Abstract
The pandemic of severe acute respiratory syndrome coronavirus 2 raised the attention towards bacterial coinfection and its role in coronavirus disease 2019 (COVID-19) disease. This study aims to systematically review and identify the pooled prevalence of bacterial coinfection in the related articles. A comprehensive search was conducted in international databases, including MEDLINE, Scopus, Web of Science, and Embase, to identify the articles on the prevalence of bacterial coinfections in COIVD-19 patients from 1 December 2019 until 30 December 2020. All observational epidemiological studies that evaluated the prevalence of bacterial coinfections in patients with COVID-19 were included without any restriction. Forty-two studies including a total sample size of 54,695 were included in the analysis. The pooled estimate for the prevalence of bacterial coinfections was 20.97% (95% CI: 15.95–26.46), and the pooled prevalence of bacterial coinfections was 5.20% (95% CI: 2.39–8.91) for respiratory subtype and 4.79% (95% CI: 0.11–14.61) for the gastrointestinal subtype. The pooled prevalence for Eastern Mediterranean Regional Office and South-East Asia Regional Office was 100% (95% CI: 82.35–100.00) and 2.61% (95% CI: 1.74–3.62). This rate of coinfection poses a great danger towards patients, especially those in critical condition. Although there are multiple complications and adverse effects related to extensive use of antibiotics to treat patients with COVID-19, it seems there is no other option except applying them, and it needs to be done carefully.
Keywords: Coinfection, coronavirus, COVID-19, meta-analysis, systematics review
Highlights
-
•
Bacterial coinfections are prevalent in coronavirus disease 2019 (COVID 19).
-
•
Respiratory bacterial coinfections are the most coinfection in COVID 19.
-
•
Eastern Mediterranean Regional Office and South-East Asia Regional Office has the most and least pooled prevalence of bacterial coinfection in COVID 19.
Introduction
Bacterial coinfection played an important role in escalating the morbidity and mortality rate during previous viral outbreaks and pandemics [1]. Most patient's death during 1918–1919 influenza pandemic was related to bacterial co-pathogens rather than the virus itself [2]. During H1N1 pandemics, several studies recorded the high prevalence of secondary and bacterial coinfection [3]. It was also reported that people with bacterial coinfection showed high number of mortalities. Critically ill patients showed greater percentage of coinfection compared with hospitalized patients [4]. Previous experience during other respiratory viral infections supported the use of antibiotics; so, at the onset of COVID-19 infection, early guidelines for COVID-19 treatment suggested the use of antibiotics in all the patients [5,6]. Identification of prevalence of bacterial coinfection is crucial for the initial empiric antibiotic treatment, in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients. The different possible complications could occur because of the extensive implication of antibiotics in patients. Antibacterial resistance is one of the challenges because of this amount of antibiotics use, which can affect the societies in the next years [7,8]. But because of similar clinical and radiological manifestation of some respiratory bacterial pathogen, such as pneumococcal, staphylococcal, and Klebsiella with COVID-19, it is difficult to decide which patients should receive antibiotics treatment, especially at the first encounter with the patients [9].
Materials and methods
All steps in this systematic review and meta-analysis study were based on preferred reporting items for systematic review and meta-analysis guidelines [10] and registered in the International Prospective Register of Systemic Reviews with CRD42021240030. Using related keywords such as “COVID-19”, “Coronavirus”, “SARS-CoV-2 infection”, “SARS-CoV-2”, “Polymicrobial Infection”, “Bacterial AND Coinfections”, “Bacterial AND Secondary Infections”, and “Mixed Infections”, all related articles were retrieved.
Method of literature search
A complete and comprehensive search without any language restrictions was conducted in international databases, including MEDLINE, Scopus, Web of Science, and Embase, to identify the articles on the prevalence of bacterial coinfections in patients with COIVD-19 from 1 December 2019 until 30 December 2020, in English and non-English language. Other sites, including Medrxiv and Social Science Research Network (SSRN), were also searched to identify the unofficially published researches. The text words and Medical Subject Headings (MeSH) terms of COIVD-19 and coinfections were used to search. The PICOTS in our study was as follows:
Population: Patients with COVID-19
Intervention: None
Comparison: None
Outcome: Prevalence of bacterial coinfections
Time: from 1 December 2019 until 30 December 2020
Study design: Observational study
The search strategy is described below that is applied based on PICOTS for MEDLINE (MeSH) and then used in other databases:
-
1.
COVID-19 [text word] OR COVID-19 [Mesh term]
-
2.
Coronavirus Disease-19 [text word] OR Coronavirus Disease-19 [Mesh term]
-
3.
SARS-CoV-2 infection [text word] OR SARS-CoV-2 infection [Mesh term]
-
4.
1 OR 2 OR 3
-
5.
Prevalence [text word] OR Prevalence [Mesh term]
-
6.
Frequency [text word] OR Frequency [Mesh term]
-
7.
Incidence [text word] OR Incidence [Mesh term]
-
8.
5 OR 6 OR 7
-
9.
Coinfection [text word] OR Coinfection [Mesh term]
-
10.
Mixed Infection [text word] OR Mixed Infection [Mesh term]
-
11.
Polymicrobial Infection [text word] OR Polymicrobial Infection [Mesh term]
-
12.
Bacterial Coinfection [text word] OR Bacterial Coinfection [Mesh term]
-
13.
9 OR 10 OR 11 OR 12
-
14.
4 AND 8 AND 13
Google Scholar was used to accessing grey literature. Also, a bacteriology expert was consulted to find relevant articles, and also, we try to find other articles by handsearching from the references list of relevant articles. Then, all data were imported to Endnote X6, and after removing the duplicated articles, the remaining studies has been screening in three steps. In the first step, the titles were reviewed, and if the article was relevant, then the abstract and then the full text of the articles were reviewed. The three steps were followed independently by two raters, “Reza Pakzad” and “Saber Soltani”, and interrater discrepancies were resolved based on the third person's opinion, “Iraj Pakzad”. Blinding and task separation were applied in study procedure selection. The interrater agreement was 89%.
Inclusion and exclusion criteria
All observational epidemiological studies, including cohort, cross-sectional, and case series studies around the world, that examined the prevalence of bacterial coinfections in patients with COVID-19 were included without any restriction. Case reports and case series with less than ten sample sizes were excluded. Also, editorials, commentaries, case–control, randomized clinical trial, and reviews were excluded.
Data extraction
In addition to general information, including the name of authors, year, country, study design, sample size or number of patients with COVID-19, age, sex, and other data including number and type of bacterial coinfections were extracted in all studies. Herein, patients with COVID-19 (confirmed cases based on molecular tests such as PCR) with even a single bacterial coinfection were considered in the study.
Variable definition
Bacteria types were classified based on transmission way and clinical signs. Countries were categorized based on the latest WHO definition that includes the following six regions: Regional Office for Africa, Regional Office of Americas (AMRO), Regional Office for the Eastern Mediterranean, Regional Office for Europe, Regional Office for South-East Asia (SEARO), and the Regional Office for the Western Pacific (WPRO).
Quality assessment
The Newcastle-Ottawa Scale for case reports/case series and observational study was used to assess the quality of the included studies [11]. This scale has three sections: 1, selection (4 items, maximum score: 4 points); 2, confounder (1 item, maximum score: 1 point); and 3, exposure (2 items, maximum score: 2 points). The studies were evaluated by two raters (Reza Pakzad and Saber Soltani) independently, and a total score was calculated for each study. The studies were then assigned to one of the following categories accordingly: very good studies: 6–7 scores; good studies: 4–5 scores; satisfactory studies: 2–3 scores; unsatisfactory studies: 0–1 score [12].
Statistical analysis
All analysis was conducted with Stata software 14.0 (College Station, TX). As previous studies [[13], [14], [15], [16]], the number of COVID-19 cases, the prevalence of bacterial coinfections in COVID-19, and its different bacterial types were extracted. Heterogeneity was determined using Cochran's Q test of heterogeneity, and the I2 index was used to quantify heterogeneity. Following the Higgins classification approach, I2 values above 0.7 were considered as high heterogeneity. The pooled prevalence with 95% CI was calculated using the “metaprop” command, and to estimate the pooled prevalence, we used the random effects model. It should be noted the “Freeman-Tukey double-arcsine transformation” method is used for estimating 95% CI to keep the value between 0% and 100%. The meta-regression analysis was used to examine age, WHO region, and sample size as factors affecting heterogeneity among studies. The “metabias” command was used to check the publication bias. If there was any publication bias, the prevalence rate was adjusted with the “meta-trim” command using the trim-and-fill method. In all analyses, a significance level of 0.05 was considered.
Results
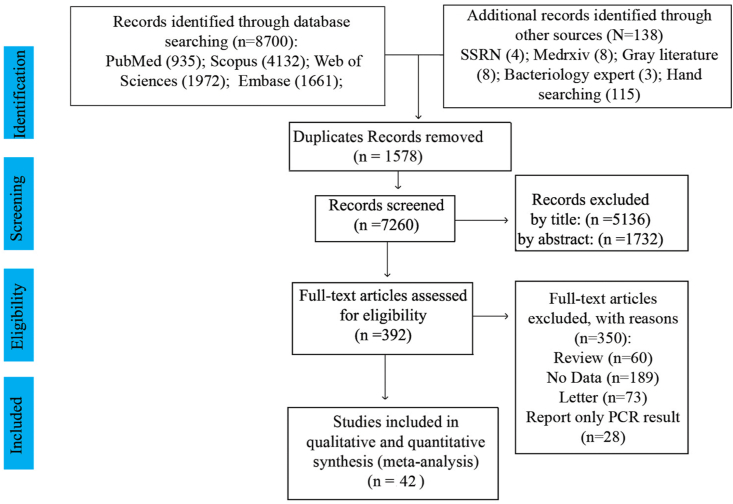
Overall, 8700 studies were found through databases, and 138 studies were identified through other sources (SSRN: 4, Medrxiv: 8, grey literature: 8, bacteriology expert: 3, and handsearching: 115). After excluding redundant articles, 7260 studies remained. Screening was done in three steps. In the first step, 5136 studies were excluded after reviewing the titles, and 2124 articles remained. After reading abstracts, 1732 studies were excluded from the list. Then, the full text of the remaining 392 studies was reviewed, and 350 studies were excluded. Finally, 42 studies [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]] with a total sample size of 54,695 were included in the analysis. The flowchart of this selection process is shown in Fig. 1, and the characteristic of the studies was showed in Table 1 and Supplement 1. European region had the highest number of studies (15 studies), and Eastern Mediterranean Region and Western Pacific had the lowest number of studies. All studies were published during the year 2020. The minimum and maximum age range of the subjects was for a study by Wu et al. (mean age = 6 years) and a study by D'Onofrio et al. (mean age = 73 years), respectively. The study setting assessment indicates 25 (59.53%) of the studies are cohort (prospective and retrospective), 12 (28.57%) are case series (prospective and retrospective), and 5 (11.9%) are cross-sectional.
Fig. 1.
PRISMA flow diagram of the process of study selection for analysis.
Table 1.
Characteristics of the included studies in present systematic review and meta-analysis
| Author | Country | Study Design | Publication year | Mean or Age | Sample Size | Bacterial Coinfections Prevalence, % (95% CI) |
|---|---|---|---|---|---|---|
| Zhu et al. [58] | China | Retrospective case series | 2020 | 51 | 257 | 91.83 (87.78–94.87) |
| Blasco et al. [17] | Spain | Retrospective case series | 2020 | 64 | 183 | 0.55 (0.10–3.10) |
| Contou et al. [20] | France | Retrospective case series | 2020 | 61 | 92 | 95.65 (89.24–98.80) |
| Sarinoglu et al. [45] | Turkey | Cross-sectional | 2020 | NA | 30 | 6.67 (0.82–22.7) |
| Chauhdary et al. [18] | Brunei | Case series | 2020 | NA | 141 | 3.55 (1.16–8.8) |
| Cheng et al. [19] | China | Retrospective cohort | 2020 | 36 | 62 | 40.32 (28.50–53.55) |
| D'Onofrio et al. [21] | Belgium | Cohort | 2020 | 73 | 110 | 2.73 (0.57–7.76) |
| Fu et al. [22] | China | Retrospective cohort | 2020 | NA | 101 | 4.95 (1.63–11.18) |
| Garcia-Vidal et al. [23] | Spain | Retrospective cohort | 2020 | 62 | 989 | 2.93 (1.97–4.18) |
| Dir et al. [24] | USA | Retrospective cohort | 2020 | 57 | 350 | 1.71 (0.63–3.69) |
| Gupta et al. [26] | India | Retrospective cohort | 2020 | 36 | 1073 | 2.50 (1.29–3.90) |
| Hazra et al. [27] | USA | Cross-sectional | 2020 | NA | 459 | 0.0 (0.0–0.80) |
| Hirotsu et al. [28] | Japan | Cross-sectional | 2020 | NA | 40 | 0.0 (0.0–8.81) |
| Hughes et al. [29] | UK | Retrospective case series | 2020 | 69.5 | 836 | 3.23 (2.14–4.66) |
| Intra et al. [30] | Italy | Retrospective cohort | 2020 | NA | 61 | 68.85 (55.71–80.10) |
| Karami et al. [31] | The Netherlands | Retrospective cohort | 2020 | 70 | 925 | 0.86 (0.37–1.70) |
| Kim et al. [32] | USA | Cross-sectional | 2020 | 46.9 | 116 | 0.0 (0.0–3.13) |
| Kimmig et al. [33] | USA | Retrospective cohort | 2020 | 46.9 | 111 | 37.84 (28.80–47.54) |
| Li et al. [34] | China | Retrospective cohort | 2020 | 66.2 | 1495 | 20.60 (18.58–22.74) |
| Li et al. [35] | China | Case series | 2020 | 57 | 32 | 31.25 (16.12–50.1) |
| Liu et al. [36] | China | Retrospective case series | 2020 | 46.5 | 20 | 20.0 (5.73–43.66) |
| Lv et al. [37] | China | Retrospective cohort | 2020 | 62 | 354 | 14.12 (10.67–18.19) |
| Ma et al. [38] | China | Case series | 2020 | 45.5 | 250 | 9.60 (6.25–13.95) |
| Massey et al. [39] | USA | Retrospective case series | 2020 | 62.3 | 790 | 55.44 (51.90–58.95) |
| Motta et al. [40] | Multiplacea | Cohort | 2020 | NA | 69 | 7.25 (2.39–16.11) |
| Neto et al. [25] | USA | Retrospective cohort | 2020 | 66 | 242 | 19.10 (14.27–24.53) |
| Verroken et al. [52] | The Netherlands | Cohort | 2020 | NA | 32 | 18.75 (7.21–36.44) |
| Nori et al. [41] | USA | Retrospective cohort | 2020 | 62 | 152 | 44.80 (36.40–52.35) |
| Pandey et al. [51] | India | Cross-sectional | 2020 | NA | 120 | 13.33 (7.82–20.75) |
| Porretta et al. [42] | Italy | Cohort | 2020 | 67.4 | 331 | 9.67 (6.71–13.37) |
| Ripa et al. [43] | Italy | Cohort | 2020 | 64 | 731 | 7.25 (5.48–9.38) |
| Rothe et al. [44] | Germany | Retrospective cohort | 2020 | 63.5 | 140 | 76.43 (68.52–83.19) |
| Sepulveda et al. [46] | USA | Retrospective cohort | 2020 | NA | 28,011 | 3.80 (3.58–4.30) |
| Sharifipour et al. [47] | Iran | Case series | 2020 | 67.1 | 19 | 100.0 (82.35–100.0) |
| Sharov et al. [48] | Russia | Retrospective case series | 2020 | NA | 147 | 75.51 (67.74–82.22) |
| Sy et al. [49] | Philippine | Cohort | 2020 | 44.21 | 12,513 | 0.90 (0.74–1.80) |
| Tadolini M et al. [50] | Multiplace | Cohort | 2020 | 48 | 49 | 85.71 (72.76–94.6) |
| Wu et al. [53] | China | Retrospective case series | 2020 | 6 | 74 | 47.30 (35.57–59.25) |
| Youngs et al. [54] | UK | Cohort | 2020 | 59 | 36 | 30.56 (16.35–48.11) |
| Yu et al. [55] | Sweden | Cohort | 2020 | NA | 2240 | 10.90 (8.87–11.41) |
| Zha et al. [56] | China | Retrospective cohort | 2020 | 57 | 874 | 2.52 (1.58–3.79) |
| Zhang et al. [57] | China | Retrospective cohort | 2020 | 64.76 | 38 | 57.89 (40.82–73.69) |
Abbreviation: NA, not available.
Belgium, Brazil, France, Italy, Russia, Singapore, Spain, and Switzerland.
Pooled prevalence of bacterial coinfections in patients with COVID-19
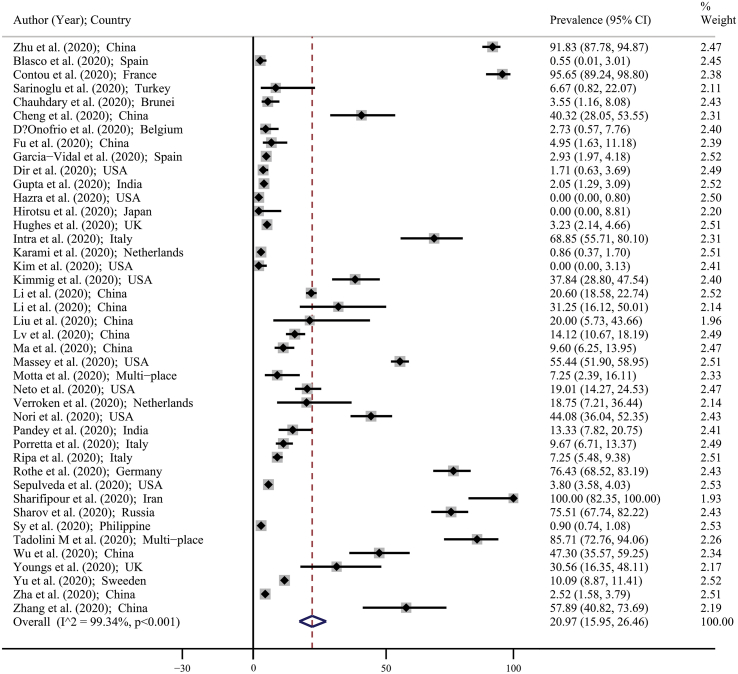
The prevalence of bacterial coinfections in all included studies was listed in Table 1. Also, Fig. 2 showed the forest plot for the prevalence of bacterial coinfections. The minimum and maximum reported prevalence of bacterial coinfections were reported by Hazra et al. (prevalence: 0%; 95% CI: 0–0.80) in Chicago [27] and by Sharifipour et al. (prevalence: 100%; 95% CI: 82.35–100) in Iran [47]. Based on Fig. 2 using random effects model approach, the pooled estimate for the prevalence of bacterial coinfections was 20.97% (95% CI: 15.95–26.46). This means that in overall, of every 100 people with COVID-19, 16–26 people have bacterial coinfections.
Fig. 2.
Forest plot for the prevalence of bacterial coinfections in patients with COVID-19 based on a random effects model. Each study identifies by the first author (year) and country. Each line segment's midpoint shows the prevalence estimate, length of line segment indicates 95% CI in each study, and diamond mark illustrates the pooled estimate.
Pooled prevalence of bacterial coinfections based on different subgroups
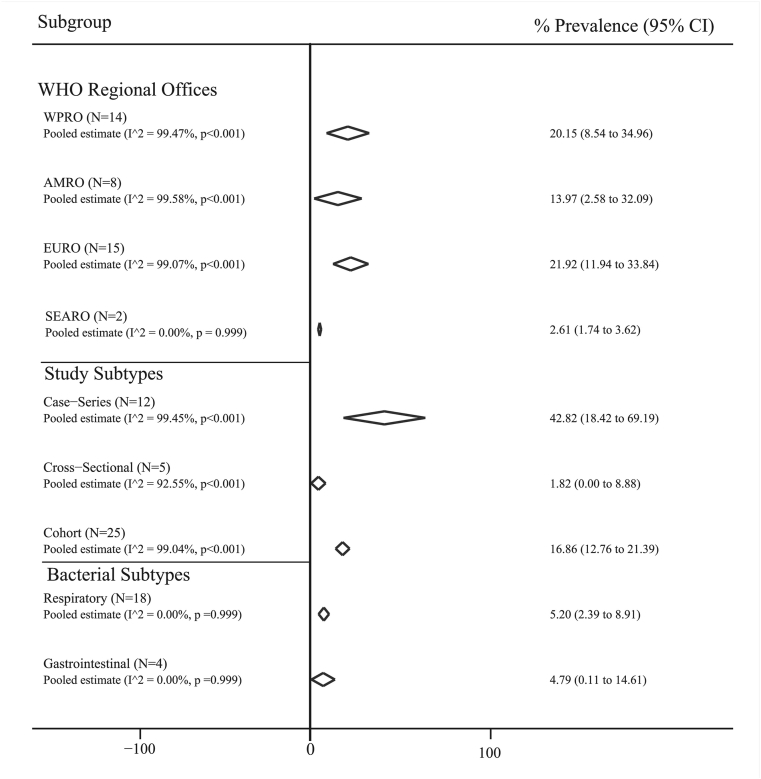
Fig. 3 shows the pool prevalence of bacterial coinfections based on bacteria subtype, different place, and study type. The pooled prevalence of bacterial coinfections was (5.20%; 95% CI: 2.39–8.91) for respiratory subtype and (4.79%; 95% CI: 0.11–14.61) for gastrointestinal subtype. The most and least pooled prevalence of bacterial coinfections based on study design was estimated in case series studies with 42.82% (95% CI: 18.42–69.19) and in cross-sectional studies with 1.82% (95% CI: 0.0–8.88), respectively. The pooled prevalence for WPRO and AMRO was 20.15% (95% CI: 8.54–34.96) and 13.97% (95% CI: 2.58–32.09), respectively. More detail was shown in Fig. 3.
Fig. 3.
Pooled prevalence with 95% CI and heterogeneity indices of bacterial coinfections in patients with COVID-19 based on the type of the bacteria, different regional places (AMRO: Regional Office of Americas; EURO: Regional Office for Europe; SEARO: Regional Office for South-East Asia; EMRO: Regional Office for the Eastern Mediterranean; WPRO; Regional Office for the Western Pacific) and the type of the study. The diamond mark illustrates the pooled prevalence, and the length of the diamond indicates the 95% CI. N is the number of the study in the analysis. The prevalence for EMRO (N = 1) was 100 % (95% CI: 82.35–100.00).
Heterogeneity and meta-regression
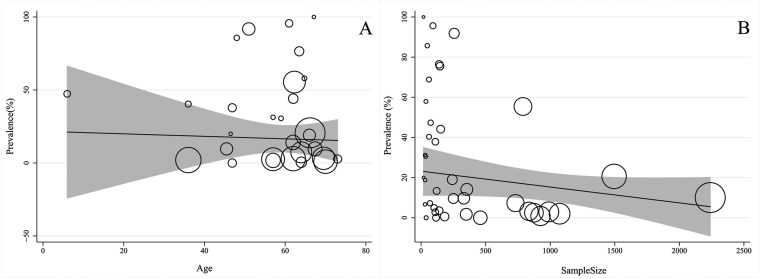
Table 2 presents the results of the heterogeneity. According to Cochran's Q test of heterogeneity, there was significant heterogeneity among studies (p < 0.001). The I2 index for total bacterial coinfections was 99%. According to meta-regression results, the age (coefficient: −0.205; p = 0.643), sample size (coefficient: −0.001; p = 0.215), and WHO region size (coefficient: −5.304; p = 0.262) had no significant effect on heterogeneity among studies (Fig. 4A and B). Type of the study (coefficient: 20.274; p = 0.007) had significant effect on heterogeneity among studies.
Table 2.
The univariate meta-regression analysis on the hertogenisity of the determinants in included studies for bacterial coinfections in patients with COVID-19.
| Variables | Coefficient | 95% CI | p value |
|---|---|---|---|
| Age (year) | −0.205 | −1.103 to 0.692 | 0.643 |
| WHO region (score) | −5.304 | −14.739 to 4.131 | 0.262 |
| Sample size (number) | −0.001 | −0.003 to 0.001 | 0.215 |
| Type of the study (score) | 20.274 | 5.768 to 34.781 | 0.007 |
Coding of WHO region: 1 = EMRO; 2 = EURO; 3 = AMRO; 4 = WPRO; 5 = SEARO; Coding of type of the study: 1 = cross-sectional; 2 = cohort; 3 = case series.
Fig. 4.
Association among prevalence of age (A) and sample size (B) with the prevalence of bacterial coinfections by means of meta-regression. The size of circles indicates the precision of each study. There is no significant association with respect to the prevalence of bacterial coinfections with age and sample size.
Publication bias
Based on the results of Begg's test, a significant publication bias was observed for total bacterial coinfections (Z score: 4.11; p < 0.001). Therefore, the trim-and-fill–adjusted pooled prevalence of bacterial coinfections (23.55%; 95% CI: 18.38–28.73) was generated, which was not significantly different from the original pooled prevalence (20.97%; 95% CI: 15.95–26.46), and the mean results have robustness.
Discussion
Critically ill patients are more prone to bacterial coinfection compared with other infected individuals. Critically ill patients demonstrated 8.1% of coinfection, which is slightly more compared with 5.9% in hospitalized individuals [59]. Another meta-analysis article showed that 7% of patients were infected with bacterial pathogens [60]. Bacterial coinfection in the meta-analysis study was observed in 3.5% of patients. Bacterial secondary infection was identified in 14.3% of patients. This meta-analysis indicated that the most common bacterial coinfection amongst patients with COVID-19 were Mycoplasma pneumonia, Pseudomonas aeruginosa, and Haemophilus influenzae. This study also mentioned 3% of the patients were coinfected with viruses. The median age ranges from 42 to 63 years in most of the studies included in this meta-analysis [60].
The overall prevalence of bacterial coinfection in patients with COVID-19 was 6.9%. Nearly all the studies indicated that the patients received some kind of antibiotics [59]. Bacterial coinfection plays an undeniable role in increasing morbidity and mortality rate in viral pandemics, such as influenza [61]. Bacterial coinfection among patients infected with influenza virus has been reported up to 30% [1].
One of the important aspects of determining the incidence and prevalence of bacterial coinfection is related to antibiotic prescription for patients with COVID-19 [25]. Although the use of antibiotics in coronavirus patients is rapidly growing, the effectiveness of them is under questioning. A number of studies have questioned the amount of prescribing antibiotics for the patients and have opinioned that this will cause us another great challenge, which is antibiotic resistance, but on the other hand, utilization of antibiotic in the pandemic situation is inevitable for different reasons, such as the difficulties of excluding bacterial coinfection and the possibility of secondary infection in patients [62].
More than 70% of patients with COVID-19 received some kind of antibiotics with a focus on broad-spectrum agents, such as fluoroquinolones and third-generation cephalosporins [59]. Bacterial coinfection was also reported in previous pandemics. During the 2009 influenza (H1N1) pandemic, patients in intensive care units showed up to 30% of bacterial coinfection. The most commonly identified pathogens were S. aureus and S. pneumoniae [1,63].
In contrast, in the recent COVID-19 pandemic, it becomes more and more clear that gram-negative and atypical bacteria are the most isolated bacteria from SARS-CoV-2 patients. A meta-analysis study showed that the most common organisms reported by the studies were Mycoplasma species, Haemophilus influenzae, and Pseudomonas aeruginosa [59].
Gram-negative microorganisms were also reported as the most frequent cause of lower respiratory tract infection. Pseudomonas aeruginosa was the most common isolated bacteria among patients with ventilator-associated pneumonia (38%) and tracheobronchitis (33%) [64]. Another systematic review and meta-analysis showed that the commonest bacteria were Mycoplasma pneumonia, Pseudomonas aeruginosa, and Haemophilus influenzae [60].
But there are controversial data about SARS-CoV-2 coinfection with these bacteria. Langford et al. showed that these bacterial pathogens are not common amongst people with COVID-19, yet another meta-analysis study reported the rate of S. aureus/COVID-19 coinfection was 25.6%, and the proportion of COVID-19/MRSA S. aureus was 53.9%, which has been collected from five different studies [59,65].
Johns’ Hopkins scientists in a multicentre study found only 1.2% of the patients had bacterial coinfection, which is less frequent than in other studies. The researchers suggested that their varied data may be related to inclusion and exclusion criteria used by them [66]. They also mentioned their sampling time could be an effective factor compared with other studies. Their study was conducted in spring, whereas other studies were implemented during winter in Europe and China. They also indicated variation in vaccination background of sample population against pneumococcal infection, and this may also affect the coinfection prevalence [67].
Although it is not the main focus of our study, it is worth mentioning the coinfection of other microorganisms, such as viruses and fungi with SARS-CoV-2. The rate of fungal coinfection with SARS-CoV-2 has been reported diversely.
A systematic review and meta-analysis conducted by Jackson S. Musuuza found the prevalence of fungal coinfections, 4% and fungal superinfections, 8% among patients with COVID-19 [68]. In contrast, another study reported that the overall pooled proportion of patients with coinfection was only 0.12 [69]. It should be mentioned that Aspergillus and Candida species were the most frequently reported among patients with COVID-19. Viral coinfections and viral superinfections were reported 10% and 4%, respectively, and the most frequently identified viruses among patients were influenza type A (22.3%), influenza type B (3.8%), and respiratory syncytial virus (3.8%) [68].
Our results showed the 5.2% pooled prevalence for respiratory bacterial coinfection and gastrointestinal subtype had 4.79% amongst patients with COVID-19, which are in consistent with previous research reported the ranged of bacterial coinfection between 3.1% and 7%. We also found that case series studies reported the highest level of coinfection compared with cross-sectional studies, which showed the lowest rate. From geographical viewpoint, we acquired some interesting results. Our analysis exhibit that the WPRO has 20.15% and AMRO had 13.97% of coinfection, which shows a great difference between these regions. Our meta-analysis showed the pooled estimate for the prevalence of bacterial coinfections was 20.97%. Our results clearly indicate the high prevalence of bacterial pathogens amongst patients with COVID-19. Therefore, we came to the conclusion that prescribing antibiotics for patients with COVID-19 based on the high percentage of bacterial coinfection is inevitable.
The currents evidence is against the massive use of antibiotics to treat patients with COVID-19 in both hospitalized and critically ill state, but it has been mentioned in this manuscript that the circumstances can be different from one to another patient situation, and it also should be noted that the data are still progressing almost every day, so it would be wise for clinicians to use antibiotics with cautions and always update themselves with the latest research.
Escalation in patient's body temperature, longer fever duration, anhelation, gastrointestinal-related symptoms, intensive care unit attending, ventilation treatment, glucocorticoid therapy, severity in disease situation, and prolongation in hospitalization time were reported as different sequences of clinical outcome linked to bacterial coinfection [67]. The data have reported the elderly patients with high level of inflammatory factors and worse lymphopenia and cardiovascular comorbidities have a higher chance of being infected with bacterial infection. In addition, these patients had worsened illness situations and showed multiple set of system failure [29,67].
The laboratory results of patients with COVID-19 have several clinical risk factors related to coinfection. A case–control study reported that C-reactive protein and median neutrophil to lymphocyte ratio were significantly higher in case compared to controls. However, there was not any statistical significance in procalcitonin levels in patients with COVID-19 with bacterial infection compared with people without bacterial infection [69]. Shengyang et al. found that patients with COVID-19 with bacterial coinfection had substantially increase in their procalcitonin. This article also confirms the increase in C-reactive protein in the patients [67].
Similar to other studies, our research had some limitations. (1) we would like to perform the gender-specific estimation, but it was not possible because of insufficient data in the primary studies; (2) we estimated the pooled prevalence based on WHO regional office and tendency to examine the spatial analysis in different geographical regions based on available methods [[70], [71], [72]], but because of the infrequent studies number, this estimation will not be robust. Also, in the SEARO subgroup, we have only two studies, and this may cause unrobust estimates. Doing a comprehensive search and estimate the pooled prevalence based on different bacteria subtypes was the present study's strengths.
Conclusion
Because of the proven track of bacterial coinfection in increasing morbidity and mortality rate in previous viral outbreaks and pandemics, proving information about the incidence and prevalence rate of them are crucial for health administrators and clinicians, but the contrary data prove that various factors affect the final output of the studies, and setting clinical guidelines or prescribing medication based on the results of different research should be done carefully and considering all the factor, which yield effect on the final results. Considering the multiple complications and adverse effects of extensive use of antibiotics in patients with COVID-19, it seems there is no other option except applying them, but it needs to be done carefully.
Authors’ contributions
S.S. contributed to study design, creation of models, and management activities to annotate (produce metadata), specifically writing the initial draft (including substantive translation).
S.F. contributed to data collection and writing the article, developed the theory, and performed the computations, specifically writing the initial draft (including substantive translation).
M.Z. contributed to data collection, writing the article, and conducting a research and investigation process.
R.S. contributed to data collection, writing the article, and conducting a research and investigation process.
A.J. contributed to data collection, writing the article, and conducting a research and investigation process.
S.A.R. contributed to data collection and writing the article.
I.P. contributed to study design and writing the article.
F.A. contributed to data collection and writing the article.
P.M. contributed to management and coordination responsibility for the research activity planning and execution, study design, data analysis, development or design of methodology, and creation of models.
R.P. contributed to design and perform the idea, data analysis, development or design of methodology, and creation of models.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None.
Footnotes
Supplementary data related to this article can be found at doi:10.1016/j.nmni.2021.100910.
Contributor Information
P. Malekifar, Email: poonehmalekifar@gmail.com.
R. Pakzad, Email: rezapakzad2010@yahoo.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rice T.W., Rubinson L., Uyeki T.M., Vaughn F.L., John B.B., Miller R.R., III Critical illness from 2009 pandemic influenza A (H1N1) virus and bacterial co-infection in the United States. Crit Care Med. 2012;40(5):1487. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taubenberger J.K., Morens D.M. The 1918 influenza pandemic and its legacy. Cold Spring Harbor Perspect Med. 2020;10(10):a038695. doi: 10.1101/cshperspect.a038695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A., Zarychanski R., Pinto R., Cook D.J., Marshall J., Lacroix J. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. Jama. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 4.Joseph C., Togawa Y., Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respirat Viruse. 2013;7:105–113. doi: 10.1111/irv.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beadling C., Slifka M.K. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dise. 2004;17(3):185–191. doi: 10.1097/00001432-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Organization W.H. World Health Organization; 2020. Clinical management of COVID-19: interim guidance, 27 May 2020. [Google Scholar]
- 7.Bengoechea J.A., Bamford C.G. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12(7) doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020:369. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 9.Chibabhai V., Duse A., Perovic O., Richards G. Collateral damage of the COVID-19 pandemic: exacerbation of antimicrobial resistance and disruptions to antimicrobial stewardship programmes? SAMJ: South African Med J. 2020;110(7):1–2. doi: 10.7196/SAMJ.2020.v110i7.14917. [DOI] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells G.A., Shea B., O’Connell Da, Peterson J., Welch V., Losos M. 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford. [Google Scholar]
- 12.Hashemi H., Pakzad R., Yekta A., Aghamirsalim M., Pakbin M., Ramin S. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye. 2020;34(8):1357–1370. doi: 10.1038/s41433-020-0806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallajzadeh J., Khoramdad M., Izadi N., Karamzad N., Almasi-Hashiani A., Ayubi E. Metabolic syndrome and its components in premenopausal and postmenopausal women: a comprehensive systematic review and meta-analysis on observational studies. Menopause. 2018;25(10):1155–1164. doi: 10.1097/GME.0000000000001136. [DOI] [PubMed] [Google Scholar]
- 14.Hashemi H., Pakzad R., Heydarian S., Yekta A., Aghamirsalim M., Shokrollahzadeh F. Global and regional prevalence of strabismus: a comprehensive systematic review and meta-analysis. Strabismus. 2019;27(2):54–65. doi: 10.1080/09273972.2019.1604773. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi H., Pakzad R., Yekta A., Bostamzad P., Aghamirsalim M., Sardari S. Global and regional estimates of prevalence of amblyopia: a systematic review and meta-analysis. Strabismus. 2018;26(4):168–183. doi: 10.1080/09273972.2018.1500618. [DOI] [PubMed] [Google Scholar]
- 16.Soltani S., Tabibzadeh A., Zakeri A., Zakeri A.M., Latifi T., Shabani M. COVID-19 associated central nervous system manifestations, mental and neurological symptoms: a systematic review and meta-analysis. Rev Neurosci. 2021:1. doi: 10.1515/revneuro-2020-0108. (ahead-of-print) [DOI] [PubMed] [Google Scholar]
- 17.Blasco M.L., Buesa J., Colomina J., Forner M.J., Galindo M.J., Navarro J. Co-detection of respiratory pathogens in patients hospitalized with Coronavirus viral disease-2019 pneumonia. J Med Virol. 2020;92(10):1799–1801. doi: 10.1002/jmv.25922. [DOI] [PubMed] [Google Scholar]
- 18.Chauhdary W.A., Chong P.L., Mani B.I., Asli R., Momin R.N., Abdullah M.S. Primary respiratory bacterial coinfections in patients with COVID-19. Am J Trop Med Hygiene. 2020;103(2):917. doi: 10.4269/ajtmh.20-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng LS-k, Chau SK-y, Tso EY-k, Tsang SW-c, Li IY-f, Wong BK-c. Bacterial co-infections and antibiotic prescribing practice in adults with COVID-19: experience from a single hospital cluster. Therapeut Adv Infect Dise. 2020;7 doi: 10.1177/2049936120978095. 2049936120978095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contou D., Claudinon A., Pajot O., Micaëlo M., Longuet Flandre P., Dubert M. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Inten Care. 2020;10(1):119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Onofrio V., Van Steenkiste E., Meersman A., Waumans L., Cartuyvels R., Van Halem K. Differentiating influenza from COVID-19 in patients presenting with suspected sepsis. Euro J Clin Microbiol Infect Dise. 2020:1–9. doi: 10.1007/s10096-020-04109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y., Yang Q., Xu M., Kong H., Chen H., Fu Y., editors. Secondary bacterial infections in critical ill patients of COVID-19. Open forum infectious diseases; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gayam V., Konala V.M., Naramala S., Garlapati P.R., Merghani M.A., Regmi N. Presenting characteristics, comorbidities, and outcomes of patients coinfected with COVID-19 and Mycoplasma pneumoniae in the USA. J Med Virol. 2020;92(10):2181–2187. doi: 10.1002/jmv.26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goncalves Mendes Neto A., Lo K.B., Wattoo A., Salacup G., Pelayo J., DeJoy R., III Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol. 2021;93(3):1489–1495. doi: 10.1002/jmv.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta N., Ish P., Gupta A., Malhotra N., Caminero J.A., Singla R. A profile of a retrospective cohort of 22 patients with COVID-19 and active/treated tuberculosis. Euro Respirator J. 2020;56(5) doi: 10.1183/13993003.03408-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazra A., Collison M., Pisano J., Kumar M., Oehler C., Ridgway J.P. Coinfections with SARS-CoV-2 and other respiratory pathogens. Infect Contr Hospit Epidemiol. 2020;41(10):1228–1229. doi: 10.1017/ice.2020.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirotsu Y., Maejima M., Shibusawa M., Amemiya K., Nagakubo Y., Hosaka K. Analysis of Covid-19 and non-Covid-19 viruses, including influenza viruses, to determine the influence of intensive preventive measures in Japan. J Clin Virol. 2020;129:104543. doi: 10.1016/j.jcv.2020.104543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Intra J., Sarto C., Beck E., Tiberti N., Leoni V., Brambilla P. Bacterial and fungal colonization of the respiratory tract in COVID-19 patients should not be neglected. Am J Infect Contr. 2020;48(9):1130–1131. doi: 10.1016/j.ajic.2020.06.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karami Z., Knoop B.T., Dofferhoff A.S., Blaauw M.J., Janssen N.A., van Apeldoorn M. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect Dise. 2021;53(2):102–110. doi: 10.1080/23744235.2020.1839672. [DOI] [PubMed] [Google Scholar]
- 32.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of Co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323(20):2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimmig L.M., Wu D., Gold M., Pettit N.N., Pitrak D., Mueller J. Il-6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Fron Med. 2020;7 doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Wang J., Yang Y., Cai P., Cao J., Cai X. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrobial Resist Infect Contr. 2020;9(1):1–7. doi: 10.1186/s13756-020-00819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., Chen Z-m, Chen L.-D., Zhan Y.-Q., Li S.-Q., Cheng J. Coinfection with SARS-CoV-2 and other respiratory pathogens in patients with COVID-19 in Guangzhou, China. J Med Virol. 2020;92(11):2381–2383. doi: 10.1002/jmv.26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Zeng W., Cao Y., Cui Y., Li Y., Yao S. Effect of a previous history of antiretroviral treatment on the clinical picture of patients with co-infection of SARS-CoV-2 and HIV: a preliminary study. Int J Infect Dise. 2020;100:141–148. doi: 10.1016/j.ijid.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv Z., Cheng S., Le J., Huang J., Feng L., Zhang B. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. 2020;22(4):195–199. doi: 10.1016/j.micinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L., Wang W., Le Grange J.M., Wang X., Du S., Li C. Coinfection of SARS-CoV-2 and other respiratory pathogens. Infect Drug Resist. 2020;13:3045. doi: 10.2147/IDR.S267238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massey B.W., Jayathilake K., Meltzer H.Y. Respiratory microbial Co-infection with SARS-CoV-2. Front Microbiol. 2020;11:2079. doi: 10.3389/fmicb.2020.02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motta I., Centis R., D’Ambrosio L., García-García J.M., Goletti D., Gualano G. Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26(4):233–240. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nori P., Cowman K., Chen V., Bartash R., Szymczak W., Madaline T. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Cont Hosp Epidemiol. 2021;42(1):84–88. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porretta A.D., Baggiani A., Arzilli G., Casigliani V., Mariotti T., Mariottini F. Increased risk of acquisition of New Delhi metallo-beta-lactamase-producing carbapenem-resistant Enterobacterales (NDM-CRE) among a cohort of COVID-19 patients in a teaching hospital in Tuscany, Italy. Pathogens. 2020;9(8):635. doi: 10.3390/pathogens9080635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ripa M., Galli L., Poli A., Oltolini C., Spagnuolo V., Mastrangelo A. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothe K., Feihl S., Schneider J., Wallnöfer F., Wurst M., Lukas M. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Euro J Clin Microbiol Infect Dise. 2020:1–11. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarınoğlu R.C., Sili U., Eryuksel E., Yildizeli S.O., Cimsit C., Yagci A.K. Tuberculosis and COVID-19: an overlapping situation during pandemic. J Infect Develop Countr. 2020;14(7):721–725. doi: 10.3855/jidc.13152. [DOI] [PubMed] [Google Scholar]
- 46.Sepulveda J., Westblade L.F., Whittier S., Satlin M.J., Greendyke W.G., Aaron J.G. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00875-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharifipour E., Shams S., Esmkhani M., Khodadadi J., Fotouhi-Ardakani R., Koohpaei A. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect Dise. 2020;20(1):1–7. doi: 10.1186/s12879-020-05374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharov K.S. SARS-CoV-2-related pneumonia cases in pneumonia picture in Russia in March-May 2020: secondary bacterial pneumonia and viral co-infections. J Glob Health. 2020;10(2) doi: 10.7189/jogh.10.-020504. 020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sy K.T.L., Haw N.J.L., Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dise. 2020;52(12):902–907. doi: 10.1080/23744235.2020.1806353. [DOI] [PubMed] [Google Scholar]
- 50.Tadolini M., Codecasa L.R., García-García J.-M., Blanc F.-X., Borisov S., Alffenaar J.-W. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Euro Respirat J. 2020;56(1) doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tiwari Pandey A., Pandey I., Zamboni P., Gemmati D., Kanase A., Singh A.V. Traditional herbal remedies with a multifunctional therapeutic approach as an implication in COVID-19 associated Co-infections. Coatings. 2020;10(8):761. [Google Scholar]
- 52.Verroken A., Scohy A., Gérard L., Wittebole X., Collienne C., Laterre P.-F. Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Crit Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q., Xing Y., Shi L., Li W., Gao Y., Pan S. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146(1) doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 54.Youngs J., Wyncoll D., Hopkins P., Arnold A., Ball J., Bicanic T. Improving antibiotic stewardship in COVID-19: bacterial co-infection is less common than with influenza. J Infect. 2020 doi: 10.1016/j.jinf.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu D., Ininbergs K., Hedman K., Giske C.G., Strålin K., Özenci V. Low prevalence of bloodstream infection and high blood culture contamination rates in patients with COVID-19. PloS One. 2020;15(11) doi: 10.1371/journal.pone.0242533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zha L., Shen J., Tefsen B., Wang Y., Lu W., Xu Q. Clinical features and outcomes of adult COVID-19 patients co-infected with Mycoplasma pneumoniae. J Infect. 2020 doi: 10.1016/j.jinf.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H., Zhang Y., Wu J., Li Y., Zhou X., Li X. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerging Microbes & Infections. 2020;9(1):1958–1964. doi: 10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu X., Ge Y., Wu T., Zhao K., Chen Y., Wu B. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kash J.C., Walters K.-A., Davis A.S., Sandouk A., Schwartzman L.M., Jagger B.W. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. MBio. 2011;2(5) doi: 10.1128/mBio.00172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goel N., Ahmad R., Fatima H., Khare S.K. New threatening of SARS-CoV-2 co-infection and strategies to fight the current pandemic. Med Drug Disc. 2021:100089. doi: 10.1016/j.medidd.2021.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a (H1N1) pdm09. BMC Infect Dise. 2018;18(1):1–20. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bardi T., Pintado V., Gomez-Rojo M., Escudero-Sanchez R., Lopez A.A., Diez-Remesal Y. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Euro J Clin Microbiol Infect Dise. 2021;40(3):495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adeiza S.S., Shuaibu A.B., Shuaibu G.M. Random effects meta-analysis of COVID-19/S. aureus partnership in co-infection. GMS Hygiene Infect Cont. 2020;15 doi: 10.3205/dgkh000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karaba S.M., Jones G., Helsel T., Smith L.L., Avery R., Dzintars K., editors. Open forum infectious diseases. Oxford University Press US; 2021. Prevalence of Co-infection at the time of hospital admission in COVID-19 patients, A multicenter study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He S., Liu W., Jiang M., Huang P., Xiang Z., Deng D. Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: a multi-center study. Plos One. 2021;16(4) doi: 10.1371/journal.pone.0249668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musuuza J.S., Watson L., Parmasad V., Putman-Buehler N., Christensen L., Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PloS One. 2021;16(5) doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nasir N., ur Rehman F., Omair S.F. Risk factors for bacterial infections in patients with moderate to severe COVID-19: a case control study. medRxiv. 2021 doi: 10.1002/jmv.27000. 2021.01. 09.21249498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holakouie-Naieni K., Mostafavi E., Boloorani A.D., Mohebali M., Pakzad R. Spatial modeling of cutaneous leishmaniasis in Iran from 1983 to 2013. Acta Trop. 2017;166:67–73. doi: 10.1016/j.actatropica.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Pakzad R., Dabbagh-Moghaddam A., Mohebali M., Safiri S., Barati M. Spatio-temporal analysis of cutaneous leishmaniasis using geographic information system among Iranian Army Units and its comparison with the general population of Iran during 2005–2014. J Parasit Dise. 2017;41(4):1114–1122. doi: 10.1007/s12639-017-0944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pakzad R., Pakzad I., Safiri S., Shirzadi M.R., Mohammadpour M., Behroozi A. Spatiotemporal analysis of brucellosis incidence in Iran from 2011 to 2014 using GIS. Int J Infect Dise. 2018;67:129–136. doi: 10.1016/j.ijid.2017.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.