Abstract
Objective:
Investigate the association between neoadjuvant treatment strategy and peri-operative complications in patients undergoing proctectomy for non-metastatic rectal cancer.
Summary Background Data:
Neoadjuvant short-course radiation with consolidation chemotherapy (SC-TNT) is an alternative to neoadjuvant chemoradiation (CRT) for rectal cancer. Some have argued that short-course radiation and extended radiation-to-surgery intervals increase operative difficulty and complication risk. However, the association between SC-TNT and surgical complications has not been previously investigated.
Methods:
This single-center retrospective cohort study included patients undergoing total mesorectal excision for non-metastatic rectal cancer after SC-TNT or CRT between 2010 and 2018. Univariate analysis of severe peri-operative morbidity (POM) and multiple secondary outcomes, including overall POM, intra-operative complications, and resection margins, was performed. Logistic regression of severe POM was also performed.
Results:
Of 415 included patients, 156 (38%) received SC-TNT and 259 (62%) received CRT. The cohorts were largely similar, though patients with higher tumors (69.9% vs. 47.5%, p<0.0001) or node-positive disease (76.9% vs. 62.6%, p=0.004) were more likely to receive SC-TNT. We found no difference in incidence of severe POM (9.6% SC-TNT vs. 12.0% CRT, p=0.46) or overall POM (39.7% SC-TNT vs. 37.5% CRT, p=0.64) between cohorts. Neoadjuvant regimen was also not associated with a difference in severe POM (OR 0.42, 95% CI 0.04 – 4.70, p=0.48) in multivariate analysis. There was no significant association between neoadjuvant regimen and any secondary outcome.
Conclusion:
In rectal cancer patients treated with SC-TNT and proctectomy, we found no significant association with POM compared to patients undergoing CRT. SC-TNT does not significantly increase the risk of POM compared to CRT.
MINI ABSTRACT
This study aimed to compare peri-operative morbidity in patients undergoing proctectomy following different neoadjuvant therapies. There was no difference in severe peri-operative complications between short-course radiation with consolidation chemotherapy (SC-TNT) recipients and those treated with long-course chemoradiation. We conclude that SC-TNT does not exacerbate the risk of peri-operative complications.
INTRODUCTION
Neoadjuvant radiation therapy in the treatment of rectal cancer has been shown to decrease the rate of local recurrence1–6. Previous investigation has shown short-course radiation therapy (SCRT) given in five fractions is equally as effective in achieving local control as conventional chemoradiation (CRT) with similar disease-free and overall survival rates7–10. While initial work demonstrated the benefits of neoadjuvant radiation and total mesorectal excision (TME), recent investigation suggests chemotherapy in the neoadjuvant setting allows for improved treatment completion and earlier initiation of systemic therapy11,12. Furthermore, combined neoadjuvant regimens – termed total neoadjuvant therapy (TNT) – have the additional advantage of facilitating nonoperative management of certain cancers13–23. At our institution, standard therapy includes short-course radiation (five fractions of 5 Gy) and consolidation chemotherapy prior to TME (SC-TNT).
We have demonstrated previously that SC-TNT provides similar disease control, tumor response rates, and treatment toxicities as CRT13. However, the impact of TNT regimens on peri-operative complication rates is unclear. Chemotherapy is immunosuppressive and can lead to malnutrition. Delivering systemic chemotherapy neoadjuvantly also increases the radiation-to-surgery interval, which may increase the extent of pelvic fibrosis, surgical difficulty, and post-operative morbidity15,24. Additionally, some studies suggest that SCRT may increase post-operative complications4,10,25. To date, the association between SC-TNT and peri-operative morbidity has not been investigated.
The objective of this study was to investigate the association between peri-operative complications and two neoadjuvant treatment strategies: SC-TNT and CRT. We hypothesized that either strategy would yield a similar incidence of peri-operative complications.
METHODS
Patient Selection
We performed a retrospective review of patients undergoing TME for rectal cancer at our institution from January 2010 to September 2018. The data were retrieved from our institutional rectal cancer database, which includes clinical and outcome data for each rectal cancer patient treated at our institution. Eligible patient records were linked to our institutional American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database for determination of 30-day peri-operative outcomes. For patients that were not captured by ACS-NSQIP, an extensive review of the medical record was performed by a single physician to identify all 30-day peri-operative complications in accordance with ACS-NSQIP guidelines. We included all adult, non-metastatic, rectal cancer patients who underwent either CRT or SC-TNT prior to TME. Procedures included low anterior resection (LAR), abdominoperineal resection (APR), and multi-organ resection (MOR). Patients who underwent local excisions were excluded. Patients were assigned to study cohorts based on the neoadjuvant therapy received.
The SC-TNT cohort were treated with 25–35 Gy of pelvic radiation fractionated over five days, followed sequentially by an intent of two to six months of platinum-based chemotherapy. The duration of neoadjuvant chemotherapy these patients received increased during the study period as our preferred regimen evolved. Those treated early in the study were intended to receive 2 months of chemotherapy, but those in the second half of the study were intended to receive 4–6 months as we tried to maximize each patient’s chances of being managed with a watch-and-wait, non-operative protocol. Patients in the CRT cohort were treated with 45–50.4 Gy of pelvic radiation fractionated over 25–28 days in conjunction with radiosensitizing single-agent chemotherapy (5-fluorouracil or capecitabine). SC-TNT was the neoadjuvant treatment of choice for all those treated at our center regardless of stage, tumor height, or medical comorbidity. However, patients who desired to receive radiation therapy at a referring institution were treated with CRT before presenting to our center for operative resection. Those treated at our center early in the study period were also more likely to receive CRT than those later in the study as our SC-TNT algorithm continued to mature. Multiagent, platinum-based chemotherapy was given in the adjuvant setting for either cohort at the discretion of the treating medical oncologist.
Baseline demographics, clinical staging, neoadjuvant treatment, final pathology, and operative details were obtained from our institutional database. Length of stay (LOS), operative time, and 30-day peri-operative complications were retrieved from the ACS-NSQIP database. All complications were assigned a Clavien-Dindo26 severity grade through direct medical record review.
Outcomes
The primary endpoint of the study was the incidence of severe peri-operative morbidity, defined as any complication of Clavien-Dindo severity grade 3a or higher. Secondary endpoints were overall POM, intra-operative complications, operative time, estimated blood loss (EBL), TME completeness, distal resection margin status, radial margin status, reoperation, LOS, and mortality. Complications collected by ACS-NSQIP included surgical site infections (superficial, deep, and organ-space), cardiopulmonary complications, sepsis, venous thromboembolism, transfusion, renal complications, Clostridium difficile infection, urinary tract infection, reoperation, and mortality. Intra-operative complications were those reported by the surgeon in the operative note. EBL was collected from the anesthesia record. All pathological variables were reported in accordance to the College of American Pathologists protocol for primary carcinoma of the colon and rectum27.
Statistical Analysis
Demographics, baseline characteristics, operative time, LOS, and mesorectal resection quality were compared. The rates of individual complications based on ACS-NSQIP definitions and Clavien-Dindo classification were compared. The occurrence of complications was monitored for 30 post-operative days for all variables except transfusion, which includes intra-operative and post-operative transfusions up to 72 hours from the procedure start time. Of note, superficial and deep surgical site infections were considered collectively as “wound infections.” SIRS and sepsis were also considered collectively as “SIRS/Sepsis.” Severe POM, overall POM, and intra-operative complications were reported as categorical variables based on the occurrence of any complication versus no complications. TME completeness was reported as “complete” or “incomplete,” with “near complete” considered as incomplete. Cohorts were compared using χ2 and t-test univariate analysis, as appropriate, followed by multivariable logistic regression modeling the risk of severe POM. Stratified analysis was performed of patients undergoing LAR, APR, and MOR to evaluate operative time, reoperation rate, LOS, mortality, TME resection quality, and POM for each operation. Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA). The study was approved by the Washington University Institutional Review Board (IRB# 201802053).
RESULTS
Patient Demographics and Baseline Characteristics
In total, 415 patients met inclusion criteria: 156 (38%) were treated with SC-TNT and 259 (62%) received CRT. Demographics and baseline characteristics were largely similar between cohorts (Table 1) with the exception of a greater prevalence of hypertension in the CRT group. The SC-TNT cohort was more likely to have private insurance than the CRT cohort (66.7% vs. 49.4%, p<0.01*). There were no differences between groups in regard to age, gender, race, BMI, ASA class, or other medical comorbidity.
TABLE 1.
Perioperative Outcomes
| Outcome | SC-TNT n = 156 | CRT n = 259 | P-value |
|---|---|---|---|
| Complications | |||
| Any complication (%) | 62 (39.7) | 97 (37.5) | 0.64 |
| Severe Complication (%) | 15 (9.6) | 31 (12.0) | 0.46 |
| Clavien-Dindo Class (%) | |||
| I | 9 (5.8) | 19 (7.3) | 0.55 |
| II | 36(23.1) | 43(16.6) | |
| IIIa | 6 (3.9) | 14 (5.4) | |
| IIIb | 4 (2.6) | 7 (2.7) | |
| IVa | 3(1.9) | 9 (3.5) | |
| IVb | 2(1.3) | 1 (0.4) | |
| V | 0(0) | 0(0) | |
| Operative time (mean minutes ± SD) | 213 ± 65 | 237 ± 93 | 0.01* |
| Intraoperative complication (%) | 7 (4.5) | 13 (5.0) | 0.81 |
| EBL (mean mL ± SD) | 342 ± 332 | 410 ± 585 | 0.17 |
| Reoperation (%) | 5 (3.2) | 13 (5.0) | 0.38 |
| 30-d mortality (%) | 0(0) | 0(0) | |
| Length of stay (mean days ± SD) | 6.5 ± 4.0 | 7.0 ± 6.3 | 0.27 |
| Quality of resection | |||
| TME complete (%) | 148 (94.9) | 242 (93.8) | 0.65 |
| Negative radial margin (%) | 153 (98.7) | 247 (96.5) | 0.18 |
| Negative distal margin (%) | 156 (100) | 253 (97.7) | 0.06 |
Denotes significante.
EBL indicates estimated blood loss: SD, standard deviation; TME, total mesorectal excision.
There were significant differences in clinical stage, tumor height, radiation-to-surgery interval, and procedure type between the two groups. Stage III disease was more prevalent in the SC-TNT group (76.9% vs. 62.6%, p=0.004). However, the CRT cohort included more low tumors (52.5% vs. 30.1%, p<0.0001) and non-sphincter sparing procedures (44.4% vs. 26.9%, p=0.001). In patients treated with SC-TNT, the median duration of neoadjuvant multiagent chemotherapy was 3 months (IQR 2 – 4 months). There were no differences in clinical T stage or operative approach between groups.
Overall Cohort Analysis
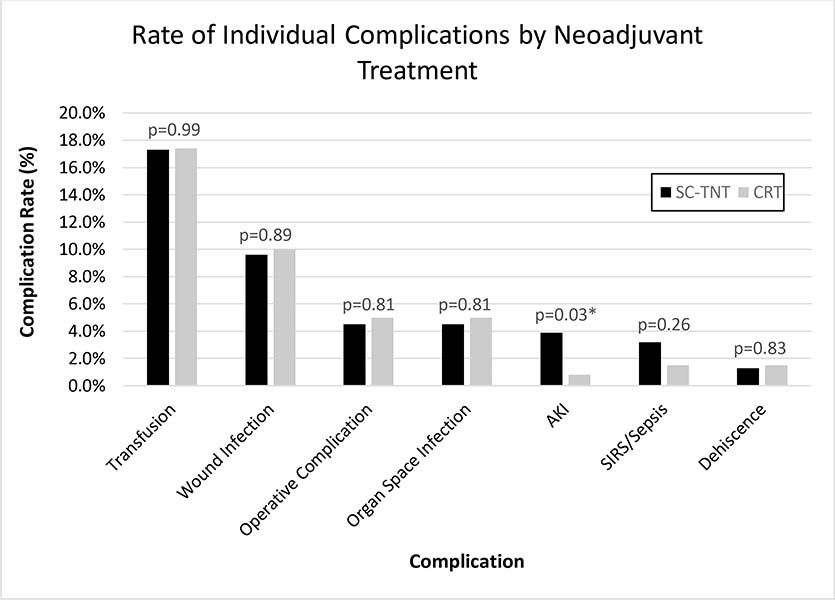
The rate of severe POM was 9.6% in the SC-TNT group and 12.0% in the CRT group (p=0.46) (Table 2). The rate of any peri-operative complication was 39.7% in the SC-TNT group and 37.5% in the CRT group (p=0.64). The most common peri-operative complications were transfusion and wound infections (Figure 1). The rate of each individual complication was similar between groups, except for the rate of acute kidney injury (AKI), which was higher in patients receiving SC-TNT (3.9% vs. 0.8%, p=0.03). There were no differences in the rates of intra-operative complications, reoperation, LOS, EBL, or mortality between the two groups. The mean operative time for the SC-TNT group was shorter than that of the CRT group (213.4 ± 65.2 minutes vs. 236.8 ± 93.3, p=0.01). In regard to resection quality, TME was complete in a similar proportion of patients between groups (SC-TNT = 94.9% vs. CRT = 93.8%, p=0.65). There was also no difference in the rates of obtaining negative distal and radial margins between the two groups.
Table 2:
Peri-Operative Outcomes
| Outcome | SC-TNT n = 156 |
CRT n= 259 |
p-value |
|---|---|---|---|
|
| |||
| Complications | |||
|
| |||
| Any complication (%) | 62 (39.7) | 97 (37.5) | 0.64 |
| Severe Complication (%) | 15 (9.6) | 31 (12.0) | 0.46 |
| Clavien-Dindo Class (%) | |||
| I | 9 (5.8) | 19 (7.3) | 0.55 |
| II | 36 (23.1) | 43 (16.6) | |
| IIIa | 6 (3.9) | 14 (5.4) | |
| IIIb | 4 (2.6) | 7 (2.7) | |
| IVa | 3 (1.9) | 9 (3.5) | |
| IVb | 2 (1.3) | 1 (0.4) | |
| V | 0 (0) | 0 (0) | |
| Operative Time (mean minutes ± SD) | 213 ± 65 | 237 ± 93 | 0.01* |
| Intra-Operative Complication (%) | 7 (4.5) | 13 (5.0) | 0.81 |
| EBL (mean mL ± SD) | 342 ± 332 | 410 ± 585 | 0.17 |
| Reoperation (%) | 5 (3.2) | 13 (5.0) | 0.38 |
| 30-day Mortality (%) | 0 (0) | 0 (0) | |
| Length of Stay (mean days ± SD) | 6.5 ± 4.0 | 7.0 ± 6.3 | 0.27 |
|
| |||
| Quality of Resection | |||
|
| |||
| TME Complete (%) | 148 (94.9) | 242 (93.8) | 0.65 |
| Negative Radial Margin (%) | 153 (98.7) | 247 (96.5) | 0.18 |
| Negative Distal Margin (%) | 156 (100) | 253 (97.7) | 0.06 |
Legend: denotes significance; SD=Standard Deviation; EBL=Estimated Blood Loss; TME=Total Mesorectal Excision
Figure 1.
Rate of Individual Complications by Neoadjuvant Treatment
The odds of suffering a severe POM did not significantly differ between cohorts after adjusting for multiple clinical factors (Table 3) (OR 0.42, 95% CI 0.04 – 4.48, p=0.48). The covariates most associated with the occurrence of a severe POM were ASA class >2 (OR 3.02, 95% CI 1.42 – 6.45, p<0.01), APR (OR 3.77, 95% CI 1.49 – 9.52, p<0.01), and MOR (OR 7.10, 95% CI 2.12 – 23.76, p=0.001). Age, gender, obesity, insurance coverage, operative approach, node positive disease, duration of neoadjuvant chemotherapy, and tumor height were among variables that were not associated with severe POM (Table 3).
Table 3:
Multivariable Logistic Regression Model of Odds of Severe Complication
| Odds Ratio (95% CI) n = 415 |
p-value | |
|---|---|---|
|
| ||
| Neoadjuvant Therapy | ||
|
| ||
| CRT | REF | |
| SC-TNT | 0.42 (0.04 – 4.48) | 0.48 |
|
| ||
| Age | ||
|
| ||
| 18–49 | REF | |
| 50–65 | 0.49 (0.20 – 1.19) | 0.12 |
| >65 | 0.48 (0.17 – 1.40) | 0.18 |
|
| ||
| Gender | ||
|
| ||
| Female | REF | |
| Male | 1.44 (0.69 – 2.99) | 0.33 |
|
| ||
| Comorbidities | ||
|
| ||
| BMI≥30 | 0.90 (0.44 – 1.87) | 0.78 |
| ASA>2 | 3.02 (1.42 – 6.45) | <0.01* |
|
| ||
| Insurance Coverage | ||
|
| ||
| Private | REF | |
| Government | 2.07 (0.93 – 4.59) | 0.07 |
| Uninsured | 1.12 (0.11 – 11.2) | 0.92 |
|
| ||
| Clinical Nodal Stage | ||
|
| ||
| Node Negative | REF | |
| Node Positive | 1.09 (0.52 – 2.29) | 0.81 |
|
| ||
| Duration of Neoadjuvant Chemotherapy | ||
|
| ||
| 0 months | REF | |
| 1 – 3 months | 2.82 (0.25 – 31.30) | 0.40 |
| 4 – 6 months | 1.96 (0.13 – 30.25) | 0.63 |
|
| ||
| Approach | ||
|
| ||
| Minimally Invasive | REF | |
| Open | 1.02 (0.49 – 2.09) | 0.96 |
|
| ||
| Tumor height (cm) | ||
|
| ||
| 11 – 15 | REF | |
| 6 – 10 | 0.70 (0.22 – 2.24) | 0.55 |
| 0 – 5 | 0.54 (0.16 – 1.84) | 0.32 |
|
| ||
| Procedure | ||
|
| ||
| LAR | REF | |
| APR | 3.77 (1.49 – 9.52) | <0.01* |
| MOR | 7.10 (2.12 – 23.76) | 0.001* |
Legend: denotes significance; LAR=Low Anterior Resection; APR=Abdominoperineal Resection; MOR=Multiorgan Resection
Stratified Analysis
Stratified analysis by procedure type also showed no difference in the rate of severe POM by neoadjuvant regimen in those undergoing LAR (SC-TNT = 7.0% vs. CRT = 5.6%, p=0.63), APR (15.6% vs. 17.0%, p=0.85), or MOR (20.0% vs. 33.3%, p=0.44) (Table 4). There were also no differences in the rates of any POM, intra-operative complications, operative time, EBL, reoperation, LOS, or mortality. There were no differences in the rates of TME completeness or negative radial margin. For patients treated with LAR, a negative distal margin was more likely to be obtained in the SC-TNT group (100% vs. 96.5%, p=0.04). Patients treated with SC-TNT and subsequent LAR were less likely to have low tumors than those treated with CRT and LAR (9.7% vs. 27.1%, p<0.0001).
Table 4:
Outcomes Stratified by Procedure Type
| LAR | APR | MOR | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Outcome | SC-TNT n = 114 |
CRT n=144 |
p-value | SC-TNT n = 32 |
CRT n = 94 |
p-value | SC-TNT n = 10 |
CRT n = 21 |
p-value |
|
| |||||||||
|
Perioperative
Outcomes
| |||||||||
| Any Complication (%) | 37 (32.5) | 45 (31.3) | 0.84 | 17 (53.1) | 36 (38.3) | 0.14 | 8 (80.0) | 16 (76.2) | 0.81 |
| Severe Complication (%) | 8 (7.0) | 8 (5.6) | 0.63 | 5 (15.6) | 16 (17.0) | 0.85 | 2 (20.0) | 7 (33.3) | 0.44 |
| Reoperation (%) | 3 (2.6) | 4 (2.8) | 0.94 | 1 (3.1) | 5 (5.3) | 0.61 | 1 (10.0) | 4 (20.0) | 0.49 |
| Operative Time (mean min.±SD) | 205±60 | 212±61 | 0.32 | 229±60 | 247±96 | 0.33 | 262±108 | 359±146 | 0.07 |
| Intra-Operative Complication (%) | 5 (4.4) | 7 (4.9) | 0.86 | 2 (6.3) | 5 (5.3) | 0.84 | 0 (0) | 1 (4.8) | 0.48 |
| EBL (mean mL±SD) | 304±343 | 282±217 | 0.61 | 356±193 | 407±623 | 0.49 | 689±5 | 1,154± 1,102 |
0.13 |
| Tumor Height | |||||||||
| 0 – 5 cm | 11 (9.7) | 39 (27.1) | <0.001* | 29 (90.6) | 84 (89.4) | 0.70 | 7 (70.0) | 13 (61.9) | 0.21 |
| 6 – 10 cm | 91 (79.8) | 76 (52.8) | 3 (9.4) | 8 (8.5) | 1 (10.0) | 7 (33.3) | |||
| 11 – 15 cm | 12 (10.5) | 29 (20.1) | 0 (0) | 2 (2.1) | 2 (20.0) | 1 (4.8) | |||
| Diverting Ostomy | 111 (99.1) | 142 (98.6) | 0.91 | 4 (100.0) | 7 (100.0) | 1.00 | |||
| 30-d Mortality (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Length of Stay (mean days±SD) | 6.3±3.8 | 6.1±4.5 | 0.74 | 6.4±3.4 | 7.1±5.3 | 0.43 | 8.8±7.3 | 13.2±14.1 | 0.26 |
|
Quality of Resection | |||||||||
| TME Complete (%) | 110 (96.5) | 137 (95.1) | 0.59 | 29 (90.6) | 88 (93.6) | 0.57 | 9 (90) | 17 (85) | 0.70 |
| Negative Radial Margin (%) | 113 (99.1) | 141 (98.6) | 0.70 | 32 (100) | 89 (94.7) | 0.18 | 8 (88.9) | 17 (89.5) | 0.96 |
| Negative Distal Margin (%) | 114 (100) | 139 (96.5) | 0.04* | 32 (100) | 93 (98.9) | 0.56 | 10 (100) | 21 (100) | 1.00 |
Legend: denotes significance; min=minutes; SD=Standard Deviation; LAR=Low Anterior Resection; APR=Abdominoperineal Resection; MOR=Multiorgan Resection; EBL=Estimated Blood Loss; TME=Total Mesorectal Excision. Reported rates of diverting ostomy in the MOR group correspond to the proportion of sphincter-sparing procedures
DISCUSSION
In the treatment of rectal cancer requiring radiation and proctectomy, the rate of peri-operative morbidity was similar in patients undergoing total neoadjuvant therapy with short-course radiation and those undergoing chemoradiation. In addition, there were no differences in the risk of intra-operative complications, operative time, reoperation, length of stay, or resection quality. It should be noted that there was an increased rate of post-operative AKI in those treated with SC-TNT. Despite the similar rates of AKI or dialysis dependence pre-operatively, as shown in Table 1, it is possible that neoadjuvant chemotherapy leaves these patients in a subclinical dehydrated state and more sensitive to intra- and post-operative fluid shifts. Fortunately, none of these patients went on to require temporary or permanent renal replacement therapy. As the pathophysiology remains unclear, this is an outcome that we will carefully follow prospectively.
Opponents of SCRT and SC-TNT have pointed to the possibility of increased post-operative complications with the short-course regimen. While any neoadjuvant radiation has been shown to increase morbidity after TME compared to TME alone1,4,25, this risk is acceptable given the improved local control and survival. When comparing radiation regimens, a subset analysis within the Stockholm III trial showed an increased risk of morbidity after SCRT when compared to CRT10. It should be noted, however, that this subset of patients was those treated with SCRT followed by immediate TME with no delay. The same trial found there was no difference in morbidity between the two regimens when the SCRT group had a 4–8-week delay between radiation and surgery. Given the delay between SCRT and TME for neoadjuvant chemotherapy in the SC-TNT regimen, immediate surgery is avoided.
Similarly, a radiation-to-surgery interval that is too long has also been called into question, with evidence suggesting increased pelvic fibrosis, surgical difficulty, and morbidity15,24. The GRECCAR-6 trial evaluated post-operative morbidity as a secondary outcome in patients undergoing chemoradiation followed by a radiation-to-surgery interval of seven or eleven weeks24. An increased rate of morbidity was reported in the eleven-week interval group, but half of these complications (in both groups) were urinary complications that are unlikely to be related to the interval difference or the procedure itself. The authors also suggested an increased technical difficulty of the procedure at eleven weeks based on higher conversion rates, surgeon-reported pelvic fibrosis, and operative time in that group; however, none of these differences were statistically significant. Similarly, the Timing of Rectal Cancer Response to Chemoradiation Consortium15 reported increased pelvic fibrosis in patients receiving CRT followed by a delay of eight to sixteen weeks for consolidation chemotherapy compared to an interval of six weeks in the group that did not receive neoadjuvant chemotherapy. Pelvic fibrosis was reported by the surgeon post-operatively, and despite the perceived increased fibrosis, surgeons also reported no difference in the technical difficulty of the procedure.
Our rate of overall POM is similar to previously published rates for CRT and SCRT which have ranged from 25–57%1,10,14–15,21–22,24. Our reported morbidity rates are reliable given the standardized and validated ACS-NSQIP definitions. Further delineating the rate of severe complications based on the Clavien-Dindo classification better identifies the proportion of patients with clinically impactful deviations from the expected post-operative course. Use of this classification will facilitate comparison to rates published in future studies. Our results are further strengthened by the long enrollment period and the comprehensive physician review performed.
Our stratified analysis accounting for procedure exemplifies the relative homogeneity between the two treatment regimens. As expected, an APR is more morbid than a LAR, and a pelvic exenteration is more morbid than either. However, when comparing SC-TNT and CRT for each procedure, there were no differences in any primary or secondary outcomes. Neither the GRECCAR-6 trial nor the Timing trial mentioned above stratified patients by procedure type, making variables such as conversion rates and operative times less reliable and suggestions of their relation to technical difficulty questionable15,24.
As with all TNT regimens, SC-TNT allows for earlier treatment of micro-metastatic disease, avoids delays in the initiation of adjuvant therapy due to surgical complications, and can increase compliance with intended systemic treatment11,12. Multiple studies, including a large meta-analysis, have shown that delays in the initiation of adjuvant chemotherapy can result in decreased disease-free and overall survival12,28. Furthermore, TNT regimens have a higher likelihood of tumor downstaging and obtaining a pathologic complete response14,15,29, which lends itself to non-operative management of rectal cancer for select patients who obtain a complete clinical response. In regard to SC-TNT specifically, it can be more convenient for patients and less costly than CRT.
Our study is limited by the number of patients in the study population, but this is the largest series of patients undergoing SC-TNT in the US. Future studies of multi-center outcomes are needed to improve generalizability to various populations. In addition, although SC-TNT is our preferred neoadjuvant regimen, many patients were treated with CRT at referring centers, and without randomization there is some degree of selection bias in the choice of neoadjuvant regimen. While the majority of morbidity data was compiled by trained data extractors according to validated ACS-NSQIP definitions, some records had to undergo physician-directed review. Still, the review was performed using the same definitions. The retrospective nature of this study is another limitation, but the results of the large, prospective RAPIDO trial should bring stronger evidence to answer a number of questions related to these neoadjuvant regimens30.
Our institution’s unique experience with SC-TNT has shown it has a similar morbidity profile as traditional CRT. When considering the advantages of a TNT model, it may be the preferred neoadjuvant regimen.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the Washington University School of Medicine Surgical Oncology Basic Science and Translational Research Training Program grant T32CA009621 from the National Cancer Institute (NCI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICTS OF INTEREST AND SOURCE OF FUNDING:
No conflicts of interest declared. Research reported in this publication was supported by the Washington University School of Medicine Surgical Oncology Basic Science and Translational Research Training Program grant T32CA009621 from the National Cancer Institute (NCI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Cammà C, Giunta M, Fiorica F, et al. Preoperative Radiotherapy for Resectable Rectal Cancer: A Meta-analysis. JAMA. 2000;284:1008–1015. [DOI] [PubMed] [Google Scholar]
- 2.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8507 patients from 22 randomised trials. Lancet. 2001;358:1291–1304. [DOI] [PubMed] [Google Scholar]
- 3.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. [DOI] [PubMed] [Google Scholar]
- 4.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 5.Van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–582. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 7.Roy A, Mahasittiwat P, Weiner AA, et al. Preoperative short-course radiation therapy for rectal cancer provides excellent disease control and toxicity: Results from a single US institution. Pract Radiat Oncol. 2017;7:e51–e58. [DOI] [PubMed] [Google Scholar]
- 8.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23. [DOI] [PubMed] [Google Scholar]
- 9.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized Trial of Short-Course Radiotherapy Versus Long-Course Chemoradiation Comparing Rates of Local Recurrence in Patients With T3 Rectal Cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol. 2012;30:3827–3833. [DOI] [PubMed] [Google Scholar]
- 10.Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–346. [DOI] [PubMed] [Google Scholar]
- 11.Marco MR, Zhou L, Patil S, et al. Consolidation mFOLFOX6 Chemotherapy After Chemoradiotherapy Improves Survival in Patients With Locally Advanced Rectal Cancer: Final Results of a Multicenter Phase II Trial. Dis Colon Rectum. 2018;61:1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tevis SE, Kohlnhofer BM, Stringfield S, et al. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum. 2013;56:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myerson RJ, Tan B, Hunt S, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys. 2014;88:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Aguilar J, Marcet J, Coutsoftides T, et al. Impact of neoadjuvant chemotherapy following chemoradiation on tumor response, adverse events, and surgical complications in patients with advanced rectal cancer treated with TME. J Clin Oncol. 2011;15:3514–3514. [Google Scholar]
- 15.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4:e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26:1722–1728. [DOI] [PubMed] [Google Scholar]
- 19.Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–248. [DOI] [PubMed] [Google Scholar]
- 20.Marechal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–1530. [DOI] [PubMed] [Google Scholar]
- 21.Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–842. [DOI] [PubMed] [Google Scholar]
- 22.Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300–3307. [DOI] [PubMed] [Google Scholar]
- 23.Chapman W Jr., Roxburgh C, Makhdoom B, et al. Rectal Cancer Downstaging is Significantly Improved with Different Regimens of Total Neoadjuvant Therapy. Int J Radiation Oncol Biol Phys. 2018;102:S65–S66. [Google Scholar]
- 24.Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol. 2016;34:3773–3780. [DOI] [PubMed] [Google Scholar]
- 25.Marignen CA, Kapiteijn E, van de Velde CJ, et al. Acute Side Effects and Complications After Short-Term Preoperative Radiotherapy Combined with Total Mesorectal Excision in Primary Rectal Cancer: Report of a Multicenter Randomized Trial. J Clin Oncol. 2002;20:817–825. [DOI] [PubMed] [Google Scholar]
- 26.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakar S, Shi C, Berho ME, et al. Protocol for the Examination of Specimens From patients With Primary Carcinoma of the Colon and Rectum. Coll Am Pathol. 2017;1–28. [Google Scholar]
- 28.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. [DOI] [PubMed] [Google Scholar]
- 29.Markovina S, Youssef F, Roy A, et al. Improved metastasis- and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: results of a matched pair analysis. Int J Radiat Oncol Biol Phys. 2017;99:417–426. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer. 2013;13:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.