Abstract
Acute lung injury (ALI) is a life-threatening clinical syndrome with high morbidity and mortality. The main pathological features of ALI are increased alveolar-capillary membrane permeability, edema, uncontrolled migration of neutrophils to the lungs, and diffuse alveolar damage, resulting in acute hypoxemic respiratory failure. Glucocorticoids, aspirin, and other anti-inflammatory drugs are commonly used to treat ALI. Respiratory supports, such as a ventilator, are used to alleviate hypoxemia. Many treatment methods are available, but they cannot significantly ameliorate the quality of life of patients with ALI and reduce mortality rates. Herbal active ingredients, such as flavonoids, terpenoids, saponins, alkaloids, and quinonoids, exhibit advantages for ALI prevention and treatment, but the underlying mechanism needs further study. This paper summarizes the role of herbal active ingredients in anti-ALI therapy and progresses in the understanding of their mechanisms. The work also provides some references and insights for the discovery and development of novel drugs for ALI prevention and treatment.
1. Introduction
Acute lung injury (ALI) is a common clinical syndrome with high morbidity and mortality and is caused by various pathological and structural changes due to direct and indirect injury factors in lung tissues. The pathological feature of this disease is damage to alveolar epithelial and microvascular endothelial cells. The former can cause edema in the alveolar cavity, exudation of fibrin and collagen, and aggregation of neutrophils, eventually leading to lung consolidation. The latter increases vascular permeability, which causes the aggregation of inflammatory cells and leads to pulmonary vascular congestion and pulmonary interstitial edema [1]. Therefore, the main factor in ALI development is the imbalance in inflammatory response, which damages diffuse alveolar and pulmonary vascular endothelial cells and may lead to oxidation/reduction imbalance [2].
The international treatment for ALI mainly includes reduction in inflammation damage and suppression of respiratory failure. Anti-inflammatory drugs, such as glucocorticoids and aspirin, are commonly used in clinics. Respiratory support, such as ventilators, is employed to relieve hypoxemia. Although many treatments are available, the patient's quality of life remains poor, and mortality is not reduced [3]. The activities of active ingredients from herbs in preventing and treating ALI have been recently explored. These substances show a broad application prospect. However, despite their widespread existence and recognized biological activities, no integral review of their mechanisms of action for ALI has been undertaken. This review is aimed at integrating and discussing the most promising anti-ALI compounds isolated from herbs over the last 10 years (2011-2020). For the collection of relevant information, reviews and single publications were searched on PubMed, SciFinder, ScienceDirect, and similar databases with relevant keywords, such as flavonoids, terpenoids, saponins, alkaloids, quinonoids, phenols, organic acids, coumarins, lignans, and ALI. Only in vivo research articles have been selected, and the mechanisms of action have been discussed. This work provides important clues and direction for the discovery of anti-ALI drugs.
2. Potential Effects of Herbal Active Ingredients on Experimental ALI
2.1. Flavonoids
Flavonoids constitute a group of compounds with 2-phenyl chromone structures; they are widely present in natural plants and have a variety of biological activities, such as antioxidant, anti-inflammatory, anticancer, antibacterial, and antiviral activities [4]. Their anti-inflammatory and antioxidant activities render them useful preventive and therapeutic candidates for inflammatory lung diseases.
Naringin is a natural 2,3-dihydro-flavonoid derived from Exocarpium Citri Grandis. Chen et al. [5] found that naringin can protect paraquat- (PQ-) induced ALI by downregulating the levels of transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), tissue inhibitor of metalloproteinase-1 (TIMP-1), and matrix metalloprotein-9 (MMP-9) and increasing the activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and heme oxygenase-1 (HO-1). The compound exhibits mucoactive effects in lipopolysaccharide- (LPS-) induced ALI in mice and beagle dogs by reducing goblet cell hyperplasia and excessive mucus secretion and promoting sputum excretion [6].
Tectorigenin, an isoflavone aglycone, is obtained from Belamcanda chinensis. Tectorigenin treatment can attenuate inflammatory cell number in bronchoalveolar lavage fluids (BALF), reduce the mRNA and protein expression levels of nuclear factor kappa b (NF-κB) p65 in the lungs, improve SOD activity, and inhibit myeloperoxidase (MPO) activity. It exhibits a protective effect on LPS-mediated ALI in mice [7].
Baicalin is a flavonoid glycoside isolated from Scutellaria baicalensis and can alleviate ALI induced by various factors. Meng et al. [8] found that it can provide protection against LPS-induced severe lung injury in mice by activating the nuclear factor E2-related factor 2- (Nrf2-) mediated HO-1 signaling pathway, which suppresses inflammation and oxidative stress. Baicalin can ameliorate severe burn-induced remote ALI in rats by suppressing the Nod-like receptor pyrin domain-containing protein 3 (NLRP3) signaling pathway [9] and avian pathogenic Escherichia coli- (E. coli-) induced ALI in chicken by restraining the activation of the NF-κB pathway [10].
Hesperidin is a flavanone glycoside, which is found mainly in citrus fruit peel. In sepsis-induced ALI models, treatment with hesperidin can provide protection against lung injury by attenuating the levels of B-cell lymphoma-2 (Bcl-2), caspase-3, heat shock protein 70 (Hsp70), toll-like receptor 4 (TLR4), and myeloid differential protein-88 (MyD88) in the lung tissues [11]. In an H1N1-induced ALI model, hesperidin exhibits efficacy in alleviating pulmonary inflammation and impairment by suppressing cytokine production through mitogen-activated protein kinase (MAPK) pathways in pulmonary microvascular endothelial cells [12]. In an LPS-induced ALI model, hesperidin pretreatment can modulate lung injury by inhibiting the infiltration of macrophages and suppressing the release of high-mobility group box-1 protein (HMGB1) [13].
Apigenin (4′,5,7-trihydroxyflavone) is a natural flavonoid compound and abundantly present in common fruits and vegetables, such as celery, apple, and onion. It can reverse ALI and immunotoxicity in PQ-induced mice by increasing GSH-Px, catalase (CAT), and SOD activities and decreasing the levels of proinflammatory cytokines and malondialdehyde (MDA) [14]. Apigenin pretreatment can protect LPS-induced ALI in mice, and the protective mechanism of apigenin might be partially due to the inhibited activation of cyclo-oxygen-ase-2 (COX-2) and NF-κB and subsequent decreased production of proinflammatory mediators [15]. Table 1 lists the potential effects of flavonoids on ALI. The structure of representative flavonoids is shown in Figure 1. In general, the key structural features of active flavonoids are unsaturation in the C ring (Δ2), the number and position of OH groups at the A and B rings, the carbonyl group at C-4 at ring C, and frequently, nonglycosylation of the molecules [16]. When these features are considered, one may infer that natural products, such as flavonoids, are useful in developing novel substances for ALI treatment.
Table 1.
Potential effects of flavonoids on ALI.
| Active ingredients | Models of ALI | Doses (mg/kg) | Relevant findings | Ref. |
|---|---|---|---|---|
| Naringin | ALI in mice induced by PQ | 60 and 120 | Reduce the levels of TNF-α, TGF-β1, TIMP-1, and MMP-9 | [5] |
| ALI in mice induced by LPS ALI in dogs induced by LPS |
15 and 60 12.4 | Reduce airway mucus secretion and goblet cell hyperplasia, promote sputum excretion | [6] | |
| Tectorigenin | ALI in mice induced by LPS | 5 and 10 | Decrease the expression of NF-κB p65 at both mRNA and protein levels, improve SOD activity, and inhibit MPO activity | [7] |
| Baicalin | ALI in mice induced by LPS | 200 | Activate Nrf2/HO-1 pathway | [8] |
| ALI in rats induced by severe burn | 80 | Modulate NLRP3 inflammasome pathways | [9] | |
| ALI in chicken induced by APEC | 50, 100, and 200 | Inhibit NF-κB pathway | [10] | |
| Hesperidin | ALI in mice induced by CLP | 10 and 20 | Induce the Hsp70/TLR4/MyD88 pathway | [11] |
| ALI in mice induced by H1N1 | 50, 200, and 500 | Reduce proinflammatory cytokine production by inhibiting MAPK pathways | [12] | |
| ALI in mice induced by LPS | 500 | Suppress HMGB1 expression and release | [13] | |
| Apigenin | ALI in mice induced by PQ | 20 and 50 | Increase the level of MDA and decrease the activity of antioxidase | [14] |
| ALI in mice induced by LPS | 10 and 20 | Inhibit the activation of COX-2 and NF-κB | [15] | |
| Rutin | ALI in mice induced by LPS | 0.61, 6.1, and 61 | Suppress the expressions of VCAM-1 and iNOS | [101] |
| Troxerutin | ALI in mice induced by LPS | 150 | Inhibit MAPK and NF-κB pathways | [102] |
| Hydroxysafflor yellow A | ALI in rats induced by OA | 15 | Activate cAMP/PKA pathway | [103] |
| ALI in mice induced by LPS | 40, 80, and 120 | Inhibit TLR4-dependent pathways | [92] | |
| ALI in mice induced by BLM | 26.7, 40, and 60 | Inhibit NF-κB activation and p38 MAPK phosphorylation | [94] | |
| EGCG | ALI in mice induced by LPS | 10 | Suppress TLR4/NF-κB pathway | [104] |
| ALI in mice induced by PQ | 5, 10, and 20 | Inhibit TLR/NF-κB pathway | [105] | |
| ALI in mice induced by H9N2 | 10 | Inhibit TLR4/NF-κB pathway | [106] | |
| Kaempferol | ALI in mice induced by LPS | 50 | Modulate TRAF6 polyubiquitination | [107] |
| ALI in mice induced by CLP | 100 | Suppress oxidative stress, iNOS, and ICAM-1 pathways | [108] | |
| ALI in mice induced by H9N2 | 15 | Inhibit TLR4/MyD88/NF-κB and MAPK pathways | [109] | |
| Luteolin | ALI in mice induced by LPS | 1 and 10 | Inhibit the activity of iNOS/NO, COX-2, HMGB1, and NF-κB | [110] |
| ALI in mice induced by HgCl2 | 100 | Prevent NF-κB activation and activate AKT/Nrf2 pathway | [111] | |
| Quercetin | ALI in mice induced by LPS | 25 and 50 | Activate cAMP-Epac pathway | [112] |
| ALI in rats induced by CLP | 30 and 50 | Inhibit ICAM-1 and MIP-2 expression | [113] | |
| ALI in mice induced by seawater instillation | 200 | Inhibit macrophage M1 polarization and proinflammatory cytokine expression | [114] | |
| Dihydromyricetin | ALI in mice induced by CLP | 50, 100, and 150 | Inhibit NLRP3 inflammasome-dependent pyroptosis | [115] |
| Isoliquiritigenin | ALI in mice induced by LPS | 30 | Activate AMPK/Nrf2/ARE signaling and inhibit NF-κB and NLRP3 activation | [116] |
Figure 1.

Chemical structures of representative flavonoids.
2.2. Terpenoids
Terpenoids are compounds with the general formula of (C5H8)n, and their oxygenated derivatives and derivatives with different degrees of saturation can be considered natural compounds linked by isoprene or isopentane in various ways. Some terpenoids have antibacterial, antioxidant, tumor suppression, hepatoprotective, anti-inflammatory, and other biological activities [17, 18].
Andrographolide is a diterpenoid lactone extracted from Andrographis paniculata and commonly used to treat respiratory infection. Peng et al. [19] found that andrographolide can ameliorate ovalbumin- (OVA-) induced ALI in mice by inhibiting the reactive oxygen species- (ROS-) mediated activation of the NF-κB and NLRP3 inflammasome signaling pathway. In addition, andrographolide contributes to the amelioration of lung inflammation and fibrosis induced by radiation and can suppress absent in melanoma 2 (AIM2) from translocating to the nucleus to sense DNA damage caused by chemotherapeutic agents or radiation in bone marrow-derived macrophages [20]. It exhibits antioxidative properties and can provide protection against cigarette smoke- (CS-) induced ALI by increasing Nrf2 activity [21].
Oridonin, isolated from Rabdosia rubescens, is an ent-kaurene diterpenoid that possesses antioxidative and anti-inflammatory effects. It can provide protection against LPS-mediated ALI by regulating Nrf2-dependent oxidative stress and Nrf2-independent NF-κB and NLRP3 pathways [22] and alleviate hyperoxia-induced lung injury. Treatment with 10 mg/kg oridonin can attenuate lung pathology, reduce lung edema, decrease TNF-α and MDA levels, and increase interleukin- (IL-) 10 and GSH levels in the lungs of model mice [23].
Betulin and betulinic acid are natural pentacyclic triterpenoids derived from various plants, including white birch bark. Zhao et al. [24] found that betulin can reduce lung damage caused by cecal ligation and puncture (CLP) and improve the survival rates of rats. This protective effect may be mediated by its anti-inflammatory effect and NF-κB and MAPK inhibition. Betulinic acid pretreatment can decrease the levels of oxidants and increase the levels of antioxidants in plasma and lungs, thereby reducing oxidative lung damage in CLP mice [25].
Asiatic acid is a pentacyclic triterpene derived from Centellae asiaticae herba. It can ameliorate LPS-induced histopathological changes in the lungs of mice by suppressing the production of inflammatory cytokines, and process is mediated by the blocked activation of TLR4-mediated NF-κB signaling [26]. Asiatic acid exerts a considerable protective effect on spinal cord injury- (SCI-) induced ALI by blocking NLRP3 inflammasome activation and oxidative stress and by upregulating Nrf2 expression [26]. The potential effects of terpenoids from herbs on ALI are shown in Table 2. The structures of representative terpenoids are shown in Figure 2. Structure-activity analysis shows that the introduction of acyl groups into the mother nuclei of terpenoids improves the anti-inflammatory activity of terpenoids, and increasing the amount of acyl groups and lengths of acyl carbon chains enhances this effect. The type of substituents on the C rings of polycyclic terpenoids is the main reason for their anti-inflammatory activity, and rosin/pimonane diterpenoids and ingenane diterpenoids have good inhibitory activities against NO production [27].
Table 2.
Potential effects of terpenoids on ALI.
| Active ingredients | Models of ALI | Doses (mg/kg) | Relevant findings | Ref. |
|---|---|---|---|---|
| Andrographolide | ALI in mice induced by OVA | 5 and 10 | Suppress ROS-mediated NF-κB signaling and NLRP3 inflammasome activation | [19] |
| ALI in mice induced by radiation | 5, 10, and 20 | Suppress AIM2 inflammasome-mediated pyroptosis in macrophage | [20] | |
| ALI in mice induced by CS | 0.1, 0.5, and 1 | Augment the activity of Nrf2 | [21] | |
| Oridonin | ALI in mice induced by LPS | 20 and 40 | Exert protective effects through Nrf2-independent anti-inflammatory and Nrf2-dependent antioxidative activities | [22] |
| ALI in mice induced by hyperoxia | 10 | Reduce MDA and TNF-α, and increase GSH and IL-10 in the lungs | [23] | |
| Betulin | ALI in rats induced by CLP | 4 and 8 | Inhibit NF-κB and MAPK pathway | [24] |
| Betulinic acid | ALI in mice induced by CLP | 3, 10, and 30 | Decrease the levels of oxidants, increase the levels of antioxidants in the lungs and plasma | [25] |
| Asiatic acid | ALI in mice induced by LPS | 25, 50, and 100 | Block the TLR4/NF-κB pathway | [26] |
| ALI in rat induced by SCI | 30 and 75 | Inhibit NLRP3 inflammasome activation and oxidative stress with the upregulation of Nrf2 | [117] | |
| Acanthoic acid | ALI in mice induced by LPS | 15, 30, and 60 | Upregulate the expression of LXRα | [100] |
| Paclitaxel | ALI in mice induced by CLP | 0.075, 0.150, 0.225, and 0.300 | Activate MUC1 and suppress TLR4/NF-κB pathway | [118] |
| Geraniol | ALI in mice induced by LPS | 12.5, 25, and 50 | Inhibit TLR4-mediated NF-κB and Bcl-2/Bax pathways | [119] |
| Triptolide | ALI in mice induced by LPS | 0.005, 0.010, and 0.015 | Activate PPAR-γ | [97] |
| Citral | ALI in mice induced by LPS | 10, 20, and 40 | Activate PPAR-γ | [98] |
| Geniposide | ALI in mice induced by LPS | 20, 40, and 80 | Block NF- κB and MAPK pathway | [120] |
| Jolkinolide B | ALI in mice induced by LPS | 2 and 10 | Suppress the activation of NF-κB and MAPK | [121] |
| Taraxasterol | ALI in mice induced by LPS | 2.5, 5, and 10 | Inhibit the NF-κB and MAPK pathways | [122] |
Figure 2.
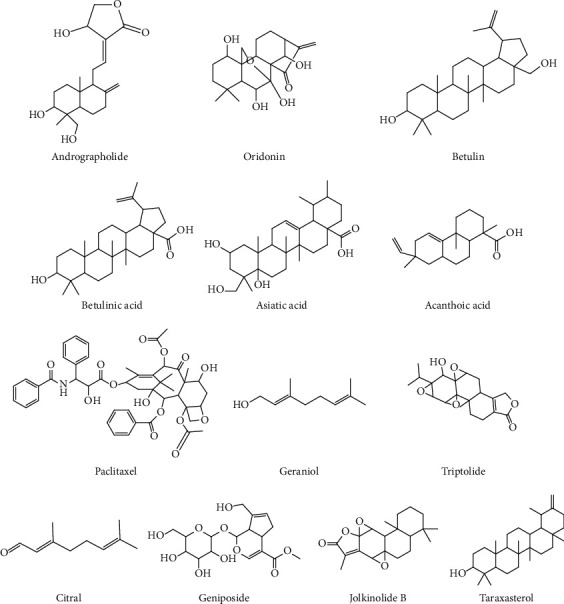
Chemical structures of representative terpenoids.
2.3. Saponins
Saponins are glycosides composed of triterpene or spiral sterane and mainly distributed in terrestrial higher plants. Saponins have multiple biological activities, such as anti-inflammatory, antibacterial, and antiviral activities, and may prevent or treat ALI [28].
Asiaticoside, a triterpenoid saponin derived from Centella asiatica, possesses potential anti-inflammatory and antioxidant activities. Qiu et al. [29] found that asiaticoside can attenuate LPS-induced ALI in a dose-dependent manner by inhibiting NF-κB p65 subunit phosphorylation and IκBα degradation. Asiaticoside can provide protection against CLP-induced ALI, and its underlying mechanisms may be related to the activation of peroxisome proliferator-activated receptor-γ (PPAR-γ) to some extent, which suppresses MAPKs and the NF-κB signaling pathway [30].
Platycodin D is the main triterpene saponin extracted from the Chinese herb Platycodonis radix and has anti-inflammatory and immunomodulatory activities. The anti-inflammatory effects of platycodin D may be associated with the activation of the liver x receptor α (LXRα)/ATP-binding cassette transporter A1 (ABCA1) signaling pathway, which disrupts the formation of lipid rafts by depleting cholesterol and preventing the translocation of TLR4 to lipid rafts, thereby blocking LPS-induced ALI [31]. Tao et al. [32] found that platycodin D pretreatment or posttreatment can improve ALI induced by LPS or BLM. Platycodin D can suppress apoptosis and inflammation by downregulating the levels of caspase-3, Bcl2-associated X protein (Bax), and NF-κB and upregulating the level of Bcl-2 in lung tissues and improve the SOD activity in BALF.
Dioscin, a natural steroid saponin mainly derived from Dioscorea villosa, can serve as an anti-inflammatory agent. Treatment with dioscin can attenuate oxidative stress, lung inflammatory response, and ALI in BLM-challenged mice [31]. Furthermore, dioscin can also alleviate LPS-induced ALI by inhibiting TLR4 signaling pathways [33].
Ginsenosides form a class of steroid glycosides and triterpene saponins. As the main active ingredients in Ginseng radix, they have various pharmacological effects, such as immunity enhancement and antiageing and antitumor effects [34–36]. Ginsenosides include Rb1, Rg1, Rg3, GRh2, and other monomeric saponins. The ginsenoside Rb1 can provide protection against Staphylococcus aureus- (S. aureus-) induced ALI in mice. It can inhibit TNF-α, IL-1β, and IL-6 production and TLR2 activation. Furthermore, Rb1 effectively downregulates the phosphorylation of extracellular regulated kinases (ERK), p65, and c-Jun N-terminal kinase (JNK). These results demonstrated that Rb1 can alleviate lung injury by inhibiting TLR2-mediated NF-κB and MAPK pathways [37]. Rg1 can relieve sepsis-induced ALI by upregulating SIRT1 to relieve endoplasmic reticulum (ER) stress and inflammation [38], and ginsenoside Rg1 pretreatment can attenuate ALI induced by hind limb ischemia-reperfusion (IR) by inhibiting the NF-κB/COX-2 signaling pathway [39]. Rg3 treatment can mitigate LPS-induced pathological damage in the lungs by increasing the production of anti-inflammatory mediators and reducing the levels of proinflammatory cytokines. This process is mediated by MerTK-dependent activation of its downstream PI3K/AKT/mTOR pathway. These findings identified a new site of the specific anti-inflammatory mechanism of ginsenoside Rg3 [40]. Rg3 exhibits a protective effect against omethoate-induced ALI in rats, and the mechanisms may be related to its antioxidant potential and anti-inflammatory effects [41]. The anti-inflammatory effect of Rh2 has made it one of the most important ginsenosides. Hsieh et al. [42] found that Rh2 can ameliorate LPS-induced lung injury by blocking the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 signaling pathways in mice.
Glycyrrhizin is an extractive component isolated from Glycyrrhiza glabra L. roots and has various pharmacological effects. It exerts protective effects in various ALI models. In a radiation-induced ALI model, glycyrrhizin mitigated radiation-induced ALI by suppressing the HMGB1/TLR4 pathway [43]. In a LPS-induced ALI model, glycyrrhizin exerted anti-inflammatory effects by suppressing TLR4/NF-κB signaling [44]. In a Streptococcus aureus-induced ALI model, glycyrrhizin mitigated lung inflammation after Streptococcus aureus infection by inhibiting NF-κB, p38/ERK pathways, and pyroptosis [45]. Table 3 shows the potential effects of saponins from herbs on ALI. The structures of representative saponins are shown in Figure 3. By summarizing the structure-activity relationship of saponins, the anti-inflammatory activities of the compounds were found to be closely related to the types of substituted sugar groups and to increase with the number of substituted sugar groups. In addition, the positions and number of hydroxyl groups on the mother nucleus have a strong influence on the anti-inflammatory activities of saponins [46].
Table 3.
Potential effects of saponins on ALI.
| Active ingredients | Models of ALI | Doses (mg/kg) | Relevant findings | Ref. |
|---|---|---|---|---|
| Asiaticoside | ALI in mice induced by LPS | 15, 30, and 45 | Inhibit NF-κB pathway | [29] |
| ALI in mice induced by CLP | 45 | Upregulate PPAR-γ expression, inhibit MAPK and NF-κB pathway | [30] | |
| Platycodin D | ALI in mice induced by LPS | 20, 40, and 80 | Activate LXRα-ABCA1 pathway | [31] |
| ALI in mice induced by LPS or BLM | 50 and 100 | Suppress apoptosis and inflammation | [32] | |
| Dioscin | ALI in mice induced by BLM | 80 | Attenuate oxidative stress and the inflammatory response | [123] |
| ALI in mice induced by LPS | 20, 40, and 80 | Inhibit NF-κB activation as well as TLR4 expression | [33] | |
| Ginsenoside Rb1 | ALI in mice induced by S. aureus | 10 and 20 | Attenuate NF-κB and MAPK activation | [37] |
| Ginsenoside Rg1 | ALI in mice induced by CLP | 10 and 20 | Upregulate SIRT1 to relieve ER stress and inflammation | [38] |
| ALI in rats induced by IR | 40 | Regulate NF-κB/COX-2 pathway | [39] | |
| Ginsenoside Rg3 | ALI in mice induced by LPS | 10, 20, and 30 | Activate MerTK-dependent PI3K/AKT/mTOR pathway | [40] |
| ALI in rats induced by omethoate | 5, 10, and 20 | Reduce inflammation and oxidation | [41] | |
| Ginsenoside Rh2 | ALI in mice induced by LPS | 5, 10, and 20 | Regulate the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 pathways | [42] |
| Glycyrrhizin | ALI in mice induced by radiation | 10 | Inhibit the HMGB1/TLR4 pathway | [43] |
| ALI in mice induced by LPS | 50 | Inhibit the TLR4/NF-κB pathway | [44] | |
| ALI in mice induced by S. aureus | 25 | Inhibit NF-κB, p38/ERK pathways, and pyroptosis | [45] | |
| Astragaloside IV | ALI in rats induced by CLP | 2.5, 5, and 10 | Improve pulmonary ventilation, decrease alveolar-capillary permeability | [88] |
| ALI in mice induced by PQ | 50 and 100 | Suppress Rho/ROCK/NF-κB pathway | [124] | |
| Saikosaponin A | ALI in mice induced by LPS | 5, 10, and 20 | Inhibit NF-κB and NLRP3 pathways | [125] |
| Tenuigenin | ALI in mice induced by LPS | 2, 4, and 8 | Inhibit NF-κB and MAPK pathways | [126] |
| Esculentoside A | ALI in mice induced by LPS | 15, 30, and 60 | Inhibit NF-κB and MAPK pathways | [127] |
Figure 3.
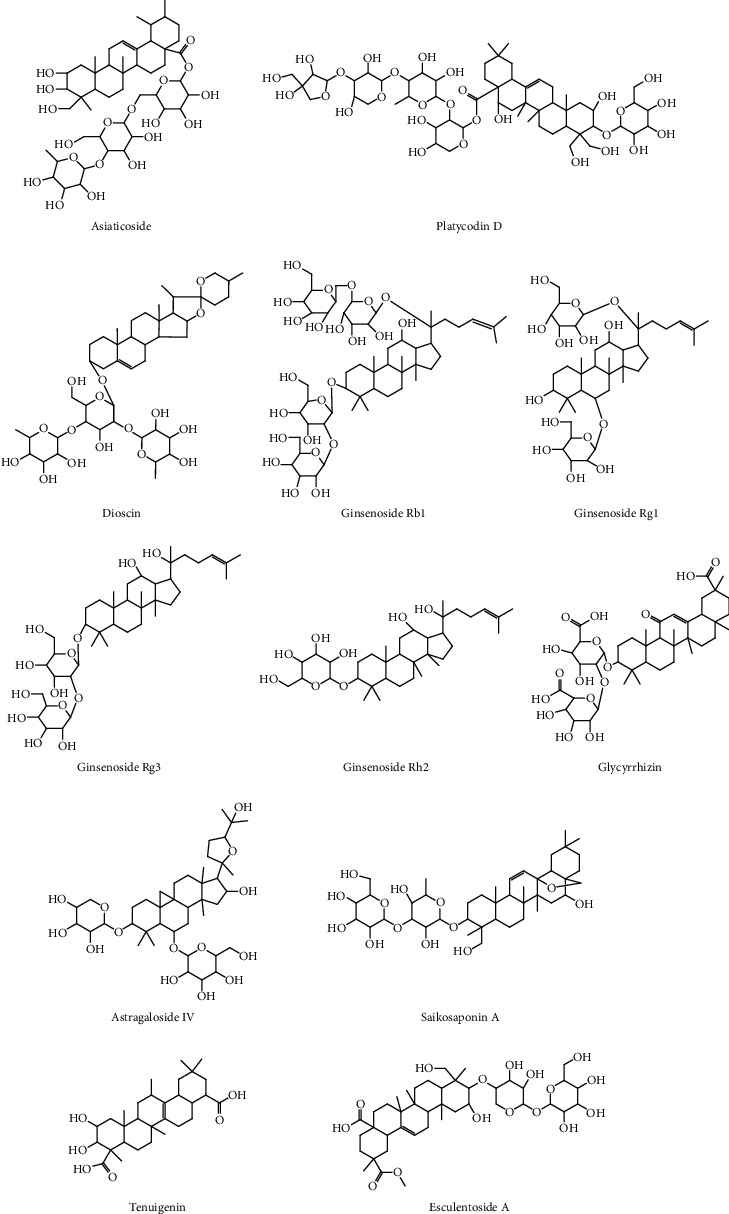
Chemical structures of representative saponins.
2.4. Alkaloids
Alkaloids are nitrogen-containing organic compounds that exist widely in nature. Most alkaloids have special and significant physiological activities, such as anti-inflammatory, antiviral, antitumor, and immune regulation activities [47, 48].
Berberine, an isoquinoline alkaloid, is extracted from Coptis chinensis and other Berberis plants. It can reduce lung histopathological changes through the protein kinase R-like endoplasmic reticulum kinase-mediated Nrf2/HO-1 signaling axis [49]. Berberine pretreatment can diminish CS-mediated lung inflammation by inhibiting the secretion of proinflammatory factors and NF-κB activity [50].
Sinomenine is an isoquinoline alkaloid extracted from Sinomenium acutum. It can provide protection against E. coli-induced ALI in mice by activating Nrf2 and inhibiting NF-κB pathways [51] and can effectively attenuate severe septic-associated ALI possibly by suppressing inflammation and oxidative damage through the activation of autophagy and Nrf2 signaling [52].
Matrine is the main active ingredient of Sophora flavescens. Liou et al. [53] found that it can prevent ALI in LPS-induced mice by decreasing the expression levels of COX-2, intercellular cell adhesion molecule-1 (ICAM-1), TNF-α, and IL-6.
Protostemonine is an active alkaloid mainly extracted from Stemona sessilifolia. In mice treated with protostemonine, heat-killed methicillin-resistant S. aureus- (HKMRSA-) mediated ALI can be alleviated through the suppression of the MAPK and NF-κB pathways [54]. Protostemonine can also effectively ameliorate LPS-induced lung inflammatory responses; the beneficial effects may be related to the downregulation of MAPK and AKT phosphorylation and reduction in the expression levels of proinflammatory mediators (such as NO, iNOS, and cytokines) [55].
Betanin is a quaternary ammonium alkaloid, mainly present in red beet roots. Treatment with betanin can protect rats from PQ-induced ALI interstitial pneumonia. The mechanisms may be associated with the increased levels of zonula occluden-1 and claudin-4, decreased levels of TNF-α and IL-1, and inhibited NF-κB activity [56]. Table 4 shows the potential effects of alkaloids from herbs on ALI. The structures of representative alkaloids are shown in Figure 4. The structural factors affecting the antioxidant activity of alkaloids are mainly stereostructure and electrical effects. The “exposure” of the nitrogen atoms in the heterocyclic ring facilitates reactions with ROS, resulting in antioxidant effects. Electron-donating groups in alkaloids or structural factors that can enrich nitrogen atoms with electrons can increase antioxidant activity [57]. Borcsa et al. [58] found that the chain length and unsaturation of the C-8 ester groups of aconitine derivatives play important roles in the anti-inflammatory activity. The toxicity of its derivative with a substituted long-chain ester group at the C-8 position is reduced relative to that of aconitine.
Table 4.
Potential effects of alkaloids on ALI.
| Active ingredients | Models of ALI | Doses (mg/kg) | Relevant findings | Ref. |
|---|---|---|---|---|
| Berberine | ALI in mice induced by LPS | 10 | Activate PERK-mediated Nrf2/HO-1 signaling axis | [49] |
| ALI in mice induced by CS | 50 | Inhibit NF-κB activation | [50] | |
| Sinomenine | ALI in mice induced by E. coli | 100 | Inhibit the activation of NF-κB and upregulate the expression of Nrf2 | [51] |
| ALI in mice induced by LPS | 100 | Activate Nrf2 and autophagy | [52] | |
| Matrine | ALI in mice induced by LPS | 10 and 20 | Decrease the expressions of COX-2 and ICAM-1 | [53] |
| Protostemonine | ALI in mice induced by HKMRSA | 20 | Inhibit MAPK and NF-κB pathways | [54] |
| ALI in mice induced by LPS | 10 | Inhibit iNOS and NO expression, and suppress MAPK and PI3K/AKT signaling transduction in macrophages | [55] | |
| Betanin | ALI in rats induced by PQ | 25 and 100 | Decrease the levels of IL-1 and TNF-α, and the activity of NF-κB | [56] |
| Tabersonine | ALI in mice induced by LPS | 20 and 40 | Reduce the K63-linked polyubiquitination of TRAF6 | [128] |
| Anisodamine | ALI in rats induced by LPS | 10 | Inhibit the levels of IL-17A and IL-17F | [129] |
| Magnoflorine | ALI in mice induced by LPS | 5, 10, and 20 | Suppress TLR4-mediated NF-κB and MAPK activation | [130] |
| Sophocarpine | ALI in mice induced by LPS | 12.5, 25, and 50 | Inhibit TLR4-mediated NF-κB and MAPK pathways | [131] |
Figure 4.

Chemical structures of representative alkaloids.
2.5. Quinonoids
Quinonoids form a large class of natural bioactive molecules with unsaturated cyclodiketone structures (quinone structures) and are mainly divided into four types: benzoquinones, phenanthrenequinones, naphthoquinones, and anthraquinones. Quinonoids exhibit multiple pharmacological activities, such as antibacterial, anti-inflammatory, antiviral, and anticancer effects, and especially play a crucial role in ALI prevention and treatment [59, 60].
Emodin is an anthraquinone derivative isolated from rhubarb and has protective effects in various lung injury models. Dong et al. [61] found that emodin can reactivate autophagy and alleviate inflammatory lung injury in mice with lethal endotoxemia. Another study showed that emodin might provide protection against severe acute pancreatitis- (SAP-) associated ALI by reducing the expression of pre-B-cell colony-enhancing factor and enhancing neutrophil apoptosis through mitochondrial and death receptor apoptotic pathways [62]. Meanwhile, emodin exhibits protective effects on CLP-induced ALI in rats, and the molecular mechanism may be associated with the suppression of p38 MAPK signaling and reduction in oxidative damage and inflammation responses [63]. Moreover, emodin can also attenuate CS-mediated oxidative damage and lung inflammation in mouse models by inhibiting ROS production [64].
Chrysophanol is one of the main active ingredients of rhubarb. Treatment with chrysophanol can attenuate PQ-induced lung inflammation by increasing PPAR-γ expression and blocking the NF-κB pathway [65].
Tanshinone IIA is a derivative of phenanthrenequinone isolated from the root of Salvia miltiorrhiza. It can prevent LPS-induced expression and proinflammatory mediator secretion, thus exerting a protective effect against ALI probably through the regulation of the Sirt1/NF-κB pathway [66]. Wang et al. [67] investigated the effect and mechanism of tanshinone IIA on PQ-induced ALI. The results indicated that tanshinone IIA exerted a therapeutic effect on ALI rats by decreasing the levels of Ang-(1-7) and angiotensin-converting enzyme 2 (ACE2) in the lungs. Tanshinone IIA also exerts a marked protective effect on seawater aspiration-mediated ALI partly through the downregulation of macrophage migration inhibitory factor and NF-κB activity and TNF-α and IL-6 expression [68].
Cryptotanshinone is one of the major active compounds of Salvia miltiorrhiza. Cryptotanshinone pretreatment can significantly inhibit LPS-induced neutrophil infiltration in lung tissues. The anti-inflammatory effects of cryptotanshinone may be due to its ability to suppress TLR4-mediated NF-κB signaling [69]. Moreover, cryptotanshinone treatment can ameliorate radiation-induced ALI by suppressing the production and secretion of inflammatory cytokines and inhibiting the activation of CC chemokine ligand 3 (CCL3)/CC chemokine receptor 1 (CCR1) [70].
Embelin is a naturally occurring hydroxybenzoquinone mainly obtained from Embelia ribes. Embelin treatment can efficiently relieve PQ-incited lung damage, and the mechanism may be related to the inhibition of oxidative stress, inflammation cascade, and MAPK/NF-κB signaling pathway [71].
Shikonin is a naphthoquinone isolated from Lithospermum. Treatment with shikonin can improve sepsis-induced ALI by increasing miRNA-140-5p expression, depressing TLR4 expression, and suppressing the expression of downstream MyD88 and NF-κB [72]. Myeloid differentiation protein 2 (MD2) plays a key role in mediating inflammation. Zhang et al. [73] found that shikonin can inhibit the formation of the MD2-TLR4 complex in an ALI mouse model, thereby reducing LPS-mediated inflammation. Table 5 shows the potential effects of quinonoids from herbs on ALI. The structures of representative quinonoids are shown in Figure 5. Structure-activity relationship analysis showed that the number and locations of OH groups on the benzene rings of quinonoids seem to be largely responsible for the observed large variations in the trends of antioxidative and anti-inflammatory properties. Nam et al. [74] found that compared with quinonoids without OH or only two OH groups on their benzene rings, purpurin with three OH groups, two of which are located in the ortho position of the anthraquinone molecule, exhibited the highest activity in antioxidative and anti-inflammatory cell assays. In addition, glycosides display lower activities than their aglycons [75].
Table 5.
Potential effects of quinonoids on ALI.
| Active ingredients | Models of ALI | Doses (mg/kg) | Relevant findings | Ref. |
|---|---|---|---|---|
| Emodin | ALI in mice induced by LPS | 20 | Upregulate the expression of BECN1 and LC3-II | [61] |
| ALI in rats induced by SAP | 10 | Decrease the expression of PBEF | [62] | |
| ALI in rats induced by CLP | 25 | Inhibit p38 MAPK pathway, and reduce oxidative stress and inflammation response | [63] | |
| ALI in mice induced by CS | 20 and 40 | Enhance the expressions and activities of HO-1 and Nrf-2 | [64] | |
| Chrysophanol | ALI in mice induced by PQ | 10 and 20 | Activate PPAR-γ and inhibit NF-κB pathway | [65] |
| Tanshinone IIA | ALI in mice induced by LPS | 10 | Modulate Sirt1/NF-κB pathway | [66] |
| ALI in rats induced by PQ | 25 | Enhance the levels of ACE2 and Ang-(1-7) | [67] | |
| ALI in rats induced by seawater aspiration | 10 | Downregulate MIF and the activity of NF-κB | [68] | |
| Cryptotanshinone | ALI in mice induced by LPS | 10, 20, and 40 | Inhibit TLR4-mediated NF-κB pathways | [69] |
| ALI in rats induced by radiation | 20 | Regulate the production and release of inflammatory cytokines especially MMP-1, and inhibit the activation of CCL3/CCR1 | [70] | |
| Embelin | ALI in rats induced by PQ | 20 | Suppress oxidative stress and inflammatory cascade by modulating MAPK/NF-κB pathway | [71] |
| Shikonin | ALI in rats induced by LPS | 12.5, 25, and 50 | Regulate miRNA-140-5p/TLR4-a | [72] |
| ALI in mice induced by LPS | 12.5 and 25 | Inhibit MD2-TLR4 complex formation | [73] | |
| Aloin | ALI in mice induced by LPS | 1.6~12.4 | Reduce inflammatory gene iNOS by inhibition activity and p-STAT-1 and NF-κB | [132] |
Figure 5.
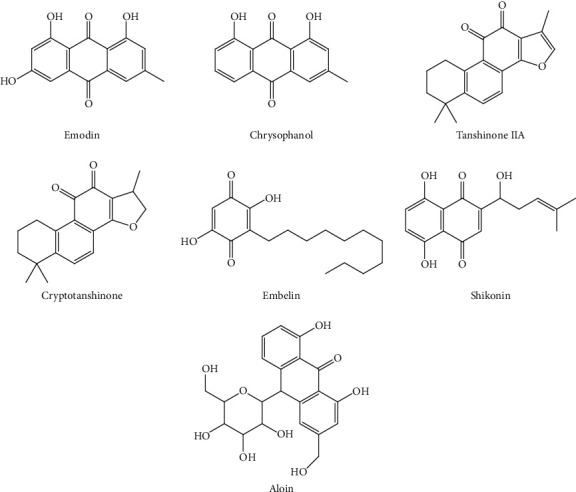
Chemical structures of representative quinonoids.
2.6. Other Components
In addition to flavonoids, saponins, terpenoids, alkaloids, and quinonoids, other active ingredients, such as phenols, organic acids, coumarins, and lignans, also play important roles in ALI prevention and treatment (Table 6). The structures of other representative components are shown in Figure 6.
Table 6.
Potential effects of other components on ALI.
| Active ingredients | Models of ALI | Doses (mg/kg) | Relevant findings | Ref. |
|---|---|---|---|---|
| Resveratrol | ALI in murine induced by LPS | 30 | Activate SOCS3 pathway | [76] |
| ALI in mice induced by CLP | 40 | Activate the VEGF-B pathway | [77] | |
| ALI in mice induced by SEB | 100 | Downregulate miR-193a that targets TGF-β pathway | [78] | |
| Curcumin | ALI in mice induced by CLP | 20 | Enhance Treg cell differentiation and increase IL-10 production | [79] |
| ALI in mice induced by BLM | 75 | Downregulate the expression of Ki 67 and EGFR | [80] | |
| Ellagic acid | ALI in mice induced by HCl | 10 | Downregulate the level of IL-6 and upregulate the level of IL-10 | [81] |
| Protocatechuic acid | ALI in mice induced by LPS | 5, 15, and 30 | Suppress p38MAPK and NF-κB pathways | [82] |
| Eugenol | ALI in mice induced by LPS | 150 | Suppress the release of inflammatory factors and the activity of antioxidant enzymes | [83] |
| Veratric acid | ALI in mice induced by LPS | 12.5, 25, and 50 | Inhibit NF-κB pathway | [84] |
| Esculin | ALI in mice induced by LPS | 20 and 40 | Inhibit TLR/NF-κB pathway | [85] |
| Arctiin | ALI in mice induced by LPS | 10, 20, and 40 | Suppress PI3K/AKT/NF-κB pathway | [86] |
| Arctigenin | ALI in mice induced by LPS | 50 | Suppress MAPK, HO-1, and iNOS pathway | [87] |
Figure 6.
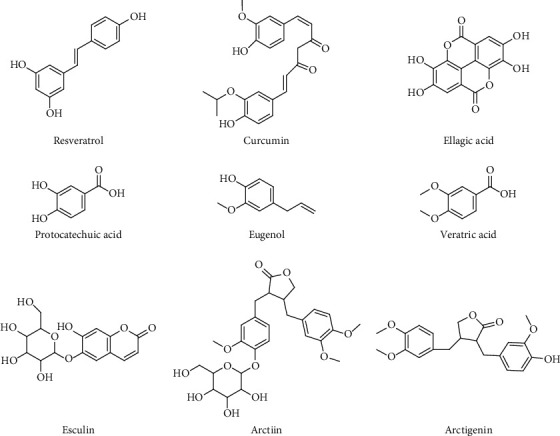
Chemical structures of other active ingredients.
Resveratrol is a stilbene derivative mainly present in Vitis viniferae fructus. It exhibits protective effects in various ALI models. In a LPS-mediated ALI model, resveratrol can counteract lung inflammation by suppressing CD45+ Siglec F− and CD45+ CD206− M1 subtype macrophages [76]. In a sepsis-induced ALI model, resveratrol can suppress the production of proinflammatory cytokines and the apoptosis of alveolar macrophages through the activation of the vascular endothelial growth factor-B (VEGF-B) pathway, thereby exhibiting anti-inflammatory and antiapoptotic effects [77]. In a staphylococcal enterotoxin B- (SEB-) induced ALI model, resveratrol can ameliorate ALI and decrease mortality through miR-193a regulation that targets the TGF-β pathway [78].
Curcumin is a natural polyphenolic compound found in Curcuma longa. Curcumin pretreatment can relieve the severity of ALI and uncontrolled inflammation in CLP-induced septic mice by enhancing the differentiation of naïve CD4+ T cells into CD4+ CD25+ FOXP3+ Treg cells [79]. Moreover, curcumin can significantly improve BLM-induced ALI by downregulating the levels of epidermal growth factor receptor (EGFR) and Ki 67 both in vitro and in vivo [80].
Ellagic acid, a naturally occurring polyphenol, is present in a variety of plants such as eucalyptus, green tea, and geranium. In preventive and therapeutic treatments, ellagic acid can restrain the development of ALI induced by HCl by reducing IL-6 levels and increasing IL-10 levels in BALF [81].
Protocatechuic acid, a dihydroxybenzoic acid, is a phenolic acid found in plants such as Alpinia oxyphylla. It can effectively ameliorate lung histopathological changes induced by LPS by suppressing the p38 MAPK and NF-κB signaling pathways [82].
Eugenol is a natural phenolic substance found in medicinal plants, such as cinnamon, clove, and bay leaves. Eugenol can alleviate LPS-induced ALI by suppressing the release of inflammatory mediators (IL-1β, TNF-α, and IL-6), NADPH oxidase activity, and antioxidant enzyme activity [83].
Veratric acid is a benzoic acid extracted from Trollius chinensis Bunge. It can attenuate inflammatory injury caused by LPS by inhibiting NF-κB signaling pathways [84].
Esculin, a coumarin compound isolated from Fraxini cortex, can inhibit LPS-induced lung inflammation in mice by regulating the TLRs/MyD88/NF-κB signaling pathways [85].
Arctiin and arctigenin are lignan compounds isolated from Arctium lappa. Arctiin can prevent LPS-induced ALI in mice by inhibiting the PI3K/AKT/NF-κB signaling pathway [86], and arctigenin can provide protection against LPS-induced lung inflammation and oxidative stress in a mouse model by suppressing the MAPK, iNOS, and HO-1 pathway [87].
3. Discussion
By organizing the active ingredients from herbs and their roles in preventing and treating ALI, the mechanisms can be summarized as follows:
3.1. Improvement of Pathological Changes
3.1.1. Inhibition of the Inflammatory Response
Under ALI, neutrophils, macrophages, vascular endothelial cells, and many other immune cells are activated in the body to release a series of inflammatory mediators, leading to an uncontrolled systemic inflammatory response [1]. This activation is one of the important mechanisms of ALI prevention and control with herbal active ingredients, regulation of the levels of inflammatory mediators, and balancing of pro- and anti-inflammatory responses [70, 81].
3.1.2. Amelioration of the Barrier Function
The main pathological features of ALI include pulmonary edema caused by the destruction of the alveolar-capillary barrier and imbalance in the inflammatory response caused by leukocyte recruitment [2]. The destruction of alveolar epithelial cells increases barrier permeability and decreases the clearance rate of alveolar fluid; injury to vascular endothelial cells leads to fluid and macromolecules entering the gap, thus causing pulmonary edema. Herbal active ingredients, such as platycodin D and astragaloside IV, can maintain the integrity of the barrier function by inhibiting the apoptosis of alveolar epithelium or endothelial cells [32, 88].
3.1.3. Alleviation of Oxidative Stress
Under ALI, activated neutrophils release a large amount of ROS. The release of considerable ROS, on the one hand, directly damages the unsaturated fatty acids in the cell membrane, reduces the fluidity of the membrane, and increases permeability. On the other hand, it can release substantial oxygen-free radicals to lung tissue, which directly damages alveolar epithelial and pulmonary vascular endothelial cells, destroys the blood barrier, and aggravates pulmonary edema [89]. Therefore, reconstruction of the balance between oxidation and antioxidation can ease ALI. Studies have shown that herbal active ingredients, including tectorigenin and arctigenin, can ease oxidative stress and pathological damage to the lung by increasing the contents of SOD and HO-1 [7, 87].
3.2. Regulation of ALI-Related Pathways
3.2.1. Inhibition of the NF-κB Pathway
When the ALI occurs, the body will produce a large number of inflammatory factors. Their transcription is mainly related to NF-κB, a key nuclear transcription factor. In resting cells, NF-κB and IκB form a compound body that exists in the cytoplasm in an inactive form. When the cell is stimulated by extracellular signals, IκB phosphorylates and free NF-κB rapidly shift to the nucleus, thereby inducing the transcription of related genes, including proinflammatory factors IL-6 and TNF-α, and aggravating ALI [90]. Many researches demonstrated that some herbal active ingredients can improve ALI by inhibiting the NF-κB pathway [29, 50, 84].
3.2.2. Inhibition of the MAPK Pathway
MAPK activation is crucial in the occurrence and development of ALI. MAPK includes three main subfamilies, namely, ERK, JNK, and p38, which can regulate cytokine release and signal transduction in ALI. JNK and p38 can regulate the occurrence of proinflammatory factors IL-6 and TNF-α. p38 can regulate the activation of NF-κB induced by LPS by inhibiting neutrophil migration and aggravate the disease condition of ALI patients [91]. Hesperidin, emodin, and other herbal active ingredients can alleviate the development of ALI by inhibiting the MAPK pathway [12, 63].
3.2.3. Other Pathway
Herbal active ingredients can also affect the occurrence and development of ALI by regulating Akt, AMPK, cAMP/PKA, or TGF-β pathways [16, 40, 78, 92]. The anti-ALI mechanisms of herbal active ingredients are summarized in Figure 7.
Figure 7.
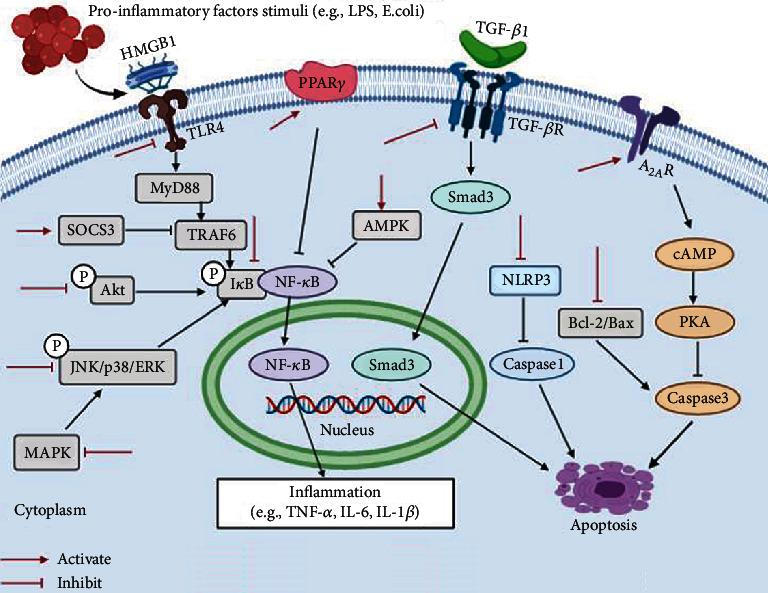
Schematic presentation of the anti-ALI mechanisms of herbal active ingredients.
3.3. Regulation of ALI-Related Receptors
3.3.1. Inhibition of TLRs
TLRs are important proteins involved in nonspecific immunity. TLR2 and TLR4 can recognize LPS and play an important role in LPS-mediated inflammatory responses. After invading the lung, LPS binds to the TLR2 or TLR4 receptor, which activates the NF-κB pathway and induces inflammation. Therefore, inhibition of TLR2 or TLR4 activation can ease immune and inflammatory responses [93]. Studies have shown that hydroxysafflor yellow A and ginsenoside Rb1 can alleviate ALI by inhibiting the expression of TLR4 or TLR2 [37, 94].
3.3.2. Upregulation of PPAR-γ
The imbalance in inflammatory response and immune regulation can promote the occurrence and development of ALI. Studies have revealed that activated PPAR-γ has anti-inflammatory and immunoregulation effects [95, 96]. Triptolide and citral can inhibit the activation of NF-κB by increasing the content of PPAR-γ, thus improving the inflammatory response of ALI [97, 98].
3.3.3. Activation of the Nuclear Receptor LXRα
LXRα, one of the main members of the nuclear receptor superfamily, can selectively reduce the expression of pulmonary inflammatory factors when activated and inhibit the activity of neutrophils and macrophages, which play a key role in the ALI inflammatory response. It can also increase the expression of several immune proteins and antioxidant enzymes in the lung. Therefore, LXRα is a potential anti-ALI target [99]. Researches have shown that acanthoic acid and platycodin D can alleviate lung inflammation by activating LXRα [31, 100].
4. Conclusion and Outlook
Herbal active ingredients for preventing and treating ALI mainly include flavonoids, terpenoids, saponins, alkaloids, and quinonoids, and animal models are used to verify their efficacy. The LPS-induced ALI model is the most widely used, and the main modeling methods include intraperitoneal injection, tracheal instillation, nasal instillation, and tail intravenous injection. The cecal ligation and puncture-induced in vivo sepsis model, retrograde pancreaticobiliary duct injection of taurocholic acid-induced severe acute pancreatitis model, tail intravenous injection of oleic acid, tracheal instillation of BLM, inhalation of influenza virus, and intraperitoneal injection of PQ are also to induce the ALI model. Although the verification of activity is simple at the cellular level, multicell interaction during ALI occurrence cannot be stimulated accurately. Most laboratories use Western blot and other methods to investigate changes in the protein contents of related pathways, but interference or knockout is rarely used in confirming the specificity of proteins and their related upstream and downstream proteins. The inhibitors of related pathways are also seldom used. Therefore, clarifying the possible targets of the active ingredients from herbs seems difficult.
In summary, herbal active ingredients possess anti-ALI activity and can ameliorate ALI by regulating immune cell function, inhibiting inflammatory response, improving barrier function, and modulating related signal transduction pathways. Further studies are needed to confirm the action target and possible structural modification and to provide insights and references for the discovery of novel drugs for ALI prevention and treatment.
Acknowledgments
This work is financially supported by the Zhejiang Natural Science Foundation (No. LY19H280012), the National Natural Science Foundation of China (No. 81603368), the Health Commission of Zhejiang Province (No. 2021KY632), and the Zhejiang Provincial Department of Science and Technology (Nos. LGF21H280008 and YS2021015).
Abbreviations
- ALI:
Acute lung injury
- ABCA1:
ATP-binding cassette transporter A1
- AIM2:
Absent in melanoma 2
- ACE2:
Angiotensin-converting enzyme 2
- BALF:
Bronchoalveolar lavage fluids
- Bcl-2:
B-cell lymphoma-2
- Bax:
Bcl2-associated X protein
- BLM:
Bleomycin
- CAT:
Catalase
- COX-2:
Cyclo-oxygen-ase-2
- CLP:
Cecal ligation and puncture
- CS:
Cigarette smoke
- CCL3:
CC chemokine ligand 3
- CCR1:
CC chemokine receptor 1
- E. coli:
Escherichia coli
- EGCG:
Epigallocatechin-3-gallate
- ERK:
Extracellular regulated kinases
- ER:
Endoplasmic reticulum
- EGFR:
Epidermal growth factor receptor
- GSH-Px:
Glutathione peroxidase
- HKMRSA:
Heat-killed methicillin-resistant Staphylococcus aureus
- Hsp70:
Heat shock protein 70
- HO-1:
Heme oxygenase-1
- HMGB1:
High mobility group box-1 protein
- IR:
Ischemia-reperfusion
- IL:
Interleukin
- ICAM-1:
Intercellular cell adhesion molecule-1
- JNK:
c-Jun N-terminal kinase
- LPS:
Lipopolysaccharides
- LXRα:
Liver x receptor α
- MD2:
Myeloid differentiation protein 2
- MMP-9:
Matrix metalloprotein-9
- MPO:
Myeloperoxidase
- MyD88:
Myeloid differential protein-88
- MAPK:
Mitogen-activated protein kinase
- MDA:
Malondialdehyde
- NF-κB:
Nuclear factor kappa b
- Nrf2:
Nuclear factor E2-related factor 2
- NLRP3:
Nod-like receptor pyrin domain-containing protein 3
- OA:
Oleic acid
- OVA:
Ovalbumin
- PQ:
Paraquat
- PPAR-γ:
Peroxisome proliferator-activated receptors-γ
- ROS:
Reactive oxygen species
- SAP:
Severe acute pancreatitis
- SCI:
Spinal cord injury
- SEB:
Staphylococcal enterotoxin B
- SOD:
Superoxide dismutase
- TGF-β:
Transforming growth factor-β
- TNF-α:
Tumor necrosis factor-α
- TIMP-1:
Tissue inhibitor of metalloproteinase-1
- TLR4:
Toll-like receptor 4
- VEGF-B:
Vascular endothelial growth factor-B.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Fanelli V., Ranieri V. M. Mechanisms and clinical consequences of acute lung injury. Annals of the American Thoracic Society. 2015;12(Supplement 1):S3–S8. doi: 10.1513/AnnalsATS.201407-340MG. [DOI] [PubMed] [Google Scholar]
- 2.Ware L. B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Seminars in Respiratory and Critical Care Medicine. 2006;27(4):337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney R. M., Griffiths M., McAuley D. Treatment of acute lung injury: current and emerging pharmacological therapies. Seminars in Respiratory and Critical Care Medicine. 2013;34(4):487–498. doi: 10.1055/s-0033-1351119. [DOI] [PubMed] [Google Scholar]
- 4.Panche A. N., Diwan A. D., Chandra S. R. Flavonoids: an overview. Journal of Nutritional Science. 2016;5 doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Nie Y. C., Luo Y. L., et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food and Chemical Toxicology. 2013;58:133–140. doi: 10.1016/j.fct.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Wu H., Nie Y. C., Li P. B., Shen J. G., Su W. W. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environmental Toxicology and Pharmacology. 2014;38(1):279–287. doi: 10.1016/j.etap.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Ma C. H., Liu J. P., Qu R., Ma S. P. Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice. Chinese Journal of Natural Medicines. 2014;12(11):841–846. doi: 10.1016/S1875-5364(14)60126-6. [DOI] [PubMed] [Google Scholar]
- 8.Meng X., Hu L., Li W. Baicalin ameliorates lipopolysaccharide-induced acute lung injury in mice by suppressing oxidative stress and inflammation via the activation of the Nrf2-mediated HO-1 signaling pathway. Naunyn-Schmiedeberg's Archives of Pharmacology. 2019;392(11):1421–1433. doi: 10.1007/s00210-019-01680-9. [DOI] [PubMed] [Google Scholar]
- 9.Bai C., Li T., Sun Q., et al. Protective effect of baicalin against severe burn induced remote acute lung injury in rats. Molecular Medicine Reports. 2018;17(2):2689–2694. doi: 10.3892/mmr.2017.8120. [DOI] [PubMed] [Google Scholar]
- 10.Peng L. Y., Yuan M., Song K., et al. Baicalin alleviated APEC-induced acute lung injury in chicken by inhibiting NF-κB pathway activation. International Immunopharmacology. 2019;72:467–472. doi: 10.1016/j.intimp.2019.04.046. [DOI] [PubMed] [Google Scholar]
- 11.Yuan X., Zhu J., Kang Q., He X., Guo D. Protective effect of hesperidin against sepsis-induced lung injury by inducing the heat-stable protein 70 (Hsp70)/toll-like receptor 4 (TLR4)/ myeloid differentiation primary response 88 (MyD88) pathway. Medical Science Monitor. 2019;25:107–114. doi: 10.12659/MSM.912490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Z., Sun G., Zhu Z. Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect. Antiviral Therapy. 2018;23(7):611–615. doi: 10.3851/IMP3235. [DOI] [PubMed] [Google Scholar]
- 13.Liu X. X., Yu D. D., Chen M. J., et al. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. International Immunopharmacology. 2015;25(2):370–376. doi: 10.1016/j.intimp.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Li Z., Xue X., Wang Y., Zhang Y., Wang J. Apigenin reverses lung injury and immunotoxicity in paraquat-treated mice. International Immunopharmacology. 2018;65:531–538. doi: 10.1016/j.intimp.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Liu Y. T., Xiao L., Zhu L., Wang Q., Yan T. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation. 2014;37(6):2085–2090. doi: 10.1007/s10753-014-9942-x. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q., Lv H., Wen Z., Ci X., Peng L. Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-κB pathway in macrophages and in acute lung injury. Frontiers in Immunology. 2017;8:p. 1518. doi: 10.3389/fimmu.2017.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan M. S. A., Khundmiri S. U. K., Khundmiri S. R., Al-Sanea M. M., Mok P. L. Fruit-derived polysaccharides and terpenoids: recent update on the gastroprotective Effects and mechanisms. Frontiers in Pharmacology. 2018;9:p. 569. doi: 10.3389/fphar.2018.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germoush M. O., Elgebaly H. A., Hassan S., Kamel E. M., Bin-Jumah M., Mahmoud A. M. Consumption of terpenoids-rich padina pavonia extract attenuates hyperglycemia, insulin resistance and oxidative stress, and upregulates PPARγ in a rat model of type 2 diabetes. Antioxidants. 2020;9(1):p. 22. doi: 10.3390/antiox9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng S., Gao J., Liu W., et al. Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget. 2016;7(49):80262–80274. doi: 10.18632/oncotarget.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J., Peng S., Shan X., et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death & Disease. 2019;10(12):p. 957. doi: 10.1038/s41419-019-2195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan S. P., Tee W., Ng D. S., et al. Andrographolide protects against cigarette smoke-induced oxidative lung injury via augmentation of Nrf2 activity. British Journal of Pharmacology. 2013;168(7):1707–1718. doi: 10.1111/bph.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H., Lv H., Li H., Ci X., Peng L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Communication and Signaling: CCS. 2019;17(1):p. 62. doi: 10.1186/s12964-019-0366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Zhang P. X., Han C. H., et al. Oridonin protects the lung against hyperoxia-induced injury in a mouse model. Undersea & Hyperbaric Medicine. 2017;44(1):33–38. doi: 10.22462/1.2.2017.6. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H., Liu Z., Liu W., Han X., Zhao M. Betulin attenuates lung and liver injuries in sepsis. International Immunopharmacology. 2016;30:50–56. doi: 10.1016/j.intimp.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Lingaraju M. C., Pathak N. N., Begum J., et al. Betulinic acid negates oxidative lung injury in surgical sepsis model. The Journal of Surgical Research. 2015;193(2):856–867. doi: 10.1016/j.jss.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Li Z., Xiao X., Yang M. Asiatic acid inhibits lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2016;39(5):1642–1648. doi: 10.1007/s10753-016-0398-z. [DOI] [PubMed] [Google Scholar]
- 27.Li H. M., Fan M., Xue Y., et al. Guaiane-type sesquiterpenoids from alismatis rhizoma and their anti-inflammatory activity. Chemical and Pharmaceutical Bulletin. 2017;65(4):403–407. doi: 10.1248/cpb.c16-00798. [DOI] [PubMed] [Google Scholar]
- 28.Lacaille-Dubois M.-A., Melzig M. Saponins: current progress and perspectives. Planta Medica. 2016;82(18):p. 1495. doi: 10.1055/s-0042-119776. [DOI] [PubMed] [Google Scholar]
- 29.Qiu J., Yu L., Zhang X., et al. Asiaticoside attenuates lipopolysaccharide-induced acute lung injury via down- regulation of NF-κB signaling pathway. International Immunopharmacology. 2015;26(1):181–187. doi: 10.1016/j.intimp.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L. N., Zheng J. J., Zhang L., et al. Protective effects of asiaticoside on septic lung injury in mice. Experimental and Toxicologic Pathology. 2011;63(6):519–525. doi: 10.1016/j.etp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Hu X., Fu Y., Lu X., et al. Protective effects of platycodin D on lipopolysaccharide-induced acute lung injury by activating LXRα-ABCA1 signaling pathway. Frontiers in Immunology. 2017;7 doi: 10.3389/fimmu.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao W., Su Q., Wang H., et al. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. International Immunopharmacology. 2015;27(1):138–147. doi: 10.1016/j.intimp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Li Q., Li T. Dioscin alleviates lipopolysaccharide-induced acute lung injury through suppression of TLR4 signaling pathways. Experimental Lung Research. 2020;46(1-2):11–22. doi: 10.1080/01902148.2020.1711830. [DOI] [PubMed] [Google Scholar]
- 34.Yu X., Zhang N., Lin W., et al. Regulatory effects of four ginsenoside monomers in humoral immunity of systemic lupus erythematosus. Experimental and Therapeutic Medicine. 2018;15(2):2097–2103. doi: 10.3892/etm.2017.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y., Wang Y. P., He Y. H., Ding J. C. Ginsenoside Rg1 performs anti-aging functions by suppressing mitochondrial pathway-mediated apoptosis and activating sirtuin 3 (SIRT3)/superoxide dismutase 2 (SOD2) pathway in Sca-1+ HSC/HPC cells of an aging rat model. Medical Science Monitor. 2020;26 doi: 10.12659/msm.920666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyu X., Xu X., Song A., Guo J., Zhang Y., Zhang Y. Ginsenoside Rh1 inhibits colorectal cancer cell migration and invasion in vitro and tumor growth in vivo. Oncology Letters. 2019;18(4):4160–4166. doi: 10.3892/ol.2019.10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaukat A., Guo Y. F., Jiang K., et al. Ginsenoside Rb1 ameliorates Staphylococcus aureus-induced acute lung injury through attenuating NF-κB and MAPK activation. Microbial Pathogenesis. 2019;132:302–312. doi: 10.1016/j.micpath.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q.-L., Yang L., Peng Y., et al. Ginsenoside Rg1 regulates SIRT1 to ameliorate sepsis-induced lung inflammation and injury via inhibiting endoplasmic reticulum stress and inflammation. Mediators of Inflammation. 2019;2019:10. doi: 10.1155/2019/6453296.6453296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Y., Shan Y., Bao C., Hu Y., Wang L. Ginsenoside Rg1 protects against hind-limb ischemia reperfusion induced lung injury via NF-κB/COX-2 signaling pathway. International Immunopharmacology. 2018;60:96–103. doi: 10.1016/j.intimp.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 40.Yang J., Li S., Wang L., et al. Ginsenoside Rg3 attenuates lipopolysaccharide-induced acute lung injury via MerTK-dependent activation of the PI3K/AKT/mTOR pathway. Frontiers in Pharmacology. 2018;9:p. 850. doi: 10.3389/fphar.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Yu X. F., Zhao J. J., Shi S. M., Fu L., Sui D. Y. Ginsenoside Rg3 attenuated omethoate-induced lung injury in rats. Human & Experimental Toxicology. 2016;35(6):677–684. doi: 10.1177/0960327115597984. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh Y.-H., Deng J.-S., Yuan-Shiun C., Huang G.-J. Ginsenoside Rh2 ameliorates lipopolysaccharide-induced acute lung injury by regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and Keap1/Nrf2/HO-1 signaling pathways in mice. Nutrients. 2018;10 doi: 10.20944/preprints201807.0426.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng L., Zhu Q., Xu C., et al. Glycyrrhizin mitigates radiation-induced acute lung injury by inhibiting the HMGB1/TLR4 signalling pathway. Journal of Cellular and Molecular Medicine. 2020;24(1):214–226. doi: 10.1111/jcmm.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S. A., Lee S. H., Kim J. Y., Lee W. S. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. Journal of Thoracic Disease. 2019;11(4):1287–1302. doi: 10.21037/jtd.2019.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao L., Sun T. Glycyrrhizin administration ameliorates Streptococcus aureus- induced acute lung injury. International Immunopharmacology. 2019;70:504–511. doi: 10.1016/j.intimp.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 46.Sun F., Ruan J., Zhao W., et al. New dammarane-type triterpenoid saponins from panax notoginseng leaves and their nitric oxide inhibitory activities. Molecules. 2020;25(1) doi: 10.3390/molecules25010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou K., Li Z., Zhang Y., et al. Advances in the study of berberine and its derivatives: a focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacologica Sinica. 2016;38:157–167. doi: 10.1038/aps.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y., Xu Y., Ji W., et al. Anti-tumor activities of matrine and oxymatrine: literature review. Tumor Biology. 2014;35(6):5111–5119. doi: 10.1007/s13277-014-1680-z. [DOI] [PubMed] [Google Scholar]
- 49.Liang Y., Fan C., Yan X., et al. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytotherapy Research. 2019;33(1):130–148. doi: 10.1002/ptr.6206. [DOI] [PubMed] [Google Scholar]
- 50.Lin K., Liu S., Shen Y., Li Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation. 2013;36(5):1079–1086. doi: 10.1007/s10753-013-9640-0. [DOI] [PubMed] [Google Scholar]
- 51.Liu S., Chen Q., Liu J., Yang X., Zhang Y., Huang F. Sinomenine protects against _E.coli_ -induced acute lung injury in mice through Nrf2-NF-κB pathway. Biomedicine & Pharmacotherapy. 2018;107:696–702. doi: 10.1016/j.biopha.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 52.Wang W., Yang X., Chen Q., et al. Sinomenine attenuates septic-associated lung injury through the Nrf2-Keap1 and autophagy. The Journal of Pharmacy and Pharmacology. 2020;72(2):259–270. doi: 10.1111/jphp.13202. [DOI] [PubMed] [Google Scholar]
- 53.Liou C.-J., Lai Y.-R., Chen Y.-L., Chang Y.-H., Li Z.-Y., Huang W.-C. Matrine attenuates COX-2 and ICAM-1 expressions in human lung epithelial cells and prevents acute lung injury in LPS-induced mice. Mediators of Inflammation. 2016;2016:12. doi: 10.1155/2016/3630485.3630485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y., Nie Y., Huang J., et al. Protostemonine alleviates heat-killed methicillin-resistant Staphylococcus aureus-induced acute lung injury through MAPK and NF-κB signaling pathways. International Immunopharmacology. 2019;77:p. 105964. doi: 10.1016/j.intimp.2019.105964. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y. X., He H. Q., Nie Y. J., Ding Y. H., Sun L., Qian F. Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacologica Sinica. 2018;39(1):85–96. doi: 10.1038/aps.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han J., Ma D., Zhang M., Yang X., Tan D. Natural antioxidant betanin protects rats from paraquat-induced acute lung injury interstitial pneumonia. BioMed Research International. 2015;2015:9. doi: 10.1155/2015/608174.608174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J. J., Chang Y. L., Teng C. M., Chen I. S. Vasorelaxing and antioxidant constituents from Hernandia nymphaeifolia. Planta Medica. 2001;67(7):593–598. doi: 10.1055/s-2001-17348. [DOI] [PubMed] [Google Scholar]
- 58.Borcsa B., Widowitz U., Csupor D., Forgo P., Bauer R., Hohmann J. Semisynthesis and pharmacological investigation of lipo-alkaloids prepared from aconitine. Fitoterapia. 2011;82(3):365–368. doi: 10.1016/j.fitote.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Dong X., Fu J., Yin X., et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytotherapy Research. 2016;30(8):1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prateeksha Y. M. A., Yusuf M. A., Singh B. N., et al. Chrysophanol: a natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules. 2019;9(2):p. 68. doi: 10.3390/biom9020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong Y., Zhang L., Jiang Y., Dai J., Tang L., Liu G. Emodin reactivated autophagy and alleviated inflammatory lung injury in mice with lethal endotoxemia. Experimental Animals. 2019;68(4):559–568. doi: 10.1538/expanim.19-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui H., Li S., Xu C., Zhang J., Sun Z., Chen H. Emodin alleviates severe acute pancreatitis-associated acute lung injury by decreasing pre-B-cell colony-enhancing factor expression and promoting polymorphonuclear neutrophil apoptosis. Molecular Medicine Reports. 2017;16(4):5121–5128. doi: 10.3892/mmr.2017.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin J. T., Wan B., Liu D. D., et al. Emodin alleviates lung injury in rats with sepsis. The Journal of Surgical Research. 2016;202(2):308–314. doi: 10.1016/j.jss.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 64.Xue W. H., Shi X. Q., Liang S. H., Zhou L., Liu K. F., Zhao J. Emodin attenuates cigarette smoke induced lung injury in a mouse model via suppression of reactive oxygen species production. Journal of Biochemical and Molecular Toxicology. 2015;29(11):526–532. doi: 10.1002/jbt.21723. [DOI] [PubMed] [Google Scholar]
- 65.Li A., Liu Y., Zhai L., Wang L., Lin Z., Wang S. Activating peroxisome proliferator-activated receptors (PPARs): a new sight for chrysophanol to treat paraquat-induced lung injury. Inflammation. 2016;39(2):928–937. doi: 10.1007/s10753-016-0326-2. [DOI] [PubMed] [Google Scholar]
- 66.Quan M., Lv Y., Dai Y., et al. Tanshinone IIA protects against lipopolysaccharide-induced lung injury through targeting Sirt1. The Journal of Pharmacy and Pharmacology. 2019;71(7):1142–1151. doi: 10.1111/jphp.13087. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Wu H., Niu W., et al. Tanshinone IIA attenuates paraquat-induced acute lung injury by modulating angiotensin-converting enzyme 2/angiotensin‑(1‑7) in rats. Molecular Medicine Reports. 2018;18(3):2955–2962. doi: 10.3892/mmr.2018.9281. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y., Zhang B., Xu D. Q., et al. Tanshinone IIA attenuates seawater aspiration-induced lung injury by inhibiting macrophage migration inhibitory factor. Biological & Pharmaceutical Bulletin. 2011;34(7):1052–1057. doi: 10.1248/bpb.34.1052. [DOI] [PubMed] [Google Scholar]
- 69.Tang Y., Chen Y., Chu Z., Yan B., Xu L. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. European Journal of Pharmacology. 2014;723:494–500. doi: 10.1016/j.ejphar.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 70.Jiang Y., You F., Zhu J., Zheng C., Yan R., Zeng J. Cryptotanshinone ameliorates radiation-induced lung injury in rats. Evidence-based Complementary and Alternative Medicine. 2019;2019:14. doi: 10.1155/2019/1908416.1908416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.SreeHarsha N. Embelin impact on paraquat-induced lung injury through suppressing oxidative stress, inflammatory cascade, and MAPK/NF-κB signaling pathway. Journal of Biochemical and Molecular Toxicology. 2020;34(4) doi: 10.1002/jbt.22456. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y. Y., Liu X., Zhang X., Zhang J. Shikonin improve sepsis-induced lung injury via regulation of miRNA-140-5p/TLR4-a vitro and vivo study. Journal of Cellular Biochemistry. 2020;121(3):2103–2117. doi: 10.1002/jcb.28199. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y., Xu T., Pan Z., et al. Shikonin inhibits myeloid differentiation protein 2 to prevent LPS-induced acute lung injury. British Journal of Pharmacology. 2018;175(5):840–854. doi: 10.1111/bph.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nam W., Kim S. P., Nam S. H., Friedman M. Structure-antioxidative and anti-inflammatory activity relationships of purpurin and related anthraquinones in chemical and cell assays. Molecules. 2017;22(2):p. 265. doi: 10.3390/molecules22020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu K., Wang P., Wang L., et al. Quinone derivatives from the genus Rubia and their bioactivities. Chemistry & Biodiversity. 2014;11(3):341–363. doi: 10.1002/cbdv.201200173. [DOI] [PubMed] [Google Scholar]
- 76.Hu L., Chen Z., Li L., Jiang Z., Zhu L. Resveratrol decreases CD45+CD206-subtype macrophages in LPS-induced murine acute lung injury by SOCS3 signalling pathway. Journal of Cellular and Molecular Medicine. 2019;23(12):8101–8113. doi: 10.1111/jcmm.14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang L., Zhang Z., Zhuo Y., et al. Resveratrol alleviates sepsis-induced acute lung injury by suppressing inflammation and apoptosis of alveolar macrophage cells. American Journal of Translational Research. 2018;10(7):1961–1975. [PMC free article] [PubMed] [Google Scholar]
- 78.Alghetaa H., Mohammed A., Sultan M., et al. Resveratrol protects mice against SEB-induced acute lung injury and mortality by miR-193a modulation that targets TGF-β signalling. Journal of Cellular and Molecular Medicine. 2018;22(5):2644–2655. doi: 10.1111/jcmm.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chai Y. S., Chen Y. Q., Lin S. H., et al. Curcumin regulates the differentiation of naive CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomedicine & Pharmacotherapy. 2020;125:p. 109946. doi: 10.1016/j.biopha.2020.109946. [DOI] [PubMed] [Google Scholar]
- 80.Shaikh S. B., Prabhu A., Bhandary Y. P. Curcumin suppresses epithelial growth factor receptor (EGFR) and proliferative protein (Ki 67) in acute lung injury and lung fibrosis in vitro and in vivo. Endocrine, Metabolic & Immune Disorders Drug Targets. 2020;20(4):558–563. doi: 10.2174/1871530319666190823160230. [DOI] [PubMed] [Google Scholar]
- 81.Favarin D. C., Teixeira M. M., de Andrade E. L., et al. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators of Inflammation. 2013;2013:13. doi: 10.1155/2013/164202.164202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X., Li C., Li J., Xu Y., Guan S., Zhao M. Protective effects of protocatechuic acid on acute lung injury induced by lipopolysaccharide in mice via p38MAPK and NF-κB signal pathways. International Immunopharmacology. 2015;26(1):229–236. doi: 10.1016/j.intimp.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 83.Magalhães C. B., Casquilho N. V., Machado M. N., et al. The anti-inflammatory and anti-oxidative actions of eugenol improve lipopolysaccharide-induced lung injury. Respiratory Physiology & Neurobiology. 2019;259:30–36. doi: 10.1016/j.resp.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 84.Ran X., Chao S., Jun-Gang Z., Yun H., Kuan-Bing C., Wen-Jun S. Protective effect of veratric acid on lipopolysaccharide-induced acute lung injury in mice. European Journal of Pharmacology. 2014;740:227–232. doi: 10.1016/j.ejphar.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Tianzhu Z., Shumin W. Esculin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of TLR/NF-κB pathways. Inflammation. 2015;38(4):1529–1536. doi: 10.1007/s10753-015-0127-z. [DOI] [PubMed] [Google Scholar]
- 86.Zhou B., Weng G., Huang Z., Liu T., Dai F. Arctiin prevents LPS-induced acute lung injury via inhibition of PI3K/AKT signaling pathway in mice. Inflammation. 2018;41(6):2129–2135. doi: 10.1007/s10753-018-0856-x. [DOI] [PubMed] [Google Scholar]
- 87.Zhang W. Z., Jiang Z. K., He B. X., Liu X. B. Arctigenin protects against lipopolysaccharide-induced pulmonary oxidative stress and inflammation in a mouse model via suppression of MAPK, HO-1, and iNOS signaling. Inflammation. 2015;38(4):1406–1414. doi: 10.1007/s10753-015-0115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang R., Li M. Protective effect of astragaloside IV against sepsis-induced acute lung injury in rats. Saudi Pharmaceutical Journal. 2016;24(3):341–347. doi: 10.1016/j.jsps.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S. M. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) Advances in Experimental Medicine and Biology. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niu X., Liu F., Li W., et al. Cavidine ameliorates lipopolysaccharide-induced acute lung injury via NF-κB signaling pathway in vivo and in vitro. Inflammation. 2017;40(4):1111–1122. doi: 10.1007/s10753-017-0553-1. [DOI] [PubMed] [Google Scholar]
- 91.Hu X., Shen H., Wang Y., Zhao M. Liver X Receptor Agonist TO901317 Attenuates Paraquat-Induced Acute Lung Injury through Inhibition of NF-κB and JNK/p38 MAPK Signal Pathways. BioMed Research International. 2017;2017:13. doi: 10.1155/2017/4652695.4652695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C., Huang Q., Wang C., et al. Hydroxysafflor yellow A suppress oleic acid-induced acute lung injury via protein kinase A. Toxicology and Applied Pharmacology. 2013;272(3):895–904. doi: 10.1016/j.taap.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 93.Ge X., Meng X., Fei D., Kang K., Wang Q., Zhao M. Lycorine attenuates lipopolysaccharide-induced acute lung injury through the HMGB1/TLRs/NF-κB pathway. Biotech. 2020;10(8):p. 369. doi: 10.1007/s13205-020-02364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y. L., Liu Y. J., Liu Y., et al. Hydroxysafflor yellow A ameliorates lipopolysaccharide-induced acute lung injury in mice via modulating toll-like receptor 4 signaling pathways. International Immunopharmacology. 2014;23(2):649–657. doi: 10.1016/j.intimp.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Zhang W., Wang G., Zhou S. Protective effects of isoliquiritigenin on LPS-induced acute lung injury by activating PPAR-γ. Inflammation. 2018;41(4):1290–1296. doi: 10.1007/s10753-018-0777-8. [DOI] [PubMed] [Google Scholar]
- 96.Ning J., Xu L., Zhao Q., Zhang Y. Y., Shen C. Q. The protective effects of terpinen-4-ol on LPS-induced acute lung injury via activating PPAR-γ. Inflammation. 2018;41(6):2012–2017. doi: 10.1007/s10753-018-0844-1. [DOI] [PubMed] [Google Scholar]
- 97.Wei D., Huang Z. Anti-inflammatory effects of triptolide in LPS-induced acute lung injury in mice. Inflammation. 2014;37(4):1307–1316. doi: 10.1007/s10753-014-9858-5. [DOI] [PubMed] [Google Scholar]
- 98.Shen Y., Sun Z., Guo X. Citral inhibits lipopolysaccharide-induced acute lung injury by activating PPAR-γ. European Journal of Pharmacology. 2015;747:45–51. doi: 10.1016/j.ejphar.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 99.Su K., Zhang G., Zhang X., Jiang W. Chikusetsusaponin V attenuates lipopolysaccharide-induced acute lung injury in mice by modulation of the NF-κB and LXRα. International Immunopharmacology. 2019;70:174–179. doi: 10.1016/j.intimp.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 100.Qiushi W., Guanghua L., Guangquan X. Acanthoic acid ameliorates lipopolysaccharide-induced acute lung injury. European Journal of Pharmacology. 2015;750:32–38. doi: 10.1016/j.ejphar.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 101.Lago J. H., Toledo-Arruda A. C., Mernak M., et al. Structure-activity association of flavonoids in lung diseases. Molecules. 2014;19(3):3570–3595. doi: 10.3390/molecules19033570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Y. C., Horng C. T., Chen S. T., et al. Rutin improves endotoxin-induced acute lung injury via inhibition of iNOS and VCAM-1 expression. Environmental Toxicology. 2016;31(2):185–191. doi: 10.1002/tox.22033. [DOI] [PubMed] [Google Scholar]
- 103.Li Y., Ma P., Fu J., Wu J., Wu X. Combining an in silico approach with an animal experiment to investigate the protective effect of troxerutin for treating acute lung injury. BMC Complementary and Alternative Medicine. 2019;19(1):p. 124. doi: 10.1186/s12906-019-2515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Y., Wang L., Jin M., Zang B. X. Hydroxysafflor yellow A alleviates early inflammatory response of bleomycin-induced mice lung injury. Biological & Pharmaceutical Bulletin. 2012;35(4):515–522. doi: 10.1248/bpb.35.515. [DOI] [PubMed] [Google Scholar]
- 105.Wang J., Fan S. M., Zhang J. Epigallocatechin-3-gallate ameliorates lipopolysaccharide-induced acute lung injury by suppression of TLR4/NF-κB signaling activation. Brazilian Journal of Medical and Biological Research. 2019;52(7) doi: 10.1590/1414-431x20198092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen H., Wu N., Liu Z., Zhao H., Zhao M. Epigallocatechin-3-gallate alleviates paraquat-induced acute lung injury and inhibits upregulation of toll-like receptors. Life Sciences. 2017;170:25–32. doi: 10.1016/j.lfs.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 107.Xu M. J., Liu B. J., Wang C. L., et al. Epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor and effectively alleviates acute lung injury induced by H9N2 swine influenza virus. International Immunopharmacology. 2017;52:24–33. doi: 10.1016/j.intimp.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 108.Qian J., Chen X., Chen X., et al. Kaempferol reduces K63-linked polyubiquitination to inhibit nuclear factor-κB and inflammatory responses in acute lung injury in mice. Toxicology Letters. 2019;306:53–60. doi: 10.1016/j.toxlet.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Rabha D. J., Singh T. U., Rungsung S., et al. Kaempferol attenuates acute lung injury in caecal ligation and puncture model of sepsis in mice. Experimental Lung Research. 2018;44(2):63–78. doi: 10.1080/01902148.2017.1420271. [DOI] [PubMed] [Google Scholar]
- 110.Zhang R., Ai X., Duan Y., et al. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomedicine & Pharmacotherapy. 2017;89:660–672. doi: 10.1016/j.biopha.2017.02.081. [DOI] [PubMed] [Google Scholar]
- 111.Park E. J., Kim Y. M., Kim H. J., Chang K. C. Luteolin activates ERK1/2- and Ca2+-dependent HO-1 induction that reduces LPS-induced HMGB1, iNOS/NO, and COX-2 expression in RAW264.7 cells and mitigates acute lung injury of endotoxin mice. Inflammation Research. 2018;67(5):445–453. doi: 10.1007/s00011-018-1137-8. [DOI] [PubMed] [Google Scholar]
- 112.Liu B., Yu H., Baiyun R., et al. Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: involvement of AKT/Nrf2 and NF-κB pathways. Food and Chemical Toxicology. 2018;113:296–302. doi: 10.1016/j.fct.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Wang X. F., Song S. D., Li Y. J., et al. Protective effect of quercetin in LPS-induced murine acute lung injury mediated by cAMP-Epac pathway. Inflammation. 2018;41(3):1093–1103. doi: 10.1007/s10753-018-0761-3. [DOI] [PubMed] [Google Scholar]
- 114.Meng L., Lv Z., Yu Z. Z., Xu D., Yan X. Protective effect of quercetin on acute lung injury in rats with sepsis and its influence on ICAM-1 and MIP-2 expression. Genetics and Molecular Research. 2016;15(3) doi: 10.4238/gmr.15037265. [DOI] [PubMed] [Google Scholar]
- 115.Wang B., Mao X. Protective effect of quercetin against seawater instillation-induced acute lung injurythrough preventing M1 polarization of macrophages in mice. Chinese Journal of Celluar and molecular immunology. 2017;33:751–755. [PubMed] [Google Scholar]
- 116.Wang Y. C., Liu Q. X., Zheng Q., et al. Dihydromyricetin alleviates sepsis-induced acute lung injury through inhibiting NLRP3 inflammasome-dependent pyroptosis in mice model. Inflammation. 2019;42(4):1301–1310. doi: 10.1007/s10753-019-00990-7. [DOI] [PubMed] [Google Scholar]
- 117.Jiang W., Li M., He F., et al. Protective effects of asiatic acid against spinal cord injury-induced acute lung injury in rats. Inflammation. 2016;39(6):1853–1861. doi: 10.1007/s10753-016-0414-3. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y. M., Ji R., Chen W. W., et al. Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-κB pathway. Drug Design, Development and Therapy. 2019;Volume 13:3391–3404. doi: 10.2147/DDDT.S222296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang K., Zhang T., Yin N., et al. Geraniol alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis. Oncotarget. 2017;8(41):71038–71053. doi: 10.18632/oncotarget.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiaofeng Y., Qinren C., Jingping H., et al. Geniposide, an iridoid glucoside derived fromGardenia jasminoides, protects against lipopolysaccharide-induced acute lung injury in mice. Planta Medica. 2012;78(6):557–564. doi: 10.1055/s-0031-1298212. [DOI] [PubMed] [Google Scholar]
- 121.Yang H., Li Y., Huo P., et al. Protective effect of Jolkinolide B on LPS-induced mouse acute lung injury. International Immunopharmacology. 2015;26(1):119–124. doi: 10.1016/j.intimp.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 122.San Z., Fu Y., Li W., et al. Protective effect of taraxasterol on acute lung injury induced by lipopolysaccharide in mice. International Immunopharmacology. 2014;19(2):342–350. doi: 10.1016/j.intimp.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 123.Wu Z. L., Wang J. Dioscin attenuates bleomycin-induced acute lung injury via inhibiting the inflammatory response in mice. Experimental Lung Research. 2019;45(8):236–244. doi: 10.1080/01902148.2019.1652370. [DOI] [PubMed] [Google Scholar]
- 124.Chen T., Wang R., Jiang W., et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation. 2016;39(1):483–492. doi: 10.1007/s10753-015-0272-4. [DOI] [PubMed] [Google Scholar]
- 125.Du Z. A., Sun M. N., Hu Z. S. Saikosaponin a ameliorates LPS-induced acute lung injury in mice. Inflammation. 2018;41(1):193–198. doi: 10.1007/s10753-017-0677-3. [DOI] [PubMed] [Google Scholar]
- 126.Lv H., Zhu C., Liao Y., et al. Tenuigenin ameliorates acute lung injury by inhibiting NF-κB and MAPK signalling pathways. Respiratory Physiology & Neurobiology. 2015;216:43–51. doi: 10.1016/j.resp.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 127.Zhong W.-t., Jiang L.-x., Wei J.-y., et al. Protective effect of esculentoside A on lipopolysaccharide-induced acute lung injury in mice. The Journal of Surgical Research. 2013;185(1):364–372. doi: 10.1016/j.jss.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 128.Zhang D., Li X., Hu Y., et al. Tabersonine attenuates lipopolysaccharide-induced acute lung injury via suppressing TRAF6 ubiquitination. Biochemical Pharmacology. 2018;154:183–192. doi: 10.1016/j.bcp.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 129.You Q. H., Zhang D., Niu C. C., et al. Expression of IL-17A and IL-17F in lipopolysaccharide-induced acute lung injury and the counteraction of anisodamine or methylprednisolone. Cytokine. 2014;66(1):78–86. doi: 10.1016/j.cyto.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 130.Guo S., Jiang K., Wu H., et al. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu Y., Xu D., Liu J., Gu L. Protective effect of sophocarpine on lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology. 2019;70:180–186. doi: 10.1016/j.intimp.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 132.Lee W., Yang S., Lee C., et al. Aloin reduces inflammatory gene iNOS via inhibition activity and p-STAT-1 and NF-κB. Food and Chemical Toxicology. 2019;126:67–71. doi: 10.1016/j.fct.2019.02.025. [DOI] [PubMed] [Google Scholar]


