Abstract
Objective
To evaluate the indirect effect of COVID-19 large-scale containment measures on the incidence of community-acquired pneumonia (CAP) in older people during the first epidemic wave of COVID-19 in Tuscany, Italy.
Methods
A population-based study was carried out on data from the Tuscany healthcare system. The outcome measures were: hospitalization rate for CAP, severity of CAP hospitalizations, and outpatient consumption of antibacterials for CAP in people aged 65 and older. Outcomes were compared between corresponding periods in 2020 (week 1 to 27) and previous years.
Results
Compared with the average of the corresponding periods in the previous 3 years, significant reductions in weekly hospitalization rates for CAP were observed from the week in which the national containment measures were imposed (week 10) until the end of the first COVID-19 wave in July (week 27). There was also a significant decrease in outpatient consumption in all antibacterial classes for CAP.
Conclusions
The implementation of large-scale COVID-19 containment measures likely reduced the incidence of CAP in older people during the first wave of the COVID-19 pandemic in Tuscany, Italy. Considering this indirect impact of pandemic containment measures on respiratory tract infections may improve the planning of health services during a pandemic in the future.
Keywords: Community-acquired pneumonia, older population, COVID-19, pandemic, containment measures, indirect effects
Introduction
Community-acquired pneumonia (CAP) is one of the leading causes of morbidity and mortality among all age groups, and especially in older adults. The most commonly identified pathogens of pneumonia in older people are bacteria, such as Streptococcus pneumoniae and Haemophilus influenza, and viruses (Bartlett and Mundy, 1995; Feldman, 1999; Fernandez-Sabé, 2003). These pathogens are mainly transmitted person-to-person via the respiratory route.
SARS-CoV-2 was identified in January 2020 as the etiological agent of COVID-19, an unusual viral pneumonia firstly observed in Wuhan Province, China, at the end of 2019 (Shereen et al., 2020; Gorbalenya et al., 2020, World Health Organization, 2020a). Since then, COVID-19 rapidly spread across the globe and was declared by the World Health Organization (WHO) as a global pandemic on 11 March 2020 (World Health Organization, 2020b).
Since the COVID-19 outbreak, large-scale measures to prevent disease transmission have been implemented worldwide. Government responses have included the prohibition of large-scale events, stay-at-home orders and national/local lockdown; furthermore, people were asked to wear face masks, respect physical distancing and take other simple precautions such as handwashing, keeping rooms well ventilated, avoiding gatherings, and coughing into a bent elbow or tissue.
If effectively implemented, these measures may have led to a reduction of the spread of other respiratory infectious diseases at the community level. However, evidence from epidemiologic studies has begun to appear only recently as containment measures implemented at such a stringent, persistent and widespread level are unprecedented in modern times (Wu et al., 2020; Sullivan et al., 2020; Kuo et al., 2021; Yamamoto et al., 2020; Chan et al., 2020; Chiu et al., 2020). To accurately evaluate the potential reduction of the burden of respiratory infectious diseases, this phenomenon needs to be measured in large and well-defined populations. Furthermore, large time frames—such as a whole intra-epidemic period—need to be considered as the SARS-CoV-2 infection in itself has the potential for increasing the incidence of bacterial pneumonia given that viral respiratory infections are usually associated with an increased risk of subsequent bacterial infections (Morens et al., 2008; Morens and Fauci 2007; Wolter et al., 2014; Salomon et al., 2020).
Thus, to date, the impact of a viral respiratory infection pandemic and the related large-scale control measures on the burden of respiratory infectious diseases at the community level remains unclear. However, its estimation would have important implications for health services planning and preparedness during a pandemic. Therefore, our study aims to evaluate the extent to which large-scale containment strategies implemented during the COVID-19 pandemic have influenced the incidence of CAP in the Tuscany Region (Italy) during the first pandemic wave (February–July 2020).
Tuscany is a region in central Italy with an area of approximately 23 000 square kilometers and a population of more than 3.7 million. Italy was one of the first Western countries severely affected by the COVID-19 pandemic. The first autochthonous Italian case was identified on 21 February 2020, but evidence suggests that SARS-CoV-2 was already circulating undetected at the end of 2019 (La Rosa et al., 2020). Indeed, a study carried out by the Italian Institute of Health documented the presence of the SARS-CoV-2 in untreated wastewaters of different geographic Italian regions during December 2019. In Tuscany, the first case of COVID-19 was observed on 24 February 2020. During the first wave of the epidemic, the Italian government imposed a 3-phase strategy to contain the circulation of SARS-CoV-2 (Lastrucci et al., 2020). In the first phase, from 11 March to 3 May 2020, a national lockdown was adopted, which included the suspension of all non-essential services and activities–including schools–and the imposition of a stay-at-home order banning all non-essential travel and contact with others. Beside these measures, physical distancing rules and the obligation to wear a face mask when leaving home were introduced. These measures were effective in containing the COVID-19 epidemic. In the second phase, from 4 May to 3 June 2020, a gradual reopening of services and business was allowed, and free movement was granted to all citizens within their region, but movement across regions for non-essential reasons was forbidden. In phase 3, free movement within the whole national territory was restored and cinemas and theatres reopened, physical distancing rules and face masks remained mandatory, and schools remained closed until September 2020.
Methods
This study was conducted in accordance with the Helsinki Declaration. According to the Italian legislation (law 211/2003) and the regional procedures, the study does not need ethical approval as it is a purely observational study on routine collected anonymous data.
Study design, population and data source
This is a cross-sectional retrospective study based on data from the Tuscany public healthcare system (TPHCS). TPHCS provides universal health coverage for all Tuscany residents. TPHCS comprises an extensive integrated healthcare delivery system with 38 secondary level hospitals, 4 university teaching hospitals and approximately 3000 general practitioners, providing comprehensive care for more than 3.7 million residents throughout the whole region.
Data used for this analysis were derived from the regional healthcare data system, which comprises several healthcare data sources. For the present study, the outpatient prescriptions and the inpatient hospitalizations registries were used. For the outpatient prescriptions registry, records of dispensed drugs for outpatient use are registered at the patient level. Records include patient demographics, information on the dispensed drug (including active substance, Anatomical Therapeutic Chemical (ATC) code, brand name, formulation), date of dispensing and the number of dispensed packages.
Measures and statistical analyses
The primary outcome was the hospitalization rate for CAP not caused by SARS-CoV-2 occurring in people aged ≥65 over the time frame corresponding to the entire first epidemic wave of COVID-19 in the Tuscany region (Week 1 to 27, 2020). In particular, the ICD-IX-CM principal diagnosis codes registered in the hospital discharge records were used to identify CAP cases; the following ICD-IX-CM principal diagnosis codes were considered: 480xx, 481xx, 482xx, 483xx, 485xx, 486xx, 4870x. The outcome was measured by week (Monday to Sunday) and by considering 2 distinct periods; the first from Week 1 to 9 (i.e., the COVID-19 pre-epidemic period) and the second from Week 10 to 27 (i.e., the COVID-19 epidemic period). The hospitalization rates for CAP in 2020 were compared with the average hospitalization rates for CAP occurring in the previous 3 years (2017–19) in the corresponding periods.
Hospitalizations were characterized accordingly to the following covariates: demographics (age and sex) and comorbidity. Comorbidity was measured using the Charlson Comorbidity Index (CCI) (Quan et al., 2011)). Furthermore, the average hospital length of stay (ALOS), percentage of hospitalizations requiring the intensive care unit (ICU), and in-hospital mortality of patients hospitalized with CAP were assessed as the secondary outcomes of the study. These outcomes were used as markers of the severity of the CAP hospitalizations.
In order to take into account a possible shift of care for less severe CAP cases from the hospital to outpatient settings, records of outpatient dispensed drugs in people aged ≥65 were assessed in the pre-epidemic and epidemic period of 2020 and compared with data of the corresponding periods of the previous year (2019). The guidelines of the TPHCS for treatment of CAP in the outpatient setting are to empirically treat almost all CAP patients with antibacterials for systemic use without further testing to ascertain the etiology. The antibacterials for systemic use indicated for the treatment of CAP in the outpatient settings were identified accordingly to the guidelines for the management of CAP in the Tuscany region and from the leading international scientific societies (Tuscany Regional Therapeutic Commission, 2017; Metlay et al., 2019; Lim et al., 2009). The following antibacterials for systemic use were evaluated: third generation cephalosporins (ATC code: J01DD), fluoroquinolones (ATC code: J01MA), penicillins with extended-spectrum and combinations of penicillins including beta-lactamase inhibitors (ATC codes: J01CA and J01CR); and macrolides (ATC code: J01FA). Dispensed drugs were then measured with the defined daily doses (DDD) assigned per ATC code using the WHO Collaborating Centre for Drug Statistics Methodology (WHO Collaborating Centre for Drug Statistics Methodology, 2020).
Percentage changes in the hospitalization rates for CAP were calculated for the corresponding periods of 2020 and 2017–19. Percentage changes in the antibacterial consumptions were calculated for corresponding periods of 2020 and 2019. 95% CI and the statistical significance of the percentage changes were calculated using the Poisson model for each period considered in the study. To compare ALOS, hospitalizations requiring ICU, and in-hospital mortality between 2017–2019 and 2020, age, sex, and CCI standardized ratios (SR) were calculated. The indirect standardization was performed using the patients hospitalized for CAP in 2017–19 as the standard population. For each analysis, an α level of 0.05 was considered as significant. The statistical software Stata 14 SE (StataCorp LP, College Station, Texas) was used for the data analyses.
Results
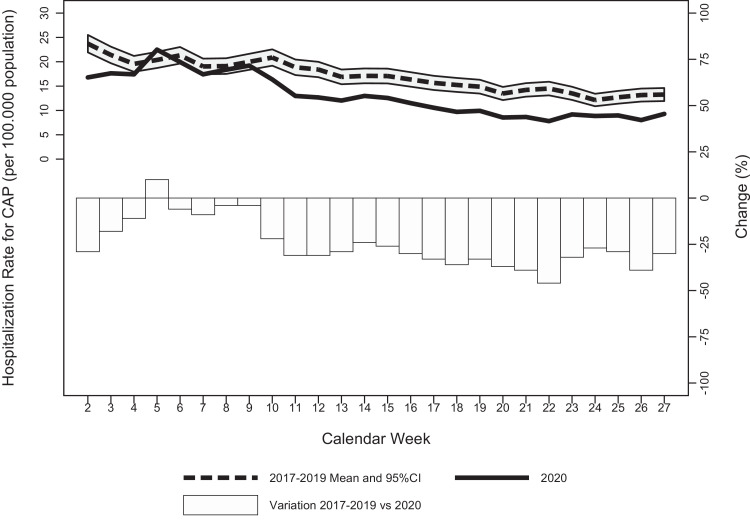
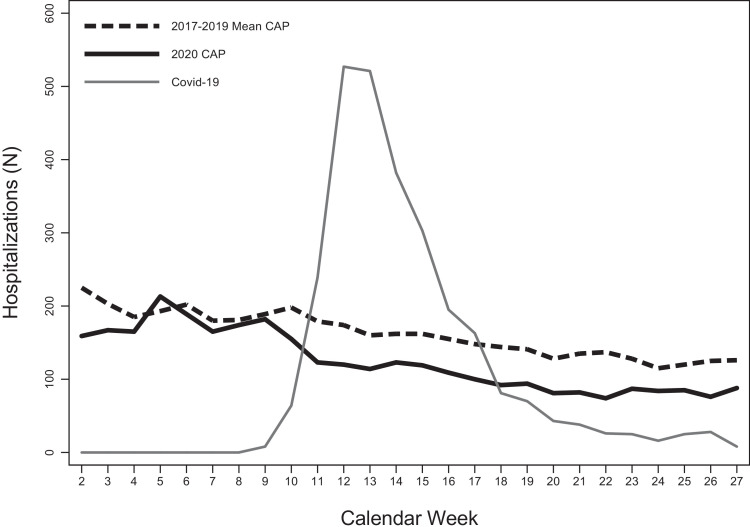
A total of 3346 new CAP hospitalizations occurred in people aged ≥65 in the study period (Week 1 to 27, 2020). Figure 1 shows the weekly hospitalization rates for CAP from Week 1 to 27 of 2020, the average weekly hospitalization rates for CAP observed in the previous 3 years, and the weekly number of hospitalizations for COVID-19 occurring in the Tuscany region. In the COVID-19 pre-epidemic period (Week 1 to 9, 2020), the weekly hospitalization rates for CAP were similar to the average weekly hospitalization rates registered in the previous 3 years, except for the first 3 weeks of the years (Figure 1A). However, for the COVID-19 epidemic period (Week 10 to 27, 2020), the weekly hospitalization rates for CAP were significantly lower than the average of the previous 3 years; the maximum decrease from the average weekly hospitalization rates of the previous 3 years was registered in Week 22 (-46%) (Figure 1A). The significant decrease in the weekly hospitalization rates for CAP started in Week 10—coinciding with the first major increase in the number of weekly COVID-19 hospitalizations (from 8 to 62) and with the implementation of the first national containment measures—and persisted after the lift of the national lockdown (Week 23) until the end of the study period (Week 27) (Figure 1A). By that time, the number of new COVID-19 hospitalizations had decreased to 7 (Figure 1B).
Figure 1.
A. Weekly hospitalization rates for CAP in 2017-2019 versus 2020 and relative changes in people aged 65 or older in Tuscany region
B. Number of hospitalizations for CAP (2017-2019 versus 2020) and for COVID-19 in people aged 65 or older in Tuscany region.
Table 1 reports the characteristics of hospitalizations and hospitalization rates for CAP in people aged ≥65 in the COVID-19 epidemic period and the corresponding period in 2017–2019. The hospitalization rate for CAP was 190.7 per 100 000 population during the COVID-19 epidemic period. Compared with the average in the corresponding period of the previous 3 years (2017–19), the hospitalization rate for CAP significantly decreased in the COVID-19 epidemic period (-31.5%; 95% CI -36%, -27%; P<0.001) (see Table 1). The decrease in the hospitalization rate for CAP was similar for men and women (-29.6% and -33.6%, respectively) and among the 3 age groups considered (-30.4%, -29.1% and -34.0% for 65–74 years, 75–84 and ≥85, respectively).
Table 1.
Temporal changes in the number of hospitalizations and hospitalization rates for CAP in people aged ≥65 in the Tuscany region during the COVID-19 epidemic period (Week 10 to 27, 2020)
| N | Hospitalization rate (x 100,000 population) | Hospitalization rate changes | |||||
|---|---|---|---|---|---|---|---|
| 2017-2019 Average | 2020 | 2017-2019 Average | 2020 | Change (%) | 95% CI | P value | |
| Total | 2636 | 1806 | 278.3 | 190.7 | -31.5 | -36; -27 | <0.001 |
| Sex | |||||||
| Male | 1379 | 971 | 335.9 | 236.5 | -29.6 | -35; -24 | <0.001 |
| Female | 1258 | 835 | 234.5 | 155.6 | -33.6 | -39; -27 | <0.001 |
| Age | |||||||
| 65–74 years | 481 | 335 | 108.0 | 75.2 | -30.4 | -39; -22 | <0.001 |
| 75–84 years | 1003 | 711 | 292.9 | 207.6 | -29.1 | -35; -22 | <0.001 |
| ≥85 years | 1152 | 760 | 722.8 | 476.9 | -34.0 | -40; -28 | <0.001 |
CAP: community-acquired pneumonia
In the pre-epidemic period, 46.1% and 9.4% of the CAP hospitalizations had a CCI of 0–1 and ≥5, respectively (see Table 2 ). The ALOS was 9.1 days, the percentage of hospitalizations requiring the ICU was 1.5%, and in-hospital mortality was 8.6%, and there were no significant differences from the same period in the previous 3 years (Table 2). In the COVID-19 epidemic period, 47% and 10.6% of the CAP hospitalizations had a CCI of 0–1 and ≥5, respectively (Table 2). The ALOS of CAP hospitalizations was 8.9 days, the percentage of hospitalizations requiring the ICU was 1.8%, and in-hospital mortality was 12.4%. Compared with the previous 3 years, the distribution of CAP hospitalizations by CCI did not significantly differ; however, significant increases in the percentage of CAP hospitalizations requiring ICU and in-hospital mortality were registered (+51%, 95% CI 1.7–122.83 and +42%, 95% CI 24.19–64.71, respectively). No significant differences in the ALOS of CAP hospitalizations were observed between the epidemic period and the corresponding period of the previous 3 years (Table 2).
Table 2.
Characteristics and clinical outcomes of CAP hospitalizations during the pre-epidemic (Week 1 to 9, 2020) and epidemic (Week 10 to 27, 2020) periods
| Pre-epidemic period | Epidemic period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2017-2019 | 2020 | Change (%) | 95% CI | P value | 2017-2019 | 2020 | Change (%) | 95% CI | P value | |
| Charlson Comorbidity Index (%) | ||||||||||
| 0–1 | 47.6 | 46.1 | -3.1 | -8.83; 3.03 | 0.316 | 45.2 | 47.0 | 3.8 | -1.6; 9.7 | 0.171 |
| 2–4 | 38.6 | 42.0 | 8.8 | 1.6; 16.4 | 0.016 | 40.4 | 40.5 | 0.1 | -5.8; 6.6 | 0.956 |
| ≥5 | 11.8 | 9.4 | -20.4 | -32.9; -5.3 | 0.027 | 11.8 | 10.6 | -10.1 | -22.3; 4.2 | 0.159 |
| Missing | 2.1 | 2.5 | 23.2 | -14.3; 77.1 | 0.261 | 2.6 | 1.9 | -24.9 | -47.5; 6.7 | 0.109 |
| Average length of stay (days) | 8.9 | 9.1 | 2.26 | -1.6; 6.2 | 0.262 | 8.9 | 8.9 | 0.2 | -3.4; 4.1 | 0.890 |
| Hospitalizations requiring ICU (%) | 1.6 | 1.5 | -6.9 | -41.2; 47.2 | 0.757 | 1.2 | 1.8 | 51.2 | 1.7; 122.8 | 0.041 |
| In-hospital mortality (%) | 10.2 | 8.6 | -15.7 | -29.9; 1.0 | 0.064 | 8.8 | 12.5 | 42.0 | 24.2; 64.7 | <0.001 |
CAP: community-acquired pneumonia; ICU: Intensive Care Unit
Table 3 reports the age, sex, and CCI standardized ratios for ALOS, hospitalizations requiring ICU, and in-hospital mortality. For these outcomes, the standardized ratios showed no significant difference between the observed and the expected results in the pre-epidemic period. In the COVID-19 epidemic period, the difference between the observed and the expected ALOS of CAP hospitalizations was not significant (SR 1.002, 95% CI 0.97–1.04, P=0.76). Although the observed CAP hospitalizations requiring ICU were higher than expected in the epidemic period, this difference was above the level of significance (SR 1.5, 95% CI 0.95–2.33 P=0.086). Lastly, the observed in-hospital mortality of CAP hospitalizations during the epidemic period was significantly higher than expected (SR 1.44, 95% CI 1.27–2.31, P<0.001).
Table 3.
Standardized ratios* for 2020 for ALOS, hospitalizations requiring ICU, and in-hospital mortality of CAP hospitalizations
| Pre-epidemic period | Epidemic period | |||||
|---|---|---|---|---|---|---|
| Standardized ratio* | 95% CI | P value | Standardized ratio* | 95% CI | P value | |
| Average length of stay | 1.02 | 0.99; 1.05 | 0.07 | 1.002 | 0.97; 1.04 | 0.76 |
| Hospitalizations requiring ICU | 0.92 | 0.77; 1.63 | 0.55 | 1.5 | 0.95; 2.33 | 0.086 |
| In-hospital mortality | 0.83 | 0.66; 1.07 | 0.15 | 1.44 | 1.27; 2.31 | <0.001 |
ALOS: average length of stay; ICU: Intensive Care Unit; CAP: community-acquired pneumonia
Ratio standardized for age, sex, and Charlson Comorbidity Index. Standard population: patients hospitalized for CAP in 2017-2019 in the two periods
Table 4 reports data concerning CAP antibiotic consumption in the outpatient setting in people aged ≥65 in the Tuscany region. The percentage change in the dispensed DDD for all the selected antibacterial drugs for the treatment of CAP from 2019 to 2020 was -13.1% (95% CI -29%, +3%) in the COVID-19 pre-epidemic period and -34.7% (95% CI -43%, -25%) in the COVID-19 epidemic period. During the COVID-19 pre-epidemic period, only fluoroquinolones showed a significant decrease in the dispensed DDD compared with the previous year (percentage change: -35.3%; 95% CI -47%, -23%). Conversely, during the COVID-19 epidemic period, all the selected antibacterial drugs showed a significant decrease in the dispensed DDD compared with the previous year; fluoroquinolones and macrolides showed the minimum and maximum reductions, respectively (percentage changes: -30.7%; 95% CI -40%, -21% and -46%; 95% CI -56%, -39%, respectively).
Table 4.
CAP Antibiotic consumption (DDD) in the outpatient setting in people aged ≥65 in the Tuscany region
| Pre-epidemic period | Epidemic period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DDD x 1000 population/ day | Change (%) | 95% CI | Pvalue | DDD x 1000 population/ day | Change (%) | 95% CI | P value | |||
| 2019 | 2020 | 2019 | 2020 | |||||||
| Overall antibacterial consumptions | 26.2 | 22.8 | -13.1 | -33; 3 | 0.026 | 18.5 | 12.0 | -34.7 | -45; -28 | 0.001 |
| 65–74 | 23.3 | 20.4 | -12.6 | -33; 2 | 0.032 | 16.4 | 10.2 | -37.6 | -48; -31 | 0.001 |
| 75–84 | 26.7 | 23.1 | -13.3 | -33; 3 | 0.025 | 18.8 | 12.2 | -35.3 | -46; -29 | 0.001 |
| ≥85 | 33.5 | 28.9 | -13.6 | -32; 3 | 0.019 | 23.5 | 16.9 | -28.3 | -39; -21 | 0.001 |
| Penicillins | 11.6 | 10.7 | -7.3 | -29; 2 | 0.082 | 8.8 | 6.0 | -31.9 | -45; -29 | 0.001 |
| 65–74 | 10.9 | 10.1 | -7.3 | -29; 3 | 0.096 | 8.3 | 5.4 | -34.0 | -47; -31 | 0.001 |
| 75–84 | 11.5 | 10.6 | -8.2 | -30; 1 | 0.062 | 8.8 | 5.9 | -32.5 | -45; -29 | 0.001 |
| ≥85 | 13.4 | 12.6 | -5.6 | -27; 3 | 0.103 | 10.2 | 7.6 | -25.9 | -40; -23 | 0.001 |
| Cephalosporins | 4.4 | 4.1 | -5.7 | -30; 2 | 0.075 | 3.1 | 2.0 | -36.7 | -50; -34 | 0.001 |
| 65–74 | 3.1 | 2.9 | -4.2 | -30; 3 | 0.101 | 2.2 | 1.2 | -43.5 | -55; -39 | 0.001 |
| 75–84 | 4.5 | 4.3 | -4.3 | -30; 3 | 0.091 | 3.2 | 2.0 | -37.1 | -51; -35 | 0.001 |
| ≥85 | 7.9 | 7.2 | -8.9 | -31; 0 | 0.045 | 5.6 | 4.0 | -28.6 | -43; -27 | 0.001 |
| Fluoroquinolones | 5.6 | 3.6 | -35.3 | -47; -23 | 0.001 | 3.6 | 2.5 | -30.7 | -40; -21 | 0.001 |
| 65–74 | 4.8 | 3.1 | -35.6 | -48; -23 | 0.001 | 3.0 | 2.0 | -33.5 | -42; -23 | 0.001 |
| 75–84 | 6.0 | 3.9 | -34.5 | -47; -22 | 0.001 | 3.8 | 2.6 | -31.6 | -40; -22 | 0.001 |
| ≥85 | 7.2 | 4.6 | -36.2 | -48; -25 | 0.001 | 4.5 | 3.5 | -23.8 | -33; -13 | 0.001 |
| Macrolides | 4.7 | 4.3 | -7.5 | -28; 7 | 0.201 | 3.0 | 1.6 | -46.0 | -56; -39 | 0.001 |
| 65–74 | 4.5 | 4.2 | -6.6 | -29; 6 | 0.175 | 2.9 | 1.5 | -47.5 | -58; -41 | 0.001 |
| 75–84 | 4.7 | 4.3 | -7.3 | -27; 8 | 0.231 | 3.0 | 1.6 | -46.5 | -55; -38 | 0.001 |
| ≥85 | 5.1 | 4.5 | -10.3 | -25; 8 | 0.247 | 3.2 | 1.9 | -41.6 | -50; -32 | 0.001 |
CAP: community-acquired pneumonia; DDD: defined daily doses
Discussion
Our study aimed to evaluate the secondary impact of large-scale containment measures for SARS-CoV-2 on the incidence of CAP in older people during the first wave of COVID-19 in the Tuscany region (February–July 2020). To assess the impact on the incidence of CAP in older people, hospital admissions with a primary diagnosis of CAP occurring in all the hospitals in the Tuscany healthcare system were evaluated and compared with the average of the previous 3 years. In addition, the average hospital length of stay, hospitalizations requiring ICU, and in-hospital mortality of patients admitted with CAP were assessed to evaluate differences in the characteristics and severity of the CAP hospitalizations. Furthermore, changes in the consumption of antibacterials (dispensed DDD) indicated for the treatment of CAP were evaluated as a proxy for less severe CAP cases occurring in the outpatient setting. Results of the study highlighted a significant reduction of the hospitalization rates for CAP during the epidemic period in the Tuscany region, compared with the average of the previous 3 years. The observed reduction of hospitalization rates during the epidemic period was similar between sexes and among different age groups. Furthermore, in-hospital mortality for CAP was significantly higher in the epidemic period than expected from previous years. Lastly, consistent reduction of consumption across all antibacterial classes indicated to treat CAP were observed in the outpatient settings during the epidemic period.
These findings suggest a significant and generalized reduction of CAP in older people during the COVID-19 epidemic period. The most plausible explanation for this reduction is the interruption of person-to-person transmission of CAP pathogens due to the effectiveness of containment measures to curb the COVID-19 pandemic. Indeed, the timing for implementation of these measures coincided with a significant reduction in the hospitalization rate for CAP, i.e., the first national containment measures such as school and university closures and movement restrictions occurred in the same week in which the first significant negative deviation in the trend of CAP hospitalization rates was observed. Interestingly, the significant deviation from previous years in the hospitalization rate for CAP remained even after the lift of the national lockdown until the end of the first wave of the COVID-19 epidemic in July. These results may indicate that prevention measures that remained mandatory after the lift of the national lockdown—namely, the physical distancing rules and obligation to wear a face mask when leaving home—may have played a role in reducing CAP incidence.
It should be noted that—especially in the first phases of the COVID-19 outbreak—the reduction of CAP hospitalizations in older people may have been influenced by a shift of the provision of care for less severe cases to the outpatient setting. Indeed, it is plausible that less severe patients, with or without consultation with their physicians, decided to defer or avoid seeking hospital care during the pandemic (Birkmeyer et al., 2020; Lu et al., 2007). However, if less seriously ill patients were disproportionately treated in the outpatient setting during the COVID-19 pandemic, an increase in antibacterial consumption would have been expected instead of the relevant and significant decrease observed. Therefore, if cases had shifted from the hospital to the outpatient setting, it was minimal and appeared to have been largely outstripped by the overall reduction in CAP cases. However, as most of the CAP antibacterials would also be used for bronchitis and acute exacerbations of chronic obstructive pulmonary disease, the observed reduction in consumption is also consistent with an overall reduced rate of lower respiratory tract infections in the community rather than CAP specifically. In light of this, it is reasonable to speculate that containment measures may have beneficially reduced the transmission of other, non-pandemic infections and that the respiratory infection burden is likely to increase as COVID-19 containment measures are lifted. Indeed, this hypothesis is confirmed by preliminary findings from infection surveillance studies reporting a significant decrease of circulation of respiratory pathogens, such as seasonal influenza, Streptococcus pneumonia and Haemophilus influenza, following the introduction of COVID-19 containment strategies and public health campaigns (Chiu et al., 2020; Brueggemann et al., 2021; Sakamoto et al., 2020).
The increased in-hospital mortality rate in patients hospitalized for CAP during the epidemic period cannot be ascribed to the lack of available resources to manage critical patients due to COVID-19 because the TPHCS never experienced a lack of availability of ICU beds. However, it may be due to patients staying away from hospitals for fear of contagion. Indeed, during the COVID-19 pandemic, people—especially older people—may have been weighing the risks of seeking out medical care against potentially contracting SARS-CoV-2; these concerns of contagion may have increased the in-hospital mortality rate for 2 different but interrelated reasons. First, less seriously ill patients disproportionately stay away from the hospital compared with more severely ill patients. Second, patients who delay seeking care put themselves at risk of serious complications and are eventually admitted to hospital with more severe illness (De rosa et al., 2020; Hafner 2020; Solomon et al., 2020). If related to a delay in seeking care, the finding of increased in-hospital mortality highlights the need to ensure that patients with CAP requiring hospital care obtain it during a pandemic. Another possible explanation for the increased in-hospital mortality rate is that a disproportionate reduction in CAP caused by pathogens associated with lower mortality and complication occurred, e.g., a reduction in the rate of viral CAP compared with bacterial CAP. However, further studies evaluating the rate of CAP by type of pathogen are needed to confirm this hypothesis.
During a pandemic, decisions should be made to balance the demands of responding directly to the pandemic with simultaneously engaging in strategic planning and coordinated action to maintain essential health service delivery, mitigating the risk of system collapse. The described indirect impact of pandemic containment measures on respiratory tract infections provides useful information for health services planning and provision during the pandemic. In particular, the findings of a reduced CAP hospital caseload may be useful to guide strategies for optimizing health workforce capacity across health services. Indeed, during pandemics, health systems face predictable health workforce challenges, including the redistribution of staff to treat increasing numbers of pandemic patients and the loss of staff who may be quarantined or infected. Furthermore, the described potential for delay in seeking care for fear of contagion suggests the need to strengthen primary care support, to implement adaptive responses (e.g., teleconsultation, integrated primary care, clear referral pathways) and ensure public awareness and sustainability of health services for CAP patients.
The present study has several strengths and limitations. A strength of the study is that the sample represents virtually the whole population of an administrative region with approximately 3.7 million inhabitants and a wide and varied geographical area. In particular, the data analyzed could be considered representative of all the hospital care that has been provided to the study population as the regional public health system provides healthcare free of charge for the entire population and private providers play a minimal role, especially in the provision of acute hospital care (Lastrucci et al., 2018). Similarly, data of antibacterial consumption in the outpatient setting could be considered representative of the overall consumption of Tuscany inhabitants, as the purchase of antibacterials requires a registered prescription from the general practitioner. Lastly, the study adopted covered a period that included the whole of the first wave of SARS-CoV-2, which allowed comparison between CAP incidence and the SARS-CoV-2 epidemic situation and related containment measures. The present study has some limitations. First, although results showed a close association between containment measures and a decrease in CAP, the study design does not allow us to assure the causality link between the two phenomena. Second, it was not possible to establish the independent effect of each of the individual containment measures as these were implemented almost simultaneously in the region. Third, although the study results accurately represent the hospitalization rate for CAP in the older population, it was not possible to accurately measure the total incidence of CAP in the region as less severe cases treated exclusively in the outpatient setting are not registered in the regional information system. The outpatient consumption of antibacterial drugs for CAP may be considered as a proxy for the incidence of CAP in the outpatient setting only to a limited extent. Nevertheless, it should be underlined that antibacterial consumption in the outpatient setting allowed us to consider CAP cases for both bacterial and viral pathogens. Indeed, empiric antibacterial treatments in the outpatient setting are recommended in almost all the patients with CAP because bacterial pathogens often coexist with viruses, and the current diagnostic tests are not accurate or fast enough to ascertain whether CAP is due solely to a virus at the time of presentation (Metlay et al., 2019). Lastly, as the study was based on routinely collected administrative data, it was not possible to control the quality of the case determination (i.e., the accuracy in case coding). However, the quality of TPHCS administrative data is very high in terms of accuracy and reliability because the data are audited each year.
Conclusion
In conclusion, significant decreases in the hospitalization rate and the outpatient consumption of antibacterials for CAP were observed in the older population during the first wave of the COVID-19 pandemic. The timing of these decreases largely coincided with the timing of implementation of initial epidemic containment measures. These findings suggest that the potential benefits of pandemic containment measures can extend beyond the prevention of COVID-19 to include reducing the burden of respiratory infectious diseases. Furthermore, results showed a significant increase in in-hospital mortality in patients hospitalized for CAP during the pandemic; such increases may be linked to a delay in seeking care by patients who are afraid of contracting SARS-CoV-2 in healthcare settings; thus, efforts should be made to ensure that patients can obtain required hospital care during a pandemic. The above-described indirect effects of SARS-CoV-2 containment measures have major implications for health services planning and provision during pandemics. Further studies are needed to confirm and characterize these indirect effects of SARS-CoV-2 containment measures on CAP in the older population and to clarify better the individual role played by each prevention measure.
Acknowledgments
Ethical approval
Not required.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995;24:1618–1624. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The Impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020;39(11):2010–2017. doi: 10.1377/hlthaff.2020.00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann AB, Jansen van Rensburg MJ, Shaw D, McCarthy N, Jolley KA, Maiden MCJ, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3(6):e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KPF, Ma TF, Kwok WC, Leung JKC, Chiang KY, Ho JCM, et al. Significant reduction in hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in Hong Kong during coronavirus disease 2019 pandemic. Respir Med. 2020;171 doi: 10.1016/j.rmed.2020.106085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu NC, Chi H, Tai YL, Peng CC, Tseng CY, Chen CC, et al. Impact of Wearing Masks, Hand Hygiene, and Social Distancing on Influenza, Enterovirus, and All-Cause Pneumonia During the Coronavirus Pandemic: Retrospective National Epidemiological Surveillance Study. J Med Internet Res. 2020;22(8):e21257. doi: 10.2196/21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman C. Pneumonia in the elderly. Clin Chest Med. 1999;20(3):563–573. doi: 10.1016/S0272-5231(05)70236-7. [DOI] [PubMed] [Google Scholar]
- Fernández-Sabé N, Carratalà J, Rosón B, Dorca J, Verdaguer R, Manresa F, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine. 2003;82(3):159–169. doi: 10.1097/01.md.0000076005.64510.87. [DOI] [PubMed] [Google Scholar]
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner K. Fear of Covid-19 leads other patients to decline critical treatment. New York Times. 2020 May 25 [Google Scholar]
- Kuo SC, Tsou HH, Wu HY, Hsu YT, Lee FJ, Shih SM, Hsiung CA, Chen WJ. Nonpolio Enterovirus Activity during the COVID-19 Pandemic, Taiwan, 2020. Emerg Infect Dis. 2021;27(1):306–308. doi: 10.3201/eid2701.203394. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. Pneumonia guidelines committee of the BTS standards of care committee. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(S3) doi: 10.1136/thx.2009.121434. iii1-55https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Lastrucci V, Lorini C, Del Riccio M, Gori E, Chiesi F, Sartor G, et al. SARS-CoV-2 Seroprevalence Survey in People Involved in Different Essential Activities during the General Lock-Down Phase in the Province of Prato (Tuscany, Italy) Vaccines. 2020;8(4):778. doi: 10.3390/vaccines8040778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastrucci V, D'Arienzo S, Collini F, Lorini C, Zuppiroli A, Forni S, et al. Diagnosis-related differences in the quality of end-of-life care: A comparison between cancer and non-cancer patients. Plos One. 2018;13(9) doi: 10.1371/journal.pone.0204458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TH, Chou YJ, Liou CS. Impact of SARS on healthcare utilization by disease categories: implications for delivery of healthcare services. Health Policy. 2007;83(2-3):375–381. doi: 10.1016/j.healthpol.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Mancini P, Ferraro GB, Veneri C, Iaconelli M, Bonadonna L, et al. SARS-CoV-2 has been circulating in northern Italy since December 2019: Evidence from environmental monitoring. Sci Total Environ. 2020;750 doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195(7):1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. American Journal of Epidemiology. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ishikane M, Ueda P. Seasonal Influenza Activity During the SARS-CoV-2 Outbreak in Japan. JAMA. 2020;323(19):1969–1971. doi: 10.1001/jama.2020.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon A, Berry I, Tuite AR, Drews S, Hatchette T, Jamieson F, et al. Influenza increases invasive meningococcal disease risk in temperate countries. Clin Microbiol Infect. 2020;26(9) doi: 10.1016/j.cmi.2020.01.004. 1257.e1-1257.e7 https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- Sullivan SG, Carlson S, Cheng AC, Chilver MB, Dwyer DE, Irwin M, Kok J, Macartney K, MacLachlan J, Minney-Smith C, Smith D, Stocks N, Taylor J, Barr IG. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25(47) doi: 10.2807/1560-7917.ES.2020.25.47.2001847. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscany Regional Therapeutic Commission. Linee di indirizzo di terapia antibiotica nella pratica del medico di Medicina Generale (paziente adulto). Commissione terapeutica regionale. Regione toscana 12/07/2017
- Wolter N, Tempia S, Cohen C, Madhi SA, Venter M, Moyes J, et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis. 2014;210(10):1649–1657. doi: 10.1093/infdis/jiu326. [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment, 2020. Oslo, Norway, 2020.
- World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it, 2020 (Accessed 21 February 2021).
- World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020, 2020 (Accessed 21 February 2021)
- Wu D, Lu J, Cao L, Ma X, Liu Q, Liu Y, Zhang Z. Positive effects of COVID-19 control measures on pneumonia prevention. Int J Infect Dis. 2020;96:548–549. doi: 10.1016/j.ijid.2020.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Komiya K, Fujita N, Okabe E, Hiramatsu K, Kadota JI. COVID-19 pandemic and the incidence of community-acquired pneumonia in elderly people. Respir Investig. 2020;58(6):435–436. doi: 10.1016/j.resinv.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]