Abstract
The pulmonary vascular endothelialitis together with the high rate of distal pulmonary embolism or thrombosis extensively reported in critically ill coronavirus disease 2019 patients may impair antibiotic diffusion in the lung parenchyma of coronavirus disease 2019 patients with ventilator-associated pneumonia leading to insufficient antibiotic concentration, thus promoting lung abscess formation. We report that 17 of 119 coronavirus disease 2019 patients (14%) with ventilator-associated pneumonia developed a lung abscess. Proportion of patients receiving corticosteroids did not differ between patients with and without lung abscess. Most of lung abscess were polymicrobial. Enterobacteriaceae, Pseudomonas aeruginosa, and Staphylococcus aureus were the leading causative bacteria. Most of lung abscesses involved the right lower lobe. Three patients had concomitant pulmonary embolism or thrombosis in the territory of lung abscess. Lung abscess was retrospectively visible on chest radiograph in 29% of the patients. As the occurrence of lung abscess impacts the duration of antibiotics therapy, chest CT scan should be easily performed in case of treatment failure of ventilator-associated pneumonia despite adequate antimicrobial therapy.
Keywords: acute respiratory distress syndrome, antibiotics therapy, coronavirus disease 2019, intensive care unit, lung abscess, ventilator-associated pneumonia
To the Editor:
More than half of coronavirus disease 2019 (COVID-19) patients with acute respiratory distress syndrome (ARDS) develop ventilator-associated pneumonia (VAP), with an increased risk as compared to ARDS from others causes (1–4). Our team noticed that several of our critically ill COVID-19 patients with a VAP subsequently developed a lung abscess during their ICU stay. Indeed, the pulmonary vascular endothelialitis (5) together with the high rate of distal pulmonary embolism or thrombosis (6) extensively reported in critically ill COVID-19 patients may impair antibiotic diffusion into the lung parenchyma leading to insufficient antibiotic concentration, thus promoting lung abscess formation (7). Furthermore, lung abscess may also result from the superinfection of a pulmonary cavitation which is a well-known complication of pulmonary infarction following pulmonary embolism or thrombosis (8). To the best of our knowledge, there is no study focusing on lung abscess among critically ill COVID-19 patient with VAP.
We therefore aimed to (1) report the proportion of our COVID-19 patients with a VAP developing a lung abscess as well as to (2) describe their clinical, radiological, and microbiological characteristics.
METHODS AND PATIENTS
All consecutive adults (≥ 18 yr old) COVID-19 patients (reverse transcriptase-polymerase chain reaction positive for severe acute respiratory syndrome coronavirus 2 on a nasopharyngeal swab) admitted to our ICU for acute respiratory failure between March 6, 2020 and April 4, 2021 and developing a VAP (first episode or recurrence) were included. Patients who did not require invasive mechanical ventilation, those without a confirmed VAP, transferred to another ICU or still hospitalized in the ICU were not included.
VAP was defined as a new onset of fever (temperature ≥ 38.3°C) or hypothermia (temperature ≤ 35°C) and/or leukocytosis (total peripheral WBC count ≥ 10,000 cells/μL)/leucopenia (total WBC count ≤ 4,500 cells/μL) or more than 15% of immature neutrophils and/or new onset of suctioned purulent respiratory secretions and/or need for ventilatory support system changes to enhance oxygenation together with a new chest radiograph infiltrate occurring more than 48 hours after initiation of invasive mechanical ventilation (9). VAPs were microbiologically documented using (blind or bronchoscopic) protected distal bronchial specimen (≥ 103 Colony-Forming Unit/mL), tracheal aspirate (≥105 Colony-Forming Unit/mL), or bronchoalveolar lavage (≥ 104 Colony-Forming Unit/mL) (9).
We retrospectively reviewed (V.B., D.C.) chest CT scans performed in COVID-19 patients with VAP in order to screen for the presence of a lung abscess. Chest CT scans were performed at the discretion of the treating clinician (all the patients with VAP did not undergo a chest CT scan). A lung abscess was defined as a lung cavitation with an air-fluid level and a thick wall on chest CT scan.
Continuous variables are reported as median (interquartile range) and compared between groups using the Student t test (data were normally distributed). Categorical variables are reported as numbers and percentages and compared using chi-square test. A p value of less than 0.05 was considered significant.
This monocenter retrospective noninterventional data-based research, using the care data collected during patients hospital stay, was conducted in accordance with the amended Declaration of Helsinki and was approved by institutional review board of the Société de Réanimation de Langue Française (SRLF 2021-54). There is no processing of indirectly identifiable data. Patients and proxies were informed, and written consent was waived.
RESULTS
During the study period, 161 COVID-19 patients required invasive mechanical ventilation, of which 119 (73%) developed a VAP. Among the 119 patients with a VAP, 68 (57%) underwent a chest CT scan which depicted a lung abscess in 17 of them (25%) (illustrative examples of lung abscesses are available in the Fig. 1) accounting for 14% (95% CI 9–22%) of the whole patients with VAP.
Figure 1.
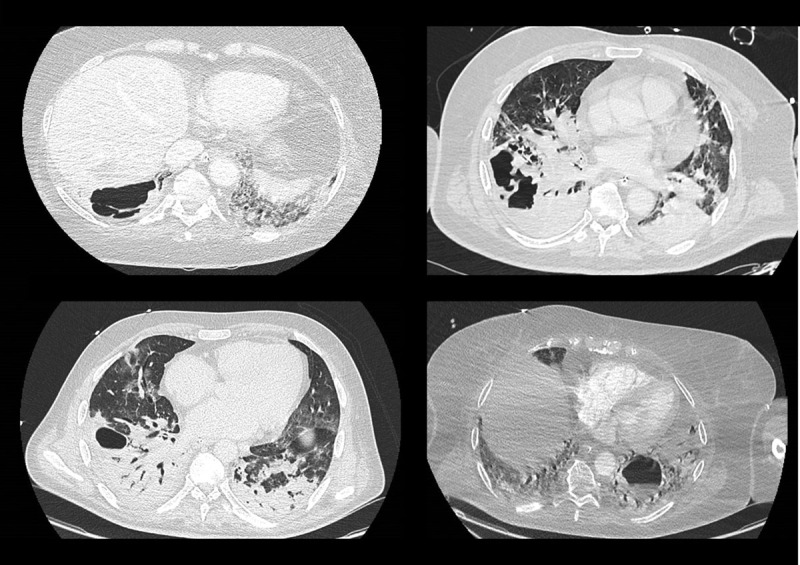
Illustrative examples of critically ill coronavirus disease 2019 patients with ventilator-associated pneumonia developing a lung abscess.
The main characteristics of the patients with a lung abscess and comparison between the patients with and without a lung abscess are detailed in the Table 1. The proportion of patients receiving corticosteroids did not differ between patients with and without a lung abscess. ICU mortality did not significantly differ between patients with and without a lung abscess (Table 1).
TABLE 1.
Comparison Between Coronavirus Disease 2019 Patients With Ventilator-Associated Pneumonia Developing (n = 17) or Not (n = 102) a Lung Abscess During ICU Stay
| Variables | Missing Data | All Patients With VAP,N = 119 | VAP Without Lung Abscess, N = 102 | VAP With Lung Abscess, N = 17 | p |
|---|---|---|---|---|---|
| Patient’s characteristics and ICU scores | |||||
| Male sex, n (%) | 0 | 91 (77) | 78 (77) | 13 (77) | 1.00 |
| Age, yr, median (interquartile range) | 0 | 65 (57–70) | 65 (57–70) | 66 (58–70) | 0.95 |
| Body mass index (kg/m2), median (interquartile range) | 0 | 31 (27–35) | 32 (26–35) | 30 (28–33) | 0.82 |
| Sequential Organ Failure Assessment, median (interquartile range) | 0 | 4 (3–7) | 4 (3–7) | 4 (3–5) | 0.50 |
| Simplified Acute Physiology Score II, median (interquartile range) | 0 | 33 (26–39) | 33 (26–40) | 32 (26–36) | 0.45 |
| First wave (from March to June 2020), n (%) | 0 | 55 (46) | 49 (48) | 6 (35) | 0.47 |
| Other waves (from August 2020 to April 2021), n (%) | 0 | 64 (54) | 53 (52) | 11 (65) | 0.47 |
| Main comorbidities, n (%) | |||||
| Obesity (body mass index ≥ 30 kg/m2) | 0 | 61 (52) | 52 (52) | 9 (53) | 1.00 |
| Diabetes mellitus | 0 | 50 (42) | 47 (46) | 3 (18) | 0.05 |
| Chronic respiratory diseases | 0 | 30 (25) | 26 (26) | 4 (24) | 1.00 |
| Chronic kidney diseases | 0 | 10 (8) | 9 (9) | 1 (6) | 1.00 |
| Immunosuppressiona | 0 | 20 (17) | 18 (18) | 2 (12) | 0.73 |
| Biological data upon ICU admission, median (interquartile range) | |||||
| Fibrinogen (g/L) | 12 | 8 (6–9) | 8 (6–8.5) | 8 (7–9) | 0.92 |
| d-dimers (µg/mL) | 18 | 2,220 (1,400–7,255) | 2,280 (1,510–7,260) | 1,920 (970–3,210) | 0.33 |
| Platelets count (G/L) | 0 | 230 (176–283) | 230 (172–279) | 228 (183–285) | 0.76 |
| Treatment administered upon ICU admission, n (%) | |||||
| Corticosteroids | 0 | 72 (61) | 61 (60) | 11 (65) | 0.90 |
| Antibiotic therapy for bacterial coinfection at ICU admission | 0 | 27 (23) | 21 (21) | 6 (35) | 0.21 |
| Antiviral drugs (lopinavir-ritonavir or remdesivir) | 0 | 0 | 0 | 0 | — |
| Tocilizumab | 0 | 0 | 0 | 0 | — |
| Organ support and outcomes in ICU | |||||
| Vasopressor support, n (%) | 0 | 94 (80) | 79 (79) | 15 (88) | 0.51 |
| Renal replacement therapy, n (%) | 0 | 36 (31) | 30 (30) | 6 (38) | 0.56 |
| Prone positioning, n (%) | 0 | 106 (89) | 90 (88) | 16 (94) | 0.69 |
| Thrombotic event during ICU stay, n (%) | 0 | 33 (28) | 27 (27) | 6 (35) | 0.55 |
| Length of ICU stay, d, median (interquartile range) | 0 | 24 (13–38) | 23 (13–35) | 35 (19–47) | 0.06 |
| ICU mortality, n (%) | 0 | 66 (56) | 55 (54) | 11 (65) | 0.57 |
VAP = ventilator-associated pneumonia.
aIncluding active solid cancer or hematologic malignancy, organ transplant, HIV, or immunosuppressive drugs.
Clinical, biological, radiological, and microbiological characteristics of the patients with a lung abscess are detailed in the Table 2. Lung abscess was diagnosed with a median time of 16 days (11–27 d) days after tracheal intubation. Lung abscess was clinically suspected before performing chest CT scan in 12 patients (71%). At diagnosis of lung abscess, median leukocytes count and procalcitonin were 25 G/L (20–30 G/L) and 12 µg/L (7–22 µg/L), respectively. Most of lung abscesses involved the right lung (71%) and the lower (76%) lobes. Lung abscesses were peripheral (in contact with the pleura) in 16 patients (94%). Three patients (18%) had pulmonary embolism or thrombosis (all segmental and all localized in the territory of the lung abscess) on the chest CT scan diagnosing lung abscess. Lung abscess was retrospectively visible on chest radiograph in five patients (29%).
TABLE 2.
Description of Biological, Radiological, and Microbiological Characteristics Among 17 Critically Ill Coronavirus Disease 2019 Patients With Lung Abscess Complicating Ventilator-Associated Pneumonia
| Variables | Missing Data | VAP With Lung Abscess, N = 17 |
|---|---|---|
| Main delays, median (interquartile range) | ||
| Days between ICU admission and diagnosis of lung abscess | 0 | 18 (14–27) |
| Days between tracheal intubation and diagnosis of lung abscess | 0 | 16 (11–27) |
| Days between diagnosis of VAP and diagnosis of lung abscess | 0 | 5 (4–10) |
| Biological data at diagnosis of lung abscess, median (interquartile range) | ||
| Leukocyte counts, G/L | 0 | 25 (20–30) |
| Procalcitonin, µg/L | 1 | 12 (7–22) |
| Fibrinogen, g/L | 3 | 7.3 (6.5–7.5) |
| d-dimers, µg/mL | 3 | 5,497 (3,887–9,385) |
| Radiological data at diagnosis of lung abscess | ||
| Reason for performing chest CT scan, n (%) | 0 | |
| Clinical or radiological suspicion of lung abscess | 12 (71) | |
| Other | 5 (29) | |
| Number of lung abscesses on chest CT scan, n (%) | 0 | |
| One | 6 (35) | |
| Two or more | 11 (65) | |
| Lateralization on chest CT scan, n (%) | 0 | |
| Right lung | 12 (71) | |
| Left lung | 4 (24) | |
| Bilateral | 1 (5) | |
| Localization on chest CT scan, n (%) | 0 | |
| Lower lobe | 13 (76) | |
| Upper lobe | 2 (12) | |
| Lower and upper lobe | 2 (12) | |
| Peripheral localization (contact with the visceral pleura), n (%) | 0 | 16 (94) |
| Larger diameter of lung abscess on chest CT scan, mm, median (interquartile range) | 0 | 33 (21–50) |
| Lung abscess visible on chest radiograph, n (%) | 0 | 5 (29) |
| Associated pulmonary thrombosis or embolism, n (%) | 0 | 3 (18) |
| Previous pulmonary thrombosis or embolism during ICU stay, n (%) | 0 | 4 (24) |
| Microbiological data, n (%) | ||
| Microbiological documentation | 0 | 15 (88) |
| Number of bacteria causing lung abscess | ||
| Monomicrobial | 3 (20) | |
| Polymicrobial | 12 (80) | |
| Isolated bacteria from pulmonary samples | 0 | N = 31 |
| Pseudomonas aeruginosa | 10/31 (32) | |
| Methicillin-sensitive Staphylococcus aureus | 4/31 (13) | |
| Enterobacteriaceae | 13/31 (42) | |
| Klebsiella aerogenes, n | 5 | |
| Escherichia coli, n | 3 | |
| Enterobacter cloacae, n | 2 | |
| Other Enterobacteriaceae, n | 3 | |
| Others bacteria | 4/31 (13) | |
| Aspergillus fumigatus isolated from pulmonary samples | 0 | 0 (0) |
| Concomitant bloodstream infection | 0 | 9 (53) |
| Bloodstream infection with a bacterium isolated from pulmonary samples | 6 (35) | |
| Bloodstream infection with another bacterium than isolated from pulmonary samples | 3 (18) | |
| Persistent (> 5 d) bacteria in consecutive pulmonary samples despite adequate antibiotic therapy | 0 | 8 (67) |
| Complications of lung abscess, n (%) | ||
| Hemoptysis | 0 | 0 (0) |
| Pneumothorax | 0 | 1 (6) |
| Pleural empyema | 0 | 0 (0) |
VAP = ventilator-associated pneumonia.
Only three of the 17 patients (18%) with a lung abscess had a bronchoscopic pulmonary sampling. Most of lung abscesses (n = 12/17; 80%) were polymicrobial. Enterobacteriaceae, Pseudomonas aeruginosa, and Staphylococcus aureus were the leading causative bacteria (Table 2). Concomitant bloodstream infection was diagnosed in nine patients (53%). Persistence (> 5 d) of the same bacteria in consecutive pulmonary samples despite adequate antimicrobial therapy occurred in eight patients (67%). None of the 17 patients with a lung abscess underwent a surgical or a chest tube drainage.
DISCUSSION AND CONCLUSIONS
We herein report that a lung abscess was diagnosed in 14% of our COVID-19 patients with VAP, which is higher than the 1.4% rate reported in a retrospective study not specifically focusing on lung abscess (10). Given that chest CT scans were not systematically performed in all patients with VAP (only 57% of them underwent a chest CT scan), our rate may even underestimate the real proportion of patients with a lung abscess.
We report on a high rate of VAP, as compared to other series (2, 3). This high rate may be due to a potential overdiagnosis of VAP among our COVID-19 patients. Indeed, we cannot exclude that some respiratory deteriorations may have been wrongly attributed to a VAP while being related to the progression of COVID-19 with a positive respiratory sample (tracheobronchial colonization). This potential overestimation of the frequency of VAP among our COVID-19 patients may also be responsible for an underestimation of the real proportion of patients with VAP developing a lung abscess.
Not surprisingly, the leading microorganisms isolated from pulmonary samples of COVID-19 patients with lung abscess complicating VAP were Enterobacteriaceae, P. aeruginosa, and S. aureus, which is consistent with the leading bacteria reported in other French series focusing on VAP among critically ill COVID-19 patients (1–4).
Our study failed to identify any predictive factor for the development of a lung abscess among COVID-19 patients with VAP. Indeed, the proportion of patients with diabetes mellitus, chronic respiratory diseases, bacterial coinfection at ICU admission, and immunosuppression including corticosteroids administration did not differ between the patients developing or not a lung abscess. Noteworthy, two third of the patients with a lung abscess had a persistence of the same bacteria in consecutive pulmonary samples despite adequate antibiotic therapy. Luyt et al (1) recently reported on a 79% rate of VAP recurrence among critically ill COVID-19 patients requiring extracorporeal membrane oxygenation, most of which (76%) being VAP “relapse” that is, VAP recurrence with the same pathogen despite adequate antimicrobial treatment. Similarly, Gragueb-Chatti et al (4) recently reported on a 37% rate of VAP recurrence among COVID-19 patients, of which 68% were due to the same pathogen. Unfortunately, none of these two studies reported on the proportion of patients with VAP “relapse” in whom a lung abscess was diagnosed. As observed in VAP occurring in ARDS from other causes than COVID-19, clinical and/or microbiological failures despite adequate antimicrobial therapy should alert the clinicians to the possibility of a lung abscess.
Our study suffers from several limitations including its monocenter retrospective design, the limited number of patients included, the lack of control with non–COVID-19 patients, and the fact that chest CT scans were not systematically performed in all the patients with VAP. However, we herein report the first study focusing on lung abscess as a complication of VAP in critically ill COVID-19 patients. Our study provides an informative picture of the clinical features of lung abscess developing in COVID-19 patients with VAP.
Intensivists should be aware that COVID-19 patients with VAP may develop lung abscesses. As the occurrence of a lung abscess impacts the duration of antibiotics therapy, chest CT scan should be easily performed (most of lung abscess were retrospectively “occult” on chest radiograph) in case of treatment failure (clinical and/or microbiological failures) despite adequate antimicrobial therapy.
Footnotes
The authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.Luyt CE, Sahnoun T, Gautier M, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: A retrospective cohort study. Ann Intensive Care. 2020; 10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razazi K, Arrestier R, Haudebourg AF, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to coronavirus 19 disease. Crit Care. 2020; 24:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouzé A, Martin-Loeches I, Povoa P, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021; 47:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gragueb-Chatti I, Lopez A, Hamidi D, et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: A multicenter retrospective study. Ann Intensive Care. 2021; 11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020; 383:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contou D, Pajot O, Cally R, et al. Pulmonary embolism or thrombosis in ARDS COVID-19 patients: A French monocenter retrospective study. PLoS One. 2020; 15:e0238413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wicky PH, Niedermann MS, Timsit JF. Ventilator-associated pneumonia in the era of COVID-19 pandemic: How common and what is the impact? Crit Care. 2021; 25:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby LS, King TE, LaForce FM, et al. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore). 1985; 64:342–348 [DOI] [PubMed] [Google Scholar]
- 9.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016; 63:e61–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blonz G, Kouatchet A, Chudeau N, et al. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit Care. 2021; 25:72. [DOI] [PMC free article] [PubMed] [Google Scholar]


