seven constructs with pain interference as outcome
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 |
|---|---|---|---|---|---|---|---|
| R2 | 0.330 | 0.343 | 0.374 | 0.498 | 0.512 | 0.520 | 0.540 |
| ΔR2 | -- | 0.013 | 0.031 | 0.124 | 0.013 | 0.008 | 0.020 |
| Disease related factors | |||||||
| Disease activity | 0.593 (0.528 to 0.659) | 0.606 (0.539 to 0.672) | 0.552 (0.483 to 0.620) | 0.323 (0.252 to 0.394) | 0.292 (0.217 to 0.368) | 0.282 (0.205 to 0.360) | 0.261 (0.180 to 0.341) |
| Organ damage | 0.248 (0.002 to 0.494) | 0.15 (−0.102 to 0.402) | 0.144 (−0.107 to 0.395) | 0.042 (−0.187 to 0.271) | 0.081 (−0.146 to 0.309) | 0.094 (−0.142 to 0.330) | 0.138 (−0.101 to 0.377) |
| Demographic factors | |||||||
| Age at survey | 0.065 (0.022 to 0.109) | 0.065 (0.022 to 0.107) | 0.065 (0.026 to 0.104) | 0.073 (0.034 to 0.112) | 0.060 (0.018 to 0.101) | 0.051 (0.008 to 0.095) | |
| Gender | |||||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref | |
| Female | −1.111 (−3.388 to 1.166) | −0.650 (−2.882 to 1.583) | −1.541 (−3.618 to 0.536) | −1.587 (−3.642 to 0.468) | −1.439 (−3.613 to 0.735) | −1.659 (−3.900 to 0.582) | |
| Marital status | |||||||
| Not single | Ref | Ref | Ref | Ref | Ref | Ref | |
| Single | 0.173 (−1.059 to 1.406) | −1.104 (−2.432 to 0.225) | −0.471 (−1.687 to 0.746) | −0.182 (−1.395 to 1.03) | −0.153 (−1.419 to 1.113) | −0.286 (−1.579 to 1.007) | |
| Race | |||||||
| Non-black | Ref | Ref | Ref | Ref | Ref | Ref | |
| Black | 1.864 (0.316 to 3.412) | 0.630 (−0.954 to 2.214) | 0.311 (−1.136 to 1.757) | 0.304 (−1.136 to 1.744) | −0.003 (−1.516 to 1.510) | 0.184 (−1.394 to 1.762) | |
| Socioeconomic factors | |||||||
| Household income | −0.494 (−0.810 to −0.178) | −0.257 (−0.546 to 0.032) | −0.254 (−0.539 to 0.032) | −0.301 (−0.599 to −0.004) | −0.264 (−0.585 to 0.056) | ||
| Education | |||||||
| High school or less | Ref | Ref | Ref | Ref | Ref | ||
| Some college | −1.003 (−2.427 to 0.422) | −0.879 (−2.173 to 0.415) | −0.703 (−1.995 to 0.588) | −0.472 (−1.828 to 0.884) | −0.388 (−1.808 to 1.031) | ||
| College or above | −2.508 (−4.156 to −0.861) | −2.195 (−3.692 to −0.698) | −2.082 (−3.569 to −0.595) | −1.717 (−3.285 to −0.150) | −1.687 (−3.306 to −0.067) | ||
| Physical factors | |||||||
| PROMIS sleep disturbance | 0.254 (0.193 to 0.315) | 0.216 (0.153 to 0.279) | 0.216 (0.150 to 0.282) | 0.200 (0.131 to 0.269) | |||
| Body mass index | 0.093 (0.028 to 0.159) | 0.089 (0.023 to 0.154) | 0.08 (0.011 to 0.148) | 0.092 (0.021 to 0.164) | |||
| PROMIS physical health | −2.415 (−3.067 to −1.762) | −2.226 (−2.885 to −1.566) | −2.379 (−3.067 to −1.692) | −2.647 (−3.369 to −1.925) | |||
| Psychological factors | |||||||
| PROMIS anxiety | 0.012 (−0.063 to 0.087) | 0.037 (−0.044 to 0.117) | 0.016 (−0.069 to 0.101) | ||||
| PROMIS depression | −0.047 (−0.115 to 0.021) | −0.026 (−0.096 to 0.045) | −0.017 (−0.091 to 0.057) | ||||
| PROMIS anger | 0.125 (0.065 to 0.186) | 0.125 (0.061 to 0.189) | 0.137 (0.069 to 0.204) | ||||
| Behavioral factors | |||||||
| Smoking | |||||||
| Not current | Ref | Ref | |||||
| Current | 0.179 (−1.441 to 1.798) | 0.297 (−1.397 to 1.991) | |||||
| Coping with religion | 0.087 (−0.186 to 0.360) | 0.185 (−0.101 to 0.471) | |||||
| Coping with substance use and alcohol | −0.305 (−0.804 to 0.194) | −0.317 (−0.824 to 0.190) | |||||
| Coping with self-blame | −0.307 (−0.689 to 0.075) | −0.424 (−0.823 to −0.026) | |||||
| Coping with denial | 0.046 (−0.325 to 0.418) | 0.047 (−0.333 to 0.427) | |||||
| Social factors | |||||||
| PROMIS emotional support | −0.016 (−0.086 to 0.054) | ||||||
| PROMIS social isolation | 0.043 (−0.034 to 0.120) | ||||||
| Financial strain | −0.013 (−0.226 to 0.199) | ||||||
| Discrimination score | −0.595 (−1.581 to 0.392) | ||||||
Model 1: Disease-related factors; Model 2: Model 1 + demographic factors; Model 3: Model 2 + socioeconomic factors; Model 4: Model 3 + physical factors; Model 5: Model 4 + psychological factors; Model 6: Model 5 + behavioral factors; Model 7: Model 6 + social factors.
Statistically significant variables in the final model are bolded
Sensitivity analyses
Supplementary Tables 1 and 2 show the findings of the sensitivity analyses using the modified disease activity measure where all of the pain-related items in the disease activity score were removed. While the inferences from Tables 4 and 5 hold, the modified disease activity measure combined with organ damage explained 25% of the variance in pain intensity and interference, which is less than the 32–33% of the variance in the original models using the full disease activity scores. After combining the other constructs, the full models explained 51–52% of the variance in both outcomes. Table 2 and Figure 1 show that the other constructs (physical, psychological, behavioral and social) explain more of the variance in pain outcomes after accounting for the pain items in disease activity.
In addition, individual contributions of SLAQ symptoms to the variance in pain outcomes varied (Supplementary Tables 3 and 4). Consistent with Supplementary Tables 1 and 2, we found that the models with constitutional, mucucutaneous and organ system symptoms explained 17–27% of the variance in pain outcomes. While the models with musculosketal symptoms contributed 38–39% of the variance in pain outcomes.
Discussion
Our findings highlight the complex and dynamic interactions of seven constructs in the perceptions of pain among patients with SLE. We found that individuals reporting increased disease activity also reported higher pain intensity and interference. There was an attenuation in these associations after accounting for other constructs, highlighting their roles in modifying the relationship between disease activity and pain intensity. The biopsychosocial model has been used in understanding the correlates of pain and fatigue in rheumatoid arthritis, multiple sclerosis, osteoarthritis and sickle cell disease (28–32). These studies found significant roles of disease activity/inflammation and psychosocial factors similar to our findings. Our findings are consistent with a cohort of patients with SLE where disease activity measured by Systemic Lupus Activity Measure (SLAM) and Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) was more than twice as high in a group of patients reporting high levels of pain compared with those reporting low levels of pain(33).
Our understanding of the mechanisms of inflammation that impact pain in SLE (and in general) is evolving. However, we confidently used SLAQ as a surrogate for inflammation because higher disease activity is correlated with higher levels of serum biomarkers of inflammation (34). When we omitted pain-related items from the SLAQ score, the association between disease activity and pain intensity persisted. Furthermore, we examined the individual contributions of constitutional, mucocutaneous, organ system, and musculoskeletal symptoms in SLAQ and found that mucocutaneous and constitutional symptoms contribute to pain to a lesser extent than musculoskeletal symptoms. Greco et al showed similar findings in a study when investigating chronic pain clustering in SLE (35). The pain clusters did not change significantly when pain symptoms were omitted from the Systemic Lupus Activity Measure–Revised (SLAM-R)(35). All these findings suggest that individual, behavioral, and social factors can potentially exacerbate or attenuate the intensity of pain caused by inflammatory mediators in SLE patients with active disease.
We found that demographic and lifestyle constructs play crucial roles in pain severity. Our finding that older age was independently associated with increased pain intensity and interference is consistent with other epidemiological studies that have found an age-related increase in the prevalence of chronic pain(1). We found that black patients reported higher pain intensity than non-black patients. These findings run parallel with gender and racial/ethnic differences in the development and outcomes of SLE (36,37). Males also seem to have more severe disease compared with females(38). However, these findings are contrary to what is known in the general population where females and whites have higher prevalence of chronic pain suggesting that pain in SLE has a different epidemiological profile(1). We found that higher socioeconomic status were protective for pain outcomes. In patients with SLE, socioeconomic status is associated with lower disease activity and damage accrual (39). In the general population, socioeconomic disadvantage is associated with almost every aspect of poorer health including increased morbidity and decreased life expectancy and not surprisingly, is also consistently associated with increased risk for pain (40).
A growing body of research suggest that obesity and poor sleep quality might play important roles in SLE outcomes and have been also associated with pain outcomes. Several studies have linked obesity with disease activity, increased risk of renal disease, cardiovascular complications, fibromyalgia, depression, poorer functional capacity, and decreased quality of life in patients with SLE(41–44). Obesity is hypothesized to cause pain through excess mechanical stresses and its proinflammtory state, and is also a marker of increased functional and psychological complications in chronic pain(45). Moreover, recent reports along with our findings suggest that complex pathways may link sleep disturbance and pain along with depression and cognitive symptoms(46). Thus, further research is needed to determine whether interventions specifically developed to tackle those factors may effectively improve pain outcomes in SLE populations.
In addition, we found that psychological, behavioral and social factors are associated with pain intensity and interference in the unadjusted models. In the fully adjusted models, these associations were no longer significant. When we omitted overt manifestations of pain from the disease activity measure (see section on sensitivity analyses), we found that psychosocial and demographic factors accounted for more variance in pain intensity and interference, highlighting their importance in pain outcomes. The psychosocial burden of SLE is immense. Compared to the general population, patients with SLE report impaired quality of life and more fatigue, fibromyalgia, anxiety and depression(47). These psychosocial factors also disproportionately increase with disease activity and organ damage. Future studies explicating the role of psychosocial factors in pain are needed for the appropriate design of targeted interventions for patients with SLE.
Our study has some limitations. First, we cannot rule out residual and unmeasured confounding due to variables not capturing the complete essence of constructs such as socioeconomic and psychological factors. Second, the survey did not capture information on fibromyalgia. Third, the cohort may not be generalizable to the US population of patients with SLE. However, as SLE affects predominantly black women - comprising 43% of SLE cases in the US according to prevalence estimates, our findings can be generalizable to a large US population, particularly the Southeastern region of the country. Fourth, given the different assessment periods for the patient reported outcomes and the cross-sectional nature of the design, we were unable to investigate the temporal relation between pain and associated factors, which in turn may impact the interpretation of our results. Finally, our findings may be confounded by the ordering of the constructs in the regression models. The choice of the order of the constructs was made prior to model building based on the assumption that the main predictors of pain in SLE are disease activity and organ damage. These findings suggest that a range of multidimensional interventions to reduce pain should continue to be directed to disease-related factors, as clinically indicated. This is the single construct that explained the largest proportion of variation in pain. However, this proportion never exceeded 33% in our models, with significant remaining proportions explained by demographic, socioeconomic, physical, psychological, behavioral, and social factors. These are constructs that are not modified directly by immunologic interventions but speak to other mechanisms that should be considered when aiming to improve the lives of those with SLE. This may include programs focusing on reducing obesity and improved sleep hygiene, as well as psychological interventions addressing anxiety, depression, and anger. Behavioral interventions, such as smoking cessation and improving coping skills, should be considered. Social programs to improve emotional support and decrease isolation may be helpful. Validated and culturally appropriate self-management programs will be an important tool in this space.
Our study has several strengths. To our knowledge, this was the first study to present a biopsychosocial model for pain intensity and interference in SLE. We leveraged a large sample of patients to make these inferences. However, this was a cross-sectional study and we were unable to answer questions about causality. Therefore future studies of longitudinal design to ascertain causality are needed. In addition, while our models elucidate a proportion, there is also a significant proportion of variance that remains undefined. Further research is need to confirm these observations as well as identify other factors that continue to remain undefined in our models.
Supplementary Material
Footnotes
Conflict of interests
The authors did not receive any financial support or other benefits from commercial sources for this work, or any other financial interests which could create a potential conflict of interest or the appearance of a conflict of interest with regard to this work.
Contributor Information
Titilola Falasinnu, Department of Epidemiology and Population Sciences, Stanford University School of Medicine, California; Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine, California.
Cristina Drenkard, Department of Medicine, Division of Rheumatology, Emory University, Atlanta, Georgia; Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia.
Gaobin Bao, Department of Medicine, Division of Rheumatology, Emory University, Atlanta, Georgia.
Sean Mackey, Department of Anesthesiology, Perioperative, and Pain Medicine, Stanford University School of Medicine.
S. Sam Lim, Department of Medicine, Division of Rheumatology, Emory University, Atlanta, Georgia; Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia.
References
- 1.Dahlhamer JM, Lucas J, Zelaya C, Nahin R, Mackey S, Debar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. Morbidity and Mortality Weekly Report Department of Health and Human Services; 2018. p. 1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borenstein DG, Hassett AL, Pisetsky DS. Pain management in rheumatology research, training, and practice. Clin Exp Rheumatol Clinical and Experimental Rheumatology S.A.S.; 2017;35:S2–7. [PubMed] [Google Scholar]
- 3.Arntsen KA, Raymond SC, Farber KM. Lupus: Patient Voices Report on Externally-led Patient-Focused Drug Development Meeting A Message of Gratitude. [Google Scholar]
- 4.Lee J, Lin J, Suter LG, Fraenkel L. Persistently Frequent Emergency Department Utilization Among Persons With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) [Internet] John Wiley and Sons Inc; 2019. [cited 2020 Mar 4];71:1410–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30295422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and Profile of High-Impact Chronic Pain in the United States. J Pain [Internet] Churchill Livingstone Inc.; 2019. [cited 2020 Mar 4];20:146–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30096445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain [Internet] Lippincott Williams and Wilkins; 2017. [cited 2020 Mar 4];158:313–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28092650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Progress in Neuro-Psychopharmacology and Biological Psychiatry Elsevier Inc; 2018. p. 168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Ahn H, Lyon D, Stechmiller J. Building a Biopsychosocial Conceptual Framework to Explore Pressure Ulcer Pain for Hospitalized Patients . Healthcare MDPI AG; 2016;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drenkard C, Rask KJ, Easley KA, Bao G, Lim SS. Primary preventive services in patients with systemic lupus erythematosus: Study from a population-based sample in Southeast U.S. Semin Arthritis Rheum [Internet] 2013. [cited 2020 Mar 26];43:209–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23731530 [DOI] [PubMed] [Google Scholar]
- 10.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus 2003;12:280–6. [DOI] [PubMed] [Google Scholar]
- 11.Yazdany J, Yelin EH, Panopalis P, Trupin L, Julian L, Katz PP. Validation of the Systemic Lupus Erythematosus Activity Questionnaire in a large observational cohort. Arthritis Care Res 2008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yazdany J, Trupin L, Gansky SA, Dall’era M, Yelin EH, Criswell LA, et al. Brief Index of Lupus damage: A patient-reported measure of damage in systemic lupus erythematosus. Arthritis Care Res NIH Public Access; 2011;63:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz P, Trupin L, Rush S, Yazdany J. Longitudinal validation of the brief index of lupus damage. Arthritis Care Res [Internet] John Wiley and Sons Inc.; 2014. [cited 2020 Mar 23];66:1057–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24376263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan J, Thompson NJ, Dunlop-Thomas C, Lim SS, Drenkard C. Relationships among organ damage, social support, and depression in African American women with systemic lupus erythematosus. Lupus [Internet] SAGE Publications Ltd; 2019. [cited 2020 Mar 23];28:253–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30482093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drenkard C, Bao G, Dennis G, Kan HJ, Jhingran PM, Molta CT, et al. Burden of Systemic Lupus Erythematosus on Employment and Work Productivity: Data From a Large Cohort in the Southeastern United States. Arthritis Care Res (Hoboken) [Internet] 2014. [cited 2019 Jan 28];66:878–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24339382 [DOI] [PubMed] [Google Scholar]
- 16.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, et al. Development of a PROMIS item bank to measure pain interference. Pain NIH Public Access; 2010;150:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, et al. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J Clin Epidemiol Elsevier USA; 2016;73:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carver CS. You want to measure coping but your protocol’s too long: Consider the brief COPE. Int J Behav Med Springer New York LLC; 1997;4:92–100. [DOI] [PubMed] [Google Scholar]
- 19.Muller L, Spitz E. [Multidimensional assessment of coping: validation of the Brief COPE among French population]. Encephale [Internet] [cited 2020 Mar 23];29:507–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15029085 [PubMed] [Google Scholar]
- 20.Hahn EA, DeVellis RF, Bode RK, Garcia SF, Castel LD, Eisen S V., et al. Measuring social health in the patient-reported outcomes measurement information system (PROMIS): Item bank development and testing. Qual Life Res [Internet] 2010. [cited 2020 Mar 23];19:1035–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20419503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes LL, Mendes De Leon CF, Wilson RS, Bienias JL, Bennett DA, Evans DA. Racial Differences in Perceived Discrimination in a Community Population of Older Blacks and Whites. J Aging Health [Internet] 2004. [cited 2020 Mar 22];16:315–37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15155065 [DOI] [PubMed] [Google Scholar]
- 22.Brown C, Matthews KA, Bromberger JT, Chang Y. The relation between perceived unfair treatment and blood pressure in a racially/ethnically diverse sample of women. Am J Epidemiol [Internet] 2006. [cited 2020 Mar 22];164:257–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16777930 [DOI] [PubMed] [Google Scholar]
- 23.Gee GC, Spencer MS, Chen J, Takeuchi D. A nationwide study of discrimination and chronic health conditions among Asian Americans. Am J Public Health [Internet] 2007. [cited 2020 Mar 22];97:1275–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17538055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunte HER, Williams DR. The association between perceived discrimination and obesity in a population-based multiracial and multiethnic adult sample. Am J Public Health [Internet] 2009. [cited 2020 Mar 22];99:1285–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18923119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor TR, Kamarck TW, Shiffman S. Validation of the detroit area study discrimination scale in a community sample of older African American adults: The Pittsburgh healthy heart project. Int J Behav Med [Internet] Springer New York LLC; 2004. [cited 2020 Mar 22];11:88–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15456677 [DOI] [PubMed] [Google Scholar]
- 26.Cutrona CE, Russell DW, Todd Abraham W, Gardner KA, Melby JN, Bryant C, et al. Neighborhood context and financial strain as predictors of marital interaction and marital quality in African American couples [Internet]. Personal Relationships Blackwell Publishing Ltd; 2003. [cited 2020 Mar 23]. p. 389–409. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17955056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faul F, Buchner A, Erdfelder E, Mayr S. A short tutorial of GPower. Tutor Quant Methods Psychol 2007; [Google Scholar]
- 28.Fifield J, Reisine ST, Grady K. Work disability and the experience of pain and depression in rheumatoid arthritis. Soc Sci Med 1991; [DOI] [PubMed] [Google Scholar]
- 29.Heitmann H, Haller B, Tiemann L, Mühlau M, Berthele A, Tölle TR, et al. Longitudinal prevalence and determinants of pain in multiple sclerosis: results from the German National Multiple Sclerosis Cohort study. Pain 2020; [DOI] [PubMed] [Google Scholar]
- 30.Day MA, Ehde DM, Charles Ward L, Hartoonian N, Alschuler KN, Turner AP, et al. An empirical investigation of a biopsychosocial model of pain in multiple sclerosis. Clin J Pain 2016; [DOI] [PubMed] [Google Scholar]
- 31.Booker SQ, Sibille KT, Terry EL, Cardoso JS, Goodin BR, Sotolongo A, et al. Psychological Predictors of Perceived Age and Chronic Pain Impact in Individuals with and without Knee Osteoarthritis. Clin J Pain 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlenz AM, Schatz J, Roberts CW. Examining biopsychosocial factors in relation to multiple pain features in pediatric sickle cell disease. J Pediatr Psychol 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldheim E, Elkan A-C, Pettersson S, van Vollenhoven R, Bergman S, Frostegård J, et al. Health-related quality of life, fatigue and mood in patients with SLE and high levels of pain compared to controls and patients with low levels of pain. Lupus [Internet] 2013. [cited 2020 Mar 4];22:1118–27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23989737 [DOI] [PubMed] [Google Scholar]
- 34.Eudy AM, Vines AI, Dooley MA, Cooper GS, Parks CG. Elevated C-reactive protein and self-reported disease activity in systemic lupus erythematosus. Lupus 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greco CM, Rudy TE, Manzi S. Adaptation to chronic pain in systemic lupus erythematosus: Applicability of the multidimensional pain inventory. Pain Med 2003;4:39–50. [DOI] [PubMed] [Google Scholar]
- 36.Drenkard C, Lim SS. Update on lupus epidemiology: Advancing health disparities research through the study of minority populations [Internet]. Current Opinion in Rheumatology Lippincott Williams and Wilkins; 2019. [cited 2020 Mar 26]. p. 689–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31436582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol (Hoboken, NJ) [Internet] NIH Public Access; 2014. [cited 2019 Mar 1];66:357–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24504808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolly M, Sequeira W, Block JA, Toloza S, Bertoli A, Blazevic I, et al. Sex Differences in Quality of Life in Patients With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) [Internet] John Wiley and Sons Inc.; 2019. [cited 2020 Apr 1];71:1647–52. Available from: 10.1002/acr.23588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol [Internet] 2016. [cited 2019 Jan 28];12:605–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27558659 [DOI] [PubMed] [Google Scholar]
- 40.Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain [Internet] 2008. [cited 2020 Mar 4];136:235–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18440703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.RIZK A GHEITA TA, NASSEF S, ABDALLAH A. The impact of obesity in systemic lupus erythematosus on disease parameters, quality of life, functional capacity and the risk of atherosclerosis. Int J Rheum Dis [Internet] 2012. [cited 2018 Oct 16];15:261–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22212605 [DOI] [PubMed] [Google Scholar]
- 42.Patterson SL, Schmajuk G, Jafri K, Yazdany J, Katz P. Obesity is Independently Associated With Worse Patient-Reported Outcomes in Women with Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) [Internet] 2019. [cited 2019 Mar 11];71:126–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29740985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaiamnuay S, Bertoli AM, Fernández M, Apte M, Vilá LM, Reveille JD, et al. The impact of increased body mass index on systemic lupus erythematosus: Data from LUMINA, a multiethnic cohort. J Clin Rheumatol 2007;13:128–33. [DOI] [PubMed] [Google Scholar]
- 44.Teh P, Zakhary B, Sandhu VK. The impact of obesity on SLE disease activity: findings from the Southern California Lupus Registry (SCOLR). Clin Rheumatol [Internet] 2 019. [cited 2019 Mar 11];38:597–600. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30357495 [DOI] [PubMed] [Google Scholar]
- 45.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res [Internet] Dove Medical Press Ltd.; 2015. [cited 2020 Mar 4];8:399–408. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26203274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lillis TA, Tirone V, Gandhi N, Weinberg S, Nika A, Sequeira W, et al. Sleep Disturbance and Depression Symptoms Mediate Relationship Between Pain and Cognitive Dysfunction in Lupus. Arthritis Care Res (Hoboken) [Internet] John Wiley and Sons Inc.; 2019. [cited 2020 Mar 23];71:acr.23593. Available from: 10.1002/acr.23593 [DOI] [PubMed] [Google Scholar]
- 47.Schmeding A, Schneider M. Fatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosus. Best Practice and Research: Clinical Rheumatology Bailliere Tindall Ltd; 2013. p. 363–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.