Abstract
Body size is emerging as a novel and clinically-relevant patient factor in bladder cancer research. Historically, a patient’s body mass index (BMI) has been used as a proxy for obesity but it shows inconsistent associations with risk of developing the disease as well as with most clinical outcomes. More specific body composition features can be derived for patients using a variety of methods. To date, skeletal muscle measurements derived from pre-operative computed tomography scans have shown the most consistent associations with clinical outcomes. Importantly, skeletal muscle can potentially be modified through resistance training and/or nutritional interventions. Large scale studies that evaluate the prognostic impact of not only body composition features at baseline but also describe changes in body composition post-treatment are needed to move the field forward to ultimately improve clinical outcomes for bladder cancer patients.
Keywords: bladder cancer, obesity, body mass index, body composition, exercise, skeletal muscle, adiposity, sarcopenia
Introduction
Bladder cancer is the tenth most common cancer worldwide and is categorized based on pathological depth of tumor invasion. Most bladder cancer patients are diagnosed with non-muscle invasive bladder cancer (NMIBC) which has the highest recurrence risk of any malignancy. Standard treatment consists of transurethral resection with intravesical immune- or chemotherapy, but a subset of patients progress to muscle-invasive bladder cancer (MIBC). Clinical management of MIBC patients include radical cystectomy with pelvic lymphadenectomy and urinary diversion as well as neoadjuvant or adjuvant chemotherapy. Unfortunately, MIBC patients experience substantial morbidity and mortality after cystectomy. Existing risk prediction models that estimate risk of recurrence, progression or survival have limited accuracy for the individual patient and rely primarily on clinicopathological variables1. Novel factors that improve risk stratification are needed. Emerging research suggests that body size constitutes a novel and clinically-relevant patient factor that potentially could be leveraged to refine risk prediction and be modified to improve clinical outcomes. The purpose of this commentary is to summarize what is known about body size (i.e., both body mass index (BMI) and body composition) in relation to bladder cancer risk and clinical outcomes, and to discuss future research directions.
Body size basics: moving beyond BMI
Historically, BMI (categorized as normal weight 18.5–25 kg/m2, overweight 25–30 kg/m2, and obese > 30 kg/m2), has been used to represent obesity. On a population level, BMI is a good marker of overall body fat content but does not accurately reflect individual distribution of body fat (subcutaneous vs. visceral) or muscle mass which may have different biological effects2,3. More specific body size features can be derived from different sources such as computerized tomography (CT) scans, magnetic resonance imaging (MRIs), dual-energy absorptiometry (DEXA), and bioelectrical impedance analysis (BIA). The most applicable for bladder cancer research are CT scans, which are obtained for staging and surveillance purposes. CT scans taken at the level of the third lumbar vertebrae (L3) are linearly related to whole body total adipose tissue and skeletal muscle mass4,5, and can be used to obtain both the cross-sectional area and the radiodensity of these tissues using established Hounsfield Units (HU) for each tissue type. Similar to BMI, the cross-sectional area variables can be divided by height in meters squared to derive the index for each (e.g., skeletal muscle index (SMI), visceral adipose tissue index (VATI) and subcutaneous adipose tissue index (SATI)). Radiodensity of muscle and adipose tissues can also be obtained but have not been widely studied in cancer. Table 1 presents common body composition variables that can be derived from CT images. As validated cut-points for high and low levels are lacking for most variables, many studies report study- and gender-specific distributions, making direct comparisons between studies challenging. Notably, low SMI alone is not synonymous with the term ‘sarcopenia’ which requires the addition of measures of strength, muscle performance, or physical performance for proper classification6 or ‘cachexia’ which requires weight loss >5% or >2% in individuals with a BMI <20 kg/m2 or sarcopenia7. Single muscle quantification (i.e., psoas) may not accurately represent overall skeletal muscle because it does not correlate with total skeletal area in cancer patients8,9. Despite these methodological issues, body composition features appear to be novel prognostic factors across several cancers, including bladder cancer.
Table 1.
Key body composition terms
| Body Composition: Area and radiodensity of muscle and adipose tissue, which cannot be assessed using measures of weight and height, but can be precisely quantified using CT scans taken for staging or surveillance of bladder cancer patients |
| Skeletal Muscle Index (SMI): Cross-sectional area of muscle divided by height squared (i.e., amount of muscle tissue) |
| Skeletal Muscle Radiodensity (SMD): Reflects fatty infiltration to the muscle (i.e., density of muscle tissue) |
| Visceral & Subcutaneous Adipose Tissue Index (VAT Index & SAT Index): Cross-sectional area of each adipose tissue divided by height squared (i.e., amount of adipose tissue) |
| Visceral & Subcutaneous Adipose Tissue Density (VAT Radiodensity & SAT Radiodensity): Reflects lipid content of adipocytes in each tissue (i.e., density of adipose tissue) |
Association of obesity defined by BMI with incidence and clinical outcomes
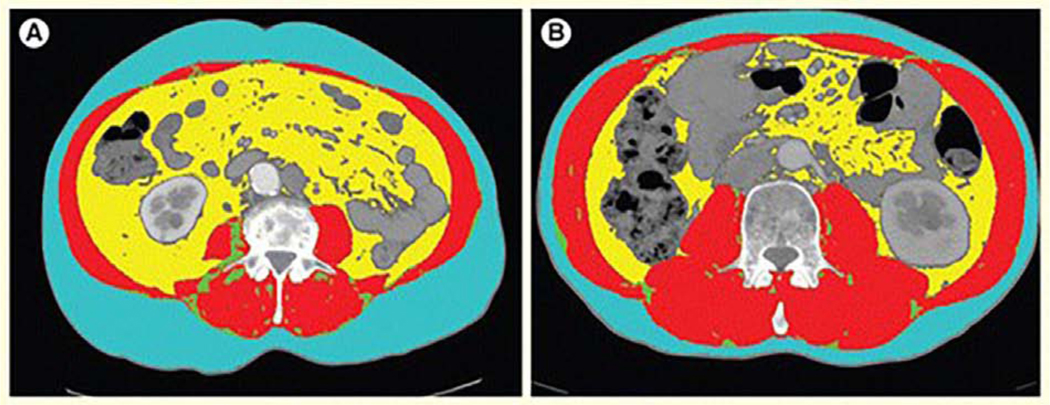
Associations between high BMI and risk of developing NMIBC and MIBC are not consistent across studies. Three prior meta-analyses each report about a 10% increased relative risk of developing bladder cancer10–12, while a recent prospective cohort of over 800,000 men and women found that the positive association between high BMI and risk was limited to males, and to those with NMIBC13. With respect to prognosis among NMIBC patients, a recent meta-analysis of 31 studies and a study across 13 institutions (n=1,155) found that patients who were overweight or obese had a significantly increased risk of recurrence (HR 5.33; p<0.001) compared to patients who were classified as normal weight at diagnosis14,15. Results for progression to MIBC were conflicting between studies. In MIBC, compared to normal weight patients, those who were overweight or obese experience longer operative times, more blood loss, and higher rates of perioperative complications16–18. Although BMI is routinely measured as part of cancer care, it is widely acknowledged that BMI is a crude measure of body size that cannot delineate body composition features such as skeletal muscle, visceral adipose and subcutaneous adipose tissues2. Indeed, cancer patients with the same BMI can vary widely in their body composition features (Figure 1).
Figure 1.
CT scans from two cancer patients with the same BMI but very different body composition features. CT scans from two different RCC patients, A and B, who have the same BMI but different body composition features. The blue area is subcutaneous fat, the red is skeletal muscle, and the yellow is visceral fat. Patient A has more subcutaneous and visceral fat and far less skeletal muscle than patient B.”
Association of body size features with clinical outcomes
Several retrospective studies have examined the relationship between body composition and bladder cancer outcomes. These studies focused largely on associations between SMI and clinical outcomes. There is limited assessment of adiposity (e.g., VATI, SATI) in relation to morbidity, mortality or systemic treatment response in bladder cancer.
Low skeletal muscle in surgically treated bladder cancer patients
A recent systematic review and meta-analysis of 12 retrospective studies by Hu, et al. 19 evaluated the association of CT-derived muscle features with oncologic outcomes in both surgically treated localized bladder (n=7 studies) and upper tract urothelial carcinoma (n=5 studies). Among localized bladder cancer patients treated with radical cystectomy (n=1,509), the overall prevalence of low SMI was 51.2% (ranging from 20–75%). Bladder cancer patients with low SMI experienced worse overall- (OS, HR 1.63, 95%CI 1.37–1.94) and cancer-specific survival (CSS, HR 1.73, 95%CI 1.35–2.21) compared to patients with high SMI.
Overall, studies evaluating the association of low pre-operative SMI in patients with localized bladder cancer treated with radical cystectomy suggest worse OS and CSS3,20,21. Mayr et al.22 conducted the largest retrospective study to date. It included 500 patients who underwent radical cystectomy with a median follow-up of 22 months (IQR 10–45 months). Patients with low SMI experienced inferior 5-year OS (38.3% vs. 50.5%, p=0.002) and CSS (49.5% vs 62.3%, p=0.016). After adjusting for clinicopathologic variables including receipt of adjuvant chemotherapy and BMI, low SMI remained significantly associated with increased all-cause mortality.
Chemotherapy and radiation therapy
Several groups have evaluated the association of body composition with chemotherapy response and toxicity. Zargar et al. evaluated 60 patients that underwent neoadjuvant chemotherapy (NAC) followed by consolidative radical cystectomy. Evaluating pre- and post-NAC CT scans, the receipt of NAC was associated with a significant 4.9% decrease in psoas muscle volume (PMV) and 0.05% in BMI; however, there was no significant association between change in PMV and pathologic response, complications, readmission, or survival outcomes. NAC dose reduction/delay (n=11, 18%) was a predictor of PMV loss (p=0.047)23. Other retrospective studies have not found a significant association between pre-operative low SMI and median time to cystectomy, chemotherapy dose reduction, delay of chemotherapy administration, toxicity or pathologic response20,24. Less is known about patients treated with definitive chemoradiation therapy. Among 68 comorbid patients unfit for radical cystectomy or systemic chemotherapy who underwent transurethral resection of bladder tumor and radiation, 72% had low SMI. In this older cohort (median age 82), having low SMI and obesity was associated with worse CSS (HR 5.0, 95%CI 1.4–16.7)25.
Perioperative outcomes
Radical cystectomy is associated with significant post-operative morbidity and mortality. Among 327 patients undergoing RC, Mayr et al. identified low SMI as an independent predictor of postoperative complications and 90-day mortality (OR 2.59, 95%CI 1.13–5.95), and Clavien-Dindo grade 4a-5 complications (p=0.003)26. Among 63 male patients, Saitoh-Maeda et al. demonstrated that low PMI (n=34) was associated with a longer post-operative length of stay and worse OS27. Albersheim, et al.28 also found that low SMI (OR 0.94, 95%CI 0.90–0.98) and high total adipose tissue mass (OR 1.24, 95%CI 1.04–1.48) were independently associated with discharge to a facility after radical cystectomy among 136 patients. Several studies also suggest that weight loss in the perioperative period can be a poor prognostic marker. The two studies that evaluated weight loss prior to or after radical cystectomy without assessment of body composition, reported increased rates of postoperative complications29 and poor survival30. Among 75 patients undergoing radical cystectomy, regardless of initial body weight, weight loss of ≥10% at one-month post-operatively was associated with decreased 5-year survival (p=0.038). Notably, by 2-months post-operatively patients had lost a mean of 17 pounds30.
Potential mechanisms underlying the associations of body composition features with oncologic outcomes
Obesity, characterized by increased adiposity, is known to contribute to malignancy via metabolic, inflammatory, and hormonal mechanisms31. Adipose tissue is an active endocrine organ secreting adipokines (e.g., leptin, adiponectin) which preliminarily have been shown to be have direct effects on bladder cancer cells in vitro32. However, other components of body size such as muscle may also contribute to cancer biology. In the absence of malignancy, age-related muscle loss is associated with a state of subclinical systemic inflammation reflected by elevated circulating inflammatory biomarkers (e.g., C-reactive protein)33,34. Among patients with localized colorectal cancer, low SMI has been associated with elevated circulating neutrophil-to-lymphocyte ratio (marker of inflammation) and the combination of these two factors doubles the risk of death35. Whether tumors induces muscle loss directly or, alternatively, whether low SMI is a marker for an environment that promotes tumor progression, is unclear.
Modifying body composition
The emerging evidence that low SMI is associated with adverse outcomes in bladder cancer provides a strong rationale to examine the efficacy of interventions to modify it. In non-cancer settings, there is a wealth of observational and randomized trial data demonstrating that exercise and/or diet interventions are associated with improvements in body size metrics36. Retrospective studies suggest that physical activity favorably impacts bladder cancer risk and survival outcomes37. To date, no prospective trials have evaluated the role of exercise or diet on bladder cancer survival outcomes, however pre-operative (pre-habilitation) exercise and dietary interventions have shown some promise in improving functional outcomes after surgery38. Insights into how body composition change with exercise/diet impact survival outcomes can be gleaned from research conducted in other solid malignancies.
Exercise and body size change: lessons learned from other cancers
Under the influence of exercise, muscle secretes myokines (e.g. myostatin, interleukin-6) which have been shown to have anti-inflammatory and immunoprotective effects39. Resistance training alone or in combination with aerobic exercise during cancer treatment has favorable effects on muscle mass (i.e. preservation or increase) among breast and prostate cancer survivors on hormone therapy40, and breast cancer survivors undergoing chemotherapy41,42. Additionally, a meta-analysis among 14 studies evaluating resistance training43 in breast and prostate cancer survivors while undergoing active neoadjuvant or adjuvant treatment reported a significant increase of muscle mass (0.85 kg, 95%CI 0.76–0.95) and decrease in body fat percentage (−1.38 kg, 95%CI −1.57−−1.19)43. Notably, significant effects favoring resistance training to promote increases in muscle mass and reductions in fat mass was observed among patients on adjuvant chemotherapy but not neoadjuvant chemotherapy43. Post-treatment, a systematic review of randomized and non-randomized controlled trials suggests resistance training may increase muscle mass in head and neck, breast, prostate, colorectal, lymphoma, and lung cancer survivors44.
Nutrition and body composition change
Numerous trials have evaluated the effects of nutrition/diet interventions on body composition in various cancers, including bladder cancer. For instance, Dawson and colleagues45 randomized prostate cancer patients on androgen deprivation therapy to one of four groups: protein supplementation coupled with resistance training, resistance training alone, protein supplementation alone, or sham exercise (control), for a 12-week intervention. Overall, the groups that participated in resistance training exhibited a significant increase in muscle mass (1.1 kg, 95%CI 0.4,1.8) and decrease in body fat percentage (−0.9%, 95%CI −1.8, −0.01); an added benefit of protein supplementation to facilitate a higher protein diet was not observed45. Madzima and colleagues46 randomized breast cancer survivors to resistance training alone or protein supplementation (aimed to promote a higher protein diet) coupled with resistance training for 12-weeks and found that both groups increased muscle mass and reduced fat mass46. Collectively, evidence supports resistance training during and after cancer treatment to promote preservation or increases in muscle mass. More evidence is needed to determine whether the addition of protein supplementation aimed at promoting a higher protein diet results in a synergistic effect on muscle mass among cancer survivors.
In a NMIBC trial (n=207), intravesical epirubicin alone or with a 12-month treatment with the probiotic Lactobacillus Casei showed an absolute ~15% decreased 3-year recurrence risk in the epirubicin and probiotic arm47. Another study found that broccoli sprout extract may help decrease recurrence risk but awaits validation in larger studies48. In the MIBC setting, Ritch, et al.,49 evaluated the role of perioperative oral nutrition supplementation (Ensure®) or twice daily multivitamin (Member’s Mark®) starting 3–4 weeks prior to radical cystectomy for MIBC and continuing for 4 weeks after surgery with a primary endpoint of 30-day hospital free days and secondary endpoints of hospital length of stay, complications, and readmissions. Notably, patients receiving nutritional supplementation had a stable prevalence of sarcopenia while in the multivitamin group there was a 20% increase in sarcopenia. These results suggest that nutritional supplementation alone in the perioperative setting may be useful to preserve muscle mass. The Southwestern Oncology Group (SWOG) currently has a phase III trial open to evaluate the role of immune-nutrition in the perioperative setting (SWOG1600, NCTN03757949) with a primary endpoint of 30-day post-operative complications based on strong preliminary data50. Pre-habilitation with exercise and nutritional interventions has also shown promise in improving functional outcomes for patients after cystectomy38.
Conclusions and future directions
Existing risk prediction models that estimate risk of recurrence, progression or survival are based predominantly on clinicopathological variables and have limited predictive accuracy for the individual patient1. Integrating additional patient-related factors such as comorbidities to nomograms is an active area of research. Using SEER-medicare data, Williams, et al.51 integrated clinicopathological, sociodemographic, and Charlson Comorbidity Index (CCI) variables to predict 3- and 5-year cancer-specific and all-cause mortality among patients undergoing RC, while Morgan et al. showed that low pre-operative serum albumin (reflection of nutritional status) and CCI significantly predicted 90-day survival among elderly patients undergoing RC52. Body composition features that are significantly associated with clinical outcomes and are independent of traditional clinicopathological variables and comorbidities may improve risk prediction but have yet to be explored.
Table 2 presents a summary of future investigations related to body composition that are needed in bladder cancer. The field should move beyond BMI when assessing the clinical impact of patient body size. Developing exercise and/or nutritional interventions that modify body composition features and actually improve clinical outcomes along the entire disease course are warranted. For example, future studies could examine how pre-habilitation affects peri-operative outcomes, or whether adjuvant interventions decrease the risk of cardiovascular mortality among survivors. Assessing body composition in the clinic and in research studies will be facilitated by the automated body composition programs that are actively being developed53. In conclusion, body composition is emerging as a novel prognostic factor that could be leveraged to potentially improve care and survival of bladder cancer patients.
Table 2.
Bladder cancer research priorities related to body composition
| Identify body composition features (alone and in combination) that are associated with clinical outcomes in NMIBC and MIBC patients |
|
Identify mechanisms underlying the associations between body composition and clinical outcomes
• Characterize local and systemic inflammatory, metabolic and hormonal differences by body size features |
| Characterize relationship between performance status, strength, and muscle and adipose tissue quantity throughout the disease course |
| Describe changes in body composition over the disease course (e.g, baseline preoperative vs. 6- and 12-months post-treatment) |
|
Develop exercise and nutritional interventions to improve bladder cancer outcomes
• Among patients with NMIBC, can these interventions decrease the risk of recurrence and progression? • Among patients with MIBC, can interventions improve perioperative, overall- and cancer-specific survival outcomes |
| Develop nutritional and exercise interventions to improve health-related quality of life and prevent long-term sequalae of bladder cancer treatments such as chronic kidney disease and cardiovascular disease |
Highlights.
BMI is an inadequate marker of a patient’s body composition features such as skeletal muscle and adiposity.
Modifiable host-factors (e.g., skeletal muscle quantity) may have important prognostic implications throughout a patient’s disease course
There is a need for well-designed exercise and nutrition interventions to improve peri-operative and long-term survival outcomes for patients with bladder cancer
Footnotes
Disclosure statement
The authors do not report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kluth LA, Black PC, Bochner BH, et al. : Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur Urol 68:238–53, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez MC, Correia M, Heymsfield SB: A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care 20:314–321, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Psutka SP, Boorjian SA, Moynagh MR, et al. : Mortality after radical cystectomy: impact of obesity versus adiposity after adjusting for skeletal muscle wasting. J Urol 193:1507–13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mourtzakis M, Prado CM, Lieffers JR, et al. : A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Shen W, Punyanitya M, Wang Z, et al. : Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 97:2333–8, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. : Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fearon K, Strasser F, Anker SD, et al. : Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–95, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Baracos VE: Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 8:527–528, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutten IJG, Ubachs J, Kruitwagen R, et al. : Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 8:630–638, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun JW, Zhao LG, Yang Y, et al. : Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One 10:e0119313, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Tian X, Duan X, et al. : Association of body mass index with bladder cancer risk: a dose-response meta-analysis of prospective cohort studies. Oncotarget 8:33990–34000, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Q, Xu X, Wang X, et al. : Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pac J Cancer Prev 14:3117–21, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Teleka S, Haggstrom C, Nagel G, et al. : Risk of bladder cancer by disease severity in relation to metabolic factors and smoking: A prospective pooled cohort study of 800,000 men and women. Int J Cancer 143:3071–3082, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Westhoff E, Witjes JA, Fleshner NE, et al. : Body Mass Index, Diet-Related Factors, and Bladder Cancer Prognosis: A Systematic Review and Meta-Analysis. Bladder Cancer 4:91–112, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferro M, Vartolomei MD, Russo GI, et al. : An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol 37:507–514, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Gierth M, Zeman F, Denzinger S, et al. : Influence of Body Mass Index on Clinical Outcome Parameters, Complication Rate and Survival after Radical Cystectomy: Evidence from a Prospective European Multicentre Study. Urol Int 101:16–24, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Arora K, Hanson KT, Habermann EB, et al. : Early Complications and Mortality following Radical Cystectomy: Associations with Malnutrition and Obesity. Bladder Cancer 4:377–388, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swalarz M, Swalarz G, Juszczak K, et al. : Correlation between malnutrition, body mass index and complications in patients with urinary bladder cancer who underwent radical cystectomy. Adv Clin Exp Med 27:1141–1147, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Dou WC, Shao YX, et al. : The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol 45:747–754, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Rimar KJ, Glaser AP, Kundu S, et al. : Changes in Lean Muscle Mass Associated with Neoadjuvant Platinum-Based Chemotherapy in Patients with Muscle Invasive Bladder Cancer. Bladder Cancer 4:411–418, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyake M, Morizawa Y, Hori S, et al. : Clinical impact of postoperative loss in psoas major muscle and nutrition index after radical cystectomy for patients with urothelial carcinoma of the bladder. BMC Cancer 17:237, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayr R, Gierth M, Zeman F, et al. : Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle 9:505–513, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zargar H, Almassi N, Kovac E, et al. : Change in Psoas Muscle Volume as a Predictor of Outcomes in Patients Treated with Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Bladder Cancer 3:57–63, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyon TD, Frank I, Takahashi N, et al. : Sarcopenia and Response to Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer 17:216–222.e5, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Stangl-Kremser J, D’Andrea D, Vartolomei M, et al. : Prognostic value of nutritional indices and body composition parameters including sarcopenia in patients treated with radiotherapy for urothelial carcinoma of the bladder. Urol Oncol 37:372–379, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Mayr R, Fritsche HM, Zeman F, et al. : Sarcopenia predicts 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J Urol 36:1201–1207, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Saitoh-Maeda Y, Kawahara T, Miyoshi Y, et al. : A low psoas muscle volume correlates with a longer hospitalization after radical cystectomy. BMC Urol 17:87, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albersheim J, Sathianathen NJ, Zabell J, et al. : Skeletal Muscle and Fat Mass Indexes Predict Discharge Disposition after Radical Cystectomy. J Urol 202:1143–1149, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Chang SS, Jacobs B, Wells N, et al. : Increased body mass index predicts increased blood loss during radical cystectomy. J Urol 171:1077–9, 2004 [DOI] [PubMed] [Google Scholar]
- 30.McDonald ML, Liss MA, Nseyo UU, et al. : Weight Loss Following Radical Cystectomy for Bladder Cancer: Characterization and Effect on Survival. Clin Genitourin Cancer 15:86–92, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Quail DF, Dannenberg AJ: The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol 15:139–154, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hariharan N, Ashcraft KA, Svatek RS, et al. : Adipose Tissue-Secreted Factors Alter Bladder Cancer Cell Migration. J Obes 2018:9247864, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bano G, Trevisan C, Carraro S, et al. : Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 96:10–15, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Can B, Kara O, Kizilarslanoglu MC, et al. : Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin Exp Res 29:745–752, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. : Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol 3:e172319, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwingshackl L, Dias S, Hoffmann G: Impact of long-term lifestyle programmes on weight loss and cardiovascular risk factors in overweight/obese participants: a systematic review and network meta-analysis. Syst Rev 3:130, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liss MA, White M, Natarajan L, et al. : Exercise Decreases and Smoking Increases Bladder Cancer Mortality. Clin Genitourin Cancer 15:391–395, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minnella EM, Awasthi R, Bousquet-Dion G, et al. : Multimodal Prehabilitation to Enhance Functional Capacity Following Radical Cystectomy: A Randomized Controlled Trial. Eur Urol Focus, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Duggal NA, Niemiro G, Harridge SDR, et al. : Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol 19:563–572, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Newton RU, Jeffery E, Galvao DA, et al. : Body composition, fatigue and exercise in patients with prostate cancer undergoing androgen-deprivation therapy. BJU Int 122:986–993, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Demark-Wahnefried W, Kenyon AJ, Eberle P, et al. : Preventing sarcopenic obesity among breast cancer patients who receive adjuvant chemotherapy: results of a feasibility study. Clin Exerc Physiol 4:44–49, 2002 [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin ML, Alvarez-Reeves M, Cadmus L, et al. : Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring) 17:1534–41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padilha CS, Marinello PC, Galvão DA, et al. : Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv 11:339–49, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Lonbro S: The effect of progressive resistance training on lean body mass in post-treatment cancer patients - a systematic review. Radiother Oncol 110:71–80, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Dawson JK, Dorff TB, Todd Schroeder E, et al. : Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer 18:368, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madzima TA, Ormsbee MJ, Schleicher EA, et al. : Effects of Resistance Training and Protein Supplementation in Breast Cancer Survivors. Med Sci Sports Exerc 49:1283–92, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Naito S, Koga H, Yamaguchi A, et al. : Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. J Urol 179:485–90, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Parsons JK, Pierce JP, Natarajan L, et al. : A randomized pilot trial of dietary modification for the chemoprevention of noninvasive bladder cancer: the dietary intervention in bladder cancer study. Cancer Prev Res (Phila) 6:971–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritch CR, Cookson MS, Clark PE, et al. : Perioperative Oral Nutrition Supplementation Reduces Prevalence of Sarcopenia following Radical Cystectomy: Results of a Prospective Randomized Controlled Trial. J Urol 201:470–477, 2019 [DOI] [PubMed] [Google Scholar]
- 50.Hamilton-Reeves JM, Stanley A, Bechtel MD, et al. : Perioperative Immunonutrition Modulates Inflammatory Response after Radical Cystectomy: Results of a Pilot Randomized Controlled Clinical Trial. J Urol 200:292–301, 2018 [DOI] [PubMed] [Google Scholar]
- 51.Williams SB, Huo J, Chu Y, et al. : Cancer and All-cause Mortality in Bladder Cancer Patients Undergoing Radical Cystectomy: Development and Validation of a Nomogram for Treatment Decision-making. Urology 110:76–83, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan TM, Keegan KA, Barocas DA, et al. : Predicting the probability of 90-day survival of elderly patients with bladder cancer treated with radical cystectomy. J Urol 186:829–34, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paris MT, Tandon P, Heyland DK, et al. : Automated body composition analysis of clinically acquired computed tomography scans using neural networks. Clin Nutr, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]