Abstract
Cerebral cavernous malformations (CCMs) are common neurovascular lesions caused by loss-of-function mutations in 1 of 3 genes, including KRIT1 (CCM1), CCM2, and PDCD10 (CCM3), and generally regarded as an endothelial cell-autonomous disease. Here we reported that proliferative astrocytes played a critical role in CCM pathogenesis by serving as a major source of VEGF during CCM lesion formation. An increase in astrocyte VEGF synthesis is driven by endothelial nitric oxide (NO) generated as a consequence of KLF2- and KLF4-dependent elevation of eNOS in CCM endothelium. The increased brain endothelial production of NO stabilized HIF-1α in astrocytes, resulting in increased VEGF production and expression of a “hypoxic” program under normoxic conditions. We showed that the upregulation of cyclooxygenase-2 (COX-2), a direct HIF-1α target gene and a known component of the hypoxic program, contributed to the development of CCM lesions because the administration of a COX-2 inhibitor significantly prevented the progression of CCM lesions. Thus, non–cell-autonomous crosstalk between CCM endothelium and astrocytes propels vascular lesion development, and components of the hypoxic program represent potential therapeutic targets for CCMs.
Keywords: Angiogenesis, Cell Biology
Keywords: Cardiovascular disease, Hypoxia, Nitric oxide
Introduction
Cerebral cavernous malformations (CCMs) are caused by gross brain endothelial changes that lead to blood-brain barrier (BBB) dysfunction, resulting in significant morbidity and mortality (1, 2). CCMs affect approximately 1 in 200 children and adults, causing a lifelong risk of chronic and acute hemorrhage and consequent disabilities from neurological deficits, for which there is no current effective pharmacologic therapy (3–6). Inherited germline and somatic loss of function mutations in the genes KRIT1 (Krev1 interaction trapped gene 1, CCM1), CCM2 (Malcavernin), PDCD10 (programmed cell death protein 10, CCM3) are associated with CCMs (5, 7–9). Notably, CCMs have been recognized as an endothelial cell-autonomous disease because endothelial-specific inactivation of murine Krit1, Ccm2, or Pdcd10, results in brain and retinal vascular lesions similar to those in patients with CCM (10–13). These murine studies are also supported by the finding of a “second hit” on the normal KRIT1 or PDCD10 allele in the CCM endothelium (14, 15). Recent studies in CCM animal models proposed an endothelial cell nonautonomous mechanism of lesion formation in the CNS (16–19). However, there has been to date no compelling mechanistic explanation for the propensity of CCM lesions to form in the central nervous system (CNS).
Recent studies have shown that loss of CCM genes in neuroglia or stromal cells may contribute to CCM pathogenesis through non–cell-autonomous mechanisms (18, 20, 21). Although CCM is a disease that affects the neurovascular unit, there is a limited understanding of the crosstalk between the CCM endothelium and astrocytes (20, 22, 23). Astrocytes are the most abundant cell in the CNS and form part of the neurovascular unit that maintains a functional BBB (24–26). Additionally, astrocytes respond to multiple forms of insults and diseases by a process called reactive astrogliosis or astrocytosis, which involves changes in morphology, function, and gene expression (27, 28). Reactive astrogliosis increases secretion of VEGF and can contribute to the development and progression of CNS disease (29–31). Previous studies have demonstrated elevated levels of angiogenic factor VEGF in CCM lesions, and in the plasma of individuals with the hereditary and sporadic form of the disease (32–35). VEGF signaling mediates disruption of the brain endothelial barrier by disassembly of inter-endothelial junctions (36) and can cause hemorrhages (37, 38), both prominent features of CCM lesions (2, 6, 10, 39, 40). We and others found that CCM led to a dramatic increase in VEGF signaling and that blocking VEGF signaling prevents subsequent formation of CCMs (6, 41). However, despite these emerging studies, the mechanism and source of VEGF production in CCM lesions in vivo remain elusive.
Endothelial nitric oxide synthase (eNOS, the product of the NOS3 gene) is an enzyme that generates nitric oxide (NO) and it is responsible for vascular remodeling, angiogenesis and vascular tone (42, 43). Previous studies indicate that eNOS contributes to VEGF-induced angiogenesis and vascular permeability (44) through NO production (45, 46). Here we show that astrocytes are a major source of VEGF during CCM pathogenesis and that depletion of proliferative astrocytes prevents CCM lesion development. We show that CCM endothelium-induced elevation of astrocyte-derived VEGF in neonatal and juvenile brains occurs through elevation of brain eNOS/NO-dependent signaling. Production of NO in CCM endothelium contributes to normoxic stability of HIF-1α in astrocytes. Our findings indicate that the eNOS increase in human CCM endothelium is ascribable to the elevation of the transcription factors KLF2 and KLF4, previously implicated in the CCM pathogenesis (6, 13, 40, 47, 48). Furthermore, genetic inactivation of one copy of the Nos3 gene is sufficient to attenuate CCM endothelial NO production, HIF-1α protein stability and astrocyte-derived VEGF expression. Our results further reveal that CCM tissue results in increased HIF activity under normoxic conditions. We show that pharmacological inhibition of a HIF-1α regulated gene, cyclooxygenase-2, ameliorates the development of CCM lesions in animal models. Overall, these findings indicate that a non–cell-autonomous mechanism mediated by CCM endothelium-driven normoxic stabilization of HIF-1α and increase of VEGF in astrocytes makes a major contribution to CCM pathogenesis. Understanding the crosstalk between dysfunctional brain vasculature and components of the neurovascular unit (e.g., astrocytes) has the potential to lead to the development of novel therapeutic strategies for CCM disease as exemplified by our finding that inhibition of HIF1-driven COX-2 by an approved and a well-tolerated drug can ameliorate murine models of CCM disease.
Results
Astrocytes contribute to cerebral cavernous malformations development.
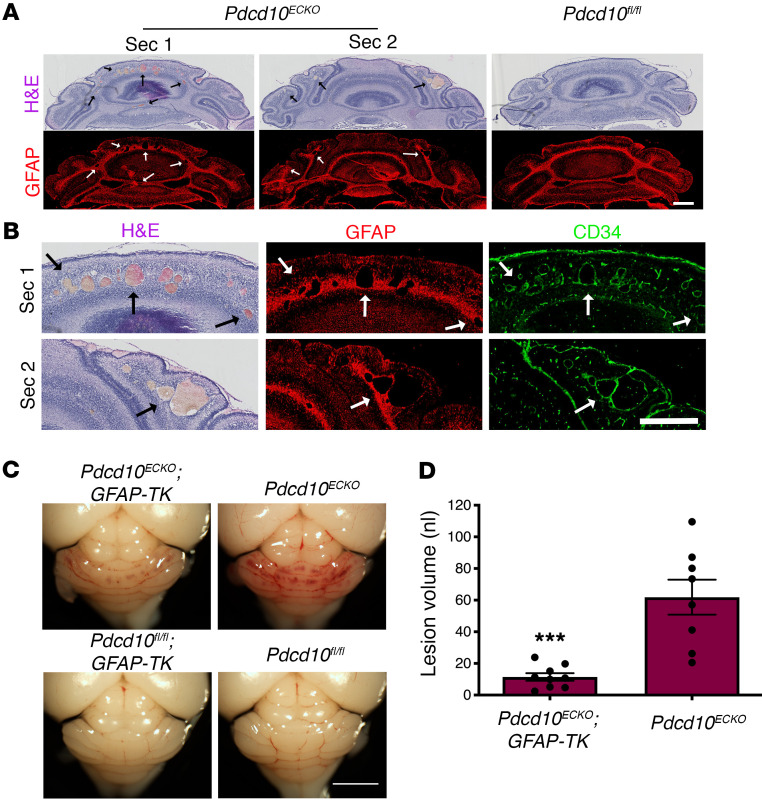
CCMs have been studied as an endothelial cell-autonomous disease (6, 14, 15), marked by changes in the CNS vasculature due to the loss of endothelial PDCD10, KRIT1, or CCM2 (10–12). Although it is a disease that affects the neurovascular unit, we know little about whether astrocytes influence CCM pathogenesis (20, 22). This prompted us to investigate the relationship between astrocytes and CCM lesion development in murine CCMs (Figure 1, A and B, and Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/JCI139570DS1). Neonatal endothelial-specific inactivation of murine Pdcd10, producing Pdcd10ECKO mice (40), results in cerebellar vascular lesions detected in sections stained by hematoxylin and eosin (Figure 1, A and B, and Supplemental Figure 1) or by observing dramatic vascular dilation by staining the endothelial marker CD34 at P9 (Figure 1, A and B). CCM lesions have high propensity to develop in GFAP-positive fibrous astrocytes (49) (Figure 1A) in the white matter of Pdcd10ECKO hindbrains (Supplemental Figure 1). Moreover, P8 Krit1ECKO hindbrains show the same spatial distribution of CCM lesions among astrocytes positive for GFAP on the white matter tract (Supplemental Figure 1). To examine the role of astrocytes, we generated Pdcd10ECKO GFAP-TK mice (Pdgfb-iCreERT2 Pdcd10fl/fl GFAP-TK [thymidine kinase] mice) in which proliferative astrocytes are selectively depleted in a time-controlled manner, by administration of antiviral agent ganciclovir (GCV) (50, 51). Administration of 20 mg/kg GCV to neonatal mice at P7 markedly reduced hindbrain vascular lesions in P9 Pdcd10ECKO GFAP-TK mice when compared with GCV-treated littermate Pdcd10ECKO controls (Figure 1C). To quantify CCM formation, we imaged P9 hindbrains using contrast-enhanced, high-resolution x-ray microcomputed tomography (micro-CT), and measured lesion volumes using semiautomated software (52). Blinded measurement of total CCM lesion volume showed that GCV-treated Pdcd10ECKO GFAP-TK mouse hindbrains exhibited significant reduction of CCM lesions compared with GCV-treated Pdcd10ECKO mouse hindbrains littermates (Figure 1D). These data suggest that in CCM disease, proliferative astrocytes participate in vascular lesion formation.
Figure 1. Astrocytes contribute to cerebral cavernous malformation development.
(A) Histological analysis of cerebellar sections from P9 Pdcd10ECKO and littermate control Pdcd10fl/fl mice. Low magnification of CCM lesions detected in sections stained by hematoxylin and eosin. CCM lesions spatially developed on fibrous astrocyte areas positive for GFAP immunostaining (red). Arrows indicate CCM lesions. (B) Magnified region from sections 1 and 2 (Sec1 and Sec2) from Pdcd10ECKO mice in (A). Dramatic vascular dilation is shown with immunohistochemistry for the endothelial marker CD34 (green). Arrows indicate CCM lesions. (C) Administration of 1.5 mg/kg GCV in neonatal mice at P7 markedly reduced vascular lesions in P9 Pdcd10ECKO GFAP-TK hindbrains when compared with GCV-treated littermate Pdcd10ECKO controls. (D) Quantification of lesion volumes by micro-CT analysis from mice in experiments showed in (C); n = 8 or 9 mice in each group. Data are mean ± SEM. ***P < 0.001, as determined by Student’s t test. Scale bars: 500 μm (A and B); 2 mm (C).
Astrocyte-derived VEGF is increased during CCM development.
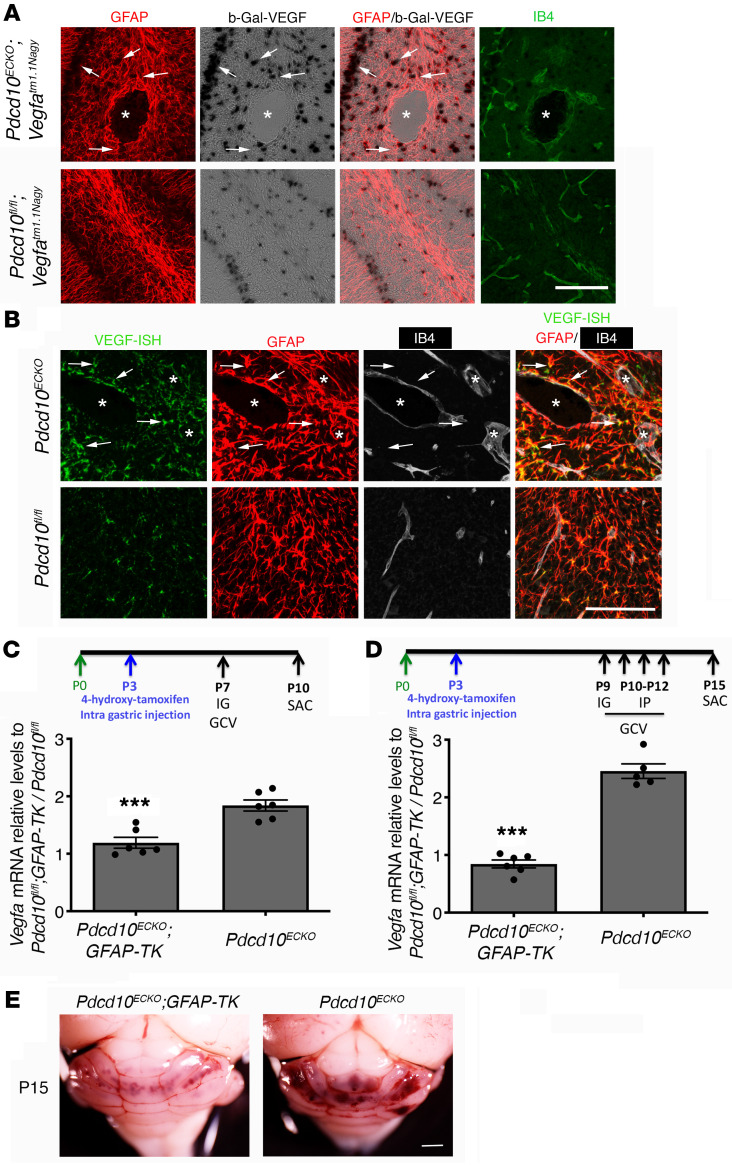
CCMs exhibit increased VEGF signaling (6, 33, 34) due to the loss of an anti-angiogenic checkpoint (6). To ascertain a potential source of increased expression of VEGF, we introduced a VEGF reporter, Vegfatm1.1Nagy, in CCM animal models. Vegfatm1.1Nagy carries a nuclear-localized beta-galactosidase (β-gal) knock-in at the 3′ UTR of the Vegfa gene locus that permits single-cell monitoring of VEGF expression (53). We observed an increase in β-gal/VEGF expression, as shown by X-gal staining, in areas surrounding CCM lesions in P10 Pdcd10ECKO Vegfatm1.1Nagy hindbrains when compared with Pdcd10fl/fl Vegfatm1.1Nagy littermate controls (Figure 2A). We found that most β-gal/VEGF+ cells colocalized with the GFAP marker of fibrous astrocytes and Bergman glia located within Purkinje cell layer (49) in Pdcd10ECKO Vegfatm1.1Nagy (Figure 2A). We also observed that calbidin-positive Purkinje neurons exhibited increased β-gal/VEGF expression near CCM lesions (data not shown). Consistent with results observed using β-gal/VEGF reporter mice, high-resolution in situ hybridization (ISH) analysis confirmed an increase in VEGF expression near and around the CCM lesion colocalized with the GFAP marker of astrocytes (Figure 2B and Supplemental Figure 2). Moreover, we observed that Pdcd10ECKO hindbrains exhibited an approximately 1.8-fold increase in Vegfa mRNA levels compared with littermate Pdcd10fl/fl controls (Figure 2C). Administration of GCV to neonatal mice at P7 significantly reduced Vegfa mRNA levels in vascular lesions in P10 Pdcd10ECKO GFAP-TK mice compared with GCV-treated littermate Pdcd10ECKO controls (Figure 2C). We next tested the effect of depletion of proliferative astrocytes at the developmental stage when vascular lesions are present in Pdcd10ECKO. Our data indicate that administration of GCV to neonatal mice between P9 to P12 significantly reduced Vegfa mRNA levels and CCM lesion burden in P15 Pdcd10ECKO GFAP-TK mice when compared with GCV-treated littermate Pdcd10ECKO controls (Figure 2, D and E). Since the retinal blood vessels is another vascular bed affected by the loss of Pdcd10 in endothelial cells, we also compared the subcellular expression of VEGF. Deletion of the endothelial Pdcd10 gene at P3 prominently increase β-gal/VEGF expression in GFAP+ retinal astrocytes at the leading edge of blood vessel growth and immediately ahead of the plexus in P9 Pdcd10ECKO Vegfatm1.1Nagy retinas (Supplemental Figures 2 and 3). We observed that the enhanced expression of β-gal/VEGF was associated with impaired extension of the developing superficial vascular plexus (54) in P9 Pdcd10ECKO Vegfatm1.1Nagy retinas when compared with Pdcd10fl/fl Vegfatm1.1Nagy littermate controls (Supplemental Figure 3). Moreover, defect on the extension of the vascular plexus in P12 Pdcd10ECKO Vegfatm1.1Nagy retinas was reduced compared with Pdcd10fl/fl Vegfatm1.1Nagy littermate controls, whereas the endothelial density and an increase in β-gal/VEGF expression in GFAP+ retinal astrocytes were elevated (Supplemental Figure 3). These data suggest that increased expression of astrocyte-derived VEGF could account for a contribution of astrocytes to CCM lesion formation.
Figure 2. Astrocyte-derived VEGF increased during cerebral cavernous malformation development.
(A) Confocal microscopy of cerebellar cortex from P10 Pdcd10ECKO and littermate control Pdcd10fl/fl mice stained for GFAP-positive astrocytes (red), β-gal/VEGF expression detected by X-gal staining (black), and endothelial marker isolectin B4 (green). Asterisk indicates vascular lumen of CCM lesion. Arrows indicate β-gal/VEGF in GFAP-positive astrocytes (n = 4). (B) ISH for VEGF (green) combined with immunohistochemistry to identify GFAP-positive astrocytes (red), endothelial marker isolectin B4 (IB4; white). Asterisk indicates vascular lumen of CCM lesion. Arrows indicate VEGF and GFAP-positive astrocyte colocalization (n = 2). (C) Quantification of Vegfa mRNA levels in P10 Pdcd10ECKO GFAP-TK and Pdcd10ECKO hindbrains when compared with littermate Pdcd10fl/fl GFAP-TK or Pdcd10fl/fl controls, respectively, as assessed by RT-qPCR. All mice received IG administration of 1.5 mg/kg GCV in neonatal at P7 (SEM, n = 6 mice in each group). (D) Quantification of Vegfa mRNA levels in P15 Pdcd10ECKO GFAP-TK and Pdcd10ECKO hindbrains when compared with littermate Pdcd10fl/fl GFAP-TK or Pdcd10fl/fl controls, respectively, as assessed by RT-qPCR. All mice received IG administration of 1.5 mg/kg GCV in neonatal at P9 and 3 consecutive doses of IP 1.5 mg/kg GCV at P10 to P12 (SEM, n = 5 or 6 mice in each group). (E) Prominent lesions are present in the cerebellum of P15 GCV-treated Pdcd10ECKO mice, whereas extensive reduction in lesions are observed in GCV-treated Pdcd10ECKO GFAP-TK littermate mice (n = 6 or 7). Data are mean ± SEM. ***P < 0.001 (comparison to Pdcd10ECKO littermates); determine by Student’s t test. Scale bars: 100 μm (A and B).
Astrocyte-derived VEGF increases during CCM formation in juvenile mice.
The previous studies showed increased VEGF expression in an acute neonatal CCM model. Recent studies have emphasized that chronic models may be more reflective of the course of the human disease (17, 55–57). We therefore, investigated the expression of VEGF increased in perilesional astrocytes in juvenile Pdcd10ECKO Vegfatm1.1Nagy mice. Similar to neonatal mice, we observed an increase in β-gal/VEGF expression in areas surrounding CCM lesions in P30 Pdcd10ECKO Vegfatm1.1Nagy cerebellar tissue (Figure 3A). We found that most β-gal/VEGF+ cells colocalized with the GFAP astrocyte marker in Pdcd10ECKO Vegfatm1.1Nagy mice (Figure 3A). Consistent with results observed using β-gal/VEGF reporter mice, Pdcd10ECKO cerebellar tissue showed a significant increase in Vegfa mRNA levels when compared with littermate Pdcd10fl/fl controls (Figure 3B). In contrast to neonatal Pdcd10ECKO mice, Pdcd10ECKO juvenile mice develop CCM lesions in the cerebrum (17, 55, 58) and these were accompanied by a marked increase in β-gal/VEGF expression in GFAP+ astrocytes and in SOX-9+ astrocytes in P30 Pdcd10ECKO Vegfatm1.1Nagy brains (Figure 3A and Supplemental Figure 3). We also observed a significant increase in Vegfa mRNA in the cerebrum of Pdcd10ECKO mice (Figure 3C). Additionally, we found elevated levels of VEGF in plasma from P30 Pdcd10ECKO mice when compared with Pdcd10fl/fl littermate controls (Figure 3D). These data support the idea that astrocyte-derived VEGF contributes to CCM lesions (32–35).
Figure 3. Astrocyte-derived VEGF increased during cerebral cavernous malformations in juvenile animal brain.
(A) Confocal microscopy of brain (cerebellum and cerebrum cortex) from Pdcd10ECKO Vegfatm1.1Nagy and Pdcd10fl/fl Vegfatm1.1Nagy littermate controls stained for β-gal/VEGF expression detected by X-gal staining (black), GFAP-positive astrocytes (red), isolectin B4 (green), and DAPI for nuclear DNA (blue). Asterisks indicate vascular lumen of CCM lesions. Arrows indicate β-gal/VEGF in GFAP-positive astrocytes. (B and C) Quantification of Vegfa mRNA levels in Pdcd10ECKO brains when compared with littermate Pdcd10fl/fl controls, as assessed by RT-qPCR (SEM, n = 4 or 6 mice in each group). (D) VEGF levels in plasma from Pdcd10ECKO mice and littermate Pdcd10fl/fl controls, as assessed by ELISA. n = 8 or 11 mice in each group. Data are mean ± SEM. *P < 0.05, as determined by Student’s t test. Scale bar: 100 μm (A).
Increase in normoxic HIF-1α stabilization in CCM astrocytes.
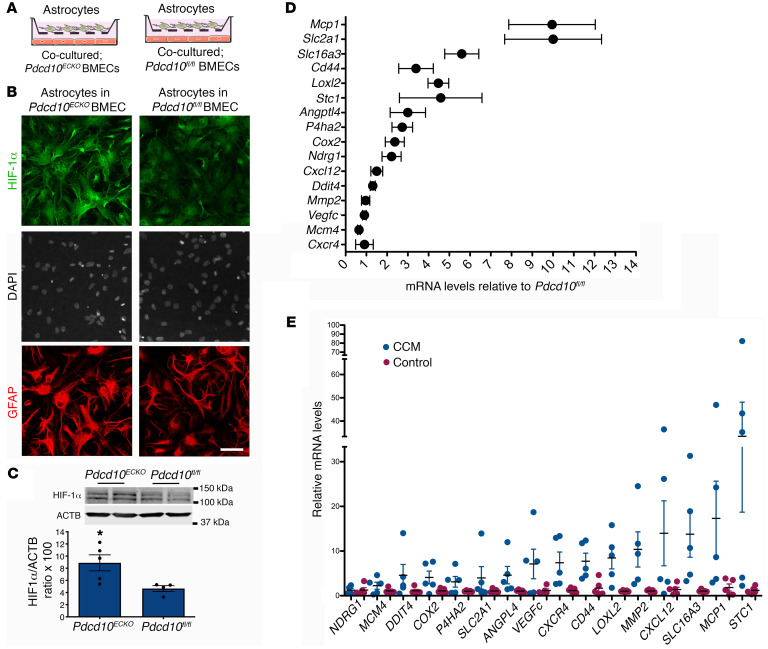
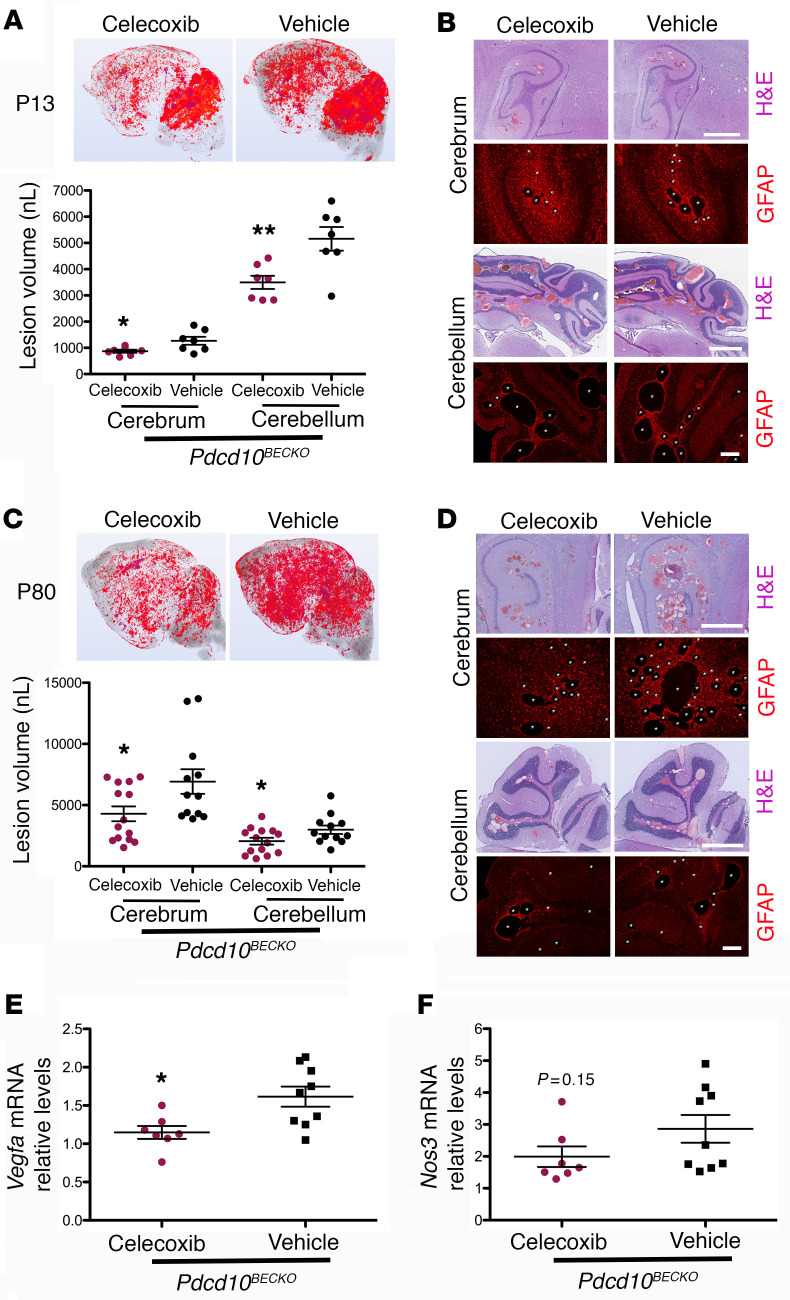
Transcription factor HIF-1α regulates expression of VEGF (30, 59–61). Therefore, we next investigated whether the significant increase of VEGF in astrocytes during CCMs is due to elevation of HIF-1α. For this study, we first prepared coculture studies to better understand the interaction between CCM endothelium and astrocytes (Figure 4A) and the level of HIF-1α in purified astrocytes (Supplemental Figure 4) cocultured with Pdcd10ECKO or Pdcd10fl/fl brain microvascular endothelial cells (BMECs) was determined. Immunocytochemistry revealed that the level of HIF-1α was elevated in astrocytes cocultured with Pdcd10ECKO BMECs compared with those cocultured with control Pdcd10fl/fl BMECs (Figure 4, A and B, and Supplemental Figure 4). Consistent with this observation, Western blot analysis of Pdcd10ECKO hindbrains at P10 confirmed an increase in HIF-1α protein levels when compared with Pdcd10fl/fl littermate controls (Figure 4C). Furthermore, we observed an increase in HIF-1α activity in Pdcd10ECKO hindbrains (Figure 4D). RT-qPCR analysis revealed a significant induction of HIF-1α regulated genes involved in inflammation (62), including cyclooxygenase-2 (Cox2) and monocyte chemoattractant protein 1 (Mcp1) as well as genes involved in metabolic reprograming such as glucose transporter 1 (solute carrier family 2, Slc2a1, GLUT-1), lactic acid, and pyruvate transporter (solute carrier family 16, Slc16a3, also known as monocarboxylate transporter 4 MCT4) (59, 63). In addition, we also observed a marked increase in HIF-1α regulated genes involved in angiogenesis (59, 63), including a cell-surface glycoprotein Cd44, Lysyl oxidase-like 2 (Loxl2) and angiopoietin-like 4 (Angptl4) (Figure 4D). Importantly, our findings in the CCM animal model were extended to human CCM because we also detected the elevation of HIF-1α regulated genes in human CCM lesions in comparison with lesion-free brain tissue from a patient with CCM or nonneurological disease control (Figure 4E). Since we observed that COX-2 is increased at mRNA (Figure 4D) and protein (data not shown) levels in Pdcd10ECKO hindbrains and at the mRNA level in human CCM lesions (Figure 4E), and it was previously showed by immunohistochemistry in human CCM tissue by Francesco Retta’s group (21), we next investigated the effect of using celecoxib (40 mg/kg), a nonsteroidal antiinflammatory drug (NSAID) that primarily inhibits COX-2 activity, in 2 preclinical CCM models. In these experiments, we used brain endothelial-specific inactivation (Slco1c1-iCreERT2) of the Pdcd10 gene, where lesions are confined to the CNS and progressively form in the cerebrum and cerebellum of P13 Pdcd10BECKO mice (Figure 5A). Blinded measurements using microCT analysis of neonatal celecoxib-treated Pdcd10BECKO mice compared with vehicle-treated littermate Pdcd10BECKO controls indicated a reduction in vascular lesion volume in both cerebellum and cerebrum (Figure 5A). Histological analysis of the hippocampal area in P13 Pdcd10BECKO mice further demonstrates the high propensity of CCM lesions to develop surrounded by GFAP+ astrocytes, and inhibition of COX-2 reduced the CCM lesions’ density in Pdcd10BECKO mice (Figure 5B and Supplemental Figure 5). Next, we investigated the effect of using celecoxib for 15 days in juvenile Pdcd10BECKO mice. Notably, blinded measurements of microCT analysis confirmed reduction in CCM in cerebrum and cerebellum of P80 celecoxib-treated Pdcd10BECKO mice (Figure 5C). Moreover, histological analysis of the cerebellum and hippocampal area in P80 celecoxib-treated Pdcd10BECKO mice confirmed a decrease in CCM lesions’ density and in GFAP-immunoreactivity (Figure 5D and Supplemental Figure 5). Similar to the CCM lesions detected in brain, we observed that P80 Pdcd10BECKO mice develop gross CCM lesions through the spinal cord (Supplemental Figure 6), and with high propensity to develop in GFAP+ astrocytes areas. In addition, P80 Pdcd10BECKO spinal cords exhibited an approximately 1.6-fold increase in Vegfa mRNA levels that it is prevented in P80 celecoxib-treated Pdcd10BECKO mice (Figure 5E), whereas the elevation of Nos3 mRNA remain unchanged (Figure 5F). Collectively, these results suggest that CCM endothelium can induce HIF-1α–dependent hypoxia and angiogenesis programs in astrocytes and that this non–cell-autonomous mechanism contributes to disease. Moreover, the capacity of celecoxib to ameliorate 2 murine CCM models underscores the potential of therapeutic strategies to emerge from these insights.
Figure 4. Increase in normoxic HIF-1α stabilization in astrocytes and COX-2 during CCM.
(A) Schematic diagram of astrocytes cocultured with Pdcd10ECKO and Pdcd10fl/fl BMECs. (B) Immunofluorescence staining for HIF-1α (green), GFAP (red), and DAPI for nuclear DNA (white) of primary astrocytes cocultured with Pdcd10ECKO BMECs compared with Pdcd10fl/fl BMEC controls for 48 hours (n = 3). (C) Quantification of HIF-1α in cerebellar tissue in P10 Pdcd10ECKO control Pdcd10fl/fl littermates, as assessed by Western blot (SEM, n = 4 mice in each group). (D) Analysis of HIF-1α target genes by RT-qPCR in cerebellar tissue from P10 Pdcd10ECKO and control Pdcd10fl/fl littermates (SEM, n = 5 or 7 mice in each group). (E) Analysis of HIF-1α target genes by RT-qPCR in human CCM lesions and control human brain tissue (SEM, n = 5). Data are mean ± SEM. *P < 0.05, as determined by Student’s t test. Scale bar: 50 μm (B).
Figure 5. COX-2 inhibition prevents CCM lesions in Pdcd10BECKO mice.
(A) Prominent lesions are present in the cerebellum and cerebrum of P13 Pdcd10BECKO mice. Intragastric administration of 40 mg/kg celecoxib for 4 consecutive days at P6 to P9 suppressed lesion formation. Quantification of lesion volumes by micro-CT analysis from mice at P13 treated with celecoxib or vehicle (SEM, n = 7 mice in each group). (B) Hematoxylin and eosin (pink and purple) or GFAP (red) staining of cerebral (hippocampal area) and cerebellar sections from Pdcd10BECKO mice after treatment with celecoxib or vehicle (n = 3). (C) Prominent lesions are present in the cerebellum and cerebrum of P80 Pdcd10BECKO mice. Oral gavage administration of 40 mg/kg celecoxib for 15 consecutive days at P55 to P70 suppressed lesion formation. Quantification of lesion volumes by micro-CT analysis from mice at P80 treated with celecoxib or vehicle (SEM, n = 12 or 14 mice in each group). (D) Hematoxylin and eosin (pink and purple) or GFAP (red) staining of cerebral (hippocampal area) and cerebellar sections from Pdcd10BECKO mice after treatment with celecoxib or vehicle (n = 3). (E and F) Quantification of Vegfa (E) or Nos3 (F) mRNA levels in P80 Pdcd10BECKO spinal cords after treatment with celecoxib or vehicle from experiments in (C) (SEM, n = 7 or 9 mice in each group). Data are mean ± SEM. *P < 0.05, **P < 0.01, as determined by Student’s t test. Scale bars: 1 mm (H&E), 200 μm (GFAP) (B and D).
Loss of brain endothelial Pdcd10 or Krit1 increases the expression of eNOS.
Previous work has demonstrated that eNOS contributes to VEGF-induced angiogenesis and vascular permeability (44) potentially through stabilization of HIF1-α by NO. Genetic inactivation of Pdcd10 (Pdcd10ECKO) or Krit1 (Krit1ECKO) results in increased Nos3 mRNA levels in BMECs (6, 64). When we cocultured Pdcd10ECKO endothelial cells with astrocytes we observed a dramatic upregulation of Nos3 mRNA (~19.2 fold increase) associated with increased eNOS protein expression (~8.9 fold increase) in Pdcd10ECKO BMECs when compared with Pdcd10fl/fl BMEC controls as assessed by Western blot analysis (Supplemental Figure 7). Similar results were observed in Krit1ECKO BMECs: an approximately 7.4 fold increase Nos3 mRNA was associated with increased eNOS protein expression (~1.9 fold increase) (Supplemental Figure 7). These results indicate that there is a significant increase in eNOS mRNA and protein upon genetic inactivation of Pdcd10 or Krit1 in BMECs.
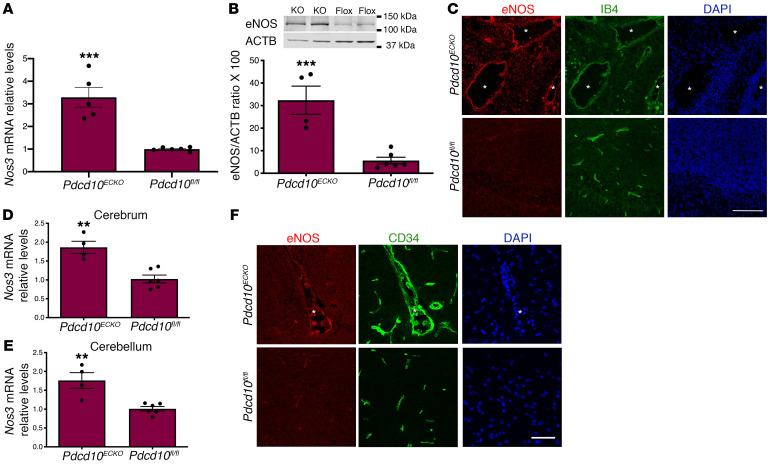
To investigate the distribution and expression of eNOS during CCMs in vivo, we first used neonatal Pdcd10ECKO mice. Consistent with results observed in vitro, Pdcd10ECKO hindbrains showed an approximately 3.3 fold increase in Nos3 mRNA levels compared with littermate Pdcd10fl/fl controls (Figure 6A). Furthermore, Western blot analysis showed an approximately 5.8 fold increase in eNOS protein expression in Pdcd10ECKO hindbrains relative to Pdcd10fl/fl hindbrains (Figure 6B). Immunohistochemistry analysis of hindbrain sections revealed that eNOS was upregulated in CCM lesions. As shown in Figure 6C, we observed that eNOS staining was increased in Pdcd10ECKO dilated vasculature as showed by colocalization of antibodies specific against eNOS and isolectin B4-FITC staining, which specifically labels the brain vasculature (Figure 6C). Moreover, analysis of the distribution and expression of eNOS in a juvenile CCM animal model indicated that Nos3 mRNA levels were elevated in brain tissue in Pdcd10ECKO mice (Figure 6, D and E). We also observed robust and focal upregulation of eNOS protein in the lesions of Pdcd10ECKO mice (Figure 6F). Together, these results demonstrate that eNOS mRNA and protein expression is increased in CCM lesions.
Figure 6. Loss of brain endothelial Pdcd10 increases the expression of eNOS in situ.
(A) Analysis of Nos3 mRNA levels by RT-qPCR in hindbrains of P10 Pdcd10ECKO and littermate Pdcd10fl/fl controls (SEM, n = 4 or 5 mice in each group). (B) Analysis of eNOS levels in hindbrains of P10 Pdcd10ECKO and littermate Pdcd10fl/fl controls, as assessed by Western blot analysis (SEM, n = 4 or 5 mice in each group). (C) Confocal microscopy of cerebellar cortex P10 stained for eNOS (red), endothelial marker isolectin B4 (green), and DAPI for nuclear DNA (blue). Asterisks indicate vascular lumen of CCM lesions (n = 4 mice in each group). (D and E) Analysis of Nos3 mRNA levels by RT-qPCR in brains of P30 Pdcd10ECKO and littermate Pdcd10fl/fl controls. (F) Immunofluorescence staining of eNOS (red), endothelial marker cd34 (green), and DAPI for nuclear DNA (blue) (n = 4 or 6 mice in each group). Data are mean ± SEM. **P < 0.01, ***P < 0.001, as determined by Student’s t test. Scale bars: 100 μm (C); 50 μm (F).
Increased eNOS expression in human CCMs is regulated by KLF2 and KLF4.
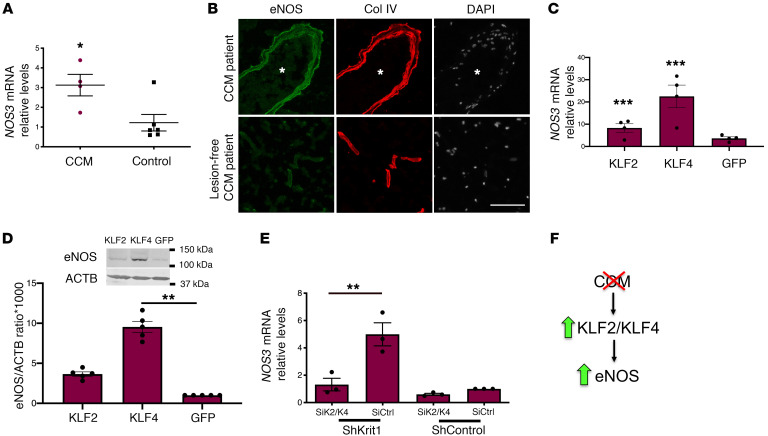
In human CCM brain tissue, there was an approximately 3 fold increase in NOS3 mRNA levels in comparison to lesion-free brain tissue (Figure 7A). We also observed that eNOS staining was increased in human CCM endothelium in comparison with lesion-free brain tissue (Figure 7B). Transcription factors KLF2 and KLF4 are regulators of eNOS (65, 66). Therefore, we confirmed that the changes in brain endothelial eNOS, at the protein and mRNA levels, were associated with an increase in transcription factors KLF2 and KLF4 (Figure 7, C and D). Moreover, we observed that reducing the expression of KLF2 and KLF4 (40) prevented increased eNOS expression in KRIT1-depleted human brain endothelial cells (Figure 7E). These results suggest that the increase in KLF2 and KLF4 increased brain endothelial eNOS in CCMs (Figure 7F).
Figure 7. KLF2 and KLF4 regulate increased eNOS expression during CCM.
(A) Expression levels of NOS3 mRNA as assessed by RT-qPCR from human CCM lesions and compared with nonneurological disease controls (SEM, n = 4 or 6 in each group). (B) Immunofluorescence staining of eNOS (green) and collagen IV (Col IV; red) of human CCM lesion matched to CCM lesion-free brain tissue (n = 3). Asterisks denotate vascular lumen of CCM lesion. Nuclei were counterstained with DAPI (white). (C) Human umbilical vein endothelial cells (HUVECs) were transduced with lentivirus encoding KLF2 or KLF4, as previously reported (6), and analysis of NOS3 mRNA levels by RT-qPCR was determined in cells overexpressing KLF2 or KLF4 and compared with lentivirus encoding GFP as a control (n = 3 or 4). (D) Analysis of eNOS protein levels in HUVECs transduced with lentivirus encoding KLF2 or KLF4, as determined by Western blot analysis (40); lentivirus encoding GFP was used as a control (SEM, n = 3). (E) Analysis of NOS3 mRNA levels by RT-qPCR in hCMEC/D3 cells transduced with lentivirus encoding shKRIT1 or scrambled control, followed by transfection with KLF2- and KLF4-specific small interfering RNAs (siRNA; siK2/K4) or small interfering RNA control (siCtrl) (SEM, n = 4). (F) Schematic model. KLF2- and KLF4-mediated elevation of eNOS in CCM endothelium by a cell-autonomous mechanism. Data are mean ± SEM. **P < 0.01, ***P < 0.001, as determined by Student’s t test and 1-way ANOVA, followed by the Tukey post hoc test. Scale bar: 100 μm (B).
Loss of brain endothelial Pdcd10 increases NO production via eNOS.
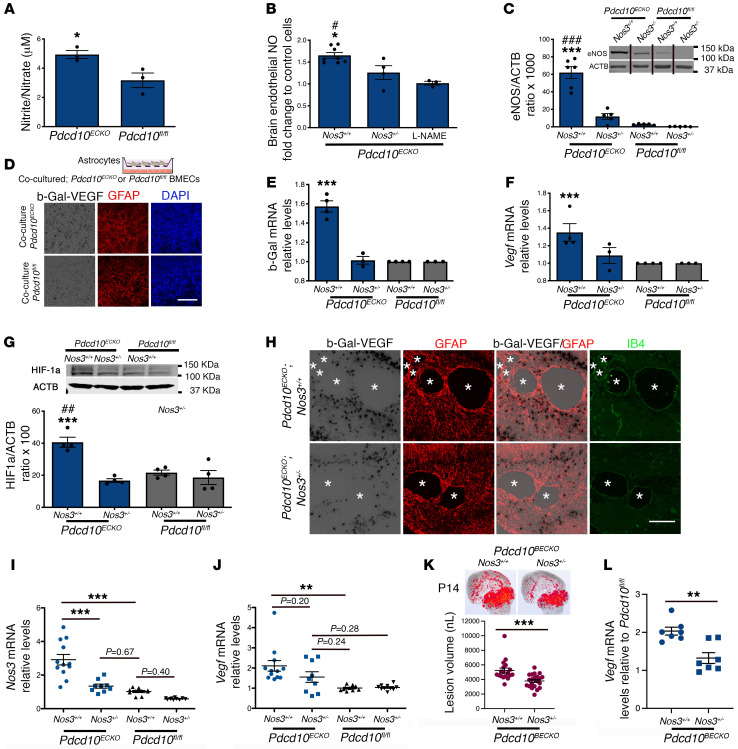
Endothelial cells metabolize l-arginine via eNOS to produce NO, therefore we next investigated if eNOS upregulation increases NO production in Pdcd10ECKO BMECs. Using a colorimetric assay to quantitatively measure total NO2 and NO3 (nitrites and nitrates) in the culture media, we observed that Pdcd10ECKO BMECs possessed an approximately 1.7 fold increase in NO release when compared with Pdcd10fl/fl BMEC controls (NO basal levels: ~3 μM) (Figure 8A). The increase in NO release is ascribable in part to the upregulation of eNOS, because genetic inactivation in one copy of the Nos3 gene (Nos3+/–) significantly reduced NO production in Pdcd10ECKO BMECs (Figure 8B). Consistent with these results, low levels of Nos3 mRNA (data not shown) and eNOS protein in Pdcd10ECKO Nos3+/– BMECs were observed (Figure 8C). Moreover, addition of a NO synthase (NOS) inhibitor, L-NAME, reduced NO production (Figure 8B) without changing eNOS mRNA and protein levels (data not shown). Taken together, these studies show that the loss of Pdcd10 in BMECs leads to an increase in eNOS expression and subsequent endothelial NO release.
Figure 8. Loss of brain endothelial Pdcd10 increases NO production and induces astrocyte-derived VEGF.
(A) Total NO production from the media of Pdcd10ECKO and Pdcd10fl/fl BMECs cultured for 36 hours (SEM, n = 7). (B) NO release in Pdcd10ECKO and Pdcd10ECKO Nos3+/– BMECs or following incubation with L-NAME (150 μM) (SEM, n = 3). (C) Quantification of eNOS protein from Pdcd10ECKO and Pdcd10ECKO Nos3+/– BMECs (SEM, n = 5). Culture media was supplemented with 500 uM l-arginine and was deficient in serum. Lanes in this panel were run on the same gel but were noncontiguous. (D) β-gal/VEGF expression (black) and staining for GFAP (red) of primary cultured astrocytes cocultured with Pdcd10ECKO BMECs compared with Pdcd10fl/fl BMEC controls for 48 hours (n = 2). (E) RT-qPCR analysis of β-gal and (F) Vegfa mRNA in primary cultured astrocytes cocultured with Pdcd10ECKO BMEC compared with Pdcd10fl/fl BMEC controls (SEM, n = 4). (G) Quantification of HIF-1α protein from primary astrocytes cocultured with Pdcd10ECKO BMEC and Pdcd10ECKO Nos3+/– BMECs (SEM, n = 4). (H) Neonatal hindbrain at P10 from Pdcd10ECKO Nos3+/+ Vegfatm1.1Nagy (Pdcd10ECKO eNOS+/+) and Pdcd10fl/fl Nos3+/– Vegfatm1.1Nagy (Pdcd10ECKO eNOS+/–) littermate controls stained for GFAP-positive astrocytes (red), β-gal/VEGF (black), isolectin B4 (green). Asterisks indicate vascular lumen of CCM lesions (n = 3). (I and J) Quantification of Nos3 (I) and Vegf (J) mRNA levels in P10 Pdcd10ECKO Nos3+/– and Pdcd10ECKO and Pdcd10fl/fl Nos3+/– hindbrains when compared with littermate Pdcd10fl/fl controls, as assessed by RT-qPCR (SEM, n = 9 or 12 mice in each group). (K) Micro-CT analysis from mice at P14 Pdcd10BECKO Nos3+/+ and Pdcd10BECKO Nos3+/– mice (SEM, n =18 or 21 mice in each group). (L) Vegf mRNA levels in P14 Pdcd10BECKO Nos3+/– and Pdcd10BECKO Nos3+/+ cerebral tissue (SEM, n = 7 mice in each group, except for Pdcd10fl/fl Nos3+/+ n = 2). Data are mean ± SEM. *, #P < 0.05, ##P < 0.01, ***, ###P < 0.001 (*comparison to Pdcd10ECKO Nos3+/– and #comparison to L-NAME or Pdcd10fl/fl eNOs+/+), as determined by Student’s t test and 1-way ANOVA, followed by the Tukey post hoc test. Scale bars: 100 μm (D and H).
Brain endothelial NO induces astrocyte-derived VEGF following loss of Pdcd10.
We showed evidence of increased levels in VEGF expression in GFAP+ brain and retinal astrocytes in Pdcd10ECKO mice (Figure 2, A, C, D; Figure 3, A and B; and Supplemental Figures 2 and 3). However, our in vivo experiments did not unequivocally exclude expression of VEGF in other CNS-resident cells during CCMs, including neurons, microglia, and pericytes. Thus, we complemented those studies by performing coculture experiments between CCM endothelium and astrocytes. First, NO donors, such as DETA-NONOate, promote brain angiogenesis by upregulation of VEGF signaling (46). To investigate whether NO stimulates the expression of VEGF in astrocytes during CCMs, we prepared primary mouse astrocyte cultures from Vegfatm1.1Nagy mice (Figure 8D). We observed that the purity of cultured astrocytes was high, as determined by cells that are double positive for specific astrocyte markers GFAP and integrin B5 (Supplemental Figure 4). We also observed that our primary astrocyte cultures respond to exogenous NO (0.5 mM DETA-NONOate [NO donor]) and that elevated levels of NO can increase astrocyte-derived VEGF (as assessed by increase in β-gal/VEGF expression) (Supplemental Figure 8). We next investigated whether elevated brain endothelial NO in Pdcd10ECKO results in increased astrocyte-derived VEGF levels. Therefore, we cocultured purified astrocytes in the presence of Pdcd10ECKO or Pdcd10fl/fl BMECs in serum-free conditions supplemented with l-arginine, and measured changes in β-gal/VEGF expression in astrocytes. We observed that Pdcd10ECKO BMECs significantly increase β-gal/VEGF expression in astrocytes in a coculture system (Figure 8, D and E). These results were consistent with increased levels of β-gal and Vegfa mRNA levels in astrocytes cocultured with Pdcd10ECKO BMECs compared with those cocultured with Pdcd10fl/fl BMEC controls (Figure 8, E and F). Furthermore, genetic inactivation of 1 copy of the Nos3 gene in Pdcd10ECKO BMECs was sufficient to prevent stability of HIF-1α and subsequent Vegfa/β-gal upregulation in astrocytes, indicating that the increase in astrocyte-derived Vegfa/β-gal mRNA levels was specific to the upregulation of eNOS (Figure 8, E and F) and dependent on HIF-1α stability (Figure 8G).
We next investigated whether the increase in astrocyte-derived VEGF during CCMs was associated with elevation of eNOS in mice. Vegfatm1.1Nagy mice were intercrossed with CCM animal models and with animals deficient in 1 copy of the Nos3 gene (eNOS+/–). We observed by histology that CCM lesions were slightly reduced and with a partial decrease in β-gal/VEGF expression in P10 Pdcd10ECKO Vegfatm1.1Nagy Nos3+/– hindbrains when compared with Pdcd10ECKO Vegfatm1.1Nagy Nos3+/+ littermate controls (Figure 8H). We confirm that the increased levels of Nos3 mRNA were significantly prevented in Pdcd10ECKO Nos3+/– hindbrains when compared with Pdcd10ECKO Nos3+/+ littermate (Figure 8I). However, we also noted that levels of Nos3 mRNA tend to be higher in Pdcd10ECKO Nos3+/– hindbrains when compared with basal levels in Pdcd10fl/fl Nos3+/+ littermate controls (Figure 8I). Consistent with results observed by histological analysis, Pdcd10ECKO Nos3+/– hindbrains showed a trend to reduced levels of Vegf mRNA and lesion genesis when compared with Pdcd10ECKO Nos3+/+ littermate (Figure 8J and Supplemental Figure 9). Notably, we observed a trend in the increase of Vegf mRNA levels in the cerebellar tissue from Pdcd10ECKO Nos3+/– compared with basal levels in Pdcd10fl/fl Nos3+/+ or Pdcd10fl/fl Nos3+/– littermate controls (Figure 8J). We next assessed CCM lesions using brain endothelial-specific inactivation (BECKO, Slco1c1-iCreERT2 line) of the Pdcd10 gene, where lesions initially form in the cerebellum (P6–P7) but progressively emerge in the cerebrum of P13 Pdcd10BECKO mice (Figure 5A). Blinded measurements using microCT analysis from P14 Pdcd10BECKO Nos3+/– whole brains and compared with brains in P14 Pdcd10BECKO Nos33+/+ mice indicate a significant reduction in brain vascular lesions volume (Figure 8K and Supplemental Figure 9). Moreover, we also observed that genetic inactivation of 1 copy of the Nos3 gene in Pdcd10BECKO mice (Pdcd10BECKO Nos3+/–) was sufficient to prevent Vegfa mRNA upregulation in cerebrum (front of brain) that correlates with a significant decrease in cerebral lesions (Figure 8, K and L). These results indicate that eNOS/NO signaling contributes to the elevation in VEGF expression and lesion genesis in Pdcd10ECKO hindbrains (Figure 8, I and J) and Pdcd10BECKO forebrains (Figure 8, K and L).
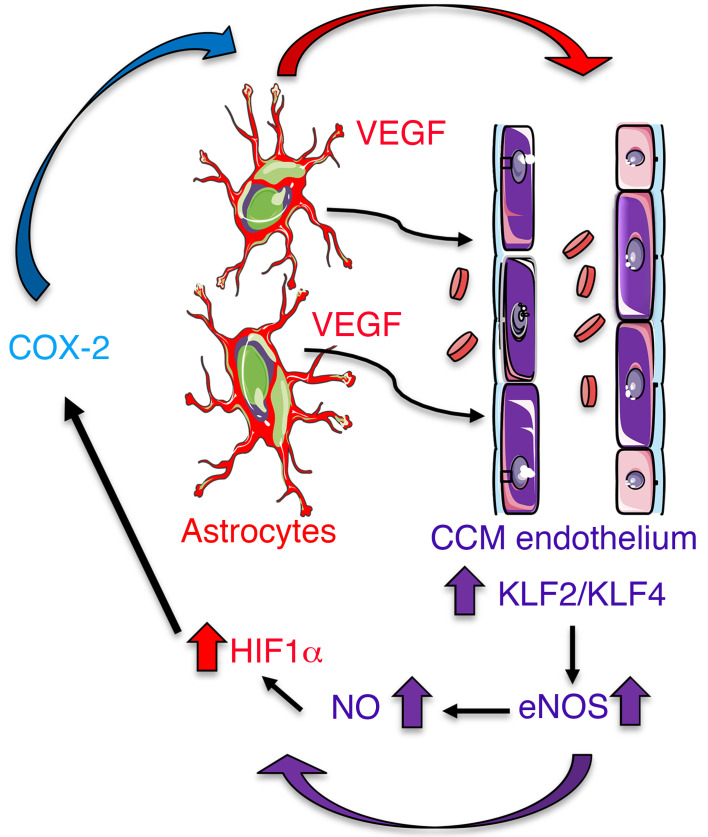
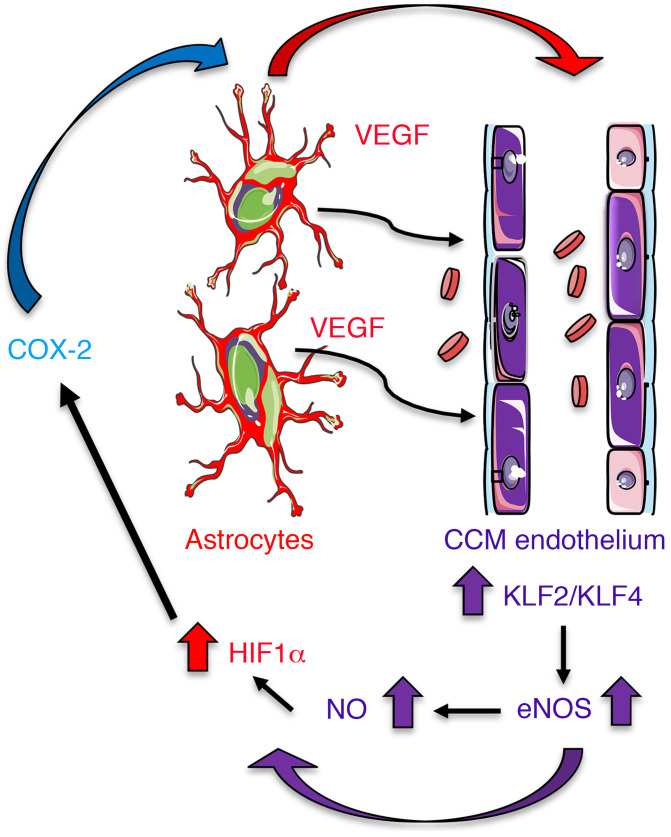
Altogether our data provide key insights into understanding a circuit of neurovascular dysfunction during CCM disease triggered by astrocyte-endothelial crosstalk (Figure 9). These data provide strong evidence that the sustained NO production as a consequence of the elevation of eNOS in CCM endothelium, through elevation of KLF2/KLF4, contributes to the normoxic stability of HIF-1α and subsequent elevation in astrocyte-derived VEGF. Therefore, astrocytes play a critical role in CCM pathogenesis by expressing hypoxia-related genes. This work establishes the principle that reciprocal communication between CCM endothelium and astrocytes drives lesion development. Our findings further support that the hypoxic program components, such as COX-2, represent potential therapeutic targets for CCM disease (Figure 9).
Figure 9. Astrocytes integrate a circuit of neurovascular dysfunction during CCM disease.
Model by which astrocytes make a substantial contribution to CCM pathogenesis. An increase in astrocyte VEGF synthesis is driven by endothelial NO generated due to KLF2- and KLF4-dependent elevation of eNOS in CCM endothelium. Production of NO in CCM endothelium stabilizes HIF-1α in astrocytes, resulting in increased VEGF production and expression of a hypoxic program under normoxic conditions. Pharmacological inhibition of HIF1-driven COX-2 can ameliorate murine models of CCM disease.
Discussion
The propensity of CCM lesions to form in the CNS parenchyma has never been mechanistically clarified. Our study shows that astrocytes respond to CCM endothelium to initiate a non–cell-autonomous enhancement of CCM formation mediated by astrocyte hypoxia and angiogenesis programs. Our experiments show that elevation of eNOS in CCM endothelium contributes to increase in astroglia-derived VEGF and HIF-1α protein stabilization in astrocytes during CCM disease. The increase in eNOS expression was ascribable to an elevation of the KLF2 and KLF4 transcription factors in CCM. eNOS production in the CCM endothelium results in a sustained elevation of NO that leads to normoxic HIF-1α protein stabilization and increase in HIF activity in CCM tissue. We therefore propose that astrocytes contribute to CCM disease by a non–cell-autonomous mechanism mediated by CCM endothelium-driven hypoxia and angiogenic programs.
Our in vivo observations in the neonatal CCM animal models, using endothelial-specific inactivation of Pdcd10 or Krit1, revealed that lesion development is spatially restricted to zones of fibrous astrocytes, which are prevalent in the white matter of the CNS (49), and that the selective ablation of proliferative astrocytes (51) markedly reduced CCM lesion and an increase in VEGF expression, prompting the suggestion that a subset of proliferative astrocytes supports CCM lesions in the developing hindbrain. Moreover, our observations using brain endothelial-specific inactivation of Pdcd10 (67), further revealed the high propensity of CCM lesions to develop surrounded by GFAP+ astrocytes throughout the CNS, including the cerebellum, cerebrum, and spinal cord. Earlier reports have shown that loss of Pdcd10 in neuroglia cells leads to formation of CCM lesions through non–cell-autonomous mechanisms, implicating a role of neural cells in the CCM pathogenesis (20). Consistent with these findings, astrogliosis has been shown to intermingle with granulation tissue in human CCM (68). However, while some subsets of reactive and proliferative astrocytes can be detrimental and contribute to CNS pathologies while other subsets of reactive astrocytes can be beneficial by supporting CNS recovery (27, 51, 69, 70). The mechanisms that contribute to functional heterogeneity in reactive astrocytes are not completely understood. Recent studies have shown that microglia control astrocyte pathogenic activities during neuroinflammation by releasing of VEGF-β and TGF-α (71), and during neurodegeneration by secreting of IL-1α, TNF and C1q (27). Our findings revealed that the CCM endothelium controls astrocyte responses through increasing the levels of eNOS and subsequent release of brain endothelial NO. Vascular eNOS has important roles in regulating endothelial homeostasis by producing tonic NO levels, but upregulation of the eNOS gene or activated form has been previously implicated in vascular permeability, angiogenesis, and neuroinflammation (29, 45, 46, 72). Here we show that eNOS is increased in human CCM endothelium and the upregulation in endothelial KLF2 and KLF4 can account for the augmented eNOS expression at the transcriptional level (66). However, these studies do not exclude the possibility that the elevation of endothelial VEGF signaling during CCM (6) may also contribute to a sustained eNOS activation (44, 73) by posttranslational modification via PI3K/AKT, Ca2+/calmodulin signaling (74) during long-term adaptations, and this notion needs to be further tested.
CCMs are hypersensitive to angiogenesis due to increase in VEGF signaling, the loss of an antiangiogenic checkpoint protein, TSP1 (6, 41), augmented secretion of angiopoietin-2 (11), and deregulation of Notch signaling (75). The increase in VEGF signaling may be associated with elevated levels of angiogenic factor VEGF in CCM lesions of individuals with the hereditary and sporadic form of the disease (32–35). We found that CCM endothelium-induced elevation of astrocyte-derived VEGF in neonatal, juvenile brains and adult spinal cords, and elevation of VEGF levels are dependent on eNOS signaling. Notably, an increase in astrocyte-derived VEGF can contribute to the development and progression of CNS disease (29–31) by disassembly of interendothelial junctions (6, 76, 77). Importantly, early studies show that eNOS contributes to VEGF-induced angiogenesis through the intercellular messenger NO (44–46, 78). Furthermore, it has also been shown that NO-mediated astrocyte HIF-1α stabilization modulates transcriptional and metabolic activities (78, 79). In agreement with this, we observed elevated levels of eNOS protein and NO bioavailability in Pdcd10ECKO BMECs that increased levels of HIF-1α and VEGF in cocultured astrocytes. Moreover, loss of 1 copy of the gene Nos3 in Pdcd10ECKO BMECs is sufficient to attenuate astrocyte HIF-1α stabilization and VEGF expression in the coculture system. In addition, using animal models of CCM disease we also observed that loss of 1 copy of Nos3 gene significantly reduces vascular lesions volume that was associated with decrease in levels of Nos3 and Vegf mRNA. Paradoxically, mice with loss of 2 copies of Nos3 gene (Pdcd10ECKO Nos3–/– mice) were not protected from CCM and instead showed early lethality (data not shown). The latter findings are consistent with previous reports in which a tonic amount of endothelial NO is responsible for the homeostasis between endothelium and surrounding parenchyma (72). Indeed, endothelial dysfunction in Nos3–/– mice is well-documented in disease models (80). On the Nos3–/– background, mice show an increase in leukocyte-endothelial interaction, platelet aggregation, thrombosis, gliosis, and vascular permeability, among other changes in these disease models (81–84). In contrast, the endothelial dysfunction in those models is not observed in the Nos3+/– mice. Thus, we ascribe the lack of protection in Nos3–/– mice to the resulting endothelial dysfunction on the Pdcd10ECKO background.
Importantly, although we found that astrocytes respond to CCM endothelium, other cells of the neurovascular unit (e.g., neurons, pericytes, and microglia) may also contribute to CCM disease in a non–cell-autonomous manner through normoxic stabilization of HIF-1α protein. It is tempting to speculate that CCM endothelium may harmonize cell activation in the neurovascular unit by elevation of NO during CCM disease. Because NO can reach cells millimeters away from where it is produced, this in turn can stabilize HIF-1α protein (85, 86) from degradation by several mechanisms directly by HIF-1α nitrosylation or also indirectly by increasing production of reactive oxidative species or by binding to the iron cofactor required for prolyl hydroxylases activity (87). HIF-1α can translocate to the nucleus where it dimerizes with HIF-1β and binds to the hypoxia-responsive element in the promoter region of several target genes (63). Our data indicate that in mouse and human CCM tissue, the HIF-1α program associated with the glycolytic pathway is activated by an increase in GLUT-1, which guarantees rapid energy production, and there is an increase in MCT4 that promotes efficient removal of lactic acid, an end product of glycolysis metabolism (63). Elevated HIF activity in CCM tissue was also associated with increases in several proangiogenic and inflammatory factors produced by glial cells, including ANGPTL4, which can induce vascular dysfunction in CNS pathologies by triggering angiogenesis (88, 89), and COX-2, an enzyme that catalyzes the biosynthesis of prostanoids during inflammation (62). We focused on studying COX-2 inhibition during CCM because it has been shown that COX-2 inhibition can suppress angiogenesis in vitro and in vivo (90, 91). Moreover, selective COX-2 inhibitors are safe and well-tolerated drugs that can be repurposed for therapy for CCM disease. Our results show that pharmacological inhibition of COX-2 significantly reduced vascular lesion formation in 2 models of CCM disease. Importantly, we noticed that the inhibition of COX-2 in the chronic CCM animal model results not only in a decrease in the density of CCM lesions but also in GFAP-immunoreactivity, suggesting that amelioration of lesion genesis counterparts astrocyte activity in the lesions. In line with these results, a recent retrospective cohort study reported that the use of NSAIDs was correlated with lower risk of hemorrhage among patients affected by CCMs (92). However, this uncontrolled association cannot be interpreted as proof of therapeutic benefit, as patients with recent CCM hemorrhages were inherently less likely to be taking NSAIDs. Cox-2 inhibitors must be investigated for safety and effectiveness in prospective controlled trials, and specific dose-effect and duration of treatment must be carefully defined. A platform for trial readiness, exploring proof-of-concept effect on lesional bleeding in human subjects, is currently being developed, and can efficiently be applied to repurposed drugs, such as NSAIDs (93). The precise role of NSAIDs in reducing CCM risk of hemorrhage version lesion formation remains incompletely understood. The COX-2/prostaglandin pathway has been implicated in vascular sprouting, migration, and induction of growth factors and proteolytic enzymes that alter angiogenesis (90, 91, 94, 95), suggesting that COX-2 could be an important therapeutic target for pathological angiogenesis (21, 90). Moreover, inhibition of COX-2–augmented VEGF pathway blockade during refractory tumor angiogenesis (96) suggests that combinatorial COX-2/VEGF pathway inhibition could be used as a potential approach to ameliorate or prevent CCMs. In addition, Sulindac metabolites, an NSAID, has been shown to inhibit b-catenin–stimulated transcription of endothelial-mesenchymal transition markers and development of CCMs (97). However, additional studies will be required to determine whether COX-2 inhibitors prevent an increase in astroglia-derived VEGF in response to CCM endothelium. Collectively, our data are consistent with the notion that reciprocal communication between CCM endothelium and astrocytes drives CCM lesion formation and contributes to neurovascular dysfunction. These observations point to the possibility of designing therapeutic approaches aimed at preventing endothelial dysfunction and astroglia activation as an intervention to reduce the burden of CCM disease in humans.
Methods
Human tissue and genetically modified mice.
Human brain samples of patients with CCM and controls without neurological disease were obtained from the Angioma Alliance Tissue Bank. Endothelial-specific conditional Pdcd10-null mice were generated by crossing a Pdgfb promoter-driven tamoxifen-regulated Cre recombinase (iCreERT2) (98) with loxP-flanked Pdcd10 (Pdcd10fl/fl, a gift from Wang Min, Yale University; Pdgfb-iCreERT2 Pdcd10fl/fl) mice. Brain endothelial-specific conditional Pdcd10-null mice were generated by crossing a Slco1c1 promoter-driven tamoxifen-regulated Cre recombinase (67) (iCreERT2, a gift from Markus Schwaninger, University of Lübeck) with Pdcd10fl/fl mice. eNOS-deficient mice (Nos3–/–) were obtained from Jackson Laboratory and crossed with Pdgfb-iCreERT2 Pdcd10fl/fl mice to generate Pdgfb-iCreERT2 Pdcd10fl/f Nos3–/– and Pdgfb-iCreERT2 Pdcd10fl/f Nos+/– mice. On postnatal day 3, mice were administered 50 μg of 4-hydroxi-tamoxifen (H7904, Sigma-Aldrich) by intragastric injection to induce genetic inactivation of the endothelial Pdcd10 gene in littermates with Pdgfb-iCreERT2 (Pdcd10ECKO), and Pdcd10fl/fl mice were used as littermate controls. For CCM in juvenile animals, on postnatal day 6, mice were administered 100 μg 4-hydroxi-tamoxifen by intragastric injection to induce genetic inactivation of the endothelial Pdcd10 gene in littermates with Pdgfb-iCreERT2 (Pdcd10ECKO), and Pdcd10fl/fl mice were used as littermate controls. On postnatal day 1, mice were administered 50 μg tamoxifen by intragastric injection to induce genetic inactivation of endothelial Pdcd10 gene in littermates with Slco1c1-iCreERT2 (Pdcd10BECKO), and Pdcd10fl/fl mice were used as littermate controls. Vegfatm1.1Nagy mice, expressing a B-galactosidase (LacZ) reporter gene inserted into the 3′ untranslated region of the Vegfa gene (53), were obtained from the Jackson Laboratory. The GFAP-TK mice line was used to selectively ablate proliferative astrocytes (glial fibrillary acidic protein-thymidine kinase, a gift from Michael V. Sofroniew, University of California, Los Angeles) (50, 51).
For celecoxib treatment, brain endothelial-specific conditional Pdcd10-null mice were randomized (by flipping a coin) to celecoxib (40 mg/kg, celebrex) or vehicle treatment (0.5% methylcellulose plus 0.025% Tween20). For neonatal experiments, 100 μL celecoxib or vehicle was administered by intragastric injection on P6, P7, P8, and P9, and animals were sacrifice at P13. For adult experiments, male and female brain endothelial-specific conditional Pdcd10-null mice were randomized (by flipping a coin) to celecoxib (40 mg/kg, Celebrex) or vehicle treatment (0.5% methylcellulose plus 0.025% tween20). Celecoxib or vehicle was administered by oral gavage administration for 15 consecutive days (P55–P70) and animals were sacrificed at P80.
Western blot analysis.
BMECs were lysed using a solution containing 1 mM sodium orthovanadate, protease inhibitor cocktail (11836170001, Roche), and 100 μL radioimmunoprecipitation assay buffer (Thermo Fisher Scientific). A Micro BCA protein assay kit (500-0116, Thermo Fisher Scientific) was used to determine the protein concentration and 25 μg cell lysates was heated for 5 minutes at 95°C to denature all proteins. The cell lysates were subjected to a 4% to 20% gradient SDS-PAGE (XP04200, Thermo Fisher Scientific) and a wet transfer was used to transfer proteins to nitrocellulose membranes (Amersham). Membranes were blocked for 1 hour at room temperature using a blocking solution (TBS 1×, 10% nonfat milk), and polyclonal rabbit antibodies directed against eNOS (1:500; PA1-037, Thermo Fisher Scientific), HIF-1α (1:150; NB100-134, Novus Biologicals) or monoclonal rabbit (1:100; 12282, Cell Signaling) and polyclonal goat (1:100; PA1-9032, Thermo Fisher Scientific) antibodies directed against COX-2 were incubated at 4°C overnight. Several washes were performed and then the membranes were incubated with appropriate IRDye/Alexa Fluor–coupled secondary antibodies (1:10,000, 926-68070; 926-32211; Li-COR) for 1 hour at room temperature. A mouse monoclonal antibody against beta-actin (1:5000; A5441, Sigma-Aldrich) was used as a control for protein loading. Membranes were imaged and analyzed using Odyssey CLx Infrared Imaging (Li-COR).
RT2-qPCR analysis.
Total RNA (10 ng) was used to produce single-stranded complementary DNA (cDNA) using random primers according to the manufacturer’s protocol (48190011; 18080093; Thermo Fisher Scientific). Briefly, 10 ng RNA was added to a master mix containing 1X First-Strand buffer, 1 μL 0.1M DTT, 1 mM dNTPs, 1 μL RNaseOUT recombinant RNase inhibitor, SuperScript III reverse transcriptase, and 0.5 μg random primers. The mixture was placed in a thermal cycler (C1000 Touch, Bio-Rad) at 25°C for 10 minutes, and then incubated at 50°C for 1 hour followed by inactivation of the reaction by heating at 70°C for 15 minutes. cDNA (300 ng) was run with the Kapa SybrFast qPCR mix (Kapa Biosystems) using 50 μM of primers to distinguish between the relative levels of genes in each condition. The PCR reaction was placed in a thermal cycler (C1000 Touch, Bio-Rad) using an initial step at 95°C for 15 minutes, followed by 40 cycles (30 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C). Analysis of the data was performed using the 2–ΔCT method.
NO determination.
Pdcd10ECKO and Pdcd10fl/fl BMECs were maintained in BMEC media supplemented with 500 μM l-arginine and deficient in fetal bovine serum and ascorbic acid for 36 hours. Then, the BMEC media were collected, and a colorimetric total NO assay was performed as specified by manufacturer’s protocol (KGE001, R&D Systems). A quantity of 50 μL undiluted BMEC media samples and nitrate (NO3) standards ranging 100 μM to 1.565 μM were plated on clear polystyrene microplates (DY990, R&D Systems). To measure total nitrites (NO2) and nitrates, 25 μL nitrate reductase and 25 μL NADH were added to each well to convert all available nitrates to nitrite. The plate was covered with an adhesive strip and incubated at 37°C for 30 minutes. After incubation, 50 μL Griess reagent I and Griess reagent II were added to all wells and incubated at room temperature for 10 minutes. A 2-step diazotization reaction occurs in which NO2– reacts with sufanilic acid to produce a diazonium ion, which is then coupled to N-(1-naphthyl) ethylenediamine to form an azo-derivative that absorbs light at 540 nm. The optical density (OD) of each well was read using a microplate reader (Infinite 200 PRO, Tecan) at 540 nm with a wavelength correction of 690 nm. Duplicate readings were averaged and normalized to the blank. A standard curve was generated for each experiment by plotting optical density against concentration (μM), and total nitrate/nitrite concentrations were determined using the linear trendline.
Statistics.
Statistical analysis for single comparisons was performed using a 2-tailed Student’s t test, and analysis for multiple comparisons was performed using ANOVA followed by Tukey’s post hoc test. The threshold for statistical significance was set at P equal to 0.05.
Study approval.
All animal experiments were performed in compliance with animal procedure protocols approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Author contributions
CCL, SIS, PH, and MALR performed and analyzed histology and gene expression experiments in culture and conditional knockout mice. CCL, SIS, and PH made substantial contributions to manuscript preparation, writing, analysis of the data, and generation of all figures. AP, RV, EJE, and SM performed histology and treatment of conditional knockout mice. RG, TM, RL, NH, RS, and IAA performed, analyzed, and interpreted the microCT studies and edited the manuscript. SM, OP, GGH, and RD contributed to data discussion and manuscript editing. BG, HS, FL, and MHG made substantial contributions to manuscript preparation, writing, and interpretation of data. MALR designed the experiments, prepared the figures, and with MHG performed overall study design to address the conceptual ideas, analysis and interpretation of the data, and writing of the manuscript.
Supplementary Material
Acknowledgments
The authors thank Angioma Alliance for providing human CCM specimens; Chelsea Hyun Ju Choi, Alyssa Castillo, Daniel Han, and Maryum Haidari for technical assistance; Jennifer Santini and Marcy Erb for microscopy technical assistance; and the UCSD School of Medicine Microscopy Core. This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants K01 HL133530, P01HL151433-01 (to MALR), HL139947 (to MHG), and National Institute of Neurological Disorders and Stroke grant P01 NS092521 (to MHG, IAA, RG, RL, NH, RS, and TM). This work was also supported by Be Brave for Life Foundation (to MALR), the Future Faculty of Cardiovascular Sciences (FOCUS) PRIDE program (to MALR), as well as by the UCSD School of Medicine Microscopy Core (P30 NS047101).
Version 1. 05/27/2021
In-Press Preview
Version 2. 07/01/2021
Electronic publication
Funding Statement
K01 HL133530 (M.A.L.-R.), HL139947 (M.H.G.)
P01 NS092521 (M.H.G., I.A.A., R.G., R.L., N.H., T.M.)
M.A.L.-R.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(13):e139570.https://doi.org/10.1172/JCI139570.
Contributor Information
Miguel Alejandro Lopez-Ramirez, Email: malopezramirez@ucsd.edu.
Catherine Chinhchu Lai, Email: clai@ucsd.edu.
Shady Ibrahim Soliman, Email: shady.soliman11@gmail.com.
Preston Hale, Email: prestonhale85@yahoo.com.
Angela Pham, Email: angelanpham@gmail.com.
Esau J. Estrada, Email: esauestrada@outlook.com.
Sara McCurdy, Email: smccurdy@health.ucsd.edu.
Romuald Girard, Email: rgirard@surgery.bsd.uchicago.edu.
Riya Verma, Email: r7verma@ucsd.edu.
Thomas Moore, Email: tmoore10@surgery.bsd.uchicago.edu.
Rhonda Lightle, Email: rlightle@surgery.bsd.uchicago.edu.
Nicholas Hobson, Email: nhobson@surgery.bsd.uchicago.edu.
Robert Shenkar, Email: rshenkar@uchicago.edu.
Orit Poulsen, Email: opoulsen@ucsd.edu.
Gabriel G. Haddad, Email: ghaddad@ucsd.edu.
Richard Daneman, Email: rdaneman@ucsd.edu.
Brendan Gongol, Email: brengong@gmail.com.
Hao Sun, Email: has073@health.ucsd.edu.
Frederic Lagarrigue, Email: frlagarrigue@gmail.com.
Issam A. Awad, Email: iawad@surgery.bsd.uchicago.edu.
Mark H. Ginsberg, Email: mhginsberg@ucsd.edu.
References
- 1.Spiegler S, et al. Cerebral cavernous malformations: an update on prevalence, molecular genetic analyses, and genetic counselling. Mol Syndromol. 2018;9(2):60–69. doi: 10.1159/000486292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer A, et al. Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis. Trends Mol Med. 2013;19(5):302–308. doi: 10.1016/j.molmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc GG, et al. Biology of vascular malformations of the brain. Stroke. 2009;40(12):e694–e702. doi: 10.1161/STROKEAHA.109.563692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gore AV, et al. Combinatorial interaction between CCM pathway genes precipitates hemorrhagic stroke. Dis Model Mech. 2008;1(4–5):275–281. doi: 10.1242/dmm.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choquet H, et al. Genetics of cerebral cavernous malformations: current status and future prospects. J Neurosurg Sci. 2015;59(3):211–220. [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Ramirez MA, et al. Thrombospondin1 (TSP1) replacement prevents cerebral cavernous malformations. J Exp Med. 2017;214(11):3331–3346. doi: 10.1084/jem.20171178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denier C, et al. Genotype-phenotype correlations in cerebral cavernous malformations patients. Ann Neurol. 2006;60(5):550–556. doi: 10.1002/ana.20947. [DOI] [PubMed] [Google Scholar]
- 8.Mikati AG, et al. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab. 2015;35(10):1632–1639. doi: 10.1038/jcbfm.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lant B, et al. Interrogating the ccm-3 gene network. Cell Rep. 2018;24(11):2857–2868. doi: 10.1016/j.celrep.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Boulday G, et al. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. J Exp Med. 2011;208(9):1835–1847. doi: 10.1084/jem.20110571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou HJ, et al. Erratum: endothelial exocytosis of angiopoietin-2 resulting from CCM3 deficiency contributes to cerebral cavernous malformation. Nat Med. 2016;22(12):1502. doi: 10.1038/nm1216-1502c. [DOI] [PubMed] [Google Scholar]
- 12.Chan AC, et al. Mutations in 2 distinct genetic pathways result in cerebral cavernous malformations in mice. J Clin Invest. 2011;121(5):1871–1881. doi: 10.1172/JCI44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, et al. Corrigendum: cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature. 2016;536(7617):488. doi: 10.1038/nature18311. [DOI] [PubMed] [Google Scholar]
- 14.McDonald DA, et al. A novel mouse model of cerebral cavernous malformations based on the two-hit mutation hypothesis recapitulates the human disease. Hum Mol Genet. 2011;20(2):211–222. doi: 10.1093/hmg/ddq433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akers AL, et al. Biallelic somatic and germline mutations in cerebral cavernous malformations (CCMs): evidence for a two-hit mechanism of CCM pathogenesis. Hum Mol Genet. 2009;18(5):919–930. doi: 10.1093/hmg/ddn430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detter MR, et al. Cerebral cavernous malformations develop through clonal expansion of mutant endothelial cells. Circ Res. 2018;123(10):1143–1151. doi: 10.1161/CIRCRESAHA.118.313970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malinverno M, et al. Endothelial cell clonal expansion in the development of cerebral cavernous malformations. Nat Commun. 2019;10(1):2761. doi: 10.1038/s41467-019-10707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman EM, et al. A conserved CCM complex promotes apoptosis non-autonomously by regulating zinc homeostasis. Nat Commun. 2019;10(1):1791. doi: 10.1038/s41467-019-09829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong CC, et al. Cerebral cavernous malformations are driven by ADAMTS5 proteolysis of versican. J Exp Med. 2020;217(10):e20200140. doi: 10.1084/jem.20200140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louvi A, et al. Loss of cerebral cavernous malformation 3 (Ccm3) in neuroglia leads to CCM and vascular pathology. Proc Natl Acad Sci U S A. 2011;108(9):3737–3742. doi: 10.1073/pnas.1012617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finetti F, et al. KRIT1 loss-mediated upregulation of NOX1 in stromal cells promotes paracrine pro-angiogenic responses. Cell Signal. 2020;68:109527. doi: 10.1016/j.cellsig.2020.109527. [DOI] [PubMed] [Google Scholar]
- 22.Choquet H, et al. Cytochrome P450 and matrix metalloproteinase genetic modifiers of disease severity in cerebral cavernous malformation type 1. Free Radic Biol Med. 2016;92:100–109. doi: 10.1016/j.freeradbiomed.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanriover G, et al. PDCD10, the gene mutated in cerebral cavernous malformation 3, is expressed in the neurovascular unit. Neurosurgery. 2008;62(4):930–938. doi: 10.1227/01.neu.0000318179.02912.ca. [DOI] [PubMed] [Google Scholar]
- 24.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss N, et al. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788(4):842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez JI, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 27.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7(4):494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argaw AT, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122(7):2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vangeison G, Rempe DA. The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist. 2009;15(6):579–588. doi: 10.1177/1073858409332405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapouly C, et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain. 2015;138(Pt 6):1548–1567. doi: 10.1093/brain/awv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maiuri F, et al. Clinical progression and familial occurrence of cerebral cavernous angiomas: the role of angiogenic and growth factors. Neurosurg Focus. 2006;21(1):e3. doi: 10.3171/foc.2006.21.1.4. [DOI] [PubMed] [Google Scholar]
- 33.Jung KH, et al. Cerebral cavernous malformations with dynamic and progressive course: correlation study with vascular endothelial growth factor. Arch Neurol. 2003;60(11):1613–1618. doi: 10.1001/archneur.60.11.1613. [DOI] [PubMed] [Google Scholar]
- 34.Abe T, et al. The association between high VEGF levels and multiple probable punctuate cavernous malformations. Acta Neurochir (Wien) 2009;151(7):855–859. doi: 10.1007/s00701-009-0410-6. [DOI] [PubMed] [Google Scholar]
- 35.Girard R, et al. Plasma biomarkers of inflammation and angiogenesis predict cerebral cavernous malformation symptomatic hemorrhage or lesional growth. Circ Res. 2018;122(12):1716–1721. doi: 10.1161/CIRCRESAHA.118.312680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argaw AT, et al. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106(6):1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng SY, et al. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci U S A. 1997;94(22):12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josko J. Cerebral angiogenesis and expression of VEGF after subarachnoid hemorrhage (SAH) in rats. Brain Res. 2003;981(1–2):58–69. doi: 10.1016/s0006-8993(03)02920-2. [DOI] [PubMed] [Google Scholar]
- 39.Awad IA, Polster SP. Cavernous angiomas: deconstructing a neurosurgical disease. J Neurosurg. 2019;131(1):1–13. doi: 10.3171/2019.3.JNS181724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Ramirez MA, et al. Cerebral cavernous malformations form an anticoagulant vascular domain in humans and mice. Blood. 2019;133(3):193–204. doi: 10.1182/blood-2018-06-856062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiStefano PV, et al. KRIT1 protein depletion modifies endothelial cell behavior via increased vascular endothelial growth factor (VEGF) signaling. J Biol Chem. 2014;289(47):33054–33065. doi: 10.1074/jbc.M114.582304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 43.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukumura D, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98(5):2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jozkowicz A, et al. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc Res. 2001;51(4):773–783. doi: 10.1016/S0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhang R, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92(3):308–313. doi: 10.1161/01.RES.0000056757.93432.8C. [DOI] [PubMed] [Google Scholar]
- 47.Cuttano R, et al. KLF4 is a key determinant in the development and progression of cerebral cavernous malformations. EMBO Mol Med. 2016;8(1):6–24. doi: 10.15252/emmm.201505433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renz M, et al. Regulation of β1 integrin-Klf2-mediated angiogenesis by CCM proteins. Dev Cell. 2015;32(2):181–190. doi: 10.1016/j.devcel.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Leto K, et al. Consensus paper: cerebellar development. Cerebellum. 2016;15(6):789–828. doi: 10.1007/s12311-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson MA, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girard R, et al. Micro-computed tomography in murine models of cerebral cavernous malformations as a paradigm for brain disease. J Neurosci Methods. 2016;271:14–24. doi: 10.1016/j.jneumeth.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miquerol L, et al. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212(2):307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- 54.Scott A, et al. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One. 2010;5(7):e11863. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardoso C, et al. Novel chronic mouse model of cerebral cavernous malformations. Stroke. 2020;51(4):1272–1278. doi: 10.1161/STROKEAHA.119.027207. [DOI] [PubMed] [Google Scholar]
- 56.Detter MR, et al. Novel murine models of cerebral cavernous malformations. Angiogenesis. 2020;23(4):651–666. doi: 10.1007/s10456-020-09736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, et al. Propranolol inhibits cavernous vascular malformations by β1 adrenergic receptor antagonism in animal models. J Clin Invest. 2021;131(3):144893. doi: 10.1172/JCI144893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. doi: 10.1101/2020.02.12.944421. Detter MR, et al. Novel hemorrhage models of cerebral cavernous malformations [preprint]. Posted on bioRxiv February 13, 2020. [DOI]
- 59.Majmundar AJ, et al. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7(8):345–350. doi: 10.1016/S1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 61.Benita Y, et al. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37(14):4587–4602. doi: 10.1093/nar/gkp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaidi A, et al. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66(13):6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 63.Simon MC. The hypoxia response pathways — hats off! N Engl J Med. 2016;375(17):1687–1689. doi: 10.1056/NEJMcibr1610065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koskimaki J, et al. Comprehensive transcriptome analysis of cerebral cavernous malformation across multiple species and genotypes. JCI Insight. 2019;4(3):e126167. doi: 10.1172/jci.insight.126167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamik A, et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282(18):13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 66.SenBanerjee S, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199(10):1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ridder DA, et al. TAK1 in brain endothelial cells mediates fever and lethargy. J Exp Med. 2011;208(13):2615–2623. doi: 10.1084/jem.20110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abe M, et al. Thrombus and encapsulated hematoma in cerebral cavernous malformations. Acta Neuropathol. 2005;109(5):503–509. doi: 10.1007/s00401-005-0994-8. [DOI] [PubMed] [Google Scholar]
- 69.Bardehle S, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16(5):580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 70.Anderson MA, et al. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothhammer V, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557(7707):724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cirino G, et al. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends Pharmacol Sci. 2003;24(2):91–95. doi: 10.1016/S0165-6147(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 73.Lin S, et al. Sustained endothelial nitric-oxide synthase activation requires capacitative Ca2+ entry. J Biol Chem. 2000;275(24):17979–17985. doi: 10.1074/jbc.275.24.17979. [DOI] [PubMed] [Google Scholar]
- 74.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol. 2003;50(1):49–59. doi: 10.18388/abp.2003_3713. [DOI] [PubMed] [Google Scholar]
- 75.Schulz GB, et al. Cerebral cavernous malformation-1 protein controls DLL4-Notch3 signaling between the endothelium and pericytes. Stroke. 2015;46(5):1337–1343. doi: 10.1161/STROKEAHA.114.007512. [DOI] [PubMed] [Google Scholar]
- 76.Stamatovic SM, et al. PDCD10 (CCM3) regulates brain endothelial barrier integrity in cerebral cavernous malformation type 3: role of CCM3-ERK1/2-cortactin cross-talk. Acta Neuropathol. 2015;130(5):731–750. doi: 10.1007/s00401-015-1479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engelhardt S, et al. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014;171(5):1210–1230. doi: 10.1111/bph.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi Q, et al. Nitric oxide from brain microvascular endothelial cells may initiate the compensatory response to mild hypoxia of astrocytes in a hypoxia-inducible factor-1α dependent manner. Am J Transl Res. 2016;8(11):4735–4749. [PMC free article] [PubMed] [Google Scholar]
- 79.Brix B, et al. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1α-mediated target gene activation. J Neurosci. 2012;32(28):9727–9735. doi: 10.1523/JNEUROSCI.0879-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atochin DN, Huang PL. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers Arch. 2010;460(6):965–974. doi: 10.1007/s00424-010-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang PL. Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep. 2003;5(6):473–480. doi: 10.1007/s11906-003-0055-4. [DOI] [PubMed] [Google Scholar]
- 82.Li Q, et al. Diabetic eNOS-knockout mice develop accelerated retinopathy. Invest Ophthalmol Vis Sci. 2010;51(10):5240–5246. doi: 10.1167/iovs.09-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakagawa T, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18(2):539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 84.Opatrilova R, et al. Nitric oxide in the pathophysiology of retinopathy: evidences from preclinical and clinical researches. Acta Ophthalmol. 2018;96(3):222–231. doi: 10.1111/aos.13384. [DOI] [PubMed] [Google Scholar]
- 85.Metzen E, et al. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14(8):3470–3481. doi: 10.1091/mbc.e02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmer LA, et al. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58(6):1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 87.Schleicher M, et al. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal. 2009;2(82):ra41. doi: 10.1126/scisignal.2000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chakraborty A, et al. Angiopoietin like-4 as a novel vascular mediator in capillary cerebral amyloid angiopathy. Brain. 2018;141(12):3377–3388. doi: 10.1093/brain/awy274. [DOI] [PubMed] [Google Scholar]
- 89.Katanasaka Y, et al. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer. 2013;12:31. doi: 10.1186/1476-4598-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iniguez MA, et al. Cyclooxygenase-2: a therapeutic target in angiogenesis. Trends Mol Med. 2003;9(2):73–78. doi: 10.1016/S1471-4914(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 91.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31(2 Suppl 7):2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 92.Flemming KD, et al. Predictors of initial presentation with hemorrhage in patients with cavernous malformations. World Neurosurg. 2020;133:e767–e773. doi: 10.1016/j.wneu.2019.09.161. [DOI] [PubMed] [Google Scholar]
- 93.Polster SP, et al. Trial readiness in cavernous angiomas with symptomatic hemorrhage (CASH) Neurosurgery. 2019;84(4):954–964. doi: 10.1093/neuros/nyy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones MK, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5(12):1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 95.Majima M, et al. Cyclo-oxygenase-2 enhances basic fibroblast growth factor-induced angiogenesis through induction of vascular endothelial growth factor in rat sponge implants. Br J Pharmacol. 2000;130(3):641–649. doi: 10.1038/sj.bjp.0703327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu L, et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6(242):242ra84. doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bravi L, et al. Sulindac metabolites decrease cerebrovascular malformations in CCM3-knockout mice. Proc Natl Acad Sci U S A. 2015;112(27):8421–8426. doi: 10.1073/pnas.1501352112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Claxton S, et al. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46(2):74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.