Abstract
Aims
Determining which patients with pericardial effusion require urgent intervention can be challenging. We sought to develop a novel, simple risk prediction score for patients with pericardial effusion.
Methods and results
Adult patients admitted through the emergency department (ED) with pericardial effusion were retrospectively evaluated. The overall cohort was divided into a derivation and validation cohort for the generation and validation of a novel risk score using logistic regression. The primary outcome was a pericardial drainage procedure or death attributed to cardiac tamponade within 24 h of ED arrival. Among 195 eligible patients, 102 (52%) experienced the primary outcome. Four variables were selected for the novel score: systolic blood pressure < 100 mmHg (1.5 points), effusion diameter [1–2 cm (0 points), 2–3 cm (1.5 points), >3 cm (2 points)], right ventricular diastolic collapse (2 points), and mitral inflow velocity variation > 25% (1 point). The need for pericardial drainage within 24 h was stratified as low (<2 points), intermediate (2–4 points), or high (≥4 points), which corresponded to risks of 8.1% [95% confidence interval (CI) 3.0–16.8%], 63.8% [95% CI 50.1–76.0%], and 93.7% [95% CI 84.5–98.2%]. The area under the curve of the simplified score was 0.94 for the derivation and 0.91 for the validation cohort.
Conclusion
Among ED patients with pericardial effusion, a four-variable prediction score consisting of systolic blood pressure, effusion diameter, right ventricular collapse, and mitral inflow velocity variation can accurately predict the need for urgent pericardial drainage. Prospective validation of this novel score is warranted.
Keywords: Pericardial effusion, Tamponade, Prediction score, Echocardiography, Pericardiocentesis
Introduction
Pericardial effusions can vary widely with respect to their clinical importance.1 Small or chronic effusions can often be expectantly monitored or medically treated while larger and rapidly accumulating effusions may result in potentially life-threatening cardiac tamponade.2 Patients with impending tamponade require urgent drainage of the pericardial fluid in order to prevent clinical decompensation and cardiac arrest.2
Although pericardial effusions are often diagnosed in the emergency department (ED), identifying patients who are at risk for developing cardiac tamponade and who require urgent drainage remains challenging.3 Traditionally, cardiac tamponade has been considered a clinical diagnosis, and multiple clinical variables as well as echocardiographic findings have been proposed as markers of tamponade physiology.1,2,4–9 However, the decision regarding whether a patient requires urgent pericardial drainage largely remains a subjective assessment, which may differ from physician to physician. A previously proposed scoring system by Halpern et al.10,11 incorporated a combination of echocardiographic measurements including right atrial collapse, right ventricular collapse, respiratory flow variation across the mitral valve and size of effusion as well as clinical variables including malignant aetiology, immunocompromise aetiology, effusion due to an unidentified cause, effusion failing medical treatment and recurrent effusion in order to identify patients who require pericardial drainage. However, this prediction score was not statistically generated and has not been independently validated.10
The objectives of this study were to create a novel, simplified score for predicting which patients with a pericardial effusion require urgent pericardial drainage, as well as to validate a previously proposed scoring system.10
Methods
Study design and selection of participants
This was a retrospective cohort study of adult patients who were admitted through the ED of an academic, tertiary centre from January 2015 to March 2018 and were given an ED or discharge ICD-9 or ICD-10 diagnosis related to pericardial effusion. Patients were eligible if they underwent a transthoracic echocardiogram performed by the cardiology department within 24 h of ED arrival that demonstrated a pericardial effusion of ≥1 cm in diastole.
Exclusion criteria were: traumatic effusion, effusion attributed to aortic dissection, septic effusion, patients who underwent pericardial drainage prior to cardiology echocardiogram, and death within 24 h of ED arrival not attributed to cardiac tamponade. This study was approved by the Partners Healthcare Institutional Review Board and informed consent was not required due to the retrospective and observational study design.
Measurements
Subjects’ electronic medical records were reviewed by trained physician investigators blinded to echocardiographic and outcomes data to collect possible covariates for the need for urgent pericardial drainage from clinical data as documented in the ED note and if missing, in the inpatient admission note. These included history, exam, laboratory, and diagnostic variables that have previously been associated with cardiac tamponade, including but not limited to those proposed by Halpern et al.1,2,4–10 To determine the aetiology of the pericardial effusion, the electronic medical records were reviewed by physician investigators blinded to the echocardiogram and clinical outcomes. Data were abstracted onto a standardized data collection form on a secure online database.
Electrocardiograms (EKGs) were reviewed by a board-certified emergency physician blinded to clinical outcomes in order to assess for the presence of low voltage (defined as QRS amplitudes of <5 mm in the limb leads and/or <10 mm in the precordial leads), atrial arrhythmia, electrical alternans (defined as alternating QRS amplitudes in one or more leads), ST-segment elevation >0.1 mV in three or more consecutive leads or PR segment depression >0.1 mV in three or more consecutive leads. The presence of cardiomegaly on chest X-ray was determined based on radiology attending interpretation.
Echocardiographic measurements
Transthoracic echocardiography was performed according to a standardized protocol using ultrasound equipment by three different manufacturers (General Electric, Milwaukee, WI, USA; Philips, Bothell, WA, USA; Siemens, Erlangen, Germany) using 2–5 MHz phased array transducers. The images were digitally stored and independently reviewed using echocardiographic software (Syngo Dynamics, Siemens, Malvern, PA, USA) by an ultrasound fellowship trained emergency physician (Y.D.) blinded to clinical outcomes following additional training by two senior physicians with expertise in echocardiography (E.P. and J.W.). Each subject’s echocardiographic images were assessed for the presence of sonographic findings that have been associated with cardiac tamponade in the literature.5–10Quantification of the diameter of pericardial effusion, right atrial and ventricular collapse, mitral and tricuspid inflow velocity variation was performed according to American Society of Echocardiography guidelines.12
Diameter of pericardial effusion was measured perpendicular to the pericardium at the widest point from the pericardium to the epicardium in diastole in the parasternal long, parasternal short, apical four-chamber, or subxiphoid views. Right atrial collapse was defined as inversion or indentation of the right atrium free wall for over 30% of the cardiac cycle in any of the above four views. Right ventricular collapse was defined as inversion or indentation of the right ventricular free wall during diastole in any of the above four views. Mitral valve inflow velocity variation was measured by calculating the percentage change between the maximum mitral valve E-wave velocity in expiration and the minimum E-wave velocity in inspiration as assessed with pulse wave Doppler in the apical four-chamber view; with variation >25% considered positive. Tricuspid valve inflow velocity variation was measured by calculating the percentage change between the maximum tricuspid E-wave velocity in inspiration and the minimum E-wave velocity in expiration as assessed with pulse wave Doppler in the apical four-chamber view; with variation >40% considered positive. inferior vena cava (IVC) diameter variation was assessed by calculating the percentage difference between the maximum and minimum IVC diameter measured just distal to the hepatic vein with variation <50% considered positive.12
Outcomes
The primary outcome was a pericardial drainage procedure or death attributed to cardiac tamponade (by review of the discharge summary) within 24 h of ED arrival. Pericardial drainage procedures included bedside pericardiocentesis, catheterization laboratory pericardiocentesis with or without insertion of a pericardial drain and surgical pericardial drainage. One of three physician investigators blinded to the clinical variables and echocardiographic image analyses reviewed each patient’s electronic medical record to assess for the primary outcome.
Secondary outcome analyses included all-cause death during inpatient hospitalization, need for intensive care unit (ICU) admission, and the need for vasopressors for blood pressure support during the hospital course.
Primary data analysis
Sample size justification
In order to achieve adequate discrimination for a logistic regression model, we sought to have 10 or more outcome events per independent predictor variable.13 We anticipated that approximately half of the subjects would experience the primary outcome. With a cohort of 200 patients, we would be able to perform multivariable logistic regression on a derivation and validation dataset of 100 patients each for the creation of a score with up to 5 predictor variables. As the Halpern et al. score included 10 independent variables, a sample size of 200 patients would also be sufficient to validate this scoring system.
Statistical analyses
In order to create a scoring system for the risk of requiring urgent pericardial drainage, a univariate screen was first performed between the clinical and echocardiographic variables and the primary outcome of pericardial drainage or death from tamponade within 24 h. Variables with a priori clinical value or found to be significant predictors were considered for selection in a multivariate logistic regression analysis. Significance was determined at the 0.1 significance level with a χ2 test of proportion, t-test, or the Wilcoxon Rank-Sum test, as appropriate. Variables expected to be collinear with more significant predictors were excluded from regression modelling. Missing data for predictor variables considered for inclusion in the multivariable logistic regression was addressed by applying multiple imputation using the Markov Chain Monte Carlo method with ten imputed datasets.14
The dataset was randomized into a derivation and a validation cohort of similar sizes by applying an equal probability selection method. Multivariable logistic regression with stepwise selection was applied on the derivation dataset to select the variables to be included in the simplified prediction score. The regression coefficient (β) for each selected variable was used to assign a point value to that predictor for the creation of risk groups as described by Han et al.15 The diagnostic accuracy of the novel prediction model for the derivation and validation datasets was assessed using area under the receiver-operating curve (AUC) (c-statistic), while the calibration of the model was assessed using the Hosmer–Lemeshow goodness-of-fit test.16
Multivariable logistic regression with AUC analysis was used to assess the accuracy of the scoring system proposed by Halpern et al.10 In their original paper, the primary outcome was a pericardial drainage procedure. We narrowed the outcome definition to drainage within 24 h in order to assess the ability of the score to predict clinically important pericardial effusions requiring urgent drainage.
Data analysis for this article was performed using SAS software, Version 9.4 of the SAS System © 2013, SAS Institute Inc., Cary, NC, USA. Findings are reported according to guidelines of the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) Statement from the Enhancing the Quality and Transparency of Health Research (EQUATOR) Network.17
Results
Characteristics of study subjects
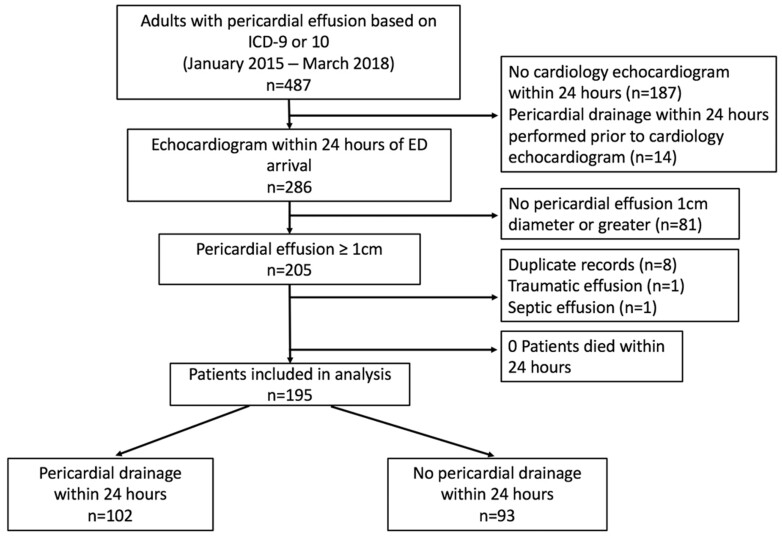
A total of 487 patients were identified by search criteria for ED or inpatient diagnoses associated with pericardial effusion (Figure 1). Among the eligible 195 patients [mean age 60, 51% men, 81% White], 102 (52%) underwent urgent pericardial drainage within 24 h. No patients died within 24 h of ED arrival. The associations between potential predictor variables and the primary outcome are displayed in Table 1.
Figure 1.
Patient selection.
Table 1.
Univariate analysis of potential predictors in patients requiring vs. not requiring pericardial drainage within 24 h
| Missing data (%) | Not drained within 24 h (N = 93) | Drained within 24 h (N = 102) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (SD) | — | 61.9 (14.4) | 58.6 (15.4) | 0.13 |
| Women, n (%) | 46 (49.5%) | 50 (49.0%) | 0.95 | |
| Race and Ethnicity | — | 0.04 | ||
| Hispanic | 5 (5.4%) | 1 (1.0%) | ||
| Non-Hispanic Black | 14 (15.1%) | 4 (3.9%) | ||
| Non-Hispanic White | 68 (73.1%) | 90 (88.2%) | ||
| Non-Hispanic Asian | 3 (3.2%) | 3 (2.9%) | ||
| Other/Unk | 3 (3.2%) | 4 (3.9%) | ||
| Signs and symptoms | ||||
| Dyspnoea | 1 (0.5%) | 71 (76.3%) | 84 (83.2%) | 0.24 |
| Chest pain | 2 (1%) | 46 (49.5%) | 40 (40.0%) | 0.19 |
| Orthopnoea | 70 (35%) | 16 (26.7%) | 15 (23.1%) | 0.64 |
| Syncope | 47 (24%) | 4 (5.6%) | 3 (3.9%) | 0.62 |
| Physical exam | ||||
| Systolic BP (mmHg) (SD) | — | 134 (29.9) | 120 (22.2) | <0.001 |
| Diastolic BP (mmHg) (SD) | — | 75.9 (16.8) | 71.1 (13.0) | 0.03 |
| Heart rate (b.p.m.) (SD) | — | 91.6 (19.7) | 101.9 (22.6) | <0.001 |
| Respiratory rate (per min) (IQR) | 1 (0.5%) | 18 (18–20) | 18 (18–20) | 0.47 |
| Pulsus paradoxus (mmHg) (SD) | 63 (32%) | 9.38 (3.7) | 12.9 (5.2) | <0.001 |
| Muffled heart sounds | 30 (15%) | 23 (31.1%) | 42 (46.2%) | 0.05 |
| Pericardial friction rub | 17 (9%) | 4 (4.8%) | 7 (7.5%) | 0.46 |
| Elevated JVP | 34 (17%) | 45 (58.4%) | 60 (71.4%) | 0.08 |
| Medical history | ||||
| Malignancy | — | 38 (40.9%) | 56 (54.9%) | 0.05 |
| Advanced renal disease | — | 11 (11.8%) | 9 (8.8%) | 0.49 |
| Autoimmune disorder | — | 8 (8.6%) | 7 (6.9%) | 0.65 |
| Anticoagulant use | — | 54 (58.1%) | 48 (47.1%) | 0.12 |
| Recurrent effusion | — | 12 (12.9%) | 18 (17.7%) | 0.36 |
| Treatment resistant effusion | — | 12 (12.9%) | 12 (11.8%) | 0.81 |
| Laboratory values | ||||
| BUN (mg/dL) (IQR) | — | 18 (12–29) | 17 (12–29) | 0.55 |
| Creatinine (mg/dL) (IQR) | — | 0.96 (0.75–1.28) | 0.90 (0.70–1.19) | 0.20 |
| Platelets (103/μL) (IQR) | — | 289 (211–381) | 312 (237–420) | 0.08 |
| Troponin T (ng/mL) | 26 (13%) | 0 | 0 | 0.98 |
| NT-proBNP (pg/mL) (IQR) | 64 (33%) | 1113 (389–3389) | 566 (261–1093) | 0.10 |
| EKG | ||||
| Low voltage | 2 (1%) | 29 (31.5%) | 44 (43.6%) | 0.08 |
| Atrial arrhythmia | 2 (1%) | 8 (8.7%) | 17 (16.8%) | 0.10 |
| Electrical alternans | 2 (1%) | 6 (6.5%) | 10 (9.9%) | 0.40 |
| ST elevation | 2 (1%) | 7 (7.61%) | 9 (8.91%) | 0.74 |
| PR depression | 2 (1%) | 8 (8.7%) | 15 (14.9%) | 0.19 |
| Chest X-ray | ||||
| Cardiomegaly, n (%) | 44 (23%) | 46 (63.9%) | 58 (73.4%) | 0.21 |
| Echocardiography | ||||
| Diameter of effusion, cm (SD) | — | 1.85 (0.75) | 2.87 (0.89) | <0.001 |
| Right atrial collapse, n (%) | 2 (1%) | 45 (49.5%) | 85 (84.2%) | <0.001 |
| Right ventricular collapse, n (%) | — | 17 (18.3%) | 79 (78.2%) | <0.001 |
| Tricuspid valve inflow variation (%) (SD) | 24 (12%) | 28.0 (8.9) | 33.9 (10.2) | <0.001 |
| Mitral valve inflow variation (%) (SD) | 13 (7%) | 19.3 (9.4) | 27.9 (8.9) | <0.001 |
| IVC respiratory variation (%) (SD) | 7 (4%) | 31.3 (21.7) | 22.2 (19.1) | 0.001 |
| Aetiology of effusion | ||||
| Pericarditis | — | 26 (28.0%) | 13 (12.8%) | 0.008 |
| Malignancy | — | 29 (31.2%) | 51 (50.0%) | 0.008 |
| Autoimmune | — | 4 (4.3%) | 3 (2.9%) | 0.61 |
| Heart failure | — | 0 | 1 (1.0%) | 0.34 |
| Renal failure | — | 5 (5.4%) | 3 (2.9%) | 0.39 |
| Post-procedural | — | 15 (16.1%) | 17 (16.7%) | 0.92 |
| Other | — | 3 (3.2%) | 8 (7.8%) | 0.16 |
| Unknown | — | 11 (11.8%) | 6 (5.9%) | 0.14 |
Data are presented as n (%) for categorical variables, mean (SD) for normally distributed continuous variables, and mean (IQR) for non-normally distributed continuous variables.
Treatment resistant effusion is defined as previously being on medical treatment for pericardial effusion without resolution of effusion. Advanced renal disease is considered creatinine > 2.5. Elevated JVP defined as greater than 8 cm H2O.
BP, blood pressure; BUN, blood urea nitrogen; IVC, inferior vena cava; JVP, jugular venous pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Novel pericardial effusion score
The incidence of the primary outcome was 57% in the derivation cohort and 48% in the validation cohort. Variables selected for inclusion in the multivariate analysis due to significant independent association with the primary outcome or a priori clinical significance were age, race, systolic blood pressure < 100, heart rate > 100, muffled heart sounds, elevated jugular venous pressure (JVP), history of malignancy, low-voltage EKG, platelets < 100, diameter of effusion (1–2 cm, 2–3 cm, >3 cm), right atrial collapse, right ventricular collapse, mitral valve inflow velocity variation > 25%, IVC respiratory variation < 50%, pericarditis aetiology of effusion, and malignancy aetiology of effusion (Table 1). Diastolic blood pressure was not included because it was collinear with systolic blood pressure. Pulsus paradoxus and tricuspid valve inflow velocity variation > 40% had P-values of <0.05 in the univariate analysis but were not included because of the high rate of missing data, and because these variables were expected to be collinear with mitral valve inflow velocity variation > 25%.
After including the above variables in a logistic regression analysis with stepwise selection in the derivation dataset, four predictors were selected for inclusion in the final scoring system: systolic blood pressure < 100 mmHg, effusion diameter (1–2 cm, 2–3 cm, or >3 cm), right ventricular diastolic collapse, and mitral valve inflow variation >25%. The point values assigned to each predictor variable are outlined in Table 2. The derivation dataset had a c-statistic of 0.94 and the validation dataset had a c-statistic of 0.91 in determining the risk of the primary outcome (Figure 2). The Hosmer–Lemeshow goodness-of-fit test resulted in scores of 8.6 (P = 0.20) and 2.4 (P = 0.78) in the derivation and validation datasets, respectively, with non-significant P-values indicating good model calibration.
Table 2.
Model selected prediction variables for requiring urgent pericardial effusion drainage
| Variables | Category | Beta (95% CI) | Odds ratio (95% CI) | P-value | Points |
|---|---|---|---|---|---|
| Systolic blood pressure <100 mmHg | No | — | — | — | 0 |
| Yes | 2.6 | 13.9 | 0.015 | 1.5 | |
| (0.5–4.8) | (1.7–117.0) | ||||
| Effusion diameter | 1–2 cm | — | — | — | 0 |
| 2–3 cm | 2.5 | 12.4 | 0.002 | 1.5 | |
| (0.9–4.1) | (2.5–60.0) | ||||
| >3 cm | 3.3 | 27.0 | 0.002 | 2 | |
| (1.3–5.3) | (3.5–209.0) | ||||
| Right ventricular collapse | No | — | — | — | 0 |
| Yes | 3.2 | 25.1 | <0.001 | 2 | |
| (1.7–4.8) | (5.4–117.2) | ||||
| Mitral valve flow variation >25% | No | — | — | — | 0 |
| Yes | 1.8 | 6.3 | 0.018 | 1 | |
| (0.3–3.3) | (1.4–28.2) |
The boldface values are the point values assigned to each variable of the prediction score.
Figure 2.

Receiver operating characteristic (ROC) curves of the novel four variable pericardial effusion scoring model in the derivation (n = 94) and validation (n = 101) datasets.
The risk of requiring pericardial drainage within 24 h was stratified as low (<2 points), intermediate (2–4 points), and high (≥4 points), which corresponded to risks of 8.1% [95% confidence interval (CI) 3.0–16.8%], 63.8% [95% CI 50.1–76.0%], and 93.7% [95% CI 84.5–98.2%] in the combined cohort. The risk of requiring urgent pericardial drainage across the three risk groups was similar in the derivation and validation cohorts (Figure 3).
Figure 3.
Comparison of the risk group stratification in the derivation and validation cohorts.
With the aim of increasing accessibility for point-of-care (POC) ultrasound practitioners, we also assessed the performance of the scoring system without the inclusion of the mitral valve inflow velocity variable. This prediction model consisting of only systolic blood pressure < 100 mmHg, effusion diameter (1–2 cm, 2–3 cm, or >3 cm) and right ventricular diastolic collapse had good model fit with a c-statistic of 0.91 in the derivation and 0.89 in the validation datasets.
Secondary analyses
All-cause death during hospital admission occurred in seven patients (3.6%). The in-hospital mortality rate was similar between patients who underwent drainage within 24 h and those who did not (3.9% vs. 3.2%, P = 0.79). There were no in-hospital deaths attributed to cardiac tamponade. Admission to an ICU was more common among those who required urgent pericardial drainage (33.3%) compared to those who did not (11.8%, P < 0.001). Similarly, patients who required urgent pericardial drainage were more likely to be treated with intravenous vasopressors (14.7%) compared to those who did not (2.15%, P = 0.002).
Validation of prior 10-point pericardial effusion score
By applying logistic regression using the variables identified in the Halpern scoring index to our full dataset of 195 subjects, their scoring index predicted the need for urgent pericardial drainage with an AUC of 0.91.
Discussion
This retrospective, observational cohort study in patients admitted through the ED with moderate or larger pericardial effusions at risk for cardiac tamponade found that a simplified prediction score with only four variables achieved the same accuracy in predicting the need for urgent pericardial drainage as a more complex scoring system that was previously proposed.
Prior investigations have sought to identify individual predictors that are associated with cardiac tamponade. In a systematic review of 300 patients with pericardial effusion, the variables with the highest sensitivities for tamponade included dyspnoea (sensitivity 88%), tachycardia (sensitivity 77%), elevated JVP (sensitivity 76%), and pulsus paradoxus (sensitivity 82%).1 Other studies have aimed to determine which echocardiographic findings are most commonly associated with cardiac tamponade. Mercé et al. proposed that the most important echocardiographic findings predicting tamponade were right atrial collapse (sensitivity 90%, specificity 66%) and right ventricular collapse (sensitivity 68%, specificity 90%), while others found that increased respiratory variation in flow across the mitral or tricuspid valves indicated impending tamponade.5–9
To the best of our knowledge, this is the first study to use statistical modelling techniques to generate and internally validate a multivariable prediction score for clinically significant pericardial effusion. Two previously proposed scoring systems with 10 and 21 variables, respectively were both created by choosing variables solely on a priori clinical significance, as opposed to statistical modelling techniques.10,18 In their original paper, Halpern et al. reported an AUC of 0.91; however, this was based on a small sample of patients (n = 48).
POC ultrasound has become an essential tool in the practice of an increasing number of medical specialties including emergency medicine and internal medicine. Although the echocardiographic data utilized in this study were collected from examinations performed by the cardiology department, the measures included in our prediction score could also be collected by practitioners at the bedside in order to make timely clinical decisions for patients with pericardial effusion.19 Diameter of effusion and right ventricular diastolic collapse are concepts traditionally taught in POC ultrasound training. Assessment of mitral valve inflow velocity variation is a more advanced ultrasound measurement which may not be within the skillset of all POC ultrasound practioners.20 A further simplified three-variable score consisting of only systolic blood pressure < 100 mmHg, effusion diameter, and right ventricular systolic collapse also performed well with only a slightly lower c-statistic (0.91 vs. 0.94) than the four-variable score in the derivation dataset. The prospective validity of our four-variable scoring criteria or the further simplified three-variable scoring criteria calculated using POC ultrasound data warrants further study.
By stratifying patients into low-, medium-, and high-risk categories, physicians may be able to better triage the treatment of patients who present to the ED with pericardial effusion. As low-risk patients are less likely to require urgent drainage, it may be appropriate to consider whether these patients could be observed for clinical progression as inpatients or in some cases as an outpatient. Intermediate-risk patients in our cohort had a significant likelihood to need pericardial drainage and would likely benefit from urgent cardiology evaluation for drainage or admission with the availability of an urgent drainage procedure. Patients in the high-risk group were extremely likely to require intervention within 24 h and should generally be treated as critical patients with resources available for an emergent pericardiocentesis. The significance of these point categories should be interpreted within each individual clinical and hospital context.
Our novel pericardial effusion prediction score includes only four variables that could be collected at the bedside by emergency medicine, internal medicine or cardiology physicians. We anticipate that this prediction score will help alleviate some of the uncertainty involved in the decisions about treatment and disposition for patients with pericardial effusion. It has been shown that a variety of prediction rules can provide improved diagnostic accuracy compared to clinical judgement alone.21 Primarily, the purpose of this prediction score is to improve patient outcomes by aiding in the identification of patients with impending cardiac tamponade so that they can undergo pericardial drainage in a timely manner.
Limitations
A limitation of this study is the retrospective nature of the design. Despite the specified search criteria, we may not have identified all patients who would have been eligible for inclusion in our study cohort. However, we used ICD codes to identify study patients and believe that this approach minimized bias. The incidence of patients who had the primary outcome was relatively high. In order to obtain sufficient echocardiographic data for building the scoring system, we only included patients who underwent an echocardiogram within 24 h of hospital arrival, which may have introduced selection bias.
This was a single-centre study, limiting its generalizability to other settings. It is possible that the prevalence of cancer-related pericardial effusions is higher at our institution due to the affiliation with a large oncology centre. It is unclear whether a prediction score created with data from this patient population will be reproducible in other populations around the country and around the world. It would be useful to attempt to validate this scoring system in other clinical settings. Although the echocardiography examinations were interpreted by an emergency physician for the purposes of this study, the images were obtained by echocardiographers from the cardiology department. This may limit the generalizability of utilizing this score with images obtained using POC ultrasound.
Our primary outcome was chosen as a marker for cardiac tamponade. However, the decision about whether to pursue urgent intervention was made by physicians who were not blinded to the history, physical exam and echocardiographic data. The perceived relevance of certain clinical and echocardiographic variables by treating physicians likely impacted the decision to perform pericardial drainage, and therefore impacted the findings of this study.
Conclusions
Among patients with moderate-size or larger pericardial effusion admitted through the ED, a novel, simple scoring system consisting of systolic blood pressure, effusion diameter, right ventricular collapse, and mitral valve inflow velocity variation by echocardiography had good accuracy in predicting the need for urgent pericardial drainage. Prospective validation of this novel scoring system is warranted.
Data availability
The data underlying this article cannot be shared due to privacy regulations from the institutional review board.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Conflict of interest: E.P. reports grants from NIH/NHLBI, grants from NIH/NIDDK, and other from Novartis, which is outside the submitted work. S.T. is currently employed by Genentech, Inc., but had no commercial associations during work on this project. The remaining authors have no commercial associations or sources of support that might pose a conflict of interest.
Supplementary Material
References
- 1. Roy CL, Minor MA, Brookhart MA, Choudhry NK.. Does this patient with a pericardial effusion have cardiac tamponade? JAMA 2007;297:1810. [DOI] [PubMed] [Google Scholar]
- 2. Spodick DH. Acute cardiac tamponade. N Engl J Med 2003;349:684–690. [DOI] [PubMed] [Google Scholar]
- 3. Argulian E, Messerli F.. Misconceptions and facts about pericardial effusion and tamponade. Am J Med 2013;126:858–861. [DOI] [PubMed] [Google Scholar]
- 4. Fowler NO. Cardiac tamponade. A clinical or an echocardiographic diagnosis? Circulation 1993;87:1738–1741. [DOI] [PubMed] [Google Scholar]
- 5. Mercé J, Sagristà-Sauleda J, Permanyer-Miralda G, Evangelista A, Soler-Soler J.. Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. Am Heart J 1999;138:759–764. [DOI] [PubMed] [Google Scholar]
- 6. Appleton CP, Hatle LK, Popp RL.. Cardiac tamponade and pericardial effusion: respiratory variation in transvalvular flow velocities studied by Doppler echocardiography. J Am Coll Cardiol 1988;11:1020–1030. [DOI] [PubMed] [Google Scholar]
- 7. Leeman DE, Levine MJ, Come PC.. Doppler echocardiography in cardiac tamponade: exaggerated respiratory variation in transvalvular blood flow velocity integrals. J Am Coll Cardiol 1988;11:572–578. [DOI] [PubMed] [Google Scholar]
- 8. D’Cruz IA, Cohen HC, Prabhu R, Glick G.. Diagnosis of cardiac tamponade by echocardiography: changes in mitral valve motion and ventricular dimensions, with special reference to paradoxical pulse. Circulation 1975;52:460–465. [DOI] [PubMed] [Google Scholar]
- 9. Materazzo C, Piotti P, Meazza R, Pellegrini MP, Viggiano V, Biasi S.. Respiratory changes in transvalvular flow velocities versus two-dimensional echocardiographic findings in the diagnosis of cardiac tamponade. Ital Heart J 2003;4:186–192. [PubMed] [Google Scholar]
- 10. Halpern DG, Argulian E, Briasoulis A, Chaudhry F, Aziz EF, Herzog E.. A novel pericardial effusion scoring index to guide decision for drainage. Crit Pathw Cardiol 2012;11:85–88. [DOI] [PubMed] [Google Scholar]
- 11. Arguilian E, Halpern DG, Aziz EF, Uretsky S, Chaudhry F, Herzog E.. Novel “CHASER” pathway for the management of pericardial disease. Crit Pathw Cardiol 2011;10:57–63. [DOI] [PubMed] [Google Scholar]
- 12. Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, Hung J, Garcia MJ, Kronzon I, Oh JK, Rodriguez ER, Schaff HV, Schoenhagen P, Tan CD, White RD.. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013;26:965–1012. [DOI] [PubMed] [Google Scholar]
- 13. Harrell FE, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 14. Johnson DR, Young R.. Toward best practices in analyzing datasets with missing data: comparisons and recommendations. J Marriage Fam 2011;73:926–945. [Google Scholar]
- 15. Han K, Song K, Choi BW.. How to develop, validate, and compare clinical prediction models involving radiological parameters: study design and statistical methods. Korean J Radiol 2016;17:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosmer DW, Lemeshow S.. Applied Logistic Regression. 2nd ed.New York: Wiley; 2000. [Google Scholar]
- 17. Collins GS, Reitsma JB, Altman DG, Moons KG.. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. Eur J Clin Invest 2015;45:204–214. [DOI] [PubMed] [Google Scholar]
- 18. Ristić AD, Imazio M, Adler Y, Anastasakis A, Badano LP, Brucato A, Caforio AL, Dubourg O, Elliott P, Gimeno J, Helio T, Klingel K, Linhart A, Maisch B, Mayosi B, Mogensen J, Pinto Y, Seggewiss H, Seferović PM, Tavazzi L, Tomkowski W, Charron P.. Triage strategy for urgent management of cardiac tamponade: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2014;35:2279–2284. [DOI] [PubMed] [Google Scholar]
- 19. Perera P, Mandavia D, Goodman A, Mailhot T.. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock 2012;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adhikari S, Fiorello A, Stolz L, Jones T, Amini R, Gross A, O'Brien K, Mosier J, Blaivas M.. Ability of emergency physicians with advanced echocardiographic experience at a single center to identify complex echocardiographic abnormalities. Am J Emerg Med 2014;32:363–366. [DOI] [PubMed] [Google Scholar]
- 21. Adams ST, Leveson SH.. Clinical prediction rules. BMJ 2012;344:d8312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared due to privacy regulations from the institutional review board.