Abstract
Background
Peripheral arterial disease (PAD) affects between 4% and 12% of people aged 55 to 70 years, and 20% of people over 70 years. A common complaint is intermittent claudication (exercise‐induced lower limb pain relieved by rest). These patients have a three‐ to six‐fold increase in cardiovascular mortality. Cilostazol is a drug licensed for the use of improving claudication distance and, if shown to reduce cardiovascular risk, could offer additional clinical benefits. This is an update of the review first published in 2007.
Objectives
To determine the effect of cilostazol on initial and absolute claudication distances, mortality and vascular events in patients with stable intermittent claudication.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL, and AMED databases, and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registries, on 9 November 2020.
Selection criteria
We considered double‐blind, randomised controlled trials (RCTs) of cilostazol versus placebo, or versus other drugs used to improve claudication distance in patients with stable intermittent claudication.
Data collection and analysis
Two authors independently assessed trials for selection and independently extracted data. Disagreements were resolved by discussion. We assessed the risk of bias with the Cochrane risk of bias tool. Certainty of the evidence was evaluated using GRADE. For dichotomous outcomes, we used odds ratios (ORs) with corresponding 95% confidence intervals (CIs) and for continuous outcomes we used mean differences (MDs) and 95% CIs. We pooled data using a fixed‐effect model, or a random‐effects model when heterogeneity was identified. Primary outcomes were initial claudication distance (ICD) and quality of life (QoL). Secondary outcomes were absolute claudication distance (ACD), revascularisation, amputation, adverse events and cardiovascular events.
Main results
We included 16 double‐blind, RCTs (3972 participants) comparing cilostazol with placebo, of which five studies also compared cilostazol with pentoxifylline. Treatment duration ranged from six to 26 weeks. All participants had intermittent claudication secondary to PAD. Cilostazol dose ranged from 100 mg to 300 mg; pentoxifylline dose ranged from 800 mg to 1200 mg. The certainty of the evidence was downgraded by one level for all studies because publication bias was strongly suspected. Other reasons for downgrading were imprecision, inconsistency and selective reporting.
Cilostazol versus placebo
Participants taking cilostazol had a higher ICD compared with those taking placebo (MD 26.49 metres; 95% CI 18.93 to 34.05; 1722 participants; six studies; low‐certainty evidence). We reported QoL measures descriptively due to insufficient statistical detail within the studies to combine the results; there was a possible indication in improvement of QoL in the cilostazol treatment groups (low‐certainty evidence). Participants taking cilostazol had a higher ACD compared with those taking placebo (39.57 metres; 95% CI 21.80 to 57.33; 2360 participants; eight studies; very‐low certainty evidence). The most commonly reported adverse events were headache, diarrhoea, abnormal stools, dizziness, pain and palpitations. Participants taking cilostazol had an increased odds of experiencing headache compared to participants taking placebo (OR 2.83; 95% CI 2.26 to 3.55; 2584 participants; eight studies; moderate‐certainty evidence).Very few studies reported on other outcomes so conclusions on revascularisation, amputation, or cardiovascular events could not be made.
Cilostazol versus pentoxifylline
There was no difference detected between cilostazol and pentoxifylline for improving walking distance, both in terms of ICD (MD 20.0 metres, 95% CI ‐2.57 to 42.57; 417 participants; one study; low‐certainty evidence); and ACD (MD 13.4 metres, 95% CI ‐43.50 to 70.36; 866 participants; two studies; very low‐certainty evidence). One study reported on QoL; the study authors reported no difference in QoL between the treatment groups (very low‐certainty evidence). No study reported on revascularisation, amputation or cardiovascular events. Cilostazol participants had an increased odds of experiencing headache compared with participants taking pentoxifylline at 24 weeks (OR 2.20, 95% CI 1.16 to 4.17; 982 participants; two studies; low‐certainty evidence).
Authors' conclusions
Cilostazol has been shown to improve walking distance in people with intermittent claudication. However, participants taking cilostazol had higher odds of experiencing headache. There is insufficient evidence about the effectiveness of cilostazol for serious events such as amputation, revascularisation, and cardiovascular events. Despite the importance of QoL to patients, meta‐analysis could not be undertaken because of differences in measures used and reporting. Very limited data indicated no difference between cilostazol and pentoxifylline for improving walking distance and data were too limited for any conclusions on other outcomes.
Plain language summary
Cilostazol for peripheral arterial disease
Background
Blockages in the arteries to the legs ‐ peripheral arterial disease ‐ affect 20% of people aged over 70 years and 4% to 12% of people aged 55 to 70 years. Approximately 40% of those with peripheral arterial disease complain of pain in the legs or buttocks that occurs with exercise and subsides with rest. This is known as intermittent claudication and these symptoms are an indicator for the development of blocked arteries elsewhere in the body. People with intermittent claudication have a three‐ to six‐fold increased chance of dying as a result of cardiovascular events compared to people of the same age without intermittent claudication.
People with intermittent claudication are treated with best medical management which includes modifying risk factors, such as stopping smoking, and doing structured exercise. Further cardiovascular risk modification includes treatment for high blood pressure, diabetes and cholesterol reduction. In practice, compliance with best medical treatment is poor and most people continue to have symptoms of intermittent claudication. Some drug therapies, such as cilostazol, are used to help improve symptoms of intermittent claudication and so we examined the evidence to see if cilostazol improved walking distance, quality of life and other important outcomes compared to placebo (dummy pill) or other drugs used for intermittent claudication.
Study characteristics and key results
We included 16 double‐blind, randomised controlled trials, with 3972 adults (search up to 9 November 2020). Participants taking cilostazol for three to six months could walk approximately 26 metres further before calf pain and 40 metres further in total compared to participants taking placebo. However, participants taking cilostazol had nearly three times the odds of experiencing headache related to study medication. There is currently not enough information about the effectiveness of cilostazol for serious events such as amputation, revascularisation and cardiovascular events. Despite its importance, only four studies reported quality of life, using different tools and ways of reporting. Very limited data indicated no difference between cilostazol and pentoxifylline for improving walking distance, and there was not enough information comparing cilostazol with pentoxifylline, for any other outcomes.
Certainty of the evidence
We judged the evidence to be 'very low' to 'low‐certainty' for all outcomes except headaches, which were 'moderate‐certainty'. All studies were downgraded because we strongly suspected publication bias from drug company involvement.
Conclusion
Cilostazol can increase the distance walked both in total and before the onset of pain, compared to placebo. Cilostazol was associated with increased headaches and there was a lack of evidence for other important outcomes such as amputation, revascularisation and cardiovascular events.
Summary of findings
Background
Description of the condition
Lower limb peripheral arterial disease (PAD) is a manifestation of atherosclerosis in the lower extremities, affecting 20% of people over 70 years of age and 4% to 12% of the population aged 55 to 70 years (Dormandy 1999; PAD 2003). Patients with PAD commonly complain of intermittent claudication, which is characterised by pain in the legs or buttocks that occurs with exercise and subsides with rest, and occurs in 40% of PAD patients (Dormandy 1999). Despite the relatively benign prognosis for the affected limb, the symptoms of intermittent claudication are an indicator for systemic atherosclerosis. Compared with age‐matched controls, people with intermittent claudication have a three‐ to six‐fold increase in cardiovascular mortality (Leng 1996). About 4% of people with intermittent claudication will require amputation over five years of follow‐up (Leng 1996).
The majority of patients with intermittent claudication are treated with best medical treatment (Khan 2005), and the mainstay of treatment for patients with PAD is cardiovascular risk factor modification. This consists of smoking cessation, prescribed exercise (Lane 2017), antiplatelet treatment, lipid‐lowering therapy and control of blood pressure and diabetes. Only two‐thirds of compliant patients will achieve symptomatic relief of intermittent claudication after three to six months. Some patients may not be able to comply with prescribed exercise due to associated comorbidity or social reasons. As angioplasty or surgery are only used in severe, disabling or progressive intermittent claudication, these symptomatic patients may benefit from adjunctive therapy.
Description of the intervention
Cilostazol, with the trade name Pletal, is a phosphodiesterase‐III inhibitor that has antiplatelet and antithrombotic actions (Sallustio 2010). Cilostazol also acts on smooth muscle cells as a vasodilator with beneficial effects on triglycerides and high‐density lipoproteins (Chapman 2003). Cilostazol is indicated for intermittent claudication but there is also evidence to suggest that cilostazol may have a role in reducing restenosis after endovascular therapy and coronary stenting (Iida 2008; Lee 2013). The suggested dose of cilostazol for intermittent claudication is 100 mg taken orally twice daily. Cilostazol is contraindicated in patients with congestive heart failure and those with renal or hepatic impairment (Chapman 2003; Dawson 2001).
How the intervention might work
Antiplatelet therapy is effective in long‐term secondary prevention of vascular events in patients at high risk of vascular disease, including those who have had ischaemic stroke or acute myocardial infarction, and a benefit of antiplatelet treatment in patients with intermittent claudication in the reduction of vascular events has been previously observed (ATT 2002; Niu 2016; PAD 2003; Robless 2001). It is unclear exactly how cilostazol works to improve claudication, but the mechanism is most likely multifactorial, involved with several of cilostazol's actions, specifically vasodilation, possible beneficial inhibition of platelet aggregation, and altering a patient's lipid profile (Chapman 2003; Rizzo 2011; Ueno 2011).
Why it is important to do this review
Treatment of intermittent claudication includes best medical treatment (BMT), lifestyle changes, physical exercise and angioplasty, if appropriate (Haile 2020). A recent review demonstrated that angioplasty and supervised exercise were 'more or less comparable treatment options' (Fakhry 2018). In practice, compliance with BMT is poor and most people remain symptomatic with intermittent claudication. There are various pharmacological agents, as well as cilostazol, used in the treatment of intermittent claudication including anticoagulants (Cosmi 2014), antiplatelets (Wong 2011), and pentoxifylline (Broderick 2020). However, there is a degree of uncertainty as to which, if any, of these medications provides the most clinical benefit. The National Institute for Health and Care Excellence (NICE) (clinical guideline 147, last updated December 2020), recommends the use of naftidrofuryl for people with intermittent claudication caused by PAD; cilostazol is licensed for the treatment of PAD in selected patients who do not respond to other treatments (NICE 2012). NICE clinical guidelines are underpinned by cost‐effectiveness analysis which is outside the remit of this review. If cilostazol is found to reduce the symptoms of claudication, as well as cardiovascular risk in patients with PAD, it would offer some patients another clinical option. This is an update of the review first published in 2007 (Robless 2007) and incorporates the most recent literature and advances in Cochrane methodology, with respect to grading of the evidence.
Objectives
To determine the effect of cilostazol on initial and absolute claudication distances, mortality and vascular events in patients with stable intermittent claudication.
Methods
Criteria for considering studies for this review
Types of studies
We included double‐blind, randomised controlled trials of cilostazol versus placebo, or versus other drugs used to improve claudication distance.
Types of participants
We included participants with stable intermittent claudication (determined by a physician or investigator). We excluded studies that identified their participants as those with peripheral arterial disease (PAD), atherosclerosis obliterans, or similar, but did not specifically state that their study population had intermittent claudication.
Types of interventions
We included studies that compared cilostazol versus placebo, or other drugs used to improve claudication distance, e.g. pentoxifylline. The interventions must have been given for at least four weeks. We excluded comparisons with exercise, anticoagulants or surgery.
Types of outcome measures
Primary outcomes
Initial claudication distance (ICD) (the distance walked on a treadmill before the onset of calf pain)
Health‐related quality of life (QoL), including general and disease‐specific QoL, measured by a validated questionnaire
Secondary outcomes
Absolute claudication distance (ACD) (the maximum distance walked on a treadmill)
Revascularisation (angioplasty or surgical bypass)
Amputation
Adverse events related to study medication
Cardiovascular events (defined as stroke, unstable angina, acute myocardial infarction (MI))
All‐cause mortality
Ankle brachial index (ABI)
Major Adverse Limb Event (MALE) defined as major vascular amputation or any vascular re‐intervention, including surgical or endovascular re‐intervention
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched from inception to 10 November 2020).
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO 2020, Issue 10).
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® 1946 to present) (searched 9 November 2020).
Embase Ovid (searched 9 November 2020).
CINAHL Ebsco (searched 9 November 2020).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1. The information Specialist also searched the following trials registries on 10 November 2020.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch)
ClinicalTrials.gov
Searching other resources
We searched the reference lists of relevant articles retrieved by the electronic searches, for additional citations.
Data collection and analysis
Selection of studies
For this update, two review authors (TB and MS), independently evaluated studies for inclusion based on selection criteria. Disagreements were resolved by discussion between the two review authors.
Data extraction and management
For this update, two review authors (TB and RBF), independently extracted the data. We identified one new eligible study for this update. We collected information regarding the trial design, participant characteristics, therapy type, dosages and treatment periods. We collected information for the primary outcomes of ICD and QoL and secondary outcomes including ACD, revascularisation, amputation, adverse events, cardiovascular events, all‐cause mortality, and ABI. We resolved disagreements through discussion between the two review authors. Data were entered into and analysed using Review Manager (RevMan Web 2019).
Assessment of risk of bias in included studies
For this update, two review authors (TB and RBF), independently assessed the methodological quality using Cochrane's risk of bias tool (Higgins 2011). We assessed the following domains: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other bias. We classified the domains as low risk, high risk, or unclear risk of bias, according to the guidelines in Higgins 2011. Disagreements were resolved by discussion between the two review authors.
Measures of treatment effect
We pooled the data on ICD, ACD and ABI, to obtain an overall estimate of the effectiveness of cilostazol therapy. We used mean change from baseline for each trial, which is more informative of treatment effect than simply comparing final walking distances because it takes baseline measures into account. Due to the differences in treadmill testing methods between the studies, mean change from baseline is the only appropriate measure for treatment effect. The results for continuous data are presented as mean differences (MDs) with 95% confidence intervals (CIs), and dichotomous data as odds ratios (ORs) with 95% CIs.
Unit of analysis issues
The unit of analysis was the individual participant in all studies included in this review. For studies with more than two treatment arms of relevance to the same meta‐analysis and with one control arm, we included data from both treatment arms. To avoid double counting of participants, we halved the number of participants in the control arm. For dichotomous outcomes, both the number of events and the total number of participants were divided up. For continuous outcomes only the total number of participants was divided up (means and standard deviations remained unchanged). This method only partially overcomes the unit of analysis error because the resulting comparisons remain correlated (Higgins 2021a). However, we were interested in evaluating all doses of drug intervention as well as drug interventions per se, so we did not feel it was appropriate to pool the intervention group data within each study.
Dealing with missing data
In previous versions of the review, when data were not available or missing, study authors were contacted to request missing data. Data imputation was not carried out and reasons for study data not being included in meta‐analyses were recorded (Table 5). All of the analyses were based on the number of participants accessed for each outcome within each study.
3. Reasons for study not being included in meta‐analyses of initial claudication distance (ICD), absolute claudication distance (ACD) and ankle brachial index (ABI).
| Study | Reason for data not included in ICD, ACD or ABI outcomes |
| Brass 2012 | Reported in peak walking time and initial claudication time with SDs, but the treadmill method was not clear, so we could not reliably convert from time to distance |
| De Albuquerque 2008 | Outcomes of interest were only broken down between non‐smokers and smokers, but not between treatment groups. Figures 2 and 3 do offer graphical information on the mean change in maximal walking distances, 'expressed as percent of control'. |
| Lee 2001 | For ACD and ABI, mean baseline and follow‐up values with SD were given; we can calculate mean change but for the imputation of SD we need the SDs associated with the change and the baseline and the post‐intervention mean, for at least one similar length study, which we do not have. Mean changes in ACD without SD or other variance were also reported in the text. |
| O'Donnell 2009 | For ICD and ACD, mean baseline and follow‐up values were given, but no SDs were given. A P value was given for the overall treatment effect but that was for the comparison between cilostazol and placebo, not between baseline and follow‐up. For ABI only, interquartile ranges were given, which could not be adequately converted to SD. |
| Otsuka Study 21‐86‐101 | Placebo‐corrected mean change from baseline was provided for the treatment group, with no SDs. Also, a ratio of the geometric means of change was calculated between cilostazol and placebo, but these data could not be recalculated to mean change and SD. |
| Otsuka Study 21‐86‐103 | Raw mean change from baseline was provided for the cilostazol and placebo groups, with no SDs. Also, a ratio of the geometric means of change was calculated between cilostazol and placebo, but these data could not be recalculated to mean change and SD. |
| Otsuka Study 21‐87‐101 | Placebo‐corrected mean change from baseline was provided for the treatment group, with no SDs. Also, a ratio of the geometric means of change was calculated between cilostazol and placebo, but these data could not be recalculated to mean change and SD. |
| Otsuka Study 21‐94‐301 | For the ICD outcome, only a ratio of the geometric means of change was calculated between cilostazol and the comparison, but these data could not be recalculated to mean change and SD. |
| Otsuka Study 21‐98‐213 | For ICD, raw mean change from baseline was provided for the cilostazol and comparison groups, with no SDs. Also, a ratio of the geometric means of change was calculated between cilostazol and comparisons, but these data could not be recalculated to mean change and SD. Mean change data with SDs were available for the ACD outcome. |
ACD: absolute claudication distance ABI: ankle brachial index ICD: initial claudication distance SD: standard deviation
Assessment of heterogeneity
We evaluated trial heterogeneity using Chi2 and I2 testing, which describe the variability in effect estimates that are due to heterogeneity between studies, rather than chance. The I2 is given as a percentage, with a measure of 0% meaning little to no variability in effect estimates between the studies, and progressing amounts of variability with increased I2 percentage values (Higgins 2021). If tests for heterogeneity found I2 > 50%, we planned to use a random‐effects model, otherwise, we planned to use a fixed‐effect model. We are aware there can be uncertainty around the value of I2 and using thresholds for interpretation, and so we also considered the direction and magnitude of effects and degree of overlap between CIs.
Assessment of reporting biases
We hoped to assess reporting bias by funnel plots if more than ten studies were included in the meta‐analysis (Higgins 2021). As we did not include more than ten studies in any analysis, we did not do this.
Data synthesis
We used a pooled fixed‐effect model meta‐analysis with subgrouping, where appropriate. We used a random‐effects model when tests for heterogeneity found I2 > 50%. We also considered the direction and magnitude of effects and degree of overlap between CI. For outcomes where we were unable to pool data, we described the results narratively.
Subgroup analysis and investigation of heterogeneity
For this update, we synthesised the data by drug comparison and so it was appropriate to subgroup by drug dose.
Sensitivity analysis
In order to determine that robust conclusions could be drawn using meta‐analyses, we removed studies of a lower methodological quality (defined as studies with five or more high‐risk or unclear‐risk ratings within the seven domains evaluated for risk of bias), from the analysis to determine the effect on the association. We planned to undertake sensitivity analysis only if sufficient studies remained in the analyses to provide a meaningful result.
Summary of findings and assessment of the certainty of the evidence
For this update, we prepared a summary of findings table to present the findings from our review for the comparisons 'Cilostazol versus placebo' (Table 1) and 'Cilostazol versus pentoxifylline' (Table 4), using GRADEpro software (GRADEpro). We used the GRADE method, to evaluate the evidence based on the risk of bias of the individual studies, inconsistency, imprecision, indirectness and publication bias (Schünemann 2021). We evaluated the following outcomes because they were the most clinically relevant:
Summary of findings 1. Cilostazol compared with placebo for intermittent claudication.
| Cilostazol compared with placebo for intermittent claudication | ||||||
| Patient or population: intermittent claudication Setting: all outpatient settings Intervention: cilostazol Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with cilostazol | |||||
|
Initial claudication distance (change in metres) 12 to 24 weeks follow‐up |
The mean change in initial claudication distance was 32.28 | MD 26.49 higher (18.93 higher to 34.05 higher) | ‐ | 1722 (6 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | |
|
Quality of life (change in points/ percentage; COM, SF‐36, VascuQol, WIQ) 16 to 24 weeks follow‐up |
There appeared to be a general improvement of cilostazol over placebo across four studies that used the SF‐36 (Beebe 1999; Dawson 2000; Money 1998; O'Donnell 2009). There were inconsistent results for walking impairment according to the WIQ (4 studies), three studies showed no difference between groups for walking impairment (Beebe 1999; Dawson 2000; O'Donnell 2009) and one study reported a 20% increase in walking speed for the cilostazol group (Money 1998). There were modest improvements across the domains of the COM in one study (Beebe 1999). There was no difference between groups in one study using the VascuQol questionnaire (O'Donnell 2009). |
‐ | 1163 (4 RCTs) |
LOW 2, 3 | Meta‐analysis was not undertaken because of differences in measures used and how they were reported. See Table 2 for further details. | |
|
Absolute claudication distance (change in metres) 12 to 24 weeks follow‐up |
The mean change in absolute claudication distance was 37.45 | MD 39.57 higher (21.8 higher to 57.33 higher) | ‐ | 2360 (8 RCTs) | ⊕⊝⊝⊝ VERY LOW 2, 4, 5 | |
|
Arterial revascularisation (number of cases) 24 weeks follow‐up |
Study population | OR 0.16 (0.01 to 4.07) | 516 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2, 6 | ||
| 6 per 1,000 | 1 per 1000 (0 to 24) | |||||
|
Amputation (number of cases) 24 weeks follow‐up |
Study population | OR 0.16 (0.01 to 4.07) | 516 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2, 6 | ||
| 6 per 1000 | 1 per 1000 (0 to 24) | |||||
|
Adverse event related to study medication ‐ headache (number of cases) 12 to 26 weeks follow‐up |
Study population | OR 2.83 (2.26 to 3.55) | 2584 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 2, 7 | ||
| 105 per 1000 | 250 per 1000 (210 to 295) | |||||
|
Cardiovascular event (number of cases) 24 to 26 weeks follow‐up |
Study population | OR 1.50 (0.51 to 4.47) | 692 (2 RCTs) | ⊕⊕⊝⊝ LOW 2, 8 | ||
| 16 per 1000 | 23 per 1000 (8 to 66) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COM: Claudication Outcome Measure; OR: odds ratio; SF‐36: self‐administered Short‐form 36; VascuQol: Vascular Quality of Life; WIQ: Walking Impairment Questionnaire. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 downgraded by one level for risk of bias because 3 studies (Dawson 1998; Otsuka Study 21‐95‐201; Strandness 2002) rated at high risk for selective reporting
2 downgraded by one level for publication bias because pharmaceutical sponsors involvement in most of these studies raises questions of whether unpublished studies that suggest no benefit exist
3 downgraded by one level for imprecision because a range of quality of life measurement tools were used and results were reported in different ways (meta‐analysis was not undertaken for these reasons)
4 downgraded by one level for risk of bias because 4 studies (Dawson 1998; Elam 1998; Otsuka Study 21‐95‐201; Strandness 2002) rated at high risk for selective reporting
5 downgraded by one level for inconsistency because of heterogeneity: I2 = 72% cilostazol 100 mg twice daily versus placebo subgroup ‐ heterogeneity reduced to 0% when 2 studies removed (Otsuka Study 21‐95‐201; Otsuka Study 21‐98‐213)
6 downgraded by two levels for imprecision due to low number of participants and events from 1 RCT (Beebe 1999)
7 see Table 3 for other adverse events related to study medication
8 downgraded by one level for imprecision due to low number of participants and events from 2 RCTs (Beebe 1999; Brass 2012)
Summary of findings 2. Cilostazol compared with pentoxifylline for intermittent claudication.
| Cilostazol 100 mg twice daily compared with pentoxifylline 400 mg twice daily for intermittent claudication | ||||||
| Patient or population: intermittent claudication Setting: all outpatient settings Intervention: cilostazol 100 mg twice daily Comparison: pentoxifylline 400 mg three times daily | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with pentoxifylline 400 mg twice daily | Risk with cilostazol 100 mg twice daily | |||||
|
Initial claudication distance (change in meters) 24 weeks follow‐up |
The mean change in initial claudication distance was 73.6 | MD 20.00 higher (2.57 lower to 42.57 higher) | ‐ | 417 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 2 | |
|
Quality of life (change in points, SF‐36, WIQ) 24 weeks follow‐up |
Quote "None of the treatments significantly affected the Medical Outcomes Scale Short Form‐36 scores on Mental Health Concepts, General Health Perception, Physical Health Concepts, or Vitality Scores. There were also no significant differences in patient‐reported walking distance or speed as determined by the Walking Impairment Questionnaire." (Dawson 2000). | ‐ | 317 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 2, 3 | ||
|
Absolute claudication distance (change in metres) 24 weeks follow‐up |
The mean change in absolute claudication distance was 70.0 | MD 13.43 higher (43.50 lower to 70.36 higher) | ‐ | 866 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2, 4, 5 | |
| Arterial revascularisation | ‐ | ‐ | ‐ | ‐ | ‐ | no studies |
| Amputation | ‐ | ‐ | ‐ | ‐ | ‐ | no studies |
|
Adverse event related to study medication ‐ headache (number of cases) 24 weeks follow‐up |
Study population | OR 2.20 (1.16 to 4.17) | 982 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 4 | ||
| 111 per 1000 | 216 per 1000 (127 to 343) | |||||
| Cardiovascular event | ‐ | ‐ | ‐ | ‐ | ‐ | no studies |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; SF‐36: self‐administered Short‐form 36; WIQ: Walking Impairment Questionnaire. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 downgraded one level for imprecision because 1 RCT had a low number of participants (Dawson 2000)
2 downgraded one level because publication bias strongly suspected
3 downgraded by two levels for imprecision because 1 RCT had a low number of participants (Dawson 2000) and imprecision could not be evaluated
4 downgraded one level for inconsistency because of heterogeneity: I2 ≥ 50%
5 downgraded one level for imprecision due to very wide CIs
ICD
health‐related QoL
ACD
revascularisation
amputation
adverse events related to study medication ‐ headache
cardiovascular events
Where meta‐analysis was not undertaken, we described the evidence using a narrative approach. GRADE assessments for the other outcomes of adverse events that were not included in the summary of findings tables are presented in an additional table (Table 3).
2. Adverse events related to study medication.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo or pentoxifylline | Risk with cilostazol | ||||
| Cilostazol compared to placebo | |||||
|
Diarrhoea 12 to 26 weeks follow‐up |
Study population | OR 2.73 (2.02 to 3.70) | 2503 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| 56 per 1000 | 140 per 1000 (108 to 181) | ||||
|
Abnormal stools 12 to 24 weeks follow‐up |
Study population | OR 3.63
(2.45 to 5.38) |
1804 (5 RCTs) | ⊕⊕⊕⊝
MODERATE 1 |
|
| 44 per 1000 | 143 per 1000 (101 to 198) | ||||
|
Dizziness 12 to 26 weeks follow‐up |
Study population | OR 2.42 (1.43 to 4.08) | 1120 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| 42 per 1000 | 97 per 1000 (60 to 153) | ||||
|
Pain 12 to 24 weeks follow‐up |
Study population | OR 0.96 (0.71 to 1.30) | 1572 (4 RCTs) | ⊕⊕⊝⊝ LOW 1, 2 | |
| 127 per 1000 | 123 per 1000 (94 to 159) | ||||
|
Palpitations 24 to 26 weeks follow‐up |
Study population | OR 7.16
(3.95 to 12.98) |
1681 (4 RCTs) | ⊕⊕⊕⊝
MODERATE 1, 3 |
|
| 16 per 1000 | 103 per 1000 (60 to 173) | ||||
| Cilostazol compared to pentoxifylline | |||||
|
Diarrhoea 24 weeks follow‐up |
Study population | OR 1.80 (0.79 to 4.12) | 982 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1, 3, 4 | |
| 97 per 1000 | 162 per 1000 (78 to 307) | ||||
|
Abnormal stools 24 weeks follow‐up |
Study population | OR 3.12 (1.57 to 6.21) | 459 (1 RCT) | ⊕⊕⊝⊝ LOW 1, 5 | |
| 52 per 1000 | 145 per 1000 (79 to 253) | ||||
|
Pain 24 weeks follow‐up |
Study population | OR 0.85 (0.57 to 1.26) | 982 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| 123 per 1000 | 107 per 1000 (74 to 151) | ||||
|
Palpitations 24 weeks follow‐up |
Study population | OR 8.35 (4.11 to 16.98) | 982 (2 RCTs) | ⊕⊕⊝⊝ LOW 1, 3 | |
| 18 per 1000 | 134 per 1000 (71 to 240) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||||
1 downgraded one level for publication bias because pharmaceutical sponsors involvement raises questions of whether unpublished studies that suggest no benefit exist
2 downgraded one level for risk of bias because 2 studies (Elam 1998; Strandness 2002) rated at high risk for selective reporting
3 downgraded one level for imprecision due to wide CIs
4 downgraded one level for inconsistency because of heterogeneity: I² = 77%
5 downgraded one level for imprecision due to data from 1 RCT with wide CIs (Dawson 2000)
Results
Description of studies
Results of the search
See Figure 1. For this update of the review, we identified one new study (Lee 2001), one additional report of a previously included study (Brass 2012), and one study is awaiting classification (Sapelkin 2013). We excluded 23 new studies. This review update involved a total of 16 included studies and 31 excluded studies.
1.
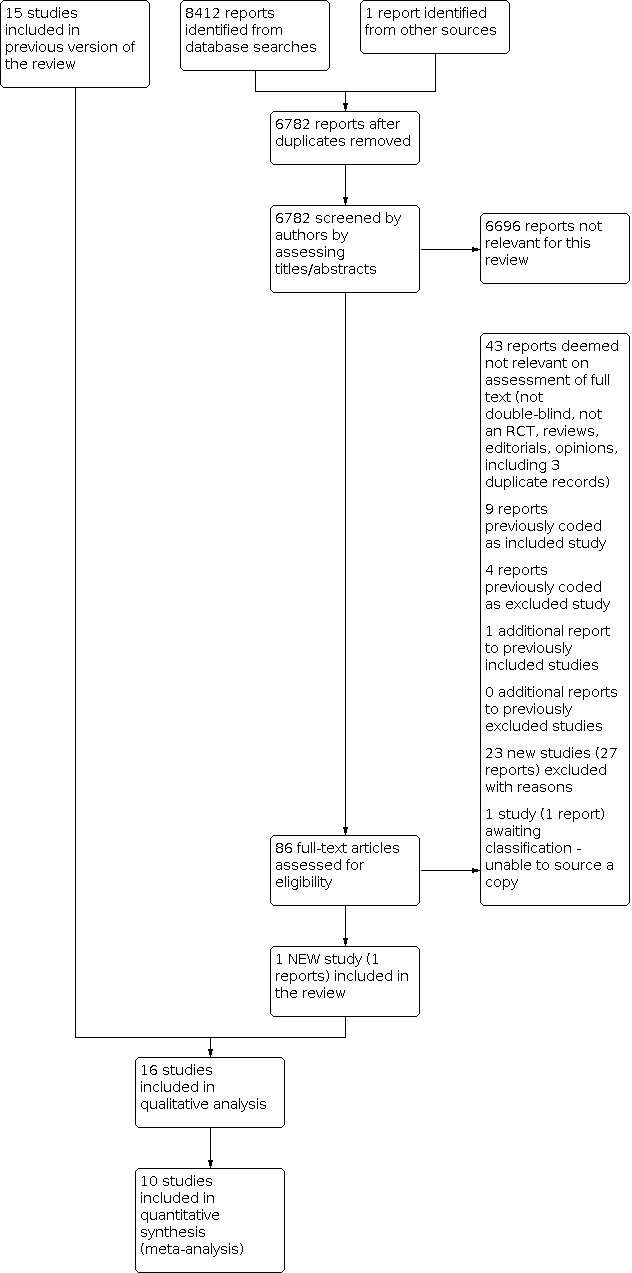
Study flow diagram.
Included studies
See Characteristics of included studies for more detail. We included 16 studies with 3972 participants. Treatment duration ranged between six and 26 weeks. All participants had intermittent claudication secondary to peripheral arterial disease (PAD). All included studies compared cilostazol 100 mg twice daily with placebo. Two studies also compared cilostazol 50 mg twice daily with placebo (Beebe 1999; Strandness 2002) and one study compared cilostazol 150 mg twice daily with placebo (Otsuka Study 21‐95‐201). Three studies also compared cilostazol 100 mg twice daily with pentoxifylline 400 mg three times daily (Dawson 2000; Otsuka Study 21‐94‐301; Otsuka Study 21‐98‐213), one study compared cilostazol 100 mg twice daily with pentoxifylline 600 mg twice daily (De Albuquerque 2008) and one study compared cilostazol 100 mg twice daily with pentoxifylline 400 mg twice daily (Lee 2001). Brass 2012 had treatment groups excluded from our analyses (K‐134, 50 mg and 100 mg twice daily) because K‐134 is not an alternative antiplatelet agent or medication currently known to increase walking distance.
Seven studies were published in journal articles and six studies were not published as journal articles, with sources of data being a medical review by the FDA in five cases (Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103; Otsuka Study 21‐87‐101; Otsuka Study 21‐94‐301; Otsuka Study 21‐95‐201), and a pharmaceutical submission to NICE in the other case (Otsuka Study 21‐98‐213). All 16 studies received funding from pharmaceutical companies, 13 of which received funding from Otsuka Pharmaceuticals, the company that formulated cilostazol. Lee 2001 was the only study to report a declaration of interest (no conflicts declared). Five studies had study authors employed by a pharmaceutical company; including Otsuka Pharmaceuticals in four cases (Dawson 2000; Elam 1998; Money 1998; Strandness 2002), and Kowa Research Institute in another case (Brass 2012). One study (O'Donnell 2009), reported that a study author received financial support from Otsuka Pharmaceuticals for travel costs to attend conferences to present data from the trial.
For two studies, the duration of treatment was six weeks (Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103), and for one study the treatment duration was eight weeks (Lee 2001). Four studies had a treatment duration of 12 weeks (Dawson 1998; Elam 1998; Otsuka Study 21‐87‐101; Otsuka Study 21‐95‐201), and one study treated participants for 16 weeks (Money 1998). The De Albuquerque 2008 study had a treatment period of 20 weeks. The most common treatment duration was 24 weeks, in six studies (Beebe 1999; Dawson 2000; O'Donnell 2009; Otsuka Study 21‐94‐301; Otsuka Study 21‐98‐213; Strandness 2002), and one study had a treatment duration of 26 weeks (Brass 2012). The number of participants in each study ranged from 19 in Otsuka Study 21‐87‐101 to 780 in Otsuka Study 21‐98‐213.
For the walking distance outcomes (initial claudication distance (ICD) and absolute claudication distance (ACD)), the treadmill test methods varied between three protocols. Five studies used a method with an immediate and constant gradient of 10% and a constant speed of 3.2 km/h (O'Donnell 2009; Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103; Otsuka Study 21‐87‐101; Otsuka Study 21‐94‐301). Six studies used a similar method with an immediate and constant gradient of 12.5% and a constant speed of 3.2 km/h (Beebe 1999; Dawson 1998; Lee 2001; Otsuka Study 21‐95‐201; Otsuka Study 21‐98‐213; Strandness 2002). Four studies adopted a delayed gradient treadmill method where the gradient began at 0% and increased by 3.5% every three minutes, with a constant speed of 3.2 km/h (Dawson 2000; De Albuquerque 2008; Elam 1998; Money 1998). It should be noted that the De Albuquerque 2008 study did not state the gradient by which the treadmill was increased, but it was assumed to be similar to the other three studies. The Brass 2012 study only described their treadmill method as "graded" and referred to another study, but we were unable to determine from this which method was used.
Excluded studies
See Characteristics of excluded studies for more detail.
Studies that were not RCTs or were not double‐blinded were judged not relevant. For this update, we excluded 23 new studies making a total of 31 excluded studies. There were nine previously excluded studies; one of these was non‐randomised and was removed from the list of studies excluded with reasons.
Briefly, 15 studies included the wrong patient population (Chao 2014; Chao 2016; Chen 2017; ChiCTR‐TRC‐09000441; Chisari 2019; Hsieh 2009; JPRN‐C000000215; JPRN‐UMIN000001198; Kim 2013; NCT00573950; NCT00886574; NCT00912756; NCT01952756; NCT01188824; Xiao 2010). Eleven studies were excluded due to the wrong intervention, for example, iloprost, olmesarten, sildenafil, ticagrelor and valsarten (Goldenberg 2012; JPRN‐UMIN000011869; JPRN‐UMIN000014307; Mazzone 2013; NCT00102050; NCT02373462; NCT02407314; NCT02636283; NCT02930811; NCT03318276; NCT03686306). We excluded the NCT00443287 study because the intervention arms were not clear, and we were unable to determine if clopidogrel was also used. We excluded one study because the duration of follow‐up far exceeded that of the other included studies, and follow‐up data at earlier time points were not available (CASTLE 2008). The NCT00300339 study was discontinued early, and no outcome data were available for the trial. We were unable to determine if the Otsuka Study PUIC‐1 was double‐blind, and the Otsuka Study PUIC‐2 abstract did not contain enough information on the methods and results of the study to be included.
One study is awaiting classification because we could not source the publication (Sapelkin 2013).
Risk of bias in included studies
Figure 2 and Figure 3 offer graphical summaries of risk of bias for the 16 included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirteen studies did not clearly describe randomisation sequence generation methods, leading to a rating of unclear risk of bias. Randomisation sequence generation was low risk in only three studies: two studies reported voice‐interactive computerised methods of randomisation (Brass 2012; Dawson 2000), and Beebe 1999 reported the use of a coded randomisation list. Eleven studies provided insufficient information to permit judgement of risk of bias and were rated as being unclear.
Five studies were rated as having low risk for allocation concealment: Brass 2012 and Dawson 2000 used computerised methods to help ensure that the participants and researchers could not determine the treatment allocation; De Albuquerque 2008, Lee 2001 and O'Donnell 2009 used coded or sealed envelopes to conceal allocation. The remaining eleven studies provided insufficient information to permit judgement of risk of bias and were rated as being unclear.
Blinding
Although all 16 included studies used a placebo control, only half (eight) of the studies adequately described their methods of blinding to ensure that both participants and researchers would not be able to determine treatment and these were rated as being at low risk of bias (Beebe 1999; Dawson 1998; Dawson 2000; De Albuquerque 2008; Lee 2001; O'Donnell 2009; Otsuka Study 21‐94‐301; Strandness 2002). The remaining eight studies were rated as being unclear.
None of the studies described blinding of assessors for all outcomes measured, but three studies (Beebe 1999; Elam 1998; Strandness 2002) did give a detailed description of assessor blinding for some of their outcomes, so we determined their risk of detection bias was low. The other 13 studies were rated as having unclear risk of detection bias.
Incomplete outcome data
Thirteen studies were at low risk of attrition bias and three studies had unclear risk of attrition bias (Lee 2001; Otsuka Study 21‐86‐103; Otsuka Study 21‐98‐213). Study authors in one study (Lee 2001) stated analysis would be performed on participants that completed the study (not intention‐to‐treat), but did not specifically state the number of participants that completed the study. The Lee 2001 study has two publications; the results tables in one reference included values that would suggest all participants were included in the analysis, and therefore completed the trial. However, the number of participants reported in the other reference had two participants missing, with no explanation. The Otsuka Study 21‐86‐103 study had an overlap of reasons for participants that dropped out, with no discussion of multiple reasons for dropouts. The data and information on the Otsuka Study 21‐98‐213 study were retrieved from a secondary NICE report, and not enough detail was provided to determine incomplete outcome data.
Selective reporting
Six studies had a low risk of reporting bias because all indicated outcomes and time points were reported on (Beebe 1999; Brass 2012; Dawson 2000; Money 1998; O'Donnell 2009; Otsuka Study 21‐87‐101). Two studies had an unclear risk of reporting bias; in one study, there was inadequate reporting of outcomes (Otsuka Study 21‐98‐213), and in another study two publications reported on different outcomes with no clear indication of what the preplanned outcomes were (Lee 2001). Eight studies had a high risk of reporting bias because they described in the methods outcomes or time points of interest that were not reported on (Dawson 1998; De Albuquerque 2008; Elam 1998; Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103; Otsuka Study 21‐94‐301; Otsuka Study 21‐95‐201; Strandness 2002).
Other potential sources of bias
All 16 studies had a low risk of other potential sources of bias.
Effects of interventions
For the primary outcome of ICD, 14 of the 16 included studies reported this outcome. However, only six of these studies were reported in an adequate and appropriate manner to be included in the meta‐analyses. This was due to methodological differences in the reporting of outcomes that did not allow us to calculate mean change and standard deviations (SD). Also, due to the large differences between studies, we deemed imputation inappropriate. Descriptions of the findings of these studies are addressed under the appropriate outcome headings. Table 5 describes the reasoning why these studies could not appropriately be included in the meta‐analyses of walking distances and ABI. Data from five studies were gathered solely from unpublished study data (Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103; Otsuka Study 21‐87‐101; Otsuka Study 21‐94‐301; Otsuka Study 21‐95‐201).
We conducted sensitivity analyses by removing studies of a lower methodological quality (defined as studies with five or more high‐risk or unclear‐risk ratings within the seven domains evaluated for risk of bias). We only performed this type of sensitivity analysis for analyses and outcomes where there were sufficient data within the meta‐analyses; this included the comparison of cilostazol versus placebo, and for the outcomes ICD, ACD, adverse events and all‐cause mortality. For the adverse events of abnormal stools and dizziness, and for ABI there were no studies defined as low quality and so these sensitivity analyses were not undertaken.
Cilostazol versus placebo
Table 1 provides a summary of the results for the comparison of cilostazol versus placebo. We carried out subgroup analysis to investigate any overall effect of cilostazol and also to compare the different cilostazol doses. Two studies compared cilostazol 50 mg twice daily with placebo (Beebe 1999; Strandness 2002), all 16 included studies compared cilostazol 100 mg twice daily with placebo (Beebe 1999; Brass 2012; Dawson 1998; Dawson 2000; De Albuquerque 2008; Elam 1998; Lee 2001; Money 1998; O'Donnell 2009; Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103; Otsuka Study 21‐87‐101; Otsuka Study 21‐94‐301; Otsuka Study 21‐95‐201; Otsuka Study 21‐98‐213; Strandness 2002) and one study compared cilostazol 150 mg twice daily with placebo (Otsuka Study 21‐95‐201).
Initial claudication distance
Two studies (400 participants) compared cilostazol 50 mg with placebo (Beebe 1999; Strandness 2002) and the fixed‐effect model found a higher ICD (MD 19.50 metres, 95% CI 6.80 to 32.21 metres) in the cilostazol treatment group compared with the placebo group (Analysis 1.1).
1.1. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 1: Initial claudication distance (ICD)
Six studies (1236 participant) comparing cilostazol 100 mg versus placebo were eligible for inclusion in the meta‐analysis (Beebe 1999; Dawson 1998; Dawson 2000; Money 1998; Otsuka Study 21‐95‐201; Strandness 2002). In the fixed‐effect model, participants taking cilostazol had a higher ICD, with a MD of 32.19 metres (95% CI 22.20 to 42.18 metres), compared with those taking placebo (Analysis 1.1).
One study (86 participants) compared cilostazol 150 mg versus placebo (Otsuka Study 21‐95‐201) and the fixed‐effect model found no observable difference between the treatment groups in ICD (MD 15.70 metres, 95% CI ‐12.20 to 43.60 metres). As only one study was included in the analysis, an overall association could not be determined (Analysis 1.1).
Overall, six studies (1722 participants) were included in the meta‐analysis that compared cilostazol (all doses) versus placebo. In the fixed‐effect model, participants taking cilostazol had a higher ICD, with a MD of 26.49 metres (95% CI 18.93 to 34.05 metres), compared with those taking placebo (Analysis 1.1). No differences were seen with subgroup analysis (test for subgroup differences: P = 0.22). When low‐quality studies were removed in sensitivity analysis, there was an additional improvement of 3.03 meters in favour of cilostazol (MD 29.52, 95% CI 21.26 to 37.78) and there was a subgroup difference (P = 0.04).
Eight additional studies reported data on change in ICD compared with baseline, but the data were not eligible for inclusion in the meta‐analysis (Brass 2012; De Albuquerque 2008; O'Donnell 2009; Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103; Otsuka Study 21‐87‐101; Otsuka Study 21‐94‐301; Otsuka Study 21‐98‐213). Brass 2012 reported change in initial claudication time, with participants in the cilostazol group showing an increase of 60 seconds ± standard deviation (SD) of 95 seconds, and the placebo group showing a smaller increase of 44 seconds ± 102 seconds. The report by De Albuquerque 2008 did not break down the ICD outcome by treatment group, and an estimate of change in ICD is meaningless for the whole study population. O'Donnell 2009 reported no difference in the change in effect between the cilostazol group and placebo, 67.0% and 51.6%, respectively, P = 0.63. Otsuka Study 21‐86‐101 reported an arithmetic placebo‐corrected mean change of 41.9 metres and a statistically significant ratio of geometric mean changes of 1.32 (95% CI 1.07 to 1.64; P = 0.01), favouring cilostazol. In contrast, Otsuka Study 21‐86‐103 reported a mean change of ‐2.5 metres for the cilostazol group and 34.4 metres for the placebo group, and a statistically significant ratio of geometric mean changes of 0.69 (95% CI 0.53 to 0.91; P = 0.01), favouring the placebo group. Otsuka Study 21‐87‐101 also reported findings favouring placebo, with an arithmetic placebo‐corrected mean change of ‐92 metres, and a non‐significant ratio of geometric mean changes of 0.69 (95% CI 0.42 to 1.13; P = 0.13). Otsuka Study 21‐94‐301 reported a placebo‐corrected mean change of 15 metres and a ratio of geometric mean changes of 1.01, favouring cilostazol. Otsuka Study 21‐98‐213 reported similar mean changes for the cilostazol and placebo groups of 47.3 metres and 45.3 metres, respectively, and a ratio of geometric mean changes of 1.02 (95% CI 0.92 to 1.13; P = 0.769).
Overall, the evidence for this outcome was of low certainty, downgraded one level because of risk of bias (selective reporting) and one level because publication bias was strongly suspected.
Health‐related quality of life
Quality of life (QoL) measures were evaluated in four studies (Beebe 1999; Dawson 2000; Money 1998; O'Donnell 2009) using the self‐administered Short‐Form 36 (SF‐36), Walking Impairment Questionnaire (WIQ), Claudication Outcome Measure (COM), and Vascular Quality of Life (VascuQol) questionnaires. The O'Donnell 2009 study reported QoL measures in normoglycaemic patients and diabetic patients, separately. Due to the differences in QoL measures, as well as how they were reported, we did not undertake a meta‐analysis. Table 2 provides information on change in QoL measures as reported in the individual studies. This table should be interpreted with caution, as no hypothesis testing has been performed, and the data format differed between studies.
1. Change in quality of life status (change in points or percentage from baseline).
| Beebe 1999 | Dawson 2000 | Money 1998 | O'Donnell 2009 | ||||||||
| Tool | Domain | Cilostazol 100 mg (n = 137) | Cilostazol 50 mg (n = 135) | Placebo (n = 141) | Cilostazol 100 mg (n = 205) | Pentox 400 mg (n = 212) | Placebo (n = 226 | Cilostazol 100 mg (n = 119) | Placebo (n = 120) | Cilostazol 100 mg ( n = 39) | Placebo (n = 41) |
| Short‐form 36 (SF‐36) | Physical function | 7.1 | 8 | 2 | 3 | 1.8 | 0.8 | 8.3 | 2.3 | 11% | ‐0.30% |
| Role‐physical | 5.3 | 4.4 | ‐2.8 | 3.7 | no improv | no improv | 3.0 | 0.1 | 7.8% | 5.4% | |
| Bodily pain | 7.2 | 4.6 | ‐1.8 | 5.2 | 1.6 | 1.0 | ‐ | ‐ | 3.7% | 10.5% | |
| Social function | 1.0 | 0.9 | 0.4 | no diff | no diff | no diff | ‐ | ‐ | ‐ | ‐ | |
| Role‐emotional | 2.9 | 0.0 | ‐1.7 | no diff | no diff | no diff | ‐ | ‐ | ‐ | ‐ | |
| Mental health | 2.5 | ‐1.5 | 0.9 | ‐0.7 | ‐0.6 | ‐1.3 | ‐ | ‐ | ‐ | ‐ | |
| General health | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 2.7% | ‐1.0% | |
| Walking Impairment Questionnaire (WIQ) | Walking speed | 0.1 | 0.2 | 0.1 | no diff | no diff | no diff | 20.0% | 0.0% | 10% | 4% |
| Walking distance | 0.2 | 0.2 | 0.1 | no diff | no diff | no diff | ‐ | ‐ | ‐1% | 3% | |
| Claudication Outcome Measure (COM) | Change in pain/discomfort | 2.8 | 2.7 | 2.4 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pain/discomfort: daily activities | 0.4 | 0.5 | 0.2 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pain/discomfort: physical activities | 0.5 | 0.5 | 0.2 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pain/discomfort: social activities | 0.3 | 0.4 | 0.3 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Walking pain/discomfort | 0.7 | 0.7 | 0.4 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Worry/concern due to pain | 0.8 | 0.6 | 0.5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Vascular Quality of Life (VascuQol) | Activity | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 7.3 | 1.8 |
| Symptom | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 3.1 | 3.2 | |
| Pain | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 10.4 | 13.2 | |
| Emotion | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 5.7 | 1.8 | |
| Social | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 1.1 | 3.4 | |
diff: difference improv: improvement Pentox: pentoxifylline
The SF‐36 is a multi‐purpose, general health questionnaire made of 36 questions from eight subscales: physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional and mental health. Each subscale is scored on a scale of zero to 100. The WIQ scale is intended for patients with intermittent claudication and gathers data on walking distance and speed using degree of difficulty scoring from zero to four, with zero representing inability to perform the task and four representing no difficulty. The COM is another disease‐specific testing method for scoring participants with intermittent claudication. It assesses severity of walking pain and discomfort with short and long distances and how participants feel the disease impacts other aspects of their life, including emotional and social. VascuQol is designed for participants with PAD and consists of 25 questions with answer options of one to seven, spanning five domains of interest: physical activity, symptoms, pain, emotion and social aspects.
There appeared to be a general improvement in QoL for cilostazol over placebo (various domains, not all domains measured within studies) (SF‐36, Beebe 1999; Dawson 2000; Money 1998; O'Donnell 2009). There were inconsistent results for walking impairment according to the WIQ (four studies), three studies showed no difference between groups for walking impairment (Beebe 1999; Dawson 2000; O'Donnell 2009) and one study reported a 20% increase in walking speed for the cilostazol group (Money 1998). There were modest improvements across the domains of the COM in one study (Beebe 1999). There was no difference between groups in one study using the VascuQol questionnaire (O'Donnell 2009).
The Strandness 2002 study also reported on QoL, with inadequate numerical data to support, but mentioned greater improvement in the cilostazol group compared with placebo, in the physical function, role‐physical and bodily pain scales. Otsuka Study 21‐95‐201 only briefly indicated no difference between the two groups for the endpoint QoL, but the authors did not indicate which questionnaires were used. Otsuka Study 21‐98‐213 reported a statistically significant difference at 12 weeks, favouring cilostazol, compared with placebo, but no data were reported.
Overall, the evidence for this outcome was of low certainty, downgraded one level for imprecision because a range of QoL measurement tools were used and results were reported in different ways and one level for strongly suspected publication bias.
Absolute claudication distance
Two studies (400 participants) compared cilostazol 50 mg with placebo (Beebe 1999; Strandness 2002), and the resulting random‐effect meta‐analysis found a higher ACD in the cilostazol arm (MD 30.84 metres, 95% CI 8.81 to 52.86 metres) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 2: Absolute claudication distance (ACD)
Eight studies (1874 participants) were included in the meta‐analysis for ACD comparing cilostazol 100 mg versus placebo (Beebe 1999; Dawson 1998; Dawson 2000; Elam 1998; Money 1998; Otsuka Study 21‐95‐201; Otsuka Study 21‐98‐213; Strandness 2002). The results of the random‐effects model showed a higher ACD in the cilostazol arm (MD 42.32 metres, 95% CI 18.12 to 66.51 metres) (Analysis 1.2).
One study of 86 participants (Otsuka Study 21‐95‐201), compared cilostazol 150 mg with placebo, and found a MD of 51.80 metres with a wide 95% CI spanning ‐10.59 to 114.19 (Analysis 1.2).
Overall, eight studies (2360 participants) were included in the meta‐analysis that compared cilostazol (all doses) versus placebo. Heterogeneity was detected so we used the random‐effects model. Participants taking cilostazol had a higher ACD, with a MD of 39.57 metres (95% CI 21.80 to 57.33 metres), compared with those taking placebo (Analysis 1.2). No differences were seen with subgroup analysis (test for subgroup differences: P = 0.70). When low‐quality studies were removed in sensitivity analysis, there was there was an additional improvement of 8.87 meters in favour of cilostazol (MD 48.44, 95% CI 34.49 to 62.39).
Eight additional studies reported on ACD, but their data were incompatible for meta‐analysis (Brass 2012; De Albuquerque 2008; Lee 2001; O'Donnell 2009; Otsuka Study 21‐86‐101; Otsuka Study 21‐86‐103; Otsuka Study 21‐87‐101; Otsuka Study 21‐94‐301). Brass 2012 measured peak walking time, similar to ACD, and found that the mean change (± SD) from baseline for the cilostazol group was 122 seconds ± 190 seconds, and for the placebo group a mean change of 72 seconds ± 196 seconds. The De Albuquerque 2008 study reported a mean change in maximal walking distance, 'expressed as per cent of control' of approximately 130% to 140%. These data were read from a graph, and no further information was given on the placebo arm. O'Donnell 2009 reported a statistically significant increased change in effect between the cilostazol group and placebo, 161.7% and 79.0%, respectively, P = 0.048. Otsuka Study 21‐86‐101 reported a placebo‐corrected arithmetic mean change for the cilostazol group of 49.7 metres, and a non‐significant ratio of geometric means of 1.17 (95% CI 0.97 to 1.42; P = 0.09). Otsuka Study 21‐86‐103's results did not support cilostazol for increased ACD, with a mean change of ‐6.9 metres for the cilostazol group and 30.3 metres for the placebo group, and a statistically significant ratio of geometric means of 0.83 (95% CI 0.70 to 0.98; P = 0.03), favouring the placebo arm. Otsuka Study 21‐87‐101 also reported ACD results that did not support cilostazol with a placebo‐corrected mean change for the cilostazol group of ‐99.1 metres, and a ratio of geometric means of 0.83 (95% CI 0.46 to 1.51; P = 0.52). Otsuka Study 21‐94‐301 reported a placebo‐corrected arithmetic mean change for the cilostazol group of 33.6 metres and a ratio of geometric means of 1.06, favouring cilostazol. Lee 2001 reported a baseline ACD for the cilostazol group of 111 metres (SD 30) and follow‐up of 145 (SD 53). The placebo group had a baseline ACD of 116 metres (SD 56) and follow‐up of 121 (SD 62), with no difference between the time points.
Overall, the evidence for this outcome was of very low certainty, downgraded one level for risk of bias (selective reporting), one level for inconsistency (heterogeneity) and one level for strongly suspected publication bias.
Revascularisation (angioplasty or surgical bypass)
One study (516 participants) compared both cilostazol 50 mg twice daily and cilostazol 100 mg twice daily versus placebo (Beebe 1999) and found no clear difference in the odds of arterial revascularisation with OR 0.16 (95% CI 0.01 to 4.07, Analysis 1.3). The evidence for this outcome was of very low certainty, downgraded by two levels for imprecision and one level for strongly suspected publication bias.
1.3. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 3: Arterial revascularisation
Amputation
One study (516 participants) compared both cilostazol 50 mg twice daily and cilostazol 100 mg twice daily versus placebo (Beebe 1999), and found no clear difference in the odds of amputation with OR 0.16 (95% CI 0.01 to 4.07, Analysis 1.4). The evidence for this outcome was of very low certainty, downgraded by two levels for imprecision and one level for strongly suspected publication bias.
1.4. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 4: Amputation
Adverse events related to study medication
Eight of the included studies recorded data on side effects in a format eligible for meta‐analysis (Beebe 1999; Brass 2012; Dawson 1998; Dawson 2000; Elam 1998; Money 1998; Otsuka Study 21‐98‐213; Strandness 2002). The side effects reported varied between the studies, but the most common events were headache, diarrhoea, abnormal stools, dizziness, pain and palpitations, which are discussed below.
The O'Donnell 2009 study reported several side effects in a combined events outcome, which was not appropriate to include in the meta‐analyses. Combined adverse events were reported in Otsuka Study 21‐86‐101, Otsuka Study 21‐86‐103, Otsuka Study 21‐87‐101, Otsuka Study 21‐94‐301 and Otsuka Study 21‐95‐201, but only for participants who dropped out of the study. These events were not considered in the meta‐analyses. Lee 2001 reported no significant subjective side effects in the cilostazol or placebo group, but did not define what they considered a side effect.
Headache
Two studies (453 participants) reported on headache when comparing cilostazol 50 mg twice daily versus placebo (Beebe 1999; Strandness 2002). Meta‐analysis using a fixed‐effect model, showed an increased odds of headache in the cilostazol 50 mg twice daily group with OR 2.02 (95% CI 1.19 to 3.43) versus the placebo group (Analysis 1.5).
1.5. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 5: Adverse event related to study medication ‐ headache
Eight studies (2131 participants) reported on headache when comparing cilostazol 100 mg twice daily versus placebo (Beebe 1999; Brass 2012; Dawson 1998; Dawson 2000; Elam 1998; Money 1998; Otsuka Study 21‐98‐213; Strandness 2002). Meta‐analysis using a fixed‐effect model, showed an increased odds of headache in the cilostazol 100 mg twice daily group with OR 3.05 (95% CI 2.38 to 3.92) versus the placebo group (Analysis 1.5).
Overall, eight studies (2584 participants) reported on headache when comparing cilostazol (all doses) versus placebo (Beebe 1999; Brass 2012; Dawson 1998; Dawson 2000; Elam 1998; Money 1998; Otsuka Study 21‐98‐213; Strandness 2002). Meta‐analysis using a fixed‐effect model, showed an increased odds of headache in the cilostazol group with OR 2.83 (95% CI 2.26 to 3.55) versus the placebo group (Analysis 1.5). Incidence rates were 380/1456 for cilostazol participants and 119/1128 for placebo participants. No differences were seen with subgroup analysis (test for subgroup differences: P = 0.17). When low‐quality studies were removed in sensitivity analysis, there was very little change in the odds of headache in the cilostazol group versus placebo, OR 2.83, (95% CI 2.21 to 3.61, Analysis 1.16).
1.16. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 16: Adverse event related to study medication ‐ headache, sensitivity analysis
Overall, the evidence for this outcome was of moderate certainty, downgraded one level for strongly suspected publication bias. Table 3 grades the evidence for other adverse events related to study medication.
Diarrhoea
Two studies (453 participants) compared cilostazol 50 mg with placebo (Beebe 1999; Strandness 2002). The fixed‐effect model found no clear difference between groups, OR 2.02 (95% CI 0.91 to 4.52) for the 50 mg comparison (Analysis 1.6).
1.6. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 6: Adverse event related to study medication ‐ diarrhoea
Seven studies (2050 participants) compared cilostazol 100 mg twice daily with placebo (Beebe 1999; Brass 2012; Dawson 2000; Elam 1998; Money 1998; Otsuka Study 21‐98‐213; Strandness 2002). The fixed‐effect model found an increased odds in the cilostazol group: OR 2.88 (95% CI 2.07 to 3.99, Analysis 1.6).
Overall, seven studies (2503 participants) compared cilostazol (all doses) with placebo (Beebe 1999; Brass 2012; Dawson 2000; Elam 1998; Money 1998; Otsuka Study 21‐98‐213; Strandness 2002). The fixed‐effect model found an increased odds in the cilostazol group: OR 2.73 (95% CI 2.02 to 3.70, Analysis 1.6). Incidence rates were 190/1402 for cilostazol participants and 62/1101 for placebo participants. No differences were seen with subgroup analysis (test for subgroup differences: P = 0.43). When low‐quality studies were removed in sensitivity analysis, there was very little change in the meta‐analyses results, OR 2.91, (95% CI 2.05 to 4.12, Analysis 1.17).
1.17. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 17: Adverse event related to study medication ‐ diarrhoea, sensitivity analysis
Dawson 1998 collected data on gastrointestinal complaints compilation, which included diarrhoea and abnormal stools, but the data was not broken down into individual adverse events and could not be used in meta‐analysis.
Abnormal stools
Two studies (453 participants) compared cilostazol 50 mg versus placebo and found no difference between the treatment groups, OR 2.48 (95% CI 1.08 to 5.71) using a fixed‐effect model (Beebe 1999; Strandness 2002) (Analysis 1.7).
1.7. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 7: Adverse event related to study medication ‐ abnormal stools
Five studies (1351 participants) compared cilostazol 100 mg with placebo and found an increased odds of abnormal stools in the cilostazol group, OR 4.04 (95% CI 2.59 to 6.31), using a fixed‐effect model (Beebe 1999; Dawson 2000; Elam 1998; Money 1998; Strandness 2002) (Analysis 1.7).
Overall, five studies (1804 participants) compared cilostazol (all doses) with placebo (Beebe 1999; Dawson 2000; Elam 1998; Money 1998; Strandness 2002). The fixed‐effect model found an increased odds in the cilostazol group: OR 3.63 (95% CI 2.45 to 5.38, Analysis 1.7). Incidence rates of abnormal stools were 150/1052 for cilostazol participants and 33/752 for placebo participants. No differences were seen with subgroup analysis (test for subgroup differences: P = 0.31).
Dizziness
A single study compared cilostazol 50 mg with placebo and found no clear difference between the two treatment groups, OR 1.95 (95% CI 0.63 to 6.06) (Beebe 1999) (Analysis 1.8).
1.8. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 8: Adverse event related to study medication ‐ dizziness
For the comparison between cilostazol 100 mg and placebo, four studies (864 participants) recorded data on dizziness (Beebe 1999; Brass 2012; Elam 1998; Money 1998). The results of the fixed‐effect meta‐analysis found an increased odds of dizziness in the cilostazol group, OR 2.57 (95% CI 1.42 to 4.63, Analysis 1.8).
Overall, four studies (1120 participants) compared cilostazol (all doses) versus placebo (Beebe 1999; Brass 2012; Elam 1998; Money 1998). The results of the fixed‐effect meta‐analysis found an increased odds of dizziness in the cilostazol group, OR 2.42 (95% CI 1.43 to 4.08, Analysis 1.8). Incidence rates were 63/649 for cilostazol participants and 20/471 for placebo participants. No differences were seen with subgroup analysis (test for subgroup differences: P = 0.67).
Pain
Pain was reported in one study (197 participants) comparing cilostazol 50 mg versus placebo (Strandness 2002); it found no clear difference between treatment groups, OR 1.53 (95% CI 0.67 to 3.48) (Analysis 1.9).
1.9. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 9: Adverse event related to study medication ‐ pain
Pain was reported in four studies (1375 participants) comparing cilostazol 100 mg versus placebo (Dawson 2000; Elam 1998; Otsuka Study 21‐98‐213; Strandness 2002). There was no clear difference in the fixed‐effect model for cilostazol 100 mg versus placebo: OR 0.88 (95% CI 0.64 to 1.23, Analysis 1.9).
Overall, four studies (1572 participants) compared cilostazol (all doses) with placebo (Dawson 2000; Elam 1998; Otsuka Study 21‐98‐213; Strandness 2002). There was no clear difference in the fixed‐effect model for cilostazol versus placebo: OR 0.96 (95% CI 0.71 to 1.30, Analysis 1.9). Incidence rates were 107/848 for cilostazol participants and 92/724 for placebo participants. No differences were seen with subgroup analysis (test for subgroup differences: P = 0.23). When low‐quality studies were removed in sensitivity analysis, there was very little change in the meta‐analyses results, OR 1.07, (95% CI 0.75 to 1.54, Analysis 1.18).
1.18. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 18: Adverse event related to study medication ‐ pain, sensitivity analysis
Palpitations
The occurrence of palpitations was measured in one study (256 participants) comparing cilostazol 50 mg versus placebo (Beebe 1999); it found a higher odds in the cilostazol group, with a very wide CI, OR 8.89 (95% CI 0.51 to 155.87), but with only a single study, an overall association could not be determined (Analysis 1.10).
1.10. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 10: Adverse event related to study medication ‐ palpitations
The occurrence of palpitations was measured in four studies (1425 participants) comparing cilostazol 100 mg versus placebo (Beebe 1999; Brass 2012; Dawson 2000; Otsuka Study 21‐98‐213). The fixed‐effects model found an increased odds of palpitations in the cilostazol group, OR 7.06 (95% CI 3.85 to 12.96, Analysis 1.10).
Overall, four studies (1681 participants) compared cilostazol (all doses) with placebo (Beebe 1999; Brass 2012; Dawson 2000; Otsuka Study 21‐98‐213). The fixed‐effects model found an increased odds of palpitations in the cilostazol group, OR 7.16 (95% CI 3.95 to 12.98, Analysis 1.10). Incidence rates were 94/923 for cilostazol participants and 12/758 for placebo participants. No differences were seen with subgroup analysis (test for subgroup differences: P = 0.88). When low‐quality studies were removed in sensitivity analysis, there was an increased odds in the cilostazol group versus placebo (OR 12.80, 95% CI 5.06 to 32.36, Analysis 1.19).
1.19. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 19: Adverse event related to study medication ‐ palpitations, sensitivity analysis
Cardiovascular events
Two studies (692 participants) reported cardiovascular events (myocardial infarction and stroke) that compared cilostazol (all doses) versus placebo (Beebe 1999; Brass 2012). Meta‐analysis using a fixed‐effect model, showed no clear difference in the odds of cardiovascular events with OR 1.50 (95% CI 0.51 to 4.47) versus the placebo group (Analysis 1.11). No differences were seen with subgroup analysis by cilostazol dose (test for subgroup differences: P = 1.0). Brass 2012 reported serious adverse cardiac events, but did not report a breakdown of the types of events included. The cilostazol group experienced one cardiac event and the placebo group had three, but they were not statistically different; P = 0.365. Overall, the evidence for this outcome was of low certainty, downgraded one level for imprecision and one level for strongly suspected publication bias.
1.11. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 11: Cardiovascular event
All‐cause mortality
All‐cause mortality was reported in eight studies (2642 participants) (Beebe 1999; Brass 2012; Dawson 1998; Dawson 2000; Money 1998; Otsuka Study 21‐94‐301; Otsuka Study 21‐98‐213; Strandness 2002). The results of the fixed‐effect model found no clear difference between the treatment groups, with an OR of 0.97 (95% CI 0.41 to 2.30, Analysis 1.12). No differences were seen with subgroup analysis by cilostazol dose (test for subgroup differences: P = 0.62). When low‐quality studies were removed, there was little change in the meta‐analyses results (OR 1.21, 95% CI 0.47 to 3.13).
1.12. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 12: All‐cause mortality
Ankle brachial index
Three studies were included in the meta‐analysis for ABI (Dawson 2000; Elam 1998; Money 1998); the results from the random‐effects model was a higher ABI in the cilostazol arm of 0.06 (95% CI 0.04 to 0.08, Analysis 1.13).
1.13. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 13: Ankle brachial index (ABI)
In addition, two studies reported ABI that could not be included in the meta‐analysis (Lee 2001; O'Donnell 2009). The O'Donnell 2009 study reported on ABI, but because they only reported interquartile range for the baseline and follow‐up measurements, and they only reported ABI in a subgroup of normoglycaemic participants, the data were not comparable. The cilostazol group had a median change of ABI of ‐0.05 on the right side of the body and median change of ‐0.04 on the left side of the body. In comparison, the placebo group had a median ABI change of ‐0.03 on the right side of the body and ‐0.08 on the left. Lee 2001 reported no differences from baseline to follow‐up for any of the treatment groups. The cilostazol treatment group had a baseline measure of 0.73 (SD 0.12) and follow‐up of 0.69 (SD 0.11), while the placebo group had a baseline of 0.69 (SD 0.12) and follow‐up of 0.71 (SD 0.13).
Major Adverse Limb Event
None of the studies reported this outcome.
Cilostazol versus pentoxifylline
Table 4 provides a summary of the results for the comparison of cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily. Five studies were included for this comparison (Dawson 2000; De Albuquerque 2008; Lee 2001; Otsuka Study 21‐94‐301; Otsuka Study 21‐98‐213).
Initial claudication distance
A single study compared cilostazol 100 mg with pentoxifylline 400 mg (Dawson 2000), and the fixed‐effect model found no observable difference between the treatment groups in ICD (MD 20.00 metres, 95% CI ‐2.57 to 42.57 metres). As only one study was included in the analysis, an overall association could not be determined (Analysis 2.1).
2.1. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 1: Initial claudication distance (ICD)
Additionally, three studies that could not be included in the meta‐analysis compared change in ICD from baseline between cilostazol 100 mg and pentoxifylline 400 mg (De Albuquerque 2008; Otsuka Study 21‐94‐301; Otsuka Study 21‐98‐213). The report by De Albuquerque 2008 did not break down the ICD outcome by treatment group, and reported only an estimate of change in ICD for the whole study population split according to smoking status. Otsuka Study 21‐94‐301 reported a placebo‐corrected mean change of 10 metres in the pentoxifylline group and a ratio of geometric mean change from baseline of 1.02, favouring pentoxifylline. The Otsuka Study 21‐98‐213 found an arithmetic mean change of 47.3 metres for the cilostazol group and 62.6 metres for the placebo group suggesting a greater increase for the pentoxifylline group. There was also a non‐significant ratio of geometric means comparing cilostazol with pentoxifylline of 0.94 (95% CI 0.95 to 1.12; P = 0.260).
Overall, the evidence for this outcome was of low certainty because of imprecision and strongly suspected publication bias.
Health‐related quality of life
One study reported this outcome (Dawson 2000). The study authors reported that none of the treatments significantly affected the scores on mental health concepts, general health perception, physical health concepts, or vitality scores (SF‐36). There were no significant differences in patient‐reported walking distance or speed (WIQ). Overall, the evidence for this outcome was of very low certainty because of very serious imprecision and strongly suspected publication bias.
Absolute claudication distance
Two studies comparing cilostazol 100 mg with pentoxifylline 400 mg could be included in the ACD meta‐analysis (Dawson 2000; Otsuka Study 21‐98‐213). The resulting random‐effects model found no difference between the treatment groups with a MD of 13.41 metres (95% CI ‐43.50 to 70.36 metres, Analysis 2.2).
2.2. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 2: Absolute claudication distance (ACD)
Additionally, three studies that could not be included in the meta‐analysis compared change in ICD from baseline between cilostazol 100 mg and pentoxifylline 400 mg (De Albuquerque 2008; Lee 2001; Otsuka Study 21‐94‐301). The De Albuquerque 2008 study did not directly compare cilostazol with pentoxifylline, but reported a mean change in maximal walking distance via a graph, 'expressed as per cent of control' of approximately 50%. Otsuka Study 21‐94‐301 found similar placebo‐corrected mean changes of 33.6 metres for the cilostazol group and 34 metres for the pentoxifylline group, with a treatment effect ratio of cilostazol to pentoxifylline of 0.99, favouring pentoxifylline. Lee 2001 reported a baseline ACD for the cilostazol group of 111 metres (SD 30) and follow‐up of 145 (SD 53). The pentoxifylline group had a baseline ACD of 114 (SD 51) and follow‐up of 147 (81). However, the authors of the study did not directly compare the change in ACD between the two treatment groups.
Overall, the evidence for this outcome was of very low certainty because of inconsistency, imprecision and strongly suspected publication bias.
Revascularisation (angioplasty or surgical bypass)
No study reported this outcome.
Amputation
No study reported this outcome.
Adverse events related to study medication
Headache
The Dawson 2000 and Otsuka Study 21‐98‐213 studies compared cilostazol 100 mg twice daily with pentoxifylline 400 mg three times daily, and the random‐effects model found an increased odds of headache in the cilostazol group, OR 2.20 (95% CI 1.16 to 4.17, Analysis 2.3). Incidence rates were 106/488 for cilostazol participants and 55/494 for placebo participants.
2.3. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 3: Adverse event related to study medication ‐ headache
Overall, the evidence for this outcome was of low certainty because of heterogeneity and strongly suspected publication bias.
Diarrhoea
The two studies comparing cilostazol 100 mg with pentoxifylline 400 mg found, with a random‐effects model, no difference in the odds of diarrhoea between the treatment groups, OR 1.80 (95% CI 0.79 to 4.12) (Dawson 2000; Otsuka Study 21‐98‐213) (Analysis 2.4). Incidence rates were 78/488 for cilostazol participants and 48/494 for placebo participants.
2.4. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 4: Adverse event related to study medication ‐ diarrhoea
Abnormal stools
Only one study (Dawson 2000) reported abnormal stools for the comparison between cilostazol and pentoxifylline; it found an increased odds of abnormal stools in the cilostazol group, OR 3.12 (95% CI 1.57 to 6.21) (Analysis 2.5). Incidence rates were 33/227 for cilostazol participants and 12/232 for placebo participants.
2.5. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 5: Adverse event related to study medication ‐ abnormal stools
Pain
Pain was reported in two studies comparing cilostazol with pentoxifylline (Dawson 2000; Otsuka Study 21‐98‐213). There was no difference in the fixed‐effect model results for cilostazol versus pentoxifylline, OR 0.85 (95% CI 0.57 to 1.26) (Analysis 2.6). Incidence rates were 52/488 for cilostazol participants and 61/494 for placebo participants.
2.6. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 6: Adverse event related to study medication ‐ pain
Palpitations
The occurrence of palpitations was measured in two studies comparing cilostazol with pentoxifylline (Dawson 2000; Otsuka Study 21‐98‐213); there was an increase in palpitations in the cilostazol group, with a fixed‐effect model, OR 8.35 (95% CI 4.11 to 16.98) (Analysis 2.7). Incidence rates were 65/488 for cilostazol participants and 9/494 for placebo participants.
2.7. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 7: Adverse event related to study medication ‐ palpitations
Subjective side effects
Additionally, one study that could not be included in the meta‐analysis (Lee 2001), reported no significant subjective side effects in the cilostazol or pentoxifylline group, but did not define what they considered a side effect.
Cardiovascular events
No study reported this outcome.
All‐cause mortality
Three studies reported on all‐cause mortality comparing cilostazol with pentoxifylline (Dawson 2000; Otsuka Study 21‐94‐301; Otsuka Study 21‐98‐213). The fixed‐effect model results found no association between the treatment groups (OR 0.58, 95% CI 0.17 to 1.98) (Analysis 2.8).
2.8. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 8: All‐cause mortality
Ankle brachial index
One study was included in the ABI meta‐analysis for the comparison of cilostazol 100 mg to pentoxifylline 400 mg (Dawson 2000); it found an ABI MD of ‐0.01 (95% CI ‐0.12 to 0.10), but no overall association could be determined (Analysis 2.9).
2.9. Analysis.

Comparison 2: Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily, Outcome 9: Ankle brachial index (ABI)
Additionally, one study that could not be included in the meta‐analysis (Lee 2001), reported no differences from baseline to follow‐up for any of the treatment groups. The cilostazol treatment group had a baseline measure of 0.73 (SD 0.12) and follow‐up of 0.69 (SD 0.11), while the pentoxifylline group had a baseline of 0.66 (SD 0.13) and follow‐up of 0.70 (SD 0.14).
Major Adverse Limb Event
No study reported this outcome.
Discussion
Summary of main results
Cilostazol versus placebo
There is very low to low‐certainty evidence that cilostazol improves walking distance, both in terms of ICD and ACD, compared to placebo. Six studies reported ICD, with a study duration ranging from 12 to 24 weeks, and cilostazol dose ranging from 100 mg to 300 mg. Participants taking cilostazol had a higher ICD, with a MD of 26.49 metres (95% CI 18.93 to 34.05; 1722 participants; 6 studies), compared with those taking placebo. When lower quality studies were removed, there was an additional improvement of three metres in favour of cilostazol (MD 29.52, 95% CI 21.26 to 37.78; 1543 participants; 5 studies) and subgroup differences according to dose (P = 0.04). Participants taking 50 mg cilostazol twice daily had a MD of 19.50 metres (95% CI 6.80 to 32.21; 400 participants; 2 studies) and participants taking 100 mg cilostazol twice daily had a MD of 32.19 metres (95% CI 22.20 to 42.18; 1236 participants; 6 studies). Participants taking cilostazol had a higher ACD, with a MD of 39.57 metres (95% CI 21.80 to 57.33; 2360 participants; 8 studies) compared with those taking placebo. When lower quality studies were removed, there was an additional improvement of nine metres in favour of cilostazol (MD 48.44, 95% CI 34.49 to 62.39; 1732 participants; 6 studies).
The value of these increases in walking distance (26 metres further before the onset of calf pain and 40 metres further in terms of total distance) are subjective and depend on the individual patient; some patients may find not much benefit from this additional walking distance but some may find the additional extra walking distance enables them to undertake more daily activities.
Four studies reported on quality of life (QoL) but, due to the differences in QoL measures as well as how they were reported, we did not undertake a meta‐analysis. Overall, cilostazol was associated with improvements in some domains of QoL compared to placebo. Very few studies reported on other outcomes making it impossible to draw any conclusions regarding the effectiveness of cilostazol versus placebo on arterial revascularisation, amputation, or cardiovascular events.
There was moderate‐certainty evidence that participants taking cilostazol had an increased odds of experiencing headache compared to participants taking placebo, during 12 to 26 weeks intervention (OR 2.83, 95% CI 2.26 to 3.55; 2584 participants; 8 studies); and an increased odds of other commonly reported adverse events including diarrhoea, abnormal stools, dizziness and palpitations.
Eight studies reported very few deaths with no difference in all‐cause mortality between cilostazol and placebo groups. Cilostazol (100 mg twice daily) improved ABI over placebo (MD 0.06, 95% CI 0.04 to 0.08; 859 participants; 3 studies).
The certainty of the evidence was downgraded by one level for all studies because publication bias was strongly suspected. Other issues that necessitated downgrading included risk of selective reporting, imprecision and inconsistency.
Cilostazol versus pentoxifylline
There is very low to low‐certainty evidence of no difference between cilostazol and pentoxifylline for improving walking distance, both in terms of ICD (MD 20.00, 95% CI ‐2.57 to 42.57; 417 participants; 1 study) and ACD (MD 13.43, 95% CI ‐43.50 to 70.36; 866 participants; 2 studies).
One study reported on QoL; the study authors reported no difference in QoL between the treatment groups. No study reported on revascularisation, amputation or cardiovascular events. There was low‐certainty evidence that cilostazol participants had an increased odds of experiencing headache compared to participants taking pentoxifylline at 24 weeks (OR 2.20, 95% CI 1.16 to 4.17; 982 participants; 2 studies); and an increased odds of experiencing abnormal stools, and palpitations, but there was no difference between treatment groups for diarrhoea or pain. There was no clear difference between treatment groups for all‐cause mortality or ABI.
Certainty of the evidence was downgraded by one level for all studies because publication bias was strongly suspected. Other issues that necessitated downgrading included imprecision and inconsistency.
Overall completeness and applicability of evidence
This review addressed whether the use of cilostazol reduced symptoms of intermittent claudication (specifically ICD and ACD) in participants with stable intermittent claudication. All 16 included studies evaluated the effects of cilostazol compared with placebo, within similar study populations. Most of the included evidence is for the comparison of cilostazol versus placebo, the two walking distance outcomes (ICD and ACD) and for the cilostazol dose of 100 mg twice daily. We identified very limited data on QoL, other serious outcomes of amputation, revascularisation and cardiovascular events, and all‐cause mortality, both in terms of the number of studies reporting these outcomes and few events reported within those studies.
Treatment duration ranged from six to 26 weeks, with the most common treatment time at 24 weeks. Most of the included studies only reported change from baseline for the final time point, so we were unable to compare studies at a common time point. Treadmill protocols ranged between three main protocols. The aberrations between the testing protocols were addressed by using change in mean walking distances, rather than absolute follow‐up distance, which does not account for baseline measures. These differences and limitations alter the strength of the applicability of the evidence, and should be kept in mind when interpreting the findings. Only two of the included studies defined their baseline treadmill test values when multiple baseline values were obtained (Brass 2012; Dawson 2000). Both studies used the highest baseline treadmill value for analysis, while the remaining studies did not indicate their methods. Possible variations in treadmill testing baseline definition could reduce the applicability of the findings.
Many included studies were quite 'old' and were carried out before best medical treatment was recommended or applied in patients with stable intermittent claudication and so a further limitation to this evidence is that it might not be an accurate representation of current practice. Also, dose recommendation for pentoxifylline is either 400 mg three times daily or 400 mg twice daily; one included study did not reflect current dosing practice as it used a pentoxifylline dose of 600 mg twice daily (De Albuquerque 2008). We only identified studies comparing cilostazol versus placebo, and cilostazol versus pentoxifylline; studies comparing cilostazol with other agents, such as naftidrofuryl, were not identified.
Quality of the evidence
We included 16 studies with 3972 participants. Using GRADE assessment, all studies (both comparisons) were downgraded one level because publication bias was strongly suspected, with pharmaceutical sponsors involved in all 16 studies (of which 13 involved the same pharmaceutical company (Otsuka)).
Using GRADE assessment, the body of evidence relating to cilostazol compared with placebo was judged to be of very low (ACD, revascularisation, amputation), low (ICD, QoL, cardiovascular outcomes) to moderate (adverse events ‐ headache) certainty. Other issues that necessitated downgrading included risk of selective reporting, imprecision and inconsistency.
Using GRADE assessment, the body of evidence relating to cilostazol compared with pentoxifylline was judged to be of very low (QoL, ACD), to low (ICD, adverse events ‐ headache) certainty. Other issues that necessitated downgrading included imprecision and inconsistency.
Certainty of the evidence was based on those studies in the summary of findings tables and (with the exception of QoL) this evidence comes from the meta‐analyses. The risk of bias assessments of those studies not included in the meta‐analyses was consistent with risk of bias assessments of studies included in the meta‐analyses. However, it should be noted that data in a significant proportion of studies was poorly reported, and we were unable to incorporate such data in any of the meta‐analyses.
Six studies were never published as journal articles, with sources of data being a medical review by the FDA in five cases and a pharmaceutical submission to NICE in another case. Seven studies were published journal articles, however, the data used for six of these studies were derived from pharmaceutical data submitted to the FDA rather than the associated publications.
Potential biases in the review process
Study selection and data extraction were performed independently by two review authors in order to minimise bias in the review process. The inclusion and exclusion criteria of the review were strictly adhered to in order to limit subjectivity.
For the primary outcome of ICD, 14 of the 16 included studies reported this outcome. However, only six of these studies were reported in an adequate and appropriate manner to be included in the meta‐analyses. Following consultation with a statistician, other forms of imputation were not carried out due to methodological differences in the reporting of outcomes that did not allow us to calculate mean change and standard deviations. Also, due to the large differences between studies, we deemed imputation inappropriate. For the Dawson 2000 study, standard deviations were provided for the ABI outcome for mean change in the comparison between cilostazol and placebo, but not for cilostazol compared to pentoxifylline. We calculated correlation coefficients using the existing mean change standard deviations and imputed values to calculate mean change standard deviations for the comparison between cilostazol and pentoxifylline.
Agreements and disagreements with other studies or reviews
The evidence presented here is consistent with the findings of two older reviews (Regensteiner 2002; Thompson 2002) which evaluated the effects of cilostazol for intermittent claudication and found similar improvements in walking distances for participants taking cilostazol. A systematic review published in 2012 comparing cilostazol, naftidrofuryl oxalate and pentoxifylline with placebo for the treatment of intermittent claudication in patients with PAD included six of the same studies as our review (Stevens 2012). For inclusion in meta‐analysis, the study authors employed imputation techniques that we ourselves did not use and they reported their findings for ICD (reported as maximum walking distance) and ACD (reported as pain‐free walking distance) as geometric mean changes compared with placebo. However, their results also found increases in both ICD and ACD for the cilostazol groups, compared with placebo, with an increase in ICD of 25% (95% credible interval 11% to 40%) and an ACD increase of 13% (95% credible interval 2% to 26%). Adverse events were not reported in the meta‐analysis, but headaches and gastrointestinal issues that were mild were noted in the intervention arms and there was no increase in cardiovascular events or deaths for cilostazol, naftidrofuryl oxalate or pentoxifylline. The authors noted that the heterogeneity of QoL reporting did not allow them to report those findings in their review.
The data from Stevens and colleagues (Stevens 2012) is also presented as part of Squires 2010 and Squires 2011 as technology assessment reports written for the National Institute for Health and Care Excellence (NICE 2011). These assessment reports continue to underpin the current NICE guideline (CG147) and there has been no major change to this guideline in relation to treatment of intermittent claudication, since its publication in 2012 and last updated in December 2020 (NICE 2012). Our review confirms that there is very little new RCT evidence for cilostazol for people with intermittent claudication. We did not identify any newer systematic reviews of cilostazol for intermittent claudication.
Two of the studies in our review that compared cilostazol versus pentoxifylline (Dawson 2000; Lee 2001) were included in another Cochrane review of pentoxifylline (Broderick 2020) where review authors concluded that the data from studies comparing cilostazol with pentoxifylline 'were too limited to allow meaningful conclusions'. In patients in whom symptoms do not improve with exercise and risk factor management, medical management using pharmacological interventions, such as cilostazol (Aboyans 2018; Gerhard‐Herman 2017; Aboyans 2018), naftidrofuryl (Aboyans 2018; NICE 2012) and pentoxifylline (Aboyans 2018; Gerhard‐Herman 2017; Aboyans 2018), are suggested by some national guidelines. A review of clinical guidelines published in 2016 showed that cilostazol was the most recommended drug (in five guidelines) as first option for pharmacological treatment (Barriocanal 2016).
Although the data supports the use of cilostazol for the treatment of intermittent claudication in people with PAD, as well as pentoxifylline and inositol nicotinate, current NICE guidelines (last updated December 2020) only recommend naftidrofuryl as treatment in this population (NICE 2012). Our review, alongside other reviews mentioned here, demonstrates that there remains a degree of uncertainty as to which, if any, of these medications provides most clinical benefit.
Authors' conclusions
Implications for practice.
Participants taking cilostazol for three to six months could walk approximately 26 metres further before the onset of calf pain and 40 metres further in terms of total distance on a treadmill compared to participants taking placebo. However, participants taking cilostazol had nearly three times the odds of experiencing headache compared to participants taking placebo. The value of these increases in walking distance will be patient‐specific. There is insufficient evidence about the effectiveness of cilostazol for serious events such as amputation, revascularisation, and cardiovascular events. Despite the importance of quality of life to patients, meta‐analysis could not be undertaken because of differences in measures used and how they were reported.
Very limited data indicated no difference between cilostazol and pentoxifylline for improving walking distance, but the data were too limited to enable any meaningful conclusions to be drawn for any of the remaining outcomes reported.
Using GRADE methods, we judged the evidence to be of very low to low certainty for all of the outcomes except for adverse events related to study medication where some events were judged as being at moderate certainty. All studies for both comparisons were downgraded one level because publication bias was strongly suspected. Other issues that necessitated downgrading included risk of selective reporting, imprecision and inconsistency.
Implications for research.
Future research on cilostazol for the treatment of intermittent claudication should ideally be performed such that comparisons can be made with other studies. Currently, there is little consensus on treatment duration, treadmill test protocol, and outcome measurement/reporting, which inhibits direct comparisons. This is apparent in this review with the significant number of studies that could not be included in the meta‐analysis due to outcome reporting being inconsistent, and other variations making imputation inappropriate. Suggestions for future research include research that is independently funded and which directly compares cilostazol with other active drugs. Quality of life is extremely important to patients and needs to be measured as a matter of course and consistently in future studies, with agreement of which tools to use and how to report the data to enable comparison across studies.
Feedback
Unpublished trials (Feedback and response added 11 September 2007),
Summary
The Cochrane review of cilostazol (1/2007) included only one study of cilostazol (CLZ) and pentoxifylline (PTX, TRENTAL), (Dawson DL 2000), and stated: "the differences in ICD and ACD showed significant improvement for the cilostazol group over patients taking pentoxifylline".
Already in 1998, eight pivotal trials with cilostazol had been analysed in the medical review by the FDA. One of these was trial 21‐94‐301 (P. 58), an unpublished trial of Otsuka with 370 patients: 247 CLZ or placebo, 123 pentoxifylline. In this study, CLZ was not statistically distinguishable from either placebo or oxpentifylline (= pentoxyfylline). A second study comparing cilostazol with pentoxifylline was the Dawson DL 2000 (trial 21‐96‐202). The FDA stated (p.231): "There is not yet a convincing basis with which to conclude that CLZ is more efficious than pentoxifylline in this regard (anti claudication efficacy)".
Pentoxifylline is not recommended for claudication in some guidelines (SIGN 10/2006, CHEST 2/2007), therefore, it is important to note that there is no difference between CLZ and PTX.
In a reply (21 March 2007) to my mail (23 February 2007) to the Cochrane peripheral vascular diseases group, Prof. Stansby stated that "the medical review (of the FDA) does not come up if you put cilostazol into the FDA web page search".
This Cochrane review was published at the same time as marketing of cilostazol started in Germany and was part of the promotional material Schwarz Pharma sent to us. Prof. Stansby declared a conflict of interest with Otsuka pharmaceuticals, the developing company. For me, this may be a problem. What does Cochrane think about it?
Submitter agrees with the default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
We agree that there appears to be an additional and unpublished trial comparing cilostazol with pentoxifylline, referred to as study 21‐94‐301 in the FDA document of 1998. We were unaware of this when we prepared our original review. It did not come to light using standard search strategies. Unfortunately, the data currently available to us are still not sufficient to allow inclusion of this trial at present. Otsuka have not made the data available to us, although it has been requested. The review has been altered to make it clear that this additional study exists and that any conclusions about a comparison with pentoxifylline should be guarded based on the one published trial. If in the future Otsuka does release further data to us, and the methodological quality is acceptable, we will consider including it in future updates.
The production of this review and its timing was entirely coincidental to the release date of cilostazol in Germany. Likewise, there was no contact with Otsuka concerning these matters. Professor Stansby has declared his conflicts of interest, but has not had any contact with Otsuka in relation to the timing and release of this review. The main conclusions of the review are not altered by this additional trial but we have updated the review to include this study under "excluded studies".
Contributors
Feedback contributed by: Dr. Heide Rose GIECK Editorial staff arznei‐telegramm A.T.I. Arzneimittelinformation Berlin GmbH Bergstr. 38A, Wasserturm, D‐12169 Berlin
Response contributed by: Professor Gerry Stansby Professor of Vascular Surgery Department of Surgery University of Newcastle upon Tyne Framlington Place Newcastle upon Tyne NE24HH UK
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2021 | New citation required but conclusions have not changed | New author joined review team. One author left review team. GRADE and Summary of Findings incorporated. Conclusions not changed. |
| 31 March 2021 | New search has been performed | Searches rerun. One new included study, 23 new excluded studies identified. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 15 April 2014 | New citation required but conclusions have not changed | New authors joined review team. Risk of bias assessments added and methods updated to reflect current standards. Conclusions not changed |
| 18 October 2013 | New search has been performed | Searches rerun; eight new studies included and seven new studies excluded |
| 9 May 2008 | Amended | Converted to new review format |
| 11 November 2007 | Feedback has been incorporated | Feedback and authors' response to feedback added. Unpublished trial Otsuka 1996b (Otsuka 21‐94‐201) is a duplicate reference to Strandness 2002 |
| 7 November 2007 | New citation required but conclusions have not changed | Two excluded studies added. No change to conclusions |
| 21 February 2007 | Amended | Edited update. Abstract edited to include unit of measurement in results section |
Acknowledgements
We would like to thank Dr Catriona Keerie for her statistical advice and Dr Rujan Shrestha for helping to screen an article written in Chinese. We would like to thank Mr Peter Robless for his input into previous versions of this review.
The review authors and the Cochrane Vascular Editorial Base are grateful to the following peer reviewers for their time and comments: Professor Stefan Acosta, Lund University, Sweden; Neal R. Barshes, M.D., M.P.H., Baylor College of Medicine, USA; Marc De Buyzere and Tine De Backer, Ghent University, Belguim; and Danial Sayyad, Iran.
Appendices
Appendix 1. Database search strategies
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Arteriosclerosis 1010 #2 MESH DESCRIPTOR Arteriolosclerosis 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 88 #4 MESH DESCRIPTOR Atherosclerosis 1362 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 882 #6 MESH DESCRIPTOR Intermittent Claudication 916 #7 MESH DESCRIPTOR Ischemia 2071 #8 MESH DESCRIPTOR Vascular Diseases 814 #9 MESH DESCRIPTOR intermittent claudication EXPLODE ALL TREES 916 #10 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 3193 #11 (atherosclero* or arteriosclero* or PVD or PAOD or PAD):TI,AB,KY 16562 #12 ((arter*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*)):TI,AB,KY 5893 #13 ((vascular) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*)):TI,AB,KY 683 #14 ((vein*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*)):TI,AB,KY 439 #15 ((veno*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*)):TI,AB,KY 313 #16 ((peripher*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*)):TI,AB,KY 1683 #17 (peripheral near/3 dis*):TI,AB,KY 0 #18 arteriopathic:TI,AB,KY 6 #19 ((claudic* or hinken*)):TI,AB,KY 2452 #20 ((isch* or CLI)):TI,AB,KY 43328 #21 dysvascular*:TI,AB,KY 28 #22 (leg near (obstruct*or steno* or block* or obliter*)):TI,AB,KY 122 #23 (limb near (obstruct*or steno* or block* or obliter*)):TI,AB,KY 309 #24 ((lower extrem*) near (obstruct* or occlus* or steno* or block* or obliter*)):TI,AB,KY 179 #25 ((aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) near (obstruct* or occlus*)):TI,AB,KY 649 #26 MESH DESCRIPTOR Femoral Artery EXPLODE ALL TREES 979 #27 MESH DESCRIPTOR Popliteal Artery EXPLODE ALL TREES 350 #28 MESH DESCRIPTOR Iliac Artery EXPLODE ALL TREES 162 #29 MESH DESCRIPTOR Iliac Vein EXPLODE ALL TREES 46 #30 MESH DESCRIPTOR Popliteal Vein EXPLODE ALL TREES 65 #31 MESH DESCRIPTOR Femoral Vein EXPLODE ALL TREES 233 #32 Fontaine:TI,AB,KY 365 #33 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 68856 #34 MESH DESCRIPTOR Vasodilator Agents EXPLODE ALL TREES 25529 #35 MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES 11085 #36 MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL TREES 7118 #37 MESH DESCRIPTOR Tetrazoles EXPLODE ALL TREES 3581 #38 vasodilator*:TI,AB,KY 6961 #39 Cilostazol:TI,AB,KY 815 #40 OPC‐13013:TI,AB,KY 6 #41 (platelet near3 inhibitor*):TI,AB,KY 4290 #42 (phosphodiesterase inhibitor*):TI,AB,KY 1041 #43 pletal:TI,AB,KY 26 #44 pletaal:TI,AB,KY 12 #45 73963‐72‐1:TI,AB,KY 3 #46 #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 42222 #47 #33 AND #46 7069 #48 01/01/2013 TO 09/11/2020:CD 1027175 #49 #47 AND #48 2477 |
Nov 2020: 2477 |
| Clinicaltrials.gov | intermittent claudication OR Peripheral Vascular Diseases | Cilostazol OR Tetrazoles OR phosphodiesterase inhibitorS OR pletal OR Vasodilator Agents OR Aggregation Inhibitors | Nov 2020: 61 |
| ICTRP Search Portal | Nov 2020: | |
| Medline (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present | 1 Arterial Occlusive Diseases/ 2 Arteriolosclerosis/ 3 Arteriosclerosis/ 4 Arteriosclerosis Obliterans/ 5 Femoral Artery/ 6 Iliac Artery/ 7 Intermittent Claudication/ 8 Ischemia/dt, et, mo, su, th [Drug Therapy, Etiology, Mortality, Surgery, Therapy] 9 Leg/bs [Blood Supply] 10 exp Peripheral Vascular Diseases/ 11 Popliteal Artery/ 12 Tibial Arteries/ 13 arteriosclero*.ti,ab. 14 arteriopathic.ti,ab. 15 claudic*.ti,ab. 16 CLI.ti,ab. 17 dysvascular*.ti,ab. 18 ischemi*.ti,ab. 19 PVD.ti,ab. 20 PAOD.ti,ab. 21 (peripheral adj3 dis*).ti,ab. 22 (("lower extrem*" or arter* or crural or femdist* or femoral or fempop* or iliac or infrainquinal or infrapopliteal or inguinal or limb or peripher* or popliteal or tibial or vascular or vein* or veno*) adj3 (block* or harden* or lesio* or obliter* or obstruct* or occlus* or reocclus* or re‐occlus* or restenos* or steno* or stiffen*)).ti,ab. 23 or/1‐22 24 Cilostazol/ 25 Vasodilator Agents/ 26 Platelet Aggregation Inhibitors/ 27 Phosphodiesterase Inhibitors/ 28 Tetrazoles/ 29 vasodilator*.ti,ab. 30 Cilostazol.ti,ab. 31 OPC‐13013.ti,ab. 32 (platelet adj3 inhibitor*).ti,ab. 33 "phosphodiesterase inhibitor*".ti,ab. 34 pletal.ti,ab. 35 pletaal.ti,ab. 36 73963‐72‐1.ti,ab. 37 or/24‐36 38 23 and 37 39 randomized controlled trial.pt. 40 controlled clinical trial.pt. 41 randomized.ab. 42 placebo.ab. 43 drug therapy.fs. 44 randomly.ab. 45 trial.ab. 46 groups.ab. 47 or/39‐46 48 exp animals/ not humans.sh. 49 47 not 48 50 38 and 49 51 (2013* or 2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020*).ed. 52 50 and 51 |
Nov 2020: 2771 |
| Embase | 1 peripheral occlusive artery disease/ 2 arteriolosclerosis/ 3 arteriosclerosis/ 4 femoral artery/ 5 iliac artery/ 6 intermittent claudication/ 7 ischemia/dt, et, su, th [Drug Therapy, Etiology, Surgery, Therapy] 8 exp peripheral vascular disease/ 9 popliteal artery/ 10 tibial artery/ 11 arteriosclero*.ti,ab. 12 arteriopathic.ti,ab. 13 claudic*.ti,ab. 14 CLI.ti,ab. 15 dysvascular*.ti,ab. 16 PVD.ti,ab. 17 PAOD.ti,ab. 18 (peripheral adj3 dis*).ti,ab. 19 (("lower extrem*" or arter* or crural or femdist* or femoral or fempop* or iliac or infrainquinal or infrapopliteal or inguinal or limb or peripher* or popliteal or tibial or vascular or vein* or veno*) adj3 (block* or harden* or lesio* or obliter* or obstruct* or occlus* or reocclus* or re‐occlus* or restenos* or steno* or stiffen*)).ti,ab. 20 or/1‐19 21 cilostazol/ 22 vasodilator agent/ 23 phosphodiesterase inhibitor/ 24 vasodilator*.ti,ab. 25 Cilostazol.ti,ab. 26 OPC‐13013.ti,ab. 27 (platelet adj3 inhibitor*).ti,ab. 28 "phosphodiesterase inhibitor*".ti,ab. 29 pletal.ti,ab. 30 pletaal.ti,ab. 31 73963‐72‐1.ti,ab. 32 or/21‐31 33 20 and 32 34 randomized controlled trial/ 35 controlled clinical trial/ 36 random$.ti,ab. 37 randomization/ 38 intermethod comparison/ 39 placebo.ti,ab. 40 (compare or compared or comparison).ti. 41 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 42 (open adj label).ti,ab. 43 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 44 double blind procedure/ 45 parallel group$1.ti,ab. 46 (crossover or cross over).ti,ab. 47 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 48 (assigned or allocated).ti,ab. 49 (controlled adj7 (study or design or trial)).ti,ab. 50 (volunteer or volunteers).ti,ab. 51 trial.ti. 52 or/34‐51 53 33 and 52 54 (2013* or 2014* or 2015* or 2016* or 2017* or 2016* or 2017* or 2018* or 2019* or 2020*).dc. 55 53 and 54 |
Nov 2020: 2218 |
| CINAHL | S54 S52 AND S53 S53 EM 2013 OR EM 2014 OR EM 2015 OR EM 2016 OR EM 2017 OR EM 2018 OR EM 2019 OR EM 2020 S52 S36 AND S51 S51 S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 S50 MH "Random Assignment" S49 MH "Triple‐Blind Studies" S48 MH "Double‐Blind Studies" S47 MH "Single‐Blind Studies" S46 MH "Crossover Design" S45 MH "Factorial Design" S44 MH "Placebos" S43 MH "Clinical Trials" S42 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S41 TX crossover OR "cross‐over" S40 AB placebo* S39 TX random* S38 TX trial* S37 TX "latin square" S36 S22 AND S35 S35 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 S34 TX 73963‐72‐1 S33 TX pletaal S32 TX pletal S31 TX "phosphodiesterase inhibitor*" S30 TX platelet N3 inhibitor* S29 TX OPC‐13013 S28 TX Cilostazol S27 TX vasodilator* S26 (MH "Phosphodiesterase Inhibitors") S25 (MH "Platelet Aggregation Inhibitors") S24 (MH "Vasodilator Agents") S23 (MH "Cilostazol") S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 S21 TX ("lower extrem*" or arter* or crural or femdist* or femoral or fempop* or iliac or infrainquinal or infrapopliteal or inguinal or limb or peripher* or popliteal or tibial or vascular or vein* or veno*) N3 (block* or harden* or lesio* or obliter* or obstruct* or occlus* or reocclus* or re‐occlus* or restenos* or steno* or stiffen*) S20 TX peripheral N3 dis* S19 TX PAOD S18 TX PVD S17 TX ischemi* S16 TX dysvascular* S15 TX CLI S14 TX claudic* S13 TX arteriopathic S12 TX arteriosclero* S11 (MH "Tibial Arteries") S10 Tibial Arteries S9 (MH "Popliteal Artery") S8 (MH "Peripheral Vascular Diseases+") S7 (MH "Leg/BS") S6 (MH "Ischemia/DT/ET/MO/SU") S5 (MH "Intermittent Claudication") S4 (MH "Iliac Artery") S3 (MH "Femoral Artery") S2 (MH "Arteriosclerosis") S1 (MH "Arterial Occlusive Diseases") |
Nov 2020: 782 |
Data and analyses
Comparison 1. Cilostazol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Initial claudication distance (ICD) | 6 | 1722 | Mean Difference (IV, Fixed, 95% CI) | 26.49 [18.93, 34.05] |
| 1.1.1 Cilostazol 50 mg twice daily | 2 | 400 | Mean Difference (IV, Fixed, 95% CI) | 19.50 [6.80, 32.21] |
| 1.1.2 Cilostazol 100 mg twice daily | 6 | 1236 | Mean Difference (IV, Fixed, 95% CI) | 32.19 [22.20, 42.18] |
| 1.1.3 Cilostazol 150 mg twice daily | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 15.70 [‐12.20, 43.60] |
| 1.2 Absolute claudication distance (ACD) | 8 | 2360 | Mean Difference (IV, Random, 95% CI) | 39.57 [21.80, 57.33] |
| 1.2.1 Cilostazol 50 mg twice daily | 2 | 400 | Mean Difference (IV, Random, 95% CI) | 30.84 [8.81, 52.86] |
| 1.2.2 Cilostazol 100 mg twice daily | 8 | 1874 | Mean Difference (IV, Random, 95% CI) | 42.32 [18.12, 66.51] |
| 1.2.3 Cilostazol 150 mg twice daily | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 51.80 [‐10.59, 114.19] |
| 1.3 Arterial revascularisation | 1 | 516 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 4.07] |
| 1.3.1 Cilostazol 50 mg twice daily | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 4.07] |
| 1.3.2 Cilostazol 100 mg twice daily | 1 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 Amputation | 1 | 516 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 4.07] |
| 1.4.1 Cilostazol 50 mg twice daily | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 4.07] |
| 1.4.2 Cilostazol 100 mg twice daily | 1 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Adverse event related to study medication ‐ headache | 8 | 2584 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [2.26, 3.55] |
| 1.5.1 Cilostazol 50 mg twice daily | 2 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.19, 3.43] |
| 1.5.2 Cilostazol 100 mg twice daily | 8 | 2131 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [2.38, 3.92] |
| 1.6 Adverse event related to study medication ‐ diarrhoea | 7 | 2503 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.73 [2.02, 3.70] |
| 1.6.1 Cilostazol 50 mg twice daily | 2 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.91, 4.52] |
| 1.6.2 Cilostazol 100 mg twice daily | 7 | 2050 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.88 [2.07, 3.99] |
| 1.7 Adverse event related to study medication ‐ abnormal stools | 5 | 1804 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.63 [2.45, 5.38] |
| 1.7.1 Cilostazol 50 mg twice daily | 2 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.08, 5.71] |
| 1.7.2 Cilostazol 100 mg twice daily | 5 | 1351 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.04 [2.59, 6.31] |
| 1.8 Adverse event related to study medication ‐ dizziness | 4 | 1120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.43, 4.08] |
| 1.8.1 Cilostazol 50 mg twice daily | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.63, 6.06] |
| 1.8.2 Cilostazol 100 mg twice daily | 4 | 864 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.42, 4.63] |
| 1.9 Adverse event related to study medication ‐ pain | 4 | 1572 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| 1.9.1 Cilostazol 50 mg twice daily | 1 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.67, 3.48] |
| 1.9.2 Cilostazol 100 mg twice daily | 4 | 1375 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.23] |
| 1.10 Adverse event related to study medication ‐ palpitations | 4 | 1681 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.16 [3.95, 12.98] |
| 1.10.1 Cilostazol 50 mg twice daily | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.89 [0.51, 155.87] |
| 1.10.2 Cilostazol 100 mg twice daily | 4 | 1425 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.06 [3.85, 12.96] |
| 1.11 Cardiovascular event | 2 | 692 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.51, 4.47] |
| 1.11.1 Cilostazol 50 mg twice daily | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.30, 7.64] |
| 1.11.2 Cilostazol 100 mg twice daily | 2 | 436 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.35, 6.52] |
| 1.12 All‐cause mortality | 8 | 2642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.41, 2.30] |
| 1.12.1 Cilostazol 50 mg twice daily | 2 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.03, 8.00] |
| 1.12.2 Cilostazol 100 mg twice daily | 8 | 2189 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.42, 2.59] |
| 1.13 Ankle brachial index (ABI) | 3 | 859 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.04, 0.08] |
| 1.13.1 Cilostazol 100 mg twice daily | 3 | 859 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.04, 0.08] |
| 1.14 Initial claudication distance (ICD) sensitivity analysis | 5 | 1543 | Mean Difference (IV, Fixed, 95% CI) | 29.52 [21.26, 37.78] |
| 1.14.1 Cilostazol 50 mg twice daily | 2 | 400 | Mean Difference (IV, Fixed, 95% CI) | 19.50 [6.80, 32.21] |
| 1.14.2 Cilostazol 100 mg twice daily | 5 | 1143 | Mean Difference (IV, Fixed, 95% CI) | 36.86 [25.98, 47.74] |
| 1.15 Absolute claudication distance (ACD) sensitivity analysis | 6 | 1732 | Mean Difference (IV, Random, 95% CI) | 48.44 [34.49, 62.39] |
| 1.15.1 Cilostazol 50 mg twice daily | 2 | 400 | Mean Difference (IV, Random, 95% CI) | 30.84 [8.81, 52.86] |
| 1.15.2 Cilostazol 100 mg twice daily | 6 | 1332 | Mean Difference (IV, Random, 95% CI) | 56.30 [40.37, 72.23] |
| 1.16 Adverse event related to study medication ‐ headache, sensitivity analysis | 7 | 2061 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [2.21, 3.61] |
| 1.16.1 Cilostazol 50 mg twice daily | 2 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.19, 3.43] |
| 1.16.2 Cilostazol 100 mg twice daily | 7 | 1608 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.10 [2.35, 4.09] |
| 1.17 Adverse event related to study medication ‐ diarrhoea, sensitivity analysis | 6 | 1980 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.91 [2.05, 4.12] |
| 1.17.1 Cilostazol 50 mg twice daily | 2 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.91, 4.52] |
| 1.17.2 Cilostazol 100 mg twice daily | 6 | 1527 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [2.15, 4.67] |
| 1.18 Adverse event related to study medication ‐ pain, sensitivity analysis | 3 | 1049 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.54] |
| 1.18.1 Cilostazol 50 mg twice daily | 1 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.67, 3.48] |
| 1.18.2 Cilostazol 100 mg twice daily | 3 | 852 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.66, 1.47] |
| 1.19 Adverse event related to study medication ‐ palpitations, sensitivity analysis | 3 | 1158 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.80 [5.06, 32.36] |
| 1.19.1 Cilostazol 50 mg twice daily | 1 | 256 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.89 [0.51, 155.87] |
| 1.19.2 Cilostazol 100 mg twice daily | 3 | 902 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.42 [5.05, 35.68] |
| 1.20 All‐cause mortality sensitivity analysis | 7 | 2119 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.47, 3.13] |
| 1.20.1 Cilostazol 50 mg twice daily | 2 | 453 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.03, 8.00] |
| 1.20.2 Cilostazol 100 mg twice daily | 7 | 1666 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.49, 3.75] |
1.14. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 14: Initial claudication distance (ICD) sensitivity analysis
1.15. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 15: Absolute claudication distance (ACD) sensitivity analysis
1.20. Analysis.

Comparison 1: Cilostazol versus placebo, Outcome 20: All‐cause mortality sensitivity analysis
Comparison 2. Cilostazol 100 mg twice daily versus pentoxifylline 400 mg three times daily.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Initial claudication distance (ICD) | 1 | 417 | Mean Difference (IV, Fixed, 95% CI) | 20.00 [‐2.57, 42.57] |
| 2.2 Absolute claudication distance (ACD) | 2 | 866 | Mean Difference (IV, Random, 95% CI) | 13.43 [‐43.50, 70.36] |
| 2.3 Adverse event related to study medication ‐ headache | 2 | 982 | Odds Ratio (M‐H, Random, 95% CI) | 2.20 [1.16, 4.17] |
| 2.4 Adverse event related to study medication ‐ diarrhoea | 2 | 982 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [0.79, 4.12] |
| 2.5 Adverse event related to study medication ‐ abnormal stools | 1 | 459 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [1.57, 6.21] |
| 2.6 Adverse event related to study medication ‐ pain | 2 | 982 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.57, 1.26] |
| 2.7 Adverse event related to study medication ‐ palpitations | 2 | 982 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.35 [4.11, 16.98] |
| 2.8 All‐cause mortality | 3 | 1229 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.17, 1.98] |
| 2.9 Ankle brachial index (ABI) | 1 | 417 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beebe 1999.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes Country: USA |
|
| Participants | Number randomised: 516 (cilostazol 100 mg, n = 175; cilostazol 50 mg, n = 171; placebo, n = 170) Age (mean years ± SE): cilostazol 100 mg = 64.3 ± 8.5; cilostazol 50 mg = 64.5 ± 9.9; placebo = 65.1 ± 9.3 Sex M/F: cilostazol 100 mg 130/45; cilostazol 50 mg = 131/40; placebo = 131/39 Inclusion criteria: ≥ 40 years of age; ≥ 6 months history of stable symptomatic IC secondary to lower extremity arterial occlusive disease; reproducible walking distances on screening treadmill tests; treadmill tests terminated solely because of claudication pain; ICD in screening period between 30 and 200 m on two consecutive tests; resting ABI of 0.90 or less and a 10 mmHg or more decrease in ankle artery blood pressure following the onset of ACD Exclusion criteria: ischaemic pain at rest; gross obesity; childbearing potential; hypertension; current metastatic malignant neoplasm; exercise‐limiting cardiac disease; history of bleeding tendencies; or concomitant use of antiplatelet, anticoagulant, vasoactive or NSAIDs |
|
| Interventions | Treatment 1: cilostazol 100 mg, twice daily, orally Treatment 2: cilostazol 50 mg twice daily, orally Control: placebo Duration: 24 weeks |
|
| Outcomes | PFWD and MWD by treadmill testing, Doppler‐measured bilateral peripheral limb pressures, patient‐based QoL questionnaires (SF‐36, WIQ, COM), patient and physician end‐of‐treatment global therapeutic assessments, cardiovascular morbidity, all‐cause mortality, amputation and adverse events. Outcomes evaluated at baseline (three times), 4, 8, 16, 20 and 24 weeks | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported | |
| Notes | "The COM questionnaire was developed by the study sponsor and has not been independently validated." Constant‐rate, constant‐grade treadmill test design, with 12.5% incline and speed of 3.2 km/h Minimum three‐week screening period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of eligible patients was stratified by each clinical center. A master randomization list of patient code assignments to the test medication… was developed using a permuted‐block design". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The master list was forwarded to the drug packaging company, where separate medication supply was prepared for each unique patient code. All 3 test medications had a similar appearance". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "For all‐cause mortality and cardiovascular morbidity assessment, an independent study committee, blinded to treatment assignment, adjudicated all patient deaths and serious adverse event…". Although it was not directly addressed for other outcomes, it was assumed blinding was adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 1 describes patient flow with all participants included in safety outcomes, and participants excluded for efficacy endpoints were similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | Although no protocol was available, all outcomes included in description of methods were reported on. |
| Other bias | Low risk | No evidence of other bias |
Brass 2012.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes; LOCF method Country: USA and Russia |
|
| Participants | Number randomised: 387 (cilostazol, n = 89; K‐134 25 mg, n = 42; K‐134 50 mg, n = 85; K‐134 100 mg, n = 84; placebo, n = 87) Age (mean years): cilostazol = 64.5; K‐134 25 mg = 63.3; K‐134 50 mg = 63.8; K‐134 100 mg = 62.8; placebo = 62.9 Sex (M%): cilostazol = 94.6; K‐134 25 mg = 83.3; K‐134 50 mg = 86.8; K‐134 100 mg = 82.3; placebo = 89.7 Inclusion criteria: aged ≥ 40 years; had PAD as documented by an ABI ≤ 0.90 or an ABI between 0.90 and 1.00 that fell by ≤ 0.20 within one minute following termination of treadmill exercise; patients with a peak walking time at baseline between one and 12 minutes Exclusion criteria: critical limb ischaemia, amputation or other non‐claudication limitation to treadmill performance; revascularisation ≤ 3 months; poorly controlled hyperlipidaemia or hypertension; major surgical procedure ≤ 6 months; myocardial infarction ≤ 4 months; history or evidence of congestive heart failure; electrocardiogram abnormalities; clinically significant laboratory or other medical conditions that pose a safety risk; use of warfarin or aspirin monotherapy, aspirin combined with clopidogrel or ticlopidine, strong inhibitors of cytochrome P3A4, use of other PDE inhibitors, use of pentoxifylline or L‐carnitine |
|
| Interventions | Treatment 1: cilostazol 100 mg, twice daily Treatment 2: K‐134 25 mg, twice daily Treatment 3: K‐134 50 mg, twice daily (initially started on 25 mg twice daily and then increased after two weeks) Treatment 4: K‐135 100 mg, twice daily (initially started on 50 mg twice daily and then increased after two weeks) Control: placebo, twice daily Duration: 26 weeks |
|
| Outcomes | Peak walking time, claudication onset time, inflammatory bio‐markers, safety and adverse events; measured at baseline (twice) and weeks 2, 4, 14 and 26 | |
| Funding | Kowa Research Institute | |
| Declaration of interests | "Dr Morgan is an employee of the study’s sponsor, Kowa Research Institute. Drs Brass, Cooper, and Hiatt were compensated by the study’s sponsor, Kowa Research Institute, for their service on the project’s steering committee. Dr Hiatt is president of the non‐profit Colorado Prevention Center, which provided academic contract research organization services (paid for by Kowa Research Institute) for the reported trial". | |
| Notes | Only data on the cilostazol and placebo groups were included in this review; the K‐134 25 mg, 50 mg and 100 mg groups were excluded from this review because K‐134 is not an alternative antiplatelet agent or medication currently known to increase walking distance. The treadmill test was only described as "graded" with a reference to another study, but we were unable to determine which of the treadmill tests from the referred paper the authors used. The K‐134 arm of 25 mg twice daily was discontinued early because it was found to be minimally informative, and no outcome data were recorded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted through a central interactive voice response system and used block randomization by site to minimize risk of imbalances". |
| Allocation concealment (selection bias) | Low risk | "Randomization was conducted through a central interactive voice response system". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The highest rates of discontinuations were observed in the 100‐mg K‐134 and cilostazol arms... but there were no statistical differences in discontinuation rates across arms". Reasons for discontinuation were similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes included in the description of methods were reported on. |
| Other bias | Low risk | No evidence of other bias |
Dawson 1998.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes; LOCF method Country: USA | |
| Participants | Number randomised: 81 (cilostazol n = 54; placebo n = 27) Age (mean years ± SE): cilostazol = 66 ± 1.1; placebo = 67 ± 2.0 Sex M/F: cilostazol = 38/16; placebo = 24/3 Inclusion criteria: ≥ 40 years; stable IC secondary to chronic occlusive arterial disease ≥ 6 months; ICD on treadmill between 30 and 200 m and had to be within ± 35% value of previous visit; confirmation of diagnosis of chronic occlusive arterial disease; doppler‐measured ankle systolic blood pressure ≥ 20 mmHg Exclusion criteria: limb‐threatening chronic limb ischaemia (ischaemic rest pain, ulceration or gangrene); lower extremity surgical or endovascular arterial reconstruction or sympathectomy in previous 6 months; uncontrolled hypertension; inability to complete the treadmill walking test for reasons other than intermittent claudication; MI within previous 6 months; DVT within previous 3 months; severe concomitant disease; substance abuse; or gross obesity | |
| Interventions | Treatment: cilostazol 100 mg, twice daily, orally Control: placebo, twice daily Duration: 12 weeks | |
| Outcomes | ICD, ACD, ABI, and subjective assessments of symptoms by patient and physician Outcomes evaluated at baseline (multiple visits), 2, 4, 8 and 12 weeks after initiation of therapy | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported | |
| Notes | Constant speed treadmill test at 3.2 km/h and a fixed incline of 12.5% Two‐week baseline period to stabilise concomitant medications, followed by a two to four‐week single‐blind placebo lead‐in phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was stratified by treatment center and patients use of calcium channel blocker". Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study used an 'identical' placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 2 gives explanations for withdrawals and exclusions, which were similar between treatment groups and unlikely to affect outcomes. |
| Selective reporting (reporting bias) | High risk | Authors only briefly mentioned ABI results in the abstract with no explicit description. |
| Other bias | Low risk | No evidence of other bias |
Dawson 2000.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes; LOCF method Country: USA | |
| Participants | Number randomised: 698 (cilostazol n = 227; pentoxifylline n = 232; placebo n = 239) Age (mean years ± SD): cilostazol = 66 ± 9; pentoxifylline = 66 ± 9; placebo = 66 ± 9 Sex M/F: cilostazol = 172/55; pentoxifylline = 181/51; placebo = 176/63 Inclusion criteria: stable, moderate to severe symptoms of IC for previous 6 months; confirmed PAD; baseline ICD ≥ 53.6 m (one minute); ACD ≤ 537.6 m (ten minutes) Exclusion criteria: patients with Buerger's disease; critical ischaemia; lower extremity surgical or endovascular reconstruction or sympathectomy in previous 3 months; limited exercise capacity due to conditions other than IC; medical problems judged likely to preclude study completion; use of pentoxifylline or any investigational drug within 30 days of study enrolment; prior use of cilostazol | |
| Interventions | Treatment: cilostazol 100 mg, twice daily with a third placebo for blinding Treatment: pentoxifylline 400 mg, three times daily Control: placebo Duration: 24 weeks | |
| Outcomes | ACD, ICD, resting doppler limb pressures, QoL questionnaires (SF‐36, WIQ); measured at baseline and weeks 2, 4,8, 12, 16, 20 and 24 | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ two of the authors (EBB, WPF) were employed by Otsuka America Pharmaceutical Inc. | |
| Notes | Standardised treadmill test, beginning at 0% incline and 3.2 km/h, increasing incline 3.5% every three minutes while maintaining 3.2 km/h speed Two‐ to three‐week baseline period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of eligible patients was stratified by clinical center, and patients were assigned to one of the three treatment regimens within each center using a permuted‐block design". "Patients were randomly assigned by using an interactive voice randomization system that blinded the investigator, patient and sponsor from treatment assignment". |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned by using an interactive voice randomization system that blinded the investigator, patient and sponsor from treatment assignment". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients were randomly assigned by using an interactive voice randomization system that blinded the investigator, patient and sponsor from treatment assignment". Study medications were identical in appearance and taken at similar intervals. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not adequately discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing patients were all accounted for and rates were similar between groups as to those who remained in the study. |
| Selective reporting (reporting bias) | Low risk | Although no protocol was available, all relevant outcomes appeared to be reported on. |
| Other bias | Low risk | No evidence of other bias |
De Albuquerque 2008.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Intention‐to‐treat: unclear Country: Brazil | |
| Participants | Number randomised: 48 (cilostazol n = 17; pentoxifylline n = 15; placebo n = 16) Age (mean years ± SD): cilostazol = 64.0 ± 9.0; pentoxifylline = 64.0 ± 10.0; placebo = 63.0 ± 9.0 Sex (% M): cilostazol = 64.7%; pentoxifylline = 60.0%; placebo = 50.0% Inclusion criteria: age 45 to 85 years; IC for at least 6 months; resting ABI ≤ 0.90; duplex evidence of PAD Exclusion criteria: critical limb ischaemia (Fontaine classification III and IV); symptomatic coronary artery disease (angina); congestive heart failure; arterial revascularisation indication; less than 6 months of diagnosed PAD | |
| Interventions | Treatment 1: cilostazol 100 mg, twice daily, orally Treatment 2: pentoxifylline 600 mg, twice daily Control: placebo, twice daily Duration: 20 weeks | |
| Outcomes | PFWD, MWD, blood analysis (CRP, triglycerides, HDL, LDL), urine analysis (8‐epi‐prostaglandin F2a), endothelial function by forearm blood flow, adverse events, change in ABI; measured at baseline and then every 4 weeks until 20 weeks | |
| Funding | "Cilostazol, pentoxifylline, and placebo were generous gifts from LIBBS, Brazil." Study supported by grants from the National Research Council (CNPq 52 1850/96‐7) and from Research Supporting Agency of Rio de Janeiro State (FAPERJ E‐26/170. 522/00) | |
| Declaration of interests | Not reported | |
| Notes | Calibrated treadmill at a constant speed of 3.2 km/h; incline was increased every 3 min. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to 20 weeks of treatment…". Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Low risk | Use of coded envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients or researchers were not able to distinguish among treatment capsules. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears all participants completed the trial; no loss‐to‐follow‐up reported |
| Selective reporting (reporting bias) | High risk | All outcomes included in description of methods were reported on, but for PFWD and MWD (Table 2); there was no breakdown for the different treatment groups. |
| Other bias | Low risk | No evidence of other bias |
Elam 1998.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes Country: USA | |
| Participants | Number randomised: 189 (cilostazol n = 95; placebo n = 94) Age (mean years): cilostazol = 66.7; placebo = 65.8 Sex M/F: cilostazol = 83/12; placebo = 76/18 Inclusion criteria: men and women > 40 years; chronic stable IC secondary to PAD Exclusion criteria: women with childbearing potential; gross obesity; poorly controlled hypertension or diabetes; history of malignancy; current alcohol or drug abuse; renal disease; bleeding tendencies | |
| Interventions | Treatment: cilostazol 100 mg, twice daily, orally Control: placebo, twice daily, orally Duration: 12 weeks | |
| Outcomes | Lipid profiles, ACD, ABI Outcomes evaluated at baseline 2, 4, 6, 8 and 12 weeks (treadmill tests conducted at two baseline visits, and weeks 8 and 12) | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ three of the authors (JH, EBB, WPF) were employed by Otsuka America Pharmaceutical Inc. | |
| Notes | "Delayed‐incline" treadmill method, where incline loading was delayed until the third minute then gradually increased by 3.5% increments every three minutes, with a constant speed of 3.2 km/h Minimum two‐week lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All lipid analyses were blinded to the investigators and patients after randomization". Although it was not directly addressed for other outcomes, it was assumed blinding was adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who completed the study were comparable between treatment groups. |
| Selective reporting (reporting bias) | High risk | In the methods, the authors stated both pain‐free and maximum walking distances as primary exercise variables, but only maximum walking distance was reported on. |
| Other bias | Low risk | No evidence of other bias |
Lee 2001.
| Study characteristics | ||
| Methods | Study design: single‐centre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: no Country: Taiwan | |
| Participants | Number randomised: 50 (cilostazol n = 17; pentoxifylline n = 17; placebo n = 16) Age (mean years (SD)): cilostazol = 66 (9); pentoxifylline = 68 (5); placebo = 69 (6) Sex M/F: cilostazol = 14/3; pentoxifylline = 14/3; placebo = 14/2 Inclusion criteria: men and women > 40 years old, IC with no symptomatic changes in previous 3 months, baseline ACD between 30 and 200 m, doppler measured ABI of ≤ 0.9, participants had to have a variance of ≤ 20% in their ACD between their two screening treadmill tests Exclusion criteria: Buerger's disease, category II or II chronic lower‐extremity ischaemia, arterial surgery/angioplasty or sympathectomy within previous 3 months | |
| Interventions | Treatment: Cilostazol 100 mg twice daily Pentoxifylline 400 mg twice daily Control: placebo twice daily Duration: 8 weeks (plus 2 weeks of placebo run‐phase) | |
| Outcomes | ABI, ACD, VEGF, IL6, neutrophils, monocytes, platelets, glucose and lipids | |
| Funding | There were multiple study drug sponsors; no further details reported. "The patient received a randomized code number, according to which the sponsor supplied the study drug". | |
| Declaration of interests | "There are neither financial nor other relations that could lead to a conflict of interest". | |
| Notes | Treadmill tests performed at 2 baseline screening visits and at 8 weeks; 3.2 km/h with 12.5% gradient, under supervision by the same person at the same time of day for a given patient | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised code number according to which sponsor supplied the study drug, but how the numbers were generated was not described. |
| Allocation concealment (selection bias) | Low risk | A sealed envelope, with information on the treatment allocated, was kept in the clinical file of each patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used special packaging to maintain blinding of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors stated analysis would be performed on participants that completed the study (not intention‐to‐treat), but did not specifically state the number that completed the study. The results tables in one reference included values that would suggest all participants were included in the analysis, and therefore completed the trial. However, the number of participants reported in the other reference had 2 patients missing (1 in the cilostazol group and 1 in the pentoxifylline group), with no explanation for the differences. |
| Selective reporting (reporting bias) | Unclear risk | The authors only stated their intention to measure ACD as a main outcome but did not specify the other outcomes they ultimately reported on. The two different references reported on different outcomes with no clear indication of what the preplanned outcomes were. |
| Other bias | Low risk | No evidence of other bias |
Money 1998.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes; LOCF method Country: USA | |
| Participants | Number randomised: 239 (cilostazol n = 119; placebo n = 120) Age (mean years ± SD): cilostazol = 64.8 ± 9.4; placebo = 64.5 ± 8.8 Sex M/F: cilostazol = 90/29; placebo = 90/30 Inclusion criteria: > 40 years; IC caused by lower extremity PAOD for at least 6 months; baseline ICD ≥ 54 m (one minute); ACD variance no greater than 20% between two screen visits and maximum allowable ACD of 805 m (15 minutes) Exclusion criteria: limb‐threatening PAOD including gangrene or ischaemic rest pain; surgical or endovascular procedures during previous 3 months; gross obesity; hypertension; current malignancy; Buerger's disease or DVT in previous 3 months; inability to complete treadmill testing for reasons unrelated to IC; bleeding problems | |
| Interventions | Treatment: 100 mg cilostazol, twice daily Control: placebo Duration: 16 weeks | |
| Outcomes | ACD, ICD, ABI, physician and patient perception of effect of study drug, QoL (SF‐36, WIQ) Treadmill tests performed at two baseline visits and weeks 8, 12 and 16 after randomisation | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported. Two of the authors (J Heckman and Dr. Forbes) were employed by Otsuka America Pharmaceutical Inc. | |
| Notes | Variable‐grade, constant‐speed treadmill test, beginning at 0% incline with a speed of 3.2 km/h, increasing by 3.5% every 3 minutes Two‐week screening period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not adequately discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for discontinuation were given and all participants accounted for. |
| Selective reporting (reporting bias) | Low risk | Although no protocol was available all relevant outcomes were reported on. |
| Other bias | Low risk | No evidence of other bias |
O'Donnell 2009.
| Study characteristics | ||
| Methods | Study design: single‐centre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes Country: Northern Ireland | |
| Participants | Number randomised: 106 (cilostazol n = 51; placebo n = 55) Age (median years): cilostazol = 64.2; placebo = 66.1 Sex (M/F): cilostazol = 34/17; placebo = 39/16 Inclusion criteria: aged 30 to 90 years (both sexes); had PAD with IC with an ABI < 0.9 stable on optimal medical therapy for 3 months Exclusion criteria: current or previous acute or critical limb ischaemia; severe claudication prohibiting treadmill testing; endovascular or surgical procedures within the preceding 6 months; non‐atherosclerotic comorbidity that had limited their walking before the onset of claudication pain; predisposition to bleeding; a history of uncontrolled cardiac, respiratory, renal or liver disease; use of omeprazole or diltiazem | |
| Interventions | Treatment: cilostazol 100 mg, twice daily, oral route Control: placebo, twice daily, oral route Duration: 24 weeks | |
| Outcomes | ICD, ACD, oxygen‐derived free‐radical generation, antioxidant consumption, other inflammatory cascade markers, QoL (SF‐36, WIQ, VascuQol); measured at baseline and weeks 6 and 24 | |
| Funding | Otsuka America Pharmaceutical Inc. provided the placebo. The study was funded by the Belfast City Hospital Vascular Research Fund and the Daisy Hill Hospital research fellowships and research grants from the Insulin Dependant Diabetes Trust and the Royal College of Surgeons Edinburgh. | |
| Declaration of interests | Otsuka America Pharmaceutical Inc. provided the placebo for use in the study. Dr O’Donnell has received financial support from Otsuka Pharmaceuticals for travel costs to attend conferences to present data from this clinical trial. | |
| Notes | Calibrated treadmill test with a constant speed of 3.2 km/h and constant 10% gradient Four‐week stabilisation run‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patient‐treatment randomisation and allocation was performed independently by the Department of Research Pharmacology in the Belfast City Hospital". Insufficient information on sequence generation |
| Allocation concealment (selection bias) | Low risk | "Both, the patient and the primary investigator, were blinded to study‐drug allocation, which was completed using the sealed‐envelope method". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both, the patient and the primary investigator, were blinded to study‐drug allocation". "Study‐drug un‐blinding was performed at the end of the study, following the completion of all clinical assessments and laboratory analyses for all patients". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not adequately discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were similar between treatment groups. |
| Selective reporting (reporting bias) | Low risk | Although no study protocol was available, all outcomes appeared to be reported on. |
| Other bias | Low risk | No evidence of other bias |
Otsuka Study 21‐86‐101.
| Study characteristics | ||
| Methods | Study design: single‐centre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes Country: USA | |
| Participants | Number randomised: 53 (cilostazol n = 28; placebo n = 25) Age (mean years): cilostazol = 62; placebo = 58 Sex (% M): cilostazol = 89%; placebo = 84% Inclusion criteria: aged 21 to 70 (both sexes), had atherosclerosis obliterans‐induced IC which was chronic (at least 6 months), stable (6 months); evidence of PAOD; ICD ≤ 100 m on a constant load treadmill (10% incline, 3.5km/h); less than 30% variation in ICD during lead‐in period Exclusion criteria: limb‐threatening PAOD including gangrene or ischaemic rest pain; surgical or endovascular procedures during previous 3 months; gross obesity; hypertension; current malignancy; Buerger's disease or DVT in previous 3 months; inability to complete treadmill testing for reasons unrelated to IC; bleeding problems | |
| Interventions | Treatment: cilostazol 100 mg, twice daily, oral administration Control: placebo Duration: 6 weeks | |
| Outcomes | ICD, ACD, subjective claudication improvement by patient, Doppler‐measured limb pressures; measured at baseline and weeks 3 and 6 | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ source of the study data was a medical review by the FDA. | |
| Notes | Immediate‐incline treadmill method: incline load started immediately at 10% and remained constant with speed constant at 3.2 km/h. Only to be stopped for claudication of sufficient severity to cause the subject to be unable to continue Three‐week placebo lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were similar between the two treatment groups, as shown in Table 31. |
| Selective reporting (reporting bias) | High risk | Subjective claudication improvement or Doppler‐measured limb pressures were not reported. |
| Other bias | Low risk | No evidence of other bias |
Otsuka Study 21‐86‐103.
| Study characteristics | ||
| Methods | Study design: single‐centre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes Country: USA | |
| Participants | Number randomised: 33 (cilostazol n = 17; placebo n = 16) Age (mean years): cilostazol = 56; placebo = 59 Sex (% M): cilostazol = 82%; placebo = 88% Inclusion criteria: aged ≥ 21 years (both sexes); had atherosclerosis obliterans‐induced IC which was chronic (at least 6 months), stable (6 months); evidence of POAD; ICD ≤ 100 m on a constant load treadmill (10% incline, 3.5km/h); less than 30% variation in ICD during lead‐in period Exclusion criteria: lower extremity ischaemic rest pain, severe ulceration or gangrene; female of childbearing potential; malignancy; cardiac valve disorder or replacement; clinically significant abnormal lab value pretreatment; renal insufficiency; a requirement for the uninterrupted use of platelet‐active or vasoactive drugs; use of an investigational drug within the past 30 days; diabetes mellitus: either insulin‐dependent or with duration > 5 years; status post‐vascular surgery, splenectomy, or gastrointestinal surgery within past 12 months | |
| Interventions | Treatment: cilostazol 150 mg, twice daily, oral administration Control: placebo Duration: 21 weeks (from the text, change in ACD and ICD were measured and reported after 6 weeks) | |
| Outcomes | Change in ACD and ICD (after 6 weeks of therapy), subjective claudication improvement as per patient, palpation of arterial pulses, Doppler‐measured limb pressure, sitting arm blood pressure; measured at baseline and then weeks 6, 9, 13, 17 and 21 | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ source of the study data was a medical review by the FDA. | |
| Notes | Immediate‐incline treadmill method: incline load started immediately at 10% and remained constant with speed constant at 3.2 km/h. Dosage of cilostazol described as "fixed 150 mg bid oral dose... formulated as 50 mg cilostazol tablets" Assumption was that authors meant tablets were taken three times daily Three‐week placebo lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts overlapped without discussion |
| Selective reporting (reporting bias) | High risk | Subjective claudication improvement, palpitation of arterial pulses, Doppler‐measured limb pressures and sitting arm blood pressure were not reported. |
| Other bias | Low risk | No evidence of other bias |
Otsuka Study 21‐87‐101.
| Study characteristics | ||
| Methods | Study design: single‐centre, randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes Country: USA | |
| Participants | Number randomised: 19 (cilostazol n = 10; placebo n = 9) Age (mean years): cilostazol = 62; placebo = 65 Sex (% M): cilostazol = 60%; placebo = 67% Inclusion criteria: aged 45 to 70 years (both sexes); IC which was stable (3 months); ICD ≤ 100 m on a constant load treadmill test with no greater than 20% variation between observations in washout period Exclusion criteria: lower extremity ischaemic rest pain, severe ulceration or gangrene; female of childbearing potential; decompensated congestive heart failure or MI within six months; cardiac valve disorder or replacement; respiratory insufficiency; vascular surgery, splenectomy, or gastrointestinal surgery within past 12 months; clinically significant abnormal lab value pretreatment; decreased mobility due to joint disorders, or chronic lumbar vertebral column syndrome; malignancy; renal insufficiency; neuropathy; history of analgesic abuse or use of an investigational drug within the past 30 days; diabetes mellitus: either requiring insulin or duration > 5 years; a requirement for the uninterrupted use of pentoxifylline, dipyridamole, certain vasodilators, acetylsalicylic acid, PDE inhibitors or prostacyclin | |
| Interventions | Treatment: cilostazol 100 mg, twice daily, oral route Control: placebo, twice daily, oral route Duration: 12 weeks | |
| Outcomes | ACD, ICD, adverse events; measured at baseline and then weeks 4, 8 and 12 | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ source of the study data was a medical review by the FDA. | |
| Notes | Immediate incline treadmill test where the incline load started immediately at 10% and remained constant with a constant 3.2 km/h. Three‐week placebo lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for all dropouts |
| Selective reporting (reporting bias) | Low risk | Although no study protocol was available, all outcomes appeared to be reported on. |
| Other bias | Low risk | No evidence of other bias |
Otsuka Study 21‐94‐301.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled Intention‐to‐treat: yes; LOCF method Country: USA | |
| Participants | Number randomised: 370 (cilostazol n = 123; pentoxifylline n = 123; placebo n = 124) Age (mean years): cilostazol = 66; pentoxifylline = 66; placebo = 66 Sex (% M): cilostazol = 70; pentoxifylline = 72; placebo = 73 Inclusion criteria: aged ≥ 40 years (both sexes); IC which was chronic (at least 6 months), stable (3 months); evidence of POAD; ACD ≤ 450 m in ≤ 8 minutes 28 seconds with no more than 20% variability in two consecutive tests during lead‐in period; ICD of at least 30 m in 34 seconds during lead‐in period; supine ABI of ≤ 0.80 after 10 minutes of rest Exclusion criteria: current use of pentoxifylline or previous discontinuation for inefficacy or adverse event; female of childbearing potential; greater than 60% above ideal body weight; supine arterial BP > 200 mmHg systolic or > 100 mmHg diastolic; sympathectomy or lower extremity arterial reparative surgery within the previous 3 months; DVT within the previous 3 months; termination of treadmill test for reasons other than IC; history or current evidence of concomitant exercise‐limiting disease other than IC; history of bleeding tendencies; history of cerebrovascular bleed, cerebral or dissecting aortic aneurysm, pericarditis, or pericardial effusion; active peptic disease; recent or anticipated surgical procedures; platelet count < 120 x 109/litre, twice the normal values for AST or ALT, or serum creatinine > 220 μmol/litre; current alcohol or other drug abuse, or use of an investigational drug within the past 30 days; a requirement for the uninterrupted use of platelet‐active, anticoagulant, NSAIDs or haemorheologic agents | |
| Interventions | Treatment 1: cilostazol 100 mg, twice daily with third placebo dose to maintain blind, oral administration Treatment 2: pentoxifylline 400 mg, three times daily Control: placebo Duration: 24 weeks | |
| Outcomes | ACD, ICD, subjective claudication improvement by physician and patient; all‐cause death, cardiovascular events, safety endpoints (vital signs, 12‐lead ECG, etc.), adverse events; measured at baseline and weeks 2, 4, 8, 12, 16, 20 and 24 | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ source of the study data was a medical review by the FDA. | |
| Notes | Immediate‐incline treadmill method: incline load started immediately at 10% and remained constant with a constant speed of 3.2 km/h Four‐ to eight‐week lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "CLZ, PTX and placebo tablets were encapsulated into identical capsule, and blinding of the dose interval was to be preserved by administered [sic] a third daily dose of placebo to CLZ‐randomized subjects". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for all dropouts |
| Selective reporting (reporting bias) | High risk | No reporting of all‐cause death or cardiovascular events |
| Other bias | Low risk | No evidence of other bias |
Otsuka Study 21‐95‐201.
| Study characteristics | ||
| Methods | Study design: randomised, double‐blind, placebo‐controlled, clinical trial Intention‐to‐treat: yes; LOCF method Country: USA | |
| Participants | Number randomised: 215 (cilostazol 150 mg, n = 73; cilostazol 100 mg, n = 72; placebo, n = 70) Age (mean years): cilostazol 150 mg = 65; cilostazol 100 mg = 68; placebo = 66 Sex M/F: cilostazol 150 mg = 81%/19%; cilostazol 100 mg = 75%/25%; placebo = 81%/19% Inclusion criteria: > 40 years; atherosclerosis obliterans‐induced IC for ≥ 6 months, stable for ≥ 3 months. Exclusion criteria: IC associated with lower extremity ischaemic rest pain, ischaemic ulceration, gangrene or Buerger's disease; women of childbearing potential; sympathectomy or lower extremity arterial reparative surgery, including endovascular procedures in previous 3 months; greater than 60% above ideal body weight; current metastatic malignancy; DVT within previous 3 months; other exercise‐limiting disease; risk of or tendency to bleeding; pericarditis or pericardial effusions; platelet count < 130,000/cm3 or haematocrit < 30%; twice the normal values for AST or ALT; serum creatinine > 2.5 mg/dL; current alcohol or other drug abuse, or use of investigational drug within the past 30 days; requirement for uninterrupted use of pentoxifylline, NSAIDs, certain antiplatelet and anticoagulant medications | |
| Interventions | Treatment 1: cilostazol 150 mg, twice daily Treatment 2: cilostazol 100 mg, twice daily Control: placebo, twice daily Duration: 12 weeks | |
| Outcomes | ACD and ICD, subjective claudication improvement as per patient and physician, Doppler‐measured limb pressures, QoL questionnaires; measured at baseline then weeks 4, 8 and 12 | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ source of the study data was a medical review by the FDA. | |
| Notes | Treadmill tests done by "immediate‐incline" method: incline load started immediately at 12.5% (and remained constant) with speed constant at 3.2km/h. Tests were only to be stopped for claudication. Two‐week lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for all dropouts |
| Selective reporting (reporting bias) | High risk | Report did not include ICD data at 4, 8 and 12 weeks, subjective claudication improvement or Doppler limb pressures. Did not report QoL results, but noted no significant differences between the groups |
| Other bias | Low risk | No evidence of other bias |
Otsuka Study 21‐98‐213.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled, clinical trial Intention‐to‐treat: yes Country: USA | |
| Participants | Number randomised: 785 (cilostazol, n = 261; pentoxifylline, n = 262; placebo, n = 262) (several of the tables in the NICE report stated 780 as the number of participants, 260 in each group, but the study characteristics on pg 177 of the report had n = 785 as the total number randomised) Age (mean years ± SE): cilostazol = 66.7 ± 9.9; pentoxifylline = 67.4 ± 9.4; placebo = not given Sex (% M): cilostazol = 75.4; pentoxifylline = 76.9; placebo = 75.4 Inclusion criteria: 40 years or older, with PAD and IC with stable symptoms for the preceding 3 months; PAD diagnosed as an abnormal resting ABI ≥ 0.4 and ≤ 0.9 in the reference leg with decline in post‐exercise ABI ≥ 10 mmHg as confirmation; symptomatic patients with normal resting ABI but with pressure drop of > 20 mmHg were also eligible; MWD varied by no more than 20% on two to three consecutive treadmill tests Exclusion criteria: limb‐threatening ischaemia; limb revascularisation within 3 months; unstable coronary artery disease; coronary revascularisation within 6 months; thromboangiitis obliterans; DVT within 3 months; symptomatic arrhythmia; conditions other than PAD that might limit exercise ability or preclude completion of the study; congestive heart failure | |
| Interventions | Treatment 1: cilostazol 100 mg, twice daily Treatment 2: pentoxifylline 400 mg, three times daily Control: placebo Duration: 24 weeks | |
| Outcomes | MWD, PFWD, all‐cause mortality, QoL (SF‐36, WIQ, COM), adverse events, vascular events; measured at baseline and then 4 weeks until 24 weeks | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported ‐ source of the study data was a pharmaceutical submission to National Institute for Care and Excellence. | |
| Notes | Constant workload treadmill test: 3.2 km/h at a constant 12.5% grade Study was also known under the trial name PACE. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although study used a placebo, there was insufficient description to determine if blinding was adequate. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No study report was available outside of the data collected from the NICE review. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol or report was available outside of the data collected from the NICE review. |
| Other bias | Low risk | No evidence of other bias |
Strandness 2002.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind, placebo‐controlled, phase III trial Intention‐to‐treat: yes; LOCF method Country: USA | |
| Participants | Number randomised: 394 (cilostazol 100 mg, n = 133; cilostazol 50 mg, n = 132; placebo, n = 129) Age (mean years ± SE): cilostazol 100 mg = 63.1 ± 10.2; cilostazol 50 mg = 63.9 ± 8.7; placebo = 64.4 ± 10.2 Sex M/F: cilostazol 100 mg = 102/31; cilostazol 50 mg = 98/34; placebo = 100/29 Inclusion criteria: ≥ 40 years; at least 6 months history of stable symptomatic IC secondary to PAD; and reproducible walking distances on screening treadmill (20% or less variation in MWD on two consecutive tests); termination of all screening treadmill tests solely for reasons of claudication pain; ability to walk between 30 and 200 m; resting ABI less than 0.90 and at least a 10 mmHg decrease in ankle systolic blood pressure in the reference leg at completion of test Exclusion criteria: ischaemic pain at rest; gross obesity; childbearing potential; hypertension; malignancy; exercise‐limiting cardiac disease; history of bleeding tendencies; concomitant use of antiplatelet, anticoagulant, haemorheologic or NSAIDs | |
| Interventions | Treatment 1: cilostazol 100 mg, twice daily Treatment 2: cilostazol 50 mg, twice daily Control: placebo, twice daily Duration: 24 weeks | |
| Outcomes | MWD, PFWD, Doppler‐measured bilateral peripheral limb pressures, QoL and functional status, end‐of‐treatment global therapeutic benefit (physician and participant), cardiovascular morbidity, all‐cause mortality; outcomes measured at baseline, weeks 2 and 4, then every 4 weeks until 24 weeks | |
| Funding | Otsuka America Pharmaceutical Inc. | |
| Declaration of interests | Not reported. Two authors (P Zhang, WP Forbes) were employed by Otsuka America Pharmaceutical Inc. | |
| Notes | Treadmill test consisted of standardised 2 mph at 12.5% incline. Two‐week lead‐in period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of sequence generation methods |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment methods |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Cilostazol was packaged as a 50 mg tablet and a placebo dummy was given to maintain double blind conditions". "The blind was reportedly not broken during the course of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent committee, blinded to treatment assignment, adjudicated all patient death and any possible cardiovascular morbid events according to the predefined morbidity criteria". Although it was not directly addressed for other outcomes, it was assumed blinding was adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "There were no clinically or statistically significant differences events, serious or adverse events, discontinuation of therapy due to adverse events and death". |
| Selective reporting (reporting bias) | High risk | ABI data were listed as a secondary outcome but not reported on, aside from a comment in the discussion for which no data were given to support it. |
| Other bias | Low risk | No evidence of other bias |
ABI: ankle brachial index ACD: absolute claudication distance ALT: alanine transaminase AST: aspartate aminotransferase bid: twice daily BP: blood pressure CLZ: cilostazol cm: centimetre COM: Claudication Outcome Measures CRP: C‐reactive protein DVT: deep vein thrombosis ECG: electrocardiogram FDA: Food and Drug Administration HDL: high‐density lipoprotein IC: intermittent claudication ICD: initial claudication distance IL6: interleukin‐6 km/h: kilometres per hour LDL: low‐density lipoprotein LOCF: last observation carried forward m: metres M/F: male/female mg: milligrams MI: myocardial infarction mph: miles per hour MWD: maximum walking distance (equivalent to ACD) NICE: National Institute for Health and Care Excellence NSAIDs: non‐steroidal anti‐inflammatory agents PAD: peripheral arterial disease PAOD: peripheral arterial occlusive disease PAR: Physical Activity Recall PDE: phosphodiesterase PFWD: pain‐free walking distance (equivalent to ICD) PTX: pentoxifylline QoL: quality of life SD: standard deviation SE: standard error SF‐36: Medical Outcomes Scale Short Form‐36 VascuQol: diseasespecific vascular quality of life VEGF: vascular endothelial growth factor WIQ: Walking Impairment Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CASTLE 2008 | This study was a safety study performed over 3.5 years, which is a much longer follow‐up than other included studies. Authors were contacted for data from earlier time points, but we received no response. |
| Chao 2014 | Wrong patient population: PAD without obvious IC |
| Chao 2016 | Wrong patient population: high risk for cardiovascular disease |
| Chen 2017 | Wrong patient population: PAD or high risk of cardiovascular disease |
| ChiCTR‐TRC‐09000441 | Wrong patient population: type 2 diabetes with ischaemic disease |
| Chisari 2019 | Wrong patient population: PAD |
| Goldenberg 2012 | Wrong intervention: L‐cartinine + cilostazol versus cilostazol |
| Hsieh 2009 | Wrong patient population: diabetic patients with POAD |
| JPRN‐C000000215 | Wrong patient population: type 2 diabetic patients with mild atherosclerosis |
| JPRN‐UMIN000001198 | Wrong patient population: patients with femoropopliteal stenting |
| JPRN‐UMIN000011869 | Wrong intervention: omega‐3 fatty acid + cilostazol versus cilostazol |
| JPRN‐UMIN000014307 | Wrong intervention: new gene transfer vector based on nontransmissible recombinant Sendai virus expressing the human fibroblast growth factor‐2 gene (DVC1‐0101) |
| Kim 2013 | Wrong patient population: type 2 diabetic patients with metabolic syndrome |
| Mazzone 2013 | Wrong intervention: iloprost + usual care versus usual care |
| NCT00102050 | Wrong intervention: phosphodiesterase inhibitor NM‐702 |
| NCT00300339 | Study was discontinued early, and no outcome data were available. |
| NCT00443287 | The specifics of the intervention arms are unclear at this time. We could not conclude which study arms also used clopidogrel. |
| NCT00573950 | Wrong patient population: type 2 diabetic patients with metabolic syndrome |
| NCT00886574 | Wrong patient population: type 2 diabetes mellitus |
| NCT00912756 | Wrong patient population: chronic arteriosclerosis obliterans afflicting the femoropopliteal artery area |
| NCT01188824 | Wrong patient population: ischaemic stroke patients with PAD |
| NCT01952756 | Wrong patient population: POAD |
| NCT02373462 | Wrong intervention: olmesartan |
| NCT02407314 | Wrong intervention: ticagrelor |
| NCT02636283 | Wrong intervention: valsartan |
| NCT02930811 | Wrong intervention: sildenafil |
| NCT03318276 | Wrong intervention: cilostazol 200 mg + ginkgo biloba leaf extract 160 mg versus cilostazol 100 mg + ginkgo biloba leaf extract 80 mg |
| NCT03686306 | Wrong intervention: sildenafil |
| Otsuka Study PUIC‐1 | Currently cannot determine if the study was double‐blinded |
| Otsuka Study PUIC‐2 | Currently not sufficient details of the study methods or outcomes to include |
| Xiao 2010 | Wrong patient population: type 2 diabetes with lower limb ischaemic disease |
IC: intermittent claudication PAD: peripheral arterial disease PAOD: peripheral arterial occlusive disease RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Sapelkin 2013.
| Methods | Only title provided |
| Participants | Only title provided |
| Interventions | Only title provided |
| Outcomes | Only title provided |
| Notes | Library unable to source this material |
Differences between protocol and review
2021 update
We restructured the review text and meta‐analyses so that the outcomes are presented according to the comparisons 'cilostazol versus placebo' and 'cilostazol versus pentoxifylline' with the cilostazol doses subgrouped. We revised the outcomes (but not the other components of the PICO) to reflect current clinical practice and clinical importance: health‐related quality of life is now a primary outcome (was a secondary outcome); we changed ‘progression to surgery’ to specifically relate to ‘revascularisation'; we added ‘amputation’ and 'major adverse limb event' (MALE); finally, we renamed ‘adverse events’ to be clearly related to study medication. Lastly, we added summary of findings tables and assessed the outcomes presented in the tables using GRADE criteria.
2014 update
The previous review version required the types of participants to be "Patients with stable intermittent claudication (Fontaine stage II) for more than six months..." In order to be as inclusive as possible, we have changed the requirement to "Patients with stable intermittent claudication (determined by a physician or investigator)".
The title of the review has been changed from 'Cilostazol for peripheral arterial disease' to 'Cilostazol for intermittent claudication' in order to reflect the change in methods; participants had been 'patients with intermittent claudication or patients undergoing bypass surgery for peripheral arterial disease' and this was changed to only 'patients with intermittent claudication'. This was done because patients undergoing surgery generally would have a more advanced disease stage than those with intermittent claudication, introducing bias and heterogeneity to the review. At the time of this update, no studies were included that had patients undergoing surgery, so no major changes had to be made. The originally planned subgroup analysis investigating differences between participants having intermittent claudication versus participants undergoing vascular surgical intervention is no longer relevant and has been removed.
Contributions of authors
TB: assessed references from the updated search, assessed risk of bias, extracted data, undertook meta‐analyses, added summary of findings tables and applied GRADE criteria, and drafted the review.
RBF: assessed risk of bias, extracted data, and assisted in drafting the review.
MC: provided clinical support, contributed to the discussion and conclusion and checked the draft review.
DPM: provided clinical support, contributed to the discussion and conclusion and checked the draft review.
GS: provided clinical support, contributed to the discussion and conclusion and checked the draft review.
MS: assessed references from the updated search, checked data analysis and assisted in drafting the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
Declarations of interest
TB: none.
RBF: none.
MC: none.
DPM: declared that he received payment to attend meetings (Amgen, Novo Nordisk), advisory boards (Novo Nordisk), and present lectures on lipids and cilostazol (Amgen, Novo Nordisk and Libytec). It is five years since his last lecture on cilostazol (Libytec). As Editor‐in‐Chief; royalties were paid to him (Informa, SAGE and Bentham publications). He has published peer‐reviewed material regarding cilostazol.
GS: none.
MS: MS is a member of the Cochrane Vascular editorial base. In order to maintain integrity, editorial tasks for this review have been carried out by other members of the editorial team.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Beebe 1999 {published data only}
- Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE Jr, Bortey EB, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Archives of Internal Medicine 1999;159(17):2041-50. [DOI] [PubMed] [Google Scholar]
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-92-202. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Brass 2012 {published data only}
- Brass EP, Cooper LT, Morgan RE, Hiatt WR. A phase II dose-ranging study of the phosphodiesterase inhibitor K-134 in patients with peripheral artery disease and claudication. Journal of Vascular Surgery 2012;55(2):381-9. [DOI] [PubMed] [Google Scholar]
- Hiatt WR, Cooper LT, Morgan RE, Brass EP. Clinical effects of the phosphodiesterase inhibitor K-134 in peripheral artery disease and claudication. Circulation 2011;124(Suppl 21):A9800. [DOI] [PubMed] [Google Scholar]
- Lewis RJC. Application of adaptive design and decision making to a phase II trial of a phosphodiesterase inhibitor for the treatment of intermittent claudication. Trials 2011;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00783081. Safety and efficacy of K-134 for the treatment of intermittent claudication. www.clinicaltrials.gov/ct2/show/NCT00783081 (first posted 31 October 2008).
Dawson 1998 {published data only}
- Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation 1998;98(7):678-86. [DOI] [PubMed] [Google Scholar]
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-90-201. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Dawson 2000 {published data only}
- Dawson DL, Cutler BS, Hiatt WR, Hobson RW 2nd, Martin JD, Bortey EB, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. American Journal of Medicine 2000;109(7):523-30. [DOI] [PubMed] [Google Scholar]
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-96-202. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
De Albuquerque 2008 {published data only}
- De Albuquerque RM, Virgini-Magalhães CE, Lencastre SF, Bottino DA, Bouskela E. Effects of cilostazol and pentoxifylline on forearm reactive hyperemia response, lipid profile, oxidative stress, and inflammatory markers in patients with intermittent claudication. Angiology 2008;59(5):549-58. [DOI] [PubMed] [Google Scholar]
Elam 1998 {published data only}
- Elam MB, Heckman J, Crouse JR, Hunninghake DB, Herd JA, Davidson M, et al. Effect of the novel antiplatelet agent cilostazol on plasma lipoproteins in patients with intermittent claudication. Arteriosclerosis, Thrombosis, and Vascular Biology 1998;18(12):1942-7. [DOI] [PubMed] [Google Scholar]
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-93-201. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Lee 2001 {published data only}
- Lee TM, Su SF, Hwang JJ, Tseng CD, Chen MF, Lee YT, et al. Differential lipogenic effects of cilostazol and pentoxifylline in patients with intermittent claudication: potential role for interleukin-6. Atherosclerosis 2001;158(2):471-6. [DOI] [PubMed] [Google Scholar]
- Lee TM, Su SF, Tsai CH, Lee YT, Wang SS. Differential effects of cilostazol and pentoxifylline on vascular endothelial growth factor in patients with intermittent claudication. Clinical Science 2001;101(3):305-11. [PubMed] [Google Scholar]
Money 1998 {published data only}
- Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, et al. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. Journal of Vascular Surgery 1998;27(2):267-74. [DOI] [PubMed] [Google Scholar]
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-94-203. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
O'Donnell 2009 {published data only}
- O'Donnell M, Badger SA, Sharif MA, Young IS, Lau LL, Lee B, et al. Randomised controlled trial assessing the effects of cilostazol on exercise-induced ischaemia-reperfusion in patients with peripheral arterial disease. In: European Society for Vascular Surgery Annual Meeting; 2008 Sep 4-7; Nice, France. 2008:43.
- O'Donnell ME, Badger SA, Makar RR, McEneny J, Young IS, Lau LL, et al. The effects of cilostazol on the attenuation of inflammatory response in patients with peripheral arterial disease. The Vascular Society of Great Britain and Ireland Yearbook 2007:49.
- O'Donnell ME, Badger SA, Makar RR, Young IS, Lau LL, Lee B, et al. The effects of cilostazol in diabetic patients. The Vascular Society of Great Britain and Ireland Yearbook 2007:82.
- O'Donnell ME, Badger SA, Sharif MA, Makar RR, McEneny J, Young IS, et al. The effects of cilostazol on exercise-induced ischaemia-reperfusion injury in patients with peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2009;37(3):326-35. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, et al. The effects of cilostazol on peripheral neuropathy in diabetic patients with peripheral arterial disease. Angiology 2008;59(6):695-704. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Badger SA, Sharif MA, Makar RR, Young IS, Lee B, et al. The vascular and biochemical effects of cilostazol in diabetic patients with peripheral arterial disease. Vascular and Endovascular Surgery 2009;43(2):132-43. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Badger SA, Sharif MA, Young IS, Lee B, Soong CV. The vascular and biochemical effects of cilostazol in patients with peripheral arterial disease. Journal of Vascular Surgery 2009;49(5):1226-34. [DOI] [PubMed] [Google Scholar]
Otsuka Study 21‐86‐101 {unpublished data only}
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-86-101. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐86‐103 {unpublished data only}
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-86-103. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐87‐101 {unpublished data only}
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-87-101. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐94‐301 {unpublished data only}
- Otsuka America. Pletal (Cilostazol) Tablets. Study 21-94-301. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐95‐201 {unpublished data only}
- Otsuka 21-95-201. A randomised double-blind study of the safety and efficacy of two different doses of cilostazol versus placebo in patients with intermittent claudication secondary to peripheral vascular disease. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study 21‐98‐213 {unpublished data only}
- Anon. Pletal (cilostazol). Study 21-98-213 PACE. Otsuka Pharmaceuticals Submission to NICE: Cilostazol for the symptomatic treatment of intermittent claudication secondary to peripheral arterial disease (accessed prior to 16 June 2021).
- European Medicines Agency. Assessment report for Cilostazol containing medicinal products. ema.europa.eu/docs/en_GB/document_library/Referrals_document/Cilostazol_31/WC500148976.pdf (accessed prior to 22 June 2021).
Strandness 2002 {published data only}
- Otsuka 21-94-201. A randomised double blind study of the effect of cilostazol versus placebo on walking distances in patients with intermittent claudication secondary to peripheral vascular disease. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
- Strandness DE Jr, Dalman RL, Panian S, Rendell MS, Comp PC, Zhang P, et al. Effect of cilostazol in patients with intermittent claudication: a randomized, double-blind, placebo-controlled study. Vascular and Endovascular Surgery 2002;36(2):83-91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
CASTLE 2008 {published data only}
- Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: a study in long-term effects). Journal of Vascular Surgery 2008;47(2):330-6. [DOI] [PubMed] [Google Scholar]
- Otsuka 21-98-214-01. CASTLE. A randomized, double-blind, placebo-controlled, multicenter, parallel-arm, study to assess the long-term effects of Pletal® (Cilostazol) versus placebo administered orally to patients with intermittent claudication secondary to peripheral arterial disease. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
- Stone WM, Demaerschalk BM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. Journal of Stroke and Cerebrovascular Diseases 2008;17(3):129-33. [DOI] [PubMed] [Google Scholar]
- Stone WM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. In: Vascular Annual Meeting; 2007 Jun 6-10; Baltimore. 2007. [DOI] [PubMed]
Chao 2014 {published data only}
- Chao TH, Tseng SY, Chen IC, Tsai YS, Huang YY, Liu PY, et al. Cilostazol enhances mobilization and proliferation of endothelial progenitor cells and collateral formation by modifying vasculo-angiogenic biomarkers in peripheral arterial disease. International Journal of Cardiology 2014;172(2):e371-4. [DOI: 10.1016/j.ijcard.2013.12.295] [DOI] [PubMed] [Google Scholar]
Chao 2016 {published data only}
- Chao TH, Chen IC, Lee CH, Chen JY, Tsai WC, Li YH, et al. Cilostazol enhances mobilization of circulating endothelial progenitor cells and improves endothelium-dependent function in patients at high risk of cardiovascular disease. Angiology 2016;67(7):638-46. [DOI: 10.1177/0003319715606249] [DOI] [PubMed] [Google Scholar]
- Chao TH, Chen IC, Liu PY, Tsai WC, Li YH, Tseng SY, et al. Cilostazol enhances mobilization of circulating endothelial progenitor cells and improves endothelium-dependent function mediated by modifying metabolic and angiogenic markers in patients with high risk for cardiovascular disease. Journal of the American College of Cardiology 2015;65(10):A1650. [Google Scholar]
- Chao TH, Liu PY, Tsai WC, Tseng SY, Li YH. The vasculo-angiogenic and vaso-protective effects of cilostazol in patients with high risk for cardiovascular disease. European Heart Journal 2015;36(Suppl 1):229. [DOI: 10.1093/eurheartj/ehv399] [DOI] [Google Scholar]
- Chao TH, Tseng SY, Tsai YS, Huang YY, Liu PY, Ou HY, et al. Cilostazol enhances mobilization and proliferation of circulating endothelial progenitor cells and collateral formation mediated by modifying vasculo-angiogenic biomarkers in patients with peripheral arterial occlusive disease. Journal of the American College of Cardiology 2013;61(10):E2052. [DOI: ] [Google Scholar]
Chen 2017 {published data only}
- Chao TH, Li YH, Tseng SY, Liu PY. The associations between the effect of cilostazol on the proprotein convertase subtilisin/kexin type 9 concentrations and circulating endothelial progenitor cells and vasculo-angiogenesis factors. European Heart Journal 2018;39(Suppl 1):523. [DOI: 10.1093/eurheartj/ehy565.P2620] [DOI] [Google Scholar]
- Chen IC, Tseng WK, Li YH, Tseng SY, Liu PY, Chao TH. Effect of cilostazol on plasma levels of proprotein convertase subtilisin/kexin type 9. Oncotarget 2017;8(64):108042-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR‐TRC‐09000441 {published data only}
- ChiCTR-TRC-09000441. Evaluation of efficacy of cilostazol on type 2 diabetes with ischemic disease of lower extremity by transcutaneous oxygen pressure measurement. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-09000441 (retrospectively registered 24 June 2009).
Chisari 2019 {published data only}
- Chisari LM, Malaguarnera S, Grasso A, Malaguarnera M, Chisari G, Borzì AM, et al. Cilostazol reduces dry eye symptoms and improve walking distance in patients with peripheral artery disease. La Clinica Terapeutica 2019;170(5):e357-63. [DOI: 10.7417/CT.2019.2160] [DOI] [PubMed] [Google Scholar]
Goldenberg 2012 {published data only}
- Goldenberg NA, Krantz MJ, Hiatt WR. L-Carnitine plus cilostazol versus cilostazol alone for the treatment of claudication in patients with peripheral artery disease: a multicenter, randomized, double-blind, placebo-controlled trial. Vascular Medicine 2012;17(3):145-54. [DOI: 10.1177/1358863X12442264] [DOI] [PubMed] [Google Scholar]
Hsieh 2009 {published data only}
- Hsieh CJ, Wang PW. Effect of cilostazol treatment on adiponectin and soluble CD40 ligand levels in diabetic patients with peripheral arterial occlusion disease. Circulation Journal 2009;73(5):948-54. [DOI] [PubMed] [Google Scholar]
JPRN‐C000000215 {published data only}
- JPRN-C000000215. Study on therapeutic effects of antiplatelet drugs in prevention of occurrence and inhibition of progression of diabetic atherosclerosis. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-C000000215 (first posted 2005).
JPRN‐UMIN000001198 {published data only}
- JPRN-UMIN000001198. Cilostazol trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000001198 (first posted 20 June 2008).
JPRN‐UMIN000011869 {published data only}
- JPRN-UMIN000011869. Relationship between arteriosclerosis obliterans and serum level of eicosapentaenoic acid as prospective interventional study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000011869 (first posted 26 September 2013).
JPRN‐UMIN000014307 {published data only}
- JPRN-UMIN000014307. Phase IIb double blinded parallel group dose response study for DVC1-0101 to treat severe intermittent claudication. http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000014307 (first posted 1 September 2014).
Kim 2013 {published data only}
- Kim NH, Kim HY, An H, Seo JA, Kim NH, Choi KM, et al. Effect of cilostazol on arterial stiffness and vascular adhesion molecules in type 2 diabetic patients with metabolic syndrome: a randomised, double-blind, placebo-controlled, crossover trial. Diabetology and Metabolic Syndrome 2013;5(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzone 2013 {published data only}
- Mazzone A, Di Salvo M, Mazzuca S, Valerio A, Gussoni G, Bonizzoni E, et al. Effects of iloprost on pain-free walking distance and clinical outcome in patients with severe stage IIb peripheral arterial disease: the FADOI 2b PILOT Study. European Journal of Clinical Investigation 2013;43(11):1163-70. [DOI: 10.1111/eci.12159] [DOI] [PubMed] [Google Scholar]
NCT00102050 {published data only}
- NCT00102050. Efficacy and safety of NM-702 tablets for the treatment of intermittent claudication. clinicaltrials.gov/show/NCT00102050 (first received 19 January 2005).
NCT00300339 {published and unpublished data}
- NCT00300339. MASCOT: mixed antagonist of serotonin for claudication optimal therapy. clinicaltrials.gov/ct2/show/NCT00300339 (first received 8 March 2006).
NCT00443287 {published and unpublished data}
- NCT00443287. Efficacy and safety of HMR1766 in patients with Fontaine Stage II peripheral arterial disease. www.clinicaltrials.gov/ct2/show/NCT00443287 (first posted 5 March 2007).
NCT00573950 {published data only}
- NCT00573950. Effects of cilostazol on plasma adipocytokine and arterial stiffness. clinicaltrials.gov/show/NCT00573950 (first posted 14 December 2007).
NCT00886574 {published data only}
- NCT00886574. Cilostazol versus aspirin for primary prevention of atherosclerotic events (CAPPA). clinicaltrials.gov/show/NCT00886574 (first posted 22 April 2009).
NCT00912756 {published data only}
- NCT00912756. Sufficient treatment of peripheral intervention by cilostazol (STOP-IC). clinicaltrials.gov/ct2/show/NCT00912756 (first posted 2 June 2009).
NCT01188824 {published data only}
- NCT01188824. The safety and efficacy of cilostazol in ischemic stroke patients with peripheral arterial disease (SPAD study). clinicaltrials.gov/ct2/show/NCT01188824 (first posted 26 August 2010).
NCT01952756 {published data only}
- NCT01952756. Effect of cilostazol endothelial progenitor cells and collateral formation in peripheral occlusive artery disease (PAOD). clinicaltrials.gov/show/NCT01952756 (first posted 21 September 2013).
NCT02373462 {published data only}
- NCT02373462. Effect of olmesaRtan on walking distancE and quaLIty of lifE in peripheral artery disease patients with hypertension treated for intermittent claudication (RELIEF). clinicaltrials.gov/show/NCT02373462 (first posted 14 February 2015).
NCT02407314 {published data only}
- NCT02407314. Ticagrelor and peripheral arterial disease. clinicaltrials.gov/ct2/show/NCT02407314 (first posted 18 March 2015).
NCT02636283 {published data only}
- NCT02636283. Use of entresto sacubitril/valsartan for the treatment of peripheral arterial disease. clinicaltrials.gov/show/NCT02636283 (first posted 29 October 2015).
NCT02930811 {published data only}
- NCT02930811. Efficacy of sildenafil on the morbi-mortality of peripheral arterial diseased patients with intermittent claudication (VALSTAR). clinicaltrials.gov/show/NCT02930811 (first posted 10 October 2016).
NCT03318276 {published data only}
- NCT03318276. Clinical trial to evaluate efficacy and safety of SID142 in patients with chronic artery occlusive disease. clinicaltrials.gov/show/NCT03318276 (first posted 18 October 2017).
NCT03686306 {published data only}
- NCT03686306. VIRTUOSE: efficiency of sildenafil on the absolute claudication distance of peripheral arterial disease patients with intermittent claudication (VIRTUOSE). clinicalTrials.gov/show/NCT03686306 (first posted 24 September 2018).
Otsuka Study PUIC‐1 {published data only}
- Otsuka America. Pletal (Cilostazol) Tablets. Study PUIC-1. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Otsuka Study PUIC‐2 {published data only}
- Otsuka America. Pletal (Cilostazol) Tablets. Study PUIC-2. www.accessdata.fda.gov/drugsatfda_docs/nda/99/20863.cfm (accessed May 2014).
Xiao 2010 {published data only}
- Xiao Z, Chen L, Yang C, Yang H, Luo B, Mai L, et al. Evaluation of cilostazol efficacy on type 2 diabetes with lower limb ischemic disease by transcutaneous oxygen pressure measurement. In: American Diabetes Association. 70th Scientific Sessions; 2010. Guangdong, China (professional.diabetes.org/abstract/evaluation-cilostazol-efficacy-type-2-diabetes-lower-limb-ischemic-disease-transcutaneous): American Diabetes Association, 2010 (accessed 1 April 2021).
References to studies awaiting assessment
Sapelkin 2013 {published data only}
- Sapelkin SV, Kharazov AF. Advanced position in the conservative treatment of patients with peripheral arterial disease. Khirurgiia 2013;4:68-73. [PubMed] [Google Scholar]
Additional references
Aboyans 2018
- Aboyans V, Ricco JB, Bartelink ME, Björck M, Brodmann M, Cohnert T, et al, ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO), The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). European Heart Journal 2018;39(9):763-816. [DOI] [PubMed] [Google Scholar]
ATT 2002
- Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324(7329):71-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barriocanal 2016
- Barriocanal AM, Lopez AM, Farre M, Montane E. Recommendations of intermittent claudication symptomatic treatment in peripheral arterial disease clinical practice guidelines. Basic and Clinical Pharmacology and Toxicology 2016;119(Suppl 1):53. [Google Scholar]
Broderick 2020
- Broderick C, Forster R, Abdel-Hadi M, Salhiyyah K. Pentoxifylline for intermittent claudication. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD005262. [DOI: 10.1002/14651858.CD005262.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2003
- Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. American Journal of Cardiovascular Drugs 2003;3(2):117-38. [DOI] [PubMed] [Google Scholar]
Cosmi 2014
- Cosmi B, Conti E, Coccheri S. Anticoagulants (heparin, low molecular weight heparin and oral anticoagulants) for intermittent claudication. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD001999. [DOI: 10.1002/14651858.CD001999.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 2001
- Dawson DL. Comparative effects of cilostazol and other therapies for intermittent claudication. American Journal of Cardiology 2001;87(12A):19D-27D. [DOI] [PubMed] [Google Scholar]
Dormandy 1999
- Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Seminars in Vascular Surgery 1999;12(2):123-37. [PubMed] [Google Scholar]
Fakhry 2018
- Fakhry F, Fokkenrood HJP, Spronk S, Teijink JAW, Rouwet EV, Hunink MGM. Endovascular revascularisation versus conservative management for intermittent claudication. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD010512. [DOI: 10.1002/14651858.CD010512.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerhard‐Herman 2017
- Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guidelines on the Management of Patients with Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2017;69:e71-e126. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 14 January 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Haile 2020
- Haile S, Linné A, Johansson UB, Joelsson-Alm E. Follow-up after surgical treatment for intermittent claudication (FASTIC): a study protocol for a multicentre randomised controlled clinical trial. BMC Nursing 2020;19(45):1-12. [DOI: 10.1186/s12912-020-00437-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021a
- Higgins JPT , Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane 2021. Available from www.training.cochrane.org/handbook.
Iida 2008
- Iida O, Nanto S, Uematsu M, Morozumi T, Kitakaze M, Nagata S. Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions. Journal of Vascular Surgery 2008;48(1):144-9. [DOI] [PubMed] [Google Scholar]
Khan 2005
- Khan S, Cleanthis M, Smout J, Flather M, Stansby G. Life-style modification in peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2005;29(1):2-9. [DOI] [PubMed] [Google Scholar]
Lane 2017
- Lane R, Harwood A, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD000990. [DOI: 10.1002/14651858.CD000990.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2013
- Lee SW, Ahn JM, Han S, Park GM, Cho YR, Lee WS, et al. Differential impact of cilostazol on restenosis according to implanted stent type (from a pooled analysis of three DECLARE randomized trials). American Journal of Cardiology 2013;112(9):1328-34. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Leng 1996
- Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral artery disease in the general population. International Journal of Epidemiology 1996;25(6):1172-81. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence (NICE). Cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. nice.org.uk/guidance/TA223/chapter/2-Clinical-need-and-practice (accessed 11 July 2014).
NICE 2012
- National Institute for Health and Care Excellence (NICE). Peripheral arterial disease: diagnosis and management (last updated December 2020). nice.org.uk/Guidance/CG147 (accessed 12 March 2021).
Niu 2016
- Niu PP, Guo ZN, Jin H, Xing YQ, Yang Y. Antiplatelet regimens in the long-term secondary prevention of transient ischaemic attack and ischaemic stroke: an updated network meta-analysis. BMJ Open 2016;6(3):e009013. [DOI: 10.1136/bmjopen-2015-009013] [DOI] [PMC free article] [PubMed] [Google Scholar]
PAD 2003
- Peripheral Arterial Diseases Antiplatelet Consensus Group. Antiplatelet therapy in peripheral arterial disease. Consensus statement. European Journal of Vascular and Endovascular Surgery 2003;26(1):1-16. [DOI] [PubMed] [Google Scholar]
Regensteiner 2002
- Regensteiner JG, Ware JE Jr, McCarthy WJ, Zhang P, Forbes WP, Heckman J, et al. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. Journal of the American Geriatrics Society 2002;50(12):1939-46. [DOI] [PubMed] [Google Scholar]
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Rizzo 2011
- Rizzo M, Corrado E, Patti AM, Rini GB, Mikhailidis DP. Cilostazol and atherogenic dyslipidemia: a clinically relevant effect? Expert Opinion on Pharmacotherapy 2011;12(4):647-55. [DOI] [PubMed] [Google Scholar]
Robless 2001
- Robless P, Mikhailidis D, Stansby G. Systematic review of antiplatelet therapy for prevention of myocardial infarction, stroke or vascular death in patients with peripheral vascular disease. British Journal of Surgery 2001;88(6):787-800. [DOI] [PubMed] [Google Scholar]
Sallustio 2010
- Sallustio F, Rotondo F, Di Legge S, Stanzione P. Cilostazol in the management of atherosclerosis. Current Vascular Pharmacology 2010;8(3):363-72. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Squires 2010
- Squires H, Simpson E, Meng Y, Harnan S, Stevens J, Wong R, National Institute for Health and Clinical Excellence. Cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for intermittent claudication in people with peripheral arterial disease. Technology Assessment Report commissioned by the NIHR HTA Programme. www.guidance.nice.org.uk/nicemedia/live/12265/51580/51580.pdf (accessed May 2014).
Squires 2011
- Squires H, Simpson E, Meng Y, Harnan S, Stevens J, Wong R, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technology Assessment 2011;15(40):1-210. [DOI: 10.3310/hta15400] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2012
- Stevens JW, Simpson E, Harnan S, Squires H, Meng Y, Thomas S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. British Journal of Surgery 2012;99(12):1630-8. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. American Journal of Cardiology 2002;90(12):1314-9. [DOI] [PubMed] [Google Scholar]
Ueno 2011
- Ueno H, Koyama H, Mima Y, Fukumoto S, Tanaka S, Shoji T, et al. Comparison of the effect of cilostazol with aspirin on circulating endothelial progenitor cells and small-dense LDL cholesterol in diabetic patients with cerebral ischemia: a randomized controlled pilot trial. Journal of Atherosclerosis and Thrombosis 2011;18(10):883-90. [DOI] [PubMed] [Google Scholar]
Wong 2011
- Wong PF, Chong LY, Mikhailidis DP, Robless P, Stansby G. Antiplatelet agents for intermittent claudication. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD001272. [DOI: 10.1002/14651858.CD001272.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bedenis 2014
- Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G. Cilostazol for intermittent claudication. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD003748. [DOI: 10.1002/14651858.CD003748.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robless 2007
- Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD003748. [DOI: 10.1002/14651858.CD003748.pub2] [DOI] [PubMed] [Google Scholar]
Robless 2008
- Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD003748. [DOI: 10.1002/14651858.CD003748.pub3] [DOI] [PubMed] [Google Scholar]


