Abstract
Objectives
To examine whether the case fatality rate (CFR) of COVID-19 decreased over time and whether the COVID-19 testing rate is a driving factor for the changes if the CFR decreased.
Methods
Analyzing COVID-19 cases, deaths and tests in Ontario, Canada, we compared the CFR between the first wave and the second wave across 26 public health units in Ontario. We also explored whether a high testing rate was associated with a large CFR decrease.
Results
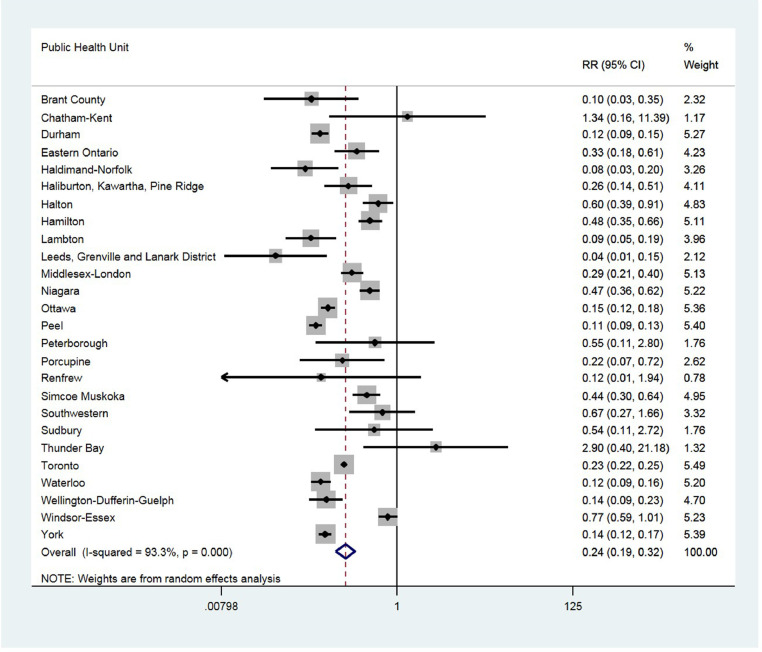
The first wave CFR ranged from 0.004 to 0.146, whereas the second wave CFR ranged from 0.003 to 0.034. The pooled RR estimate of second wave COVID-19 case fatality, compared with first wave, was 0.24 (95% CI: 0.19-0.32). Additionally, COVID-19 testing percentages were not associated with the estimated relative risk (P=0.246).
Conclusions
The COVID-19 CFR decreased significantly in Ontario during the second wave, and COVID-19 testing was not a driving factor for this decrease.
Introduction
The COVID-19 pandemic continues to surge globally. Recently, many countries experienced a substantial second wave of COVID-19 (James et al., 2021). While there were much higher infection numbers and in some countries more deaths than those in the first wave, a substantial decrease in the case fatality rate (CFR) was observed (https://www.ft.com/content/b3801b63-fbdb-433b-9a46-217405b1109f). Interestingly, researchers have reported that CFR decreased during the first wave indicating that COVID-19 severity might lessen with time (Schmidt et al., 2021; Anesi et al., 2021). However, prior studies only examined declining CFR in hospitalized patients during the first wave. Because there was a higher likelihood of detecting asymptomatic and mildly symptomatic infections during the second wave, health experts suspect that increased testing numbers may partially explain the decline in CFR (https://www.ft.com/content/b3801b63-fbdb-433b-9a46-217405b1109f). However, little is known regarding the CFR changes between the first and second waves at the population level or whether high testing rates were associated with the CFR decrease. Determining the magnitude of the CFR decrease and the association between COVID-19 testing rates and CFR changes could have implications for future public health interventions.
Methods
We conducted a cross-sectional study using publicly reported COVID-19 cases, deaths, and tests available on the Ontario Public Health website as of February 10, 2021 (https://covid-19.ontario.ca/data). In Ontario, Canada, the peak 7-day moving average during the first and second waves was 640 in April 2020 and 4249 in January 2021. As the number of new cases in Ontario dropped below 200 per day between late June and early September 2020, we used July 31 to separate the first and second waves. We also conducted sensitivity analyses using June 30, 2020, and August 31, 2020, as the cut-off dates. We determined the CFR per public health unit (PHU) for both the first and second waves, defined as the number of deaths divided by the number of new cases. PHUs which had no COVID-19 deaths in the first or second wave were excluded. We calculated the relative risk (RR) of COVID-19 CFR between the 2 waves (the second versus the first wave) and the corresponding 95% CI for each PHU. Using a random-effects meta-analysis with inverse variance weighting, we estimated the pooled RR on population-level CFR across PHUs. Using meta-regression techniques, we regressed the logarithm form of effect size by the second wave testing percentage (the number of tests divided by the total population). Acknowledging that second-wave patients were younger than in the first wave, we also analyzed individual-level data. Applying hierarchical generalized linear models, we estimated the adjusted odds ratio (AOR), clustering by PHUs and controlling for patient age, wave, and testing percentage.
Results
Of the 34 PHUs in Ontario, Canada, 26 PHUs had more than 1 death in the first and second waves and were therefore included in the analyses. The first wave CFR ranged from 0.004 to 0.146, whereas the second wave CFR ranged from 0.003 to 0.034. There was substantial variation in the RRs between the 2 waves across PHUs (Figure 1 ). The pooled RR estimate of second wave COVID-19 case fatality, compared with first wave, was 0.24 (95% CI: 0.19-0.32). Meta-regression models showed that COVID-19 testing percentages were not statistically significantly associated with the estimated RR (P=0.246) despite a trend that the higher the percentage of COVID-19 testing, the smaller the effect size, i.e., RR close to 1 (Appendix Figure). Individual-level data analyses, adjusting for age, revealed that the AOR of the second wave, compared with the first wave, was 0.41 (95% CI: 0.38-0.43; Table 1 ). Sensitivity analyses using 2 different dates to define the second wave reached similar conclusions (data not shown).
Figure 1.
COVID-19 case fatality in the first versus the second wave in Ontario, Canada
RR: relative risk
Table 1.
Unadjusted and adjusted odds ratio for the analyses using individual-level data
| Individual-level analysis |
|||
|---|---|---|---|
| Unadjusted | Model 1* | Model 2* | |
| 2nd wave (ref: 1st wave) | 0.21 (0.20, 0.23) |
0.42 (0.39, 0.44) |
0.42 (0.39, 0.44) |
| Public health unit-level testing percentage (continuous variable) | 0.38 (0.05, 2.81) |
||
Model 1 adjusted for patient age and wave; model 2 adjusted for age, wave, and public health unit-level second-wave testing percentage.
Discussion
We found that the COVID-19 CFR decreased significantly in Ontario during the second wave. Young age contributed to this decrease, but high COVID-19 testing was not a driving factor. Potential reasons for the decrease in COVID-19 CFR may be attributed to improvement in clinical care: for example, there was increasing evidence to support corticosteroid use (Chris Baraniuk, 2021), and Canada approved effective treatments, such as Remdesivir and Bamlanivimab in July and November 2020, respectively (https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/list-drugs.html). Additionally, our findings suggest that SARS-CoV-2, despite becoming more transmissible, might decline in virulence over time. Indeed, researchers have demonstrated a dynamic shift in SARS-CoV-2 variants to those with increased survival (Esper et al., 2021). As SARS-CoV-2 genome sequencing in Ontario during the study period is lacking, research on the CFR of variants is needed. Increased public awareness during the second-wave period, such as compliance with wearing masks, could also decrease the viral inoculum, leading to milder and asymptomatic infection manifestations (Gandhi et al., 2020). While our random-effects models account for PHU-level characteristics, we acknowledge that we are unable to control for confounding factors. To evaluate COVID-19 intervention programs, researchers and policy decision-makers should account for the changes in COVID-19 severity.
Declaration of Competing of Interest
Dr. Gross reported receiving research grants from the National Comprehensive Cancer Network (funded by Pfizer and AstraZeneca), funding from Johnson and Johnson to assist with developing new approaches to sharing clinical trial data (through the Yale Open Data Access Project), and funding from Flatiron, Inc., for travel/speaking, outside the submitted work. No other disclosures were reported.
Acknowledgments
Acknowledgments/Funding/Support
None
Ethical Approval
Not applicable
References
- Anesi GL, Jablonski J, Harhay MO, et al. Characteristics, Outcomes, and Trends of Patients With COVID-19-Related Critical Illness at a Learning Health System in the United States. Ann Intern Med. 2021 doi: 10.7326/M20-5327. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniuk C. Where are we with drug treatments for covid-19? BMJ. 2021;373:n1109. doi: 10.1136/bmj.n1109. http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- Cookson C, Burn-Murdoch J. Why the second wave of COVID-19 appears to be less lethal. Financial Times. October 21, 2020 https://www.ft.com/content/b3801b63-fbdb-433b-9a46-217405b1109f Available at. Accessed on March 24, 2021. [Google Scholar]
- COVID-19 (coronavirus) in Ontario. Available at https://covid-19.ontario.ca/data. Accessed on January 31, 2021.
- Drug and vaccine authorizations for COVID-19: List of authorized drugs, vaccines and expanded indications. https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/list-drugs.html. Assessed on June 14, 2021.
- Esper FP, Cheng Y, Adhikari TM, et al. Genomic Epidemiology of SARS-CoV-2 Infection During the Initial Pandemic Wave and Association With Disease Severity. JAMA Network Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020 Oct; 35;(10):3063–3066. doi: 10.1007/s11606-020-06067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James N, Menzies M, Radchenko P. COVID-19 second wave mortality in Europe and the United States. Chaos. 2021;31 doi: 10.1063/5.0041569. [DOI] [PubMed] [Google Scholar]
- Public Health Ontario . 2021. Summary Report. SARS-CoV-2 Variants of Concern: Results of Point Prevalence Study.https://www.publichealthontario.ca/-/media/documents/ncov/voc/2021/02/sars-cov-2-variants-point-prevalence.pdf?la=en Available at. Accessed on March 24, 2021. [Google Scholar]
- Schmidt M, Hajage D, Demoule A, et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]