Abstract
Aims
Major adverse cardiac events (MACE) triggered by non-cardiac surgery are prognostically important perioperative complications. However, due to often asymptomatic presentation, the incidence and timing of postoperative MACE are incompletely understood.
Methods and results
We conducted a prospective observational study implementing a perioperative screening for postoperative MACE [cardiovascular death (CVD), acute heart failure (AHF), haemodynamically relevant arrhythmias, spontaneous myocardial infarction (MI), and perioperative myocardial infarction/injury (PMI)] in patients at increased cardiovascular risk (≥65 years OR ≥45 years with history of cardiovascular disease) undergoing non-cardiac surgery at a tertiary hospital. All patients received serial measurements of cardiac troponin to detect asymptomatic MACE. Among 2265 patients (mean age 73 years, 43.4% women), the incidence of MACE was 15.2% within 30 days, and 20.6% within 365 days. CVD occurred in 1.2% [95% confidence interval (CI) 0.9–1.8] and in 3.7% (95% CI 3.0–4.5), haemodynamically relevant arrhythmias in 1.2% (95% CI 0.9–1.8) and in 2.1% (95% CI 1.6–2.8), AHF in 1.6% (95% CI 1.2–2.2) and in 4.2% (95% CI 3.4–5.1), spontaneous MI in 0.5% (95% CI 0.3–0.9) and in 1.6% (95% CI 1.2–2.2), and PMI in 13.2% (95% CI 11.9–14.7) and in 14.8% (95% CI 13.4–16.4) within 30 days and within 365 days, respectively. The MACE-incidence was increased above presumed baseline rate until Day 135 (95% CI 104–163), indicating a vulnerable period of 3–5 months.
Conclusion
One out of five high-risk patients undergoing non-cardiac surgery will develop one or more MACE within 365 days. The risk for MACE remains increased for about 5 months after non-cardiac surgery.
Trial registration
https://www.clinicaltrials.gov. Unique identifier: NCT02573532.
Keywords: Acute myocardial infarction, Heart failure, Arrhythmia, Death, Surgery
Introduction
More than 300 million surgeries are performed worldwide per year.1 Despite the undisputed benefits of surgery, surgical procedures are possible triggers for major adverse cardiac events (MACE), including myocardial infarction (MI)/injury, acute heart failure (AHF), haemodynamically relevant arrhythmias, and cardiovascular death (CVD).2–5 Several complex pathophysiological processes triggered by the surgical and anaesthetic stress including an increase in sympathetic and neurohumoral activity, pro-coagulant factors, intravascular volume load, and systemic inflammation,6,7 as well as several periprocedural factors such as intraoperative tachycardia,8 intraoperative hypertension,9 perioperative hypotension,8,10 and perioperative anaemia,11 seem to contribute to MACE following non-cardiac surgery. Especially perioperative myocardial infarction/injury (PMI) is increasingly recognized as major contributor to perioperative morality, yet often remains undetected as PMI most commonly present without typical symptoms of myocardial ischaemia such as chest pain, angina pectoris, or dyspnoea.2,12–14 Therefore, PMI is often missed without systematic screening in clinical routine as well as observational research,2–15 leading to an underestimation of incidence of perioperative MACE.
Furthermore, the time frame in which MACE are more frequent following non-cardiac surgery is unclear. Knowledge about the time window in which patients might require closer follow-up might facilitate the development of strategies for the prevention and early detection of MACE.16,17
In order to contribute to closing these major gaps in knowledge, we conducted a prospective study with a MACE screening programme embedded in clinical routine aiming to reliably estimate incidence of MACE following non-cardiac surgery and to define the postoperative period during which MACE rate is still increased by the surgical procedure.
Methods
Study design and patient population
We conducted a prospective cohort single-centre study (BASEL-PMI) including consecutive high-risk patients undergoing non-cardiac surgery at the University Hospital Basel, Switzerland between October 2014 and November 2015. Written general consent to registration in a dedicated prospective database was obtained from all individual participants included in the study after ethics approval (EKNZ 2015-301, NCT02573532) and in accordance with the ethical standards laid down in the Declaration of Helsinki. We adhered to the STROBE reporting guidelines, with further information found in the Supplementary material online.
High-risk patients were defined as aged between 65 and 85 years OR between 45 to 64 years AND a history of cardiovascular disease (coronary artery disease, peripheral artery disease, or stroke). Patients had elective, urgent, or emergency non-cardiac surgery and stayed in hospital at least one night after surgery. Non-cardiac surgery involved visceral, orthopaedic, trauma, vascular, urologic, spinal, and thoracic surgical procedures. We excluded patients who declined general consent, were incorrectly screened (<45 years, hospitalization <24 h, surgery involving the heart), had their surgery cancelled or were enrolled more than once within 1 year after surgery (Supplementary material online, Figure S1).
Procedures and outcome adjudication
MACE screening
Patients meeting the eligibility criteria received a cardiac troponin-based screening for PMI as well as MACE embedded in clinical routine. Patients were followed for 1 year to assess occurrence of CVD, AHF, haemodynamically relevant arrhythmias, spontaneous MI, and PMI.14 CVD included death attributable to MI, sudden cardiac death, AHF, stroke, cardiovascular procedure, cardiovascular haemorrhage (e.g. ruptured aortic aneurysm or dissection), and pulmonary embolism.14 AHF was defined as AHF requiring admission to the hospital or the in-hospital transfer to an intensive care unit or the use of intravenous diuretics. Haemodynamically relevant arrhythmias were defined as cardiac arrest, sustained ventricular tachycardia, atrial fibrillation with the need of treatment (electrical or chemical cardioversion, or a heart rate >120 b.p.m. with the need of rate control such as beta-blockers or calcium channel blockers), and atrioventricular block III. Spontaneous MI was defined according to the universal definition of MI.18,19 In order to differentiate between PMI vs. pre-existing elevations of high-sensitivity cardiac troponin T (hs-cTnT) due to chronic or even acute cardiac disorders, the routine screening consisted of a preoperative hs-cTnT measurement as baseline concentration, and two postoperative measurements on postoperative Days 1 and 2.14,20 In case of a PMI, defined as an absolute increase in hs-cTnT concentrations of ≥14 ng/L above preoperative concentrations, or between two postoperative concentrations, if the preoperative concentration was missing,14 a study physician prospectively recorded patient symptoms and a 12-lead electrocardiogram. Additionally, a cardiology consultation was triggered. PMI were also recorded during subsequent operations. If deemed indicated clinically, further measurements of hs-cTnT were made.
Follow-up
Patients and family physicians were contacted 365 days after the surgical procedure by mail or telephone calls. In addition, electronic health records of the University Hospital Basel and all other institutions mentioned during follow-up were reviewed for MACE. Moreover, the national registry on mortality was reviewed for all patients.
Aims
The primary aim was to define the 30-day and 365-day incidence of MACE. The secondary aim was to define the postoperative period during which the MACE rate is still increased by the surgical procedure.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges, categorical variables as numbers and percentages. The Mann–Whitney U test was used to compare continuous variables, and the Fisher’s exact test for categorical variables. The 30-day and 365-day incidence of the combined endpoint MACE were calculated as the amount of patients suffering at least one MACE with the associated 95% confidence interval (95% CI). The 30-day incidence of MACE was stratified by surgical discipline and European Society of Cardiology/European Society of Anesthesiology (ESC/ESA) surgical risk.21
Time to first MACE, and the univariable association between PMI and the occurrence of further MACE during 365 days of follow-up were shown by Kaplan–Meier Curves. Differences were assessed using the log-rank test. Total number of events, with multiple events allowed per patient, was calculated because total MACE even better reflects total harm and total costs from a societal perspective.
To determine the postoperative period during which the MACE rate is still increased by the surgical procedure, we investigated the time of occurrence of MACE within the follow-up period of 365 days for our patient cohort. We constructed a model with days after surgery on the x-axis, and pending MACE on the y-axis. We investigated changes in the slope of the curve, and assumed that after postoperative Day 300 the MACE rate in so longer affected by the surgical procedure, referring to a large observational study of intermediate to high-risk vascular surgery patients.22 By comparison of the slope of piece-wise linear regression of pending MACE, a change in trend in MACE rate was estimated. We performed this analysis for the entire patient cohort, and for the subgroup of patients with initial PMI, and without initial PMI.
All hypothesis testing was two-tailed, and P-values of <0.05 were considered to be statistically significant. IBM SPSS Statistics, version 22.0, and R Studio, version 3.4.1, were used to perform all statistical analyses.
Results
Baseline characteristics
The patient flow is shown in Supplementary material online, Figure S1. Baseline characteristics of the 2265 patients eligible for the 30-day analysis are presented in Table 1. Briefly, median age was 73 years and 43.3% (984/2265) of patients were women. Patients developing MACE were older, more often had a history of cardiac disease, cardiovascular risk factors, and chronic kidney disease, and more often underwent emergency surgery. Moreover, the incidence of MACE differed according to surgical specialty.
Table 1.
Baseline characteristics shown for all patients and classified according to the occurrence of MACE within 30 days after surgery
| Overall (n = 2265; 100%) | No 30-day MACE (n = 1921; 84.8%) | 30-day MACE (n = 344; 15.2%) | p-value | |
|---|---|---|---|---|
| Age (years) | 73 (68–79) | 73 (68–79) | 77 (70–82) | <0.001 |
| Sex, male | 1281 (56.6) | 1086 (56.7) | 192 (55.8) | 0.77 |
| Medical history | ||||
| Coronary artery disease | 621 (27.4) | 489 (25.5) | 132 (38.4) | <0.001 |
| Prior myocardial infarction | 303 (13.4) | 230 (12.0) | 73 (21.2) | <0.001 |
| Congestive heart failure | 244 (10.9) | 177 (9.2) | 67 (20.7) | <0.001 |
| Atrial fibrillation | 340 (15.0) | 251 (13.1) | 89 (25.9) | <0.001 |
| Valvular heart disease | 249 (11.0) | 180 (9.4) | 69 (20.1) | <0.001 |
| Peripheral artery disease | 385 (17.0) | 298 (15.5) | 87 (25.3) | <0.001 |
| Prior stroke | 210 (9.3) | 173 (9.0) | 37 (10.8) | 0.31 |
| Hypertension | 1453 (64.2) | 1209 (62.9) | 244 (70.9) | 0.005 |
| Diabetes mellitus | 521 (23.0) | 410 (21.3) | 111 (32.3) | <0.001 |
| Chronic kidney disease (CKD ≥ III) | 330 (14.6) | 236 (12.3) | 94 (27.3) | <0.001 |
| Active cancer | 568 (25.1) | 501 (26.1) | 67 (19.5) | 0.008 |
| Type of surgery | <0.001 | |||
| Elective surgery | 1492 (65.9) | 1306 (68.0) | 186 (54.13) | |
| Urgent surgery (>24 h) | 339 (15.0) | 265 (13.8) | 74(21.5) | |
| Emergency surgery (≤24 h) | 434 (19.2) | 350 (18.2) | 84 (24.4) | |
| Surgical speciality | <0.001 | |||
| Orthopaedic | 262 (11.6) | 219 (11.4) | 43 (12.5) | |
| Trauma | 417 (18.4) | 338 (17.6) | 79 (23.0) | |
| Spinal | 355 (15.7) | 309 (16.1) | 46 (13.4) | |
| Thoracic | 192 (8.5) | 149 (7.8) | 43 (12.5) | |
| Urologic | 358 (15.8) | 327 (17.0) | 31 (9.0) | |
| Vascular | 279 (12.3) | 220 (11.5) | 59 (17.2) | |
| Visceral | 316 (14.0) | 286 (14.9) | 30 (8.7) | |
| Other | 86 (3.8) | 73 (3.8) | 13 (3.8) |
Continuous variables are presented as medians (1st and 3rd quartile), categorical variables are presented as numbers (%). Continuous variables were compared with the Mann–Whitney U test, and categorical variables with the Fisher’s exact test.
CKD-EPI, chronic kidney disease-epidemiology collaboration; MACE, major adverse cardiac events.
Incidence of MACE
Follow-up was complete at 365 days in 99.5% of patients. Preoperative measurements of hs-cTnT were available in 2116 of 2265 patients (93.4%).
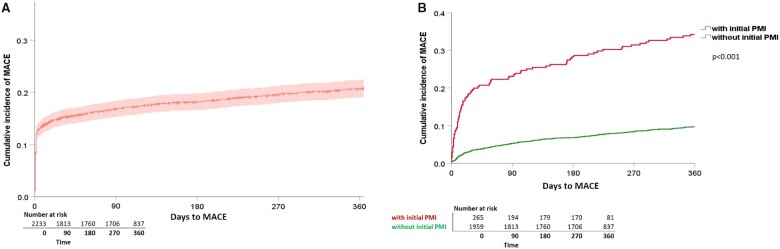
At least one MACE occurred in 344/2265 patients (15.2%; 95% CI 13.8–16.8%) within 30 days (Table 2) and in 466/2265 patients (20.6%; 95% CI 19–22.3%) within 365 days after the surgical procedure (Figure 1A). CVD occurred in 1.2% (95% CI 0.9–1.8) and in 3.7% (95% CI 3.0–4.5), haemodynamically relevant arrhythmias in 1.2% (95% CI 0.9–1.8) and in 2.1% (95% CI 1.6–2.8), AHF in 1.6% (95% CI 1.2–2.2) and in 4.2% (95% CI 3.4–5.1), spontaneous MI in 0.5% (95% CI 0.3–0.9) and in 1.6% (95% CI 1.2–2.2), and PMI in 13.2% (95% CI 11.9–14.7) and in 14.8% (95% CI 13.4–16.4), within 30 and 365 days, respectively (Table 2).
Table 2.
30-day and 365-day incidences shown for the combined endpoint MACE with its single components, and for the secondary endpoint, all-cause mortality
| 30-day incidence (95% CI) | 365-day incidence (95% CI) | |
|---|---|---|
| Combined endpoint MACE | 15.2% (13.8–16.8) (344/2265) | 20.6% (19.0–22.3) (466/2265) |
| Cardiovascular death | 1.2% (0.9–1.8) (28/2265) | 3.7% (3.0–4.5) (83/2265) |
| Haemodynamically-relevant arrhythmias | 1.2% (0.9–1.8) (28/2265) | 2.1% (1.6–2.8) (47/2265) |
| Acute heart failure | 1.6% (1.2–2.2) (36/2265) | 4.2% (3.4–5.1) (95/2265) |
| Perioperative myocardial infarction/injurya | 13.2% (11.9–14.7) (300/2265) | 14.8% (13.4–16.4) (336/2265) |
| Spontaneous myocardial infarction | 0.5% (0.3–0.9) (12/2265) | 1.6% (1.2–2.2) (37/2265) |
| Secondary endpoint | ||
| All-cause mortality | 3.0% (2.3–3.7) (67/2265) | 11.5% (10.3–12.9) (261/2265) |
Data are presented as percentage (95% confidence interval), in brackets () absolute number/total number of patients.
CI, confidence interval; MACE, major adverse cardiac events.
Initial PMI and PMI occurring during subsequent surgical procedures within 365 days of follow-up.
Figure 1.
Cumulative incidence of MACE within 365 days of follow-up. Kaplan–Meier curves displaying the cumulative incidence of MACE within 365 days of follow-up for (A) all patients (n = 2265, 464 events), and (B) split for patients with PMI (n = 274 with 90 events in red), and without PMI (n = 1989 with 190 events in green). Differences between groups (with, and without PMI) were assessed using the log-rank test. MACE, major adverse cardiac events; PMI, perioperative myocardial injury/infarction.
In total, with multiple events allowed per patient, within 7 days after the surgical procedure 363 events and from postoperative Day 8 until Day 365, 403 events were recorded, resulting in a total MACE number of 766 events in 2265 patients (Supplementary material online, Figure S2). Within the first 7 postoperative days 47.4% (95% CI 43.9–50.9), and within the first 30 days after surgery 62.5% (95% CI 59.1–65.9) of the total event rate were recorded. For each single component of MACE, the proportion out of the total event rate was as followed: for CVD 1.3% (95% CI 0.7–2.4) and 3.7% (95% CI 2.5–5.2), for haemodynamically relevant arrhythmias 4.3% (95% CI 3.1–6.0) and 7.3% (95% CI 5.7–9.4), for AHF 4.4% (95% CI 3.2–6.1) and 8.1% (95% CI 6.4–10.2), for spontaneous MI 0.7% (95% CI 0.3–1.5) and 1.6% (95% CI 0.9–2.7), and for PMI 36.7% (95% CI 33.4–40.2) and 41.9% (95% CI 38.5–45.4) within 7 days and within 30 days after surgery respectively. The absolute numbers are presented in Supplementary material online, Figure S2.
The incidence of MACE increased with higher ESC/ESA surgical risk category, ranging from 6% (95% CI 2–15%) in the lowest (<1% risk) to 57% (95% CI 25–84) in the highest (>5% risk) category. The 30-day MACE incidence varied among different types of surgery. The highest 30-day incidence was observed in thoracic surgery patients with 22% (95% CI 17–29), followed by vascular and trauma surgery patients with 21% (95% CI 17–26) and 19% (95% CI 15–23), respectively (Supplementary material online, Table S1).
Association of PMI with other MACE occurring within 365 days
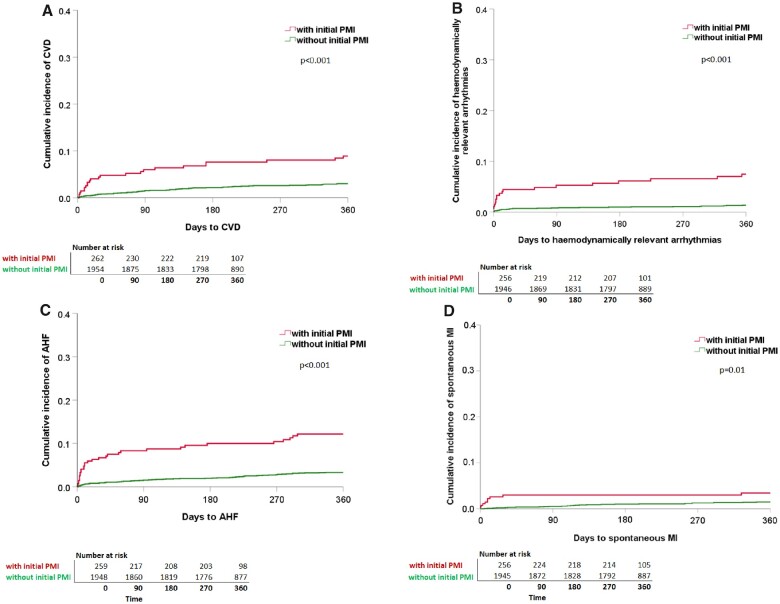
Within 365 days, 90/274 patients with PMI (32.9%; 95% CI 27.6–38.6) suffered from at least one further MACE, compared to 190/1989 patients without PMI (9.6%; 95% CI 8.3–10.9) (Figure 1B). Of all 445 events occurring after Day 3, 128 events (28.8%) occurred in patients with initial PMI. Furthermore, CVD, haemodynamically relevant arrhythmias, AHF, and spontaneous MI occurred significantly more frequent in patients with initial PMI as compared to patients without initial PMI (Figure 2A–D).
Figure 2.
Cumulative incidence of the single components of MACE within 365 days of follow-up split for patients with and without PMI. Kaplan–Meier curves showing the cumulative incidence of the single components of MACE for (A) CVD (with PMI: n = 275, 23 events; without PMI: n = 1990; 60 events). (B) Haemodynamically relevant arrhythmias (with PMI: n = 274, 19 events; without PMI: n = 1989, 26 events). (C) AHF (with PMI: n = 275, 31 events; without PMI: n = 1990, 64 events). (D) Spontaneous MI (with PMI: n = 275; 9 events; without PMI: n = 1990, 28 events). Each split for patients with PMI (red line) and without PMI (green line). Differences between groups (with, and without PMI) were assessed using the log-rank test. Patients with initial PMI are more likely to suffer from CVD, haemodynamically relevant arrhythmias, AHF, and spontaneous MI during 365 days of follow-up. AHF, acute heart failure; CVD, cardiovascular death; MACE, major adverse cardiac events; MI, myocardial infarction; PMI, perioperative myocardial injury/infarction.
Time to MACE
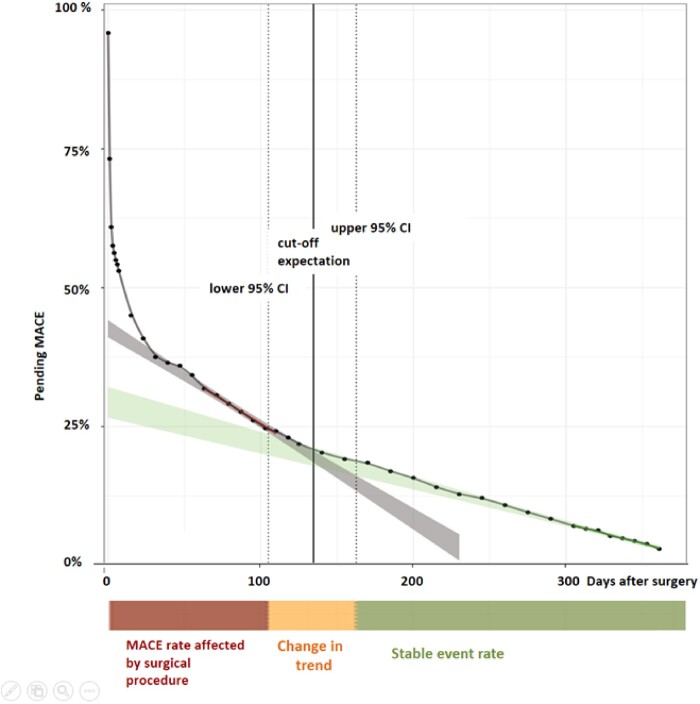
The time of occurrence of MACE within the follow-up period of 365 days for our entire patient cohort is shown in Figure 3. The rapid drop in the curve within the first postoperative days is mainly related to PMI detected during the perioperative hs-cTnT-screening period. Following this, a change in trend is seen at Day 135 (95% CI 104–163), with a steeper slope from Day 0–103 vs. Day 164–365. From this, we concluded that the occurrence of MACE is more frequent within the first 3–5 months after surgical procedure. The MACE risk is highest within the first 30 days after surgery and in particular high within the first 7 days after surgery (Figure 3, Supplementary material online, Figure S2).
Figure 3.
Time of occurrence of MACE within 365 days of follow-up. The time of occurrence of MACE within 365 days of follow-up shown for the entire patient cohort (n = 2265). Days after surgery on the x-axis, and pending events (in percent) on the y-axis. The green line was derived by linear regression of pending events from postoperative Day 300 to 365, the red line was derived by linear regression of pending events from postoperative Day 30 to 118. 95% confidence intervals (CIs) are shown as ribbons. The intersection area represents the overlap of the derived linear regression lines and their 95% CI. The intersection area is at 135 (95% CI 104–163) days of follow-up and is also shown by the orange coloured bar below with the label ‘Change in trend’. The steeper slope from postoperative Day 0 to 103 is illustrated by the red coloured bar with the label ‘MACE rate affected by the surgical procedure’, and the time period from postoperative Day 164 to 365 is illustrated by the green coloured bar with the label ‘stable event rate’. MACE, major adverse cardiac events.
When applying the same analysis for the subgroup of patients with initial PMI (n = 275), and for the subgroup of patients without initial PMI (n = 1990), the change in trend is seen at postoperative Day 142 (95% CI 55–209), and postoperative Day 130 (95% CI 94–162), respectively, with no significant difference between groups (Supplementary material online, Figure S3 and Table S2).
Discussion
This prospective cohort study enrolling consecutive patients at increased cardiovascular risk undergoing non-cardiac surgery screened for perioperative MACE. We report three major findings:
First, the incidence of MACE was high. CVD occurred in 1.2%, haemodynamically relevant arrhythmias in 1.2%, AHF in 1.6%, and spontaneous MI in 0.5% of patients. Including also the emerging entity of PMI, one out of seven patients developed at least one MACE within 30 days. The MACE rate, particularly AHF and PMI, was much higher than expected and reported in previous studies, that did not systematically screen for MACE.4,5,23 Other components of MACE less affected by ascertainment bias including all-cause mortality had similar incidence as compared to previous studies2,5,12,24,25 which supports the generalizability of our findings. In other recent screening studies also including younger low-risk patients12,26,27 at 30 days the rate of cardiovascular mortality was 1.2%27 and 1.9%,12 MI 4.2%, AHF 0.9%, and clinically relevant atrial fibrillation 1,6%.25 In POISE-2, death or MI occurred in 11.8% of patients at 365 days.28
The incidence of MACE varied among different types of surgery. The 30-day MACE incidence was highest in thoracic surgery patients with 22%, followed by vascular surgery with 21%. This extends and corroborates findings of a recently published observational study including cardiovascular high-risk patients undergoing elective thoracic surgery.3 The extent of lung resection was identified as an independent risk factor for developing myocardial injury, which suggests that mechanism like systemic inflammatory processes or an elevation of the right ventricular afterload might have an impact on perioperative cardiovascular outcomes in thoracic surgery patients.3 Additionally to the specific surgical procedure performed, there seem to be several other factors effecting cardiovascular outcomes, such as the obesity paradox13,29 or preoperative anaemia,30 which was identified as a strong predictor for cardiac events in vascular surgery patients.16
Second, patients with PMI, of which the majority did not experience symptoms during the event due to accompanying anaesthesia, are not only at increased risk of death within 1 year14,31,32 but also at increased risk of future AHF, haemodynamically relevant arrhythmias, and spontaneous MI. This extends and corroborates important studies showing that postoperative myocardial ischaemia detected within the first 48 h after non-cardiac surgery by continuous electrocardiographic monitoring was independently associated with an increased risk of adverse cardiac events,33 and 70% of all adverse cardiac events were preceded by postoperative ischaemia.2,4 Considering our findings, a MACE Screening using hs-cTnT measurements preoperatively and on postoperative Day 1 and 2 for patients meeting the high-risk criteria used in this study might provide an opportunity to improve perioperative care.
Third, we report that the vulnerable period after non-cardiac surgery with increased risk of MACE lasts about 5 months, and is similar in length in patients with vs. without initial PMI. A recently published large international, randomized, placebo-controlled trial supports this concept.34 Similarly, in a large observational study of intermediate- or high-risk vascular surgery patients, the hazard of mortality was greatest in the first months and persisted up to 10 months after surgery.22 Our findings show that the MACE risk is especially high within the first 30 days after surgery, and in particular within the first postoperative 7 days, which has important clinical implications when considering the optimal length of therapeutic interventions aimed at reducing the MACE rate after non-cardiac surgery. Furthermore, this finding should inform the design of future clinical trials aimed at preventing MACE after non-cardiac surgery.
Strengths
To the best of our knowledge, this is the first prospective study investigating long-term MACE after non-cardiac surgery in consecutive patients incorporating systematic screening for PMI and AHF. The large sample size of 2265 patients, central adjudication of MACE, 365 days follow-up and very high completeness of long-term follow-up with 99.5% (95% CI 99.2–99.7%) are important additional strengths.
Limitations
Several limitations should be considered when interpreting these findings. First, this was a single-centre study. As the 30-day rate of AHF, haemodynamically relevant arrhythmias, and CVD was comparable to that observed in the international VISION study12,25,27 our main findings likely also apply to most other institutions. Still, future studies need to externally validate the point estimates for the different MACE components derived in this study. Second, until now controversies exist regarding the definition of PMI.13,14,35 Changes in the definition of PMI will invariably also affect overall MACE rate.3,12,26,27,36Third, although using a very stringent methodology to detect and adjudicate MACE, we likely still have underestimated the MACE rate as the screening for PMI was performed only on the first and second postoperative day. Fourth, in the absence of a fully-matched control population with comparable cardiac and non-cardiac comorbidities, but not undergoing surgery, the quantification of the vulnerable period after non-cardiac surgery required assumptions and should therefore be considered a range rather than a precise point-estimate. It definitely requires external validation in future studies.
Conclusion
One out of five high-risk patients undergoing non-cardiac surgery will develop one or more MACE within 365 days. The risk for MACE remains increased for about 5 months after non-cardiac surgery. As this incidence is much higher than commonly expected, novel strategies to reduce cardiac complications seem warranted.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Supplementary Material
Acknowledgements
We wish to thank the patients who participated in this study, as well as the clinical teams caring for them.
Additional BASEL-PMI investigators and contributors to this manuscript
Stella Marbot1, Joan Walter1, Michael Freese1, Thomas Nestelberger1, Jasper Boeddinghaus1, Jeanne du Fay de Lavallaz1, Raphael Twerenbold1, Patrick Badertscher1, Luca Koechlin1, Tobias Zimmermann1, Valentina Troester1, Eleni Michou1, Stefan Osswald1, Esther Seeberger, MD1; Manfred Seeberger, MD2; Daniel Rickli3; Desiree Wussler, MD1; Julia Dinort, MD1; Alexandra Prepoudis, MD1; Kathrin Meissner, RN1; Gregor Fahrni, MD1; Raban Jeger, MD1; Christoph Kaiser, MD1; Laura Infanti, MD4
1Department of Cardiology and Cardiovascular Research Institute Basel (CRIB), University Hospital Basel, University Basel, Basel, Switzerland; 2Department of Anaesthesiology, Hirslanden Clinic, Witellikerstrasse 40, 8032 Zürich, Switzerland 3Department of Traumatology & Orthopedics, University Hospital Basel, University of Basel, Basel, Switzerland 4Blood Bank and Department of Hematology, University Hospital Basel, University Basel, Basel, Switzerland
Funding
This study was funded by the University Basel, the University Hospital Basel, the Swiss Heart Foundation, the Swiss National Science Foundation, Abbott, Astra Zeneca, Roche, the PhD Educational Platform for Health Sciences, the Forschungsfond Kantonsspital Aarau, and the Cardiovascular Research Foundation Basel. The funders had no role in the design, data collection, statistical analysis, writing of this manuscript, or decision to publish.
Consent to participate
Written general consent to registration in a dedicated prospective database was obtained from all individual participants included in the study.
Conflict of interest: Dr. Puelacher reports grants from PhD Educational Platform for Health Sciences, Roche Diagnostics and the University Hospital Basel as well as chaired an advisory board for Roche Diagnostics, during the conduct of the study. Dr. Gualandro has received research grants from FAPESP (Sao Paulo Research Foundation) for the submitted work, and speaker or consulting honoraria from Servier and Roche, outside the submitted work. Dr. Lurati Buse reports grants from University of Basel as well as cochaired an advisory board for Roche Diagnostics, during the conduct of the study. Dr. Hammerer-Lercher reports speaker or consulting honoraria from Roche, Abbott and Beckman, outside the submitted work. Dr. Kindler reports grants from Forschungsfond Kantonsspital Aarau, during the conduct of the study. Dr. Mueller reports grants from the Swiss Heart Foundation and grants and non-financial support from several diagnostic companies during the conduct of the study, as well as grants, personal fees and non-financial support from several diagnostic companies outside the submitted work. All other authors report no conflicts of interest.
Contributor Information
for the BASEL-PMI Investigators:
Stella Joan Marbot, Michael Walter, Thomas Freese, Jasper Nestelberger, Jeanne Boeddinghaus, Raphael du Fay de Lavallaz, Patrick Twerenbold, Luca Badertscher, Tobias Koechlin, Valentina Zimmermann, Eleni Troester, Stefan Michou, Esther Osswald, Manfred Seeberger, Daniel Seeberger, Desiree Rickli, Julia Wussler, Alexandra Dinort, Kathrin Prepoudis, Gregor Meissner, Raban Fahrni, Christoph Jeger, Laura Kaiser, and Infanti
References
- 1. Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR, Gawande AA. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385:S11. [DOI] [PubMed] [Google Scholar]
- 2. Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, Leslie K, Rao-Melacini P, Chrolavicius S, Yang H, Macdonald C, Avezum A, Lanthier L, Hu W, Yusuf S. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011;154:523–528. [DOI] [PubMed] [Google Scholar]
- 3. González-Tallada A, Borrell-Vega J, Coronado C, Morales P, Miguel M de, Ferreira-González I, Nadal M de. Myocardial injury after noncardiac surgery: incidence, predictive factors, and outcome in high-risk patients undergoing thoracic surgery: an observational study. J Cardiothorac Vasc Anesth 2019;34:426–432. [DOI] [PubMed] [Google Scholar]
- 4. Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM.. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992;268:233–239. [DOI] [PubMed] [Google Scholar]
- 5. Sabaté S, Mases A, Guilera N, Canet J, Castillo J, Orrego C, Sabaté A, Fita G, Parramón F, Paniagua P, Rodríguez A, Sabaté M, ANESCARDIOCAT Group B, Rivilla M, Gine M, Sadurni M, Fau M, Arroyo R, Rojo A, Pujol Rosa E, Rovira I, Alcon A, Lacambra M, Pi A, Campello D, Sierra P, Arnal A, Llorente C, Mazo V, Lopez S. Incidence and predictors of major perioperative adverse cardiac and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2011;107:879–890. [DOI] [PubMed] [Google Scholar]
- 6. Kehlet H. The modifying effect of anesthetic technique on the metabolic and endocrine responses to anesthesia and surgery. Acta Anaesthesiol Belg 1988;39:143–146. [PubMed] [Google Scholar]
- 7. Gualandro DM, Campos CA, Calderaro D, Yu PC, Marques AC, Pastana AF, Lemos PA, Caramelli B. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012;222:191–195. [DOI] [PubMed] [Google Scholar]
- 8. Abbott TEF, Pearse RM, Archbold RA, Ahmad T, Niebrzegowska E, Wragg A, Rodseth RN, Devereaux PJ, Ackland GL. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: Results of the VISION study. Anesth Analg 2018;126:1936–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reich DL, Bennett-Guerrero E, Bodian CA, Hossain S, Winfree W, Krol M.. Intraoperative tachycardia and hypertension are independently associated with adverse outcome in noncardiac surgery of long duration. Anesth Analg 2002;95:273–277, table of contents. [DOI] [PubMed] [Google Scholar]
- 10. Roshanov PS, Sheth T, Duceppe E, Tandon V, Bessissow A, Chan MTV, Butler C, Chow BJW, Khan JS, Devereaux PJ. Relationship between perioperative hypotension and perioperative cardiovascular events in patients with coronary artery disease undergoing major noncardiac surgery. Anesthesiology 2019;130:756–766. [DOI] [PubMed] [Google Scholar]
- 11. Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM.. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res 2002;102:237–244. [DOI] [PubMed] [Google Scholar]
- 12.Devereaux PJ, Chan MT V, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati Buse G, Botto F, Guyatt G, Heels-Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307:2295–2304. [DOI] [PubMed] [Google Scholar]
- 13. Hidvegi R, Puelacher C, Gualandro DM, Lampart A, Lurati Buse G, Hammerer-Lerchner A, Walter J, Liffert M, Bolliger D, Steiner L, Kindler C, Espinola J, Strebel I, Gueckel J, Marbot S, Arslani K, Boeddinghaus J, Nestelberger T, Zimmermann T, Freese M, Guerke L, Mujagic E, Rikli D, Buser A, Mueller C, Wolff T, Wildi K, Genini A, Twerenbold R, Sazgary L. Obesity paradox and perioperative myocardial infarction/injury in non-cardiac surgery. Clin Res Cardiol 2020;doi:10.1007/s00392-020-01605-0. [DOI] [PubMed] [Google Scholar]
- 14. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery. Circulation 2018;137:1221–1232. [DOI] [PubMed] [Google Scholar]
- 15. Pearse R, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, Vallet B, Vincent JL, Hoeft A, Rhodes A, Fagnoul D, Obbergh L van, Al-Subaie N, Arif F, Cashman J, Cecconi M, Edsell M, Fossati N, Hammond SJ, Hamilton M, Lonsdale D, Moran C, Rhodes A, Siegmueller C, Velzeboer F, Wong P, Awlakpui E, Scheidemann M, Wittmann M, Damster S. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012;380:1059–1065.22998715 [Google Scholar]
- 16. Gualandro DM, Puelacher C, LuratiBuse G, Llobet GBGB, Yu PCPC, Cardozo FA, Glarner N, Zimmerli A, Espinola J, Corbière S, Calderaro D, Marques ACAC, Casella IBIB, Luccia N de, Oliveira MTMT, Lampart A, Bolliger D, Steiner L, Seeberger M, Kindler C, Osswald S, Gürke L, Caramelli B, Mueller C, GREAT network. Prediction of major cardiac events after vascular surgery. J Vasc Surg 2017;66:1826–1835.e1. [DOI] [PubMed] [Google Scholar]
- 17. Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, Graham M, Tandon V, Styles K, Bessissow A, Sessler DI, Bryson G, Devereaux PJ. Canadian Cardiovascular Society Guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33:17–32. [DOI] [PubMed] [Google Scholar]
- 18. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Glob Heart 2012;7:275–295. [DOI] [PubMed] [Google Scholar]
- 19. Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267–315.26320110 [Google Scholar]
- 20. Gualandro DM, Puelacher C, Mueller C.. High-sensitivity cardiac troponin in acute conditions. Curr Opin Crit Care 2014;20:472–477. [DOI] [PubMed] [Google Scholar]
- 21. Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert S De, Ford I, Juanatey JRG, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Luescher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Uva MS, Voudris V, Funck-Brentano C. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 2014;31:517–573. [DOI] [PubMed] [Google Scholar]
- 22. Reed GW, Horr S, Young L, Clevenger J, Malik U, Ellis SG, Michael Lincoff A, Nissen SE, Menon V. Associations between cardiac troponin, mechanism of myocardial injury, and long-term mortality after noncardiac vascular surgery. J Am Heart Assoc 2017;6:e005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Helwani MA, Amin A, Lavigne P, Rao S, Oesterreich S, Samaha E, Brown JC, Nagele P. Etiology of acute coronary syndrome after noncardiac surgery. Anesthesiology 2018;128:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Waes JAR, Grobben RB, Nathoe HM, Kemperman H, Borst GJ De, Peelen LM, Klei WA Van. One-year mortality, causes of death, and cardiac interventions in patients with postoperative myocardial injury. Anesth Analg 2016;doi:10.1213/ANE.0000000000001313. [DOI] [PubMed] [Google Scholar]
- 25.Bhandari M, Buckley N, Cinà CS, Cook DJ, Beer J de, Devereaux PJ, Guyatt GH, Haynes RB, Heels-Ansdell D, Julian JA, Marcaccio M, Mrkobrada M, Paul J, Pettit S, Simunovic N, Srinathan S, Thorlund K, Worster A, Villar JC, Walsh M, Yusuf S, Chan MTV, Chan PLM, Choi GYS, Gin T, Lit LCW, Multi PC, Schünemann H, Vizza E, Agnes MB. , , An international prospective cohort study evaluating major vascular complications among patients undergoing noncardiac surgery: the VISION Pilot Study. Open Med 2011. [PMC free article] [PubMed] [Google Scholar]
- 26. Devereaux PJ, Goldman L, Yusuf S, Gilbert K, Leslie K, Guyatt GH.. Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: a review\r10.1503/cmaj.050316. CMAJ 2005;173:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MT V., Srinathan SK, Walsh M, Abraham V, Pearse R, Wang CY, Sessler DI, Kurz A, Szczeklik W, Berwanger O, Villar JC, Malaga G, Garg AX, Chow CK, Ackland G, Patel A, Borges FK, Belley-Cote EP, Duceppe E, Spence J, Tandon V, Williams C, Sapsford RJ, Polanczyk CA, Tiboni M, Alonso-Coello P. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642. [DOI] [PubMed] [Google Scholar]
- 28. Sessler DI, Conen D, Leslie K, Yusuf S, Popova E, Graham M, Kurz A, Villar JC, Mrkobrada M, Sigamani A, Biccard BM, Meyhoff CS, Parlow JL, Guyatt G, Xavier D, Chan MT V., Kumar PA, Forget P, Malaga G, Fleischmann E, Amir M, Torres D, Wang CY, Paniagua P, Berwanger O, Srinathan S, Landoni G, Manach Y Le, Whitlock R, Lamy A. One-year results of a factorial randomized trial of aspirin versus placebo and clonidine versus placebo in patients having noncardiac surgery. Anesthesiology 2020;132:692–701. [DOI] [PubMed] [Google Scholar]
- 29. Yoshihisa A, Sato T, Kajimoto K, Sato N, Takeishi Y.. Heterogeneous impact of body mass index on in-hospital mortality in acute heart failure syndromes: an analysis from the ATTEND Registry. Eur Hear J Acute Cardiovasc Care 2019;8:589–598. [DOI] [PubMed] [Google Scholar]
- 30. Kajimoto K, Minami Y, Otsubo S, Sato N.. Association of admission and discharge anemia status with outcomes in patients hospitalized for acute decompensated heart failure: differences between patients with preserved and reduced ejection fraction. Eur Hear J Acute Cardiovasc Care 2019;8:606–614. [DOI] [PubMed] [Google Scholar]
- 31. Shen JT, Xu M, Wu Y, et al. Association of pre-operative troponin levels with major adverse cardiac events and mortality after noncardiac surgery: A systematic review and meta-analysis. Eur J Anaesthesiol 2018;35:815–824. [DOI] [PubMed] [Google Scholar]
- 32. Thomas S, Borges F, Bhandari M, Beer J De, Urrútia Cuchí G, Adili A, Winemaker M, Avram V, Chan MTV, Lamas C, Cruz P, Aguilera X, Garutti I, Alonso-Coello P, Villar JC, Jacka M, Wang CY, Berwanger O, Chow C, Srinathan S, Pettit S, Heels-Ansdell D, Rubery P, Devereaux PJ, Thomas S, Walsh M, Tiboni M, Guyatt G, Heels-Ansdell D, Thorlund K. Association between myocardial injury and cardiovascular outcomes of orthopaedic surgery: a Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) substudy. J Bone Joint Surg Am 2020;102:880–888. [DOI] [PubMed] [Google Scholar]
- 33. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM, Group S of perioperative ischemia research.. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990;323:1781–1788. [DOI] [PubMed] [Google Scholar]
- 34. Devereaux PJ, Duceppe E, Guyatt G, Tandon V, Rodseth R, Biccard BM, Xavier D, Szczeklik W, Meyhoff CS, Vincent J, Franzosi MG, Srinathan SK, Erb J, Magloire P, Neary J, Rao M, Rahate PV, Chaudhry NK, Mayosi B, Nadal M de, Iglesias PP, Berwanger O, Villar JC, Botto F, Eikelboom JW, Sessler DI, Kearon C, Pettit S, Sharma M, Connolly SJ. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018;391:2325–2334. [DOI] [PubMed] [Google Scholar]
- 35. Du Fay De Lavallaz J, Puelacher C, Lurati Buse G, Bolliger D, Germanier D, Hidvegi R, Walter JE, Twerenbold R, Strebel I, Badertscher P, Sazgary L, Lampart A, Espinola J, Kindler C, Hammerer-Lercher A, Thambipillai S, Guerke L, Rentsch K, Buser A, Gualandro D, Jakob M, Mueller C. Daytime variation of perioperative myocardial injury in non-cardiac surgery and effect on outcome. Heart 2019;105:826–833 [DOI] [PubMed] [Google Scholar]
- 36. Botto F, Alonso-Coello P, Chan MTV, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120:564–578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.