Abstract
Background:
Many factors affect outcomes after congenital cardiac surgery.
Objective:
The Residual Lesion Score (RLS) Study explored the impact of severity of residual lesions on postoperative outcomes across operations of varying complexity.
Methods:
In a prospective, multicenter, observational study, 17 sites enrolled 1149 infants undergoing five common operations: tetralogy of Fallot repair (TOF, n=250), complete atrioventricular septal defect repair (CAVSD, n=249), arterial switch operation (ASO, n=251), coarctation/interrupted arch with ventricular septal defect repair (Arch/VSD, n=150), and Norwood operation (n=249). RLS was assigned based on postoperative echocardiography and clinical events: RLS 1, trivial/none; RLS 2, minor; or RLS 3, reintervention for or major residual lesions before discharge. The primary outcome was days alive and out of hospital within 30 postoperative days (60 for Norwood). Secondary outcomes assessed postoperative course, including major medical events and days in hospital.
Results:
RLS 3 (vs. RLS 1) was an independent risk factor for fewer days alive and out of hospital (p≤0.008) and longer postoperative hospital stay (p≤0.02) for all 5 operations, and for all secondary outcomes after Arch/VSD and Norwood (p≤0.03). Outcomes for RLS 1 vs. 2 did not differ consistently. RLS alone explained 5% (TOF) to 20% (Norwood) of variation in the primary outcome.
Conclusions:
Adjusting for preoperative factors, residual lesions after congenital cardiac surgery impacted in-hospital outcomes across operative complexity with greatest impact following complex operations. Minor residual lesions had minimal impact. These findings may provide guidance for surgeons when considering short-term risks and benefits of returning to bypass to repair residual lesions.
Keywords: Residual Lesion Score, outcomes, days alive and out of the hospital
Condensed Abstract
The Residual Lesion Score (RLS), a quality improvement tool, classifies residual lesion after congenital heart surgery as: RLS 1, trivial/none; RLS 2, minor; or RLS 3, major residual lesions or reintervention for such lesions before discharge. In 1149 subjects enrolled from 17 centers, major residual lesions (RLS 3) are independently associated with fewer days alive and out of hospital following congenital heart surgery across a spectrum of complexity. In more complex procedures, major residual lesions are associated with secondary measures of greater morbidity. The RLS may identify higher-risk patients for closer surveillance, explain inter-institutional variability in outcomes, and improve performance.
INTRODUCTION
Outcomes after congenital cardiac surgery are affected by patient characteristics, preoperative status and perioperative factors (1–4). The presence of residual lesions after cardiac surgery, i.e., the degree to which a congenital cardiac operation achieved its intended result, is generally considered one of the most important factors determining short- and long-term outcomes.
In prior studies, the Technical Performance Score (TPS) measured the technical success of congenital cardiac operations and demonstrated its usefulness in predicting both early and mid-term outcomes(5–24). However, the generalizability of findings has been limited by score sub-components designed at a single center, absence of adjudication by a core echocardiography laboratory, and participation by a small group of surgical centers. These early studies highlighted that residual lesions after surgery were not always related to surgical technique, but were sometimes unavoidable due to anatomic variation or suboptimal preoperative diagnosis. To analyze the degree to which residual lesions impact outcome, regardless of the cause, a national panel of experts refined the TPS using the modified RAND-Delphi technique to examine validity and feasibility of score components and renamed it the Residual Lesion Score (RLS). The RLS is a tool for assessing surgical results based upon postoperative echocardiographic findings and unplanned surgical or catheter-based reinterventions prior to discharge in the anatomic areas that were manipulated at surgery.
The RLS Study is a large, prospective, multicenter study designed to investigate the association of RLS with early and mid-term outcomes following five common congenital cardiac operations. We hypothesized that the presence of major residual lesions, or reinterventions for residual lesions prior to discharge from the index hospitalization, would be associated with greater postoperative morbidity.
METHODS
Study design
The RLS Study was performed by the NHLBI’s Pediatric Heart Network as a multicenter prospective observational cohort study with enrollment between 2015 and 2017 and the study design has been published (25). The protocol was approved by the Institutional Review Board or Research Ethics Board at each participating site, as well as at the Data Coordinating Center, and informed consent was obtained from parents/guardians.
Patients
We enrolled infants <1 year of age who underwent one of five common congenital cardiac operations of increasing complexity according to the Society of Thoracic Surgeon - European Association for Cardio-Thoracic Surgery Congenital Heart Surgery (STAT) mortality category (26, 27): tetralogy of Fallot (TOF) with pulmonary stenosis repair (STAT 1–2); complete atrioventricular septal defect (CAVSD, STAT 3) repair, arterial switch operation with or without ventricular septal defect (VSD) closure (ASO, STAT 3–4), coarctation/hypoplastic or interrupted arch with VSD closure (Arch/VSD, STAT 4), and Norwood operation (STAT 5). The Norwood procedure resulted in a palliated/univentricular circulation. All other procedures resulted in a biventricular circulation. Exclusion criteria were any previous open or closed heart surgery and/or prespecified interventional catheterization procedure prior to index surgery (25). The five procedures selected were the most common neonatal and infant procedures performed across centers in the United States and Canada, and represented a spectrum of complexity in congenital heart surgery from simple to highly complex. Imaging and surgical techniques were fairly standardized across centers for these procedures (26, 27).
Residual Lesion Score
The RLS was refined from the original TPS (5) using the RAND-Delphi methodology as previously described (25). Briefly, each procedure was divided into subcomponents. A panel of experts each voted on the feasibility and validity of individual subcomponents of each operation as a measure of completeness of repair (the first round by email review, and the second round by an in-person meeting to discuss and review). The votes of the individual panel members were collated and analyzed to finalize the RLS.
The RLS is derived from the findings of the postoperative echocardiogram performed before hospital discharge or reintervention, whichever occurred earlier. Any unplanned reinterventions for residual lesions in the anatomic area of repair during the same hospitalization automatically resulted in assignment of RLS 3. Echocardiograms were analyzed by a core laboratory for four operations. For the ASO group, postoperative echocardiograms were more standardized across sites so local interpretations were used to derive RLS, lowering study costs.
The RLS for each of the five operations (25) was the composite of multiple relevant sub-components (Supplemental Tables S1A-S1E) Each sub-component was classified as RLS 1 (no/trivial residual lesions), RLS 2 (minor residual lesions), or RLS 3 (major residual lesions or unplanned reintervention for residual lesions in anatomic area of repair prior to discharge following the index operation). The sub-component scores were then combined to derive an overall score based upon the highest sub-component score. Two additional classes were created: RLS 4 for patients with incomplete echocardiograms that precluded scoring as RLS 1–3, and RLS 5 for patients who did not have a pre-discharge/pre-reintervention echocardiogram.
Outcomes
This manuscript provides data on short-term outcomes of the RLS Study. The primary outcome was number of days alive and out of hospital within 30 days after surgery (60 days for Norwood). This outcome not only serves as a measure of global morbidity and resource utilization, but also accounts for any bias introduced by death within the first few days following surgery(25). For patients who remained in the hospital longer than 30/60 days and for patients who died during index hospitalization or within the 30-day (60-day for Norwood) window, the primary outcome was set to zero. Secondary outcomes included total postoperative hospital length of stay, initial and total postoperative duration of mechanical ventilation, initial and total length of postoperative intensive care unit (ICU) stay, and incidence of major postoperative medical events, including mortality/transplant, cardiac arrest requiring cardiopulmonary resuscitation and medications, cardiopulmonary insufficiency requiring mechanical circulatory support, low cardiac output state or cardiac failure, mediastinitis requiring surgical debridement, bleeding requiring exploration, neurologic deficit, renal failure requiring dialysis, necrotizing enterocolitis requiring laparotomy, diaphragmatic paralysis requiring plication, vocal cord paralysis, prolonged ventilation for >7 days, sepsis, multi-system organ failure, unplanned hospital readmission within 30 (or 60) days, unplanned non-cardiac reoperation, non-routine open sternum, and hemothorax (26, 27).
Statistical methods
All analyses were conducted using SAS v9.4 (SAS Institute Inc., Cary, NC). Estimated power for the primary outcome ranged from 84%−99% across the five operations,25 with each considered an independent study and analyzed separately.
Continuous outcomes were compared across RLS 1–3 using analysis of variance (ANOVA) and Kruskal-Wallis tests. Categorical outcomes were compared via Fisher’s exact test. Statistical modeling used a generalized linear mixed model (GLMM) approach: random effects linear regression for continuous outcomes and random effects logistic regression for binary outcomes (25). Models included center as a random effect to account for potential correlation among patients from the same center. Competing risks proportional hazards Cox models were utilized for time-to-event outcomes, with death before event as a competing risk. If the RLS score was found to violate the proportional hazards assumption, the interaction between RLS and time was included in the model.
For each outcome, baseline patient and procedural characteristics associated with the outcome at p<0.20 in bivariate analysis were identified. Where feasible, log transformation of continuous outcomes was performed to better approximate normal distributions. A multivariable model comprised of significant predictors (p<0.05) was then constructed using backwards elimination. RLS (1 vs. 2 vs. 3, with RLS 1 as the reference category) was then added to the multivariable model, and estimates of effects were obtained for RLS and all significant predictors in the combined model. For the random effects linear and logistic regression models, Rβ*2 statistics (28), which also offers semi-partial R2 statistics to assess the relative importance of each predictor, were reported. For the competing risks proportional hazards Cox models, generalized R2 statistics (29) were reported. Typical measures of R2 are not available for the models used in this study, and the reported R2 statistics do not have a strict interpretation of percent variance explained. However, comparison of the Rβ*2 statistics to the traditional R2 statistic from models excluding random effects showed excellent agreement. Estimates of R2 for RLS were obtained first in a model where RLS was the only predictor, then obtained for the multivariable model comprised of RLS and other significant predictors.
RLS 4 and 5 were not included in analyses as these categories represent patients for whom an RLS could not be assigned. No missing data were imputed for the primary outcome (n=12 missing). For total mechanical ventilation time and total postoperative ICU stay, in order to avoid bias of death-related short hospital length of stay, for each operation, the highest value +1 was imputed (30). Sensitivity analyses excluded patients who died, as well as Norwood patients who remained in the hospital until Stage II surgery (n=20). Interactions of RLS with covariates were tested in exploratory analyses.
RESULTS
Seventeen sites enrolled 1149 patients from 2015–2017: TOF repair, n=250; CAVSD repair, n=249; ASO, n=251; Arch/VSD repair, n=150; and Norwood operation, n=249 (Supplemental Figure S1). Baseline characteristics and study outcomes for each operation are described in Tables 1 and 2. For these operations, RLS ranged as follows: RLS 1 in 11–33%, RLS 2 in 30–53% and RLS 3 in 15–35% of patients (Table 2, central illustration).
Table 1.
Baseline Characteristics by Operation
| TOF | CAVSD | ASO | Arch/VSD | Norwood* | |
|---|---|---|---|---|---|
| Total N | 250 | 249 | 251 | 150 | 249 |
| Age at surgery (days), median (25th,75th) | 150 (99, 188) | 141 (105, 179) | 6 (4, 8) | 8 (5, 15) | 5 (4, 7) |
| Weight (kilogram), median (25th,75th) | 6.2 (5.3, 7.2) | 5.2 (4.4, 5.8) | 3.4 (3.1, 3.8) | 3.3 (3.0, 3.8) | 3.3 (2.9, 3.6) |
| Male Gender | 144 (58%) | 109 (44%) | 185 (74%) | 83 (55%) | 159 (64%) |
| Race | |||||
| White/Caucasian | 166 (66%) | 157 (63%) | 170 (68%) | 107 (71%) | 186 (75%) |
| Black/African American | 24 (10%) | 41 (17%) | 13 (5%) | 9 (6%) | 27 (11%) |
| Other Race | 25 (10%) | 18 (7%) | 24 (10%) | 14 (9%) | 20 (8%) |
| Unknown or Not Reported | 35 (14%) | 33 (13%) | 44 (18%) | 20 (13%) | 16 (6%) |
| Ethnicity | |||||
| Hispanic/Latino | 27 (11%) | 28 (11%) | 28 (11%) | 26 (17%) | 23 (9%) |
| Not Hispanic/Latino | 191 (76%) | 190 (76%) | 183 (73%) | 110 (73%) | 209 (84%) |
| Unknown | 32 (13%) | 31 (12%) | 40 (16%) | 14 (9%) | 17 (7%) |
| Pregnancy term | |||||
| Full term | 216 (86%) | 212 (85%) | 234 (93%) | 139 (93%) | 237 (95%) |
| Pre-term | 26 (10%) | 32 (13%) | 17 (7%) | 11 (7%) | 12 (5%) |
| Unknown | 8 (3%) | 5 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Antenatal diagnosis of CHD | |||||
| No | 120 (48%) | 66 (27%) | 96 (38%) | 81 (54%) | 35 (14%) |
| Yes | 118 (47%) | 171 (69%) | 155 (62%) | 68 (45%) | 213 (86%) |
| Unknown | 12 (5%) | 12 (5%) | 0 (0%) | 1 (1%) | 1 (0%) |
| Non cardiac congenital anomaly, syndromes, genetic abnormalities †,‡ | |||||
| 0 | 204 (82%) | 33 (13%) | 238 (95%) | 108 (72%) | 208 (84%) |
| 1 | 32 (13%) | 185 (74%) | 8 (3%) | 28 (19%) | 33 (13%) |
| >1 | 14 (6%) | 31 (12%) | 5 (2%) | 14 (9%) | 8 (3%) |
| Timing of Surgery § | |||||
| Elective | 218 (87%) | 216 (87%) | 90 (36%) | 75 (50%) | 100 (40%) |
| Urgent | 31 (12%) | 33 (13%) | 152 (61%) | 74 (49%) | 142 (57%) |
| Emergent | 1 (0%) | 0 (0%) | 9 (4%) | 1 (1%) | 7 (3%) |
| Pre-operative risk factor †,║ | |||||
| 0 | 206 (82%) | 162 (65%) | 130 (52%) | 92 (61%) | 168 (68%) |
| 1 | 39 (16%) | 67 (27%) | 82 (33%) | 34 (23%) | 54 (22%) |
| >1 | 5 (2%) | 20 (8%) | 39 (16%) | 24 (16%) | 27 (11%) |
| Procedure-specific factor †,# | |||||
| 0 | 154 (62%) | 186 (75%) | 51 (20%) | 38 (25%) | 52 (21%) |
| 1 | 79 (32%) | 48 (19%) | 54 (22%) | 56 (37%) | 104 (42%) |
| >1 | 17 (7%) | 15 (6%) | 146 (58.2%) | 56 (37%) | 93 (37%) |
| Institutional practice to discharge directly from ICU | 26 (10%) | 11 (4%) | 41 (16.3%) | 16 (11%) | 34 (14%) |
| Tertiles of site volume | |||||
| 13 – 29 | 23 (9%) | 19 (8%) | 22 (8.8%) | 6 (4%) | 25 (10%) |
| 40 – 82 | 86 (34%) | 69 (28%) | 89 (35.5%) | 46 (31%) | 80 (32%) |
| 89 – 153 | 141 (56%) | 161 (65%) | 140 (55.8%) | 98 (65%) | 144 (58%) |
| Categories of surgeon volume | |||||
| 1 – 10 | 27 (11%) | 17 (7%) | 25 (10.0%) | 13 (9%) | 27 (11%) |
| 11 – 28 | 103 (41%) | 95 (38%) | 115 (45.8%) | 50 (33%) | 100 (40%) |
| 30 – 43 | 120 (48%) | 137 (55%) | 111 (44.2%) | 87 (58%) | 122 (49%) |
| Intraoperative findings different from preoperative echocardiogram ** | |||||
| No | 225 (90%) | 224 (90%) | 212 (84.5%) | 133 (89%) | 236 (95%) |
| Yes, better | 3 (1%) | 1 (0%) | 4 (1.6%) | 4 (3%) | 0 (0%) |
| Yes, worse | 22 (9%) | 24 (10%) | 35 (13.9%) | 13 (9%) | 13 (5%) |
N (%), unless otherwise specified
ventricular dominance:83% RV dominant, 8% LV dominant, 5% balanced, 4% indeterminate; single ventricle anatomy: 30% AA/MA, 25% AA/MS, 6% AS/MA, 20% AS/MS, 19% other
outcome modelling compared between 3-categories (0,1,>1) and 2-categories (yes/no); the more significantly associated grouping was chosen for multivariable modelling
non-cardiac congenital abnormalities, genetic defects, and syndromes combined as a single category
emergent combined with urgent in outcome modelling due to low N. Choice for elective neonatal surgery (ASO, Arch/VSD, Norwood) was site-dependent with some sites selecting “Elective” for neonatal surgery for index operations performed after postnatal stabilization as a scheduled case, rather than as an urgent or emergent case for hemodynamic instability.
CPR, AV block, mechanical support, shock (persistent or resolved at time of surgery), mechanical ventilator support up to surgery, renal failure, hepatic failure, neurologic deficit, sepsis, NEC, noncardiac surgery, tracheostomy, other preoperative risk factors (e.g., coagulation disorder, respiratory syncytial virus)
see Nathan, et. al.25 for a list for each surgery
“yes, better” combined with “no” in outcome modelling due to low N
AA = aortic atresia; Arch/VSD = coarctation/hypoplastic or interrupted arch with VSD; AS = aortic stenosis; ASO = arterial switch operation; AV = atrioventricular block; CAVSD = complete atrioventricular septal defect; CHD = congenital heart disease; CPR = cardio pulmonary resuscitation; ICU = intensive care unit; MA = mitral atresia; MS = mitral stenosis; N = number; NEC=necrotizing enterocolitis; TOF = tetralogy of Fallot with pulmonary stenosis
Table 2.
Residual Lesion Score (RLS) and Study Outcomes by Operation
| TOF N=250 |
CAVSD N=249 |
ASO N=251 |
Arch/VSD N=150 |
Norwood N=249 |
|
|---|---|---|---|---|---|
| Residual Lesion Score (RLS) * | |||||
| RLS 1. No/trivial residual lesions | 49 (20%) | 27 (11%) | 49 (20%) | 49 (33%) | 51 (20%) |
| RLS 2. Minor residual lesions | 129 (52%) | 131 (53%) | 127 (51%) | 77 (51%) | 74 (30%) |
| RLS 3. Major residual lesions or reintervention for residual lesions prior to discharge | 59 (24%) | 86 (35%) | 58 (23%) | 23 (15%) | 82 (33%) |
| RLS 3 based on pre-discharge reintervention | 5 (2%) | 12 (5%) | 10 (4%) | 4 (3%) | 71 (29%) |
| RLS 3 based on pre-discharge echocardiogram alone | 54 (22%) | 74 (30%) | 48 (19%) | 19 (13%) | 11 (4%) |
| RLS 4. Incomplete echocardiogram | 11 (4%) | 2 (1%) | 14 (6%) | 1 (1%) | 38 (15%) |
| RLS 5. No echocardiogram | 2 (1%) | 3 (1%) | 3 (1%) | 0 (0%) | 4 (2%) |
| Days Alive and Out of Hospital (Days) †,‡ | 24 (21,25) | 23 (17,25) | 18 (12,21) | 16 (6,20) | 17.5 (0,39) |
| Postoperative hospital length of stay (Days) †,§ | 6 (5,9) | 7 (5,13) | 12 (9,17) | 14 (10,23) | 33 (19,59) |
| Time to Initial Extubation (Days) †,║ | 0.6 (0.1,1.2) | 0.9 (0.5,1.9) | 2.8 (1.6,4.8) | 3.7 (2.2,4.9) | 5.9 (3.8,10.2) |
| Total Ventilation Time (Days) †,# | 0.7 (0.2,1.3) | 1.0 (0.6,2.7) | 2.9 (1.8,5.0) | 3.7 (2.2,5.0) | 7.2 (4.6,20.1) |
| Initial postoperative length of ICU stay (Days) †,** | 3.1 (2.0,5.1) | 3.8 (2.1,6.8) | 6.9 (4.9,11.8) | 6.9 (5.0,11.0) | 16.1 (9.9,32.8) |
| Total postoperative length of ICU stay (Days) †,†† | 3.1 (2.0,5.1) | 3.8 (2.1,6.9) | 7.0 (5.0,11.9) | 6.9 (5.0,11.0) | 21.8 (11.8,59.3) |
| Incidence of Major Medical Events *,‡‡ | 45 (18%) | 66 (27%) | 118 (47%) | 82 (55%) | 204 (82%) |
| Mortality * | |||||
| In-hospital Death | 2 (1%) | 1 (0%) | 2 (1%) | 1 (1%) | 36 (15%) |
| Post-discharge within 30/60 days §§ | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (2%) |
| From 30/60 days to 12 months §§ | 1 (0%) | 5 (2%) | 0 (0%) | 0 (0%) | 15 (6%) |
n (%)
median (25th percentile, 75th percentile)
within 30 days for TOF, CAVSD, ASO, Arch/VSD; within 60 days for Norwood. N=12 missing, 5 due to missing vital status after discharge and 7 due to discharge to another hospital ward/floor with discharge home date unavailable.
after excluding censored values (death – N=42, transfer to another hospital/hospice with no discharge home date available – N=9), median (IQR) were identical except for Norwood: 34 (19, 55.5).
N=1 missing due to initial extubation time missing in electronic medical record. After excluding censored values (death – N=20), median (IQR) were virtually identical except for Norwood: 5.8 (3.8, 9.1).
N=1 missing due to initial extubation time missing in electronic medical record. Before imputation of highest value + 1 for deaths before discharge, median (IQR) were virtually identical except for Norwood: 6.9 (4.5, 15.1). After excluding deaths before discharge, means (SD) by procedure are: 1.9 (4.3), 2.6 (5.0), 4.3 (6.5), 5.3 (7.2), and 10.7 (14.3), respectively; median (IQR) were virtually identical except for Norwood: 6.2 (4.0, 11.8).
After excluding censored values (death – N=33, transfer to another hospital ward/floor with discharge home date not available – N=3), median (IQR) were virtually identical except for Norwood: 15.9 (9.9, 30.6).
Before imputation of highest value + 1 for deaths before discharge, median (IQR) were virtually identical except for Norwood: 18.8 (10.5,36.9). After excluding deaths before discharge, respectively; median (IQR) were virtually identical except for Norwood: 17.8 (10.1,35.5).
occurring <30 days (TOF, CAVSD, ASO, Arch/VSD) or <60 days (Norwood) from surgery or before hospital discharge: defined as including mortality/transplant, cardiac arrest requiring cardiopulmonary resuscitation (CPR) and medications, cardiopulmonary insufficiency requiring extracorporeal membrane oxygenation (ECMO), low cardiac output state or cardiac failure, mediastinitis requiring surgical debridement, bleeding requiring exploration, major neurologic event, renal failure requiring dialysis, necrotizing enterocolitis (NEC) requiring laparotomy, diaphragmatic paralysis requiring plication, vocal cord paralysis, prolonged ventilation for > 7 days, sepsis, multisystem organ failure (MSOF), non-routine open sternum, unplanned non-cardiac reoperation, hemothorax requiring drainage, and unplanned hospital readmission
within 30 days for TOF, CAVSD, ASO, Arch/VSD; within 60 days for Norwood.
Arch/VSD = coarctation/hypoplastic or interrupted arch with VSD; ASO = arterial switch operation; CAVSD = complete atrioventricular septal defect; ICU = intensive care unit; IQR = interquartile range; N = number; RLS = residual lesion score; TOF = tetralogy of Fallot with pulmonary stenosis
Central Illustration. Distribution of Residual Lesion Score by Operation.
RLS 1 is represented in green, RLS 2 in amber and RLS 3 in red. RLS ranged as follows: RLS 1 in 11–33%, RLS 2 in 30–53% and RLS 3 in 15–35% of patients. Arch/VSD = coarctation/hypoplastic or interrupted arch with VSD; ASO = arterial switch operation; CAVSD = complete atrioventricular septal defect; RLS = residual lesion score; TOF = tetralogy of Fallot with pulmonary stenosis.
Primary outcome
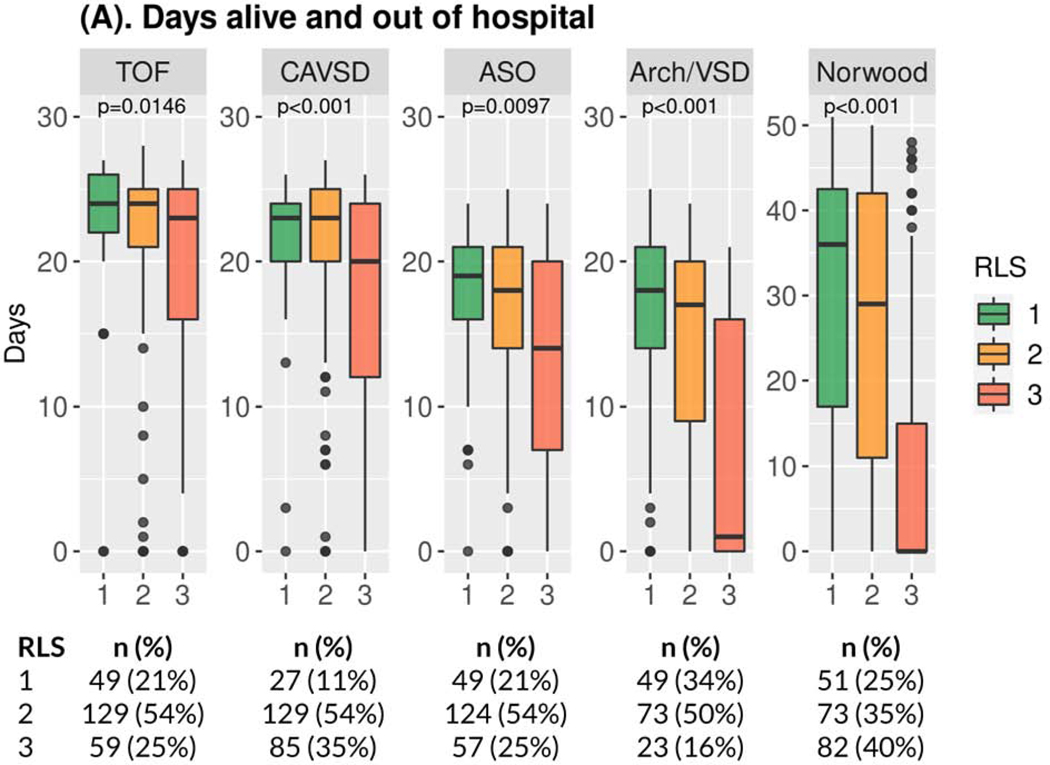
For the primary outcome of number of days alive and out of hospital within 30 days after surgery (60 days for Norwood), a higher number is more favorable. RLS 3 was consistently associated with worse outcomes (Figure 1A, Supplemental Table S2A) in 3-way analysis as compared with RLS 1 and 2. Across all operations, those patients who met criteria for RLS 3 had significantly fewer days alive and out of hospital in multivariable analyses, compared with those who had RLS 1 (Tables 3 and 4, Supplemental Table S5A). RLS 3 was the only independent risk factor for fewer (worse) days alive and out of hospital common to all five operations (Table 4, Supplemental Table S5A). Days alive and out of hospital did not differ between RLS 1 vs. RLS 2 for any operation. Sensitivity analyses found consistent results and no significant interactions for any of the five operations.
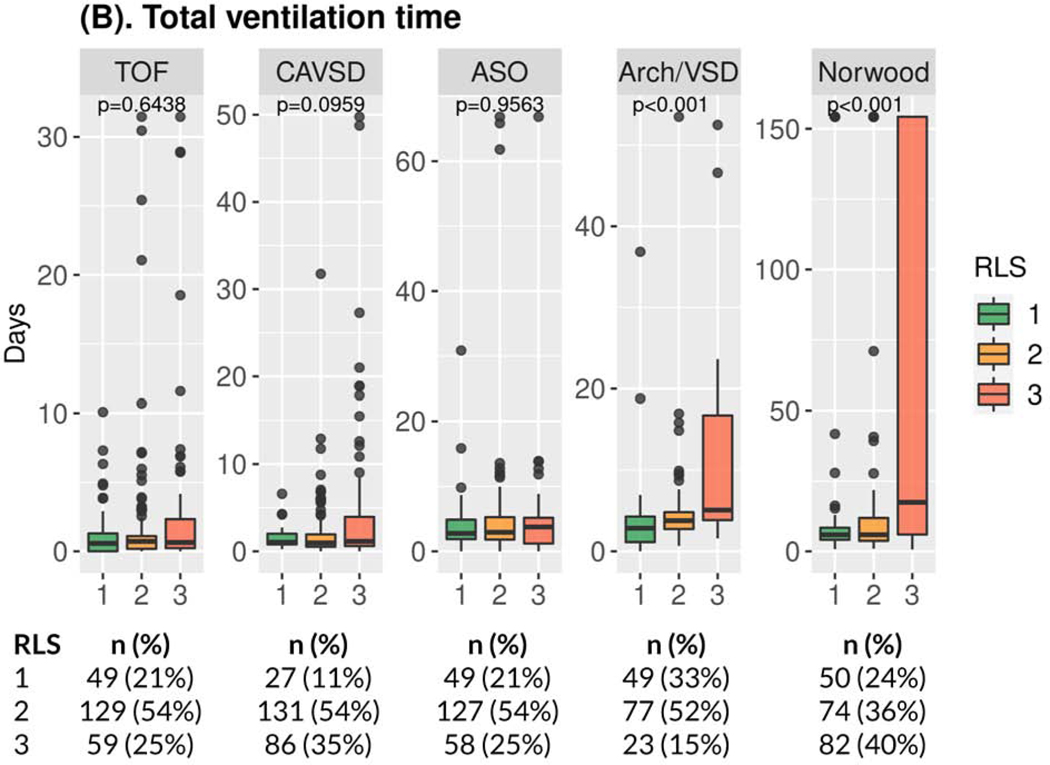
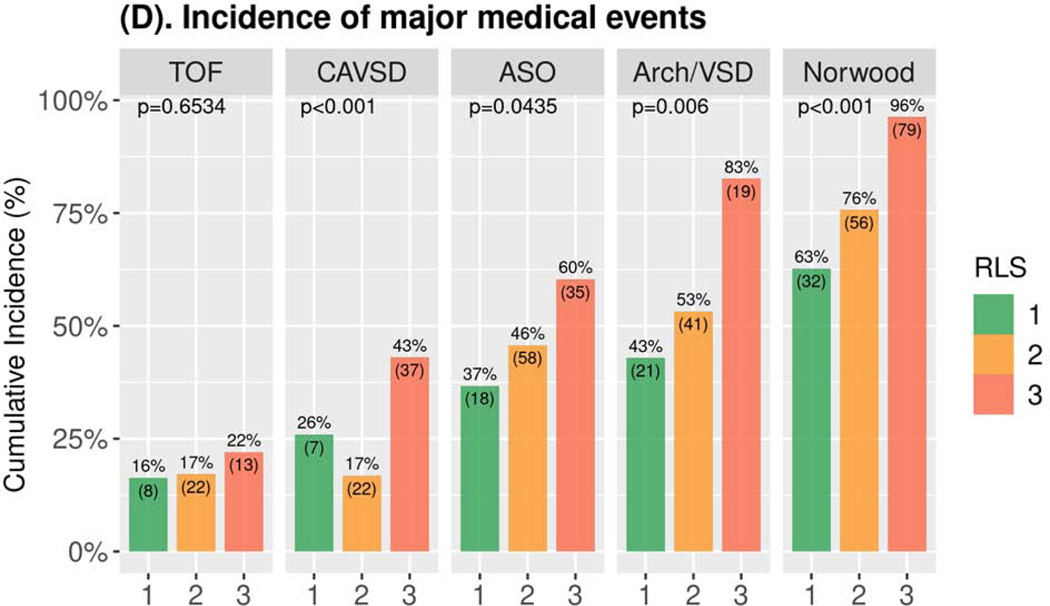
Figure 1. Study Outcomes by Operation*.
(A) Boxplot of Days Alive and Out of Hospital†,‡; (B) Boxplot of Total Ventilation Time †; (C) Boxplot of Total Postoperative Length of ICU Stay†; (D) Bar Chart of Incidence of Major Medical Events‡,§,║* Number and percentage of patients in the three RLS classes are provided below the boxplot. † p-values Kruskal-Wallis test ‡ within 30 days for TOF, CAVSD, ASO, Arch/VSD; within 60 days for Norwood. § p-values Fisher’s exact test. ║ Includes mortality/transplant, cardiac arrest, extracorporeal membrane oxygenation, cardiac failure, mediastinitis, bleeding, neurologic event, renal failure, necrotizing enterocolitis, diaphragmatic paralysis, vocal cord paralysis, prolonged ventilation, sepsis, multisystem organ failure, non-cardiac reoperation, hemothorax, and readmission. Arch/VSD = coarctation/hypoplastic or interrupted arch with VSD; ASO = arterial switch operation; CAVSD = complete atrioventricular septal defect; ICU = intensive care unit; RLS = residual lesion score; TOF = tetralogy of Fallot with pulmonary stenosis.
Table 3.
Effect of RLS and Amount of Variance Explained (R2), Results of Multivariable Modelling
| TOF | CAVSD | ASO | Arch/VSD | Norwood | |
|---|---|---|---|---|---|
| Days alive and out of hospital (days)* | |||||
| Effect of RLS 3: major residual lesions † | −3.6 (1.2) ## | −4.2 (1.4) ## | −4.4 (1.3) ## | −6.9 (1.9) ## | −16.6 (2.9) ## |
| R2 of RLS only ‡ | 0.05 | 0.09 | 0.08 | 0.11 | 0.20 |
| R2 of covariates only § | 0.18 | 0.16 | 0.08 | 0.15 | 0.09 |
| R2 of RLS + covariates ║ | 0.21 | 0.23 | 0.12 | 0.23 | 0.26 |
| Postoperative hospital length of stay (days)# | |||||
| Effect of RLS 3: major residual lesions † | 0.6 (0.4, 0.9) ## | 0.5 (0.4, 0.7) ## | 0.6 (0.4, 0.8) ## | 0.4 (0.2, 0.6) ## | 0.3 (0.2, 0.4) ## |
| R2 of RLS only ‡ | 0.03 | 0.09 | 0.03 | 0.08 | 0.18 |
| R2 of covariates only § | 0.12 | 0.12 | 0.06 | 0.15 | 0.06 |
| R2 of RLS + covariates ║ | 0.14 | 0.20 | 0.10 | 0.22 | 0.25 |
| Time to initial extubation (Initial postoperative duration of mechanical ventilation) (days)# | |||||
| Effect of RLS 3: major residual lesions † | 0.9 (0.6, 1.3) | 1.2 (0.6, 2.3) | 1.0 (0.6, 1.8) | 0.3 (0.1, 0.6) ## | 0.3 (0.2, 0.6) ## |
| R2 of RLS only ‡ | 0.01 | 0.01 | 0.00 | 0.12 | 0.10 |
| R2 of covariates only § | 0.24 | 0.13 | 0.03 | 0.20 | 0.20 |
| R2 of RLS + covariates ║ | 0.24 | 0.17 | 0.04 | 0.29 | 0.30 |
| Total ventilation (Total postoperative duration of mechanical ventilation) (days, log-scale)** | |||||
| Effect of RLS 3: major residual lesions † | 1.0 (0.7) | 0.3 (0.6) | −0.2 (0.5) | 1.5 (0.4) ## | 1.1 (0.2) ## |
| R2 of RLS only ‡ | 0.01 | 0.01 | 0.00 | 0.10 | 0.14 |
| R2 of covariates only § | 0.15 | 0.13 | 0.06 | 0.03 | 0.13 |
| R2 of RLS + covariates ║ | 0.17 | 0.14 | 0.06 | 0.12 | 0.26 |
| Time to initial ICU discharge (Initial postoperative length of ICU stay) (days) # | |||||
| Effect of RLS 3: major residual lesions † | 0.7 (0.5, 1.1) | 0.6 (0.4, 0.9) ## | 0.7 (0.4, 1.1) ## | 0.5 (0.3, 0.7) ## | 0.4 (0.3, 0.6) ## |
| R2 of RLS only ‡ | 0.02 | 0.09 | 0.02 | 0.09 | 0.12 |
| R2 of covariates only § | 0.19 | 0.14 | 0.08 | 0.08 | 0.08 |
| R2 of RLS + covariates ║,†† | 0.20 | 0.22 | 0.09 | 0.17 | 0.21 |
| Total ICU stay (Total postoperative length of ICU stay) (days, log-scale) ** | |||||
| Effect of RLS 3: major residual lesions † | 0.2 (0.1) | 0.4 (0.2) ## | 0.2 (0.1) | 0.8 (0.2) ## | 1.0 (0.2) ## |
| R2 of RLS only ‡ | 0.01 | 0.09 | 0.02 | 0.17 | 0.19 |
| R2 of covariates only § | 0.25 | 0.42‡‡ | 0.37‡‡ | 0.11 | 0.08 |
| R2 of RLS + covariates ║,†† | 0.26 | 0.44‡‡ | 0.26 ‡‡ | 0.25 | 0.26 |
| Incidence of major events (odds ratio) §§ | |||||
| Effect of RLS 3: major residual lesions † | 1.3 (0.5, 3.8) | 2.1 (0.8, 5.6) | 2.6 (1.0, 6.6) | 5.8 (1.6, 21.1) ## | 16.2 (4.4, 59.7) ## |
| R2 of RLS only ‡ | 0.00 | 0.06 | 0.02 | 0.05 | 0.13 |
| R2 of covariates only § | 0.11 | 0.00 ║║ | 0.06 | 0.00║║ | 0.03 |
| R2 of RLS + covariates║ | 0.11 | 0.06║║ | 0.07 | 0.05║║ | 0.15 |
slope (SE) from random effects linear regression; R2 computed using the Rβ*2 statistic26
comparison of RLS 3 vs. RLS 1, controlling for significant covariates. The model compared RLS 1, 2, and 3, with RLS 1 as the reference category. For simplicity, only results for RLS 3 vs. 1 are presented; the only significant effects of minor residual lesions (RLS 2 vs. RLS 1) were for time to initial extubation [CAVSD: 1.9 (1.0,3.4); Arch/VSD: 0.3 (0.2,0.6)], and total ventilation [Arch/VSD: 0.9 (0.3)]
from unadjusted models with RLS as only predictor
from models with all significant covariates (without RLS)
from final adjusted models with RLS and all significant covariates
hazard ratio (95% CI) from competing risks proportional hazards Cox model, with hospital discharge (for postoperative hospital length of stay), initial extubation, or initial ICU discharge as the event of interest and death as a competing risk; a HR<1 represents lower likelihood of the event. R2 computed using a generalized R2 statistic27
slope (SE) from random effects linear regression model, after log-transformation of outcome; β coefficients can be interpreted as a percentage difference in the outcome between RLS 3 vs. 1 (the reference), calculated as ((exp(β) − 1) * 100) (https://data.library.virginia.edu/interpreting-log-transformations-in-a-linear-model/). For total ventilation, RLS 3 patients had 3.5 times (348%) the number of total ventilation days for Arch/VSD and 2 times (200%) for Norwood, as compared to RLS 1 patients. For total ICU stay, RLS 3 patients had 0.5 times (49%) the number of ICU days for CAVSD, 1.2 times (123%) for Arch/VSD, and 1.7 times (172%) for Norwood, as compared with RLS 1 patients. R2 computed using the Rβ*2 statistic26
excluding the covariate of institutional practice to discharge directly from the ICU, which is not of interest in the comparison of variance explained by RLS vs. other factors
R2 without the random effect of site is 0.12 (without RLS) and 0.21 (with RLS) for CAVSD, and 0.13 (without RLS) and 0.14 (with RLS) for ASO. The large increase in R2 with addition of the random effect of site suggests considerable site variation, not related solely to significant effects of site volume tertile
OR (95% CI) from random effects logistic regression; R2 computed using the Rβ*2 statistic26
p<0.05 on post-hoc testing after significant overall p-value
no covariates significant in final model
Arch/VSD = coarctation/hypoplastic or interrupted arch with VSD; ASO = arterial switch operation; CAVSD = complete atrioventricular septal defect; CI = confidence interval; HR = hazard ratio; ICU = intensive care unit; RLS = residual lesion score; TOF = tetralogy of Fallot with pulmonary stenosis; SE = standard error
Table 4.
| TOF R2 = 0.21 |
CAVSD R2 = 0.23 |
ASO R2 = 0.12 |
Arch/VSD R2 = 0.23 |
Norwood R2 = 0.26 |
|
|---|---|---|---|---|---|
| semi-partial R2 ‡ | |||||
| Major residual lesions (RLS 3) (worse outcome) | 0.03 | 0.04 | 0.05 | 0.09 | 0.14 |
| Older age at surgery (better outcome) | 0.09 | 0.02 | |||
| Higher weight at surgery (better outcome) | 0.05 | 0.05 | |||
| Male gender (better outcome) | 0.03 | ||||
| Black race (vs. White) (worse outcome) | 0.02 | ||||
| Urgent/emergent Surgery (worse outcome) | 0.02 | ||||
| Pre-operative risk factors (worse outcome) | 0.07 | 0.04 | 0.06 | ||
| Procedure-specific factors (worse outcome) | 0.03 | ||||
| Intraoperative findings worse than pre-op echo (worse outcome) | 0.04 | ||||
| Antenatal diagnosis of CHD (worse outcome) | 0.02 | ||||
the following were not significantly associated with outcome for any surgery: ethnicity, pregnancy term, non-cardiac congenital anomaly/syndromes/genetic abnormalities, site volume, surgeon volume, ventricular dominance (Norwood only), single ventricle anatomy (Norwood only).
within 30 days for TOF, CAVSD, ASO, Arch/VSD; within 60 days for Norwood; better outcome = more days alive and out of hospital; worse outcome = fewer days alive and out of hospital
calculated using the method of Jaeger (2017); represents the additional contribution of the predictor, after accounting for all other predictors, thereby providing a means of assessing the relative importance of each predictor
Arch/VSD = coarctation/hypoplastic or interrupted arch with VSD; ASO = arterial switch operation; CAVSD = complete atrioventricular septal defect; CH = congenital heart disease; RLS = residual lesion score; TOF = tetralogy of Fallot with pulmonary stenosis
Secondary outcomes
RLS 3 was consistently associated with longer postoperative hospital length of stay across all operations in both bivariate (Supplemental Figure S2, Supplemental Table S2B) and multivariable (Table 3, Supplemental Table S5B) analyses. The relationship of RLS 3 to other secondary outcomes varied by operation (Table 3, Supplemental Tables S5C-S5G). RLS 3 was consistently associated with worse results for each secondary outcome after the Norwood operation and Arch/VSD repair. For CAVSD, RLS 3 vs. 1 was associated only with longer initial and total duration of ICU stay. For TOF and ASO, RLS was not associated with any secondary outcome other than postoperative hospital length of stay. Covariates other than RLS that achieved significance are presented in supplemental tables (Supplemental Tables S4, S5) and varied across the operations and outcomes. There was no consistent effect of RLS 2 vs. RLS 1 on primary or secondary outcomes (Supplemental Tables S2, S5).
Effects of RLS 3 based on discharge echocardiogram vs. unplanned pre-discharge reintervention
RLS 3 could be assigned either because the discharge echocardiogram showed evidence of major residual lesions in one or more subcomponents of surgery or because the patient underwent an unplanned reintervention for residual lesion in an anatomic area repaired at index surgery before hospital discharge. Assignment of RLS 3 based upon unplanned reintervention before discharge was associated with much worse outcomes compared with RLS 3 based on discharge echocardiogram findings across procedures (Supplemental Table S3A-E); however, with the exception of Norwood patients, frequencies of unplanned reinterventions were low. Among 82 Norwood procedures classified as RLS 3, 71 (87%) were based upon pre-discharge reintervention, most commonly shunt or arch interventions. In contrast, RLS 3 based on pre-discharge reintervention was between 8% and 17% for the other procedures: 5/59 (8%) for TOF, 12/86 (14%) for CAVSD, 10/58 (17%) for ASO and 4/23 (17%) for Arch/VSD.
Percent variance in outcomes explained by RLS
The variance explained by RLS for days alive and out of hospital varied according to complexity of surgery. For the highly complex Norwood operation, the R2 for RLS in bivariate analysis was 20% (Table 3). The models including only patient/preoperative factors had an R2 of 9%, and the final multivariable model including RLS had an R2 of 26%. Additionally, the semi-partial R2 for RLS alone was 14%, compared with 3–6% for each of the other predictors (Table 4). In contrast, for the least complex operation, TOF, RLS accounted for an R2 of only 5% in bivariate analysis; in multivariable analysis, patient/preoperative factors had an R2 of 18%; when RLS was added to this model, the R2 increased to 21%, with a semi-partial R2 for RLS of only 3%. The impact of RLS on other operations was intermediate between those in Norwood and TOF. For the six secondary outcomes (Table 3), RLS fairly consistently had the highest R2 for the Norwood operation (10–19% in bivariate analyses) and generally contributed the lowest R2 in secondary outcomes for TOF, CAVSD, and ASO (0–3% for TOF and ASO, 1–9% for CAVSD in bivariate analyses). The greatest contribution of patient/preoperative factors was for total ICU stay in CAVSD and ASO, with site volume accounting for the largest proportion of variance, and for time to initial extubation in TOF and Arch/VSD.
DISCUSSION
The RLS Study is the first prospective, multicenter study to analyze the impact of residual lesions on early postoperative outcomes in a well-defined subset of common congenital cardiac operations performed with relatively similar operative technique across institutions. We found that minor residual lesions (RLS 2) had little effect on in-hospital outcomes, but the presence of major residual lesions (RLS 3) was associated with fewer days alive and out of hospital at 30 days (60 for Norwood) and with longer hospital length of stay, even after controlling for patient and preoperative factors in all procedural categories. The relationship of major residual lesions to other in-hospital outcomes varied by operative complexity. For the two most technically complex operations, the Norwood and Arch/VSD, major residual lesions were significantly associated with worse outcomes, including duration of mechanical ventilation, ICU stay, and major medical events. For the remaining operations, RLS contributed less to the variance in secondary postoperative outcomes than patient/preoperative covariates, with the exception of major medical events after CAVSD repair, for which no covariates were significant in the final model.
Significant residual lesions after congenital cardiac surgery have been previously associated with greater mortality and morbidity, longer ICU stay, greater hospital cost, and worse neurodevelopmental outcomes in retrospective studies (7,12,13,15,16,18–24). The current multicenter study builds upon this work in several ways. First, the RLS for each lesion was created by a national panel of experts in congenital cardiac surgery or pediatric cardiology and two facilitators using a modified RAND-Delphi technique (25). Second, the study was performed prospectively, so that missing, incomplete and inconsistent data were minimized. Finally, although operations were performed in accordance with local center practices, an echocardiography core laboratory was used to assess residual lesions for four of the five operations, facilitating a more uniform and unbiased classification.
A number of factors may contribute to the greater impact of major residual lesions on operations of greater complexity, i.e., greatest after Norwood operation, lower after Arch/VSD repair and ASO, and lowest after TOF and CAVSD repair. We speculate that postoperative hemodynamic status may be so tenuous in patients with univentricular circulation (Norwood) that those with residual lesions do poorly. In support of this theory, RLS 3 classification among Norwood procedures derived largely from reintervention prior to discharge rather than from findings on discharge echocardiography. It follows that patients who require reoperation prior to discharge will generally have a longer postoperative length of stay. Differences in the impact of RLS among lesions could also be attributable, in part, to omission of important operative sub-components by the RLS designed by the RAND-Delphi panel. Indeed, the RLS developed by our expert panel accounted for <10% of the variance in our primary perioperative outcome for all operations except the Norwood. Moreover, no model explained more than about half of the variance for the study’s primary or secondary outcomes, suggesting an important role for as-yet unidentified factors. Future studies will compare consensus-based RLS sub-components and cut-points with those derived from the data for each of the five operations.
Limitations
Certain limitations to our study should be noted. Whereas every effort was made to capture all data elements, a small amount of missing data occurred due to lack of documentation of clinical details. Centers may have varied in their detection and reporting of certain adverse events, such as laryngeal nerve paralysis. Additionally, major medical events were diverse, and may have differed on their impact on clinical course. Bias was minimized by enrolling consecutive eligible patients from the participating centers who consented over a two-year period. For those patients who died postoperatively before consent was obtained, a waiver of consent was obtained to avoid selection bias. We used local rather than core laboratory interpretation for the ASO due to funding constraints, having noted that the echocardiographic elements for the ASO RLS are standard in clinical practice and center interpretation was expected to be less variable. Whereas RLS captured the components of the cardiac repair, other components related to conduct of surgery, such as preservation of the phrenic nerve (for all five procedures) and recurrent laryngeal nerve (for Arch/VSD and Norwood), were not part of RLS. It is well known that injury to these structures can affect duration of ventilation, intensive care, and hospital length of stay (31). The RLS was derived from echocardiograms performed prior to discharge (or reinterventions) at index hospitalization to reflect residual lesions without the hemodynamic lability seen in the immediate perioperative period. However, to impact management in the operating room and immediate postoperative period, data on residual lesions must be obtained from intraoperative transesophageal echocardiography (TEE); the impact of residual lesions by TEE on outcomes will be analyzed in the future. Decisions on whether or not to return to the operating room because of residual lesions generally consider a child’s clinical well-being as well as residual lesions. We analyzed five common index operations across a spectrum of complexity, but generalizability of our findings to other operations is untested. Finally, this study focused on assessing the implications of residual lesions on early postoperative outcomes; future studies will assess the impact of residual lesions on medium- and long-term outcome.
Conclusion
Adjusting for patient and preoperative factors, major residual lesions were most strongly associated with worse in-hospital outcomes in the most complex operations, but had less predictive value for operations of lower complexity. Minor or trivial residual lesions had little impact on perioperative outcomes. In the future, measurement of RLS by intraoperative TEE together with data-driven analyses, possibly including machine learning approaches, may further refine the RLS as a tool that can guide intraoperative management and postoperative surveillance, provide a uniform platform to explain inter-institutional variability in outcomes after CHD surgery, and help surgeons and care teams improve performance.
Supplementary Material
Clinical Perspectives.
Competency in Patient Care and Procedural Skills:
Adjusted for pre-operative clinical features, residual lesions after congenital cardiac surgery, assessed using a residual lesion score (RLS), are related to in-hospital outcomes, and the impact is greatest following more complex operations.
Translational Outlook:
Future studies should evaluate the utility of the residual lesion score to identify high-risk patients with congenital heart disease undergoing other types of invasive procedures and guide other aspects of long-term management.
Acknowledgements:
Presented at the 100th Annual Meeting of the American Association for Thoracic Surgery, May 23, 2020. We would like to thank the study coordinators, surgeons, echocardiographers, echocardiogram uploaders, and registry coordinators from the 17 sites, and data coordinating center staff for their invaluable contributions to this study. (Appendix: Residual Lesion Score (RLS) Study Investigators). A special thanks to Ed Marcus who played a key role in the RLS echocardiogram corelab to ensure that all echocardiograms were uploaded fully deidentified and appropriately formatted, and developed a specialized reporting system for ease of data transfer to the data coordinating center.
Funding
This study was supported by grants (U24HL135691, U10HL068270, HL109818, HL109778, HL109816, HL109743, HL109741, HL109673, HL068270, HL109781, HL135665, HL135680) from the National Heart, Lung, and Blood Institute, National Institutes of Health. Meena Nathan was supported by a K23 grant-NHLBI/NIH HL119600. Brett Anderson was supported by a K23 grant-NHLBI/NIH HL133454.
Abbreviations:
- ASO
Arterial Switch Operation
- CAVSD
Complete Atrio Ventricular Septal Defect
- ICU
Intensive Care Unit
- RLS
Residual Lesion Score
- STAT
Society of Thoracic Surgeon - European Association for Cardio-Thoracic Surgery Congenital Heart Surgery mortality category
- TOF/PS
Tetralogy of Fallot with Pulmonary Stenosis
- TPS
Technical Performance Score
- VSD
Ventricular Septal Defect
Footnotes
Disclosures/Conflicts of Interest: Authors have no disclosures or conflicts of interest to report. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the US Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Connor JA, Gauvreau K, Jenkins KJ. Factors associated with increased resource utilization for congenital heart disease. Pediatrics 2005;116(3);689–695. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 2002; 123(1):110–118. [DOI] [PubMed] [Google Scholar]
- 3.Jenkin KJ. Risk adjustment for congenital heart surgery: The RACHS-1 method Seminars in Thorac and Cardiovasc Surg: Pediatric Cardiac Surgery Annual. 2004; 7(1):180–184. [DOI] [PubMed] [Google Scholar]
- 4.Connor JA and Jenkins KJ. Factors associated with increased resource utilization for congenital heart disease. In: Congenital Heart Defects: From Origin to Treatment. Editors Wyszynski D and Correa A. Oxford University, New York, 2010. [Google Scholar]
- 5.Nathan M, Karamichalis J. Quality Improvement: Surgical Performance. Technical Performance Score as a performance measure for congenital heart surgery. In Sellke/Sabiston and Spencer’s Surgery of the Chest, 9th edition, 2015; Chapter 134. [Google Scholar]
- 6.Karamichalis JM, Colan SD, Nathan M, et al. Technical performance scores in congenital cardiac operations: a quality assessment initiative. Ann Thorac Surg 2012; 94(4):1317–23. [DOI] [PubMed] [Google Scholar]
- 7.Shuhaiber J, Gauvreau K, Thiagarjan R, et al. Congenital heart surgeon’s technical proficiency affects neonatal hospital survival. J Thorac Cardiovasc Surg 2012; 144(5):1119–1124. [DOI] [PubMed] [Google Scholar]
- 8.Nathan M, Pigula FA, Liu H, et al. Inadequate Technical Performance Scores are associated with late mortality and need for late reintervention in a 13-month cohort of patients Followed for 4 years. Annals Thorac Surg 2013; 96(2):664–669. [DOI] [PubMed] [Google Scholar]
- 9.Nathan M, Sadhwani A, Gauvreau K, et al. Association between Technical Performance Scores and neurodevelopmental outcomes after congenital cardiac surgery. J Thorac Cardiovasc Surg. 2014. 147(1):389–94. [DOI] [PubMed] [Google Scholar]
- 10.Nathan M, Gauvreau K, Samnaliev M, et al. Technical Performance Score predicts resource utilization in congenital cardiac procedures. J Am Coll Cardiol. 2016; 67(22): 2696–8. [DOI] [PubMed] [Google Scholar]
- 11.Howard TS, Kalish BT, Wigmore D, et al. Association of ECMO support adequacy and residual lesions on outcomes in neonates supported after cardiac surgery. Ped Crit Care Med. 2016; 17(11): 1045–1054. [DOI] [PubMed] [Google Scholar]
- 12.Karamichalis JM, Thiagarajan RR, Liu H, Mamic P, Gauvreau K, Bacha EA. Stage I Norwood: Optimal Technical Performance improves outcomes irrespective of preoperative physiologic status or case-complexity. J Thorac Cardiovasc Surg 2010; 139(4):962–968. [DOI] [PubMed] [Google Scholar]
- 13.Nathan M, Karamichalis J, Liu H, et al. Intra-operative adverse events can be compensated in infants after cardiac surgery: A prospective study. J Thorac Cardiovasc Surg 2011; 142(5):1098–1107. [DOI] [PubMed] [Google Scholar]
- 14.Nathan M, Karamichalis JM, Colan S, et al. Surgical Technical Performance Scores are predictors for late mortality and unplanned reinterventions in infants after cardiac surgery. J Thorac Cardiovasc Surg 2012; 144(5):1095–1101. [DOI] [PubMed] [Google Scholar]
- 15.Nathan M, Karamichalis J, Liu H, et al. Technical Performance Scores are strongly associated with early mortality, postoperative adverse events and ICU length of stay – analysis of consecutive discharges over 2 years. J Thorac Cardiovasc Surg 2014; 147(1):389–396. [DOI] [PubMed] [Google Scholar]
- 16.Blinder JJ, Thiagarajan R, Williams K, Nathan M, Mayer J, Kulik TJ. Duration of mechanical ventilation and perioperative care quality after neonatal cardiac operations. Ann Thorac Surg. 2017; 103(6):1956–1962. [DOI] [PubMed] [Google Scholar]
- 17.Martin E, Del Nido PJ, Nathan M. Technical performance scores are predictors of midterm mortality and reinterventions following congenital mitral valve repair. Eur J Cardiothorac Surg. 2017; 52(2):218–224. [DOI] [PubMed] [Google Scholar]
- 18.Ijsselhof R, Gauvreau, del Nido P, Nathan M. Technical Performance Score is a predictor for post-discharge reinterventions following complete atrioventricular septal defect repair. Ann Thorac Surg. 2017; 104(4):1371–1377. [DOI] [PubMed] [Google Scholar]
- 19.Tishler B, Gauvreau K, Colan SD, Del Nido P, Nathan M. Technical Performance Score predicts partial/transitional atrioventricular septal defect outcomes. Ann Thorac Surg. 2018; 105(5):1461–1468. [DOI] [PubMed] [Google Scholar]
- 20.Nathan M, Sleeper L, Ohye R, et al. al for the Pediatric Heart Network Investigators. Technical Performance Score is associated with outcomes after the Norwood procedure. J Thorac Cardiovasc Surg. 2014; 148(5):2208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham MEA, Donofrio MT, Peer SM, Zurakowski D, Jonas R, Sinha P. Influence of age and weight on technical repair of tetralogy of Fallot. Ann Thorac Surg. 2016;102(3): 864–869. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham MEA, Donofrio MT, Peer SM, et al. Optimal timing for elective early primary repair of tetralogy of Fallot: analysis of intermediate term outcomes. Ann Thorac Surg. 2017;103(3): 845–852. [DOI] [PubMed] [Google Scholar]
- 23.Lushaj EB, Bartlett HL, Lamers LJ, Arndt S, Hermsen J, Ralphe JC, Anagnostopoulos PV. Technical performance score predicts perioperative outcomes in complex congenital heart surgery performed in a small to medium volume program. Pediatr Cardiol. 2020. 41(1):88–93 [DOI] [PubMed] [Google Scholar]
- 24.Nathan M, Marshall A, Kerstein J, et al. Technical Performance Score as predictor for post discharge reintervention in valve sparing Tetralogy of Fallot repair. Semin in Thorac and Cardio Vasc Surg 2014 Winter; 26(4): 297–303. [DOI] [PubMed] [Google Scholar]
- 25.Nathan M, Trachtenberg FL, Van Rompay MI, et al. The Pediatric Heart Network Residual Lesion Score Study: Design and Objectives. J Thorac Cardiovasc Surg 2020; 160(1):218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138(5):1139–53. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs JP, Mayer JE Jr, Pasquali SK, Hill KD, Overman DM, St Louis JD, Kumar SR, Backer CL, Tweddell JS, Dearani JA, Jacobs ML The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2019 Update on Outcomes and Quality. Ann Thorac Surg . 2019. March;107(3):691–704 [DOI] [PubMed] [Google Scholar]
- 28.Jaeger Byron C., Edwards Lloyd J., Das Kalyan & Sen Pranab K. An statistic for fixed effects in the generalized linear mixed model. Journal of Applied Statistics. Taylor & Francis Journals, 2017; 44(6):1086–1105. [Google Scholar]
- 29.Allison Paul D. 1995. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Institute Inc. [Google Scholar]
- 30.Brock GN, Barnes C, Ramirez JA, Myers J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med Res Methodol. 2011;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornik CP, He X, Jacobs JP, et al. Complications after the Norwood operation: An analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg 2011;92:1734–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.