Abstract
Background
The recombinant tetravalent live-attenuated dengue vaccine based on the YF 17D vaccine virus backbone (CYD-TDV) demonstrated vaccine efficacy (VE) against symptomatic, virologically confirmed dengue of any serotype from month 13 to month 25 (VCD-DENV-AnyM13→M25) in the CYD14 (2–14-y-olds) and CYD15 (9–16-y-olds) phase 3 trials. Fifty percent plaque reduction neutralization test (PRNT50) titers are a potential surrogate for immunobridging VE to adults.
Methods
Using PRNT50 calibration datasets, we applied immunobridging approaches using baseline and/or M13 PRNT50 titers to estimate VE against VCD-DENV-AnyM0→M25 and against hospitalized VCD (HVCD)-DENV-AnyM0→M72 in hypothetical 18–45-y-old and 46–50-y-old CYD14 and CYD15 cohorts.
Results
Baseline and M13 geometric mean PRNT50 titers were greater in 18–45-y-olds and in 46–50-y-olds vs 9–16-y-olds for most comparisons. Estimated VE (95% CIs against VCD-DENV-AnyM0→M25 ranged from 75.3% to 90.9% (52.5% to 100%) for 18–45-y-olds and 74.8% to 92.0% (53.4% to 100%) for 46–50-y-olds. Estimated VE (95% CIs) against HVCD-DENV-AnyM0→M72 ranged from 58.8% to 78.1% (40.9 to 98.9%) for 18–45-y-olds and 57.2% to 78.4% (40.5 to 97.6%) for 46–50-y-olds. Corresponding predictions among baseline-seropositive individuals yielded comparable or higher VE estimates.
Conclusions
VE M0→M25 against DENV-Any and VE against HVCD-DENV-AnyM0→M72 are both expected to be higher in 18–45 and 46–50-y-olds vs CYD14 and CYD15 9–16-y-olds.
Keywords: CYD-TDV, dengue vaccine, immunobridging, neutralizing antibodies, surrogate endpoint, vaccine efficacy
Introduction
The global health and economic burdens of dengue include 390 million (including 96 clinically significant) estimated annual infections1 and an estimated annual cost of US 8.9 billion.2 Approximately 40% of the world's population is at risk of dengue; climate change, population growth and urbanization are projected to increase this figure to 60% by 2080.3 Dengue and severe dengue can occur in individuals of any age, and severe dengue is a leading cause of hospitalization and death in both children and adults in many countries in Asia and Latin America.4 An effective preventive dengue vaccine licensed for adults could reduce global dengue morbidity and mortality.
8.9 billion.2 Approximately 40% of the world's population is at risk of dengue; climate change, population growth and urbanization are projected to increase this figure to 60% by 2080.3 Dengue and severe dengue can occur in individuals of any age, and severe dengue is a leading cause of hospitalization and death in both children and adults in many countries in Asia and Latin America.4 An effective preventive dengue vaccine licensed for adults could reduce global dengue morbidity and mortality.
The recombinant tetravalent live-attenuated dengue vaccine based on the YF 17D vaccine virus backbone [CYD-TDV (Dengvaxia); Sanofi Pasteur, Lyon, France] is administered in three doses (M0, M6 and M12) (‘Mx’ denotes time since first vaccination, where x=the number of months). The CYD-TDV vaccine demonstrated vaccine efficacy (VE) against symptomatic, virologically confirmed dengue (VCD) of any serotype (DENV-Any) between M13 and M25 (VCD-DENV-AnyM13→M25) in two phase 3 trials: Estimated VE in CYD14 (2–14-y-olds in Asia) was 56.5%; estimated VE in CYD15 (9–16-y-olds in Latin America) was 60.8%. VE against VCD through M25 was higher in baseline-seropositive than baseline-seronegative participants,5,6 and vaccination decreased the risk of severe VCD and hospitalized VCD (HVCD) through M60 in baseline-seropositive individuals.7 However, vaccination increased the risk of severe VCD and HVCD through M60 in baseline-seronegative individuals.7 The WHO recommends ‘a pre-vaccination screening strategy, in which only dengue-seropositive persons are vaccinated’.8
No direct efficacy data are available for CYD-TDV in individuals aged >16 y. Neutralizing antibodies are mechanistic correlates of protection for many licensed vaccines9 and baseline and M13 PRNT50 titers strongly associate with VE against VCD-DENV-AnyM13→M25 in both baseline-seropositive and baseline-seronegative subgroups.10 We previously used epidemiological immunobridging methods and PRNT50 titer measurements in adult CYD-TDV vaccine recipient populations not included in the CYD14 and CYD15 trials as surrogate endpoints to estimate the VE of CYD-TDV in adult populations. We concluded that, in dengue-endemic settings, VE against VCD-DENV-AnyM0→M25 is expected to be higher in 18–45-y-olds compared with that observed in 9–16-y-olds in CYD14 and CYD15 and that overall VE against HVCD-DENV-AnyM0→M72 is expected to be positive in 18–45-y-olds.11
Here, we assess the robustness and replicability of Gilbert et al.11 by including an additional bridging population and an additional calibration study. We bridge CYD-TDV VE against VCD-DENV-AnyM0→M25 and against VCD of each serotype separately and also bridge CYD-TDV VE against HVCD-DENV-AnyM0→M72 to hypothetical CYD14 and CYD15 18–45-y-old and 46–50-y-old cohorts, using different calibration studies. We also bridge VE separately to baseline-seropositive individuals. One potential application of such immunobridging analyses is to provide information on age groups for whom efficacy data are not available to extend the target population for whom CYD-TDV vaccination is indicated.
Methods
Study objectives
First, baseline and M13 PRNT50 titer distributions were compared between 18–45-y-olds or 46–50-y-olds with 9–16-y-olds in the CYD65 cohorts, with non-inferiority assessed by the lower 95% confidence bound (LB) about the ratio (adults vs children) of geometric mean titer (rGMT). No acceptability limit was prespecified; nevertheless, LBs >1.0 indicate non-inferiority, as they imply the stronger conclusion of superiority.
Second, we used the Gilbert and Huang method12 (see Gilbert et al.11) to estimate VE against VCD-DENV-Any and against each serotype-specific VCD endpoint occurring between M0 and M25 in a target population. For the VCD-DENV-Any endpoint, this multiplicative VE parameter is one minus the ratio (vaccine vs placebo) of the cumulative probabilities of VCD occurrence by M25. For the separate serotype (v)-specific endpoints, we estimated VE(v) for v=1, 2, 3 and 4, where VE(v) is defined as having any VCD occurrence of serotype-v by M25. We also estimated corresponding additive–difference versions of the VE parameters, VEd and VEd(v), which are the differences in cumulative probabilities between vaccine and placebo. We further estimated VE against HVCD-DENV-Any occurring between M0 and M72 in the target population. Multiplicative and additive-difference versions of the VE parameters were studied.
For bridging, the VE parameters were estimated for 18–45 and 46–50-y-olds in the hypothetical setting where the entire CYD14 and CYD15 study (all countries in CYD14 and CYD15) had included an 18–45-y-old or a 46–50-y-old cohort under the same inclusion rules and identical follow-up conditions as for the 9–16-y-old cohort. Prediction of VE in 18–45-y-olds or 46–50-y-olds was performed using the corresponding age cohort in CYD22 and/or CYD65 as a ‘calibration study’.
Calibration studies
CYD22 (NCT00875524) was a phase 2 trial of the CYD-TDV vaccine in Vietnam that enrolled 9–45-y-olds.13 CYD65 (NCT02628444) is a phase 2 trial of the CYD-TDV vaccine being conducted in Colombia (CYD-COL cohort) and the Philippines (CYD-65 cohort) that enrolled 9–50-y-olds. Both studies measured M0 and M13 PRNT50 titers (CYD22: 9–16-y-olds and 18–45-y-olds, CYD65: 9–16-y-olds and 18–50-y-olds) according to a previously described method.14 These PRNT50 measurements allow estimation of how PRNT50 titer distributions change with age. Our previous analysis11 used CYD22 as a ‘calibration study’. Here, we considered multiple calibration studies:
CYD65 Philippines (CYD65-PHL): 9–16 vs 18–45 and vs 46–50.
CYD65 Colombia (CYD65-COL): 9–16 vs 18–45 and vs 46–50.
CYD65 (CYD65-Overall, Philippines and Colombia): 9–16 vs 18–45 and vs 46–50.
CYD65 (Philippines and Colombia) and CYD22 (Vietnam): 9–16 vs 18–45.
Assuming that the age differences in titer distributions in these calibration studies would be the same in CYD14 and CYD15 allows using the M0 and M13 PRNT50 titers from CYD14 and CYD15 9–16-y-olds plus calibrations from these studies to estimate PRNT50 titer distributions by age in hypothetical CYD14 and CYD15 18–45 and/or 46–50-y-old cohorts.11 Note that CYD65 consisted of three randomized treatment arms where individuals were assigned to receive one, two or three vaccinations. The distribution of M13 PRNT50 titer in CYD65 was constructed only based on individuals randomized to the arm with three vaccinations. Estimation of the M0 PRNT50 titer in CYD65 was pooled over all vaccine arms to improve precision.
Bridging approaches
The immunobridging approaches are based on estimating different VE curves in CYD14 and CYD15 9–16-y-olds based on M0 and/or M13 serotype-specific or average PRNT50 titer. We estimated serotype-specific VE curves and the overall VE curve for VCDM0→M25 as functions of M13 homologous titers or average titer using the Juraska, Huang and Gilbert method15 with a hinge correlate of risk model used in Moodie et al.10 We estimated the four serotype-specific VE curves and the overall VE curve through M13 or M25 as functions of baseline homologous titers or average titer using the Huang, Gilbert and Janes logistic regression method.16
For predicting VE against VCDM0→M25, the following approaches were used:
Serotype-specific VE approach 1: based on VE(25, v) curves by M13 serotype v titers and VE(13, v) curves by M0 serotype v titers.
Serotype-specific VE approach 2: based on VE(25, v) curves by M0 serotype v titers.
Overall VE approach 1: based on VE(25, v) curves by M13 serotype v titers and VE(13, v) curves by M0 serotype v titers; aggregate the serotype-specific results to get overall VE.
Overall VE approach 2: based on VE(25, v) curves by M0 serotype v titers; aggregate the serotype-specific results to get overall VE.
Overall VE approach 1 average titer (AT): based on VE(25) curves by M13 average titers and VE(13) curves by M0 average titers.
Overall VE approach 2 AT: based on VE(25) curves by M0 average titers.
For predicting VE against HVCD-DENV-Any through M72, overall VE approach 1 AT and approach 2 AT were used.
Point estimates and 95% bootstrap percentile CIs are presented for each VE parameter of interest.
Key assumptions
Here, we briefly summarize the key assumptions: (1) the relevant VE-by-baseline and/or -month 13 titer curve in the older target cohort is the same as the corresponding VE-by-titer curve in the 9–16-y-old cohort; (2) the cumulative distribution functions of each titer variable in the older target cohort and in the 9–16-y-old cohort are linked by a mixed binary and continuous location shift model, where the OR of positive response and the location shift parameter comparing the older cohort and 9–16-y-old cohort are the same between the CYD14–15 study and the calibration study; and (3) after accounting for baseline titer and/or month 13 titer, age does not affect background/unvaccinated risk. Further details are available in Supplementary Text 1 and Gilbert et al.11
Sensitivity analysis
We conducted a sensitivity analysis (Supplementary Text 2) to possible violations of assumptions 1 and 3 by varying two sensitivity parameters, ɸ (ratio of the VCD-DENV-v VE curves between the two age cohorts) and ρ (ratio of the background [unvaccinated] incidence of VCD-DENV-v between the two age cohorts), for serotype-specific VE approaches 1 and 2. Parallel approaches were used for the other methods.
Results
Bridging cohorts
Tables 1 and 2 show the sample sizes and distributions of M13 (Table 1) and M0 (Table 2) PRNT50 titers in four cohorts (CYD22, CYD65-Overall, CYD65-PHL and CYD65-COL) across the three age subgroups, with the rGMTs of PRNT50 titers across age groups.
Table 1.
Comparison of month 13 neutralizing antibody titers (50% plaque reduction neutralization test) in CYD-TDV vaccine recipients receiving all three vaccinations between age cohorts (18–45 vs 9–16 y; 46–50 vs 9–16 y) in the CYD22, CYD65-Overall, CYD65-PHL, CYD65-COL, and CYD14 and CYD15 studies
| CYD22* | CYD65-Overall | CYD65-PHL | CYD65-COL | CYD14 and CYD15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 18–45 | 9–16 | 18–45 | 46–50 | 9–16 | 18–45 | 46–50 | 9–16 | 18–45 | 46–50 | 9–16 | 9–16 | |
| Sample size | 18 | 32 | 173 | 43 | 97 | 88 | 26 | 51 | 85 | 17 | 46 | 1870 | |
| Average titer | Response rate (95% CI) | 100.0% (82.4 to 100.0%) | 100.0% (89.3 to 100.0%) | 100.0% (97.8 to 100.0%) | 100.0% (91.8 to 100.0%) | 100.0% (96.2 to 100.0%) | 100.0% (95.8 to 100.0%) | 100.0% (87.1 to 100.0%) | 100.0% (93.0 to 100.0%) | 100.0% (95.7 to 100.0%) | 100.0% (81.6 to 100.0%) | 100.0% (92.3 to 100.0%) | 99.8% (99.5 to 99.9%) |
| GM (95% CI) | 549.6 (392.6 to 769.3) | 204.5 (125.6 to 333.0) | 633.3 (544.0 to 737.2) | 582.9 (413.0 to 822.7) | 255.4 (195.2 to 334.2) | 692.8 (601.8 to 797.5) | 733.8 (600.8 to 896.4) | 371.3 (268.3 to 514.0) | 577.0 (438.3 to 759.6) | 409.9 (176.4 to 952.4) | 168.7 (111.1 to 256.1) | 374.8 (352.5 to 398.5) | |
| GM ratio (95% CI) | 2.69 (1.51 to 4.79) | Ref | 2.48 (1.82 to 3.37) | 2.28 (1.48 to 3.52) | Ref | 1.87 (1.31 to 2.65) | 1.98 (1.36 to 2.88) | Ref | 3.42 (2.08 to 5.61) | 2.43 (0.97 to 6.12) | Ref | - | |
| GM ratio (95% CI) | 1.47 (1.04 to 2.06) | - | 1.69 (1.43 to 1.99) | 1.56 (1.10 to 2.21) | - | 1.85 (1.59 to 2.15) | 1.96 (1.59 to 2.41) | - | 1.54 (1.16 to 2.04) | 1.09 (0.47 to 2.55) | - | Ref | |
| DENV-1 | Response rate (95% CI) | 100.0% (82.4 to 100.0%) | 96.9% (84.3 to 99.4%) | 97.7% (94.2 to 99.1%) | 93.0% (81.4 to 97.6%) | 94.8% (88.5 to 97.8%) | 100.0% (95.8 to 100.0%) | 100.0% (87.1 to 100.0%) | 100.0% (93.0 to 100.0%) | 95.3% (88.5 to 98.2%) | 82.4% (59.0 to 93.8%) | 89.1% (77.0 to 95.3%) | 94.8% (93.7 to 95.7%) |
| GM (95% CI) | 695.5 (335.3 to 1442.7) | 176.7 (87.3 to 357.7) | 767.1 (607.3 to 968.9) | 698.9 (396.2 to 1232.9) | 193.2 (134.9 to 276.7) | 866.4 (689.1 to 1089.5) | 901.2 (600.9 to 1351.4) | 339.5 (210.4 to 547.9) | 676.3 (446.2 to 1025.0) | 473.8 (120.5 to 1863.2) | 103.4 (62.9 to 170.0) | 352.0 (321.1 to 385.8) | |
| GM ratio (95% CI) | 3.94 (1.47 to 10.54) | Ref | 3.97 (2.59 to 6.09) | 3.62 (1.86 to 7.04) | Ref | 2.55 (1.51 to 4.32) | 2.65 (1.43 to 4.91) | Ref | 6.54 (3.44 to 12.43) | 4.58 (1.09 to 19.32) | Ref | - | |
| GM ratio (95% CI) | 1.98 (0.95 to 4.12) | - | 2.18 (1.70 to 2.80) | 1.99 (1.12 to 3.53) | - | 2.46 (1.92 to 3.15) | 2.56 (1.69 to 3.87) | - | 1.92 (1.26 to 2.94) | 1.35 (0.34 to 5.31) | - | Ref | |
| DENV-2 | Response rate (95% CI) | 100.0% (82.4 to 100.0%) | 100.0% (89.3 to 100.0%) | 98.3% (95.0 to 99.4%) | 97.7% (87.9 to 99.6%) | 97.9% (92.8 to 99.4%) | 100.0% (95.8 to 100.0%) | 100.0% (87.1 to 100.0%) | 98.0% (89.7 to 99.7%) | 96.5% (90.1 to 98.8%) | 94.1% (73.0 to 99.0%) | 97.8% (88.7 to 99.6%) | 98.6% (97.9 to 99.0%) |
| GM (95% CI) | 825.2 (492.5 to 1382.6) | 350.6 (187.1 to 656.9) | 763.3 (620.1 to 939.5) | 734.3 (489.1 to 1102.5) | 283.0 (206.6 to 387.7) | 815.5 (668.1 to 995.3) | 889.2 (663.2 to 1192.1) | 357.2 (237.9 to 536.4) | 712.7 (490.6 to 1035.4) | 548.0 (206.5 to 1454.3) | 218.6 (133.5 to 357.8) | 565.3 (527.2 to 606.1) | |
| GM ratio (95% CI) | 2.35 (1.07 to 5.19) | Ref | 2.70 (1.85 to 3.93) | 2.59 (1.56 to 4.32) | Ref | 2.28 (1.46 to 3.58) | 2.49 (1.52 to 4.08) | Ref | 3.26 (1.77 to 6.01) | 2.51 (0.86 to 7.33) | Ref | - | |
| GM ratio (95% CI) | 1.46 (0.87 to 2.46) | - | 1.35 (1.08 to 1.68) | 1.30 (0.86 to 1.96) | - | 1.44 (1.17 to 1.78) | 1.57 (1.16 to 2.12) | - | 1.26 (0.86 to 1.84) | 0.97 (0.36 to 2.58) | - | Ref | |
| DENV-3 | Response rate (95% CI) | 100.0% (82.4 to 100.0%) | 96.9% (84.3 to 99.4%) | 100.0% (97.8 to 100.0%) | 97.7% (87.9 to 99.6%) | 95.9% (89.9 to 98.4%) | 100.0% (95.8 to 100.0%) | 100.0% (87.1 to 100.0%) | 100.0% (93.0 to 100.0%) | 100.0% (95.7 to 100.0%) | 94.1% (73.0 to 99.0%) | 91.3% (79.7 to 96.6%) | 97.9% (97.2 to 98.5%) |
| GM (95% CI) | 423.8 (286.3 to 627.4) | 157.1 (94.9 to 260.1) | 526.6 (444.3 to 624.1) | 482.9 (338.1 to 689.8) | 271.3 (193.5 to 380.6) | 595.5 (490.0 to 723.7) | 649.2 (482.2 to 874.0) | 449.1 (293.0 to 688.5) | 463.7 (349.6 to 615.0) | 307.1 (140.4 to 671.6) | 155.2 (94.1 to 256.0) | 431.6 (400.4 to 465.2) | |
| GM ratio (95% CI) | 2.70 (1.45 to 5.02) | Ref | 1.94 (1.33 to 2.83) | 1.78 (1.09 to 2.89) | Ref | 1.33 (0.83 to 2.11) | 1.45 (0.87 to 2.41) | Ref | 2.99 (1.69 to 5.28) | 1.98 (0.80 to 4.90) | Ref | - | |
| GM ratio (95% CI) | 0.98 (0.66 to 1.46) | - | 1.22 (1.01 to 1.47) | 1.12 (0.78 to 1.61) | - | 1.38 (1.12 to 1.70) | 1.50 (1.11 to 2.04) | - | 1.07 (0.80 to 1.44) | 0.71 (0.32 to 1.56) | - | Ref | |
| DENV-4 | Response rate (95% CI) | 100.0% (82.4 to 100.0%) | 96.9% (84.3 to 99.4%) | 100.0% (97.8 to 100.0%) | 100.0% (91.8 to 100.0%) | 99.0% (94.4 to 99.8%) | 100.0% (95.8 to 100.0%) | 100.0% (87.1 to 100.0%) | 100.0% (93.0 to 100.0%) | 100.0% (95.7 to 100.0%) | 100.0% (81.6 to 100.0%) | 97.8% (88.7 to 99.6%) | 97.8% (97.0 to 98.3%) |
| GM (95% CI) | 375.0 (250.7 to 561.0) | 179.7 (106.8 to 302.4) | 521.6 (447.7 to 607.6) | 465.9 (338.6 to 641.0) | 287.0 (225.4 to 365.4) | 547.5 (445.2 to 673.5) | 557.5 (380.1 to 817.7) | 349.1 (265.5 to 458.9) | 496.0 (394.6 to 623.5) | 354.0 (197.7 to 633.9) | 231.0 (153.1 to 348.5) | 229.8 (216.9 to 243.5) | |
| GM ratio (95% CI) | 2.09 (1.10 to 3.96) | Ref | 1.82 (1.37 to 2.42) | 1.62 (1.09 to 2.41) | Ref | 1.57 (1.12 to 2.20) | 1.60 (1.01 to 2.54) | Ref | 2.15 (1.35 to 3.43) | 1.53 (0.76 to 3.07) | Ref | - | |
| GM ratio (95% CI) | 1.63 (1.09 to 2.45) | - | 2.27 (1.93 to 2.67) | 2.03 (1.47 to 2.80) | - | 2.38 (1.92 to 2.95) | 2.43 (1.65 to 3.57) | - | 2.16 (1.71 to 2.73) | 1.54 (0.86 to 2.77) | - | Ref | |
DENV, dengue virus; GM, geometric mean; Ref, reference group in the denominator.
The CYD22 response rates, GMs and GM ratios were previously reported in11 and are included here for comparison. CYD14 and CYD15 results shown are for controls only (i.e. VCD-free through month 25).
Table 2.
Comparison of month 0 neutralizing antibody titers (50% plaque reduction neutralization test) in the CYD-TDV vaccine and placebo recipients (pooled) between age cohorts (18–45 vs 9–16 y; 46–50 vs 9–16 y) in the CYD22, CYD65-Overall, CYD65-PHL, CYD65-COL, and CYD14 and CYD15 studies
| CYD22* | CYD65-Overall | CYD65-PHL | CYD65-COL | CYD14 and CYD15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 18–45 | 9–16 | 18–45 | 46–50 | 9–16 | 18–45 | 46–50 | 9–16 | 18–45 | 46–50 | 9–16 | 9–16 | |
| Sample size | 30 | 52 | 504 | 144 | 282 | 258 | 75 | 142 | 246 | 69 | 140 | 2728 | |
| Average titer | Response rate (95% CI) | 93.3% (78.7 to 98.2%) | 75.0% (61.8 to 84.8%) | 92.9% (90.3 to 94.8%) | 90.3% (84.3 to 94.1%) | 74.8% (69.4 to 79.5%) | 99.6% (97.8 to 99.9%) | 100.0% (95.1 to 100.0%) | 83.1% (76.1 to 88.4%) | 85.8% (80.9 to 89.6%) | 79.7% (68.8 to 87.5%) | 66.4% (58.3 to 73.7%) | 80.1% (78.5 to 81.5%) |
| GM (95% CI) | 156.2 (93.2 to 262.0) | 39.0 (23.7 to 64.1) | 229.8 (201.9 to 261.6) | 207.2 (158.2 to 271.6) | 56.5 (45.0 to 70.8) | 272.3 (242.8 to 305.5) | 277.1 (228.6 to 335.9) | 76.2 (57.0 to 101.7) | 192.4 (152.1 to 243.3) | 151.1 (89.7 to 254.5) | 41.7 (29.5 to 58.8) | 94.1 (87.6 to 101.1) | |
| GM ratio (95% CI) | 4.00 (1.98 to 8.10) | Ref | 4.07 (3.14 to 5.28) | 3.67 (2.58 to 5.22) | Ref | 3.58 (2.62 to 4.88) | 3.64 (2.58 to 5.14) | Ref | 4.62 (3.05 to 6.99) | 3.63 (1.95 to 6.75) | Ref | - | |
| GM ratio (95% CI) | 1.66 (0.99 to 2.80) | - | 2.44 (2.11 to 2.83) | 2.20 (1.67 to 2.91) | - | 2.89 (2.53 to 3.31) | 2.94 (2.40 to 3.62) | - | 2.04 (1.60 to 2.61) | 1.61 (0.95 to 2.72) | - | Ref | |
| DENV-1 | Response rate (95% CI) | 90.0% (74.4 to 96.5%) | 61.5% (48.0 to 73.5%) | 89.9% (86.9 to 92.2%) | 87.5% (81.1 to 91.9%) | 61.0% (55.2 to 66.5%) | 97.7% (95.0 to 98.9%) | 98.7% (92.8 to 99.8%) | 68.3% (60.3 to 75.4%) | 81.7% (76.4 to 86.0%) | 75.4% (64.0 to 84.0%) | 53.6% (45.3 to 61.6%) | 71.5% (69.8 to 73.2%) |
| GM (95% CI) | 215.5 (107.1 to 433.6) | 44.9 (24.0 to 83.9) | 286.0 (242.2 to 337.7) | 243.3 (176.1 to 336.1) | 58.7 (44.3 to 77.8) | 295.7 (249.7 to 350.2) | 308.9 (229.7 to 415.5) | 65.7 (45.8 to 94.3) | 276.2 (206.2 to 369.8) | 187.6 (103.3 to 340.6) | 52.3 (33.8 to 80.9) | 116.1 (106.3 to 126.9) | |
| GM ratio (95% CI) | 4.80 (1.91 to 12.09) | Ref | 4.87 (3.52 to 6.75) | 4.14 (2.70 to 6.35) | Ref | Ref | 5.28 (3.13 to 8.91) | 3.59 (1.72 to 7.47) | Ref | - | |||
| GM ratio (95% CI) | 1.86 (0.92 to 3.75) | - | 2.46 (2.04 to 2.97) | 2.09 (1.50 to 2.93) | - | 2.55 (2.10 to 3.08) | 2.66 (1.95 to 3.62) | - | 2.38 (1.75 to 3.23) | 1.62 (0.88 to 2.95) | - | Ref | |
| DENV-2 | Response rate (95% CI) | 93.3% (78.7 to 98.2%) | 63.5% (49.9 to 75.2%) | 92.1% (89.4 to 94.1%) | 88.2% (81.9 to 92.5%) | 67.4% (61.7 to 72.6%) | 4.50 (3.02 to 6.69%) | 4.70 (2.95 to 7.47%) | 76.1% (68.4 to 82.3%) | 84.6% (79.5 to 88.5%) | 75.4% (64.0 to 84.0%) | 58.6% (50.3 to 66.4%) | 76.0% (74.3 to 77.5%) |
| GM (95% CI) | 290.3 (163.6 to 515.0) | 70.6 (37.3 to 133.8) | 301.1 (260.1 to 348.5) | 278.3 (202.4 to 382.7) | 67.4 (52.3 to 86.9) | 376.6 (329.0 to 430.9) | 372.7 (292.7 to 474.6) | 95.9 (68.5 to 134.2) | 238.1 (183.2 to 309.4) | 202.6 (110.0 to 373.1) | 47.2 (32.4 to 68.7) | 137.6 (126.6 to 149.5) | |
| GM ratio (95% CI) | 4.11 (1.77 to 9.56) | Ref | 4.47 (3.33 to 5.98) | 4.13 (2.75 to 6.20) | Ref | 3.93 (2.73 to 5.64) | 3.89 (2.57 to 5.87) | Ref | 5.05 (3.20 to 7.97) | 4.29 (2.10 to 8.76) | Ref | - | |
| GM ratio (95% CI) | 2.11 (1.18 to 3.76) | - | 2.19 (1.85 to 2.59) | 2.02 (1.46 to 2.81) | - | 2.74 (2.34 to 3.21) | 2.71 (2.10 to 3.50) | - | 1.73 (1.31 to 2.28) | 1.47 (0.79 to 2.73) | - | Ref | |
| DENV-3 | Response rate (95% CI) | 93.3% (78.7 to 98.2%) | 65.4% (51.8 to 76.8%) | 92.1% (89.4 to 94.1%) | 89.6% (83.5 to 93.6%) | 69.1% (63.5 to 74.3%) | 98.8% (96.6 to 99.6%) | 98.7% (92.8 to 99.8%) | 77.5% (69.9 to 83.6%) | 85.0% (80.0 to 88.9%) | 79.7% (68.8 to 87.5%) | 60.7% (52.4 to 68.4%) | 75.0% (73.4 to 76.6%) |
| GM (95% CI) | 132.2 (76.7 to 227.7) | 33.4 (20.4 to 54.8) | 253.3 (219.5 to 292.3) | 226.7 (170.9 to 300.8) | 76.1 (58.1 to 99.6) | 314.4 (270.4 to 365.5) | 320.5 (246.2 to 417.1) | 117.4 (81.6 to 168.8) | 202.0 (158.0 to 258.1) | 155.6 (93.6 to 258.9) | 49.1 (33.2 to 72.4) | 108.3 (99.8 to 117.6) | |
| GM ratio (95% CI) | 3.95 (1.92 to 8.15) | Ref | 3.33 (2.45 to 4.51) | 2.98 (2.02 to 4.40) | Ref | 2.68 (1.81 to 3.97) | 2.73 (1.75 to 4.27) | Ref | 4.12 (2.60 to 6.51) | 3.17 (1.68 to 6.00) | Ref | - | |
| GM ratio (95% CI) | 1.22 (0.70 to 2.11) | - | 2.34 (1.98 to 2.76) | 2.09 (1.56 to 2.81) | - | 2.90 (2.44 to 3.45) | 2.96 (2.25 to 3.90) | - | 1.86 (1.44 to 2.41) | 1.44 (0.86 to 2.41) | - | Ref | |
| DENV-4 | Response rate (95% CI) | 83.3% (66.4 to 92.7%) | 50.0% (36.9 to 63.1%) | 89.3% (86.3 to 91.7%) | 88.9% (82.7 to 93.0%) | 61.3% (55.5 to 66.8%) | 97.3% (94.5 to 98.7%) | 98.7% (92.8 to 99.8%) | 72.5% (64.7 to 79.2%) | 80.9% (75.5 to 85.3%) | 78.3% (67.2 to 86.4%) | 50.0% (41.8, 58.2) | 68.0% (66.3 to 69.8%) |
| GM (95% CI) | 72.1 (38.4 to 135.3) | 21.9 (13.9 to 34.6) | 127.9 (112.2 to 145.9) | 120.2 (92.5 to 156.2) | 33.7 (27.2 to 41.8) | 157.1 (136.6 to 180.7) | 159.8 (123.3 to 207.1) | 45.5 (34.2 to 60.4) | 103.1 (82.5 to 128.9) | 88.2 (55.4 to 140.6) | 24.9 (18.1 to 34.2) | 45.3 (42.3 to 48.5) | |
| GM ratio (95% CI) | 3.29 (1.53 to 7.08) | Ref | 3.79 (2.95 to 4.87) | 3.56 (2.54 to 4.99) | Ref | 3.46 (2.52 to 4.74) | 3.51 (2.40 to 5.15) | Ref | 4.14 (2.81 to 6.09) | 3.54 (2.02 to 6.20) | Ref | - | |
| GM ratio (95% CI) | 1.59 (0.85 to 3.00) | - | 2.83 (2.44 to 3.28) | 2.66 (2.03 to 3.48) | - | 3.47 (2.97 to 4.05) | 3.53 (2.70 to 4.61) | - | 2.28 (1.80 to 2.88) | 1.95 (1.22 to 3.12) | - | Ref | |
DENV, dengue virus; GM, geometric mean; Ref, reference group in the denominator.
The CYD22 response rates, GMs and GM ratios were previously reported in11 and are included here for comparison. CYD14 and CYD15 results shown are for controls only (i.e. VCD-free through month 25).
For CYD65 (that did not have a placebo group), titers were pooled over all vaccine groups (1, 2 or 3 vaccinations).
We first compared PRNT50 titers for each age subgroup across CYD22 and CYD65. Among 18–45-y-olds, CYD65-Overall vs CYD22 average GMT was not significantly different, at M13 or baseline (t-test p=0.43 and p=0.15); and among 9–16-y-olds, CYD65-Overall vs CYD22 average GMT was not significantly different, at M13 or baseline (t-test p=0.42 and p=0.18). Similarly, among the same two age groups, CYD65-Overall vs CYD22 serotype-specific GMTs were not significantly different at baseline or M13, except that baseline CYD65-Overall DENV-3 GMT tended to be higher than baseline CYD22 DENV-3 GMT, in 18–45-y-olds and in 9–16-y-olds (t-test p=0.004 and p=0.02). The empirical estimates of reverse cumulative distribution functions (rcdfs) showed similar results, with the CYD65-COL and CYD65-PHL 18–45-y-old and 9–16-y-old curves generally shifted rightwards from the corresponding CYD22 curves (Figure 1).
Figure 1.
Reverse cumulative distribution functions (rcdfs) of month 0 and month 13 50% plaque reduction neutralization test (PRNT50) neutralizing antibodies by serotype. (A) Month 0 (top row) and month 13 (bottom row) PRNT50 titers by age cohort in CYD14 and CYD15, CYD22 and CYD65-COL. (B) Month 0 (top row) and month 13 (bottom row) PRNT50 titers by age cohort in CYD14 and CYD15, CYD22 and CYD65-PHL. Month 0 PRNT50 titers: vaccine and placebo groups; Month 13 PRNT50 titers: vaccine group (participants who were randomized to receive three vaccinations). The CYD22 and CYD14 and CYD15 rcdfs were previously shown in11 and are included here for comparison. CYD14 and CYD15 results shown are for controls only (i.e. VCD-free through month 25). For CYD65 (that did not have a placebo group), baseline titers were pooled over all vaccine groups (1, 2 or 3 vaccinations).
Non-inferiority analysis of M13 titers
For CYD22, we have shown the non-inferiority of M13 and baseline titers in 18–45–y-olds vs 9–16-y-olds.11 For the CYD65-Overall, CYD65-COL and CYD65-PHL bridging cohorts, we first assessed whether M13 titers were non-inferior in 18–45–y-olds and in 46–50–y-olds vs those in (1) 9–16-y-olds in the same bridging cohort and (2) CYD14 and CYD15 9–16-y-olds. Within CYD65-Overall, M13 average titer was significantly higher in 18–45-y-olds (rGMT 2.48, LB 1.82) and in 46–50-y-olds (rGMT 2.28, LB 1.48) vs 9–16-y-olds; M13 serotype-specific titers were also significantly higher in 18–45-y-olds (Table 1). Comparing across age subgroups and trials, M13 average titer was significantly higher in CYD65-Overall 18–45-y-olds (rGMT 1.69, LB 1.43) and in CYD65-Overall 46–50-y-olds (rGMT 1.56, LB 1.10) vs CYD14 and CYD15 9–16-y-olds (Table 1). For CYD65-COL and CYD65-PHL, M13 average and serotype-specific titers were generally higher in 18–45-y-olds and in 46–50-y-olds vs 9–16-y-olds, for both the within-cohort and CYD14 and CYD15 comparisons, although many differences were not statistically significant due to the smaller sample sizes (Table 1).
Within CYD65-COL, the 18–45-y-old and 46–50-y-old curves were shifted rightwards from the 9–16-y-old curves (Figure 1). Similar results were seen for the CYD65-COL 18–45-y-old and 46–50-y-old curves vs CYD14 and CYD15 9–16-y-old curves, except with less or no right-shifts for DENV-3 and DENV-4. Within CYD65-PHL, similar results were seen. Cumulatively, the rGMT and rcdf results support non-inferiority (and usually a stronger conclusion of superiority) of M13 titers in 18–45–y-olds and in 46–50–y-olds in each CYD65 study cohort vs M13 titers in 9–16-y-olds, for most comparisons.
Non-inferiority analysis of baseline titers
Within CYD65-Overall, baseline average titer was significantly higher in 18–45-y-olds (rGMT 4.07, LB 3.14) and in 46–50-y-olds (rGMT 3.67, LB 2.58) vs 9–16-y-olds (Table 2). Comparing across age subgroups and trials, baseline average titer was significantly higher in CYD65-Overall 18–45-y-olds (rGMT 2.44, LB 2.11) and in CYD65-Overall 46–50-y-olds (rGMT 2.20, LB 1.67) vs CYD14 and CYD15 9–16-y-olds (Table 2). Baseline serotype-specific titers were also all significantly higher in CYD65-Overall 18–45-y-olds and in CYD65-Overall 46–50-y-olds vs CYD14 and CYD15 9–16-y-olds (Table 2). For CYD65-COL and CYD65-PHL, baseline average and serotype-specific titers were nearly all higher in 18–45-y-olds and in 46–50-y-olds vs 9–16-y-olds, for both the within-cohort and CYD14 and CYD15 comparisons, although once again many differences were not statistically significant due to the smaller sample sizes.
Within CYD65-COL, all baseline 18–45-y-old curves and 46–50-y-old curves were shifted rightwards from the baseline 9–16-y-old curves for titers <1000 and rightwards from the baseline CYD14 and CYD15 9–16-y-old curves (Figure 1). Similarly, within CYD65-PHL, all baseline 18–45-y-old curves and 46–50-y-old curves were shifted rightwards of the baseline 9–16-y-old curves and rightwards of the CYD14 and CYD15 9–16-y-old curves. Cumulatively, the rGMT and rcdf results support the non-inferiority of baseline titers in 18–45–y-olds and in 46–50–y-olds in each CYD65 study cohort vs baseline titers in 9–16-y-olds.
Bridging predictions of VE for hypothetical CYD14 and CYD15 18–45 and 46–50-y-old cohorts
Bridging VE against VCD through M25
We employed four approaches to predict overall VE and two approaches to predict serotype-specific VE against VCD by M25 [VE(25)] in two hypothetical CYD14 and CYD15 cohorts (18–45 and 46–50-y-olds). The time point M25 matches the active surveillance follow-up period for VCD in the CYD14 and CYD15 trials. Three approaches used baseline and M13 titers and three used only baseline titers. Table 3 presents multiplicative VCD-DENV-Any VE(25) estimates obtained using different combinations of CYD22/CYD65 calibration data. VE estimates exceeded 74% for all approaches and calibration studies, with the union of the bootstrap 95% CIs extending from 52.5% to 100%. Moreover, VE estimates were similar between 18–45 and 46–50-y-olds for all approaches and calibration studies. Supplementary Table 2 provides corresponding additive-difference VE estimates, with similar point estimates across approaches and between 18–45 and 46–50-y-olds.
Table 3.
Predicted point estimates and 95% CIs of multiplicative VE for the VCD-DENV-Any endpoint between month 0 and month 25 in the hypothetical CYD14 and CYD15 18–45-y-old and 46–50-y-old cohorts, fixing ρ=ɸ=1
| Data | Age (y) | Approach 1 | Approach 2 | Approach 1 AT | Approach 2 AT |
|---|---|---|---|---|---|
| CYD22* | 18–45 | 86 (52.5 to 100) | 84.7 (66.6 to 90.9) | 75.3 (59.3 to 100) | 84.0 (68.6 to 92.7) |
| CYD65-PHL | 18–45 | 90.9 (59.7 to 100) | 88.6 (73.1 to 92.6) | 79.1 (63.6 to 100) | 87.7 (77.6 to 93.8) |
| CYD65-COL | 18–45 | 81.1 (58.1 to 100) | 82.3 (66.8 to 88.3) | 75.0 (61.4 to 94.9) | 82.8 (71.2 to 89.9) |
| CYD65 | 18–45 | 84.4 (58.4 to 100) | 84.4 (69.2 to 89.4) | 74.7 (62.1 to 94.9) | 83.7 (73.3 to 90.6) |
| CYD22 and CYD65 | 18–45 | 83.4 (56.9 to 100) | 84.3 (69.2 to 89.5) | 75.3 (62.4 to 95.5) | 83.9 (73.2 to 9 0.7) |
| CYD65-PHL | 46–50 | 92.0 (62.2 to 100) | 89.5 (73.5 to 93.5) | 79.2 (63.4 to 100) | 87.9 (77.9 to 94.1) |
| CYD65-COL | 46–50 | 74.8 (53.4 to 99.8) | 79.8 (62.7 to 86.6) | 72.6 (59.8 to 91.7) | 80.2 (65.7 to 88.3) |
| CYD65 | 46–50 | 84.4 (58.4 to 100) | 84.4 (69.2 to 89.4) | 74.7 (62.1 to 94.9) | 83.7 (73.3 to 90.6) |
AT, average titer; VCD-DENV-Any, symptomatic, virologically confirmed dengue of any serotype; VE, vaccine efficacy.
The CYD22 results were also reported in11 and are shown here for comparison.
Table 4 presents the multiplicative serotype-specific VE(25) estimates. VE point estimates against DENV-3 and DENV-4 VCD exceeded 82% (DENV-3) and 92% (DENV-4) for all approaches and calibration studies, with the union of the bootstrap 95% CIs extending from 47.6% to 100% (DENV-3) and from 72.2% to 100% (DENV-4). VE point estimates against DENV-1 and DENV-2 VCD tended to be slightly lower and exceeded 35% (DENV-1) and 66% (DENV-2) for all approaches and calibration studies, with the union of the bootstrap 95% CIs extending from 19.1% to 100% (DENV-1) and from -58.5% to 100% (DENV-4). Supplementary Table 3 shows corresponding additive-difference serotype-specific VE estimates.
Table 4.
Predicted point estimates and 95% CIs of multiplicative VE for serotype-specific VCD between month 0 and month 25 in the hypothetical CYD14 and CYD15 18–45-y-old and 46–50-y-old cohorts, fixing ρ=ɸ=1
| Data | Age (y) | DENV-1 | DENV-2 | DENV-3 | DENV-4 | |
|---|---|---|---|---|---|---|
| CYD22* | 18–45 | Approach 1 | 56.9 (26.7 to 100) | 85.8 (−58.5 to 100) | 91.4 (49.5 to 100) | 100 (72.2 to 100) |
| Approach 2 | 76.9 (39.3 to 92.9) | 68.3 (−34.1 to 91.4) | 95 (68.1 to 97.6) | 93.2 (75.3 to 97.1) | ||
| CYD65-PHL | 18–45 | Approach 1 | 56.0 (30.4 to 100) | 92.2 (3.80 to 100) | 99.5 (58.0 to 100) | 100 (77.0 to 100) |
| Approach 2 | 84.3 (43.7 to 94.1) | 75.2 (−23.2 to 94.4) | 96.0 (68.6 to 97.4) | 94.6 (76.1 to 97.1) | ||
| CYD65-COL | 18–45 | Approach 1 | 44.4 (23.8 to 99.5) | 100 (40.7 to 100) | 84.8 (47.6 to 100) | 100 (72.8 to 100) |
| Approach 2 | 72.3 (35.5 to 86.7) | 66.0 (−27.5 to 89.5) | 93.5 (68.4 to 97.0) | 93.0 (75.6 to 97.1) | ||
| CYD65 | 18–45 | Approach 1 | 49.8 (27.9 to 100) | 92.9 (22.3 to 100) | 90.9 (55.0 to 100) | 100 (74.1 to 100) |
| Approach 2 | 76.4 (40.1 to 88.7) | 68.2 (−24.7 to 90.4) | 94.3 (68.6 to 97.1) | 93.3 (75.7 to 97.1) | ||
| CYD22 and CYD65 | 18–45 | Approach 1 | 48.9 (27.6 to 100) | 91.5 (17.4 to 100) | 89.2 (53.4 to 100) | 100 (74.2 to 100) |
| Approach 2 | 76.5 (40.1 to 88.9) | 68.1 (−25.4 to 90.5) | 94.4 (68.6 to 97.1) | 93.4 (75.6 to 97.1) | ||
| CYD65-PHL | 46–50 | Approach 1 | 56.4 (30.1 to 100) | 94.6 (15.1 to 100) | 99.3 (55.4 to 100) | 100 (74.9 to 100) |
| Approach 2 | 86.5 (44.1 to 95.8) | 76.1 (−22.1 to 95.3) | 96.1 (68.6 to 97.6) | 95.0 (76.0 to 97.2) | ||
| CYD65-COL | 46–50 | Approach 1 | 35.1 (19.1 to 82.3) | 95.9 (−20.0 to 100) | 82.3 (50.7 to 100) | 100 (73.4 to 100) |
| Approach 2 | 69.7 (32.9 to 85.0) | 59.7 (−34.7 to 87.8) | 92.8 (67.7 to 97.0) | 92.6 (75.4 to 97.1) | ||
| CYD65 | 46–50 | Approach 1 | 49.8 (27.9 to 100) | 92.9 (22.3 to 100) | 90.9 (55.0 to 100) | 100 (74.1 to 100) |
| Approach 2 | 76.4 (40.1 to 88.7) | 68.2 (−24.7 to 90.4) | 94.3 (68.6 to 97.1) | 93.3 (75.7 to 97.1) | ||
VCD, symptomatic, virologically confirmed dengue; VE, vaccine efficacy.
The CYD22 results were also reported in11 and are shown here for comparison.
Sensitivity analyses for bridging VE against VCD through M25
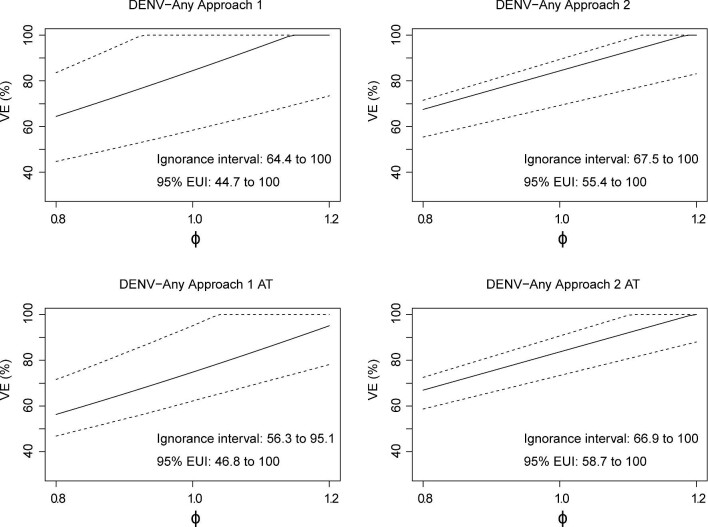
Figures 2 (ρ=1.0) and 3 (ρ=0.8) present sensitivity analyses for 18–45-y-olds with ɸ varying from 0.8 to 1.2, using CYD65 as the calibration study. The results are near identical; thus, the ratio of background VCD incidence in the two unvaccinated age cohorts has little effect on the VE estimates. Similar results based on calibration with CYD22 and CYD65 are presented in Supplementary Figures 1 and 2. Sensitivity analyses for multiplicative VE in 46–50-y-olds are presented in Supplementary Figures 3 and 4, and for additive-difference VE against VCD-DENV-Any in Supplementary Figures 5–10. The choice of ρ (1.0 or 0.8) and the calibration cohort (CYD65 or CYD22 and CYD65) had little effect on the multiplicative VE estimates; moreover, VE estimates on the multiplicative or additive-difference scale did not differ between 18–45-y-olds and 46–50-y-olds. The estimates of multiplicative VEs exceed 40% at ɸ=0.8 and increase as ɸ increases.
Figure 2.
Results bridging VE(25) to the hypothetical CYD14 and CYD15 18–45-y-old cohort based on CYD65 data. Estimated multiplicative VE against VCD-DENV-Any through month 25 is shown with 95% bootstrap CIs with the sensitivity parameter ɸ (ratio of VE curves) varying from 0.8 to 1.2 and ρ (ratio of background risk curves) equal to 1.0. Estimated ignorance intervals and 95% estimated uncertainty intervals (EUIs) are given in the lower-right corner of each plot.
Figure 3.
Bridging VE(25) to the hypothetical CYD14 and CYD15 18–45-y-old cohort based on CYD65 data. Estimated multiplicative VE against VCD-DENV-Any through month 25 is shown with 95% bootstrap CIs with the fixed sensitivity parameter ɸ (ratio of VE curves) varying from 0.8 to 1.2 and ρ (ratio of background risk curves) equal to 0.8. Estimated ignorance intervals and 95% estimated uncertainty intervals (EUIs) are given in the lower-right corner of each plot.
We also conducted bridging analyses among baseline-seropositive individuals (defined as PRNT50>10 for at least one serotype) utilizing baseline-titer based approaches. Approach 2 and Approach 2 AT were used for this analysis given that the small number of serotype-v VCD events among baseline seropositive individuals made Approach 1 infeasible. The VE estimates among baseline-seropositive individuals (Supplementary Tables 5 and 6) were higher than or comparable with those for when the bridging was performed regardless of baseline sero-status (Tables 3 and 4; Supplementary Tables 2 and 3).
Bridging VE against HVCD through M72
We previously found that VE against HVCD-DENV-Any through M72 increases with average M13 PRNT50 titer in 9–16-y-old vaccine recipients across CYD14 and CYD15.11 Next, we addressed the objective of bridging VE against HVCD through M72 [VE(72)] to the hypothetical CYD14 and CYD15 cohorts. This time point (M72) represents complete follow-up for HVCD in CYD14 and CYD15. Table 5 presents the multiplicative HVCD-DENV-Any VE(72) estimates. Within both approaches and across all calibration studies, VE estimates were similar between 18–45 and 46–50-y-olds, with slightly higher estimates obtained using Overall VE Approach 2 AT. Across both approaches and all calibration studies, VE estimates in both age cohorts exceeded 57% (95% CI LBs ranging from 40.5% to 52.6%). The additive-difference VE estimates (Supplementary Table 4) show similar results.
Table 5.
Predicted point estimates and 95% CIs of multiplicative VE for HVCD-DENV-Any endpoint between month 0 and month 72 in the hypothetical CYD14 and CYD15 18–45-y-old and 46–50-y-old cohorts, fixing ρ=ɸ=1
| Data | Age (y) | Approach 1 AT | Approach 2 AT |
|---|---|---|---|
| CYD22* | 18–45 | 59.1 (40.9 to 92.2) | 73.5 (44.7 to 89.0) |
| CYD65-PHL | 18–45 | 62.3 (43.7 to 98.9) | 78.1 (52.3 to 91.5) |
| CYD65-COL | 18–45 | 60.2 (43.9 to 88.7) | 72.2 (47.5 to 86.3) |
| CYD65 | 18–45 | 58.8 (43.9 to 85.9) | 73.2 (48.4 to 87.6) |
| CYD22 and CYD65 | 18–45 | 59.5 (44.0 to 87.2) | 73.4 (48.9 to 87.5) |
| CYD65-PHL | 46–50 | 62.2 (43.4 to 97.6) | 78.4 (52.6 to 91.6) |
| CYD65-COL | 46–50 | 57.2 (40.5 to 81.1) | 69.1 (44.1 to 84.5) |
| CYD65 | 46–50 | 58.8 (43.9 to 85.9) | 73.2 (48.4 to 87.6) |
AT, average titer; HVCD-DENV-Any, hospitalized, symptomatic, virologically confirmed dengue of any serotype; VE, vaccine efficacy.
The CYD22 results were also reported in11 and are shown here for comparison.
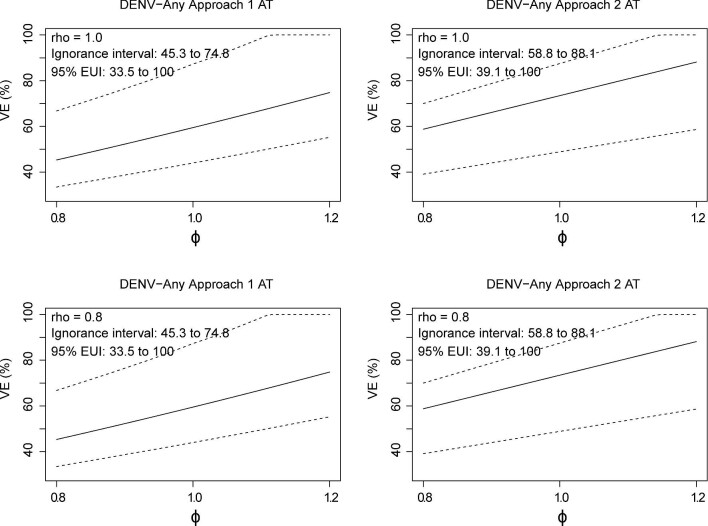
Sensitivity analyses for bridging VE against HVCD through M72
Next, we performed sensitivity analyses for VE(72) against HVCD-DENV-Any, varying ρ and ɸ. Figures 4 and 5 present sensitivity analyses for multiplicative VE in 18–45-y-olds, using CYD65 or CYD22 and CYD65 as the calibration study. Similar results were obtained across the different calibration cohorts. Corresponding results in 46–50-y-olds based on CYD65 calibration data are presented in Supplementary Figure 11. The results are nearly identical to those shown in Figure 4. Sensitivity analyses for additive-difference VE are provided in Supplementary Figures 12–14. Again, VE estimates on the additive-difference scale did not differ between 18–45-y-olds and 46–50-y-olds.
Figure 4.
Bridging VE(72) to the hypothetical CYD14 and CYD15 18–45-y-old cohort based on CYD65 data. Estimated multiplicative VE against HVCD-DENV-Any through month 72 with 95% bootstrap CIs for the sensitivity parameter ɸ (ratio of VE curves) varying from 0.8 to 1.2 and ρ (ratio of background risk curves) equal to 1.0 or 0.8. Estimated ignorance intervals and 95% estimated uncertainty intervals (EUIs) are given in the upper-left corner of each plot.
Figure 5.
Bridging VE(72) to the hypothetical CYD14 and CYD15 18–45-y-old cohort based on CYD22 and CYD65 data. Estimated multiplicative VE against HVCD-DENV-Any through month 72 is shown with 95% bootstrap CIs with the sensitivity parameter ɸ (ratio of VE curves) varying from 0.8 to 1.2 and ρ (ratio of background risk curves) equal to 1.0 or 0.8. Estimated ignorance intervals and 95% estimated uncertainty intervals (EUIs) are given in the upper-left corner of each plot.
We also used Approach 2 AT for bridging analyses among baseline-seropositive individuals. VE estimates among baseline-seropositive individuals (Supplementary Table 7) were higher than or comparable with those for when the bridging was performed regardless of baseline sero-status (Table 5, Supplementary Table 4).
Discussion
Three important additions of this work to Gilbert et al.11 are: (1) extrapolation of VE to an additional hypothetical older cohort not included in Gilbert et al.11; (2) VE bridging separately for the baseline-seropositive subgroup; and (3) the use of an additional calibration study. Next, we elaborate on the importance of these additions: (1) for the additional hypothetical older cohort (CYD14 and CYD15 46–50-y-olds), VE estimates against VCDM0→M25 and against HVCD-DENV-AnyM0→M72 were remarkably similar compared with those for hypothetical CYD14 and CYD15 18–45-y-olds, suggesting that VE would be expected to be similar between these two adult cohorts; (2) as the US Food and Drug Administration indication is for individuals ‘with laboratory-confirmed previous dengue infection’, our finding that VE estimates are in general comparable or higher in baseline seropositive adults vs estimates in adults regardless of baseline sero-status (consistent with the patterns observed in 9–16-y-olds7) is important; and (3) the additional calibration study constitutes the first use of PRNT50 data in a Latin American population for calibration.
Additional strengths of our study include the use of multiple immunobridging methods, using different combinations of baseline and M13 titers, along with the use of multiple calibration studies including PRNT50 data measured in populations in three different countries. Moreover, some of the calibration data were obtained from countries included in the CYD14 or CYD15 trial, i.e. Vietnam (CYD22 and CYD14), the Philippines (CYD65 and CYD14) and Colombia (CYD65 and CYD15). The fact that our results were remarkably consistent across bridging methods and across calibration studies—including across the geographical region of the calibration study (Asia vs Latin America)—increases the robustness of our bridging inferences.
The present work generated results that were highly consistent with those from our previous work,11 i.e. remarkably similar VE estimates against VCD-DENV-AnyM0→M25 in a hypothetical 18–45-y-old CYD14 and CYD15 cohort (present: 74.7% to 90.9% [95% CI LB 56.9%, UB 100.0%]; previous: 75.3% to 86.0% [95% CI LB 52.5%, UB 100.0%]) and remarkably similar VE estimates against HVCD-DENV-AnyM0→M72 in the hypothetical 18–45-y-old CYD14 and CYD15 cohort (present: 59.5% to 78.1% [95% CI LB 43.7%, UB 98.9%]; previous: 59.1% to 73.5% [95% CI LB 40.9%, UB 92.2%]). Considering that the present work used four new calibration datasets, the current study makes an important contribution in bolstering the robustness and replicability of our previous findings.
The current study has limitations. First, we could not perform immunobridging analyses to extrapolate VE against serotype-specific HVCD through M72 in the hypothetical CYD14 and CYD15 cohorts, because the small numbers of serotype-specific HVCD endpoints precluded generation of the relevant serotype-specific VE curves used in the immunobridging methods. Second, the follow-up time (M25) for which VE against VCD was extrapolated was relatively short, meaning that our results do not provide direct support for extrapolation of longer-term VE against VCD. The long period (median 27.1 mo) between the end of the 25-mo active surveillance phase of follow-up for VCD and the reinitiation of active surveillance at approximately M507 after an intervening passive surveillance phase hindered the extrapolation of VE against VCD through M72.
Conclusions
Our results support the conclusions of our previous immunobridging analysis, demonstrating its reproducibility with additional calibration datasets, and extend the results to 46–50-y-olds and baseline-seropositives. The VE of CYD-TDV against symptomatic, virologically confirmed dengue through month 25 is expected to be similarly higher in 18–45 and 46–50-y-olds than in CYD14 and CYD15 9–16-y-olds, for the overall and all serotype-specific VCD endpoints. Likewise, the VE of CYD-TDV against hospitalized, symptomatic, virologically confirmed dengue through month 72 is expected to be higher in 18–45 and 46–50-y-olds than in CYD14 and CYD15 9–16-y-olds.
Supplementary Material
Acknowledgements
We thank the CYD14, CYD15, CYD22 and CYD65 study participants and site staff.
Contributor Information
Ying Huang, Vaccine and Infec tious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA; Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA.
Zoe Moodie, Vaccine and Infec tious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA.
Michal Juraska, Vaccine and Infec tious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA.
Youyi Fong, Vaccine and Infec tious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA; Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA.
Lindsay N Carpp, Vaccine and Infec tious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA.
Laurent Chambonneau, Global Biostatistical Sciences, Sanofi Pasteur, Marcy-l'Etoile, 69280, France.
Diana L Coronel, Clinical Sciences, Sanofi Pasteur, Mexico City, 04000, Mexico.
Gustavo H Dayan, Sanofi Pasteur, Swiftwater, PA, 18370, USA.
Carlos A DiazGranados, Sanofi Pasteur, Marcel Mérieux, Marcy l'Etoile, 69280, France.
Peter B Gilbert, Vaccine and Infec tious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, WA, 98109, USA; Department of Biostatistics, University of Washington, Seattle, WA, 98195, USA.
Authors’ contributions
YH led the bridging study analyses, wrote the Statistical Analysis Plan, conducted analyses and made major contributions to the writing and interpretation of results; ZM conducted analyses and contributed to the writing and interpretation of results; MJ conducted analyses and contributed to the writing and interpretation of results; YF conducted analyses and contributed to the writing and interpretation of results; LNC made major contributions to the writing and interpretation of results; LC reviewed the SAP/analysis and checked accuracy and integrity; DLC led the CYD65 study and participated in data acquisition and data analysis; GHD led the CYD15 study used for the analyses and contributed to interpretation of the results; CAD contributed to the design of the analytical plan and interpreted the results; PBG led the bridging study analyses, wrote the SAP, conducted analyses and made major contributions to the writing and interpretation of results. All the authors read and approved the final manuscript. YH and PBG are guarantors of the paper.
Funding:
This work was supported by a contract from Sanofi Pasteur to PBG and by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Department of Health and Human Services, under award number R37AI054165 to PBG.
Potential conflicts of interest:
YH, ZM, MJ, YF, LNC and PBG received salary support via a contract from Sanofi Pasteur to perform the statistical analysis and submit the work for publication. LC, DLC, GHD and CAD are full-time employees and shareholders at Sanofi Pasteur.
Ethical approval:
The CYD14, CYD15, CYD22 and CYD65 study protocols were approved by all relevant regulatory bodies.
Data availability:
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, Statistical Analysis Plan and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
References
- 1. Bhatt S, Gething PW, Brady OJet al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shepard DS, Undurraga EA, Halasa YAet al. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16(8):935–41. [DOI] [PubMed] [Google Scholar]
- 3. Messina JP, Brady OJ, Golding Net al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4(9):1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Dengue and severe dengue. Available at https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue [accessed April 22, 2020]. [Google Scholar]
- 5. Capeding MR, Tran NH, Hadinegoro SRet al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384(9951):1358–65. [DOI] [PubMed] [Google Scholar]
- 6. Villar L, Dayan GH, Arredondo-Garcia JLet al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372(2):113–23. [DOI] [PubMed] [Google Scholar]
- 7. Sridhar S, Luedtke A, Langevin Eet al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379(4):327–40. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . Meeting of the Strategic Advisory Group of Experts on immunization, April 2018—conclusions and recommendations. Wkly Epidemiol Rec.2018;23(93):339. [Google Scholar]
- 9. Plotkin SA, Gilbert PB.. Correlates of Protection. Chapter 3, pp. 35–40. In Plotkin's Vaccines, 7th edition. 2018. Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Philadelphia, PA: Elsevier, Inc. [Google Scholar]
- 10. Moodie Z, Juraska M, Huang Yet al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J Infect Dis. 2018;217(5):742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbert PB, Huang Y, Juraska Met al. Bridging efficacy of a tetravalent dengue vaccine from children/adolescents to adults in highly endemic countries based on neutralizing antibody response. Am J Trop Med Hyg. 2019;101(1):164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilbert PB, Huang Y. Predicting overall vaccine efficacy in a new setting by re-calibrating baseline covariate and intermediate response endpoint effect modifiers of type-specific vaccine efficacy. Epidemiol Methods. 2016;5(1):93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tran N, Luong C, Vu Tet al. Safety and immunogenicity of recombinant, live attenuated tetravalent dengue vaccine (CYD-TDV) in healthy Vietnamese adults and children. J Vaccines Vaccination. 2012;3(7):162. [Google Scholar]
- 14. Timiryasova TM, Bonaparte MI, Luo Pet al. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg. 2013;88(5):962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juraska M, Huang Y, Gilbert PB. Inference on treatment effect modification by biomarker response in a three-phase sampling design. Biostatistics. 2018;21(3):545–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Gilbert PB, Janes H. Assessing treatment-selection markers using a potential outcomes framework. Biometrics. 2012;68(3):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, Statistical Analysis Plan and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies and process for requesting access can be found at https://www.clinicalstudydatarequest.com.